
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Funded articles
- Page Path
- HOME > Browse > Funded articles
Original Articles
- Basic Research
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
- Diabetes Metab J. 2024;48(2):231-241. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0366
- Funded: National Research Foundation of Korea, Ministry of Science and ICT

- 1,483 View
- 128 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Administration of pancreatic endoplasmic reticulum kinase inhibitor (PERKi) improved insulin secretion and hyperglycemia in obese diabetic mice. In this study, autophagic balance was studied whether to mediate it.
Methods
Human islets were isolated from living patients without diabetes. PERKi GSK2606414 effects were evaluated in the islets under glucolipotoxicity by palmitate. Islet insulin contents and secretion were measured. Autophagic flux was assessed by microtubule associated protein 1 light chain 3 (LC3) conversion, a red fluorescent protein (RFP)-green fluorescent protein (GFP)- LC3 tandem assay, and P62 levels. For mechanical analyses, autophagy was suppressed using 3-methyladenine in mouse islets. Small interfering RNA for an autophagy-related gene autophagy related 7 (Atg7) was transfected to interfere autophagy.
Results
PERKi administration to mice decreased diabetes-induced P62 levels in the islets. Glucolipotoxicity significantly increased PERK phosphorylation by 70% and decreased insulin contents by 50% in human islets, and addition of PERKi (40 to 80 nM) recovered both. PERKi also enhanced glucose-stimulated insulin secretion (6-fold). PERKi up-regulated LC3 conversion suppressed by glucolipotoxicity, and down-regulated P62 contents without changes in P62 transcription, indicating enhanced autophagic flux. Increased autophagosome-lysosome fusion by PERKi was visualized in mouse islets, where PERKi enhanced ATG7 bound to LC3. Suppression of Atg7 eliminated PERKi-induced insulin contents and secretion.
Conclusion
This study provided functional changes of human islets with regard to autophagy under glucolipotoxicity, and suggested modulation of autophagy as an anti-diabetic mechanism of PERKi.
- Basic Research
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Ji-Hae Park, Soyeon Kwon, Young Mi Park
- Diabetes Metab J. 2024;48(2):215-230. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0332
- Funded: National Research Foundation of Korea, Ministry of Science, ICT and Future Planning
- 2,054 View
- 169 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
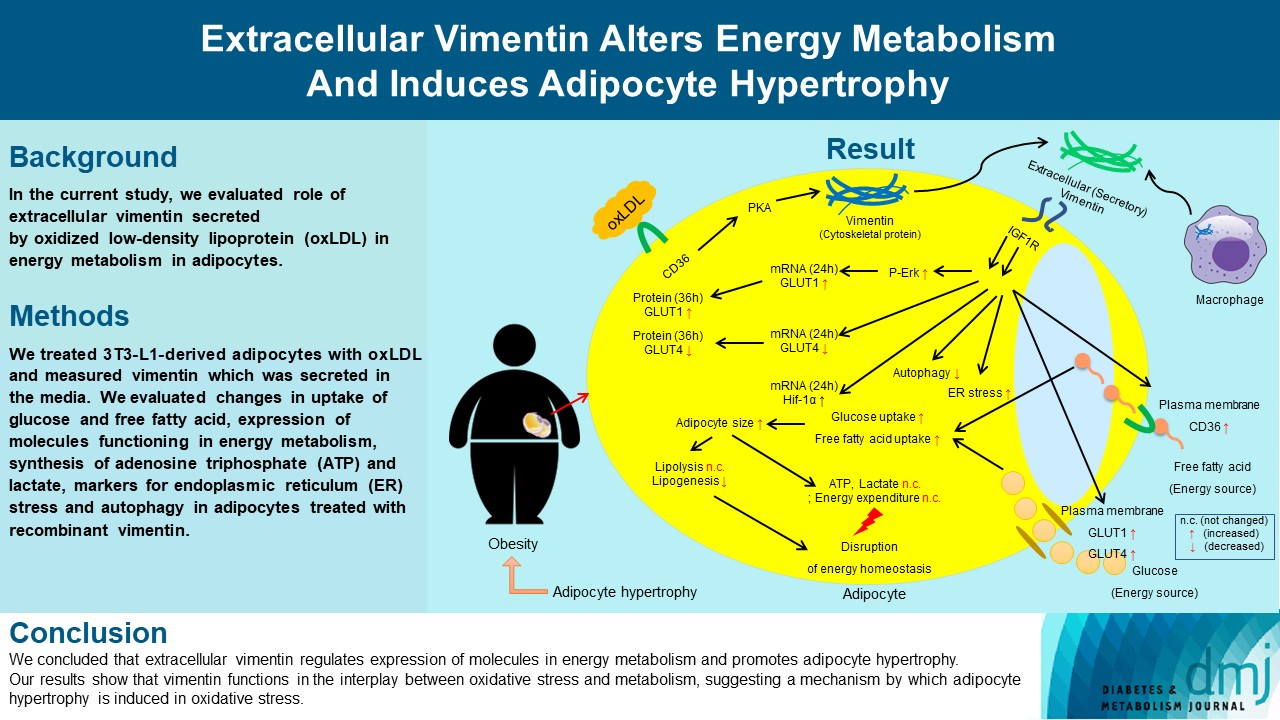
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
- Complications
- Impact of Hyperglycemia on Complication and Mortality after Transarterial Chemoembolization for Hepatocellular Carcinoma
- Sun Joon Moon, Chang Ho Ahn, Yun Bin Lee, Young Min Cho
- Diabetes Metab J. 2024;48(2):302-311. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0255
- Funded: Sanofi, Daewoong Pharmaceutical Company, LG Chemical
- 641 View
- 93 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
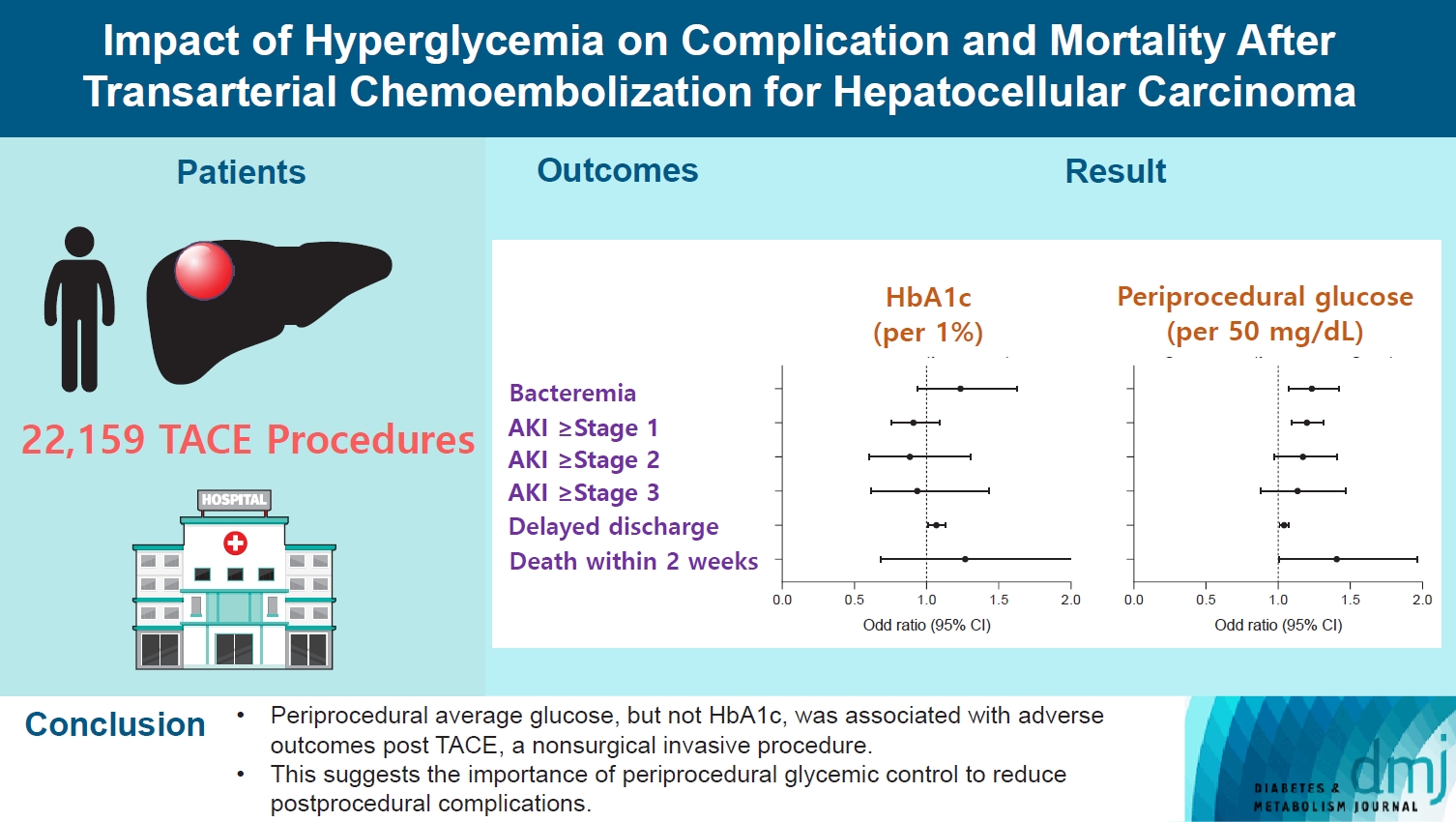
Current guidelines regarding periprocedural glycemic control to prevent complications after nonsurgical invasive procedures are insufficient. Transarterial chemoembolization (TACE) is a widely used treatment for unresectable hepatocellular carcinoma. We aimed to investigate the association between diabetes mellitus (DM) per se and the degree of hyperglycemia with postprocedural complications after TACE.
Methods
A total of 22,159 TACE procedures performed at Seoul National University Hospital from 2005 to 2018 were retrospectively analyzed. The associations between DM, preprocedural glycosylated hemoglobin (HbA1c), and periprocedural average glucose with postprocedural adverse outcomes were evaluated. The primary outcome was occurrence of postprocedural bacteremia. Secondary outcomes were acute kidney injury (AKI), delayed discharge and death within 14 days. Periprocedural glucose was averaged over 3 days: the day of, before, and after the TACE procedures. Propensity score matching was applied for procedures between patients with or without DM.
Results
Periprocedural average glucose was significantly associated with bacteremia (adjusted odds ratio per 50 mg/dL of glucose, 1.233; 95% confidence interval, 1.071 to 1.420; P=0.004), AKI, delayed discharge, and death within 14 days. DM per se was only associated with bacteremia and AKI. Preprocedural HbA1c was associated with delayed discharge. Average glucose levels above 202 and 181 mg/dL were associated with a significantly higher risk of bacteremia and AKI, respectively, than glucose levels of 126 mg/dL or lower.
Conclusion
Periprocedural average glucose, but not HbA1c, was associated with adverse outcomes after TACE, which is a nonsurgical invasive procedure. This suggests the importance of periprocedural glycemic control to reduce postprocedural complications.
- Complications
- Risk of Depression according to Cumulative Exposure to a Low-Household Income Status in Individuals with Type 2 Diabetes Mellitus: A Nationwide Population- Based Study
- So Hee Park, You-Bin Lee, Kyu-na Lee, Bongsung Kim, So Hyun Cho, So Yoon Kwon, Jiyun Park, Gyuri Kim, Sang-Man Jin, Kyu Yeon Hur, Kyungdo Han, Jae Hyeon Kim
- Diabetes Metab J. 2024;48(2):290-301. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0299
- Funded: National Research Foundation of Korea, Ministry of Science and ICT
- 782 View
- 113 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
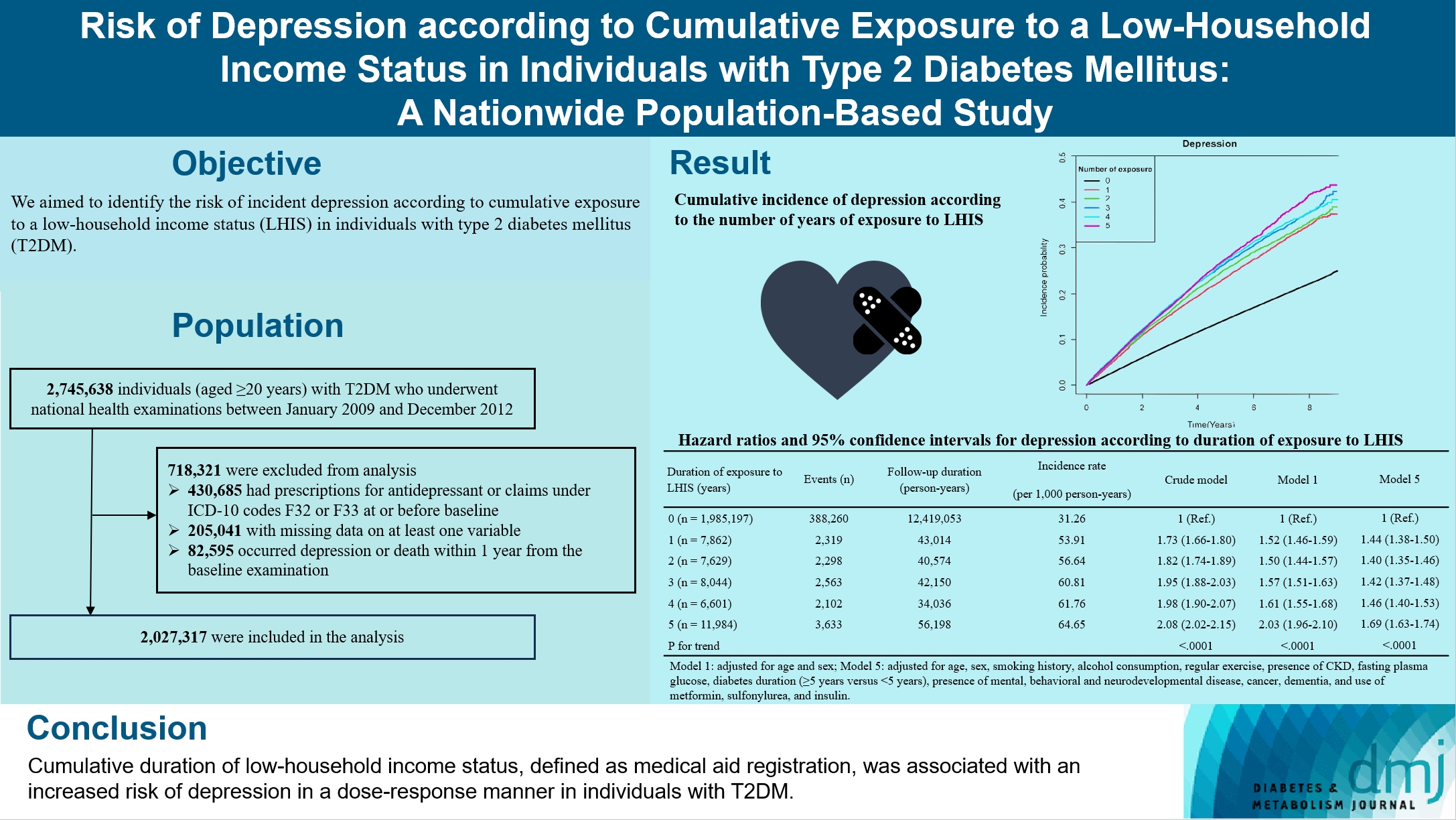
We aimed to identify the risk of incident depression according to cumulative exposure to a low-household income status in individuals with type 2 diabetes mellitus (T2DM).
Methods
For this retrospective longitudinal population-based cohort study, we used Korean National Health Insurance Service data from 2002 to 2018. Risk of depression was assessed according to cumulative exposure to low-household income status (defined as Medical Aid registration) during the previous 5 years among adults (aged ≥20 years) with T2DM and without baseline depression who underwent health examinations from 2009 to 2012 (n=2,027,317).
Results
During an average 6.23 years of follow-up, 401,175 incident depression cases occurred. Advance in cumulative number of years registered for medical aid during the previous 5 years from baseline was associated with an increased risk of depression in a dose-dependent manner (hazard ratio [HR], 1.44 [95% confidence interval (CI), 1.38 to 1.50]; HR, 1.40 [95% CI, 1.35 to 1.46]; HR, 1.42, [95% CI, 1.37 to 1.48]; HR, 1.46, [95% CI, 1.40 to 1.53]; HR, 1.69, [95% CI, 1.63 to 1.74] in groups with 1 to 5 exposed years, respectively). Insulin users exposed for 5 years to a low-household income state had the highest risk of depression among groups categorized by insulin use and duration of low-household income status.
Conclusion
Cumulative duration of low-household income status, defined as medical aid registration, was associated with an increased risk of depression in a dose-response manner in individuals with T2DM.
- Drug/Regimen
- Two-Year Therapeutic Efficacy and Safety of Initial Triple Combination of Metformin, Sitagliptin, and Empagliflozin in Drug-Naïve Type 2 Diabetes Mellitus Patients
- Young-Hwan Park, Minji Sohn, So Yeon Lee, Soo Lim
- Diabetes Metab J. 2024;48(2):253-264. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0128
- Funded: Seoul National University Bundang Hospital
- 1,513 View
- 252 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
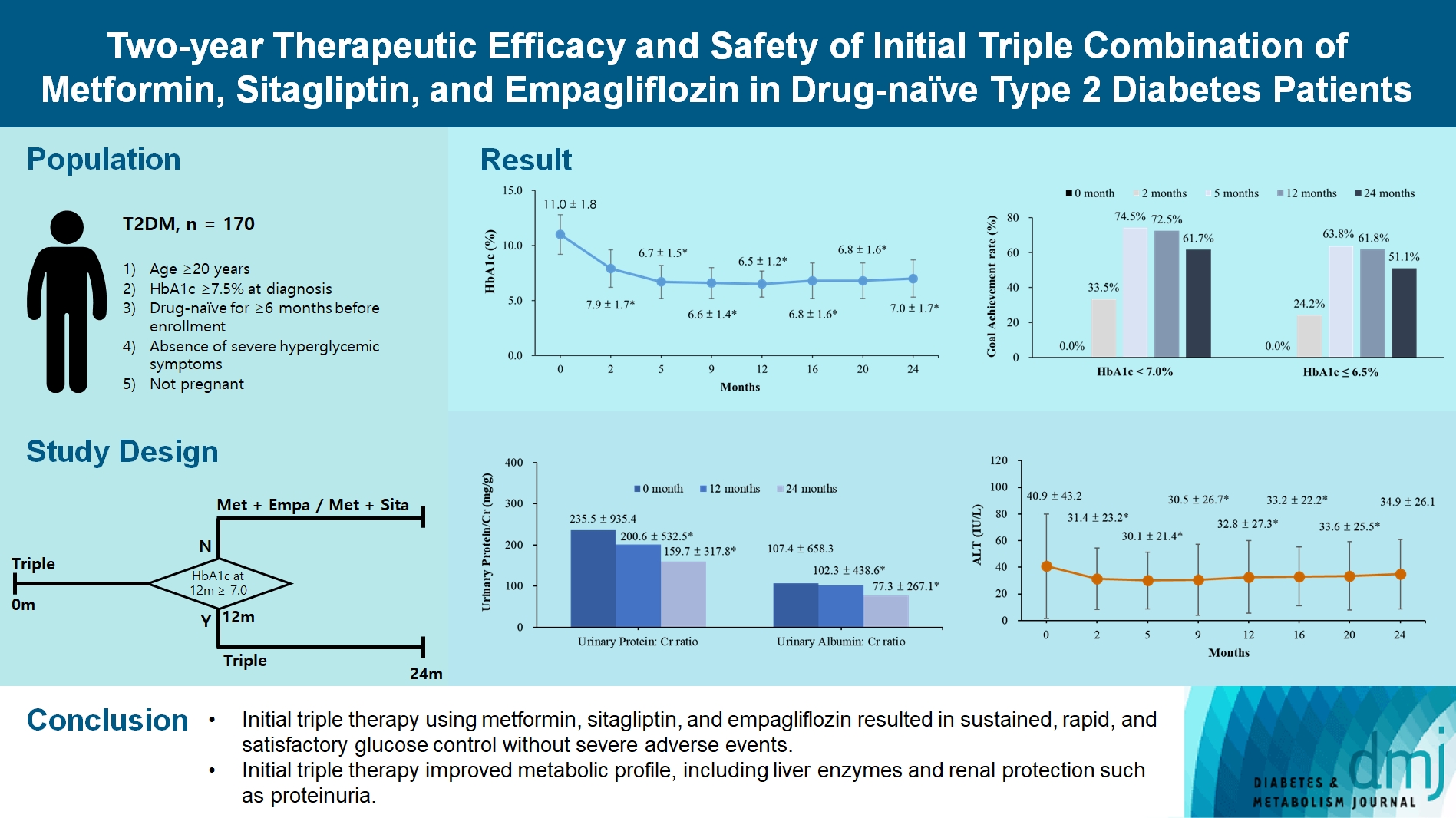
We investigated the long-term efficacy and safety of initial triple therapy using metformin, a dipeptidyl peptidase-4 inhibitor, and a sodium-glucose cotransporter-2 inhibitor, in patients with type 2 diabetes mellitus.
Methods
We enrolled 170 drug-naïve patients with glycosylated hemoglobin (HbA1c) level >7.5% who had started triple therapy (metformin, sitagliptin, and empagliflozin). Glycemic, metabolic, and urinary parameters were measured for 24 months.
Results
After 24 months, HbA1c level decreased significantly from 11.0%±1.8% to 7.0%±1.7%. At 12 and 24 months, the rates of achievement of the glycemic target goal (HbA1c <7.0%) were 72.5% and 61.7%, respectively, and homeostasis model assessment of β-cell function and insulin resistance indices improved. Whole-body fat percentage decreased by 1.08%, and whole-body muscle percentage increased by 0.97% after 24 months. Fatty liver indices and albuminuria improved significantly. The concentration of ketone bodies was elevated at the baseline but decreased after 24 months. There were no serious adverse events, including ketoacidosis.
Conclusion
Initial triple combination therapy with metformin, sitagliptin, and empagliflozin led to achievement of the glycemic target goal, which was maintained for 24 months without severe hypoglycemia but with improved metabolic function and albuminuria. This combination therapy may be a good strategy for drug-naïve patients with type 2 diabetes mellitus.
Reviews
- Metabolic Risk/Epidemiology
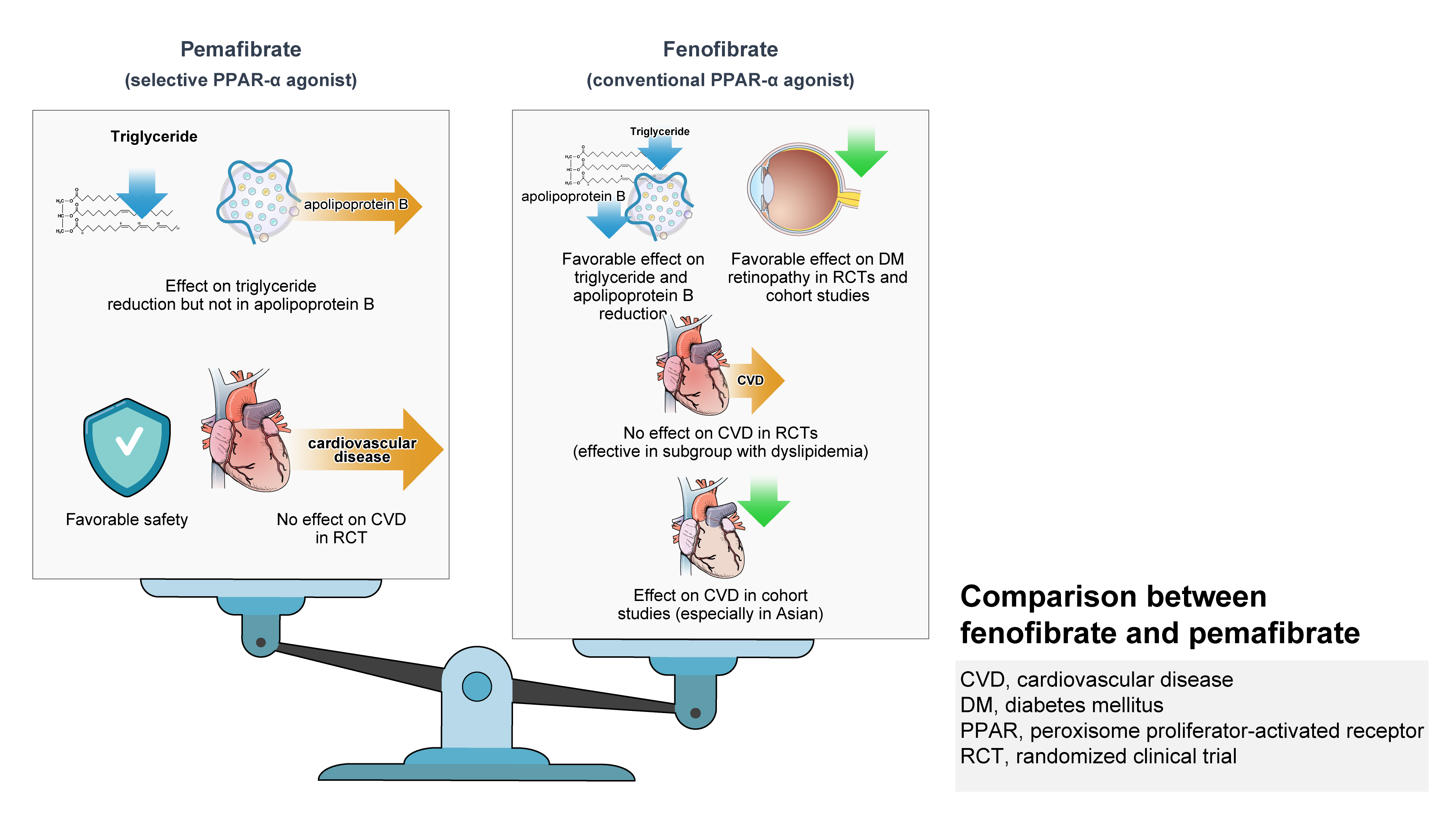
- Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
- Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
- Diabetes Metab J. 2024;48(2):184-195. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0168
- Funded: Abbott Laboratories Ltd.
- 2,007 View
- 302 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Hypertriglyceridemia and decreased high-density lipoprotein cholesterol (HDL-C) persist despite statin therapy, contributing to residual atherosclerotic cardiovascular disease (ASCVD) risk. Asian subjects are metabolically more susceptible to hypertriglyceridemia than other ethnicities. Fenofibrate regulates hypertriglyceridemia, raises HDL-C levels, and is a recommended treatment for dyslipidemia. However, data on fenofibrate use across different Asian regions are limited. This narrative review summarizes the efficacy and safety data of fenofibrate in Asian subjects with dyslipidemia and related comorbidities (diabetes, metabolic syndrome, diabetic retinopathy, and diabetic nephropathy). Long-term fenofibrate use resulted in fewer cardiovascular (CV) events and reduced the composite of heart failure hospitalizations or CV mortality in type 2 diabetes mellitus. Fenofibrate plays a significant role in improving irisin resistance and microalbuminuria, inhibiting inflammatory responses, and reducing retinopathy incidence. Fenofibrate plus statin combination significantly reduced composite CV events risk in patients with metabolic syndrome and demonstrated decreased triglyceride and increased HDL-C levels with an acceptable safety profile in those with high CV or ASCVD risk. Nevertheless, care is necessary with fenofibrate use due to possible hepatic and renal toxicities in vulnerable individuals. Long-term trials and real-world studies are needed to confirm the clinical benefits of fenofibrate in the heterogeneous Asian population with dyslipidemia.
- Others
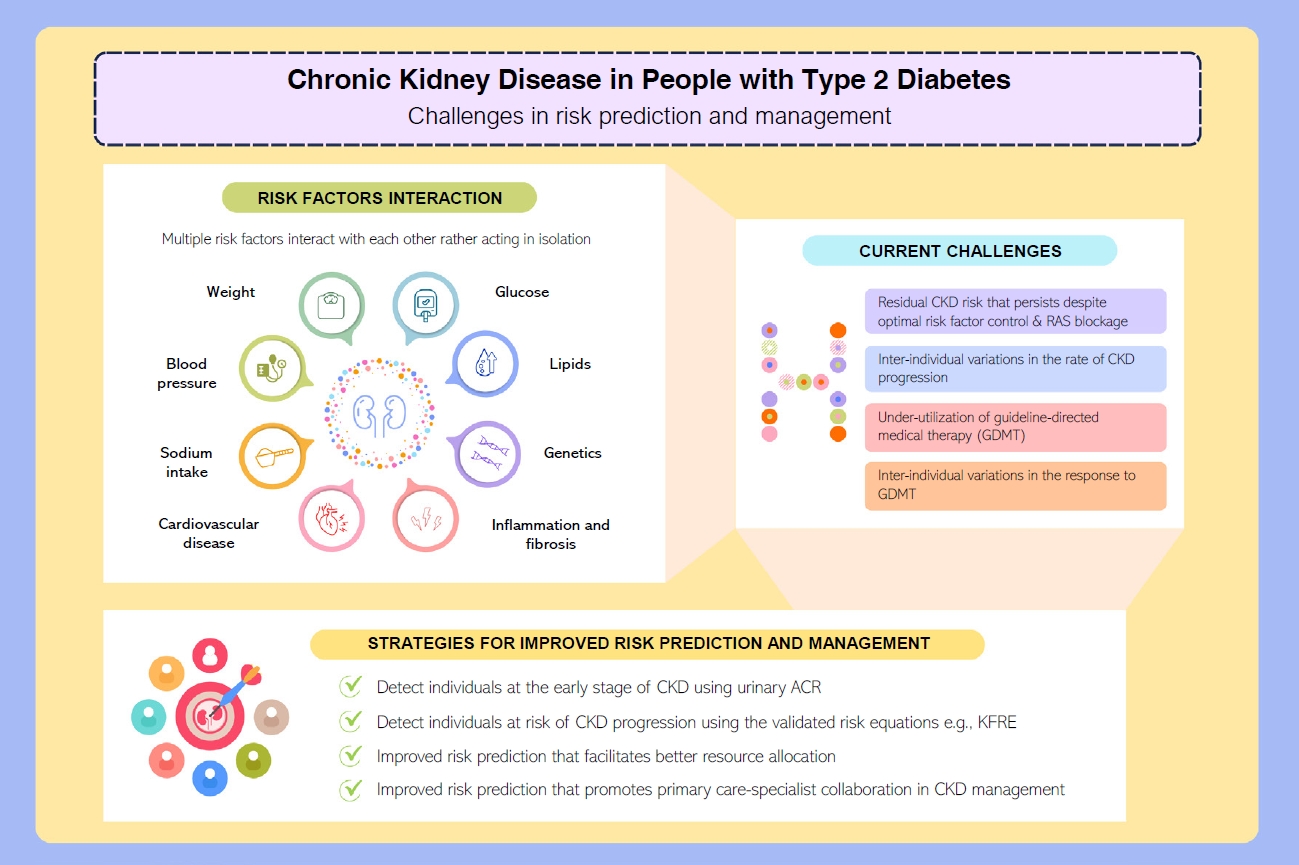
- Risk Prediction and Management of Chronic Kidney Disease in People Living with Type 2 Diabetes Mellitus
- Ying-Guat Ooi, Tharsini Sarvanandan, Nicholas Ken Yoong Hee, Quan-Hziung Lim, Sharmila S. Paramasivam, Jeyakantha Ratnasingam, Shireene R. Vethakkan, Soo-Kun Lim, Lee-Ling Lim
- Diabetes Metab J. 2024;48(2):196-207. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0244
- Funded: University of Malaya
- 1,657 View
- 322 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - People with type 2 diabetes mellitus have increased risk of chronic kidney disease and atherosclerotic cardiovascular disease. Improved care delivery and implementation of guideline-directed medical therapy have contributed to the declining incidence of atherosclerotic cardiovascular disease in high-income countries. By contrast, the global incidence of chronic kidney disease and associated mortality is either plateaued or increased, leading to escalating direct and indirect medical costs. Given limited resources, better risk stratification approaches to identify people at risk of rapid progression to end-stage kidney disease can reduce therapeutic inertia, facilitate timely interventions and identify the need for early nephrologist referral. Among people with chronic kidney disease G3a and beyond, the kidney failure risk equations (KFRE) have been externally validated and outperformed other risk prediction models. The KFRE can also guide the timing of preparation for kidney replacement therapy with improved healthcare resources planning and may prevent multiple complications and premature mortality among people with chronic kidney disease with and without type 2 diabetes mellitus. The present review summarizes the evidence of KFRE to date and call for future research to validate and evaluate its impact on cardiovascular and mortality outcomes, as well as healthcare resource utilization in multiethnic populations and different healthcare settings.
Original Article
- Others
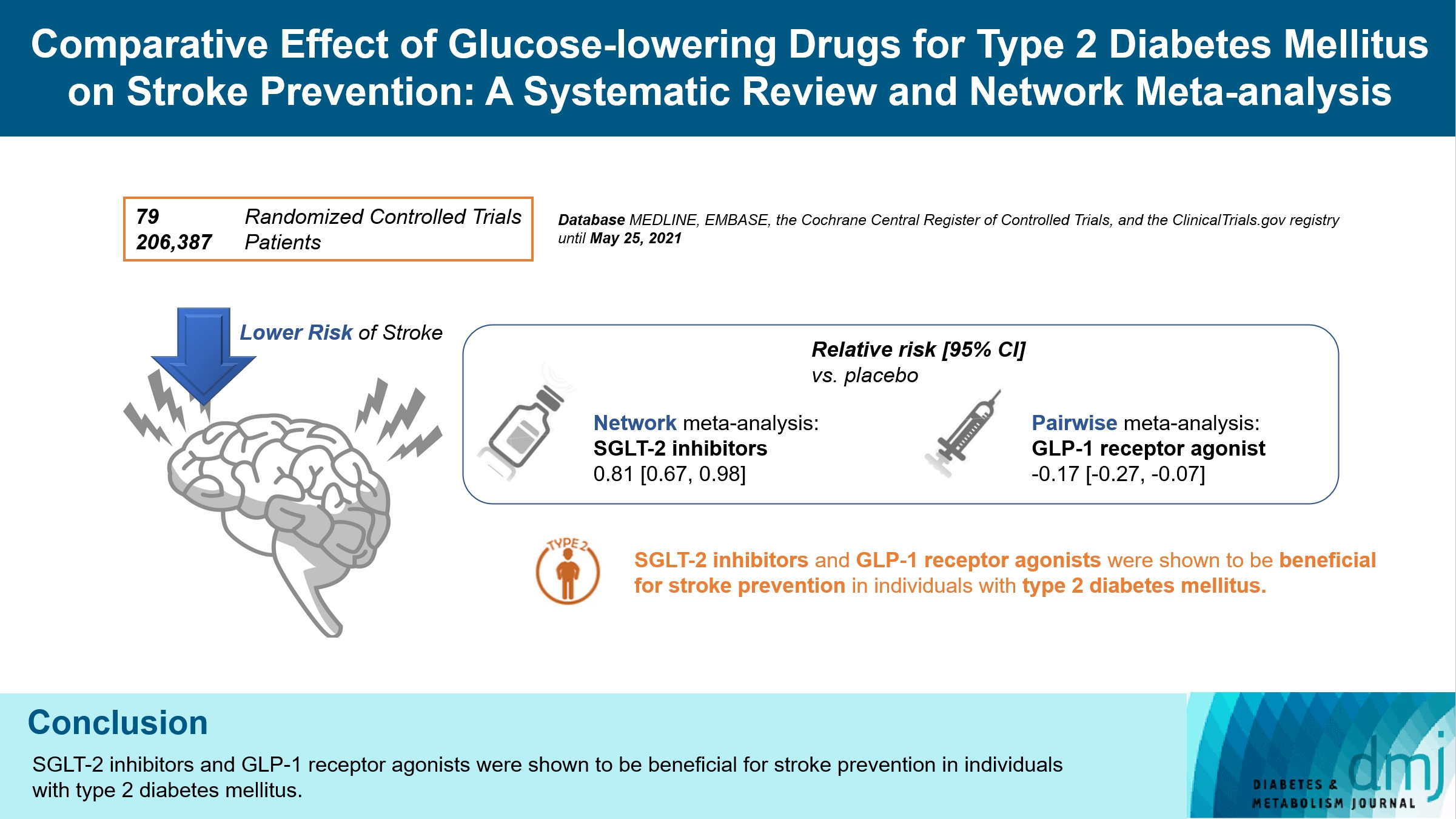
- Comparative Effect of Glucose-Lowering Drugs for Type 2 Diabetes Mellitus on Stroke Prevention: A Systematic Review and Network Meta-Analysis
- Ji Soo Kim, Gyeongsil Lee, Kyung-Il Park, Seung-Won Oh
- Diabetes Metab J. 2024;48(2):312-320. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0421
- Funded: Seoul National University Hospital
- 1,073 View
- 183 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There is still a lack of research on which diabetic drugs are more effective in preventing stroke. Our network metaanalysis aimed to compare cerebrovascular benefits among glucose-lowering treatments.
Methods
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the ClinicalTrials.gov registry for clinical trials from inception through May 25, 2021. We included both prespecified cerebrovascular outcomes and cerebrovascular events reported as severe adverse events. Subgroup analyses were conducted by stroke subtype, publication type, age of patients, baseline glycosylated hemoglobin (HbA1c), duration of type 2 diabetes mellitus, and cardiovascular risks.
Results
Of 2,861 reports and 1,779 trials screened, 79 randomized controlled trials comprising 206,387 patients fulfilled the inclusion criteria. In the pairwise meta-analysis, the use of glucagon-like peptide-1 (GLP-1) agonist was associated with a lower risk of total stroke compared with placebo (relative risk [RR], –0.17; 95% confidence interval [CI], –0.27 to –0.07). In the network meta- analysis, only the use of sodium-glucose cotransporter-2 (SGLT-2) inhibitor was associated with a reduction of total stroke, compared with placebo (RR, 0.81; 95% CI, 0.67 to 0.98). In the subgroup analyses, the use of SGLT-2 inhibitor and GLP-1 agonist was associated with a lower risk of stroke in those with high HbA1c (≥8.0) and low-risk of cardiovascular disease, respectively.
Conclusion
SGLT-2 inhibitors and GLP-1 agonists were shown to be beneficial for stroke prevention in patients with type 2 diabetes mellitus. -
Citations
Citations to this article as recorded by- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes
Jae-Han Jeon
Diabetes & Metabolism Journal.2024; 48(2): 213. CrossRef - Reply to comment on: Association of glucose-lowering drugs with incident stroke and transient ischaemic attacks in primary care patients with type 2 diabetes: disease analyser database
Wolfgang Rathmann, Karel Kostev
Acta Diabetologica.2024;[Epub] CrossRef
- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes
Reviews
- Metabolic Risk/Epidemiology
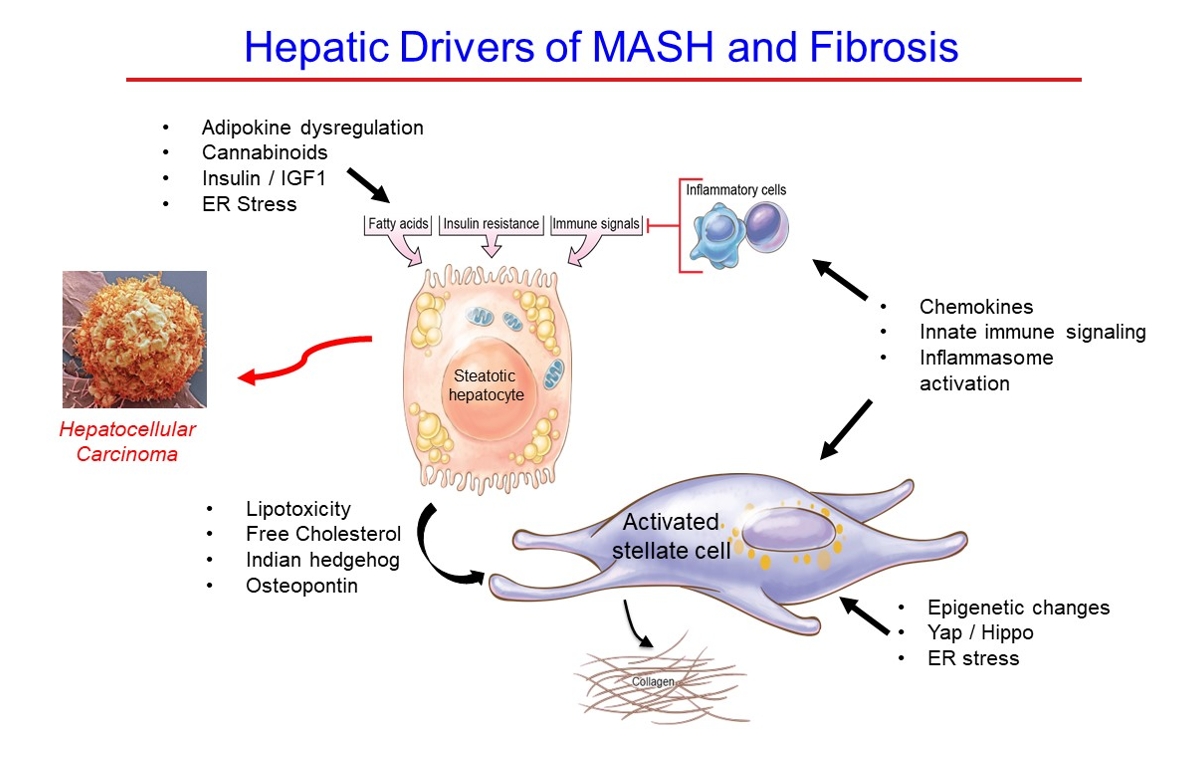
- Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
- Scott L. Friedman
- Diabetes Metab J. 2024;48(2):161-169. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0240
- Funded: National Institute of Diabetes, Digestive and Kidney Diseases
- 1,981 View
- 243 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolic dysfunction-associated steatotic (fatty) liver disease (MASLD), previously termed non-alcoholic fatty liver disease, is a worldwide epidemic that can lead to hepatic inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). The disease is typically a component of the metabolic syndrome that accompanies obesity, and is often overlooked because the liver manifestations are clinically silent until late-stage disease is present (i.e., cirrhosis). Moreover, Asian populations, including Koreans, have a higher fraction of patients who are lean, yet their illness has the same prognosis or worse than those who are obese. Nonetheless, ongoing injury can lead to hepatic inflammation and ballooning of hepatocytes as classic features. Over time, fibrosis develops following activation of hepatic stellate cells, the liver’s main fibrogenic cell type. The disease is usually more advanced in patients with type 2 diabetes mellitus, indicating that all diabetic patients should be screened for liver disease. Although there has been substantial progress in clarifying pathways of injury and fibrosis, there no approved therapies yet, but current research seeks to uncover the pathways driving hepatic inflammation and fibrosis, in hopes of identifying new therapeutic targets. Emerging molecular methods, especially single cell sequencing technologies, are revolutionizing our ability to clarify mechanisms underlying MASLD-associated fibrosis and HCC.
- Metabolic Risk/Epidemiology
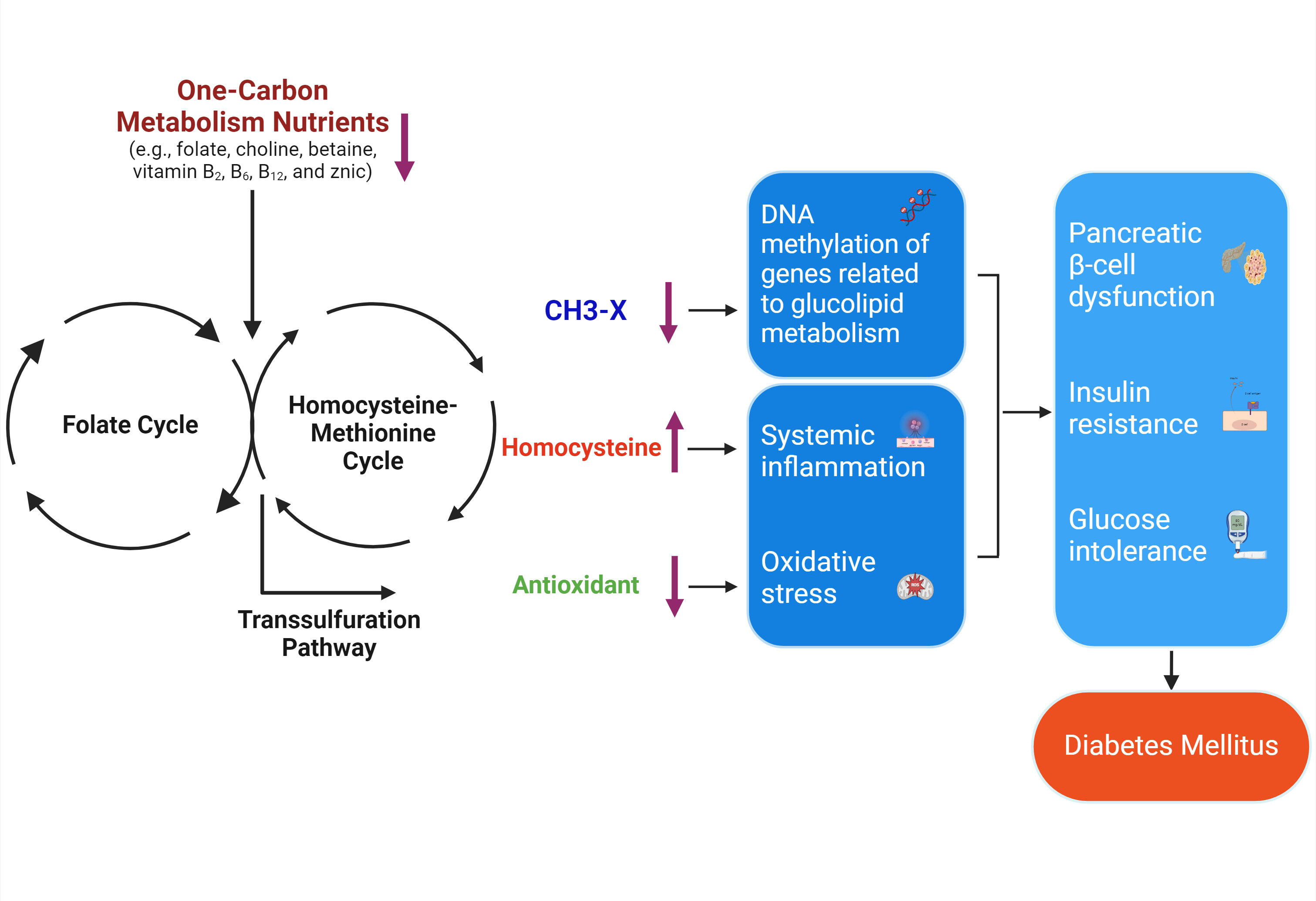
- One-Carbon Metabolism Nutrients, Genetic Variation, and Diabetes Mellitus
- Jie Zhu, Gunjana Saikia, Xiaotao Zhang, Xiaoxi Shen, Ka Kahe
- Diabetes Metab J. 2024;48(2):170-183. Published online March 12, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0272
- Funded: National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases, 2022 Multidisciplinary Internal Research Grant, Translational Health Research Center/Community Health and Economic Resilience Research, Texas State University, Icahn School of Medicine at Mount Sinai Institute Start-Up Grant
- 929 View
- 131 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Diabetes mellitus (DM) affects about 9.3% of the population globally. Hyperhomocysteinemia (HHcy) has been implicated in the pathogenesis of DM, owing to its promotion of oxidative stress, β-cell dysfunction, and insulin resistance. HHcy can result from low status of one-carbon metabolism (OCM) nutrients (e.g., folate, choline, betaine, vitamin B6, B12), which work together to degrade homocysteine by methylation. The etiology of HHcy may also involve genetic variation encoding key enzymes in OCM. This review aimed to provide an overview of the existing literature assessing the link between OCM nutrients status, related genetic factors, and incident DM. We also discussed possible mechanisms underlying the role of OCM in DM development and provided recommendations for future research and practice. Even though the available evidence remains inconsistent, some studies support the potential beneficial effects of intakes or blood levels of OCM nutrients on DM development. Moreover, certain variants in OCM-related genes may influence metabolic handling of methyl-donors and presumably incidental DM. Future studies are warranted to establish the causal inference between OCM and DM and examine the interaction of OCM nutrients and genetic factors with DM development, which will inform the personalized recommendations for OCM nutrients intakes on DM prevention.
- Pathophysiology
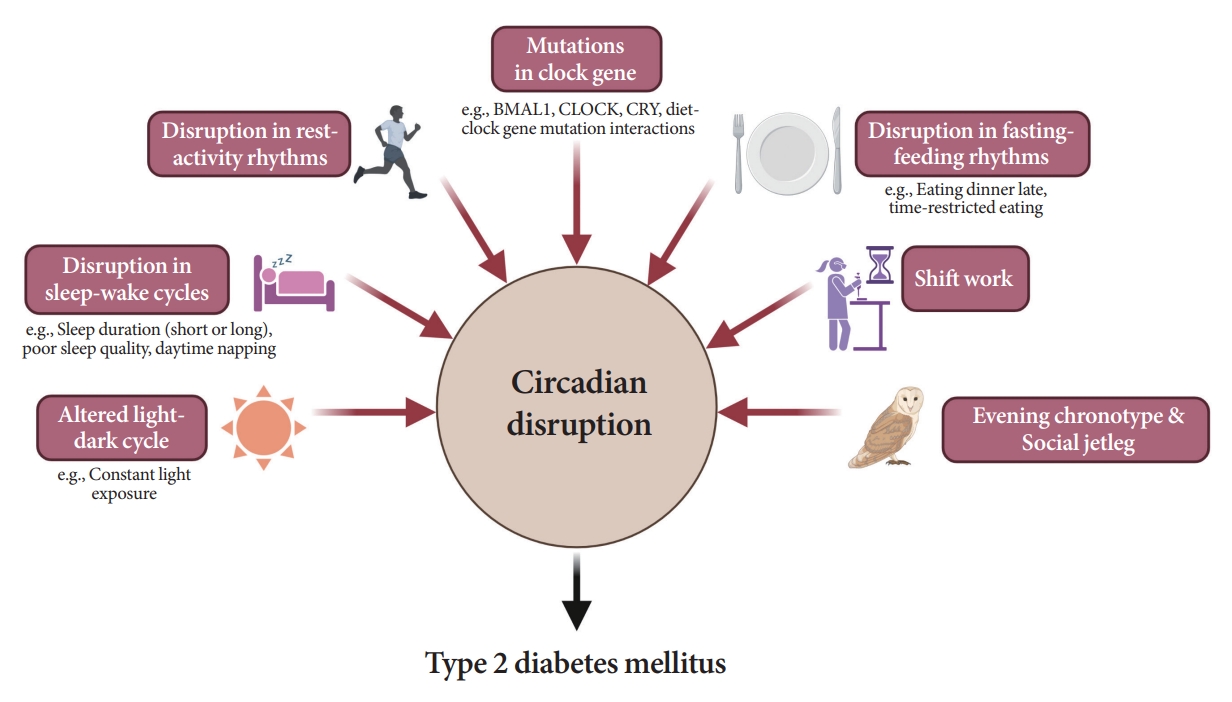
- Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
- Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
- Diabetes Metab J. 2024;48(1):37-52. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0193
- Funded: National Research Foundation of Korea, Ministry of Science and ICT, Ministry of Education, National IT Industry Promotion Agency, Korea Health Industry Development Institute, Korea University
- 2,044 View
- 214 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Novel strategies are required to reduce the risk of developing diabetes and/or clinical outcomes and complications of diabetes. In this regard, the role of the circadian system may be a potential candidate for the prevention of diabetes. We reviewed evidence from animal, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of diabetes. The circadian clock governs genetic, metabolic, hormonal, and behavioral signals in anticipation of cyclic 24-hour events through interactions between a “central clock” in the suprachiasmatic nucleus and “peripheral clocks” in the whole body. Currently, circadian rhythmicity in humans can be subjectively or objectively assessed by measuring melatonin and glucocorticoid levels, core body temperature, peripheral blood, oral mucosa, hair follicles, rest-activity cycles, sleep diaries, and circadian chronotypes. In this review, we summarized various circadian misalignments, such as altered light-dark, sleep-wake, rest-activity, fasting-feeding, shift work, evening chronotype, and social jetlag, as well as mutations in clock genes that could contribute to the development of diabetes and poor glycemic status in patients with diabetes. Targeting critical components of the circadian system could deliver potential candidates for the treatment and prevention of type 2 diabetes mellitus in the future.
- Pathophysiology
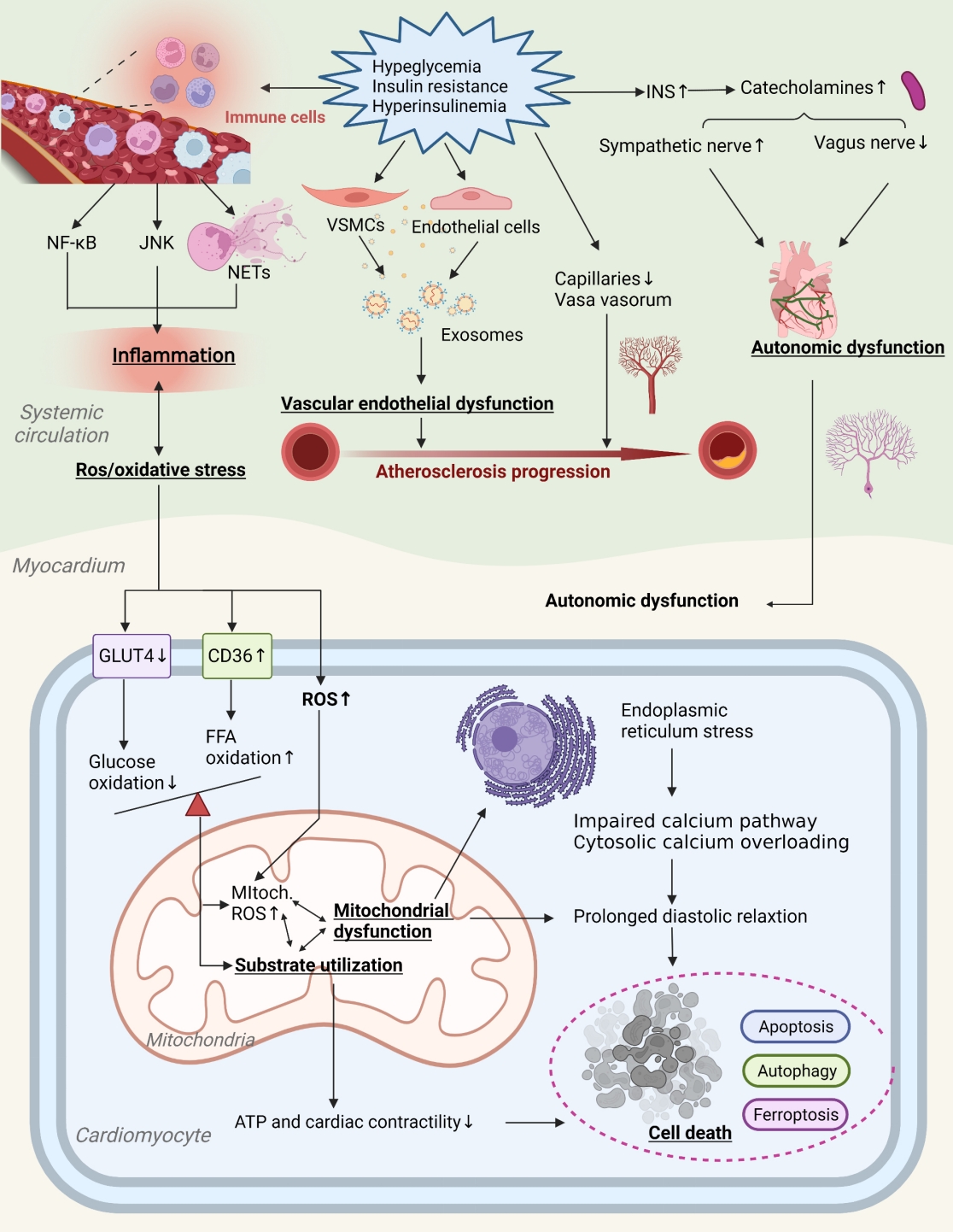
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- Funded: Chinese Medicine Inheritance and Innovation QI Huang Scholar", Tianjin Famous Chinese Medicine Doctor Inheritance Studio Special Grant, National Natural Science Foundation of China
- 2,048 View
- 178 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Basic Research
- Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases
- Benyuan Zhang, Joon Young Chang, Min Hee Lee, Sang-Hyeon Ju, Hyon-Seung Yi, Minho Shong
- Diabetes Metab J. 2024;48(1):1-18. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0115
- Funded: National Research Foundation of Korea, Ministry of Science and ICT

- 1,929 View
- 253 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Mitochondrial stress and the dysregulated mitochondrial unfolded protein response (UPRmt) are linked to various diseases, including metabolic disorders, neurodegenerative diseases, and cancer. Mitokines, signaling molecules released by mitochondrial stress response and UPRmt, are crucial mediators of inter-organ communication and influence systemic metabolic and physiological processes. In this review, we provide a comprehensive overview of mitokines, including their regulation by exercise and lifestyle interventions and their implications for various diseases. The endocrine actions of mitokines related to mitochondrial stress and adaptations are highlighted, specifically the broad functions of fibroblast growth factor 21 and growth differentiation factor 15, as well as their specific actions in regulating inter-tissue communication and metabolic homeostasis. Finally, we discuss the potential of physiological and genetic interventions to reduce the hazards associated with dysregulated mitokine signaling and preserve an equilibrium in mitochondrial stress-induced responses. This review provides valuable insights into the mechanisms underlying mitochondrial regulation of health and disease by exploring mitokine interactions and their regulation, which will facilitate the development of targeted therapies and personalized interventions to improve health outcomes and quality of life.
Original Articles
- Basic Research
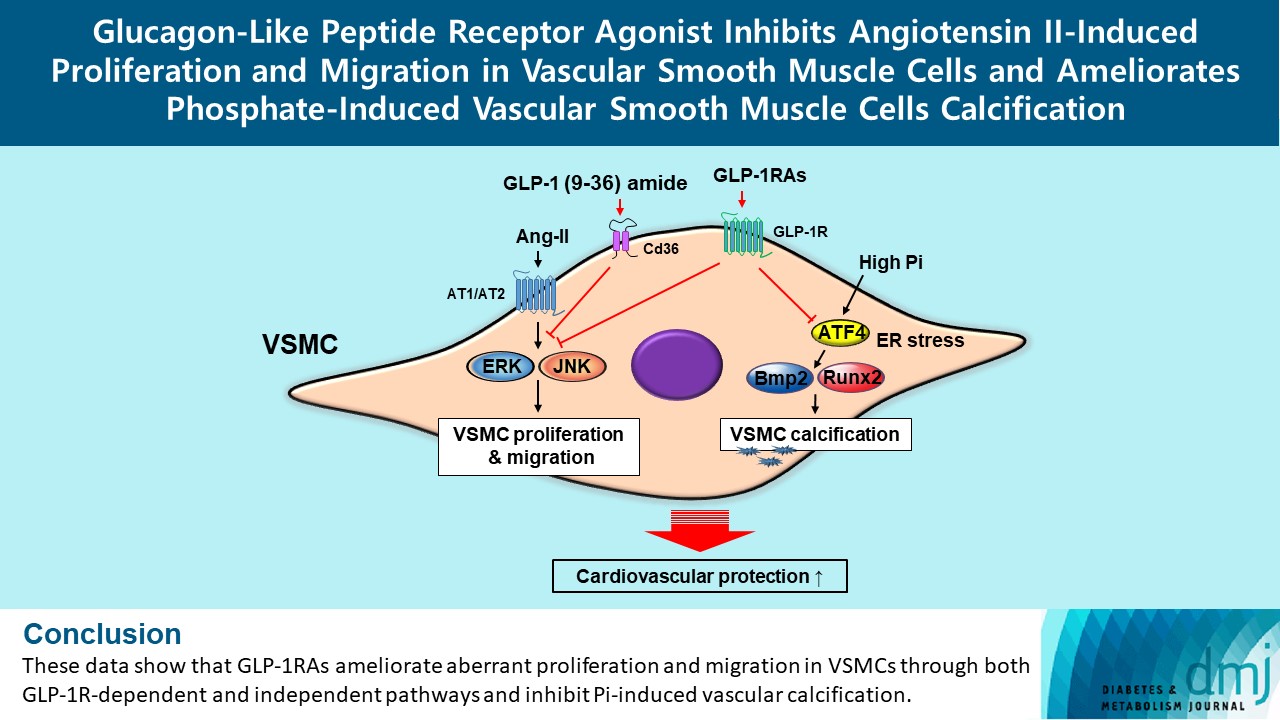
- Glucagon-Like Peptide Receptor Agonist Inhibits Angiotensin II-Induced Proliferation and Migration in Vascular Smooth Muscle Cells and Ameliorates Phosphate-Induced Vascular Smooth Muscle Cells Calcification
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2024;48(1):83-96. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0363
- Funded: National Research Foundation of Korea, Daewoong Foundation
- 1,616 View
- 165 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Glucagon-like peptide-1 receptor agonist (GLP-1RA), which is a therapeutic agent for the treatment of type 2 diabetes mellitus, has a beneficial effect on the cardiovascular system.
Methods
To examine the protective effects of GLP-1RAs on proliferation and migration of vascular smooth muscle cells (VSMCs), A-10 cells exposed to angiotensin II (Ang II) were treated with either exendin-4, liraglutide, or dulaglutide. To examine the effects of GLP-1RAs on vascular calcification, cells exposed to high concentration of inorganic phosphate (Pi) were treated with exendin-4, liraglutide, or dulaglutide.
Results
Ang II increased proliferation and migration of VSMCs, gene expression levels of Ang II receptors AT1 and AT2, proliferation marker of proliferation Ki-67 (Mki-67), proliferating cell nuclear antigen (Pcna), and cyclin D1 (Ccnd1), and the protein expression levels of phospho-extracellular signal-regulated kinase (p-Erk), phospho-c-JUN N-terminal kinase (p-JNK), and phospho-phosphatidylinositol 3-kinase (p-Pi3k). Exendin-4, liraglutide, and dulaglutide significantly decreased the proliferation and migration of VSMCs, the gene expression levels of Pcna, and the protein expression levels of p-Erk and p-JNK in the Ang II-treated VSMCs. Erk inhibitor PD98059 and JNK inhibitor SP600125 decreased the protein expression levels of Pcna and Ccnd1 and proliferation of VSMCs. Inhibition of GLP-1R by siRNA reversed the reduction of the protein expression levels of p-Erk and p-JNK by exendin-4, liraglutide, and dulaglutide in the Ang II-treated VSMCs. Moreover, GLP-1 (9-36) amide also decreased the proliferation and migration of the Ang II-treated VSMCs. In addition, these GLP-1RAs decreased calcium deposition by inhibiting activating transcription factor 4 (Atf4) in Pi-treated VSMCs.
Conclusion
These data show that GLP-1RAs ameliorate aberrant proliferation and migration in VSMCs through both GLP-1Rdependent and independent pathways and inhibit Pi-induced vascular calcification. -
Citations
Citations to this article as recorded by- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
Andrea Etrusco, Mislav Mikuš, Antonio D’Amato, Fabio Barra, Petar Planinić, Trpimir Goluža, Giovanni Buzzaccarini, Jelena Marušić, Mara Tešanović, Antonio Simone Laganà
Biomedicines.2024; 12(3): 653. CrossRef
- Incretin Hormone Secretion in Women with Polycystic Ovary Syndrome: Roles of Obesity, Insulin Sensitivity and Treatment with Metformin and GLP-1s
- Basic Research
- DWN12088, A Prolyl-tRNA Synthetase Inhibitor, Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis
- Dong-Keon Lee, Su Ho Jo, Eun Soo Lee, Kyung Bong Ha, Na Won Park, Deok-Hoon Kong, Sang-In Park, Joon Seok Park, Choon Hee Chung
- Diabetes Metab J. 2024;48(1):97-111. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0367
- Funded: National Research Foundation of Korea, Ministry of Science, ICT and Future Planning, Ministry of Education, Ministry of Science and ICT
- 1,656 View
- 177 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Nonalcoholic steatohepatitis (NASH) is a liver disease caused by obesity that leads to hepatic lipoapoptosis, resulting in fibrosis and cirrhosis. However, the mechanism underlying NASH is largely unknown, and there is currently no effective therapeutic agent against it. DWN12088, an agent used for treating idiopathic pulmonary fibrosis, is a selective prolyl-tRNA synthetase (PRS) inhibitor that suppresses the synthesis of collagen. However, the mechanism underlying the hepatoprotective effect of DWN12088 is not clear. Therefore, we investigated the role of DWN12088 in NASH progression.
Methods
Mice were fed a chow diet or methionine-choline deficient (MCD)-diet, which was administered with DWN12088 or saline by oral gavage for 6 weeks. The effects of DWN12088 on NASH were evaluated by pathophysiological examinations, such as real-time quantitative reverse transcription polymerase chain reaction, immunoblotting, biochemical analysis, and immunohistochemistry. Molecular and cellular mechanisms of hepatic injury were assessed by in vitro cell culture.
Results
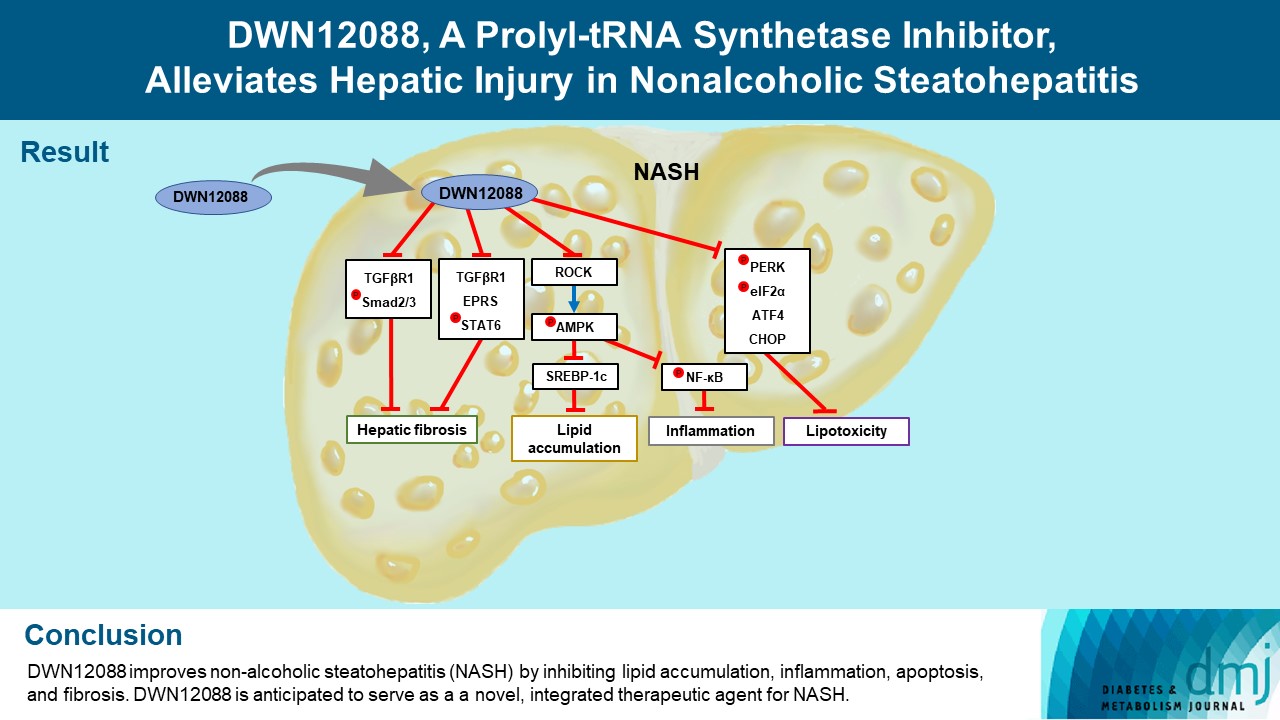
DWN12088 attenuated palmitic acid (PA)-induced lipid accumulation and lipoapoptosis by downregulating the Rho-kinase (ROCK)/AMP-activated protein kinase (AMPK)/sterol regulatory element-binding protein-1c (SREBP-1c) and protein kinase R-like endoplasmic reticulum kinase (PERK)/α subunit of eukaryotic initiation factor 2 (eIF2α)/activating transcription factor 4 (ATF4)/C/EBP-homologous protein (CHOP) signaling cascades. PA increased but DWN12088 inhibited the phosphorylation of nuclear factor-κB (NF-κB) p65 (Ser536, Ser276) and the expression of proinflammatory genes. Moreover, the DWN12088 inhibited transforming growth factor β (TGFβ)-induced pro-fibrotic gene expression by suppressing TGFβ receptor 1 (TGFβR1)/Smad2/3 and TGFβR1/glutamyl-prolyl-tRNA synthetase (EPRS)/signal transducer and activator of transcription 6 (STAT6) axis signaling. In the case of MCD-diet-induced NASH, DWN12088 reduced hepatic steatosis, inflammation, and lipoapoptosis and prevented the progression of fibrosis.
Conclusion
Our findings provide new insights about DWN12088, namely that it plays an important role in the overall improvement of NASH. Hence, DWN12088 shows great potential to be developed as a new integrated therapeutic agent for NASH.

 KDA
KDA
 First
First Prev
Prev





