
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Metabolic Risk/Epidemiology
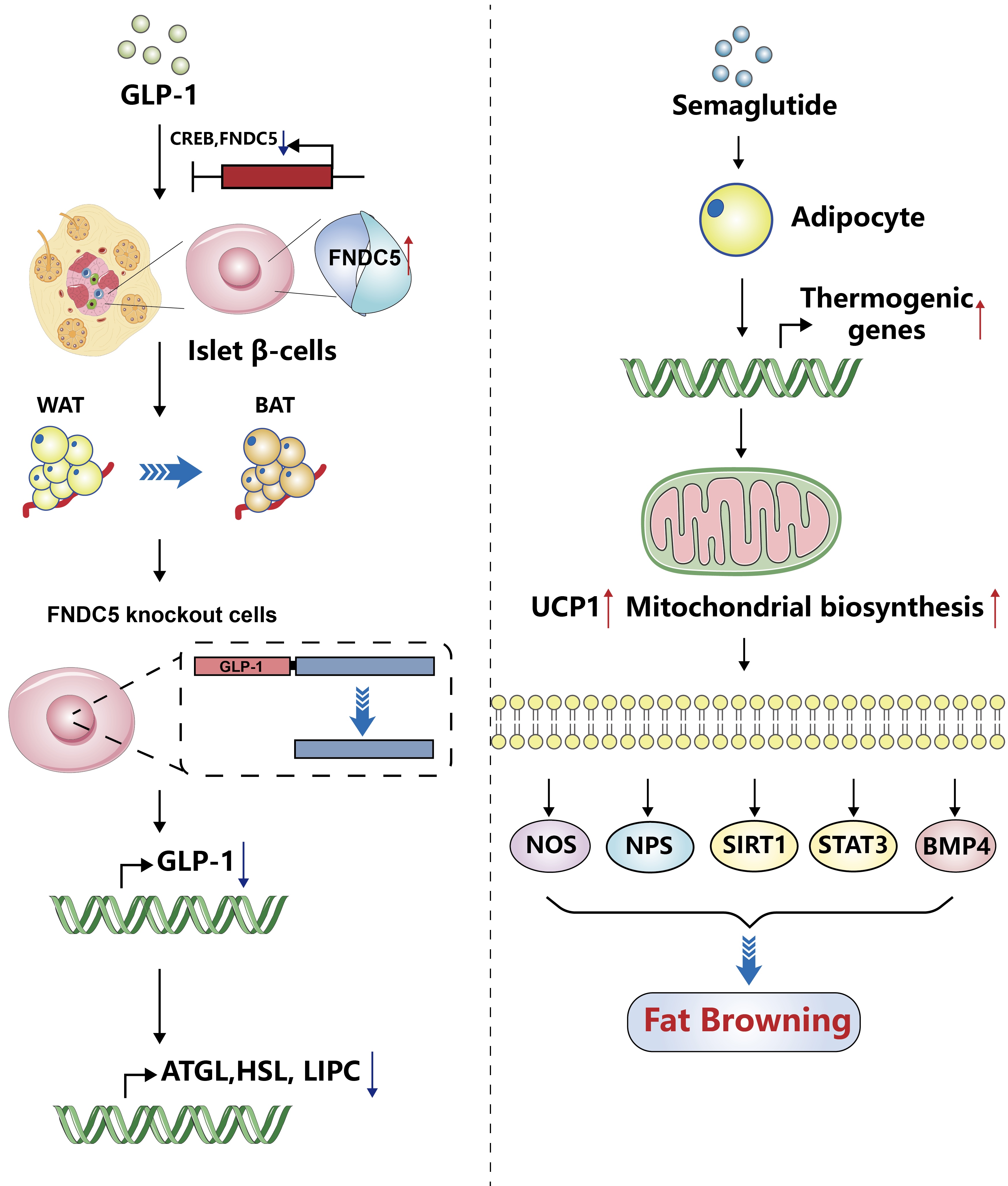
- Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism
- Tong Bu, Ziyan Sun, Yi Pan, Xia Deng, Guoyue Yuan
- Received August 14, 2023 Accepted January 1, 2024 Published online April 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0277 [Epub ahead of print]
- 487 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Glucagon-like peptide-1 (GLP-1) is a 30-amino acid peptide hormone that is mainly expressed in the intestine and hypothalamus. In recent years, basic and clinical studies have shown that GLP-1 is closely related to lipid metabolism, and it can participate in lipid metabolism by inhibiting fat synthesis, promoting fat differentiation, enhancing cholesterol metabolism, and promoting adipose browning. GLP-1 plays a key role in the occurrence and development of metabolic diseases such as obesity, nonalcoholic fatty liver disease, and atherosclerosis by regulating lipid metabolism. It is expected to become a new target for the treatment of metabolic disorders. The effects of GLP-1 and dual agonists on lipid metabolism also provide a more complete treatment plan for metabolic diseases. This article reviews the recent research progress of GLP-1 in lipid metabolism.
- Metabolic Risk/Epidemiology
- Biologically Informed Polygenic Scores for Brain Insulin Receptor Network Are Associated with Cardiometabolic Risk Markers and Diabetes in Women
- Jannica S. Selenius, Patricia P. Silveira, Mikaela von Bonsdorff, Jari Lahti, Hannu Koistinen, Riitta Koistinen, Markku Seppälä, Johan G. Eriksson, Niko S. Wasenius
- Received February 10, 2023 Accepted November 25, 2023 Published online March 25, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0039 [Epub ahead of print]

- 709 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate associations between variations in the co-expression-based brain insulin receptor polygenic score and cardiometabolic risk factors and diabetes mellitus.
Methods
This cross-sectional study included 1,573 participants from the Helsinki Birth Cohort Study. Biologically informed expression-based polygenic risk scores for the insulin receptor gene network were calculated for the hippocampal (hePRS-IR) and the mesocorticolimbic (mePRS-IR) regions. Cardiometabolic markers included body composition, waist circumference, circulating lipids, insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding protein 1 and 3 (IGFBP-1 and -3). Glucose and insulin levels were measured during a standardized 2-hour 75 g oral glucose tolerance test and impaired glucose regulation status was defined by the World Health Organization 2019 criteria. Analyzes were adjusted for population stratification, age, smoking, alcohol consumption, socioeconomic status, chronic diseases, birth weight, and leisure-time physical activity.
Results
Multinomial logistic regression indicated that one standard deviation increase in hePRS-IR was associated with increased risk of diabetes mellitus in all participants (adjusted relative risk ratio, 1.17; 95% confidence interval, 1.01 to 1.35). In women, higher hePRS-IR was associated with greater waist circumference and higher body fat percentage, levels of glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, triglycerides, apolipoprotein B, insulin, and IGFBP-1 (all P≤0.02). The mePRS-IR was associated with decreased IGF-1 level in women (P=0.02). No associations were detected in men and studied outcomes.
Conclusion
hePRS-IR is associated with sex-specific differences in cardiometabolic risk factor profiles including impaired glucose regulation, abnormal metabolic markers, and unfavorable body composition in women.
- Basic Research
- Roles of Histone Deacetylase 4 in the Inflammatory and Metabolic Processes
- Hyunju Kang, Young-Ki Park, Ji-Young Lee, Minkyung Bae
- Received June 5, 2023 Accepted February 7, 2024 Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0174 [Epub ahead of print]

- 637 View
- 33 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Histone deacetylase 4 (HDAC4), a class IIa HDAC, has gained attention as a potential therapeutic target in treating inflammatory and metabolic processes based on its essential role in various biological pathways by deacetylating non-histone proteins, including transcription factors. The activity of HDAC4 is regulated at the transcriptional, post-transcriptional, and post-translational levels. The functions of HDAC4 are tissue-dependent in response to endogenous and exogenous factors and their substrates. In particular, the association of HDAC4 with non-histone targets, including transcription factors, such as myocyte enhancer factor 2, hypoxia-inducible factor, signal transducer and activator of transcription 1, and forkhead box proteins, play a crucial role in regulating inflammatory and metabolic processes. This review summarizes the regulatory modes of HDAC4 activity and its functions in inflammation, insulin signaling and glucose metabolism, and cardiac muscle development.
- Pathophysiology
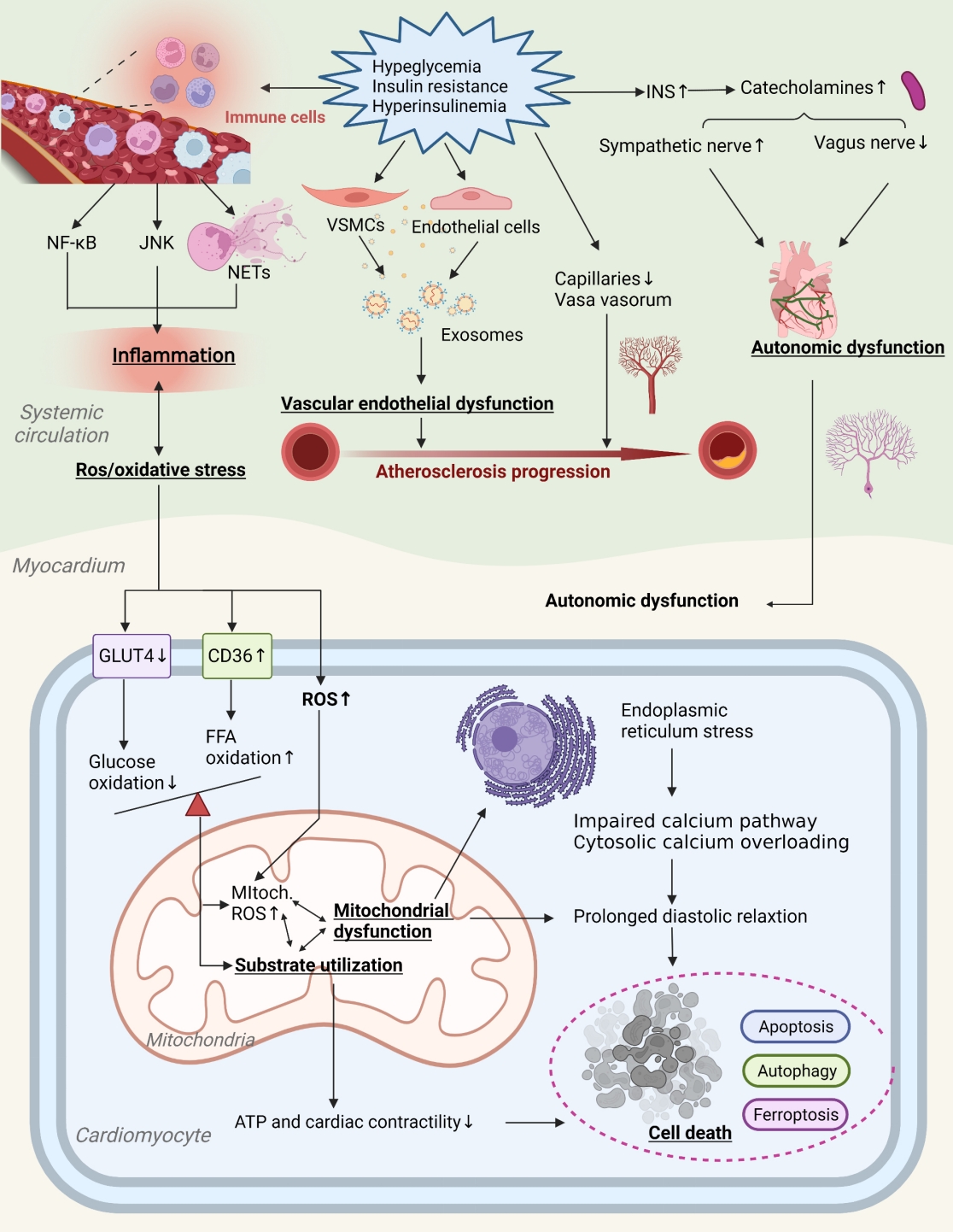
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- 2,167 View
- 182 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Basic Research
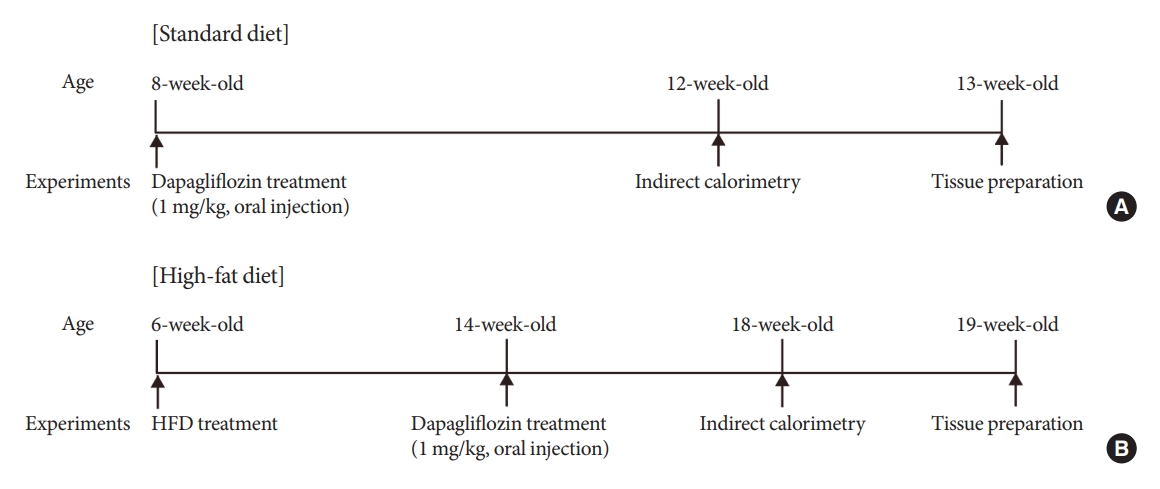
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2023;47(6):784-795. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0261
- 1,410 View
- 149 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are currently used to treat patients with diabetes. Previous studies have demonstrated that treatment with SGLT-2 inhibitors is accompanied by altered metabolic phenotypes. However, it has not been investigated whether the hypothalamic circuit participates in the development of the compensatory metabolic phenotypes triggered by the treatment with SGLT-2 inhibitors.
Methods
Mice were fed a standard diet or high-fat diet and treated with dapagliflozin, an SGLT-2 inhibitor. Food intake and energy expenditure were observed using indirect calorimetry system. The activity of hypothalamic neurons in response to dapagliflozin treatment was evaluated by immunohistochemistry with c-Fos antibody. Quantitative real-time polymerase chain reaction was performed to determine gene expression patterns in the hypothalamus of dapagliflozin-treated mice.
Results
Dapagliflozin-treated mice displayed enhanced food intake and reduced energy expenditure. Altered neuronal activities were observed in multiple hypothalamic nuclei in association with appetite regulation. Additionally, we found elevated immunosignals of agouti-related peptide neurons in the paraventricular nucleus of the hypothalamus.
Conclusion
This study suggests the functional involvement of the hypothalamus in the development of the compensatory metabolic phenotypes induced by SGLT-2 inhibitor treatment. -
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
Diabetes & Metabolism Journal.2024; 48(1): 159. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Basic Research
- Adipose Tissue and Metabolic Health
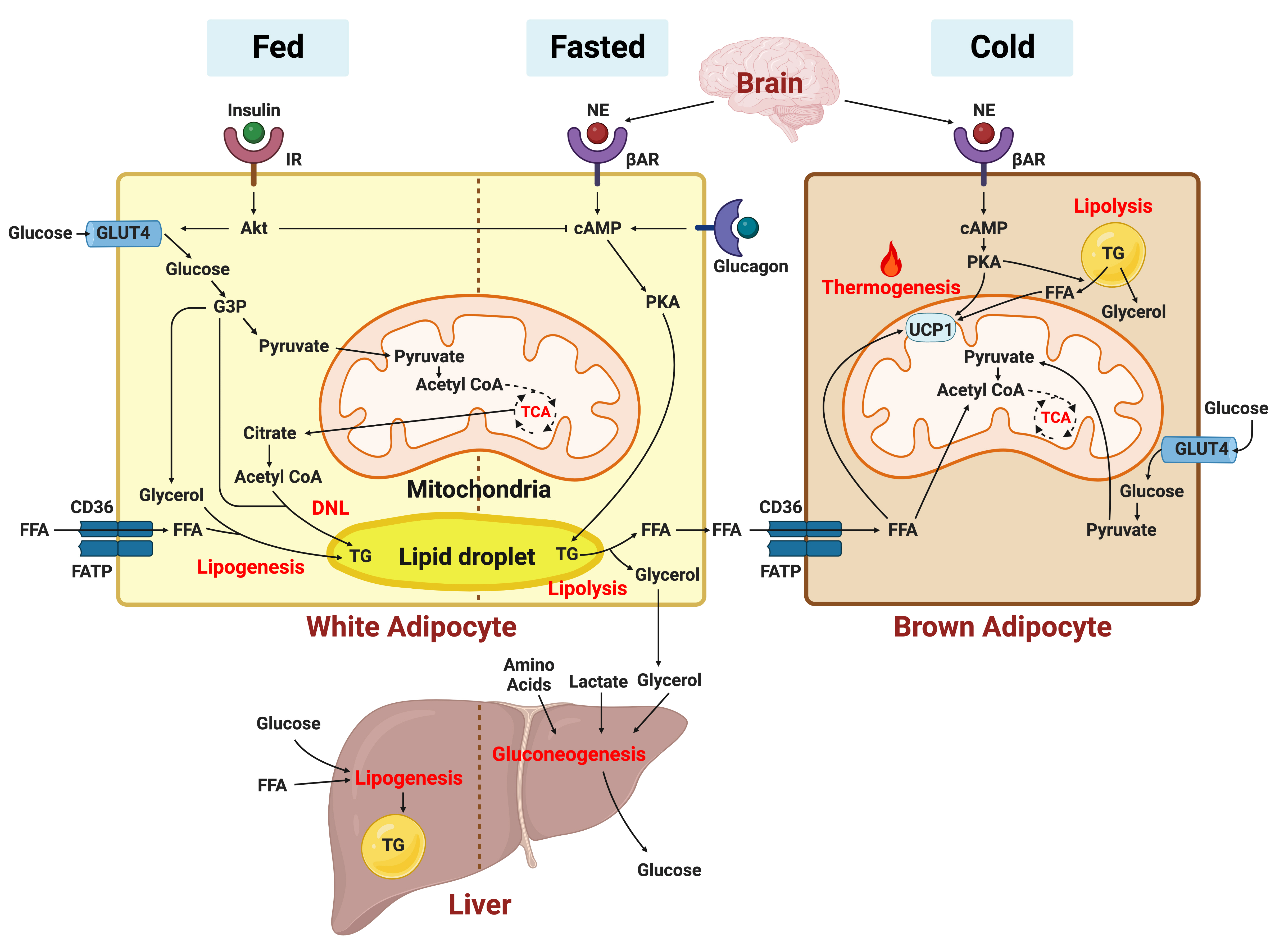
- Sung-Min An, Seung-Hee Cho, John C. Yoon
- Diabetes Metab J. 2023;47(5):595-611. Published online July 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0011
- 3,854 View
- 442 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - In this review, we provide a brief synopsis of the connections between adipose tissue and metabolic health and highlight some recent developments in understanding and exploiting adipocyte biology. Adipose tissue plays critical roles in the regulation of systemic glucose and lipid metabolism and secretes bioactive molecules possessing endocrine, paracrine, and autocrine functions. Dysfunctional adipose tissue has a detrimental impact on metabolic health and is intimately involved in key aspects of metabolic diseases such as insulin resistance, lipid overload, inflammation, and organelle stress. Differences in the distribution of fat depots and adipose characteristics relate to divergent degrees of metabolic dysfunction found in metabolically healthy and unhealthy obese individuals. Thermogenic adipocytes increase energy expenditure via mitochondrial uncoupling or adenosine triphosphate-consuming futile substrate cycles, while functioning as a metabolic sink and participating in crosstalk with other metabolic organs. Manipulation of adipose tissue provides a wealth of opportunities to intervene and combat the progression of associated metabolic diseases. We discuss current treatment modalities for obesity including incretin hormone analogs and touch upon emerging strategies with therapeutic potential including exosome-based therapy, pharmacological activation of brown and beige adipocyte thermogenesis, and administration or inhibition of adipocyte-derived factors.
-
Citations
Citations to this article as recorded by- Pharmacological targets at the lysosomal autophagy–NLRP3 inflammasome crossroads
Srinivasa Reddy Bonam, Dylan Mastrippolito, Philippe Georgel, Sylviane Muller
Trends in Pharmacological Sciences.2024; 45(1): 81. CrossRef - Senescent adipocytes and type 2 diabetes – current knowledge and perspective concepts
Weronika Kruczkowska, Julia Gałęziewska, Mateusz Kciuk, Adrianna Gielecińska, Elżbieta Płuciennik, Zbigniew Pasieka, Lin-Yong Zhao, Yi-Jin Yu, Damian Kołat, Żaneta Kałuzińska-Kołat
Biomolecular Concepts.2024;[Epub] CrossRef - Visceral Adipose Tissue: The Hidden Culprit for Type 2 Diabetes
Sneha Dhokte, Krzysztof Czaja
Nutrients.2024; 16(7): 1015. CrossRef - Beyond the Cold: Activating Brown Adipose Tissue as an Approach to Combat Obesity
Cristina Elena Negroiu, Iulia Tudorașcu, Cristina Maria Bezna, Sanziana Godeanu, Marina Diaconu, Raluca Danoiu, Suzana Danoiu
Journal of Clinical Medicine.2024; 13(7): 1973. CrossRef
- Pharmacological targets at the lysosomal autophagy–NLRP3 inflammasome crossroads
- Others
- Change Profiles and Functional Targets of MicroRNAs in Type 2 Diabetes Mellitus Patients with Obesity
- Guanhua Lu, Huanhuan Gao, Zhiyong Dong, Shuwen Jiang, Ruixiang Hu, Cunchuan Wang
- Diabetes Metab J. 2023;47(4):559-570. Published online April 25, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0226
- 1,707 View
- 76 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
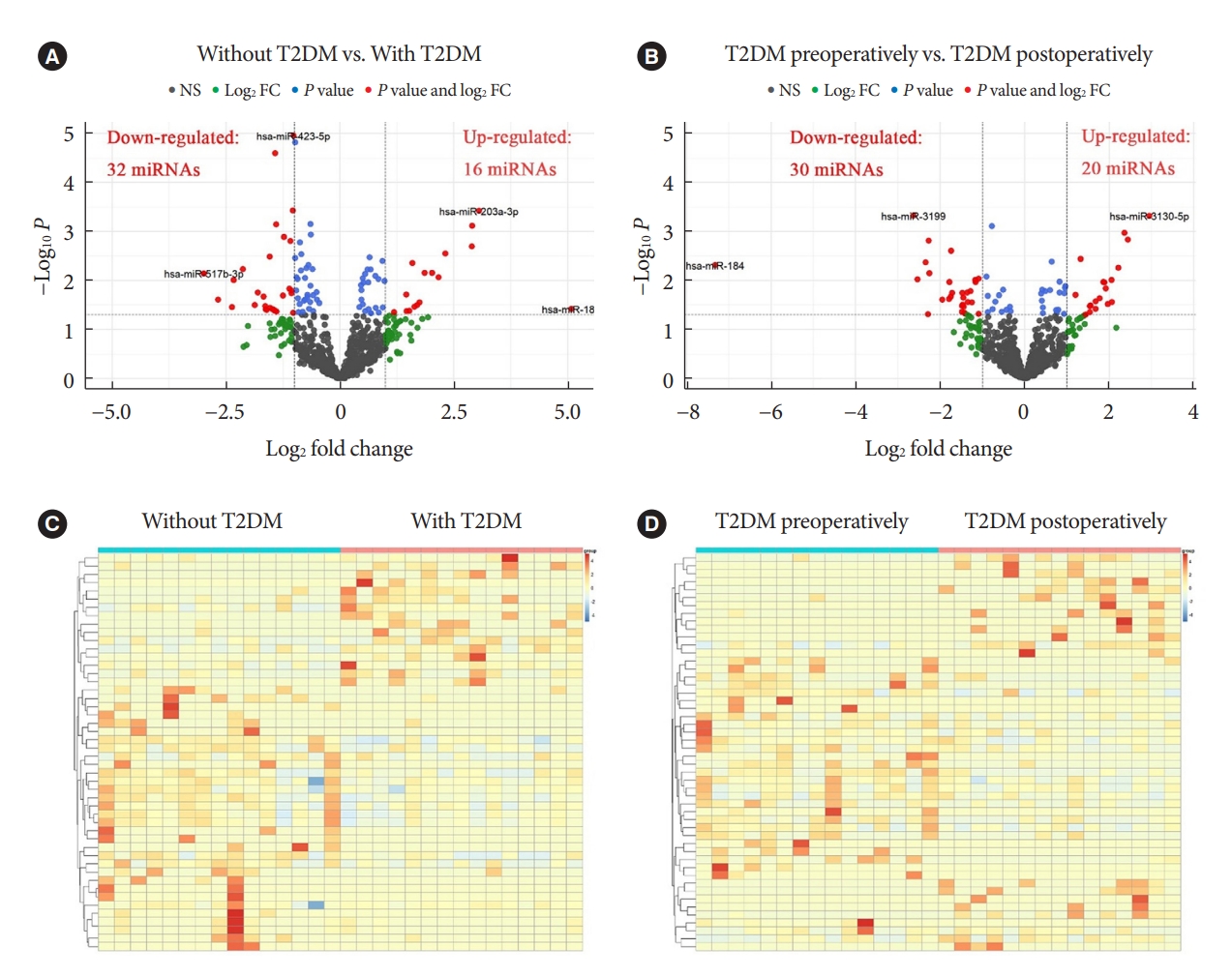
MicroRNAs (miRNAs) exert an essential contribution to obesity and type 2 diabetes mellitus (T2DM). This study aimed to investigate the differences of miRNAs in the presence and absence of T2DM in patients with obesity, as well as before and after bariatric surgery in T2DM patients with obesity. Characterization of the common changes in both was further analyzed.
Methods
We enrolled 15 patients with obesity but without T2DM and 15 patients with both obesity and T2DM. Their preoperative clinical data and serum samples were collected, as well as 1 month after bariatric surgery. The serum samples were analyzed by miRNA sequencing, and the miRNAs profiles and target genes characteristics were compared.
Results
Patients with T2DM had 16 up-regulated and 32 down-regulated miRNAs compared to patients without T2DM. Improvement in metabolic metrics after bariatric surgery of T2DM patients with obesity was correlated with changes in miRNAs, as evidenced by the upregulation of 20 miRNAs and the downregulation of 30 miRNAs. Analysis of the two miRNAs profiles identified seven intersecting miRNAs that showed opposite changes. The target genes of these seven miRNAs were substantially enriched in terms or pathways associated with T2DM.
Conclusion
We determined the expression profiles of miRNAs in the obese population, with and without diabetes, before and after bariatric surgery. The miRNAs that intersected in the two comparisons were discovered. Both the miRNAs discovered and their target genes were closely associated with T2DM, demonstrating that they might be potential targets for the regulation of T2DM.
- Others
- Current Trends of Big Data Research Using the Korean National Health Information Database
- Mee Kyoung Kim, Kyungdo Han, Seung-Hwan Lee
- Diabetes Metab J. 2022;46(4):552-563. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0193
- 5,727 View
- 277 Download
- 32 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
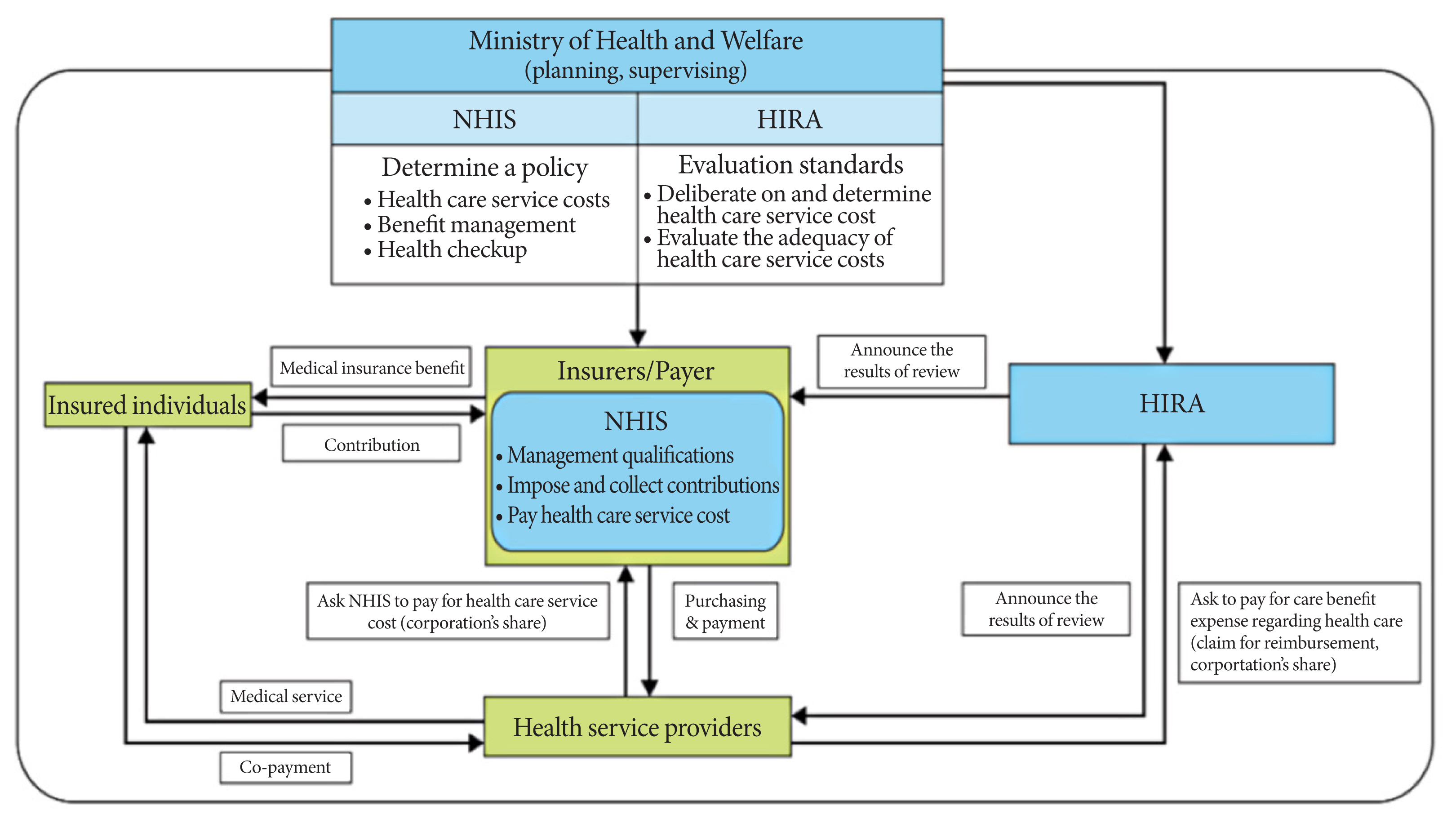
ePub - Recently, medical research using big data has become very popular, and its value has become increasingly recognized. The Korean National Health Information Database (NHID) is representative of big data that combines information obtained from the National Health Insurance Service collected for claims and reimbursement of health care services and results obtained from general health examinations provided to all Korean adults. This database has several strengths and limitations. Given the large size, various laboratory data, and questionnaires obtained from medical check-ups, their longitudinal nature, and long-term accumulation of data since 2002, carefully designed studies may provide valuable information that is difficult to obtain from other forms of research. However, consideration of possible bias and careful interpretation when defining causal relationships is also important because the data were not collected for research purposes. After the NHID became publicly available, research and publications based on this database have increased explosively, especially in the field of diabetes and metabolism. This article reviews the history, structure, and characteristics of the Korean NHID. Recent trends in big data research using this database, commonly used operational diagnosis, and representative studies have been introduced. We expect further progress and expansion of big data research using the Korean NHID.
-
Citations
Citations to this article as recorded by- Weight change in patients with new‐onset type 2 diabetes mellitus and its association with remission: Comprehensive real‐world data
Jinyoung Kim, Bongseong Kim, Mee Kyoung Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024; 26(2): 567. CrossRef - Repeated detection of non‐alcoholic fatty liver disease increases the incidence risk of type 2 diabetes in young adults
Jin Hwa Kim, Young Sang Lyu, Mee Kyoung Kim, Sang Yong Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024; 26(1): 180. CrossRef - Diabetes severity and the risk of depression: A nationwide population-based study
Yunjung Cho, Bongsung Kim, Hyuk-Sang Kwon, Kyungdo Han, Mee Kyoung Kim
Journal of Affective Disorders.2024; 351: 694. CrossRef - Diabetes Duration, Cholesterol Levels, and Risk of Cardiovascular Diseases in Individuals With Type 2 Diabetes
Mee Kyoung Kim, Kyu Na Lee, Kyungdo Han, Seung-Hwan Lee
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Remnant cholesterol is an independent risk factor for the incidence of chronic kidney disease in newly-diagnosed type 2 diabetes: A nationwide population-based study
Soo Yeon Jang, Minwoong Kang, Eyun Song, Ahreum Jang, Kyung Mook Choi, Sei Hyun Baik, Hye Jin Yoo
Diabetes Research and Clinical Practice.2024; 210: 111639. CrossRef - Association of the Intensive Blood Pressure Target and Cardiovascular Outcomes in the Population With Chronic Kidney Disease: A Retrospective Study in Korea
Soo‐Young Yoon, Ji Yoon Kong, Su Jin Jeong, Jin Sug Kim, Hyeon Seok Hwang, Kyunghwan Jeong
Journal of the American Heart Association.2024;[Epub] CrossRef - Risk of Depression according to Cumulative Exposure to a Low-Household Income Status in Individuals with Type 2 Diabetes Mellitus: A Nationwide Population- Based Study
So Hee Park, You-Bin Lee, Kyu-na Lee, Bongsung Kim, So Hyun Cho, So Yoon Kwon, Jiyun Park, Gyuri Kim, Sang-Man Jin, Kyu Yeon Hur, Kyungdo Han, Jae Hyeon Kim
Diabetes & Metabolism Journal.2024; 48(2): 290. CrossRef - Risk of Cause-Specific Mortality across Glucose Spectrum in Elderly People: A Nationwide Population-Based Cohort Study
Joonyub Lee, Hun-Sung Kim, Kee-Ho Song, Soon Jib Yoo, Kyungdo Han, Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(5): 525. CrossRef - A nationwide cohort study on diabetes severity and risk of Parkinson disease
Kyungdo Han, Bongsung Kim, Seung Hwan Lee, Mee Kyoung Kim
npj Parkinson's Disease.2023;[Epub] CrossRef - Predicting the Risk of Insulin-Requiring Gestational Diabetes before Pregnancy: A Model Generated from a Nationwide Population-Based Cohort Study in Korea
Seung-Hwan Lee, Jin Yu, Kyungdo Han, Seung Woo Lee, Sang Youn You, Hun-Sung Kim, Jae-Hyoung Cho, Kun-Ho Yoon, Mee Kyoung Kim
Endocrinology and Metabolism.2023; 38(1): 129. CrossRef - Big Data Research in the Field of Endocrine Diseases Using the Korean National Health Information Database
Sun Wook Cho, Jung Hee Kim, Han Seok Choi, Hwa Young Ahn, Mee Kyoung Kim, Eun Jung Rhee
Endocrinology and Metabolism.2023; 38(1): 10. CrossRef - Comparison of Operational Definition of Type 2 Diabetes Mellitus Based on Data from Korean National Health Insurance Service and Korea National Health and Nutrition Examination Survey
Jong Ha Baek, Yong-Moon Park, Kyung Do Han, Min Kyong Moon, Jong Han Choi, Seung-Hyun Ko
Diabetes & Metabolism Journal.2023; 47(2): 201. CrossRef - Comorbidity Differences by Trajectory Groups as a Reference for Identifying Patients at Risk for Late Mortality in Childhood Cancer Survivors: Longitudinal National Cohort Study
Hyery Kim, Hae Reong Kim, Sung Han Kang, Kyung-Nam Koh, Ho Joon Im, Yu Rang Park
JMIR Public Health and Surveillance.2023; 9: e41203. CrossRef - Diabetes severity is strongly associated with the risk of active tuberculosis in people with type 2 diabetes: a nationwide cohort study with a 6-year follow-up
Ji Young Kang, Kyungdo Han, Seung-Hwan Lee, Mee Kyoung Kim
Respiratory Research.2023;[Epub] CrossRef - Investigation of the Relationship Between Psychiatry Visit and Suicide After Deliberate Self-harm: Longitudinal National Cohort Study
Hye Hyeon Kim, Chanyoung Ko, Ji Ae Park, In Han Song, Yu Rang Park
JMIR Public Health and Surveillance.2023; 9: e41261. CrossRef - Reply
Yeonghee Eun, Hyungjin Kim, Jaejoon Lee
Arthritis & Rheumatology.2023; 75(6): 1081. CrossRef - Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017
Eugene Han, Kyung-Do Han, Yong-ho Lee, Kyung-Soo Kim, Sangmo Hong, Jung Hwan Park, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(3): 347. CrossRef - Comparison of Cefepime with Piperacillin/Tazobactam Treatment in Patients with Hospital-Acquired Pneumonia
Bo-Guen Kim, Danbee Kang, Kyung Hoon Min, Juhee Cho, Kyeongman Jeon
Antibiotics.2023; 12(6): 984. CrossRef - Cumulative exposure to metabolic syndrome increases thyroid cancer risk in young adults: a population-based cohort study
Jinyoung Kim, Kyungdo Han, Mee Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
The Korean Journal of Internal Medicine.2023; 38(4): 526. CrossRef - Risk of developing chronic kidney disease in young-onset Type 2 diabetes in Korea
Joonyub Lee, Seung-Hwan Lee, Kun-Ho Yoon, Jae Hyoung Cho, Kyungdo Han, Yeoree Yang
Scientific Reports.2023;[Epub] CrossRef - Factors Affecting High Body Weight Variability
Kyungdo Han, Mee Kyoung Kim
Journal of Obesity & Metabolic Syndrome.2023; 32(2): 163. CrossRef - Physical activity and reduced risk of fracture in thyroid cancer patients after thyroidectomy — a nationwide cohort study
Jinyoung Kim, Kyungdo Han, Jin-Hyung Jung, Jeonghoon Ha, Chaiho Jeong, Jun-Young Heu, Se-Won Lee, Jeongmin Lee, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Ki-Hyun Baek
Frontiers in Endocrinology.2023;[Epub] CrossRef - The impact of diabetes status on total and site-specific cancer risk in the elderly population: A nationwide cohort study
Kyuho Kim, Bongseong Kim, Hyunho Kim, Hyung Soon Park, Yu-Bae Ahn, Seung-Hyun Ko, Kyungdo Han, Jae-Seung Yun
Diabetes Research and Clinical Practice.2023; 203: 110866. CrossRef - Response to comments of Lai et al. “Proposal of one option for patient-centered, heterogeneous selection of antidiabetic drug”
Sunyoung Kim, Sang Youl Rhee
Diabetes Research and Clinical Practice.2023; 203: 110864. CrossRef - Risk of Pancreatic Cancer and Use of Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes: A Propensity Score-Matching Analysis
Mee Kyoung Kim, Kyungdo Han, Hyuk-Sang Kwon, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(4): 426. CrossRef - Increased risk of ischemic stroke associated with elevated gamma-glutamyl transferase level in adult cancer survivors: a population-based cohort study
Kyuwoong Kim, Hyeyun Jung, Edvige Di Giovanna, Tae Joon Jun, Young-Hak Kim
Scientific Reports.2023;[Epub] CrossRef - Real-world data analysis on effectiveness of integrative therapies: A practical guide to study design and data analysis using healthcare databases
Ye-Seul Lee, Yoon Jae Lee, In-Hyuk Ha
Integrative Medicine Research.2023; 12(4): 101000. CrossRef - Possible Applications of the Korean Experience in the Development of Croatian Healthcare System
Predrag Bejakovic, Romina P Družeta, Ohmin Kwon
Science, Art and Religion.2023; 2(1--2): 26. CrossRef - Cumulative effect of impaired fasting glucose on the risk of dementia in middle-aged and elderly people: a nationwide cohort study
Jin Yu, Kyu-Na Lee, Hun-Sung Kim, Kyungdo Han, Seung-Hwan Lee
Scientific Reports.2023;[Epub] CrossRef - Alcohol consumption and the risk of liver disease: a nationwide, population-based study
Sang Yi Moon, Minkook Son, Yeo Wool Kang, Myeongseok Koh, Jong Yoon Lee, Yang Hyun Baek
Frontiers in Medicine.2023;[Epub] CrossRef - Long-Term Cumulative Exposure to High γ-Glutamyl Transferase Levels and the Risk of Cardiovascular Disease: A Nationwide Population-Based Cohort Study
Han-Sang Baek, Bongseong Kim, Seung-Hwan Lee, Dong-Jun Lim, Hyuk-Sang Kwon, Sang-Ah Chang, Kyungdo Han, Jae-Seung Yun
Endocrinology and Metabolism.2023; 38(6): 770. CrossRef - Sodium-glucose cotransporter 2 inhibitors for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: A nationwide propensity-score matched cohort study
Jinyoung Kim, Kyungdo Han, Bongsung Kim, Ki-Hyun Baek, Ki-Ho Song, Mee Kyoung Kim, Hyuk-Sang Kwon
Diabetes Research and Clinical Practice.2022; 194: 110187. CrossRef - Chronic viral hepatitis accelerates lung function decline in smokers
Suh-Young Lee, Sun-Sin Kim, So-Hee Lee, Heung-Woo Park
Clinical and Experimental Medicine.2022; 23(6): 2159. CrossRef
- Weight change in patients with new‐onset type 2 diabetes mellitus and its association with remission: Comprehensive real‐world data
- Metabolic Risk/Epidemiology
- Iron Overload and the Risk of Diabetes in the General Population: Results of the Chinese Health and Nutrition Survey Cohort Study
- He Gao, Jinying Yang, Wenfei Pan, Min Yang
- Diabetes Metab J. 2022;46(2):307-318. Published online March 7, 2022
- DOI: https://doi.org/10.4093/dmj.2020.0287
- 5,035 View
- 196 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Recent studies have found that there are significant associations between body iron status and the development of diabetes. In the present study, we aimed to analyze the association among iron overload (IO), insulin resistance (IR), and diabetes in Chinese adults, and to explore the sex difference.
Methods
Men and women (age >19 years) who participated in the Chinese Health and Nutrition Survey and did not have diabetes at baseline were followed between 2009 and 2015 (n=5,779). Over a mean of 6 years, 75 participants were diagnosed with incident diabetes. Logistic regression was used to assess the risk factors associated with IO. Cox proportional hazard regression was used to estimate the risk of incident diabetes and to determine whether the risk differed among subgroups. Causal mediation analysis (CMA) was used to explore the mechanism linking IO and diabetes.
Results
According to sex-stratified multivariable-adjusted Cox proportional hazards regression, IO increased the risk of incident diabetes. Women with IO had a higher risk of diabetes than men. Subgroup analysis with respect to age showed that the association between IO and diabetes was stronger in older women and younger men (P<0.001). CMA showed that liver injury (alanine transaminase) and lipid metabolism abnormalities (triglyceride, apolipoprotein B) contributed to the association between IO and diabetes.
Conclusion
IO is associated with diabetes and this association is sex-specific. IO may indirectly induce IR via liver injury and lipid metabolism abnormalities, resulting in diabetes. -
Citations
Citations to this article as recorded by- Quantitative susceptibility mapping for iron monitoring of multiple subcortical nuclei in type 2 diabetes mellitus: a systematic review and meta-analysis
Sana Mohammadi, Sadegh Ghaderi, Fatemeh Sayehmiri, Mobina Fathi
Frontiers in Endocrinology.2024;[Epub] CrossRef - Plasma Ferritin Concentrations in the General Population: A Cross-Sectional Analysis of Anthropometric, Metabolic, and Dietary Correlates
Cara Övermöhle, Sabina Waniek, Gerald Rimbach, Katharina Susanne Weber, Wolfgang Lieb
The Journal of Nutrition.2023; 153(5): 1524. CrossRef - Association of Body Iron Metabolism with Type 2 Diabetes Mellitus in Chinese Women of Childbearing Age: Results from the China Adult Chronic Disease and Nutrition Surveillance (2015)
Jie Feng, Xiaoyun Shan, Lijuan Wang, Jiaxi Lu, Yang Cao, Lichen Yang
Nutrients.2023; 15(8): 1935. CrossRef - Iron overload induces islet β cell ferroptosis by activating ASK1/P-P38/CHOP signaling pathway
Ling Deng, Man-Qiu Mo, Jinling Zhong, Zhengming Li, Guoqiao Li, Yuzhen Liang
PeerJ.2023; 11: e15206. CrossRef - The role of ferroptosis in metabolic diseases
Ling Xie, Bin Fang, Chun Zhang
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research.2023; 1870(6): 119480. CrossRef - Epidemiological and transcriptome data identify potential key genes involved in iron overload for type 2 diabetes
Xuekui Liu, Xiu Hong, Shiqiang Jiang, Rui Li, Qian Lv, Jie Wang, Xiuli Wang, Manqing Yang, Houfa Geng, Yang Li
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Association between serum iron and liver transaminases based on a large adult women population
Andong He, Zhuoping Zhou, Lili Huang, Ka Cheuk Yip, Jing Chen, Ruiling Yan, Ruiman Li
Journal of Health, Population and Nutrition.2023;[Epub] CrossRef - Association between serum ferritin and uric acid levels and nonalcoholic fatty liver disease in the Chinese population
Fangli Zhou, Xiaoli He, Dan Liu, Yan Ye, Haoming Tian, Li Tian
PeerJ.2023; 11: e16267. CrossRef - The Role of Iron Overload in Diabetic Cognitive Impairment: A Review
Ji-Ren An, Qing-Feng Wang, Gui-Yan Sun, Jia-Nan Su, Jun-Tong Liu, Chi Zhang, Li Wang, Dan Teng, Yu-Feng Yang, Yan Shi
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3235. CrossRef - The Association Between METS-IR and Serum Ferritin Level in United States Female: A Cross-Sectional Study Based on NHANES
Han Hao, Yan Chen, Ji Xiaojuan, Zhang Siqi, Chu Hailiang, Sun Xiaoxing, Wang Qikai, Xing Mingquan, Feng Jiangzhou, Ge Hongfeng
Frontiers in Medicine.2022;[Epub] CrossRef - Research Progress on Relationship Between Iron Overload and Lower Limb Arterial Disease in Type 2 Diabetes Mellitus
Zhongjing Wang, Shu Fang, Sheng Ding, Qin Tan, Xuyan Zhang
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 2259. CrossRef - Iron deficiency in cardiac surgical patients
L Hof, O Old, A.U. Steinbicker, P Meybohm, S Choorapoikayil, K Zacharowski
Acta Anaesthesiologica Belgica.2022; 73(4): 235. CrossRef
- Quantitative susceptibility mapping for iron monitoring of multiple subcortical nuclei in type 2 diabetes mellitus: a systematic review and meta-analysis
- Basic Research
- Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis
- Okamatsu-Ogura Yuko, Masayuki Saito
- Diabetes Metab J. 2021;45(6):840-852. Published online June 25, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0291

- 9,101 View
- 507 Download
- 17 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- Brown adipose tissue (BAT) is a specialized tissue for nonshivering thermogenesis to dissipate energy as heat. Although BAT research has long been limited mostly in small rodents, the rediscovery of metabolically active BAT in adult humans has dramatically promoted the translational studies on BAT in health and diseases. Moreover, several remarkable advancements have been made in brown fat biology over the past decade: The molecular and functional analyses of inducible thermogenic adipocytes (socalled beige adipocytes) arising from a developmentally different lineage from classical brown adipocytes have been accelerated. In addition to a well-established thermogenic activity of uncoupling protein 1 (UCP1), several alternative thermogenic mechanisms have been discovered, particularly in beige adipocytes. It has become clear that BAT influences other peripheral tissues and controls their functions and systemic homeostasis of energy and metabolic substrates, suggesting BAT as a metabolic regulator, other than for thermogenesis. This notion is supported by discovering that various paracrine and endocrine factors are secreted from BAT. We review the current understanding of BAT pathophysiology, particularly focusing on its role as a metabolic regulator in small rodents and also in humans.
-
Citations
Citations to this article as recorded by- Brown adipose tissue evaluation using water and triglyceride as indices by diffuse reflectance spectroscopy
Tomomi Iida, Yukio Ueda, Hideo Tsukada, Dai Fukumoto, Takafumi Hamaoka
Journal of Biophotonics.2024;[Epub] CrossRef - White-brown adipose tissue interplay in polycystic ovary syndrome: Therapeutic avenues
Khadijeh Abbasi, Reza Zarezadeh, Amir Valizadeh, Amir Mehdizadeh, Hamed Hamishehkar, Mohammad Nouri, Masoud Darabi
Biochemical Pharmacology.2024; 220: 116012. CrossRef - Brown Adipose Tissue, Batokines, and Bioactive Compounds in Foods: An Update
Fabiane Ferreira Martins, Bruna Cadete Martins, Ananda Vitoria Silva Teixeira, Matheus Ajackson, Vanessa Souza‐Mello, Julio Beltrame Daleprane
Molecular Nutrition & Food Research.2024;[Epub] CrossRef - Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis
Yanqiu Peng, Lixia Zhao, Min Li, Yunfei Liu, Yuke Shi, Jian Zhang
Biomolecules.2024; 14(4): 483. CrossRef - Homotaurine exhibits contrasting effects of DRD1-mediated thermogenesis-related regulators in C2C12 myoblasts and 3T3−L1 white adipocytes
Kiros Haddish, Jong Won Yun
Biotechnology and Bioprocess Engineering.2024;[Epub] CrossRef - Thermogenic Brown Fat in Humans: Implications in Energy Homeostasis, Obesity and Metabolic Disorders
Masayuki Saito, Yuko Okamatsu-Ogura
The World Journal of Men's Health.2023; 41(3): 489. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - White adipose tissue undergoes browning during preweaning period in association with microbiota formation in mice
Anju Tsukada, Yuko Okamatsu-Ogura, Emi Futagawa, Yuki Habu, Natsumi Takahashi, Mira Kato-Suzuki, Yuko Kato, Satoshi Ishizuka, Kei Sonoyama, Kazuhiro Kimura
iScience.2023; 26(7): 107239. CrossRef - In situ fluorescence-photoacoustic measurement of the changes of brown adipose tissue in mice under hindlimb unloading
Baojie Gong, Jianxin Tang, Xiaoxiao Jiang, Zhe Zhang, Shiying Li, Hongjun Jin, Liming Nie, Guojia Huang
Journal of Applied Physiology.2023; 135(2): 251. CrossRef - Age-Related Expression Dynamics of Uncoupling Protein 1 in Adipose Tissues of ICR Outbred Mice during Postnatal Ontogenesis
A. V. Yakunenkov, E. I. Elsukova, I. O. Natochy
Journal of Evolutionary Biochemistry and Physiology.2023; 59(4): 1020. CrossRef - UNCOUPLING PROTEIN UCP1 EXPRESSION DYNAMICS IN ADIPOSE TISSUES OF THE OUTBRED ICR MICE IN POSTNATAL ONTOGENESIS
A. V. Yakunenkov, E. I. Elsukova, I. O. Natochy
Журнал эволюционной биохимии и физиологии.2023; 59(4): 255. CrossRef - Antibodies Regulate Dual-Function Enzyme IYD to Induce Functional Synergy between Metabolism and Thermogenesis
Sunghyun Kang, Hwan-Woo Park, Kyung Ho Han
International Journal of Molecular Sciences.2022; 23(14): 7834. CrossRef - Machine learning-featured Secretogranin V is a circulating diagnostic biomarker for pancreatic adenocarcinomas associated with adipopenia
Yunju Jo, Min-Kyung Yeo, Tam Dao, Jeongho Kwon, Hyon‐Seung Yi, Dongryeol Ryu
Frontiers in Oncology.2022;[Epub] CrossRef - Possible roles of exercise and apelin against pregnancy complications
Hamed Alizadeh Pahlavani
Frontiers in Endocrinology.2022;[Epub] CrossRef - Relationships between the expression of adipose genes and profiles of hospitalized dogs
Yukina Sugiyama, Fumie Shimokawa, Kazutoshi Sugiyama, Takashi Kobayashi, Yusuke Yamashita, Kei Kazama, Ken Onda, Masayuki Funaba, Masaru Murakami
Veterinary Research Communications.2022; 46(4): 1239. CrossRef - Garlic (Allium sativum L.) in diabetes and its complications: Recent advances in mechanisms of action
Yayi Jiang, Rensong Yue, Guojie Liu, Jun Liu, Bo Peng, Maoyi Yang, Lianxue Zhao, Zihan Li
Critical Reviews in Food Science and Nutrition.2022; : 1. CrossRef - Fruit of Gardenia jasminoides Induces Mitochondrial Activation and Non-Shivering Thermogenesis through Regulation of PPARγ
Woo Yong Park, Gahee Song, Ja Yeon Park, Kwan-Il Kim, Kwang Seok Ahn, Hyun Jeong Kwak, Jungtae Leem, Jae-Young Um, Jinbong Park
Antioxidants.2021; 10(9): 1418. CrossRef
- Brown adipose tissue evaluation using water and triglyceride as indices by diffuse reflectance spectroscopy
- Basic Research
- Revisiting the Bacterial Phylum Composition in Metabolic Diseases Focused on Host Energy Metabolism
- Yeonmi Lee, Hui-Young Lee
- Diabetes Metab J. 2020;44(5):658-667. Published online July 9, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0220
- 9,013 View
- 131 Download
- 19 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
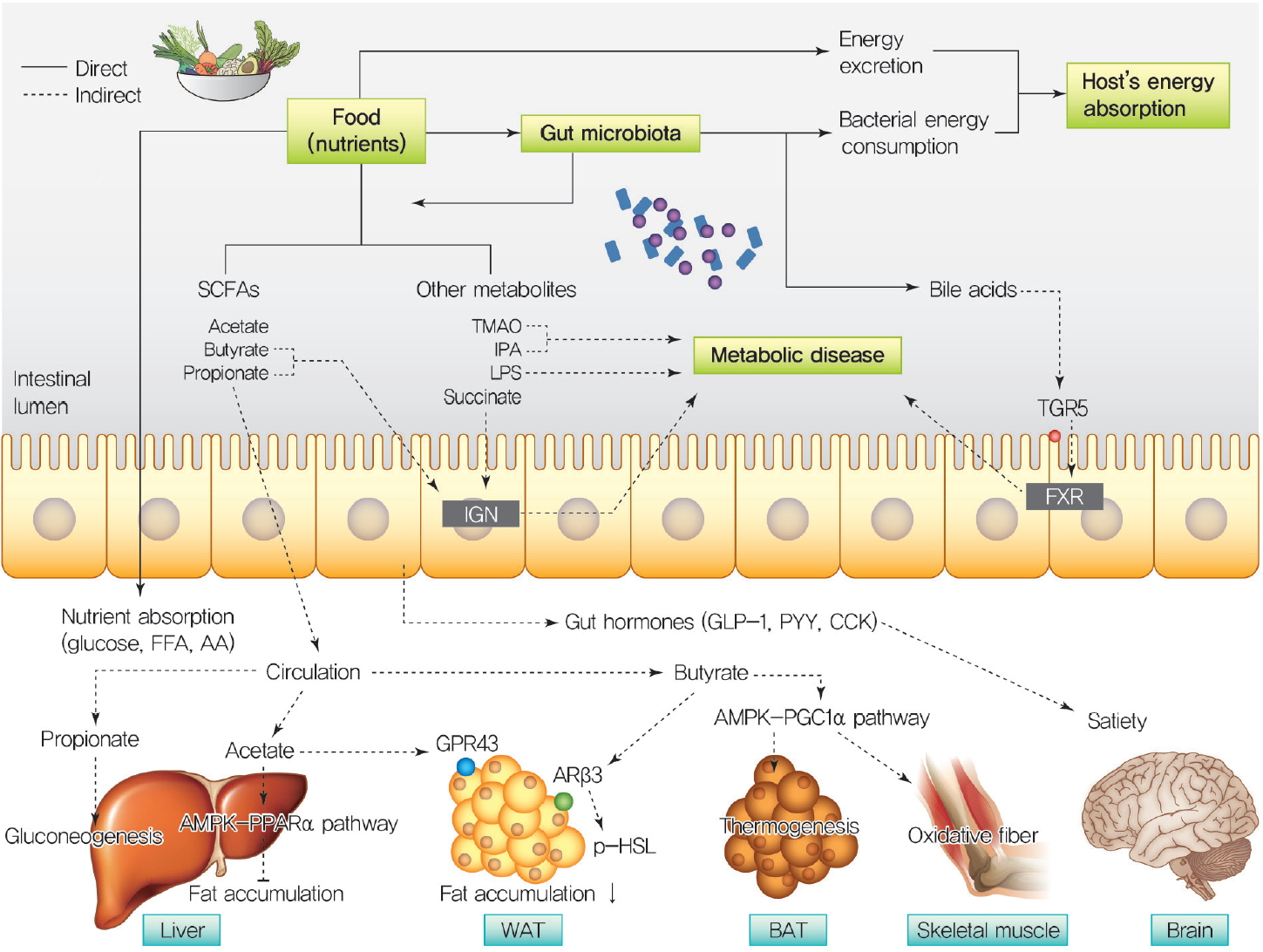
ePub Over a hundred billion bacteria are found in human intestines. This has emerged as an environmental factor in metabolic diseases, such as obesity and related diseases. The majority of these bacteria belong to two dominant phyla,
Bacteroidetes andFirmicutes . Since the ratio ofFirmicutes toBacteroidetes increases in people with obesity and in various animal models, it has been assumed that phylum composition causes the increase in occurrence of metabolic diseases over the past decade. However, this assumption has been challenged by recent studies that have found even an opposite association of phylum composition within metabolic diseases. Moreover, the gut microbiota affects host energy metabolism in various ways including production of metabolites and interaction with host intestinal cells to regulate signaling pathways that affect energy metabolism. However, the direct effect of gut bacteria on host energy intake, such as energy consumption by the bacteria itself and its effects on intestinal energy absorption, has been underestimated. This review aims to discuss whether increased ratio ofFirmicutes toBacteroidetes is associated with the development of metabolic diseases, and whether energy competition between the bacteria and host is a missing part of the mechanism linking gut microbiota to metabolic diseases.-
Citations
Citations to this article as recorded by- Behavior, intestinal health, and growth of small sea cucumbers Apostichopus japonicus in different color morphs
Peng Ding, Yushi Yu, Zihe Zhao, Xiang Li, Xiajing Wang, Huiyan Wang, Xiyuan Huang, Jun Ding, Chong Zhao
Marine Environmental Research.2024; 193: 106300. CrossRef - Traditional Chinese Medicine formula Dai-Zong-Fang alleviating hepatic steatosis in db/db mice via gut microbiota modulation
Li-Wei Zhang, Li-Li Zhu, Xiao-Yun Zhu, Shou-Qiang Fu, Xi-Ming Liu
Frontiers in Pharmacology.2024;[Epub] CrossRef - Repeated inoculation with rumen fluid accelerates the rumen bacterial transition with no benefit on production performance in postpartum Holstein dairy cows
Fanlin Kong, Feiran Wang, Yijia Zhang, Shuo Wang, Wei Wang, Shengli Li
Journal of Animal Science and Biotechnology.2024;[Epub] CrossRef - Identification of oncogenic signatures in the inflammatory colon of C57BL/6 mice fed a high-fat diet
Huawei Zeng, Bryan D. Safratowich, Wen-Hsing Cheng, Michael R. Bukowski
The Journal of Nutritional Biochemistry.2023; 111: 109188. CrossRef - Evaluation of the gut microbiome alterations in healthy rats after dietary exposure to different synthetic ZnO nanoparticles
Xinyi Zhu, Henghui Li, Liuzhu Zhou, Huijun Jiang, Minghui Ji, Jin Chen
Life Sciences.2023; 312: 121250. CrossRef - Microplastic-induced gut microbiota and serum metabolic disruption in Sprague-Dawley rats
Nan Zhao, Meirong Zhao, Hangbiao Jin
Environmental Pollution.2023; 320: 121071. CrossRef - Effects of neutral polysaccharide from Platycodon grandiflorum on high-fat diet-induced obesity via the regulation of gut microbiota and metabolites
Jing Song, Qin liu, Mengqi Hao, Xiaohu Zhai, Juan Chen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Metabolite interactions between host and microbiota during health and disease: Which feeds the other?
Yan Zhang, Rui Chen, DuoDuo Zhang, Shuang Qi, Yan Liu
Biomedicine & Pharmacotherapy.2023; 160: 114295. CrossRef - Connecting Gut Microbial Diversity with Plasma Metabolome and Fecal Bile Acid Changes Induced by the Antibiotics Tobramycin and Colistin Sulfate
Aishwarya Murali, Varun Giri, Franziska Maria Zickgraf, Philipp Ternes, Hunter James Cameron, Saskia Sperber, Volker Haake, Peter Driemert, Hennicke Kamp, Dorothee Funk-Weyer, Shana J. Sturla, Ivonne M.C.M. Rietjens, Bennard van Ravenzwaay
Chemical Research in Toxicology.2023; 36(4): 598. CrossRef - Short-Term Alternate Feeding between Terrestrially Sourced Oil- and Fish Oil-Based Diets Modulates the Intestinal Microecology of Juvenile Turbot
Xiuhua Ma, Yaoyao Kong, Houguo Xu, Qingzhu Bi, Mengqing Liang, Kangsen Mai, Yanjiao Zhang
Biology.2023; 12(5): 650. CrossRef - Effects and action mechanisms of lotus leaf (Nelumbo nucifera) ethanol extract on gut microbes and obesity in high-fat diet-fed rats
Zhang Yanan, Ma Lu, Zhang Lu, Huo Jinhai, Wang Weiming
Frontiers in Nutrition.2023;[Epub] CrossRef - Effects of coffee with different roasting degrees on obesity and related metabolic disorders
Claudia I. Gamboa-Gómez, Laura J. Barragán-Zúñiga, Fernando Guerrero-Romero, Gerardo Martínez-Aguilar, José Luis Gónzalez, Almendra A. Valenzuela-Ramírez, Juan A. Rojas-Contreras, Monica Anese, Maribel Cervantes Flores, Marilisa Alongi
Journal of Functional Foods.2023; 111: 105889. CrossRef - Gut Microbiota and Bacterial Translocation in the Pathogenesis of Liver Fibrosis
Roman Maslennikov, Elena Poluektova, Oxana Zolnikova, Alla Sedova, Anastasia Kurbatova, Yulia Shulpekova, Natyia Dzhakhaya, Svetlana Kardasheva, Maria Nadinskaia, Elena Bueverova, Vladimir Nechaev, Anna Karchevskaya, Vladimir Ivashkin
International Journal of Molecular Sciences.2023; 24(22): 16502. CrossRef - Eugenol, A Major Component of Clove Oil, Attenuates Adiposity, and Modulates Gut Microbiota in High‐Fat Diet‐Fed Mice
Mengjie Li, Yuhan Zhao, Yanan Wang, Ruixuan Geng, Jingjing Fang, Seong‐Gook Kang, Kunlun Huang, Tao Tong
Molecular Nutrition & Food Research.2022;[Epub] CrossRef - Heimao tea polysaccharides ameliorate obesity by enhancing gut microbiota-dependent adipocytes thermogenesis in mice fed with high fat diet
Yu Wang, Ting Li, Yueyue Liu, Chengcheng Yang, Lei Liu, Xiangnan Zhang, Xingbin Yang
Food & Function.2022; 13(24): 13014. CrossRef - The Interplay of Sex Steroids, the Immune Response, and the Intestinal Microbiota
Fernanda Pace, Paula I. Watnick
Trends in Microbiology.2021; 29(9): 849. CrossRef - Heat stress on microbiota composition, barrier integrity, and
nutrient transport in gut, production performance, and its amelioration in farm
animals
Amlan Kumar Patra, Indrajit Kar
Journal of Animal Science and Technology.2021; 63(2): 211. CrossRef - Mechanisms linking gut microbial metabolites to insulin resistance
Hye Rim Jang, Hui-Young Lee
World Journal of Diabetes.2021; 12(6): 730. CrossRef - The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health
Lenka Tomasova, Marian Grman, Karol Ondrias, Marcin Ufnal
Nutrition & Metabolism.2021;[Epub] CrossRef
- Behavior, intestinal health, and growth of small sea cucumbers Apostichopus japonicus in different color morphs
- Pathophysiology
- The Role of Growth Differentiation Factor 15 in Energy Metabolism
- Joon Young Chang, Hyun Jung Hong, Seul Gi Kang, Jung Tae Kim, Ben Yuan Zhang, Minho Shong
- Diabetes Metab J. 2020;44(3):363-371. Published online June 29, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0087
- 8,135 View
- 226 Download
- 15 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Growth differentiation factor 15 (GDF15) is receiving great interest beyond its role as an aging and disease-related biomarker. Recent discovery of its receptor, glial cell line-derived neurotrophic factor (GDNF) family receptor α-like (GFRAL), suggests a central role in appetite regulation. However, there is also considerable evidence that GDF15 may have peripheral activity through an as-of-yet undiscovered mode of action. This raises the question as to whether increased GDF15 induction during pathophysiologic conditions also suppresses appetite. The present review will briefly introduce the discovery of GDF15 and describe the different contexts under which GDF15 is induced, focusing on its induction during mitochondrial dysfunction. We will further discuss the metabolic role of GDF15 under various pathophysiological conditions and conclude with possible therapeutic applications.
-
Citations
Citations to this article as recorded by- Growth differentiation factor-15 is an IFN-γ regulated mediator of infection-induced weight loss and the hepatic FGF21 response
Jojo Reyes, Yanlin Zhao, Krushang Pandya, George S. Yap
Brain, Behavior, and Immunity.2024; 116: 24. CrossRef - Biomarkers of disability worsening in inactive primary progressive multiple sclerosis
Maria-Elizabeth Baeva, Isabelle Tottenham, Marcus Koch, Carlos Camara-Lemarroy
Journal of Neuroimmunology.2024; 387: 578268. CrossRef - Tricarboxylic Acid Cycle Intermediates and Individual Ageing
Natalia Kurhaluk
Biomolecules.2024; 14(3): 260. CrossRef - A long‐acting GDF15 analog causes robust, sustained weight loss and reduction of food intake in an obese nonhuman primate model
Songmao Zheng, David Polidori, Yuanping Wang, Brian Geist, Xiefan Lin‐Schmidt, Jennifer L. Furman, Serena Nelson, Andrea R. Nawrocki, Simon A. Hinke
Clinical and Translational Science.2023; 16(8): 1431. CrossRef - GDF15 enhances body weight and adiposity reduction in obese mice by leveraging the leptin pathway
Samuel N. Breit, Rakesh Manandhar, Hong-Ping Zhang, Michelle Lee-Ng, David A. Brown, Vicky Wang-Wei Tsai
Cell Metabolism.2023; 35(8): 1341. CrossRef - Serum growth differentiation factor-15 (GDF-15) is a biomarker of cardiac manifestations in children with COVID-19
Sally Raafat Ishak, Mona Mostafa El Ganzoury, Eman Mahmoud Fouda, Maha Ahmad Anwar, Amany Moustafa Kamal, Heba Mostafa Hamza, Nehad Ahmed Bakry
European Journal of Medical Research.2023;[Epub] CrossRef - Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia
Eunbyul Yeom, Kweon Yu
Experimental & Molecular Medicine.2022; 54(4): 426. CrossRef - Investigating the combination of plasma amyloid-beta and geroscience biomarkers on the incidence of clinically meaningful cognitive decline in older adults
Wan-Hsuan Lu, Kelly Virecoulon Giudici, John E. Morley, Sophie Guyonnet, Angelo Parini, Geetika Aggarwal, Andrew D. Nguyen, Yan Li, Randall J. Bateman, Bruno Vellas, Philipe de Souto Barreto, Bruno Vellas, Sophie Guyonnet, Isabelle Carrié, Lauréane Brigit
GeroScience.2022; 44(3): 1489. CrossRef - The Potential Role of Growth Differentiation Factor 15 in COVID-19: A Corollary Subjective Effect or Not?
Ahmad O. Babalghith, Hayder M. Al-kuraishy, Ali I. Al-Gareeb, Michel De Waard, Jean-Marc Sabatier, Hebatallah M. Saad, Gaber El-Saber Batiha
Diagnostics.2022; 12(9): 2051. CrossRef - Metformin and growth differentiation factor 15 (GDF15) in type 2 diabetes mellitus: A hidden treasure
Hayder M. Al‐kuraishy, Ali I. Al‐Gareeb, Athanasios Alexiou, Marios Papadakis, Eman Hassan Nadwa, Sarah M. Albogami, Mohammed Alorabi, Hebatallah M. Saad, Gaber El‐Saber Batiha
Journal of Diabetes.2022; 14(12): 806. CrossRef - Effects of Exercise Intervention on Mitochondrial Stress Biomarkers in Metabolic Syndrome Patients: A Randomized Controlled Trial
Jae Seung Chang, Jun Namkung
International Journal of Environmental Research and Public Health.2021; 18(5): 2242. CrossRef - Neurological & psychological aspects of Barth syndrome: Clinical manifestations and potential pathogenic mechanisms
Melissa Olivar-Villanueva, Mindong Ren, Colin K.L. Phoon
Mitochondrion.2021; 61: 188. CrossRef - The metabolic role of spermidine in obesity: Evidence from cells to community
Yanee Choksomngam, Sintip Pattanakuhar, Nipon Chattipakorn, Siriporn C. Chattipakorn
Obesity Research & Clinical Practice.2021; 15(4): 315. CrossRef - The Role of GDF15 as a Myomitokine
Kornelia Johann, Maximilian Kleinert, Susanne Klaus
Cells.2021; 10(11): 2990. CrossRef
- Growth differentiation factor-15 is an IFN-γ regulated mediator of infection-induced weight loss and the hepatic FGF21 response
- Basic Research
- Effects of Microbiota on the Treatment of Obesity with the Natural Product Celastrol in Rats
- Weiyue Hu, Lingling Wang, Guizhen Du, Quanquan Guan, Tianyu Dong, Ling Song, Yankai Xia, Xinru Wang
- Diabetes Metab J. 2020;44(5):747-763. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0124
- 9,328 View
- 136 Download
- 16 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
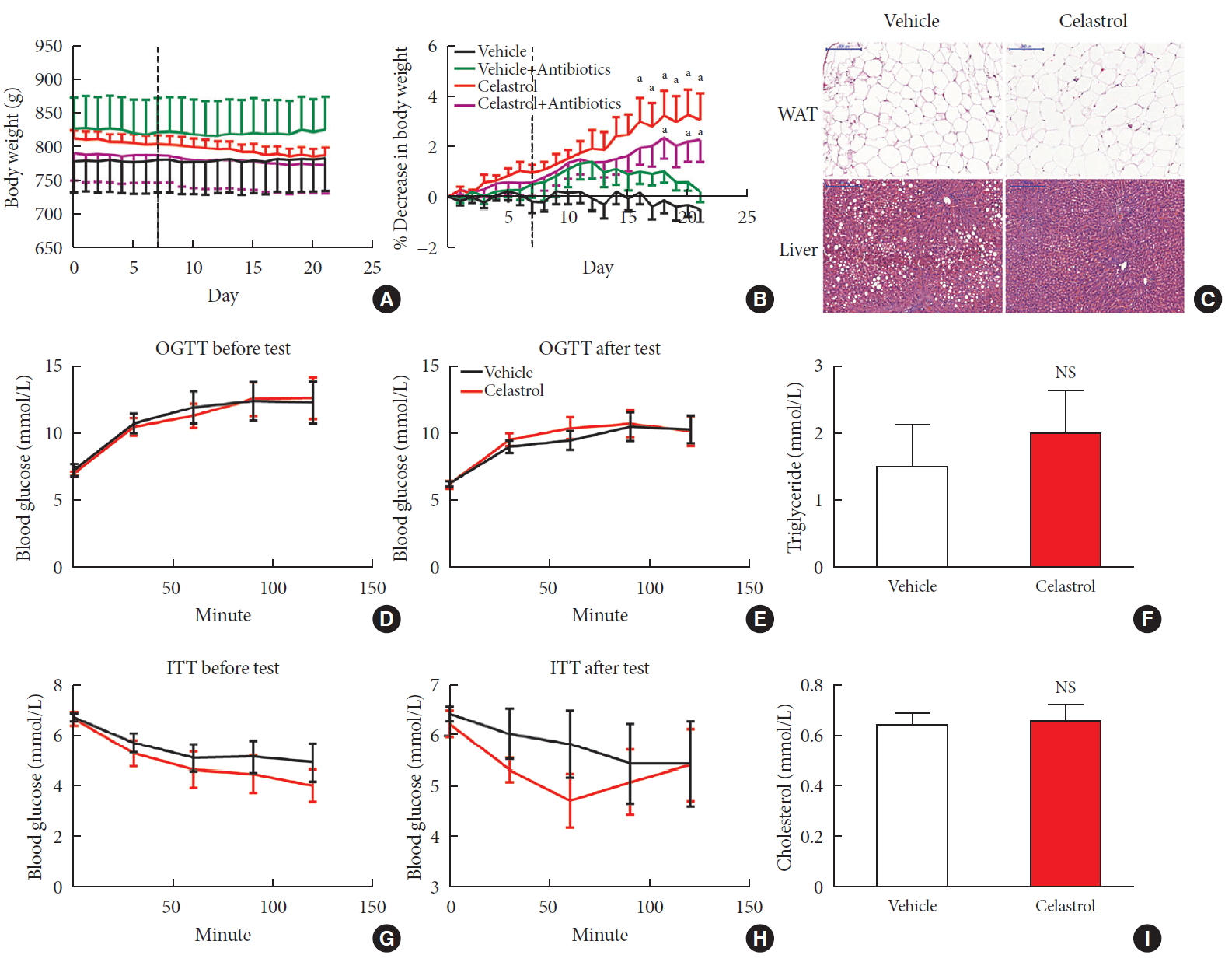
ePub Background Obesity has become one of the most serious issues threatening the health of humankind, and we conducted this study to examine whether and how celastrol protects against obesity.
Methods We fed male Sprague-Dawley rats a high-fat diet and administered celastrol to obese rats for 3 weeks. By recording body weight (BW) and other measures, we identified the effective dose of celastrol for obesity treatment. Feces were collected to perform 16S rRNA sequencing, and hypothalami were extracted for transcriptome sequencing. We then treated leptin knockout rats with celastrol and explored the changes in energy metabolism. Male Institute of Cancer Research (ICR) mice were used to test the acute toxicity of celastrol.
Results We observed that celastrol reduced BW and promoted energy expenditure at a dose of 500 µg/kg BW but that food intake was not changed after administration. The diversity of the gut microbiota was improved, with an increased ratio of
Bacteroidetes toFirmicutes , and the gut microbiota played an important role in the anti-obesity effects of celastrol. Hypothalamic transcriptome analysis showed a significant enrichment of the leptin signaling pathway, and we found that celastrol significantly enhanced energy expenditure, which was mediated by the leptin signaling pathway. Acute lethal toxicity of celastrol was not observed at doses ranging from 0 to 62.5 mg/kg BW.Conclusion Our study revealed that celastrol decreased the BW of obese rats by enhancing energy expenditure but not by suppressing food intake and that this effect was mediated by the improvement of the gut microbiota and the activation of the hypothalamic leptin signaling pathway.
-
Citations
Citations to this article as recorded by- Natural compounds as obesity pharmacotherapies
Xin‐Yuan Zhao, Ji‐Qiu Wang, G. Gregory Neely, Yan‐Chuan Shi, Qiao‐Ping Wang
Phytotherapy Research.2024; 38(2): 797. CrossRef - Celastrol functions as an emerging manager of lipid metabolism: Mechanism and therapeutic potential
Jia Gu, Ya-Ning Shi, Neng Zhu, Hong-Fang Li, Chan-Juan Zhang, Li Qin
Biomedicine & Pharmacotherapy.2023; 164: 114981. CrossRef - Tripterygium hypoglaucum extract ameliorates adjuvant-induced arthritis in mice through the gut microbiota
Jianghui HU, Jimin NI, Junping ZHENG, Yanlei GUO, Yong YANG, Cheng YE, Xiongjie SUN, Hui XIA, Yanju LIU, Hongtao LIU
Chinese Journal of Natural Medicines.2023; 21(10): 730. CrossRef - Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation
Lili Su, Zhifan Hong, Tong Zhou, Yuanyuan Jian, Mei Xu, Xuanping Zhang, Xiaoyan Zhu, Jiayin Wang
Scientific Reports.2022;[Epub] CrossRef - Tripterygium hypoglaucum (Levl.) Hutch: A systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology
Jiangping Wei, Liyun Chen, Sijia Gao, Jirui Wang, Yunhong Wang, Zhiwei Zhang, Yuyu Zhang, Xiaomei Zhang, Yong Yang, Dajian Yang
Pharmacological Research - Modern Chinese Medicine.2022; 3: 100094. CrossRef - Celastrol: An Update on Its Hepatoprotective Properties and the Linked Molecular Mechanisms
Mengzhen Li, Faren Xie, Lu Wang, Guoxue Zhu, Lian-Wen Qi, Shujun Jiang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Celastrol inhibits the proliferation and migration of MCF-7 cells through the leptin-triggered PI3K/AKT pathway
Pingping Chen, Bin Wang, Meng Li, Chunxue Cui, Fei Liu, Yonggang Gao
Computational and Structural Biotechnology Journal.2022; 20: 3173. CrossRef - Investigating Celastrol’s Anti-DCM Targets and Mechanisms via Network Pharmacology and Experimental Validation
Rui Xi, Yongxin Wan, Lihong Yang, Jingying Zhang, Liu Yang, Shuai Yang, Rui Chai, Fengchen Mu, Qiting Sun, Rui Yan, Zhifang Wu, Sijin Li, Zhijun Liao
BioMed Research International.2022; 2022: 1. CrossRef - Celastrol inhibits TXNIP expression to protect pancreatic β cells in diabetic mice
Si-wei Wang, Tian Lan, Fang Zheng, Hui Huang, Hang-fei Chen, Qi Wu, Feng Zhang
Phytomedicine.2022; 104: 154316. CrossRef - Celastrol: A Promising Agent Fighting against Cardiovascular Diseases
Zhexi Li, Jingyi Zhang, Xulei Duan, Guoan Zhao, Min Zhang
Antioxidants.2022; 11(8): 1597. CrossRef - Celastrol: A lead compound that inhibits SARS‐CoV‐2 replication, the activity of viral and human cysteine proteases, and virus‐induced IL‐6 secretion
Carlos A. Fuzo, Ronaldo B. Martins, Thais F. C. Fraga‐Silva, Martin K. Amstalden, Thais Canassa De Leo, Juliano P. Souza, Thais M. Lima, Lucia H. Faccioli, Débora Noma Okamoto, Maria Aparecida Juliano, Suzelei C. França, Luiz Juliano, Vania L. D. Bonato,
Drug Development Research.2022; 83(7): 1623. CrossRef - In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates
Mahmoud Saad Abdel-Halim, Momen Askoura, Basem Mansour, Galal Yahya, Amira M. El-Ganiny
The Journal of Antibiotics.2022; 75(12): 679. CrossRef - Celastrol alleviates metabolic disturbance in high‐fat diet‐induced obese mice through increasing energy expenditure by ameliorating metabolic inflammation
Xueping Yang, Fan Wu, Lingli Li, Ernest C. Lynch, Linglin Xie, Yan Zhao, Ke Fang, Jingbin Li, Jinlong Luo, Lijun Xu, Xin Zou, Fuer Lu, Guang Chen
Phytotherapy Research.2021; 35(1): 297. CrossRef - Celastrol in metabolic diseases: Progress and application prospects
Shaohua Xu, Yaqian Feng, Weishen He, Wen Xu, Wei Xu, Hongjun Yang, Xianyu Li
Pharmacological Research.2021; 167: 105572. CrossRef - The Anti-Obesity Effect of Traditional Chinese Medicine on Lipid Metabolism
Qijing Fan, Furong Xu, Bin Liang, Xiaoju Zou
Frontiers in Pharmacology.2021;[Epub] CrossRef - Serum Metabolome Mediates the Antiobesity Effect of Celastrol-Induced Gut Microbial Alterations
Shaohua Xu, Liwei Lyu, Huaichang Zhu, Xiaoqiang Huang, Wei Xu, Wen Xu, Yaqian Feng, Yong Fan
Journal of Proteome Research.2021; 20(10): 4840. CrossRef - Interrelated Mechanism by Which the Methide Quinone Celastrol, Obtained from the Roots of Tripterygium wilfordii, Inhibits Main Protease 3CLpro of COVID-19 and Acts as Superoxide Radical Scavenger
Francesco Caruso, Manrose Singh, Stuart Belli, Molly Berinato, Miriam Rossi
International Journal of Molecular Sciences.2020; 21(23): 9266. CrossRef
- Natural compounds as obesity pharmacotherapies
- Basic Research
- Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
- Mahesh Raj Nepal, Mi Jeong Kang, Geon Ho Kim, Dong Ho Cha, Ju-Hyun Kim, Tae Cheon Jeong
- Diabetes Metab J. 2020;44(6):908-918. Published online April 6, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0147
- 5,667 View
- 114 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
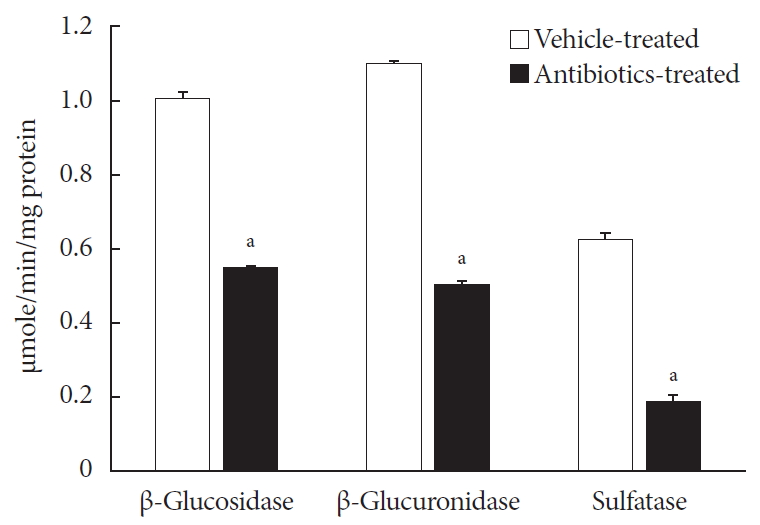
ePub Background Voglibose, an α-glucosidase inhibitor, inhibits breakdown of complex carbohydrates into simple sugar units in intestine. Studies showed that voglibose metabolism in the liver might be negligible due to its poor intestinal absorption. Numerous microorganisms live in intestine and have several roles in metabolism and detoxification of various xenobiotics. Due to the limited information, the possible metabolism of voglibose by intestinal microbiota was investigated
in vitro andin vivo .Methods For the
in vitro study, different concentrations of voglibose were incubated with intestinal contents, prepared from both vehicle- and antibiotics-treated mice, to determine the decreased amount of voglibose over time by using liquid chromatography-mass spectrometry. Similarly,in vivo pharmacodynamic effect of voglibose was determined following the administration of voglibose and starch in vehicle- and antibiotic-pretreated non-diabetic and diabetic mice, by measuring the modulatory effects of voglibose on blood glucose levels.Results The
in vitro results indicated that the remaining voglibose could be significantly decreased when incubated with the intestinal contents from normal mice compared to those from antibiotic-treated mice, which had less enzyme activities. Thein vivo results showed that the antibiotic pretreatment resulted in reduced metabolism of voglibose. This significantly lowered blood glucose levels in antibiotic-pretreated mice compared to the control animals.Conclusion The present results indicate that voglibose would be metabolized by the intestinal microbiota, and that this metabolism might be pharmacodynamically critical in lowering blood glucose levels in mice.
-
Citations
Citations to this article as recorded by- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
Liyang Jia, Shiqiong Huang, Boyu Sun, Yongguang Shang, Chunsheng Zhu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Phenolics from endophytic fungi as natural α-glucosidase inhibitors: A comprehensive review
Muhammad Imran Tousif, Saba Tauseef, Sadeer Nabeelah, Jugreet Sharmeen, Gokhan Zengin, Lesetja Legoabe, Muhammad Imran, Mohamad Fawzi Mahomoodally
Journal of Molecular Structure.2023; 1291: 135852. CrossRef - Ligand-targeted fishing of α-glucosidase inhibitors from Tribulus terrestris L. based on chitosan-functionalized multi-walled carbon nanotubes with immobilized α-glucosidase
Xin Meng, Hou Zong, Zhong Zheng, Junpeng Xing, Zhiqiang Liu, Fengrui Song, Shu Liu
Analytical and Bioanalytical Chemistry.2023; 415(14): 2677. CrossRef - Isolation, structure elucidation, and biological activities of sesquiterpenes and phthalides from two edible mushrooms Pleurotus species
Jewel C De Padua, Emi Fukushima-Sakuno, Kotomi Ueno, Thomas Edison E dela Cruz, Atsushi Ishihara
Bioscience, Biotechnology, and Biochemistry.2023; 87(12): 1429. CrossRef - Effects of Oral Glucose-Lowering Agents on Gut Microbiota and Microbial Metabolites
Dongmei Wang, Jieying Liu, Liyuan Zhou, Qian Zhang, Ming Li, Xinhua Xiao
Frontiers in Endocrinology.2022;[Epub] CrossRef - 18:0 Lyso PC, a natural product with potential PPAR-γ agonistic activity, plays hypoglycemic effect with lower liver toxicity and cardiotoxicity in db/db mice
Yiming Ma, Xinyi Du, Dandan Zhao, Kegong Tang, Xiaona Wang, Shaoting Guo, Xiaobei Li, Song Mei, Na Sun, Jiaqi Liu, Chengyu Jiang
Biochemical and Biophysical Research Communications.2021; 579: 168. CrossRef
- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
- Basic Research
- Role of CRTC2 in Metabolic Homeostasis: Key Regulator of Whole-Body Energy Metabolism?
- Hye-Sook Han, Yongmin Kwon, Seung-Hoi Koo
- Diabetes Metab J. 2020;44(4):498-508. Published online March 5, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0200
- 6,944 View
- 163 Download
- 14 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
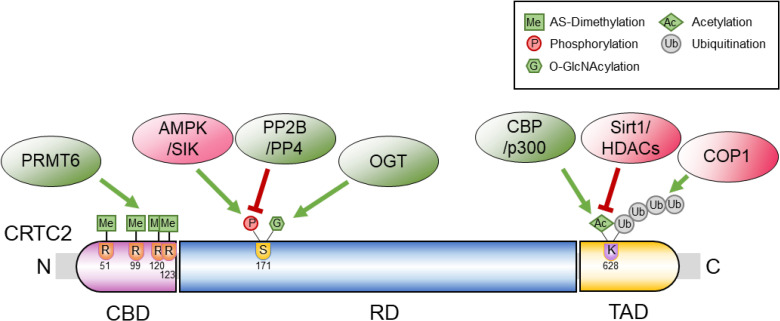
ePub Cyclic adenosine monophosphate (cAMP) signaling is critical for regulating metabolic homeostasis in mammals. In particular, transcriptional regulation by cAMP response element-binding protein (CREB) and its coactivator, CREB-regulated transcription coactivator (CRTC), is essential for controlling the expression of critical enzymes in the metabolic process, leading to more chronic changes in metabolic flux. Among the CRTC isoforms, CRTC2 is predominantly expressed in peripheral tissues and has been shown to be associated with various metabolic pathways in tissue-specific manners. While initial reports showed the physiological role of CRTC2 in regulating gluconeogenesis in the liver, recent studies have further delineated the role of this transcriptional coactivator in the regulation of glucose and lipid metabolism in various tissues, including the liver, pancreatic islets, endocrine tissues of the small intestines, and adipose tissues. In this review, we discuss recent studies that have utilized knockout mouse models to delineate the role of CRTC2 in the regulation of metabolic homeostasis.
-
Citations
Citations to this article as recorded by- Integration of genomic and transcriptomic data of inbred mouse models for polygenic obesity and leanness revealed “obese” and “lean” candidate alleles in polyadenylation signals
Martin Šimon, Špela Mikec, Nicholas M. Morton, Santosh S. Atanur, Simon Horvat, Tanja Kunej
Gene Reports.2024; 35: 101903. CrossRef - Mylabris phalerata induces the apoptosis and cell cycle delay in HCC, and potentiates the effect of sorafenib based on the molecular and network pharmacology approach
Young Woo Kim, Seon Been Bak, Su Youn Baek, Il Kon Kim, Won-Yung Lee, Un-Jung Yun, Kwang-Il Park
Molecular & Cellular Toxicology.2023; 19(4): 731. CrossRef - Emerging Role of SMILE in Liver Metabolism
Nanthini Sadasivam, Kamalakannan Radhakrishnan, Hueng-Sik Choi, Don-Kyu Kim
International Journal of Molecular Sciences.2023; 24(3): 2907. CrossRef - PIMT regulates hepatic gluconeogenesis in mice
Bandish Kapadia, Soma Behera, Sireesh T. Kumar, Tapan Shah, Rebecca Kristina Edwin, Phanithi Prakash Babu, Partha Chakrabarti, Kishore V.L. Parsa, Parimal Misra
iScience.2023; 26(3): 106120. CrossRef - Biological functions of CRTC2 and its role in metabolism-related diseases
Hong-Yu Zheng, Yan-Xia Wang, Kun Zhou, Hai-Lin Xie, Zhong Ren, Hui-Ting Liu, Yang-Shao Ou, Zhi-Xiang Zhou, Zhi-Sheng Jiang
Journal of Cell Communication and Signaling.2023; 17(3): 495. CrossRef - An insulin-regulated arrestin domain protein controls hepatic glucagon action
Sezin Dagdeviren, Megan F. Hoang, Mohsen Sarikhani, Vanessa Meier, Jake C. Benoit, Marinna C. Okawa, Veronika Y. Melnik, Elisabeth M. Ricci-Blair, Natalie Foot, Randall H. Friedline, Xiaodi Hu, Lauren A. Tauer, Arvind Srinivasan, Maxim B. Prigozhin, Sudha
Journal of Biological Chemistry.2023; 299(8): 105045. CrossRef - The Pleiotropic Face of CREB Family Transcription Factors
Md. Arifur Rahman Chowdhury, Jungeun An, Sangyun Jeong
Molecules and Cells.2023; 46(7): 399. CrossRef - It is a branched road to adipose tissue aging
N. Touitou, B. Lerrer, H. Y. Cohen
Nature Aging.2023; 3(8): 911. CrossRef - Impaired BCAA catabolism in adipose tissues promotes age-associated metabolic derangement
Hye-Sook Han, Eunyong Ahn, Eun Seo Park, Tom Huh, Seri Choi, Yongmin Kwon, Byeong Hun Choi, Jueun Lee, Yoon Ha Choi, Yujin L. Jeong, Gwang Bin Lee, Minji Kim, Je Kyung Seong, Hyun Mu Shin, Hang-Rae Kim, Myeong Hee Moon, Jong Kyoung Kim, Geum-Sook Hwang, S
Nature Aging.2023; 3(8): 982. CrossRef - Exploring the diagnostic value, prognostic value, and biological functions of NPC gene family members in hepatocellular carcinoma based on a multi-omics analysis
Keheng Chen, Xin Zhang, Huixin Peng, Fengdie Huang, Guangyu Sun, Qijiang Xu, Lusheng Liao, Zhiyong Xing, Yanping Zhong, Zhichao Fang, Meihua Liao, Shihua Luo, Wencheng Chen, Mingyou Dong
Functional & Integrative Genomics.2023;[Epub] CrossRef - MicroRNA regulation of AMPK in nonalcoholic fatty liver disease
Hao Sun, Jongsook Kim Kemper
Experimental & Molecular Medicine.2023; 55(9): 1974. CrossRef - Serine active site containing protein 1 depletion alters lipid metabolism and protects against high fat diet-induced obesity in mice
Miaomiao Du, Xueyun Li, Fangyi Xiao, Yinxu Fu, Yu Shi, Sihan Guo, Lifang Chen, Lu Shen, Lan Wang, Huang Cheng, Hao Li, Anran Xie, Yaping Zhou, Kaiqiang Yang, Hezhi Fang, Jianxin Lyu, Qiongya Zhao
Metabolism.2022; 134: 155244. CrossRef - cAMP Signaling in Cancer: A PKA-CREB and EPAC-Centric Approach
Muhammad Bilal Ahmed, Abdullah A. A. Alghamdi, Salman Ul Islam, Joon-Seok Lee, Young-Sup Lee
Cells.2022; 11(13): 2020. CrossRef - Hepatic Sam68 Regulates Systemic Glucose Homeostasis and Insulin Sensitivity
Aijun Qiao, Wenxia Ma, Ying Jiang, Chaoshan Han, Baolong Yan, Junlan Zhou, Gangjian Qin
International Journal of Molecular Sciences.2022; 23(19): 11469. CrossRef - The Role of Small Heterodimer Partner-Interacting Leucine Zipper
(SMILE) as a Transcriptional Corepressor in Hepatic Glucose and Lipid
Metabolism
Woo-Ram Park, Byungyoon Choi, Nanthini Sadasivam, Don-Kyu Kim
Trends in Agriculture & Life Sciences.2022; 60: 7. CrossRef - AMPK Localization: A Key to Differential Energy Regulation
Qonita Afinanisa, Min Kyung Cho, Hyun-A Seong
International Journal of Molecular Sciences.2021; 22(20): 10921. CrossRef
- Integration of genomic and transcriptomic data of inbred mouse models for polygenic obesity and leanness revealed “obese” and “lean” candidate alleles in polyadenylation signals

 KDA
KDA
 First
First Prev
Prev





