- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 48(1); 2024 > Article
-
ReviewPathophysiology Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
-
Yajie Fan1,2
 , Zhipeng Yan1, Tingting Li1, Aolin Li1, Xinbiao Fan1, Zhongwen Qi3
, Zhipeng Yan1, Tingting Li1, Aolin Li1, Xinbiao Fan1, Zhongwen Qi3 , Junping Zhang1
, Junping Zhang1
-
Diabetes & Metabolism Journal 2024;48(1):19-36.
DOI: https://doi.org/10.4093/dmj.2023.0110
Published online: January 3, 2024
- 2,218 Views
- 185 Download
1Department of Cardiovascular, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
2Department of Cardiovascular, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
3Institute of Gerontology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
-
Corresponding authors: Zhongwen Qi
 Institute of Gerontology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, No. 1 Caochang Road, Haidian District, Beijing 100091, China E-mail: 13820596855@163.com
Institute of Gerontology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, No. 1 Caochang Road, Haidian District, Beijing 100091, China E-mail: 13820596855@163.com -
Junping Zhang
 Department of cardiovascular, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, No. 88 Changling Road, Xiqing District, Tianjin 300381, China E-mail: tjzhtcm@163.com
Department of cardiovascular, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, No. 88 Changling Road, Xiqing District, Tianjin 300381, China E-mail: tjzhtcm@163.com
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Diabetes heart disease (DHD) is a structural and functional heart disease associated with diabetes mellitus (DM) and occurs in the absence of conventional cardiovascular disease (CVD). DHD mainly includes coronary artery disease, heart failure (HF), autonomic heart disease, and diabetes cardiomyopathy. Specifically, the functional features are dominated by left ventricular diastolic dysfunction, and the structural features include myocardial fibrosis, cardiac hypertrophy, and impairment of coronary microvascular perfusion [1]. Recent studies have shown that DHD is the most common and dangerous complication of DM, affecting more than half of all DM patients [2]. Despite the fact that glycemia control alone can exacerbate myocardial injury, CVD is progressing. In contrast, metformin reduces the incidence of cardiovascular damage [3]. This indicates that enhanced insulin sensitivity, rather than plasma glucose levels, plays an important role in improving DHD outcomes. Insulin resistance is the pivotal link in metabolic disorders and crucial factor in the progression of DHD [4]. Some clinical trials suggest that patients with insulin resistance are twice as likely to develop coronary heart disease as those without insulin resistance [5,6]. The incidence of CVDs in hyperinsulinemic patients exceeds three times that of non-hyperinsulinemic patients. Improving insulin resistance can reduce the risk of CVDs in DM patients by about 55% [3].
- The pathophysiology of insulin resistance leading to DHD has been the topic of research since its first description. Insulin resistance can exacerbate atherosclerosis, myocardial fibrosis and ventricular hypertrophy, leading to cardiac diastolic dysfunction and eventual development of HF. The aim of this review is to provide a comprehensive and updated overview of the clinical, pathogenetic, and molecular aspects of DHD. Firstly, we introduce the pathways of insulin signaling and the development of insulin resistance. We also summarize the mechanisms of insulin resistance to DHD, including myocardial energy metabolism disorders, abnormal calcium cycling, microRNAs (miRNAs), cell cycle processes, vascular injury, cardiac autonomic dysfunction, and inflammation. Finally, we outline the potential targeted therapy for improving insulin resistance during DHD.
INTRODUCTION
- Insulin resistance is a state where the body reduces insulin sensitivity and glucose absorption and processing capacity. Oxidative stress, inflammation, lipid metabolism disorders, epigenetic changes, and intestinal microorganisms can contribute to the development of insulin resistance. Although insulin responses in different cell types vary, this is largely due to different lateral effects, as all insulin-responsive cells have very similar peripheral components. To date, two major insulin signal pathways have been identified: the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway and Ras/mitogen-activated protein kinase (RAS/MAPK) pathway (Fig. 1). The PI3K/Akt signaling pathway is the most classical insulin signaling pathway. A PI3K enzyme comprises two subunits, the catalytic subunit p110 and the regulatory subunit p85. It catalyzes the production of phosphate (3,4,5)-triphosphate (PIP3), which activates Akt by phosphoinositide-dependent kinase 1 (PDPK1) [7,8]. Phosphorylation of Akt plays an instrumental role in cell growth and proliferation, glucose and fatty acid metabolism [7,9]. Insulins also phosphorylate proteins containing SH2 structural domains (SHCs), activate RAS/MAPK signal pathways, and regulate various physiological processes such as cell growth, proliferation, differentiation, and apoptosis, without involvement in metabolism [10].
- Imbalances in insulin signaling consists of two main aspects: on the one hand, inactivation of insulin receptor substrate (IRS) proteins, including down-regulation of receptor structure, number and binding affinity, and on the other hand, dysfunction of insulin signaling pathways and impairment of their signaling capacity. Any abnormal insulin signaling site can cause insulin resistance, for example, IRS degradation, phosphorylation, distribution, blockade, and odd expression of PI3K, Akt, and downstream proteins. Additionally, the effects of insulin resistance on intracellular signaling appear to be pathway-specific [11]. PI3K/Akt signaling is impaired in individuals in a state of insulin resistance, but insulin-activated MAPK pathways are often preserved and are involved in atherosclerotic (AS) lesions [12].
INSULIN RESISTANCE
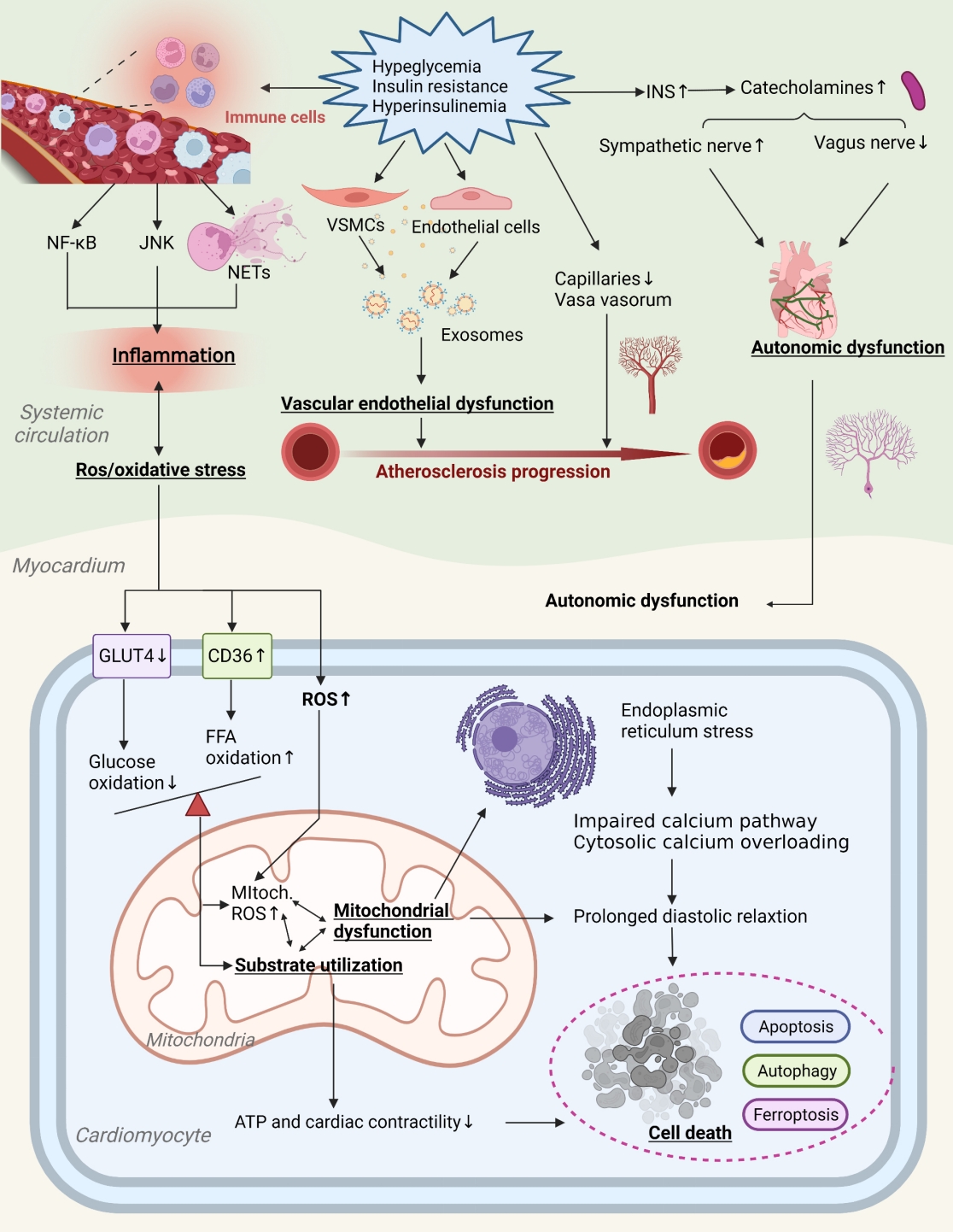
- Potential contributory mechanisms to insulin resistance-induced DHD are gradually expanding. Currently, maturation mediators identified include myocardial metabolic substrates, impaired calcium handling, miRNAs, cell cycle processes, vascular damage, autonomic nervous system of the heart, and inflammation [8,13,14]. Fig. 2 provides a more comprehensive illustration of these contributing mechanisms and the places where they may overlap.
- Insulin resistance affects DHD through myocardial metabolic substrates
- The heart derives approximately two-thirds of its energy requirements from fatty acid oxidation and one-third from glucose oxidation, maintaining a relative balance between the two. As DHD advances, cardiac energy substrate metabolism is altered: cardiomyocytes shift from using both glucose and fatty acid oxidation to relying almost exclusively on fatty acid β-oxidation for energy, resulting in impaired synthesis of high-energy phosphate compounds in cardiac muscle tissue [15]. Insulin resistance plays a role in this change. Depending on the daytime indoor lighting environment, insulin-resistant patients affect the metabolism of the body’s substrate and energy consumption in a time-dependent manner, including an increase in fatty acid oxidation, a reduction in glucose oxidation and glycolysis [16].
- Free fatty acid (FFA) is the primary energy source of the myocardium. Increased expression of peroxisome proliferator-activated receptor α (PPARα) in cardiomyocytes from diabetic patients is associated with intake of FFA, accumulation of triacylglycerol and reduced use of glucose [15]. Furthermore, increased FFA release from fat tissue in turn affects FFA transport in heart cells. Cluster differentiation protein 36 (CD36) promotes the absorption of FFA in cardiomyocytes and increases cardiac lipotoxicity. It was found that insulin resistance induces CD36 upregulation in H9C2 cells and promotes inflammation and lipid accumulation, exacerbating diabetic myocardial metabolic disorders [17,18]. An AMP-activated kinase (AMPK) is an energy-sensing enzyme that is compensatively increased during low cellular energy levels [15]. Insulin resistance status stimulates AMPK, which increases FFA uptake by the heart via Fat -CD36 and increases DHD burden [19]. Abnormalities in lipid metabolism tend to accelerate the development of DHD. Insulin resistance makes insulin ineffective in inhibiting adipocyte lipolysis and increase FFA in the liver, which further stimulates the accumulation and secretion of low-density lipoproteins, leading to disorders of lipid metabolism, lipotoxicity, cardiac steatosis and even myocardial cell necrosis, resulting in diabetic myocardial damage [20].
- The oxidation of glucose and pyruvate is an important factor in the formation and storage of myocardial energy. Pyruvate dehydrogenase (PDH) is an important enzyme that regulates the balance of heart carbohydrates and lipid metabolism. In patients with DHD, PDH activity is reduced and pyruvate oxidation is impaired, which seems to be associated with insulin resistance [21]. The dissociation of glycolysis and pyruvate oxidation in the diabetic heart leads to the accumulation of glycolytic intermediates, which activates specific glucose-sensitive transcription factors. Glucose enters cardiomyocytes through glucose transporters 1 and 4, where GLUT1 is located on the cell membrane, and GLUT4 is transferred to the cell membrane under the activation of Akt, and regulates genes such as glycogen synthase kinase-3 (GSK-3) and forkhead box protein O1 (FoxO1) which promote glucose uptake in cardiomyocytes and inhibit glycogen synthesis [22]. Under conditions of hyperglycemia and insulin resistance, the PI3K/Akt signaling pathway that stimulates the GLUT4 recruitment to plasma membrane is impaired, causing the production of advanced glycation end products (AGEs) and the activation of the chronic hexosamine biosynthesis pathway, leading to heart glucotoxicity and myocardial dysfunction [23,24].
- Ketone bodies are easily oxidized by the heart. Therefore, when the metabolism of other energy substrates is dilated, the ketone body may represent an additional source of energy for the diabetic heart. In fact, increased regulation of enzymes involved in ketone metabolism has been reported in both patients with DHD and advanced HF mice models. Increasing circulating ketone bodies by intravenous infusion of ketone bodies significantly improves cardiac function in patients with DHD [25]. A ketogenic diet extends the lifespan in mice with insulin resistance and improves memory in aged mice [26,27]. In addition, some researchers found that the concentration of ketone bodies was negatively correlated with insulin resistance and triglycerides [28]. Consequently, insulin resistance can affect energy metabolism in the diabetic heart by regulating ketones metabolism.
- Insulin resistance affects DHD through impaired calcium handling
- In cardiomyocytes, the calcium cycle maintains physiological excitation-contraction couplings. The sarcoplasmic reticulum (SR) is the main site of the cardiac calcium cycle. Many Ca2+ enter the cardiomyocytes through L-type calcium channels, induces SR calcium release through the ryanodine receptor (Ryr), and regulates myocardial contractility. Additionally, the SR reuptake of cardiomyocyte Ca2+ is mediated by increased activity of the sarcoplasmic reticulum Ca2+ pump (SERCA), the Na+/Ca2+ exchanger (NCX), and the Ca2+ATPase [23,29]. In diabetes cardiomyopathy, abnormal expressions or activities of Ryr receptors, SERCA2a and NCX allows impaired Ca2+ transients as well as Ca2+ uptake by the SR, affecting myocardial contractile function. At the same time, impaired Ca2 efflux from the extracellular matrix, impaired relaxation of cardiomyocytes, and sustained increase in action potential time can lead to diastolic dysfunction [15,23,30]. These data indicate that weakened Ca2+ treatment in the cardiovascular cells plays an important role in the development of diastolic dysfunction characteristics of early diabetes cardiomyopathy.
- Insulin can enhance the function of the calcium channel through the PI3K/Akt signaling pathway, activate SERCA2a and improve myocardial contraction [8]. On the other hand, a state of cardiac insulin resistance attenuates PI3K/Akt signaling, and decreased glucose uptake can reduce Ca2+ ATPase activity, making it challenging to move Ca2+ back into the SR, resulting in impaired calcium handling [23,29]. A study finds that heart insulin resistance can impair Ca2+ homeostasis through the protein kinase B/striated muscle preferentially expressed protein kinase/SERCA2a (PKB/SPEG/SERCA2a) pathway and promote the development of diabetes cardiomyopathy [31]. The state of insulin resistance also significantly promotes calcium ion and calcium/calmodulin-dependent protein kinase II phosphorylation, thereby affecting calcium metabolism [32]. In addition, reduced nitric oxide (NO) bioavailability can affect T-type Ca2+ channels through the cyclic guanosine monophosphate/protein kinase G (cGMP/PKG) signaling pathway, aggravate myocardial fibrosis caused by hyperglycemia, and lead to contraction deformation of myocytes [33,34].
- Insulin resistance affects DHD through MiRNAs
- MiRNAs are small single-stranded RNA molecules of the noncoding RNA family, which interfere with the repression of mRNA and degradation of protein production by target genes at the posttranscriptional level.
- Diabetes cardiomyopathy is highly correlated with dysregulated expression of miRNAs. Some miRNAs, such as miR-1 and miR-133a, which are abundantly expressed in cardiomyocytes, are significantly reduced in DM patients, thus contributing to the development of diabetes cardiomyopathy [35]. In streptozotocin-induced DM rats, the overexpression of miR133a improved myocardial contractility and mitigated myocardial injury by upregulating tyrosine aminotransferase [36]. In addition, insulin resistance affects the expression levels of miR-155-5p and miR-143-3p, exacerbating diabetes-related vascular disease. MiR-155-5p was overexpressed and miR-143-3p was weakened in atherosclerosis in mice and humans, causing plaque instability and accelerating the progression of atherosclerosis [37]. Insulin resistance is clearly responsible for affecting epigenetics and contributing to DHD development. MiR-690 is an exosome-derived miRNA from M2-polarized macrophages. Recent studies have shown that miR-690 improves glucose tolerance and insulin in obese mice, and it can be a novel insulin sensitizer for metabolic heart disease [38].
- Insulin resistance affects DHD through cell cycle processes
- Mature cardiomyocytes are non-regenerative. Cell death is the main form of cardiomyocyte reduction and is a fundamental cause of cardiac hypertrophy, myocardial fibrosis and ventricular remodeling, leading to progressive HF.
- Apoptosis is a programmatic death process regulated by various genes and proteases. The researchers found that elevated levels of apoptosis markers such as TNF receptor 1 (TNFR-1), TRAIL receptor 2 (TRAILR-2), and Fas increased the risk of myocardial infarction and stroke in patients with DHD [39]. In the PI3K/Akt signaling pathway, in addition to the phosphorylation of mammalian target of rapamycin (mTOR) protein, the GSK-3β/β-catenin signaling pathway can also improve cardiomyocyte apoptosis and improve myocardial injury in rats with myocardial ischemia-reperfusion [40,41]. However, in insulin resistance, the PI3K/Akt signaling pathway is inhibited, but the RAS/MAPK signaling pathway still functions, inducing apoptosis and necrosis in cardiac myocytes and accelerating the progression of diabetes cardiomyopathy [42]. It has been confirmed that the apoptosis of cardiomyocytes induced by insulin resistance depends on dose [43]. In addition, lipotoxicity due to insulin resistance may also lead to increased apoptosis [44]. Endoplasmic reticulum stress (ERS) is involved in apoptosis. In mice fed a high-fat diet, down-regulating ERSrelated proteins and inhibiting the pathways that regulate ERS and sterol regulatory element-binding protein (SREBP)-1c/SREBP-2 improved insulin resistance [45]. Therefore, insulin resistance can play an important role in activating the apoptosis pathway of the ERS cell, which could become an essential path for studying the DHD apoptosis mechanism.
- Autophagy is a highly preserved catabolic process, which plays a key role in maintaining cell homeostasis by degrading and recycling the cytoplasmic content, but this process is often impaired in diabetic hearts [46]. In preclinical tests, dysregulation of autophagy was observed in the diabetic heart [47]. Insulin is an essential regulator of myocardial autophagy, and the unc-51-like kinase 1 (ULK1) dot is an early marker of autophagy formation. It was found that insulin can regulate autophagy by altering ULK1 phosphorylation and FoxO1/3 activation through the Akt/mTOR signaling pathway [48]. Insulin resistance exacerbates diabetes-related cardiac injury by affecting ULK1 expression and altering the expression levels of p62 and light chain 3I (LC3I)/LC3II proteins, leading to dysregulation of cardiomyocyte autophagy [49]. The mTOR mediates autophagy inhibition, and its inhibitor rapamycin improves insulin resistance, alleviates glucose and lipid metabolism disorders, reduces inflammatory reactions, and promotes autophagy [50]. Thus, rapamycin may be a potential treatment for diabetes-related myocardial autophagy. In addition, autophagy can also adversely affect insulin resistance, and a lack of the autophagy related 16-like 1 (ATG16L1) autophagy gene can cause insulin resistance by destroying insulin receptor substrates-1 (IRS1) through Kelch-like 9/Kelchlike 13/complex with cullin 3 (KLHL9/KLHL13/CUL3) mechanisms [51]. Visceral adipose tissue-derived serine protease inhibitor (vaspin) reduces insulin resistance, metabolic disorders and hepatic steatosis [52]. Vaspin was found to protect against myocardial ischemia/reperfusion by upregulating the autophagy flux dependent on AMPK-mTOR and the recovery of lysosomal functions [53]. Vaspin also increased the level of myocardial autophagy and decreased the rate of apoptosis and fibrosis of cardiomyocytes in diabetic rats [54]. Vaspin may be a potential target of autophagy in DHD patients.
- Ferroptosis is caused by the accumulation of lipid peroxides dependent on iron. A study found that the ferroptosis is an important pathogen for the development of diabetes cardiomyopathy, using transmission electron microscopy to study the expression of key regulated ferroptosis in mice, and to observe typical morphological changes in ferroptosis in cardiomyocytes [55]. Glutathione (GSH) and glutathione peroxidase 4 (GPX4) are involved in ferroptosis. Targeting the guanine-rich sequence binding factor 1 (Grsf1)/GPX4 axis improves insulin resistance, inhibits oxidative stress and iron reduction, and protects damaged myocardium [56,57]. GSH formation is dependent on solute carrier family 7 member 11 (SLC7A11), which is an important molecule for the mitigation of lipid peroxidation and ferroptosis. In the hearts of mice with insulin resistance, SLC7A11 and GSH levels were significantly downregulated, leading to ferroptosis in cardiomyocytes [58]. These confirm that insulin resistance disrupts normal cellular function and enhances lipid peroxidation, leading to iron overload in cardiomyocytes and participating in the progression of DHD mechanisms. Nuclear factor erythroid 2-related factor 2 (Nrf2) plays an important role in maintaining the cell redox through the regulation of multiple antioxidants. Recent studies suggest that activation of Nrf2 to inhibit ferroptosis can be a potential therapeutic target for DHD [59]. However, it is not yet clear how Nrf2 activation alters DHD pathogenesis and ferroptosis during development.
- In addition to myocardial cell death pathways, insulin resistance hampers the ability of adult heart stem cells and progenitor cells to expand and differentiate, thereby further deteriorating their senescence phenomena and accelerating the development of DHD [60,61]. Eliminating adipocytes in mice with high-fat diets improves insulin sensitivity, inflammation and glycemic status [62]. It is clear that the senescent cells accumulate in the process of insulin resistance that causes DHD. Another feature of senescent cells is the activation of the senescence-associated secretory phenotype (SASP). Removal of senescent cells using senolytics could decrease cardiac fibrosis and hypertrophy [63]. Therefore, the removal of senolytic-mediated senescent cells can be a means of improving insulin resistance, reducing cardiac remodeling, and preventing DHD progression.
- Insulin resistance affects DHD through vascular damage
- Vascular damage is a major link between insulin resistance and DHD. Vascular endothelial dysfunction (VED), including reduced bioavailability of NO, higher intracellular adhesion molecule-1 (ICAM-1) and endothelin-1 (ET-1), results in reduced vasodilator activity and instability of lipid plaques, which severely affects vascular function and structure. Insulin resistance is positively correlated with VED and can exacerbate vascular damage related to diabetes [64]. In insulin-resistant states, aldosterone and insulin activate glucocorticoid kinase1intheserum by the corticoid salt receptor and insulin receptor, triggering synergistic activity of the sodium channel of the endothelial vascular endothelium, reducing NO production and exacerbating vascular sclerosis [65]. By modeling the dynamics of insulin signaling in the vascular endothelium, insulin resistance is further confirmed to affect PI3K/NO and MAPK/ET-1 pathways, thereby inhibiting endothelial nitric oxide synthase (eNOS) and increasing ET-1 and adhesion molecules [66].
- Exosomes are nano-scale vesicles released by cells. They consist of a lipid bilayer and contain several biomolecules that act as intercellular communication by focusing on receptor cells to release their content. Exosomes secreted by endothelial cells and vascular smooth muscle cells (VSMC) are important in maintaining vessel wall homeostasis. Exosomes secreted by oxidized LDL-treated human arteries (human umbilical vein endothelial cells [HUVECs]) cause hyperlipidemia and increase the expression of the transcripts of metastatic lung adenocarcinoma 1, which increases local inflammation and causes the formation and progression of AS plaques [67]. The exosome plays a role in the mechanisms of insulin resistance causing DHD. The expression of arginase 1 in serum exosomes of diabetic mice and diabetic patients increased, and it was found that it was captured by endothelial cells, inhibiting endothelial NO production and thus promoting DHD-related endothelial functional impairment [68]. Furthermore, circRNA-0077930, delivered by HUVEC exosomes in insulin-resistant states, induces VSMC senescence by lowering the expression of miR-622 and regulating Kras, p21, p53, and p16, which can accelerate the pathological progression of diabetic vascular dysfunction [69]. Exosomes are clearly involved in pathology of vascular damage associated with insulin resistance.
- An endogenous repair process can alleviate vascular endothelial injury. Circulated endothelial progenitor cells (EPCs) are mediators of endothelial repair and contribute to angiogenesis and functional recovery in ischemic tissues in DHD patients [70,71]. Insulin resistance can disrupt EPC function and increase cardiovascular and metabolic risk [71]. The number and location of EPCs is inversely linked to insulin resistance [72]. The use of insulin sensitive metformin and rosiglitazone inhibitors prevents the activation of nuclear factor-κB (NF-κB) and inflammation in the local concentration of PM2.5, improves insulin resistance, and restores the circulating EPC level [73]. Additionally, Nrf2 regulates the survival and angiogenesis functions of EPC. It was found that the expression level of Nrf2 and its downstream genes decreased in the EPCs of DHD patients and db/db mice, and that the increase in the expression of NRF2 increased the resistance of EPCs to the oxidative damage caused by diabetes [74]. Therefore, Nrf2 is a potential therapeutic target to improve insulin resistance, restore EPC function, and inhibit the progression of DHD.
- Vasa vasorum (VV) surrounds the middle and outer layers of the walls of large and medium blood vessels, providing oxygen and nutrients to the walls of blood vessels and surrounding tissues, and expelling cellular metabolic waste. Patients with DHD have impaired VV, resulting in coronary microcirculation disturbances and consequently impaired coronary flow reserve. Several researchers have analyzed cardiac capillary density in patients with end-stage HF and found reduced capillary diameter and higher vascular permeability in patients with combined DM [75]. Insulin resistance states can damage the VV and rare microvasculature, thereby causing vasospasm [76]. Studies have shown that in insulin-resistant environments, factors such as tumor necrosis factor (TNF) and vascular endothelial growth factor (VEGF) can impair vascular maturation and result in smaller, weaker, potentially fracturing vessels, thrombosis and cardiovascular events [77,78]. Another study showed that insulin resistance adipocyte-derived exosomes (IRADEs) exacerbated the insulin resistance status of diabetic ApoE-/- mice, promoted VV production, and increased plaque burden and AS plaque susceptibility index [79]. More research is still needed to demonstrate the relationship between VV and diabetic vascular dysfunction.
- Insulin resistance affects DHD through cardiac autonomic nerves
- Cardiac adipose tissue possesses rich neural distribution. Autonomic dysregulation in patients with DHD may induce arrhythmias, such as ventricular fibrillation and atrial fibrillation. Insulin resistance is closely related to the function of the autonomous nervous system. This was confirmed in a study by using electrophysiological methods to observe autonomic tone in rats fed a high-fat diet [80]. Heart rate variability (HRV) is currently the most common non-invasive index for evaluating autonomic function. Patients with insulin resistance have impaired cardiac autonomic function and reduced HRV indicators [81]. The reason may be that insulin resistance increases the level of insulin and the abnormal increase in catecholamine levels, which affects the four phase depolarization of the heart and electrical activity of the heart, causing the decrease in vagal activity and the increase in sympathetic activity.
- The nerve growth factor (NGF) is a nutritional factor for sympathetic nerve survival and is vital for sympathetic nerve damage repair. Starting from day 3 after myocardial infarction, the level of intramyocardial NGF increased significantly, and the level of sympathetic regeneration was consistent with the level of intramyocardial NGF. NGF reduces insulin resistance, activates IRS1, and improves glucose metabolism and c-Fos expression [82]. It was shown that NGF levels in diabetic mice have decreased and sirtuin 1 (SIRT1) and NGF expression in neuronal tissues have increased, protecting diabetic animals from neuronal damage caused by hyperglycemia [83]. Purinergic ligand-gated ion channel 3 (P2X3) receptors are mainly expressed in primary sensory neurons and are associated with various pathological pains, especially inflammatory pain. P2X3 was found to be involved in the desensitization of afferent neurons in chronic HF and the expression of P2X3 receptors in the L4/5 dorsal root ganglion of rats with HF was 2.8-fold higher than in controls [84]. P2X3 shRNA can reduce sympathetic activity by interfering with P2X3 receptor expression in stellate ganglia, thereby alleviating diabetic cardiac autonomic neuropathy [85]. In addition, a study has found that hyperglycemia can be involved in autonomic injury in DM patients by regulating the activation of the metabolism and/or oxidative states of neuronal cells, such as increased production of the polyol pathway, hexosamine pathway, and AGE [86]. Additionally, renin-angiotensin-aldosterone system (RAAS) activity in the paraventricular nucleus of the hypothalamus raises the signaling of extracellular signal-regulated kinase 1/2 (ERK1/2) MAPK and plays an important role in mediating the sympathetic movement of HF mice [87]. These studies initially suggest that in insulin resistance, autonomic dysfunction is an important part of diabetic cardiac autonomic neuropathy.
- Insulin resistance affects DHD through inflammation
- The role of inflammation in insulin resistance-induced CVDs has been investigated. DHD is often accompanied by high levels of circulating proinflammatory cytokines, such as TNFα, interleukin 6 (IL-6), and IL-18, and cytokine receptors [88]. Insulin resistance is a chronic low-level inflammation state that activates the NF-κB in flammatory pathway, induces NLR family pyrin domain containing 3 (NLRP3) inflammasome expression, and improves chronic inflammatory responses in the system [89]. Furthermore, activation of the NLRP3 inflammasome can exacerbate caspase-1 recruitment, promote cystatin-1 activation, and cleave IL-1β and IL-18 precursors, thereby enhancing the proinflammatory cascade [90,91]. In the type 2 diabetes mellitus (T2DM) rat model, activated NLRP3 inflammasome leads to inflammatory cell expansion and infiltration, which plays a crucial role in the pathogenesis of HF, and reduced NLRP3 inflammasome causes cardiomyopathy in T2DM rats [92]. Moreover, insulin resistance can also induce the c-Jun N-terminal kinases (JNKs) inflammatory pathway.
- An important feature of the inflammatory response is the migration and aggregation of neutrophils. NETosis is a specific neutrophil cell death process in which neutrons release their nucleiin the form of neutron extracellular traps (NETs) [93]. NETosis plays a role in insulin resistance-induced inflammatory heart disease, including myocardial injury and vascular disease. An animal model established by Wnt5a-mediated infiltration of neutrophils into the heart confirmed that NETs lead to excessive inflammation and cardiac insufficiency [94]. Another study has also confirmed the potential role of excess or dysregulated NETosis in atherosclerosis development [95]. NETs release products are significantly increased in insulin-resistant patients [96]. Therefore, NETs may mediate the inflammatory response between insulin resistance and DHD. Furthermore, insulin resistance leads to a switch from a proinflammatory M2 to an anti-inflammatory M1 macrophage polarity, thereby escalating inflammatory responses [91]. Early anti-inflammatory or insulin sensitization treatment strategies have potential benefits in individuals with features of insulin resistance syndrome.
- In the state of insulin resistance, reactive oxygen species increased dramatically, and endogenous antioxidant factors such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were decreased in cardiomyocytes, exacerbating the high oxidative stress state [97]. The family protein nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (Nox1) is involved in the oxidative stress process. This protein is up-regulated in diabetic mice, and knocking down it can delay the progression of atherosclerosis [98]. Therefore, Nox1 could be a potential target for improving insulin resistance and delaying the progression of DHD.
IMPACT OF INSULIN RESISTANCE ON DIABETES HEART DISEASE
Free fatty acid
Glucose
Ketone bodies
Apoptosis
Autophagy
Ferroptosis
Exosomes
Endothelial progenitor cells
Vasa vasorum
- Here, we mainly aimed to identify agents that both improve insulin resistance and have cardiovascular benefits. These agents can directly or indirectly target IRS1 and IRS2 and their associated protein kinases and gene expression to activate insulin signaling pathways, which may provide potential therapeutic options for the prevention and treatment of insulin resistance and its cardiovascular complications (Table 1).
- Hypoglycemic agents are currently the first choice of drugs for clinical improvement of insulin resistance. Biguanides is currently the first-line drug for the treatment of DM with HF [99-101]. A 49-year prospective open randomized study of patients with metabolic syndrome and diabetes showed that the use of metformin (1,000 mg two times a day) in addition to an improvement in lifestyle significantly reduced insulin resistance and improved diabetes [102]. Thiazolidinediones are PPARγ agonists that increase tissue sensitivity to insulin, enhance glucose utilization, and reduce blood FFA concentrations to improve myocardial energy metabolism [103-105]. However, edema is a potential side effect and should be used with caution in subjects at risk for HF, and the degree of edema should be monitored regularly. Exenatide, a glucagon-like peptide 1 receptor (GLP-1R) agonist, improves calcium circulation, cardiac systolic function and endothelial function, and has the potential to treat T2DM combined with HF [106,107]. However, this class of drugs may directly affect sinus node myocytes and enhance sympathetic nervous system activity, and the therapeutic effect in HF clinical treatment is unstable. Dipeptide-IV inhibitors inhibit the MAPK and NF-κB signal pathways, reduce inflammatory responses and improve insulin resistance [108]. However, in the EXAMINE trial, alogliptin increased the incidence of HF in patients who had signs of HF at the time of randomization grouping [109]. Further evidence is needed to draw reliable conclusions about the cardiovascular safety of alogliptin in patients with T2DM. Sodium glucose co-transporter 2 inhibitor (SGLT-2i) are novel glucose-lowering agents that promise to break the vicious cycle of insulin resistance and HF. Englestrin improves β-cell function and insulin sensitivity, regulates mitochondrial function, reduces plasma volume and osmotic diuresis, attenuates ventricular remodeling, myocardial energy metabolism, and adipokine kinetics, and mediates cardioprotective and renoprotective effects [110-113]. Further clinical trials are needed to verify long-term safety and effectiveness.
- Some lipid lowering agents have shown have shown numerous effects beyond the ability to control blood lipids and improved insulin resistance. Statins can increase AMPK activation and inhibit FoxO1 to counteract cardiac hypertrophy. However, some studies have found that statins can cause insulin resistance, possibly due to differences in statin doses [114,115]. Fibrates downregulates phosphokinase and phosphatase-1 activity in mitochondria and improves insulin sensitivity [116]. However, such drugs have the disadvantages of low bioavailability, poor patient compliance, and individual differences. There is no clinical study of fibrates in the treatment of T2DM complicated by HF. Antiplatelet agents help to improve islet survival and function, islet graft outcome, and insulin gene expression [117-120]. However, platelet reactivity is enhanced in patients with insulin resistance, and the risk of thrombosis and bleeding should be taken into account. Trimetazidine is an anti-anginal drug. Studies have shown that trimetazidine activates insulin signaling pathways, reduces cardiomyocyte apoptosis and enhances anti-inflammatory and antioxidant capacity, significantly improving glycated hemoglobin, blood glucose, myocardial velocity and other indices [121]. RAAS inhibitors RAAS inhibitors can resist islet fibrosis, improve islet β-cell function and protect the kidney [122]. Early intervention can prevent the development of microalbuminuria in patients with T2DM. Angiotensin-converting enzyme inhibitors (ACEIs) have a more advantageous cardioprotective effect than angiotensin receptor blockers (ARBs) [123]. New compounds of angiotens inreceptor enkephalinase inhibitors (ARNis) improve cardiac function, promote energy expenditure, and reduce hospitalization rates of HF patients with DM [124,125].
- Recent clinical trials have demonstrated the benefits of non-steroidal mineralocorticoid receptor antagonists (MRAs) in DM patients. Finerenone, a non-steroidal highly selective MRA, was developed based on the structure of dihydropyridine [126]. The secondary analysis of the FIGARO-DKD Trial on HF has confirmed that finerenone can significantly reduce the incidence of new HF, improve HF-related prognosis, and reduce cardiovascular death or the composite endpoint of hospitalization for HF, for patients with T2DM and chronic kidney disease, regardless of their history of HF [127]. The HF subgroup analysis of the FIDELITY-DKD Trial supports this statement [128]. Finerenone brings a new therapeutic option for the prevention and treatment of cardiovascular risk management in patients with DHD. α-1 Adrenergic receptor antagonists can be used alone or in combination with other drug groups in a broad range of hypertensive patients. They can also improve insulin sensitivity and adverse blood lipids in many hypertensive patients [129,130]. β-Blockers are crucial to the treatment of patients with HF with reduced ejection fraction [131]. Currently, the evidence that selective α/β blockers improve insulin sensitivity is still very minimal, and more research is needed to verify this.
- In addition to the above agents, some targets strengthen the link between insulin resistance and CVD, such as O-GlcNAcase (OGA), SPEG, fibroblast growth factor 2 (FGF2), and exosomes like vesicles (ELVs). Researchers have discovered that DM patients are also at risk for heart disease from the hyperglycemia-related hexosamine biosynthesis pathway and the OGlcNAcylation pathway. Chronic elevation of O-GlcNAcylation induces mitochondrial dysfunction and impaired left ventricular function [132]. Moreover, reducing excess O-GlcNAcylation is beneficial for restoring Ca2+ handling and cardiac contractile function in cardiomyocytes [133]. To prevent diabetes cardiomyopathy, targeting hexosamine biosynthesis or O-GlcNAcylation may be a promising approach. SPEG, a member of the myosin light chain kinase family, can regulate SERCA2a activation and is a new target for treating diabetes cardiomyopathy [132,134]. In addition to these new targets, FGF2 produced by skeletal muscle has similar effects to FGF1 in adipocytes, both promoting lipolysis and acting through FGF receptors. Studies have found that FGF2 can work directly on cardiomyocytes to maintain myocardial integrity and function and prevent damage during oxidative stress [135], which may serve as a potential target for insulin sensitization and cardiovascular protection. In addition, ELV dysregulation plays a functional role in various metabolic, autoimmune, and CVDs. ELV quantity is positively correlated with insulin resistance, and macrophages treated with ELV can inhibit the phosphorylation of Akt in human adipocytes [136,137]. Targeting ELV may be a novel mediator and therapeutic target for treating insulin resistance and β-cell exhaustion.
TARGETING INSULIN RESISTANCE IN DHD
- Insulin resistance and subsequent metabolic disorders are the main drivers of the pathological processes specific to DHD. Although many current studies are underway, the pathology of insulin resistance, which affects the structure and function of the heart, has not yet been fully explained, including mitochondrial dysfunction, amino acid transport and metabolism, additional signaling molecules, hormones, kinases, phosphatases, and modifications of gene expression [138]. It was found that the oxidative capacity of mitochondria in T2DM mice was reduced by dysregulating mitochondrial fission/fusion proteins, including dynamin-related protein-1 (DRP1) and optic atrophy-1 (OPA1) [56,139,140]. Insulin resistance is involved in the dysfunction of the mitochondria in the heart through the AktmTOR-NF-κB signaling pathway, resulting in a reduction in the potential of the mitochondrial membrane, a decrease in the activity of the electron transport chain, and abnormal mitochondrial biogenesis [8]. In the future, as a research methodology, different signaling pathways of physiological effects of insulin can be used as mainline modules, and the interrelationships between each module and the upstream and downstream of different pathways can be analyzed to further clarify the specific pathological mechanisms of insulin resistance to DHD. In addition, the link between intestine dysbiosis and insulin resistance has gained attention [141]. Researchers found that the intake of probiotics in rats significantly improved insulin resistance caused by high sugar diets, reduced low-level chronic inflammation and oxidative stress, and regulated the body’s energy metabolism [142]. Intestinal flora imbalance is a breakthrough in the study of insulin resistance and the DHD mechanisms. In addition to medication, regular exercise is an important modifiable factor in improving cardiac function in patients with DHD. A nationwide retrospective cohort study in Korea found an association between sustained active physical exercise and a reduced risk of major adverse cardiovascular events in patients with DM, regardless of the type or amount of exercise [143].
- Since subclinical cardiac disorders can be reversible when detected early, insulin replacement therapy and insulin sensitive chemotherapy are essential to reduce the risk of HF in DHD patients. Future urgent research is needed to rigorously test this possibility. Presently, there are still no ideal clinical interventions to combat insulin resistance in childhood [144,145]. Careful stratification or phenotyping of subjects may help to identify which signaling pathways are disturbed and facilitate the development of target-specific therapies. In addition, exosomes as a breakthrough with corresponding inhibitors will be a new target for the prevention or treatment of insulin resistance and DHD.
CONCLUSION AND PERSPECTIVE
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This work was supported by the “Hundred Million” Talent Project of Chinese Medicine Inheritance and Innovation (QI Huang Project) QI Huang Scholar (Zhang Junping) Special Grant (No. 2021); Tianjin Famous Chinese Medicine Doctor (Zhang Junping) Inheritance Studio Special Grant (No. 2020); National Natural Science Foundation of China Project (30672734).
NOTES
-
Acknowledgements
- None

| Category | Mechanisms of action | Effects on cardiac function | Reference |
|---|---|---|---|
| Biguanide | ↑ Glu uptake and FA oxidation | ↓ Exacerbation of diabetes cardiomyopathy | [99-102] |
| ↓ NF-κB and inflammatory factors | |||
| ↓ Cardiomyocytes and fibroblast LV remodeling | |||
| Thiazolidinedione | ↑ The sensitivity to insulin and the utilization of glucose | ↓ The risk of recurrent MACE, stroke, or myocardial infarction in DHD patients | [103-105] |
| ↑ Myocardial energy metabolism | |||
| ↓ Inflammation, lipid and protein metabolism | |||
| ↓ Atherosclerotic plaque volume | |||
| GLP-1 receptor agonist | ↓ Inflammatory myocardial remodeling | ↑ LVEF and 6-minute walk test | [106,107] |
| ↑ Calcium and cardiac systolic | ↓ HF worsening hospitalization rate | ||
| ↑ Microcirculation perfusion and angiogenesis | Renal protective effect | ||
| Dipeptidyl peptidase-IV inhibitor | ↑ IRS2 mRNA and IRS2/PI3K | ↓ The occurrence of MACE | [108,109] |
| ↓ NF-κB | Use of alogliptin and saxagliptin in patients with pre-existing HF is controversial | ||
| =/↑ diastolic function | ↓ Risk of hospitalization for HF | ||
| SGLT-2 inhibitor | ↑ β-Cell and insulin sensitivity | Renal protective effect | [110-113] |
| ↑ Osmotic diuresis and natriuresis | |||
| ↓ Sodium-hydrogen exchanger | |||
| ↓ The reabsorption of glucose | |||
| ↑ Urinary glucose excretion | |||
| Statin | ↑ PI3K/Akt/eNOS | ↓ The occurrence of adverse cardiovascular events | [114,115] |
| ↑ AMPK | |||
| ↑ LVEF and HR | |||
| ↓ Myocardial inflammatory factors | |||
| Fibrate | ↑ The cardiac PPARα and FoxO1 | ↑ Cardiac function | [116] |
| ↑ Myocardial energy metabolism | |||
| ↓ Ventricular remodeling | |||
| Antiplatelet agent | ↑ Islet survival and function | ↓ The incidence of CVD and all-cause mortality in DM patients | [117-120] |
| ↑ Islet transplantation outcomes and insulin gene expression | |||
| ↓ Lipid oxidation | |||
| Trimetazidine | ↑ P38MAPK and Akt; | ↑ LVEF | [121] |
| ↓ Cardiomyocytes apoptosis | ↓ The occurrence of adverse cardiovascular events | ||
| ↑ Anti-inflammatory and antioxidant capacity | |||
| Angiotensin-converting enzyme inhibitor | ↓ MAPK and oxidative stress | ↓ The incidence of CVD and all-cause mortality in DM patients | [122-124] |
| ↑ Anti-islet fibrosis, islet β-cell function | ↓ Hospitalization rate of HF patients with DM | ||
| Renal protective effect | ↓ Proteinuria | ||
| Angiotensin receptor blocker | ↑ PI3K/Akt/eNOS | ↓ Proteinuria | [122,123] |
| ↓ Oxidative stress | Weaker cardioprotective effect than ACEI | ||
| Renal protective effect | |||
| Non-steroidal mineralocorticoid receptor antagonist | ↓ Inflammation | ↓ The occurrence of MACE | [126-128] |
| ↓ Specific pro-fibrotic cardiac genes | ↓ Risk of hospitalization for HF | ||
| ↓ Hypertrophy of cardiomyocytes | Renal protective effect |
Symbol ↑ indicates that the activation of pathways or effects and symbol ↓ indicates that the inhibition of pathways or effects.
DHD, diabetes heart disease; Glu, glucose; FA, fatty acid; NF-κB, nuclear factor-κB; LV, left ventricular; IRS2, insulin receptor substrate 2; PI3K, phosphatidylinositol 3-kinase; MACE, major adverse cardiovascular events; GLP-1, glucagon-like peptide 1; LVEF, left ventricular ejection fraction; HF, heart failure; SGLT-2, sodium glucose co-transporter 2; Akt, protein kinase B; eNOS, endothelial nitric oxide synthase; AMPK, AMPactivated kinase; HR, heart rate; PPARα, peroxisome proliferator-activated receptor α; FoxO1, forkhead box protein O1; CVD, cardiovascular disease; DM, diabetes mellitus; MAPK, mitogen-activated protein kinase; ACEI, angiotensin-converting enzyme inhibitor.
- 1. Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res 2020;126:1501-25.ArticlePubMedPMC
- 2. Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease. Part 1: the epidemiology and risk factors. Circ Res 2017;121:677-94.ArticlePubMed
- 3. Adeva-Andany MM, Martinez-Rodriguez J, Gonzalez-Lucan M, Fernandez-Fernandez C, Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr 2019;13:1449-55.ArticlePubMed
- 4. Laakso M. Is insulin resistance a feature of or a primary risk factor for cardiovascular disease? Curr Diab Rep 2015;15:105.ArticlePubMedPDF
- 5. Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care 2003;26:1513-7.PubMed
- 6. Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 2002;25:1177-84.PubMed
- 7. Saltiel AR. Insulin signaling in health and disease. J Clin Invest 2021;131:e142241.ArticlePubMedPMC
- 8. Ye H, He Y, Zheng C, Wang F, Yang M, Lin J, et al. Type 2 diabetes complicated with heart failure: research on therapeutic mechanism and potential drug development based on insulin signaling pathway. Front Pharmacol 2022;13:816588.ArticlePubMedPMC
- 9. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 2018;98:2133-23.ArticlePubMedPMC
- 10. Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta 2011;1813:1619-33.ArticlePubMed
- 11. Harmancey R, Haight DL, Watts KA, Taegtmeyer H. Chronic hyperinsulinemia causes selective insulin resistance and downregulates uncoupling protein 3 (UCP3) through the activation of sterol regulatory element-binding protein (SREBP)-1 transcription factor in the mouse heart. J Biol Chem 2015;290:30947-61.ArticlePubMedPMC
- 12. Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes 2003;52:2562-9.ArticlePubMedPDF
- 13. Paneni F, Costantino S, Cosentino F. Insulin resistance, diabetes, and cardiovascular risk. Curr Atheroscler Rep 2014;16:419.ArticlePubMedPDF
- 14. Gargiulo P, Perrone-Filardi P. “Heart failure, whole-body insulin resistance and myocardial insulin resistance: an intriguing puzzle”. J Nucl Cardiol 2018;25:177-80.ArticlePubMedPDF
- 15. Wang LY, Chen C. Energy metabolism homeostasis in cardiovascular diseases. J Geriatr Cardiol 2021;18:1044-57.PubMedPMC
- 16. Harmsen JF, Wefers J, Doligkeit D, Schlangen L, Dautzenberg B, Rense P, et al. The influence of bright and dim light on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals depends on time of day. Diabetologia 2022;65:721-32.ArticlePubMedPMCPDF
- 17. Han B, Wang J, Wu J, Yan F, Wang Y, Li J. High glucose-induced upregulation of CD36 promotes inflammation stress via NF-κB in H9c2 cells. Mol Med Rep 2021;24:764.ArticlePubMedPMC
- 18. Shu H, Peng Y, Hang W, Nie J, Zhou N, Wang DW. The role of CD36 in cardiovascular disease. Cardiovasc Res 2022;118:115-29.ArticlePubMedPMCPDF
- 19. Song F, Mao YJ, Hu Y, Zhao SS, Wang R, Wu WY, et al. Acacetin attenuates diabetes-induced cardiomyopathy by inhibiting oxidative stress and energy metabolism via PPAR-α/AMPK pathway. Eur J Pharmacol 2022;922:174916.ArticlePubMed
- 20. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 2018;17:122.ArticlePubMedPMCPDF
- 21. Karwi QG, Wagg CS, Altamimi TR, Uddin GM, Ho KL, Darwesh AM, et al. Insulin directly stimulates mitochondrial glucose oxidation in the heart. Cardiovasc Diabetol 2020;19:207.ArticlePubMedPMCPDF
- 22. Longo M, Scappaticcio L, Cirillo P, Maio A, Carotenuto R, Maiorino MI, et al. Glycemic control and the heart: the tale of diabetic cardiomyopathy continues. Biomolecules 2022;12:272.ArticlePubMedPMC
- 23. Salvatore T, Pafundi PC, Galiero R, Albanese G, Di Martino A, Caturano A, et al. The diabetic cardiomyopathy: the contributing pathophysiological mechanisms. Front Med (Lausanne) 2021;8:695792.ArticlePubMedPMC
- 24. Daniels MC, McClain DA, Crook ED. Transcriptional regulation of transforming growth factor β1 by glucose: investigation into the role of the hexosamine biosynthesis pathway. Am J Med Sci 2020;359:79-83.ArticlePubMed
- 25. Lopaschuk GD, Karwi QG, Ho KL, Pherwani S, Ketema EB. Ketone metabolism in the failing heart. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158813.ArticlePubMed
- 26. Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 2018;27:1156.ArticlePubMedPMC
- 27. Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab 2017;26:547-57.ArticlePubMedPMC
- 28. Garcia E, Shalaurova I, Matyus SP, Oskardmay DN, Otvos JD, Dullaart RP, et al. Ketone bodies are mildly elevated in subjects with type 2 diabetes mellitus and are inversely associated with insulin resistance as measured by the lipoprotein insulin resistance index. J Clin Med 2020;9:321.ArticlePubMedPMC
- 29. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin 2012;8:609-17.PubMedPMC
- 30. Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes 2002;51:1166-71.ArticlePubMedPDF
- 31. Quan C, Du Q, Li M, Wang R, Ouyang Q, Su S, et al. A PKBSPEG signaling nexus links insulin resistance with diabetic cardiomyopathy by regulating calcium homeostasis. Nat Commun 2020;11:2186.ArticlePubMedPMCPDF
- 32. Roe ND, He EY, Wu Z, Ren J. Folic acid reverses nitric oxide synthase uncoupling and prevents cardiac dysfunction in insulin resistance: role of Ca2+/calmodulin-activated protein kinase II. Free Radic Biol Med 2013;65:234-43.ArticlePubMedPMC
- 33. Qin L, Zang M, Xu Y, Zhao R, Wang Y, Mi Y, et al. Chlorogenic acid alleviates hyperglycemia-induced cardiac fibrosis through activation of the NO/cGMP/PKG pathway in cardiac fibroblasts. Mol Nutr Food Res 2021;65:e2000810.ArticlePubMedPDF
- 34. Li Q, Zhang Y, Wu N, Yin N, Sun XH, Wang Z. Activation of somatostatin receptor 5 suppresses T-type Ca2+ channels through NO/cGMP/PKG signaling pathway in rat retinal ganglion cells. Neurosci Lett 2019;708:134337.ArticlePubMed
- 35. de Gonzalo-Calvo D, van der Meer RW, Rijzewijk LJ, Smit JW, Revuelta-Lopez E, Nasarre L, et al. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci Rep 2017;7:47.PubMedPMC
- 36. Nandi SS, Zheng H, Sharma NM, Shahshahan HR, Patel KP, Mishra PK. Lack of miR-133a decreases contractility of diabetic hearts: a role for novel cross talk between tyrosine aminotransferase and tyrosine hydroxylase. Diabetes 2016;65:3075-90.ArticlePubMedPMCPDF
- 37. Gonzalez-Lopez P, Ares-Carral C, Lopez-Pastor AR, InfanteMenendez J, Gonzalez Illaness T, Vega de Ceniga M, et al. Implication of miR-155-5p and miR-143-3p in the vascular insulin resistance and instability of human and experimental atherosclerotic plaque. Int J Mol Sci 2022;23:10253.ArticlePubMedPMC
- 38. Ying W, Gao H, Dos Reis FC, Bandyopadhyay G, Ofrecio JM, Luo Z, et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab 2021;33:781-90.ArticlePubMedPMC
- 39. Mattisson IY, Bjorkbacka H, Wigren M, Edsfeldt A, Melander O, Fredrikson GN, et al. Elevated markers of death receptoractivated apoptosis are associated with increased risk for development of diabetes and cardiovascular disease. EBioMedicine 2017;26:187-97.ArticlePubMedPMC
- 40. Gong DD, Yu J, Yu JC, Jiang XD. Effect of miR-26a targeting GSK-3β/β-catenin signaling pathway on myocardial apoptosis in rats with myocardial ischemia-reperfusion. Eur Rev Med Pharmacol Sci 2019;23:7073-82.PubMed
- 41. Tong L, Li W, Zhang Y, Zhou F, Zhao Y, Zhao L, et al. Tacrolimus inhibits insulin release and promotes apoptosis of Min6 cells through the inhibition of the PI3K/Akt/mTOR pathway. Mol Med Rep 2021;24:658.ArticlePubMed
- 42. Forzisi E, Yu W, Rajwade P, Sesti F. Antagonistic roles of RasMAPK and Akt signaling in integrin-K+ channel complexmediated cellular apoptosis. FASEB J 2022;36:e22292.ArticlePubMedPMCPDF
- 43. Adel FW, Zheng Y, Wan SH, Greason C, Pan S, Ameenuddin S, et al. Insulin therapy is associated with increased myocardial interstitial fibrosis and cardiomyocyte apoptosis in a rodent model of experimental diabetes. Front Physiol 2022;13:890907.ArticlePubMedPMC
- 44. Park MJ, Han HJ, Kim DI. Lipotoxicity-induced PRMT1 exacerbates mesangial cell apoptosis via endoplasmic reticulum stress. Int J Mol Sci 2017;18:1421.ArticlePubMedPMC
- 45. Wu L, Guo T, Deng R, Liu L, Yu Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 cells and high-fat diet-fed mice. J Pharmacol Exp Ther 2021;377:146-56.ArticlePubMed
- 46. Dewanjee S, Vallamkondu J, Kalra RS, John A, Reddy PH, Kandimalla R. Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev 2021;68:101338.ArticlePubMed
- 47. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 2018;122:624-38.ArticlePubMedPMC
- 48. Frendo-Cumbo S, Tokarz VL, Bilan PJ, Brumell JH, Klip A. Communication between autophagy and insulin action: at the crux of insulin action-insulin resistance? Front Cell Dev Biol 2021;9:708431.ArticlePubMedPMC
- 49. Han K, Jia N, Zhong Y, Shang X. S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J Cell Biochem 2018;119:3111-7.ArticlePubMedPDF
- 50. Zhou W, Ye S. Rapamycin improves insulin resistance and hepatic steatosis in type 2 diabetes rats through activation of autophagy. Cell Biol Int 2018;42:1282-91.ArticlePubMedPDF
- 51. Frendo-Cumbo S, Jaldin-Fincati JR, Coyaud E, Laurent EM, Townsend LK, Tan JM, et al. Deficiency of the autophagy gene ATG16L1 induces insulin resistance through KLHL9/KLHL13/CUL3-mediated IRS1 degradation. J Biol Chem 2019;294:16172-85.ArticlePubMedPMC
- 52. Jaganathan R, Ravindran R, Dhanasekaran S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can J Diabetes 2018;42:446-56.ArticlePubMed
- 53. Yang F, Xue L, Han Z, Xu F, Cao S, Dai S, et al. Vaspin alleviates myocardial ischaemia/reperfusion injury via activating autophagic flux and restoring lysosomal function. Biochem Biophys Res Commun 2018;503:501-7.ArticlePubMed
- 54. Li X, Ke X, Li Z, Li B. Vaspin prevents myocardial injury in rats model of diabetic cardiomyopathy by enhancing autophagy and inhibiting inflammation. Biochem Biophys Res Commun 2019;514:1-8.ArticlePubMed
- 55. Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B 2022;12:708-22.ArticlePubMedPMC
- 56. Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, et al. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients 2020;12:2954.ArticlePubMedPMC
- 57. Schwarzler J, Mayr L, Radlinger B, Grabherr F, Philipp M, Texler B, et al. Adipocyte GPX4 protects against inflammation, hepatic insulin resistance and metabolic dysregulation. Int J Obes (Lond) 2022;46:951-9.ArticlePubMedPDF
- 58. Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res 2020;127:486-501.ArticlePubMed
- 59. Wang G, Song X, Zhao L, Li Z, Liu B. Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. Biomed Res Int 2018;2018:2150218.ArticlePubMedPMCPDF
- 60. Cianflone E, Torella M, Biamonte F, De Angelis A, Urbanek K, Costanzo FS, et al. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells 2020;9:1558.ArticlePubMedPMC
- 61. Vecellio M, Spallotta F, Nanni S, Colussi C, Cencioni C, Derlet A, et al. The histone acetylase activator pentadecylidenemalonate 1b rescues proliferation and differentiation in the human cardiac mesenchymal cells of type 2 diabetic patients. Diabetes 2014;63:2132-47.ArticlePubMedPDF
- 62. Rouault C, Marcelin G, Adriouch S, Rose C, Genser L, Ambrosini M, et al. Senescence-associated β-galactosidase in subcutaneous adipose tissue associates with altered glycaemic status and truncal fat in severe obesity. Diabetologia 2021;64:240-54.ArticlePubMedPDF
- 63. Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016;15:973-7.ArticlePubMedPMCPDF
- 64. Westergren HU, Svedlund S, Momo RA, Blomster JI, Wahlander K, Rehnstrom E, et al. Insulin resistance, endothelial function, angiogenic factors and clinical outcome in non-diabetic patients with chest pain without myocardial perfusion defects. Cardiovasc Diabetol 2016;15:36.ArticlePubMedPMC
- 65. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021;119:154766.ArticlePubMed
- 66. Muniyappa R, Chen H, Montagnani M, Sherman A, Quon MJ. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: insights from mechanistic modeling. Am J Physiol Endocrinol Metab 2020;319:E629-46.ArticlePubMedPMC
- 67. Gao H, Wang X, Lin C, An Z, Yu J, Cao H, et al. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol Chem 2020;401:367-76.ArticlePubMed
- 68. Zhang H, Liu J, Qu D, Wang L, Wong CM, Lau CW, et al. Serum exosomes mediate delivery of arginase 1 as a novel mechanism for endothelial dysfunction in diabetes. Proc Natl Acad Sci U S A 2018;115:E6927-36.ArticlePubMedPMC
- 69. Wang S, Zhan J, Lin X, Wang Y, Wang Y, Liu Y. CircRNA-0077930 from hyperglycaemia-stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochem Funct 2020;38:1056-68.PubMed
- 70. Jain R, Awal H, Sen S. Using adult stem cells to monitor endothelial dysfunction in diabetes mellitus. J Diabetes Complications 2020;34:107588.ArticlePubMed
- 71. Peyter AC, Armengaud JB, Guillot E, Yzydorczyk C. Endothelial progenitor cells dysfunctions and cardiometabolic disorders: from mechanisms to therapeutic approaches. Int J Mol Sci 2021;22:6667.ArticlePubMedPMC
- 72. Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J 2006;27:2247-55.ArticlePubMed
- 73. Haberzettl P, McCracken JP, Bhatnagar A, Conklin DJ. Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am J Physiol Heart Circ Physiol 2016;310:H1423-38.ArticlePubMedPMC
- 74. Dai X, Wang K, Fan J, Liu H, Fan X, Lin Q, et al. Nrf2 transcriptional upregulation of IDH2 to tune mitochondrial dynamics and rescue angiogenic function of diabetic EPCs. Redox Biol 2022;56:102449.ArticlePubMedPMC
- 75. Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, et al. Diabetes mellitus-induced microvascular destabilization in the myocardium. J Am Coll Cardiol 2017;69:131-43.ArticlePubMed
- 76. Shikama M, Sonoda N, Morimoto A, Suga S, Tajima T, Kozawa J, et al. Association of crossing capillaries in the finger nailfold with diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Investig 2021;12:1007-14.ArticlePubMedPMCPDF
- 77. O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 1996;93:672-82.ArticlePubMed
- 78. Owusu J, Barrett E. Early microvascular dysfunction: is the vasa vasorum a “missing link” in insulin resistance and atherosclerosis. Int J Mol Sci 2021;22:7574.ArticlePubMedPMC
- 79. Wang F, Chen FF, Shang YY, Li Y, Wang ZH, Han L, et al. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE-/- mice. Int J Cardiol 2018;265:181-7.ArticlePubMed
- 80. Tanida M, Imanishi K, Akashi H, Kurata Y, Chonan O, Naito E, et al. Injection of Lactobacillus casei strain Shirota affects autonomic nerve activities in a tissue-specific manner, and regulates glucose and lipid metabolism in rats. J Diabetes Investig 2014;5:153-61.PubMed
- 81. Lundqvist MH, Almby K, Wiklund U, Abrahamsson N, Kamble PG, Pereira MJ, et al. Altered hormonal and autonomic nerve responses to hypo- and hyperglycaemia are found in overweight and insulin-resistant individuals and may contribute to the development of type 2 diabetes. Diabetologia 2021;64:641-55.ArticlePubMedPDF
- 82. Sposato V, Canu N, Fico E, Fusco S, Bolasco G, Ciotti MT, et al. The medial septum is insulin resistant in the AD presymptomatic phase: rescue by nerve growth factor-driven IRS1 activation. Mol Neurobiol 2019;56:535-52.ArticlePubMedPDF
- 83. Oza MJ, Kulkarni YA. Formononetin ameliorates diabetic neuropathy by increasing expression of SIRT1 and NGF. Chem Biodivers 2020;17:e2000162.ArticlePubMedPDF
- 84. Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 2010;588(Pt 24s):5033-47.ArticlePubMedPMC
- 85. Xu X, Liu B, Yang J, Zou Y, Sun M, Li Z, et al. Glucokinase in stellate ganglia cooperates with P2X3 receptor to develop cardiac sympathetic neuropathy in type 2 diabetes rats. Brain Res Bull 2020;165:290-7.ArticlePubMed
- 86. Limberg JK, Soares RN, Padilla J. Role of the autonomic nervous system in the hemodynamic response to hyperinsulinemia-implications for obesity and insulin resistance. Curr Diab Rep 2022;22:169-75.ArticlePubMedPMCPDF
- 87. Yu Y, Wei SG, Zhang ZH, Weiss RM, Felder RB. ERK1/2 MAPK signaling in hypothalamic paraventricular nucleus contributes to sympathetic excitation in rats with heart failure after myocardial infarction. Am J Physiol Heart Circ Physiol 2016;310:H732-9.ArticlePubMedPMC
- 88. Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev 2010;15:331-41.ArticlePubMedPDF
- 89. Festa A, D’Agostino R Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42-7.ArticlePubMed
- 90. Fuentes-Antras J, Ioan AM, Tunon J, Egido J, Lorenzo O. Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int J Endocrinol 2014;2014:847827.PubMedPMC
- 91. Orliaguet L, Ejlalmanesh T, Alzaid F. Metabolic and molecular mechanisms of macrophage polarisation and adipose tissue insulin resistance. Int J Mol Sci 2020;21:5731.ArticlePubMedPMC
- 92. Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 2014;9:e104771.ArticlePubMedPMC
- 93. Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 2015;52:497-503.ArticlePubMedPDF
- 94. Wang Y, Sano S, Oshima K, Sano M, Watanabe Y, Katanasaka Y, et al. Wnt5a-mediated neutrophil recruitment has an obligatory role in pressure overload-induced cardiac dysfunction. Circulation 2019;140:487-99.ArticlePubMedPMC
- 95. Nahrendorf M, Swirski FK. Immunology: neutrophil-macrophage communication in inflammation and atherosclerosis. Science 2015;349:237-8.ArticlePubMed
- 96. Njeim R, Azar WS, Fares AH, Azar ST, Kfoury Kassouf H, Eid AA. NETosis contributes to the pathogenesis of diabetes and its complications. J Mol Endocrinol 2020;65:R65-76.ArticlePubMed
- 97. Wilson AJ, Gill EK, Abudalo RA, Edgar KS, Watson CJ, Grieve DJ. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 2018;104:293-9.ArticlePubMed
- 98. Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013;127:1888-902.ArticlePubMed
- 99. Kinsara AJ, Ismail YM. Metformin in heart failure patients. Indian Heart J 2018;70:175-6.ArticlePubMedPMC
- 100. Sardu C, Paolisso P, Sacra C, Mauro C, Minicucci F, Portoghese M, et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: the CODYCE Multicenter Prospective Study. Diabetes Care 2019;42:1946-55.ArticlePubMedPDF
- 101. Top WM, Kooy A, Stehouwer CD. Metformin: a narrative review of its potential benefits for cardiovascular disease, cancer and dementia. Pharmaceuticals (Basel) 2022;15:312.ArticlePubMedPMC
- 102. Ladeiras-Lopes R, Sampaio F, Leite S, Santos-Ferreira D, Vilela E, Leite-Moreira A, et al. Metformin in non-diabetic patients with metabolic syndrome and diastolic dysfunction: the METDIME randomized trial. Endocrine 2021;72:699-710.ArticlePubMedPDF
- 103. Dandona P, Ghanim H, Chaudhuri A, Mohanty P. Thiazolidinediones-improving endothelial function and potential long-term benefits on cardiovascular disease in subjects with type 2 diabetes. J Diabetes Complications 2008;22:62-75.ArticlePubMed
- 104. de Jong M, van der Worp HB, van der Graaf Y, Visseren FL, Westerink J. Pioglitazone and the secondary prevention of cardiovascular disease: a meta-analysis of randomized-controlled trials. Cardiovasc Diabetol 2017;16:134.PubMedPMC
- 105. Tian Y, Chen T, Wu Y, Yang L, Wang L, Fan X, et al. Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanisms. Cardiovasc Diabetol 2017;16:140.ArticlePubMedPMCPDF
- 106. Liakos A, Lambadiari V, Bargiota A, Kitsios K, Avramidis I, Kotsa K, et al. Effect of liraglutide on ambulatory blood pressure in patients with hypertension and type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2019;21:517-24.ArticlePubMedPDF
- 107. Koseoglu D, Koparal SS, Ozdemir Baser O, Berker D. Exenatide improves cardiovascular risk factors in obese patients with type 2 diabetes mellitus: a prospective study. Turk J Med Sci 2021;51:167-74.ArticlePubMedPMC
- 108. Zheng W, Zhou J, Song S, Kong W, Xia W, Chen L, et al. Dipeptidyl-peptidase 4 inhibitor sitagliptin ameliorates hepatic insulin resistance by modulating inflammation and autophagy in ob/ob Mice. Int J Endocrinol 2018;2018:8309723.ArticlePubMedPMCPDF
- 109. Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067-76.ArticlePubMed
- 110. Wang X, Ni J, Guo R, Li L, Su J, He F, et al. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail Rev 2022;27:961-80.ArticlePubMedPDF
- 111. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509-14.ArticlePubMedPMC
- 112. Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 2017;8:416-27.ArticlePubMedPMCPDF
- 113. Bonaventura A, Carbone S, Dixon DL, Abbate A, Montecucco F. Pharmacologic strategies to reduce cardiovascular disease in type 2 diabetes mellitus: focus on SGLT-2 inhibitors and GLP-1 receptor agonists. J Intern Med 2019;286:16-31.ArticlePubMedPDF
- 114. Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis 2011;215:1-8.ArticlePubMed
- 115. Yandrapalli S, Malik A, Guber K, Rochlani Y, Pemmasani G, Jasti M, et al. Statins and the potential for higher diabetes mellitus risk. Expert Rev Clin Pharmacol 2019;12:825-30.ArticlePubMed
- 116. Duhaney TA, Cui L, Rude MK, Lebrasseur NK, Ngoy S, De Silva DS, et al. Peroxisome proliferator-activated receptor alpha-independent actions of fenofibrate exacerbates left ventricular dilation and fibrosis in chronic pressure overload. Hypertension 2007;49:1084-94.ArticlePubMed
- 117. Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, et al. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: insights from the COMPASS Trial. Circulation 2020;141:1841-54.ArticlePubMedPMC
- 118. Brown E, Ozawa K, Moccetti F, Vinson A, Hodovan J, Nguyen TA, et al. Arterial platelet adhesion in atherosclerosis-prone arteries of obese, insulin-resistant nonhuman primates. J Am Heart Assoc 2021;10:e019413.ArticlePubMedPMC
- 119. Liu M, Zhuang X, Chen X, Zhang S, Yang D, Zhong X, et al. Antiplatelet strategy in primary and secondary prevention of cardiovascular disease in patients with type 2 diabetes mellitus: a perspective from the guideline appraisal. J Diabetes Investig 2021;12:99-108.ArticlePubMedPMCPDF
- 120. Nemati M, Karbalaei N, Mokarram P, Dehghani F. Effects of platelet-rich plasma on the pancreatic islet survival and function, islet transplantation outcome and pancreatic pdx1 and insulin gene expression in streptozotocin-induced diabetic rats. Growth Factors 2020;38:137-51.ArticlePubMed
- 121. Zhang W, Dun Y, You B, Qiu L, Ripley-Gonzalez JW, Cheng J, et al. Trimetazidine and exercise offer analogous improvements to the skeletal muscle insulin resistance of mice through Nrf2 signaling. BMJ Open Diabetes Res Care 2022;10:e002699.ArticlePubMedPMC
- 122. Murarka S, Movahed MR. Diabetic cardiomyopathy. J Card Fail 2010;16:971-9.ArticlePubMed
- 123. Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 2010;6:319-30.ArticlePubMedPDF
- 124. Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail 2016;9:e002560.PubMedPMC
- 125. Wu W, Shi F, Liu D, Ceddia RP, Gaffin R, Wei W, et al. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal 2017;10:eaam6870.ArticlePubMedPMC
- 126. Gonzalez-Juanatey JR, Gorriz JL, Ortiz A, Valle A, Soler MJ, Facila L. Cardiorenal benefits of finerenone: protecting kidney and heart. Ann Med 2023;55:502-13.ArticlePubMedPMC
- 127. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252-63.ArticlePubMed
- 128. Filippatos G, Anker SD, Agarwal R, Ruilope LM, Rossing P, Bakris GL, et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: analyses from the FIGARO-DKD Trial. Circulation 2022;145:437-47.ArticlePubMedPMC
- 129. Ramsay LE, Yeo WW, Jackson PR. Influence of diuretics, calcium antagonists, and alpha-blockers on insulin sensitivity and glucose tolerance in hypertensive patients. J Cardiovasc Pharmacol 1992;20 Suppl 11:S49-54.PubMed
- 130. Reid JL. The place of alpha blockers in the treatment of hypertension. Clin Exp Hypertens 1993;15:1291-7.ArticlePubMed
- 131. Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure?: a meta-analysis of large-scale clinical trials. Am Heart J 2003;146:848-53.PubMed
- 132. Wang X, Feng Z, Wang X, Yang L, Han S, Cao K, et al. O-GlcNA case deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia 2016;59:1287-96.ArticlePubMedPDF
- 133. Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 2005;96:1006-13.ArticlePubMed
- 134. Campbell H, Aguilar-Sanchez Y, Quick AP, Dobrev D, Wehrens XH. SPEG: a key regulator of cardiac calcium homeostasis. Cardiovasc Res 2021;117:2175-85.ArticlePubMedPMCPDF
- 135. Chen Q, Chen X, Han C, Wang Y, Huang T, Du Y, et al. FGF-2 transcriptionally down-regulates the expression of BNIP3L via PI3K/Akt/FoxO3a signaling and inhibits necrosis and mitochondrial dysfunction induced by high concentrations of hydrogen peroxide in H9c2 cells. Cell Physiol Biochem 2016;40:1678-91.ArticlePubMedPDF
- 136. Ge Q, Xie XX, Xiao X, Li X. Exosome-like vesicles as new mediators and therapeutic targets for treating insulin resistance and β-cell mass failure in type 2 diabetes mellitus. J Diabetes Res 2019;2019:3256060.ArticlePubMedPMCPDF
- 137. Kranendonk ME, Visseren FL, van Balkom BW, Nolte-‘t Hoen EN, van Herwaarden JA, de Jager W, et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring) 2014;22:1296-308.ArticlePubMedPDF
- 138. James DE, Stockli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol 2021;22:751-71.ArticlePubMedPDF
- 139. Hayden MR. The mighty mitochondria are unifying organelles and metabolic hubs in multiple organs of obesity, insulin resistance, metabolic syndrome, and type 2 diabetes: an observational ultrastructure study. Int J Mol Sci 2022;23:4820.ArticlePubMedPMC
- 140. Kwon B, Gamache T, Lee HK, Querfurth HW. Synergistic effects of β-amyloid and ceramide-induced insulin resistance on mitochondrial metabolism in neuronal cells. Biochim Biophys Acta 2015;1852:1810-23.ArticlePubMed
- 141. Wang H, Chen X, Chen C, Pan T, Li M, Yao L, et al. Electroacupuncture at lower he-sea and front-mu acupoints ameliorates insulin resistance in type 2 diabetes mellitus by regulating the intestinal flora and gut barrier. Diabetes Metab Syndr Obes 2022;15:2265-76.ArticlePubMedPMCPDF
- 142. Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond) 2013;10:35.ArticlePubMedPMCPDF
- 143. Kim D, Seo J, Ha KH, Kim DJ. Maintaining physical activity is associated with reduced major adverse cardiovascular events in people newly diagnosed with diabetes. J Obes Metab Syndr 2022;31:187-95.ArticlePubMedPMC
- 144. Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther 2022;7:216.ArticlePubMedPMCPDF
- 145. Andreadi A, Bellia A, Di Daniele N, Meloni M, Lauro R, Della-Morte D, et al. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: a target for new therapies against cardiovascular diseases. Curr Opin Pharmacol 2022;62:85-96.ArticlePubMed
REFERENCES
Figure & Data
References
Citations


 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite