
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
- Page Path
- HOME > Browse
- Guideline/Fact Sheet
- 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
- Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae Jin Kim, Hyun Min Kim, Jung Hae Ko, Nam Hoon Kim, Chong Hwa Kim, Jeeyun Ahn, Tae Jung Oh, Soo-Kyung Kim, Jaehyun Kim, Eugene Han, Sang-Man Jin, Won Suk Choi, Min Kyong Moon, Committee of Clinical Practice Guidelines, Korean Diabetes Association
- Diabetes Metab J. 2023;47(5):575-594. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0282
- 5,016 View
- 628 Download
- 7 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - In May 2023, the Committee of Clinical Practice Guidelines of the Korean Diabetes Association published the revised clinical practice guidelines for Korean adults with diabetes and prediabetes. We incorporated the latest clinical research findings through a comprehensive systematic literature review and applied them in a manner suitable for the Korean population. These guidelines are designed for all healthcare providers nationwide, including physicians, diabetes experts, and certified diabetes educators who manage patients with diabetes or individuals at risk of developing diabetes. Based on recent changes in international guidelines and the results of a Korean epidemiological study, the recommended age for diabetes screening has been lowered. In collaboration with the relevant Korean medical societies, recently revised guidelines for managing hypertension and dyslipidemia in patients with diabetes have been incorporated into this guideline. An abridgment containing practical information on patient education and systematic management in the clinic was published separately.
-
Citations
Citations to this article as recorded by- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
Eugene Han, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Sang Hoon Ahn, Yong-ho Lee, Seung Up Kim
Metabolism.2024; 152: 155789. CrossRef - Letter by In-Kyung Jeong Regarding Article, Trends in Prevalence of Hypertriglyceridemia and Related Factors in Korean Adults: A Serial Cross-Sectional Study
In-Kyung Jeong
Journal of Lipid and Atherosclerosis.2024; 13(1): 80. CrossRef - Association between cardiovascular disease risk and incident type 2 diabetes mellitus in individuals with prediabetes: A retrospective cohort study
Myung Jin Kim, Yun Kyung Cho, Chang Hee Jung, Woo Je Lee
Diabetes Research and Clinical Practice.2024; 208: 111125. CrossRef - Korea Hypertension Fact Sheet 2023: analysis of nationwide population-based data with a particular focus on hypertension in special populations
Hyeon Chang Kim, Hokyou Lee, Hyeok-Hee Lee, Dasom Son, Minsung Cho, Sojung Shin, Yeeun Seo, Eun-Jin kim, Song Vogue Ahn, Sun Ha Jee, Sungha Park, Hae-Young Lee, Min Ho Shin, Sang-Hyun Ihm, Seung Won Lee, Jong Ku Park, Il Suh, Tae-Yong Lee
Clinical Hypertension.2024;[Epub] CrossRef - Diabetes Duration, Cholesterol Levels, and Risk of Cardiovascular Diseases in Individuals With Type 2 Diabetes
Mee Kyoung Kim, Kyu Na Lee, Kyungdo Han, Seung-Hwan Lee
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Effect of Adding Apolipoprotein B Testing on the Prevalence of Dyslipidemia and Risk of Cardiovascular Disease in the Korean Adult Population
Rihwa Choi, Sang Gon Lee, Eun Hee Lee
Metabolites.2024; 14(3): 169. CrossRef - A self-powered and supercapacitive microneedle continuous glucose monitoring system with a wide range of glucose detection capabilities
Hye-Jun Kil, Jang Hyeon Kim, Kanghae Lee, Tae-Uk Kang, Ju-Hyun Yoo, Yong-ho Lee, Jin-Woo Park
Biosensors and Bioelectronics.2024; 257: 116297. CrossRef - Cardiorenal outcomes and mortality after sodium‐glucose cotransporter‐2 inhibitor initiation in type 2 diabetes patients with percutaneous coronary intervention history
Jin Hwa Kim, Young Sang Lyu, BongSeong Kim, Mee Kyung Kim, Sang Yong Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Recommendations for Pharmacological Treatment of Type 2 Diabetes
Junghyun Noh
The Journal of Korean Diabetes.2023; 24(3): 127. CrossRef - 2023 Clinical Practice Guidelines for Diabetes
Min Kyong Moon
The Journal of Korean Diabetes.2023; 24(3): 120. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk Factors
Ye Seul Yang
The Journal of Korean Diabetes.2023; 24(3): 135. CrossRef - A 33-Year-Old Man Presented with Abdominal Pain and Vomiting Starting a Day Ago
Jong Han Choi
The Korean Journal of Medicine.2023; 98(6): 289. CrossRef - Comorbidity Patterns and Management in Inpatients with Endocrine Diseases by Age Groups in South Korea: Nationwide Data
Sung-Soo Kim, Hun-Sung Kim
Journal of Personalized Medicine.2023; 14(1): 42. CrossRef
- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
- Guideline/Fact Sheet
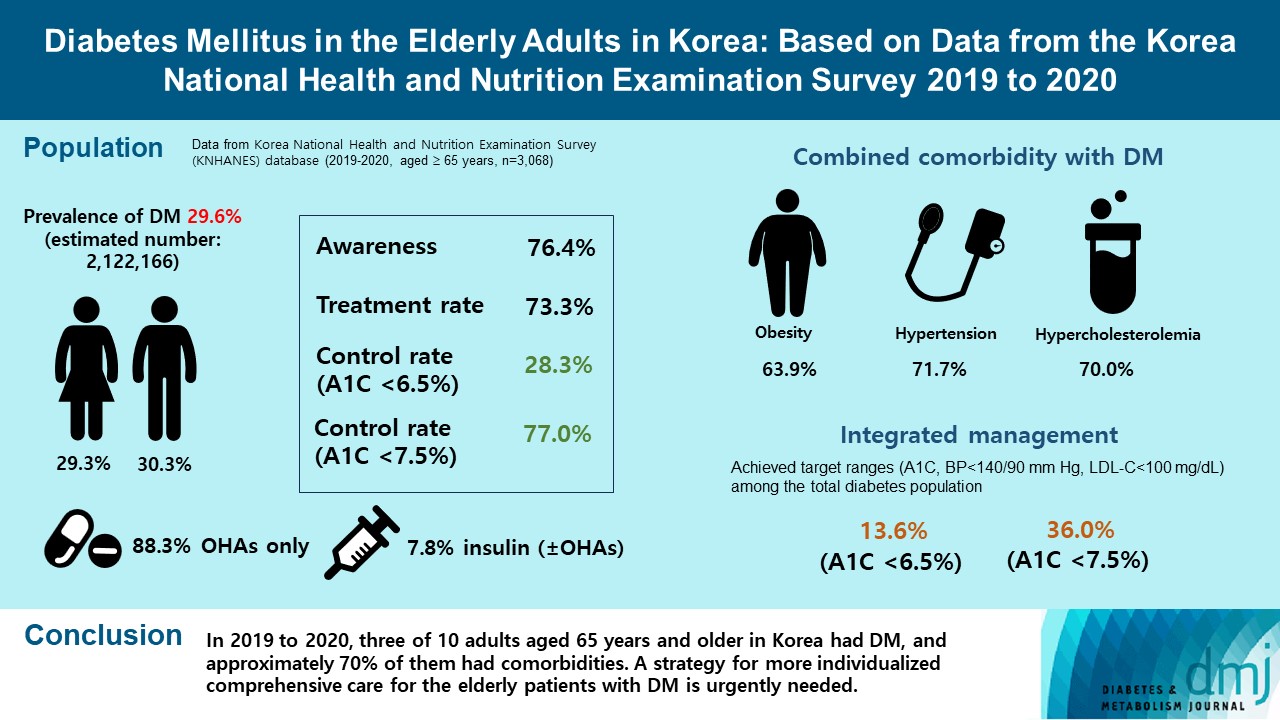
- Diabetes Mellitus in the Elderly Adults in Korea: Based on Data from the Korea National Health and Nutrition Examination Survey 2019 to 2020
- Seung-Hyun Ko, Kyung Do Han, Yong-Moon Park, Jae-Seung Yun, Kyuho Kim, Jae-Hyun Bae, Hyuk-Sang Kwon, Nan-Hee Kim
- Diabetes Metab J. 2023;47(5):643-652. Published online August 7, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0041
- 2,262 View
- 207 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We evaluated the prevalence and management of diabetes mellitus (DM) in elderly Korean patients based on data from the Korea National Health and Nutrition Examination Survey (KNHANES).
Methods
A total of 3,068 adults aged 65 years and older (19.8% of total population) were analyzed using KNHANES from 2019 to 2020. Prevalence, awareness, treatment, and control rates, and comorbidities were analyzed. Lifestyle behaviors and energy intake were also measured.
Results
The prevalence of DM and prediabetes was 29.6% and 50.5%, respectively. The awareness, treatment and control rates were 76.4%, 73.3%, and 28.3%, respectively. The control rate was 77.0% if A1C <7.5% criteria was used. The mean A1C value of individuals with known DM was 7.1%, and 14.5% of the known DM patients had A1C ≥8.0%. Abdominal obesity, hypertension, and hypercholesterolemia were combined with DM in 63.9%, 71.7%, and 70.7%, respectively, and the rate of integrated management was 36.0% (A1C <7.5% criteria). A total of 40.1% of those with DM walked regularly. The percentage of energy intake from carbohydrates was higher in those with DM than in those without DM (P=0.044), while those of fat (P=0.003) and protein (P=0.025) were lower in those with DM than in those without DM in women.
Conclusion
In 2019 to 2020, three of 10 adults aged 65 years and older in Korea had DM, and approximately 70% of them had comorbidities. A strategy for more individualized comprehensive care for the elderly patients with DM is urgently needed. -
Citations
Citations to this article as recorded by- Association Between High Blood Folate Levels and Glaucoma in a Representative Korean Population
Ji Young Lee, Jin A. Choi, Sung Pyo Park, Donghyun Jee
Investigative Opthalmology & Visual Science.2024; 65(1): 6. CrossRef - The Growing Challenge of Diabetes Management in an Aging Society
Seung-Hwan Lee
Diabetes & Metabolism Journal.2023; 47(5): 630. CrossRef
- Association Between High Blood Folate Levels and Glaucoma in a Representative Korean Population
- Guideline/Fact Sheet
- Comparison of Operational Definition of Type 2 Diabetes Mellitus Based on Data from Korean National Health Insurance Service and Korea National Health and Nutrition Examination Survey
- Jong Ha Baek, Yong-Moon Park, Kyung Do Han, Min Kyong Moon, Jong Han Choi, Seung-Hyun Ko
- Diabetes Metab J. 2023;47(2):201-210. Published online February 8, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0375

- 3,259 View
- 217 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We evaluated the validity and reliability of the operational definition of type 2 diabetes mellitus (T2DM) based on the Korean National Health Insurance Service (NHIS) database.
Methods
Adult subjects (≥40 years old) included in the Korea National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2017 were merged with those from the NHIS health check-up database, producing a cross-sectional dataset. We evaluated the sensitivity, specificity, accuracy, and agreement of the NHIS criteria for defining T2DM by comparing them with the KNHANES criteria as a standard reference.
Results
In the study population (n=13,006), two algorithms were devised to determine from the NHIS dataset whether the diagnostic claim codes for T2DM were accompanied by prescription codes for anti-diabetic drugs (algorithm 1) or not (algorithm 2). Using these algorithms, the prevalence of T2DM was 14.9% (n=1,942; algorithm 1) and 20.8% (n=2,707; algorithm 2). Good reliability in defining T2DM was observed for both algorithms (Kappa index, 0.73 [algorithm 1], 0.63 [algorithm 2]). However, the accuracy (0.93 vs. 0.89) and specificity (0.96 vs. 0.90) tended to be higher for algorithm 1 than for algorithm 2. The validity (accuracy, ranging from 0.91 to 0.95) and reliability (Kappa index, ranging from 0.68 to 0.78) of defining T2DM by NHIS criteria were independent of age, sex, socioeconomic status, and accompanied hypertension or dyslipidemia.
Conclusion
The operational definition of T2DM based on population-based NHIS claims data, including diagnostic codes and prescription codes, could be a valid tool to identify individuals with T2DM in the Korean population. -
Citations
Citations to this article as recorded by- Metabolic dysfunction-associated fatty liver disease increases the risk of type 2 diabetes mellitus in young Korean adults
Junchul Ha, Oak-Kee Hong, Kyungdo Han, Hyuk-Sang Kwon
Diabetes Research and Clinical Practice.2024; : 111584. CrossRef - Risk of Cause-Specific Mortality across Glucose Spectrum in Elderly People: A Nationwide Population-Based Cohort Study
Joonyub Lee, Hun-Sung Kim, Kee-Ho Song, Soon Jib Yoo, Kyungdo Han, Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(5): 525. CrossRef
- Metabolic dysfunction-associated fatty liver disease increases the risk of type 2 diabetes mellitus in young Korean adults
- Guideline/Fact Sheet
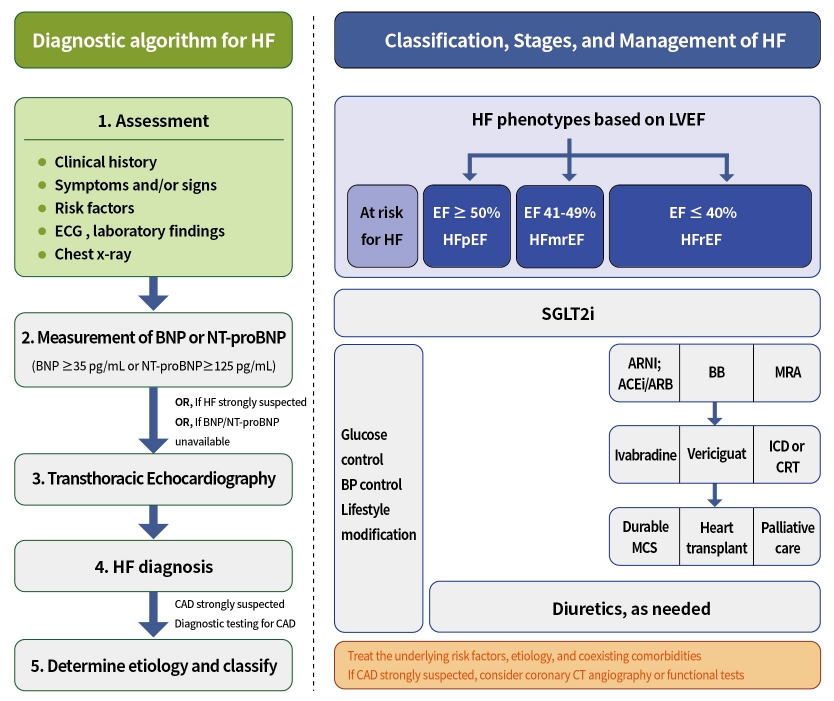
- Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
- Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon, The Committee of Clinical Practice Guidelines, Korean Diabetes Association and Committee of Clinical Practice Guidelines, Korean Society of Heart Failure
- Diabetes Metab J. 2023;47(1):10-26. Published online January 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0420
- 4,335 View
- 411 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Diabetes mellitus is a major risk factor for the development of heart failure. Furthermore, the prognosis of heart failure is worse in patients with diabetes mellitus than in those without it. Therefore, early diagnosis and proper management of heart failure in patients with diabetes mellitus are important. This review discusses the current criteria for diagnosis and screening tools for heart failure and the currently recommended pharmacological therapies for heart failure. We also highlight the effects of anti-diabetic medications on heart failure.
-
Citations
Citations to this article as recorded by- A Multicenter, Randomized, Open-Label Study to Compare the Effects of Gemigliptin Add-on or Escalation of Metformin Dose on Glycemic Control and Safety in Patients with Inadequately Controlled Type 2 Diabetes Mellitus Treated with Metformin and SGLT-2 Inh
Hae Jin Kim, Jung Hyun Noh, Min Kyong Moon, Sung Hee Choi, Seung-Hyun Ko, Eun-Jung Rhee, Kyu Yeon Hur, In-Kyung Jeong, Mark Yorek
Journal of Diabetes Research.2024; 2024: 1. CrossRef - Comparison of the effects of gemigliptin versus glimepiride on cardiac function in patients with type 2 diabetes uncontrolled with metformin: The gemi‐heart study
Seung Min Chung, Jun Sung Moon, Jun Hwa Hong, In‐Chang Hwang, Soo Lim
Diabetes, Obesity and Metabolism.2023; 25(8): 2181. CrossRef - Optimization of guideline-directed medical treatment for heart failure patients with reduced ejection fraction
Minjung Bak, Jin-Oh Choi
The Korean Journal of Internal Medicine.2023; 38(5): 595. CrossRef
- A Multicenter, Randomized, Open-Label Study to Compare the Effects of Gemigliptin Add-on or Escalation of Metformin Dose on Glycemic Control and Safety in Patients with Inadequately Controlled Type 2 Diabetes Mellitus Treated with Metformin and SGLT-2 Inh
- Guideline/Fact Sheet
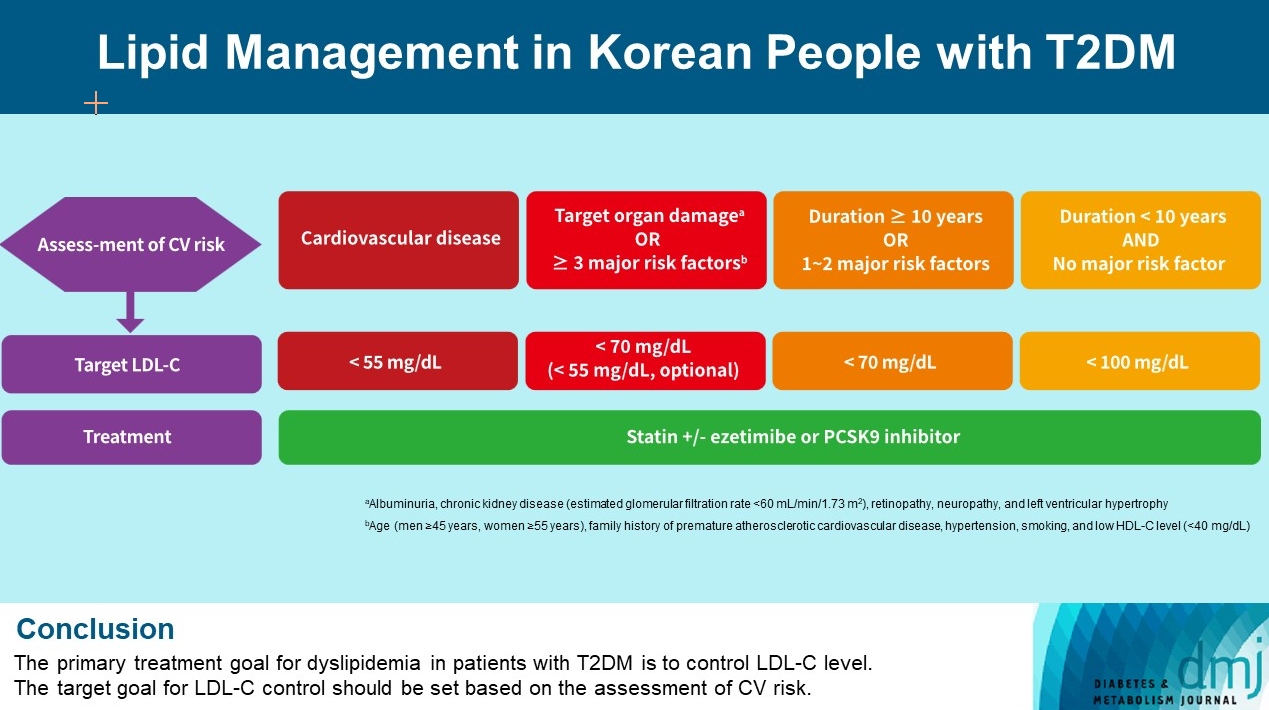
- Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
- Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon, on Behalf of Committee of Clinical Practice Guideline, Korean Diabetes Association and Clinical Practice Guideline Committee, Korean Society of Lipid and Atherosclerosis
- Diabetes Metab J. 2023;47(1):1-9. Published online January 20, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0448
- 3,556 View
- 378 Download
- 3 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Dyslipidemia in patients with diabetes is an important treatment target as a modifiable risk factor for cardiovascular disease (CVD). Although the primary treatment goal for dyslipidemia is to control low-density lipoprotein cholesterol (LDL-C), achieving this goal remains suboptimal according to recent studies. It is important to set the target goal for LDL-C control based on an accurate risk assessment for CVD. Here, we summarize the latest evidence on lipid management in patients with diabetes and present a consensus of the Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis on the treatment goals of LDL-C according to the duration of diabetes, presence of CVD, target organ damage, or major cardiovascular risk factors. In patients with type 2 diabetes mellitus (T2DM) and CVD, an LDL-C goal of <55 mg/dL and a reduction in LDL-C level by 50% or more from the baseline is recommended. For the primary prevention of CVD in patients with T2DM with a duration of diabetes ≥10 years, major cardiovascular risk factors, or target organ damage, an LDL-C goal of <70 mg/dL is recommended. In patients with T2DM with a duration of diabetes <10 years and no major cardiovascular risk factors, an LDL-C goal of <100 mg/dL is recommended.
-
Citations
Citations to this article as recorded by- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ji Yoon Kim, Jimi Choi, Sin Gon Kim
European Heart Journal - Cardiovascular Pharmacotherapy.2024; 10(2): 118. CrossRef - Clinical Characteristics of Patients With Statin Discontinuation in Korea: A Nationwide Population-Based Study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Journal of Lipid and Atherosclerosis.2024; 13(1): 41. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(5): 632. CrossRef - 2023 Clinical Practice Guidelines for Diabetes
Min Kyong Moon
The Journal of Korean Diabetes.2023; 24(3): 120. CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk Factors
Ye Seul Yang
The Journal of Korean Diabetes.2023; 24(3): 135. CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Journal of Lipid and Atherosclerosis.2023; 12(3): 237. CrossRef
- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
- Guideline/Fact Sheet
- Screening for Prediabetes and Diabetes in Korean Nonpregnant Adults: A Position Statement of the Korean Diabetes Association, 2022
- Kyung Ae Lee, Dae Jung Kim, Kyungdo Han, Suk Chon, Min Kyong Moon, on Behalf of the Committee of Clinical Practice Guideline of Korean Diabetes Association
- Diabetes Metab J. 2022;46(6):819-826. Published online November 24, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0364
- 4,264 View
- 268 Download
- 4 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
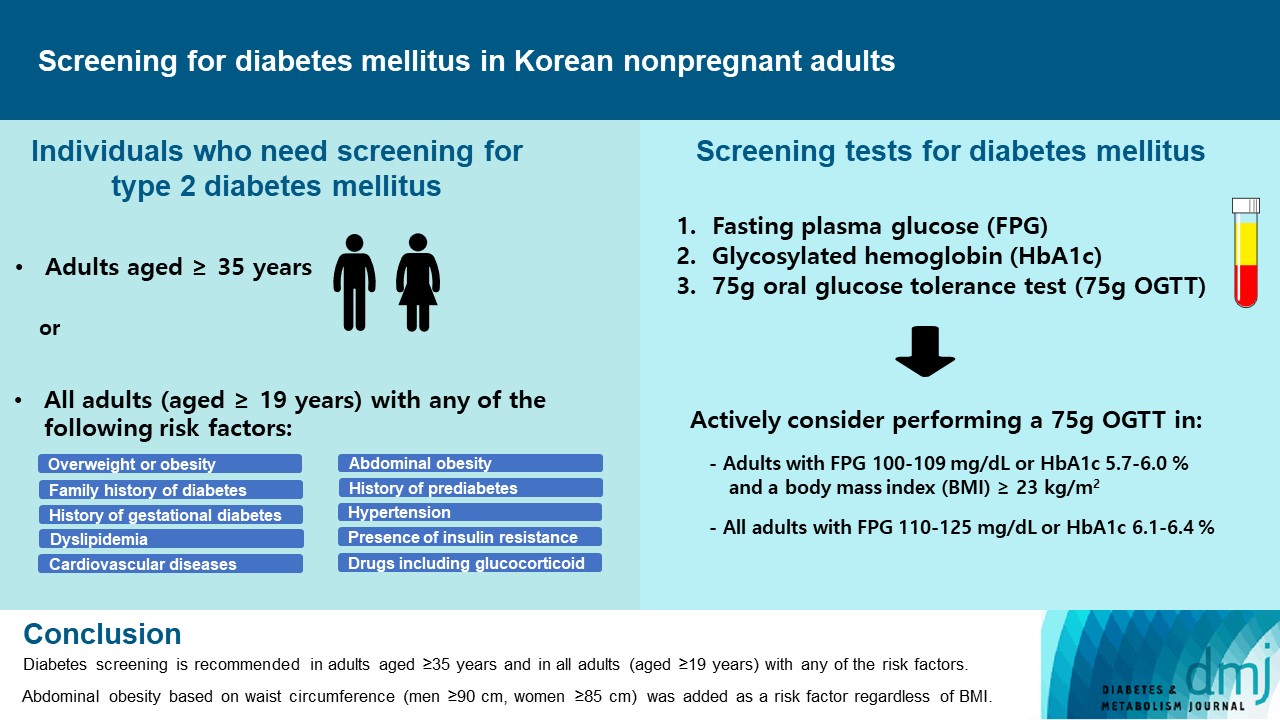
ePub - Diabetes screening serves to identify individuals at high-risk for diabetes who have not yet developed symptoms and to diagnose diabetes at an early stage. Globally, the prevalence of diabetes is rapidly increasing. Furthermore, obesity and/or abdominal obesity, which are major risk factors for type 2 diabetes mellitus (T2DM), are progressively increasing, particularly among young adults. Many patients with T2DM are asymptomatic and can accompany various complications at the time of diagnosis, as well as chronic complications develop as the duration of diabetes increases. Thus, proper screening and early diagnosis are essential for diabetes care. Based on reports on the changing epidemiology of diabetes and obesity in Korea, as well as growing evidence from new national cohort studies on diabetes screening, the Korean Diabetes Association has updated its clinical practice recommendations regarding T2DM screening. Diabetes screening is now recommended in adults aged ≥35 years regardless of the presence of risk factors, and in all adults (aged ≥19) with any of the risk factors. Abdominal obesity based on waist circumference (men ≥90 cm, women ≥85 cm) was added to the list of risk factors.
-
Citations
Citations to this article as recorded by- Oxidative Balance Score and New-Onset Type 2 Diabetes Mellitus in Korean Adults without Non-Alcoholic Fatty Liver Disease: Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) Cohort
Mid-Eum Moon, Dong Hyuk Jung, Seok-Jae Heo, Byoungjin Park, Yong Jae Lee
Antioxidants.2024; 13(1): 107. CrossRef - Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin as Metformin Add-on in a Korean Population with Type 2 Diabetes
Byung-Wan Lee, Young Min Cho, Sin Gon Kim, Seung-Hyun Ko, Soo Lim, Amine Dahaoui, Jin Sook Jeong, Hyo Jin Lim, Jae Myung Yu
Diabetes Therapy.2024; 15(2): 547. CrossRef - Triglyceride-glucose index predicts type 2 diabetes mellitus more effectively than oral glucose tolerance test-derived insulin sensitivity and secretion markers
Min Jin Lee, Ji Hyun Bae, Ah Reum Khang, Dongwon Yi, Mi Sook Yun, Yang Ho Kang
Diabetes Research and Clinical Practice.2024; 210: 111640. CrossRef - Cumulative muscle strength and risk of diabetes: A prospective cohort study with mediation analysis
Shanhu Qiu, Xue Cai, Yan Liang, Wenji Chen, Duolao Wang, Zilin Sun, Bo Xie, Tongzhi Wu
Diabetes Research and Clinical Practice.2023; 197: 110562. CrossRef - Revisiting the Diabetes Crisis in Korea: Call for Urgent Action
Jun Sung Moon
The Journal of Korean Diabetes.2023; 24(1): 1. CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - 2023 Clinical Practice Guidelines for Diabetes
Min Kyong Moon
The Journal of Korean Diabetes.2023; 24(3): 120. CrossRef
- Oxidative Balance Score and New-Onset Type 2 Diabetes Mellitus in Korean Adults without Non-Alcoholic Fatty Liver Disease: Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) Cohort
- Guideline/Fact Sheet
- Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension
- Jong Han Choi, Jee-Hyun Kang, Suk Chon
- Diabetes Metab J. 2022;46(3):377-390. Published online May 25, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0051
- 4,945 View
- 249 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The Joint Committee of the Korean Diabetes Association, the Korean Society for the Study of Obesity, and the Korean Society of Hypertension announced a consensus statement on carbohydrate-restricted diets and intermittent fasting, representing an emerging and popular dietary pattern. In this statement, we recommend moderately-low-carbohydrate or low-carbohydrate diets, not a very-low-carbohydrate diet, for patients with type 2 diabetes mellitus. These diets can be considered a dietary regimen to improve glycemic control and reduce body weight in adults with type 2 diabetes mellitus. This review provides the detailed results of a meta-analysis and systematic literature review on the potential harms and benefits of carbohydrate-restricted diets in patients with diabetes. We expect that this review will help experts and patients by fostering an in-depth understanding and appropriate application of carbohydrate-restricted diets in the comprehensive management of diabetes.
-
Citations
Citations to this article as recorded by- Efficacy of convenience meal-type foods designed for diabetes in the management of metabolic syndrome based on a 3-week trial
Do Gyeong Lee, In Gyeong Kang, Tae Seok Kim, Yun Ahn, Sang Yun Lee, Hye Jin Ahn, Yoo Kyoung Park
Nutrition.2024; 118: 112287. CrossRef - Long-Term Results of a Digital Diabetes Self-Management and Education Support Program Among Adults With Type 2 Diabetes: A Retrospective Cohort Study
Ashley Berthoumieux, Sarah Linke, Melinda Merry, Alison Megliola, Jessie Juusola, Jenna Napoleone
The Science of Diabetes Self-Management and Care.2024; 50(1): 19. CrossRef - Medical nutrition therapy for diabetes mellitus
Suk Chon
Journal of the Korean Medical Association.2023; 66(7): 421. CrossRef
- Efficacy of convenience meal-type foods designed for diabetes in the management of metabolic syndrome based on a 3-week trial
- Guideline/Fact Sheet
- Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hypertension
- Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim, Committee of Clinical Practice Guidelines, Korean Society for the Study of Obesity (KSSO), Committee of Clinical Practice Guidelines and Committee of Food and Nutrition, Korean Diabetes Association (KDA), Policy Committee of Korean Society of Hypertension (KSH), Policy Development Committee of National Academy of Medicine of Korea (NAMOK)
- Diabetes Metab J. 2022;46(3):355-376. Published online May 25, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0038
- 11,006 View
- 588 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
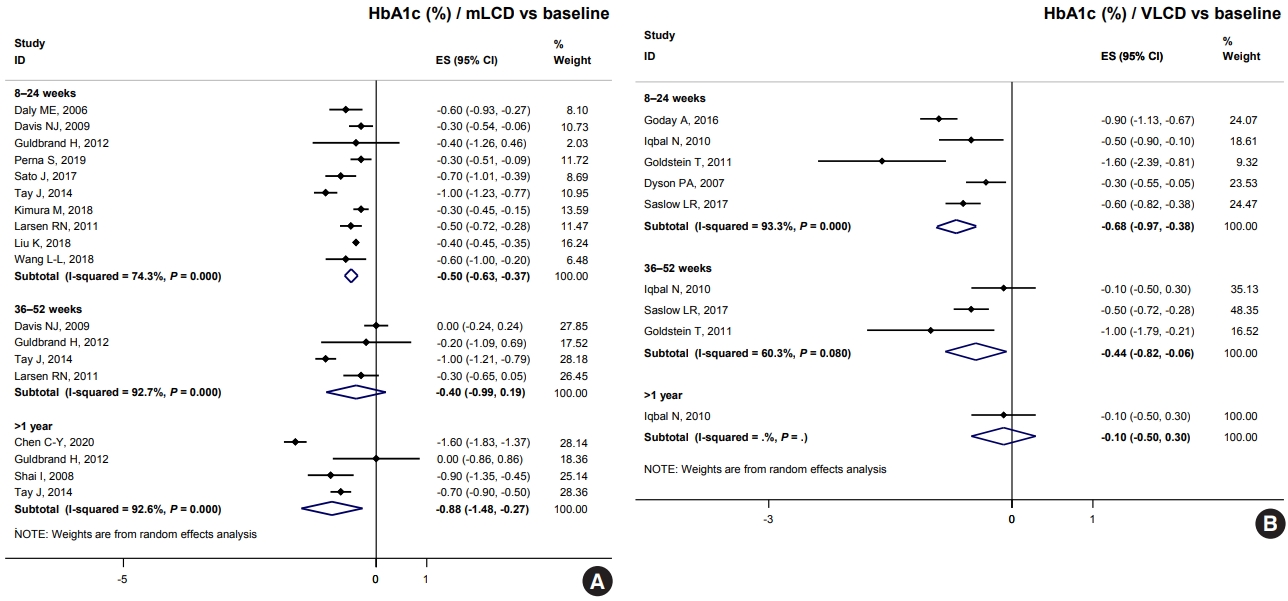
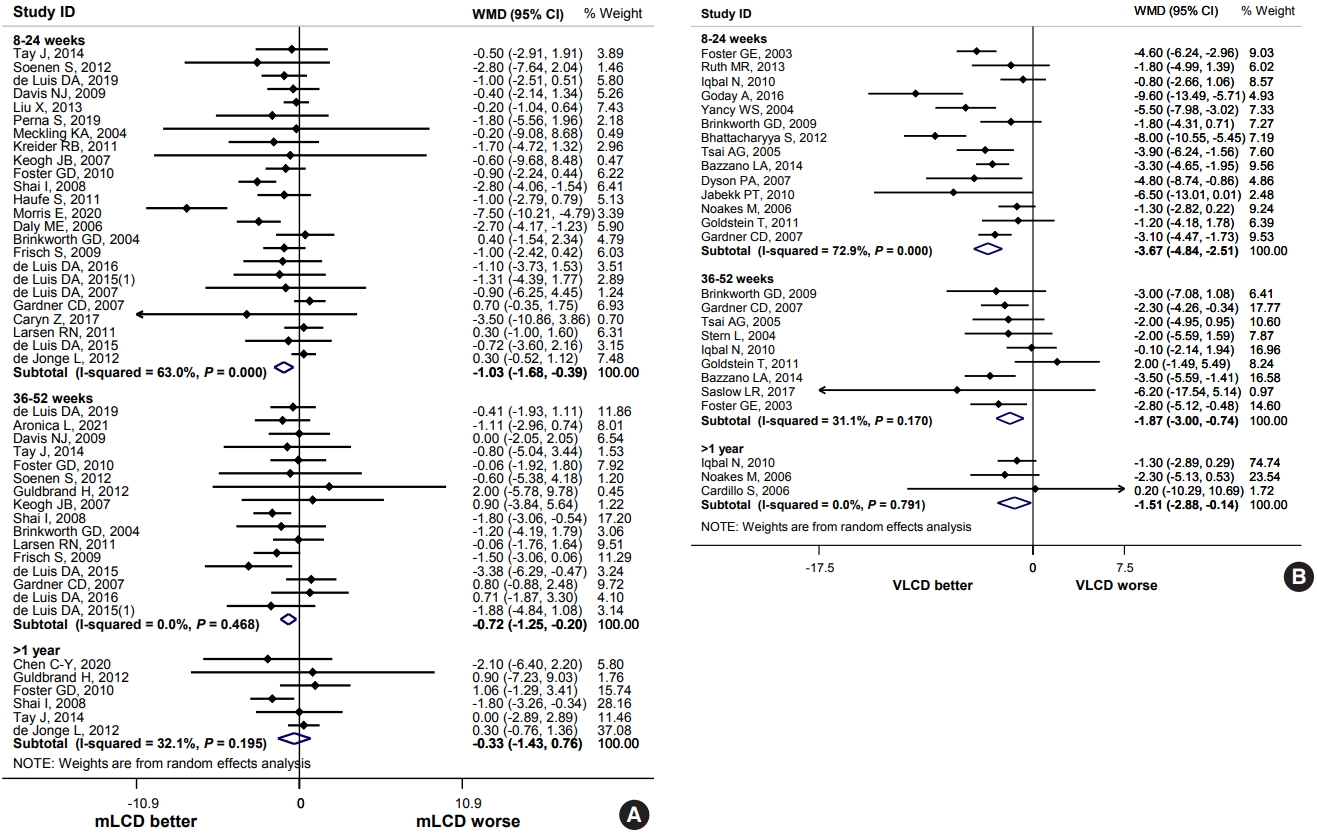
ePub - Carbohydrate-restricted diets and intermittent fasting (IF) have been rapidly gaining interest among the general population and patients with cardiometabolic disease, such as overweight or obesity, diabetes, and hypertension. However, there are limited expert recommendations for these dietary regimens. This study aimed to evaluate the level of scientific evidence on the benefits and harms of carbohydrate-restricted diets and IF to make responsible recommendations. A meta-analysis and systematic literature review of 66 articles on 50 randomized controlled trials (RCTs) of carbohydrate-restricted diets and 10 articles on eight RCTs of IF was performed. Based on the analysis, the following recommendations are suggested. In adults with overweight or obesity, a moderately-low carbohydrate or low carbohydrate diet (mLCD) can be considered as a dietary regimen for weight reduction. In adults with type 2 diabetes mellitus, mLCD can be considered as a dietary regimen for improving glycemic control and reducing body weight. In contrast, a very-low carbohydrate diet (VLCD) and IF are recommended against in patients with diabetes. Furthermore, no recommendations are suggested for VLCD and IF in adults with overweight or obesity, and carbohydrate-restricted diets and IF in patients with hypertension. Here, we describe the results of our analysis and the evidence for these recommendations.
-
Citations
Citations to this article as recorded by- Metabolic changes with intermittent fasting
Maria G. Lange, Alice A. Coffey, Paul C. Coleman, Thomas M. Barber, Thijs Van Rens, Oyinlola Oyebode, Sally Abbott, Petra Hanson
Journal of Human Nutrition and Dietetics.2024; 37(1): 256. CrossRef - Papel do Jejum Intermitente e da Dieta Restrita em Carboidratos na Prevenção de Doenças Cardiovasculares em Pacientes Pré-Diabéticos
Mohamed Khalfallah, Basma Elnagar, Shaimaa S. Soliman, Ahmad Eissa, Amany Allaithy
Arquivos Brasileiros de Cardiologia.2023;[Epub] CrossRef - Medical nutrition therapy for diabetes mellitus
Suk Chon
Journal of the Korean Medical Association.2023; 66(7): 421. CrossRef - Euglycemic diabetic ketoacidosis development in a patient with type 2 diabetes receiving a sodium-glucose cotransporter-2 inhibitor and a carbohydrate-restricted diet
Gwanpyo Koh, Jisun Bang, Soyeon Yoo, Sang Ah Lee
Journal of Medicine and Life Science.2023; 20(3): 126. CrossRef - Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hyperte
Jong Han Choi, Jee-Hyun Kang, Suk Chon
Diabetes & Metabolism Journal.2022; 46(3): 377. CrossRef - The Related Metabolic Diseases and Treatments of Obesity
Ming Yang, Shuai Liu, Chunye Zhang
Healthcare.2022; 10(9): 1616. CrossRef - Updated Meta-Analysis of Studies from 2011 to 2021 Comparing the Effectiveness of Intermittent Energy Restriction and Continuous Energy Restriction
Kyoung-Kon Kim, Jee-Hyun Kang, Eun Mi Kim
Journal of Obesity & Metabolic Syndrome.2022; 31(3): 230. CrossRef
- Metabolic changes with intermittent fasting
- Guideline/Fact Sheet
- 2021 Clinical Practice Guidelines for Diabetes Mellitus in Korea
- Kyu Yeon Hur, Min Kyong Moon, Jong Suk Park, Soo-Kyung Kim, Seung-Hwan Lee, Jae-Seung Yun, Jong Ha Baek, Junghyun Noh, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Ye Seul Yang, Jang Won Son, Jong Han Choi, Kee Ho Song, Nam Hoon Kim, Sang Yong Kim, Jin Wha Kim, Sang Youl Rhee, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim, Chong Hwa Kim, Dae Jung Kim, SungWan Chun, Eun-Jung Rhee, Hyun Min Kim, Hyun Jung Kim, Donghyun Jee, Jae Hyun Kim, Won Seok Choi, Eun-Young Lee, Kun-Ho Yoon, Seung-Hyun Ko, Committee of Clinical Practice Guidelines, Korean Diabetes Association
- Diabetes Metab J. 2021;45(4):461-481. Published online July 30, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0156
- 23,950 View
- 1,626 Download
- 121 Web of Science
- 140 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
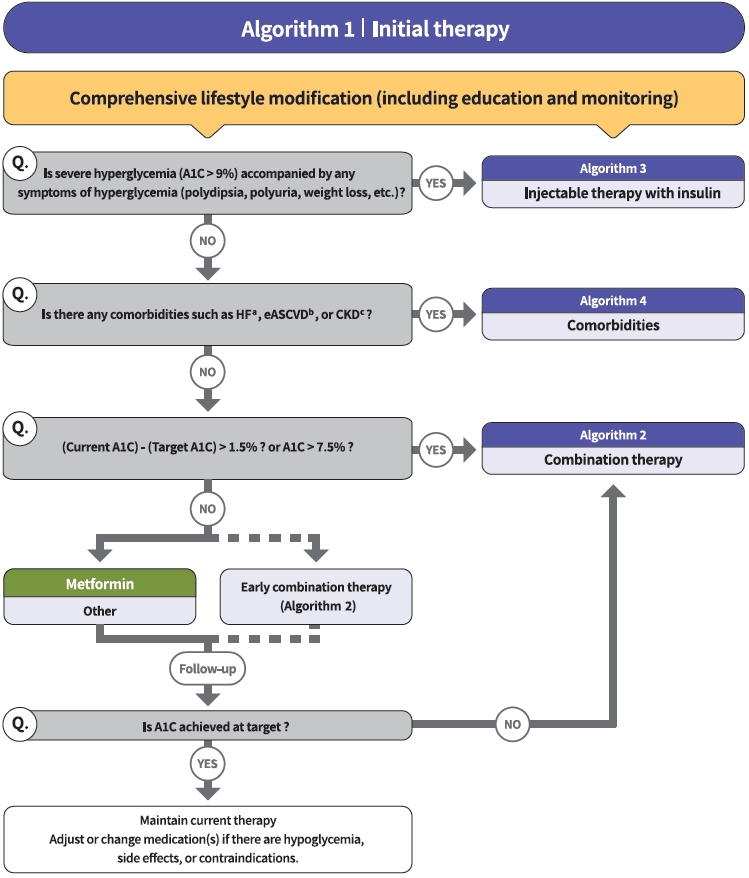
- The Committee of Clinical Practice Guidelines of the Korean Diabetes Association (KDA) updated the previous clinical practice guidelines for Korean adults with diabetes and prediabetes and published the seventh edition in May 2021. We performed a comprehensive systematic review of recent clinical trials and evidence that could be applicable in real-world practice and suitable for the Korean population. The guideline is provided for all healthcare providers including physicians, diabetes experts, and certified diabetes educators across the country who manage patients with diabetes or the individuals at the risk of developing diabetes mellitus. The recommendations for screening diabetes and glucose-lowering agents have been revised and updated. New sections for continuous glucose monitoring, insulin pump use, and non-alcoholic fatty liver disease in patients with diabetes mellitus have been added. The KDA recommends active vaccination for coronavirus disease 2019 in patients with diabetes during the pandemic. An abridgement that contains practical information for patient education and systematic management in the clinic was published separately.
-
Citations
Citations to this article as recorded by- Impact of Subclinical Atrial Function on the Prognosis of Patients With Atrial Fibrillation and Metabolic Syndrome
Hyun-Jin Kim
CardioMetabolic Syndrome Journal.2024; 4(1): 36. CrossRef - Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ji Yoon Kim, Jimi Choi, Sin Gon Kim
European Heart Journal - Cardiovascular Pharmacotherapy.2024; 10(2): 118. CrossRef - A Multicenter, Randomized, Open-Label Study to Compare the Effects of Gemigliptin Add-on or Escalation of Metformin Dose on Glycemic Control and Safety in Patients with Inadequately Controlled Type 2 Diabetes Mellitus Treated with Metformin and SGLT-2 Inh
Hae Jin Kim, Jung Hyun Noh, Min Kyong Moon, Sung Hee Choi, Seung-Hyun Ko, Eun-Jung Rhee, Kyu Yeon Hur, In-Kyung Jeong, Mark Yorek
Journal of Diabetes Research.2024; 2024: 1. CrossRef - Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin as Metformin Add-on in a Korean Population with Type 2 Diabetes
Byung-Wan Lee, Young Min Cho, Sin Gon Kim, Seung-Hyun Ko, Soo Lim, Amine Dahaoui, Jin Sook Jeong, Hyo Jin Lim, Jae Myung Yu
Diabetes Therapy.2024; 15(2): 547. CrossRef - Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Association between Dyslipidemia and Glycated Hemoglobin in a Population-Based Study
Purum Kang, Ka Young Kim, Hye Young Shin
Metabolites.2024; 14(2): 92. CrossRef - Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease
Heejoon Jang, Yeonjin Kim, Dong Hyeon Lee, Sae Kyung Joo, Bo Kyung Koo, Soo Lim, Woojoo Lee, Won Kim
JAMA Internal Medicine.2024; 184(4): 375. CrossRef - View on Metformin: Antidiabetic and Pleiotropic Effects, Pharmacokinetics, Side Effects, and Sex-Related Differences
Guglielmina Froldi
Pharmaceuticals.2024; 17(4): 478. CrossRef - Comparison between a tubeless, on-body automated insulin delivery system and a tubeless, on-body sensor-augmented pump in type 1 diabetes: a multicentre randomised controlled trial
Ji Yoon Kim, Sang-Man Jin, Eun Seok Kang, Soo Heon Kwak, Yeoree Yang, Jee Hee Yoo, Jae Hyun Bae, Jun Sung Moon, Chang Hee Jung, Ji Cheol Bae, Sunghwan Suh, Sun Joon Moon, Sun Ok Song, Suk Chon, Jae Hyeon Kim
Diabetologia.2024;[Epub] CrossRef - Efficacy of intermittent short‐term use of a real‐time continuous glucose monitoring system in non‐insulin–treated patients with type 2 diabetes: A randomized controlled trial
Sun Joon Moon, Kyung‐Soo Kim, Woo Je Lee, Mi Yeon Lee, Robert Vigersky, Cheol‐Young Park
Diabetes, Obesity and Metabolism.2023; 25(1): 110. CrossRef - Therapeutic Effects of Switching to Anagliptin from Other DPP-4 Inhibitors in T2DM Patients with Inadequate Glycemic Control: A Non-interventional, Single-Arm, Open-Label, Multicenter Observational Study
Sang-Yong Kim, Sungrae Kim
Diabetes Therapy.2023; 14(1): 109. CrossRef - Low Skeletal Muscle Mass Accompanied by Abdominal Obesity Additively Increases the Risk of Incident Type 2 Diabetes
Ji Eun Jun, Seung-Eun Lee, You-Bin Lee, Gyuri Kim, Sang-Man Jin, Jae Hwan Jee, Jae Hyeon Kim
The Journal of Clinical Endocrinology & Metabolism.2023; 108(5): 1173. CrossRef - Diabetes screening in South Korea: a new estimate of the number needed to screen to detect diabetes
Kyoung Hwa Ha, Kyung Ae Lee, Kyung-Do Han, Min Kyong Moon, Dae Jung Kim
The Korean Journal of Internal Medicine.2023; 38(1): 93. CrossRef - Justicia carnea extracts ameliorated hepatocellular damage in streptozotocin-induced type 1 diabetic male rats via decrease in oxidative stress, inflammation and increasing other risk markers
John Adeolu Falode, Oluwaseun Igbekele Ajayi, Tolulope Victoria Isinkaye, Akinwunmi Oluwaseun Adeoye, Basiru Olaitan Ajiboye, Bartholomew I. C. Brai
Biomarkers.2023; 28(2): 177. CrossRef - Sex differences in the impact of diabetes mellitus on tuberculosis recurrence: a retrospective national cohort study
Dararat Eksombatchai, Dawoon Jeong, Jeongha Mok, Doosoo Jeon, Hee-Yeon Kang, Hee Jin Kim, Hee-Sun Kim, Hongjo Choi, Young Ae Kang
International Journal of Infectious Diseases.2023; 127: 1. CrossRef - The Predictive Ability of C-Peptide in Distinguishing Type 1 Diabetes From Type 2 Diabetes: A Systematic Review and Meta-Analysis
Sajid Iqbal, Abdulrahim Abu Jayyab, Ayah Mohammad Alrashdi, Silvia Reverté-Villarroya
Endocrine Practice.2023; 29(5): 379. CrossRef - Anagliptin twice‐daily regimen improves glycaemic variability in subjects with type 2 diabetes: A double‐blind, randomized controlled trial
Yong‐ho Lee, Doo‐Man Kim, Jae Myung Yu, Kyung Mook Choi, Sin Gon Kim, Kang Seo Park, Hyun‐Shik Son, Choon Hee Chung, Kyu Jeung Ahn, Soon Hee Lee, Ki‐Ho Song, Su Kyoung Kwon, Hyeong Kyu Park, Kyu Chang Won, Hak Chul Jang
Diabetes, Obesity and Metabolism.2023; 25(5): 1174. CrossRef - Implementation of five machine learning methods to predict the 52-week blood glucose level in patients with type 2 diabetes
Xiaomin Fu, Yuhan Wang, Ryan S. Cates, Nan Li, Jing Liu, Dianshan Ke, Jinghua Liu, Hongzhou Liu, Shuangtong Yan
Frontiers in Endocrinology.2023;[Epub] CrossRef - Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Journal of Lipid and Atherosclerosis.2023; 12(1): 12. CrossRef - The Efficacy of Treatment Intensification by Quadruple Oral Therapy Compared to GLP-1RA Therapy in Poorly Controlled Type 2 Diabetes Mellitus Patients: A Real-world Data Study
Minyoung Kim, Hosu Kim, Kyong Young Kim, Soo Kyoung Kim, Junghwa Jung, Jong Ryeal Hahm, Jaehoon Jung, Jong Ha Baek
Diabetes & Metabolism Journal.2023; 47(1): 135. CrossRef - Safety and Effectiveness of Empagliflozin in Korean Patients with Type 2 Diabetes Mellitus: Results from a Nationwide Post-Marketing Surveillance
Jun Sung Moon, Nam Hoon Kim, Jin Oh Na, Jae Hyoung Cho, In-Kyung Jeong, Soon Hee Lee, Ji-Oh Mok, Nan Hee Kim, Dong Jin Chung, Jinhong Cho, Dong Woo Lee, Sun Woo Lee, Kyu Chang Won
Diabetes & Metabolism Journal.2023; 47(1): 82. CrossRef - Evaluation and Management of Patients With Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
International Journal of Heart Failure.2023; 5(1): 1. CrossRef - Influenza vaccination trend and related factors among patients with diabetes in Korea: Analysis using a nationwide database
Dong-Hwa Lee, Bumhee Yang, Seonhye Gu, Eung-Gook Kim, Youlim Kim, Hyung Koo Kang, Yeong Hun Choe, Hyun Jeong Jeon, Seungyong Park, Hyun Lee
Frontiers in Endocrinology.2023;[Epub] CrossRef - Optimal Low-Density Lipoprotein Cholesterol Level for Primary Prevention in Koreans with Type 2 Diabetes Mellitus
Ji Yoon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2023; 47(1): 42. CrossRef - Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 10. CrossRef - Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 1. CrossRef - Association between Low-Density Lipoprotein Cholesterol Level and Cardiovascular Outcomes in Korean Adults: A Nationwide Cohort Study
Junghyun Noh, Min Kyong Moon, Eun-Jung Rhee, Sang Hyun Park, Hyeon Chang Kim, Byung Jin Kim, Hae Jin Kim, Seonghoon Choi, Jin Oh Na, Young Youl Hyun, Bum Joon Kim, Kyung-Do Han, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(1): 59. CrossRef - Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer
Su Jung Lee, Chulho Kim, Hyunjae Yu, Dong-Kyu Kim
Cancers.2023; 15(4): 1094. CrossRef - The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension
Hack-Lyoung Kim, Eun Mi Lee, Shin Young Ahn, Kwang-il Kim, Hyeon Chang Kim, Ju Han Kim, Hae-Young Lee, Jang Hoon Lee, Jong-Moo Park, Eun Joo Cho, Sungha Park, Jinho Shin, Young-Kwon Kim
Clinical Hypertension.2023;[Epub] CrossRef - Consistency of 1-day and 3-day average dietary intake and the relationship of dietary intake with blood glucose, hbA1c, BMI, and lipids in patients with type 2 diabetes
DaeEun Lee, Haejung Lee, Sangeun Lee, MinJin Lee, Ah Reum Khang
Journal of Korean Biological Nursing Science.2023; 25(1): 20. CrossRef - Efficacy and safety of enavogliflozin versus dapagliflozin added to metformin plus gemigliptin treatment in patients with type 2 diabetes: A double-blind, randomized, comparator-active study: ENHANCE-D study
Kyung-Soo Kim, Kyung Ah Han, Tae Nyun Kim, Cheol-Young Park, Jung Hwan Park, Sang Yong Kim, Yong Hyun Kim, Kee Ho Song, Eun Seok Kang, Chul Sik Kim, Gwanpyo Koh, Jun Goo Kang, Mi Kyung Kim, Ji Min Han, Nan Hee Kim, Ji Oh Mok, Jae Hyuk Lee, Soo Lim, Sang S
Diabetes & Metabolism.2023; 49(4): 101440. CrossRef - Effect of olmesartan and amlodipine on serum angiotensin-(1–7) levels and kidney and vascular function in patients with type 2 diabetes and hypertension
Kyuho Kim, Ji Hye Moon, Chang Ho Ahn, Soo Lim
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Menopausal hormone therapy and the risk of type 2 diabetes mellitus: Health Insurance Database in South Korea–based retrospective cohort study
Jin-Sung Yuk, Jung Min Kim
Menopause.2023; 30(5): 497. CrossRef - Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus
Takayoshi Sasako, Toshimasa Yamauchi, Kohjiro Ueki
Diabetes & Metabolism Journal.2023; 47(2): 185. CrossRef - Association between antidiabetic drugs and the incidence of atrial fibrillation in patients with type 2 diabetes: A nationwide cohort study in South Korea
Sunyoung Kim, So Young Park, Bongseong Kim, Chanyang Min, Wonyoung Cho, Dong Keon Yon, Joo Young Kim, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee, Sang Youl Rhee
Diabetes Research and Clinical Practice.2023; 198: 110626. CrossRef - Totally robotic Roux-en-Y gastric bypass in a morbidly obese patient in Korea: a case report
Ji Won Seo, Kyong-Hwa Jun
Journal of Minimally Invasive Surgery.2023; 26(1): 40. CrossRef - Effect of diabetes-specific oral nutritional supplements with allulose on weight and glycemic profiles in overweight or obese type 2 diabetic patients
Jihye Tak, Minkyung Bok, Hyunkyung Rho, Ju Hyun Park, Yunsook Lim, Suk Chon, Hyunjung Lim
Nutrition Research and Practice.2023; 17(2): 241. CrossRef - Associations Between Modifiable Risk Factors and Changes in Glycemic Status Among Individuals With Prediabetes
Salma Nabila, Ji-Eun Kim, Jaesung Choi, JooYong Park, Aesun Shin, Sang-Ah Lee, Jong-koo Lee, Daehee Kang, Ji-Yeob Choi
Diabetes Care.2023; 46(3): 535. CrossRef - Efficacy and safety of enavogliflozin, a novel SGLT2 inhibitor, in Korean people with type 2 diabetes: A 24‐week, multicentre, randomized, double‐blind, placebo‐controlled, phase III trial
Soo Heon Kwak, Kyung Ah Han, Kyung‐Soo Kim, Jae Myung Yu, EunSook Kim, Jong Chul Won, Jun Goo Kang, Choon Hee Chung, Seungjoon Oh, Sung Hee Choi, Kyu Chang Won, Sin Gon Kim, Seung Ah Cho, Bo Young Cho, Kyong Soo Park
Diabetes, Obesity and Metabolism.2023; 25(7): 1865. CrossRef - Adjusting the Use of Glucose-Lowering Agents in the Real-World Clinical Management of People with Type 2 Diabetes: A Narrative Review
Siew Pheng Chan, Lee-Ling Lim, Juliana C. N. Chan, David R. Matthews
Diabetes Therapy.2023; 14(5): 823. CrossRef - The association of perfluoroalkyl substances (PFAS) exposure and kidney function in Korean adolescents using data from Korean National Environmental Health Survey (KoNEHS) cycle 4 (2018–2020): a cross-sectional study
Jisuk Yun, Eun-Chul Jang, Soon-Chan Kwon, Young-Sun Min, Yong-Jin Lee
Annals of Occupational and Environmental Medicine.2023;[Epub] CrossRef - A Comparison of the Pharmacokinetics and Safety of Dapagliflozin Formate, an Ester Prodrug of Dapagliflozin, to Dapagliflozin Propanediol Monohydrate in Healthy Subjects
Hyun Chul Kim, Sangmi Lee, Siyoung Sung, Eunjin Kim, In-Jin Jang, Jae-Yong Chung
Drug Design, Development and Therapy.2023; Volume 17: 1203. CrossRef - Efficacy and safety of monotherapy with enavogliflozin in Korean patients with type 2 diabetes mellitus: Results of a 12‐week, multicentre, randomized, double‐blind, placebo‐controlled, phase 2 trial
Ye Seul Yang, Kyung Wan Min, Seok‐O Park, Kyung‐Soo Kim, Jae Myung Yu, Eun‐Gyoung Hong, Sung Rae Cho, Kyu Chang Won, Yong Hyun Kim, Seungjoon Oh, Sung Hee Choi, Gwanpyo Koh, Wan Huh, Su Young Kim, Kyong Soo Park
Diabetes, Obesity and Metabolism.2023; 25(8): 2096. CrossRef - An Integrated Digital Health Care Platform for Diabetes Management With AI-Based Dietary Management: 48-Week Results From a Randomized Controlled Trial
You-Bin Lee, Gyuri Kim, Ji Eun Jun, Hyunjin Park, Woo Je Lee, You-Cheol Hwang, Jae Hyeon Kim
Diabetes Care.2023; 46(5): 959. CrossRef - Performance of Simple Fibrosis Score in Non-Alcoholic Fatty Liver Disease with and without Type 2 Diabetes
Seung Min Chung, Min Kyu Kang, Jun Sung Moon, Jung Gil Park
Endocrinology and Metabolism.2023; 38(2): 277. CrossRef - Correlation analysis of cancer incidence after pravastatin treatment
Jin Yu, Raeun Kim, Jiwon Shinn, Man Young Park, Hun-Sung Kim
Cardiovascular Prevention and Pharmacotherapy.2023; 5(2): 61. CrossRef - Comparison of the effects of gemigliptin versus glimepiride on cardiac function in patients with type 2 diabetes uncontrolled with metformin: The gemi‐heart study
Seung Min Chung, Jun Sung Moon, Jun Hwa Hong, In‐Chang Hwang, Soo Lim
Diabetes, Obesity and Metabolism.2023; 25(8): 2181. CrossRef - The era of continuous glucose monitoring and its expanded role in type 2 diabetes
Jin Yu, Jae‐Hyoung Cho, Seung‐Hwan Lee
Journal of Diabetes Investigation.2023; 14(7): 841. CrossRef - Impact of continuous glucose monitoring on glycemic control and its derived metrics in type 1 diabetes: a longitudinal study
So Hyun Cho, Seohyun Kim, You-Bin Lee, Sang-Man Jin, Kyu Yeon Hur, Gyuri Kim, Jae Hyeon Kim
Frontiers in Endocrinology.2023;[Epub] CrossRef - Impact of mental disorders on the risk of heart failure among Korean patients with diabetes: a cohort study
Tae Kyung Yoo, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
Cardiovascular Diabetology.2023;[Epub] CrossRef - Asia-Pacific consensus recommendations for application of continuous glucose monitoring in diabetes management
Alice P.S. Kong, Soo Lim, Seung-Hyun Yoo, Linong Ji, Liming Chen, Yuqian Bao, Ester Yeoh, Siew-Pheng Chan, Chih-Yuan Wang, Viswanathan Mohan, Neale Cohen, Margaret J. McGill, Stephen M. Twigg
Diabetes Research and Clinical Practice.2023; 201: 110718. CrossRef - Chronic disease management program applied to type 2 diabetes patients and prevention of diabetic complications: a retrospective cohort study using nationwide data
Min Kyung Hyun, Jang Won Lee, Seung-Hyun Ko
BMC Public Health.2023;[Epub] CrossRef - Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017
Eugene Han, Kyung-Do Han, Yong-ho Lee, Kyung-Soo Kim, Sangmo Hong, Jung Hwan Park, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(3): 347. CrossRef - Opening the Precision Diabetes Care through Digital Healthcare
Joonyub Lee, Jin Yu, Kun-Ho Yoon
Diabetes & Metabolism Journal.2023; 47(3): 307. CrossRef - Glycemia according to the Use of Continuous Glucose Monitoring among Adults with Type 1 Diabetes Mellitus in Korea: A Real-World Study
You-Bin Lee, Minjee Kim, Jae Hyeon Kim
Diabetes & Metabolism Journal.2023; 47(3): 405. CrossRef - Navigating the Seas of Glycemic Control: The Role of Continuous Glucose Monitoring in Type 1 Diabetes Mellitus
Jun Sung Moon
Diabetes & Metabolism Journal.2023; 47(3): 345. CrossRef - Lost in translation: assessing the nomenclature change for diabetic kidney disease in Japan
Tetsuya Babazono, Tatsumi Moriya
Diabetology International.2023; 14(4): 319. CrossRef - Effects of dapagliflozin compared with glimepiride on body composition in Asian patients with type 2 diabetes inadequately controlled with metformin: The BEYOND study
Hyeong Kyu Park, Kyoung‐Ah Kim, Kyung‐Wan Min, Tae‐Seo Sohn, In Kyung Jeong, Chul Woo Ahn, Nan‐Hee Kim, Ie Byung Park, Ho Chan Cho, Choon Hee Chung, Sung Hee Choi, Kang Seo Park, Seoung‐Oh Yang, Kwan Woo Lee
Diabetes, Obesity and Metabolism.2023; 25(9): 2743. CrossRef - Topic Modeling Analysis of Diabetes-Related Health Information during the Coronavirus Disease Pandemic
Soyoon Min, Jeongwon Han
Healthcare.2023; 11(13): 1871. CrossRef - Screening Test for Evaluation of Cardiovascular Disease in Patients with Diabetes
Ji-Oh Mok, Chan-Hee Jung
The Journal of Korean Diabetes.2023; 24(2): 76. CrossRef - Paradigm Shift in Management of Hyperglycemia in Patients with Type 2 Diabetes: Glucocentric versus Organ Protection
Jong Chul Won
The Journal of Korean Diabetes.2023; 24(2): 59. CrossRef - Association between type 2 diabetes mellitus and depression among Korean midlife women: a cross-sectional analysis study
You Lee Yang, Eun-Ok Im, Yunmi Kim
BMC Nursing.2023;[Epub] CrossRef - Fibrotic Burden in the Liver Differs Across Metabolic Dysfunction-Associated Fatty Liver Disease Subtypes
Tae Seop Lim, Ho Soo Chun, Soon Sun Kim, Ja Kyung Kim, Minjong Lee, Hyo Jung Cho, Seung Up Kim, Jae Youn Cheong
Gut and Liver.2023; 17(4): 610. CrossRef - Association between the number of pregnancies and cardiac target organ damages: a cross-sectional analysis of data from the Korean women’s chest pain registry (KoROSE)
Hack-Lyoung Kim, Hyun-Jin Kim, Mina Kim, Sang Min Park, Hyun Ju Yoon, Young Sup Byun, Seong-Mi Park, Mi-Seung Shin, Kyung-Soon Hong, Myung-A Kim
BMC Women's Health.2023;[Epub] CrossRef - Exercise therapy for diabetes mellitus
Chaiho Jeong, Tae-Seo Sohn
Journal of the Korean Medical Association.2023; 66(7): 427. CrossRef - Medical nutrition therapy for diabetes mellitus
Suk Chon
Journal of the Korean Medical Association.2023; 66(7): 421. CrossRef - Identification of individuals at risk of hepatocellular carcinoma: screening for clinically significant liver fibrosis in patients with T2DM
Tina Reinson, Ryan M Buchanan, Christopher D Byrne
Expert Review of Endocrinology & Metabolism.2023; 18(5): 355. CrossRef - Additive impact of diabetes and sarcopenia on all-cause and cardiovascular mortality: A longitudinal nationwide population-based study
Eyun Song, Soon Young Hwang, Min Jeong Park, Ahreum Jang, Kyeong Jin Kim, Ji Hee Yu, Nam Hoon Kim, Hye Jin Yoo, Ji A. Seo, Sin Gon Kim, Nan Hee Kim, Sei Hyun Baik, Kyung Mook Choi
Metabolism.2023; 148: 155678. CrossRef - Exposure to perfluoroalkyl and polyfluoroalkyl substances and risk of stroke in adults: a meta-analysis
Min Cheol Chang, Seung Min Chung, Sang Gyu Kwak
Reviews on Environmental Health.2023;[Epub] CrossRef - Risk of Pancreatic Cancer and Use of Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes: A Propensity Score-Matching Analysis
Mee Kyoung Kim, Kyungdo Han, Hyuk-Sang Kwon, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(4): 426. CrossRef - Incident infection risks depending on oral antidiabetic exposure in insulin-treated type 2 diabetes patients
Sanghwa Park, Jiseon Jeong, Yunna Woo, Yeo Jin Choi, Sooyoung Shin
Scientific Reports.2023;[Epub] CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Journal of Lipid and Atherosclerosis.2023; 12(3): 237. CrossRef - Diabetes Mellitus in the Elderly Adults in Korea: Based on Data from the Korea National Health and Nutrition Examination Survey 2019 to 2020
Seung-Hyun Ko, Kyung Do Han, Yong-Moon Park, Jae-Seung Yun, Kyuho Kim, Jae-Hyun Bae, Hyuk-Sang Kwon, Nan-Hee Kim
Diabetes & Metabolism Journal.2023; 47(5): 643. CrossRef - Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review
Wah-Kheong Chan, Kee-Huat Chuah, Ruveena Bhavani Rajaram, Lee-Ling Lim, Jeyakantha Ratnasingam, Shireene Ratna Vethakkan
Journal of Obesity & Metabolic Syndrome.2023; 32(3): 197. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk Factors
Ye Seul Yang
The Journal of Korean Diabetes.2023; 24(3): 135. CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(5): 632. CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Riesgo residual. Conclusiones
Ángel Cequier, José Luis Zamorano
Revista Española de Cardiología Suplementos.2023; 23: 25. CrossRef - Intake of Fruit and Glycemic Control in Korean Patients with Diabetes Mellitus Using the Korea National Health and Nutrition Examination Survey
Eunju Yoon, Ji Cheol Bae, Sunghwan Suh
Endocrinology and Metabolism.2023; 38(5): 538. CrossRef - 2023 Clinical Practice Guidelines for Diabetes
Min Kyong Moon
The Journal of Korean Diabetes.2023; 24(3): 120. CrossRef - Cumulative effect of impaired fasting glucose on the risk of dementia in middle-aged and elderly people: a nationwide cohort study
Jin Yu, Kyu-Na Lee, Hun-Sung Kim, Kyungdo Han, Seung-Hwan Lee
Scientific Reports.2023;[Epub] CrossRef - Coleus forskohlii Root Extract (ForcslimTM) as a Prospective Antidiabetic Agent: In vitro Glucose Uptake Stimulation and α-Amylase Inhibitory Effects
Firoz Hirehal Hussain Mi, Channangihalli Thimmegowda Sadashiva, Neethumol Benny, Sreedrisya Ayippakkari Kuttiattu, Ravi Subban
International Journal of Pharmacology.2023; 19(5): 730. CrossRef - Comparison of on-Statin Lipid and Lipoprotein Levels for the Prediction of First Cardiovascular Event in Type 2 Diabetes Mellitus
Ji Yoon Kim, Jimi Choi, Sin Gon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2023; 47(6): 837. CrossRef - Differential Impact of Obesity on the Risk of Diabetes Development in Two Age Groups: Analysis from the National Health Screening Program
Tae Kyung Yoo, Kyung-Do Han, Yang-Hyun Kim, Ga Eun Nam, Sang Hyun Park, Eun-Jung Rhee, Won-Young Lee
Diabetes & Metabolism Journal.2023; 47(6): 846. CrossRef - Strategies to Maintain the Remission of Diabetes Following Metabolic Surgery
Mi Kyung Kim, Hye Soon Kim
Journal of Metabolic and Bariatric Surgery.2023; 12(2): 26. CrossRef - East Asian perspectives in metabolic and bariatric surgery
Tae Jung Oh, Hyuk‐Joon Lee, Young Min Cho
Journal of Diabetes Investigation.2022; 13(5): 756. CrossRef - Recent Updates to Clinical Practice Guidelines for Diabetes Mellitus
Jin Yu, Seung-Hwan Lee, Mee Kyoung Kim
Endocrinology and Metabolism.2022; 37(1): 26. CrossRef - Association between Physical Exercise and Glycated Hemoglobin Levels in Korean Patients Diagnosed with Diabetes
Il Yun, Hye Jin Joo, Yu Shin Park, Eun-Cheol Park
International Journal of Environmental Research and Public Health.2022; 19(6): 3280. CrossRef - Effectiveness and safety of teneligliptin added to patients with type 2 diabetes inadequately controlled by oral triple combination therapy: A multicentre, randomized, double‐blind, and placebo‐controlled study
Minyoung Lee, Woo‐je Lee, Jae Hyeon Kim, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2022; 24(6): 1105. CrossRef - Trends of severe hypoglycemia in patients with type 2 diabetes in Korea: A longitudinal nationwide cohort study
Jae‐Seung Yun, Kyungdo Han, Seung‐Hyun Ko
Journal of Diabetes Investigation.2022; 13(8): 1438. CrossRef - GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions
Ji Hee Yu, So Young Park, Da Young Lee, Nan Hee Kim, Ji A Seo
Kidney Research and Clinical Practice.2022; 41(2): 136. CrossRef - Cardiorenal Risk Profiles Among Data-Driven Type 2 Diabetes Sub-Phenotypes: A Post-Hoc Analysis of the China Health and Nutrition Survey
Hui Gao, Kan Wang, Wensui Zhao, Jianlin Zhuang, Yu Jiang, Lei Zhang, Qingping Liu, Fariba Ahmadizar
Frontiers in Endocrinology.2022;[Epub] CrossRef - Individualized Medical Nutrition Therapy for Diabetic Patients according to Diabetes Medication
Juyeon Park
The Journal of Korean Diabetes.2022; 23(1): 50. CrossRef - Critical shear stress of red blood cells as a novel integrated biomarker for screening chronic kidney diseases in cases of type 2 diabetes
Il Rae Park, Jimi Choi, Eun Young Ha, Seung Min Chung, Jun Sung Moon, Sehyun Shin, Sin Gon Kim, Kyu Chang Won
Clinical Hemorheology and Microcirculation.2022; 81(4): 293. CrossRef - Effects of exercise on reducing diabetes risk in Korean women according to menopausal status
Jung-Hwan Cho, Hye-Mi Kwon, Se-Eun Park, Ju-Hwan Yoo, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(2): 75. CrossRef - Novel Glycemic Index Based on Continuous Glucose Monitoring to Predict Poor Clinical Outcomes in Critically Ill Patients: A Pilot Study
Eun Yeong Ha, Seung Min Chung, Il Rae Park, Yin Young Lee, Eun Young Choi, Jun Sung Moon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Free Versus Fixed-Ratio Combination of Basal Insulin and GLP-1 Receptor Agonists in Type 2 Diabetes Uncontrolled With GLP-1 Receptor Agonists: A Systematic Review and Indirect Treatment Comparison
Han Na Jung, Yun Kyung Cho, Se Hee Min, Hwi Seung Kim, Ye-Jee Kim, Joong-Yeol Park, Woo Je Lee, Chang Hee Jung
Frontiers in Endocrinology.2022;[Epub] CrossRef - Obesity, hypertension, diabetes mellitus, and hypercholesterolemia in Korean adults before and during the COVID-19 pandemic: a special report of the 2020 Korea National Health and Nutrition Examination Survey
Ga Bin Lee, Yoonjung Kim, Suyeon Park, Hyeon Chang Kim, Kyungwon Oh
Epidemiology and Health.2022; 44: e2022041. CrossRef - Adherence to healthy lifestyle behaviors as a preventable risk factor for severe hypoglycemia in people with type 2 diabetes: A longitudinal nationwide cohort study
Jae‐Seung Yun, Kyungdo Han, Yong‐Moon Park, Eugene Han, Yong‐ho Lee, Seung‐Hyun Ko
Journal of Diabetes Investigation.2022; 13(9): 1533. CrossRef - Diabetes Fact Sheet in Korea 2021
Jae Hyun Bae, Kyung-Do Han, Seung-Hyun Ko, Ye Seul Yang, Jong Han Choi, Kyung Mook Choi, Hyuk-Sang Kwon, Kyu Chang Won
Diabetes & Metabolism Journal.2022; 46(3): 417. CrossRef - Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hyperte
Jong Han Choi, Jee-Hyun Kang, Suk Chon
Diabetes & Metabolism Journal.2022; 46(3): 377. CrossRef - Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Diabetes & Metabolism Journal.2022; 46(3): 355. CrossRef - Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: consensus statement of the Korean Society for the Study of obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Clinical Hypertension.2022;[Epub] CrossRef - Efficacy of Personalized Diabetes Self-care Using an Electronic Medical Record–Integrated Mobile App in Patients With Type 2 Diabetes: 6-Month Randomized Controlled Trial
Eun Young Lee, Seon-Ah Cha, Jae-Seung Yun, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, Min Kyung Hyun, Seung-Hyun Ko
Journal of Medical Internet Research.2022; 24(7): e37430. CrossRef - A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - Hypoglycemic agents and glycemic variability in individuals with type 2 diabetes: A systematic review and network meta-analysis
SuA Oh, Sujata Purja, Hocheol Shin, Minji Kim, Eunyoung Kim
Diabetes and Vascular Disease Research.2022; 19(3): 147916412211068. CrossRef - Tolerability and Effectiveness of Switching to Dulaglutide in Patients With Type 2 Diabetes Inadequately Controlled With Insulin Therapy
Youngsook Kim, Ji Hye Huh, Minyoung Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Frontiers in Endocrinology.2022;[Epub] CrossRef - Factors Influencing the Utilization of Diabetes Complication Tests Under the COVID-19 Pandemic: Machine Learning Approach
Haewon Byeon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Association of prediabetes with death and diabetic complications in older adults: the pros and cons of active screening for prediabetes
Giwoong Choi, Hojun Yoon, Hyun Ho Choi, Kyoung Hwa Ha, Dae Jung Kim
Age and Ageing.2022;[Epub] CrossRef - Recent information on test utilization and intraindividual change in anti-glutamic acid decarboxylase antibody in Korea: a retrospective study
Rihwa Choi, Wonseo Park, Gayoung Chun, Jiwon Lee, Sang Gon Lee, Eun Hee Lee
BMJ Open Diabetes Research & Care.2022; 10(3): e002739. CrossRef - Extra-Glycemic Effects of Anti-Diabetic Medications: Two Birds with One Stone?
Eun-Jung Rhee
Endocrinology and Metabolism.2022; 37(3): 415. CrossRef - Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Antidiabetic Agents
Kyung-Soo Kim
The Journal of Korean Diabetes.2022; 23(2): 83. CrossRef - Maintaining Physical Activity Is Associated with Reduced Major Adverse Cardiovascular Events in People Newly Diagnosed with Diabetes
Duhoe Kim, Jaehun Seo, Kyoung Hwa Ha, Dae Jung Kim
Journal of Obesity & Metabolic Syndrome.2022; 31(2): 187. CrossRef - Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Journal of Obesity & Metabolic Syndrome.2022; 31(2): 100. CrossRef - Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus
Jeongmin Lee, Jae-Seung Yun, Seung-Hyun Ko
Nutrients.2022; 14(15): 3086. CrossRef - Severe hypoglycemia as a risk factor for cardiovascular outcomes in patients with type 2 diabetes: is it preventable?
Seung-Hyun Ko
Cardiovascular Prevention and Pharmacotherapy.2022; 4(3): 106. CrossRef - New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
Diabetes & Metabolism Journal.2022; 46(4): 517. CrossRef - Current status of obesity treatment in Korea: based on the 2020 Korean Society for the Study of Obesity guidelines for obesity management
Eun-Jung Rhee
Journal of the Korean Medical Association.2022; 65(7): 388. CrossRef - Experiences of Using Wearable Continuous Glucose Monitors in Adults With Diabetes: A Qualitative Descriptive Study
Hee Sun Kang, Hyang Rang Park, Chun-Ja Kim, Savitri Singh-Carlson
The Science of Diabetes Self-Management and Care.2022; 48(5): 362. CrossRef - 젊은 2형 당뇨병 환자의 관리
재현 배
Public Health Weekly Report.2022; 15(35): 2474. CrossRef - Real-World Prescription Patterns and Barriers Related to the Use of Sodium-Glucose Cotransporter 2 Inhibitors among Korean Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease
Jong Ha Baek, Ye Seul Yang, Seung-Hyun Ko, Kyung Do Han, Jae Hyeon Kim, Min Kyong Moon, Jong Suk Park, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Jong Han Choi, Kyu Yeon Hur
Diabetes & Metabolism Journal.2022; 46(5): 701. CrossRef - Low-Density Lipoprotein Cholesterol Level, Statin Use and Myocardial Infarction Risk in Young Adults
Heekyoung Jeong, Kyungdo Han, Soon Jib Yoo, Mee Kyoung Kim
Journal of Lipid and Atherosclerosis.2022; 11(3): 288. CrossRef - Blood Pressure Target in Type 2 Diabetes Mellitus
Hyun-Jin Kim, Kwang-il Kim
Diabetes & Metabolism Journal.2022; 46(5): 667. CrossRef - Association of underweight status with the risk of tuberculosis: a nationwide population-based cohort study
Su Hwan Cho, Hyun Lee, Hyuktae Kwon, Dong Wook Shin, Hee-Kyung Joh, Kyungdo Han, Jin Ho Park, Belong Cho
Scientific Reports.2022;[Epub] CrossRef - Exploring the risk factors of impaired fasting glucose in middle-aged population living in South Korean communities by using categorical boosting machine
Haewon Byeon
Frontiers in Endocrinology.2022;[Epub] CrossRef - External validation and clinical application of the predictive model for severe hypoglycemia
Jae-Seung Yun, Kyungdo Han, Soo-Yeon Choi, Seon-Ah Cha, Yu-Bae Ahn, Seung-Hyun Ko
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effect of Euonymus alatus Extracts on Diabetes Related Markers in Pancreatic β-Cells and C57BL/Ksj-db/db Mice
Ye Rin Kim, Eun-young Kim, Seong Uk Lee, Young Wan Kim, Yoon Hee Kim
Journal of the Korean Society of Food Science and Nutrition.2022; 51(9): 894. CrossRef - Muscle fat contents rather than muscle mass determines nonalcoholic steatohepatitis and liver fibrosis in patients with severe obesity
Eugene Han, Mi Kyung Kim, Hye Won Lee, Seungwan Ryu, Hye Soon Kim, Byoung Kuk Jang, Youngsung Suh
Obesity.2022; 30(12): 2440. CrossRef - Correlation between shift work and non-alcoholic fatty liver disease among male workers in the steel manufacturing company of Korea: a cross-sectional study
Kiseok Kim, Yong-Jin Lee, Soon-Chan Kwon, Young-Sun Min, Hyun Kyo Lee, Gwangin Baek, Sang Hyeon Kim, Eun-Chul Jang
Annals of Occupational and Environmental Medicine.2022;[Epub] CrossRef - FGM-based remote intervention for adults with type 1 diabetes: The FRIEND randomized clinical trial
Jinju Lee, Myeong Hoon Lee, Jiyun Park, Kyung-Soo Kim, Soo-Kyung Kim, Yong-Wook Cho, Hyun Wook Han, Young Shin Song
Frontiers in Endocrinology.2022;[Epub] CrossRef - Screening for Prediabetes and Diabetes in Korean Nonpregnant Adults: A Position Statement of the Korean Diabetes Association, 2022
Kyung Ae Lee, Dae Jung Kim, Kyungdo Han, Suk Chon, Min Kyong Moon
Diabetes & Metabolism Journal.2022; 46(6): 819. CrossRef - Blood Pressure Control in Patients with Diabetic Kidney Disease
Yaeni Kim, Won Kim, Jwa-Kyung Kim, Ju Young Moon, Samel Park, Cheol Whee Park, Hoon Suk Park, Sang Heon Song, Tae-Hyun Yoo, So-Young Lee, Eun Young Lee, Jeonghwan Lee, Kyubok Jin, Dae Ryong Cha, Jin Joo Cha, Sang Youb Han
Electrolytes & Blood Pressure.2022; 20(2): 39. CrossRef - The Gangwon Obesity and Metabolic Syndrome Study: Methods and Initial Baseline Data
Yoon Jeong Cho, Sohyun Park, Sung Soo Kim, Hyo Jin Park, Jang Won Son, Tae Kyung Lee, Sangmo Hong, Jee-Hyun Kang, Seon Mee Kim, Yang-Hyun Kim, Won Jun Kim, Young Eun Seo, Yoosuk An, Sang Youl Rhee, Suk Chon, Sookyoung Jeon, Kyungho Park, Bong-Soo Kim, Cha
Journal of Obesity & Metabolic Syndrome.2022; 31(4): 303. CrossRef - Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes?
Hwi Seung Kim, Chang Hee Jung
International Journal of Molecular Sciences.2021; 22(18): 9936. CrossRef - Long-term effectiveness and safety of quadruple combination therapy with empagliflozin versus dapagliflozin in patients with type 2 diabetes: 3-year prospective observational study
Eu Jeong Ku, Dong-Hwa Lee, Hyun Jeong Jeon, Tae Keun Oh
Diabetes Research and Clinical Practice.2021; 182: 109123. CrossRef - Albuminuria Is Associated with Steatosis Burden in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2021;45:698-707)
Mi-kyung Kim
Diabetes & Metabolism Journal.2021; 45(6): 968. CrossRef - Incidence and Risk Factors for Progression to Diabetes Mellitus: A Retrospective Cohort Study
Min Kyung Hyun, Jong Heon Park, Kyoung Hoon Kim, Soon-Ki Ahn, Seon Mi Ji
International Journal of Environmental Research and Public Health.2021; 19(1): 123. CrossRef - 2021 Clinical Practice Guidelines for Diabetes: Pharmacotherapy and the Korean Diabetes Association Support System
Kyu Yeon Hur
The Journal of Korean Diabetes.2021; 22(4): 250. CrossRef - 2021 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk
Min Kyong Moon
The Journal of Korean Diabetes.2021; 22(4): 259. CrossRef
- Impact of Subclinical Atrial Function on the Prognosis of Patients With Atrial Fibrillation and Metabolic Syndrome
- Guideline/Fact Sheet
- Sodium-Glucose Cotransporter-2 Inhibitor for Renal Function Preservation in Patients with Type 2 Diabetes Mellitus: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
- Tae Jung Oh, Ju-Young Moon, Kyu Yeon Hur, Seung Hyun Ko, Hyun Jung Kim, Taehee Kim, Dong Won Lee, Min Kyong Moon, The Committee of Clinical Practice Guideline, Korean Diabetes Association and Committee of the Cooperative Studies, Korean Society of Nephrology
- Diabetes Metab J. 2020;44(4):489-497. Published online August 21, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0172
- 7,803 View
- 167 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
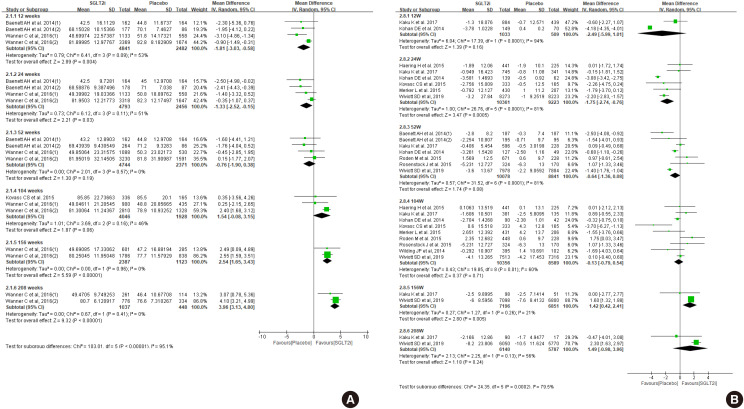
ePub Diabetes is a leading cause of end-stage renal disease. Therefore, prevention of renal dysfunction is an important treatment goal in the management of diabetes. The data of landmark cardiovascular outcome trials of sodium-glucose cotransporter-2 (SGLT2) inhibitor showed profound reno-protective effects. The Korean Diabetes Association and the Korean Society of Nephrology reviewed clinical trials and performed meta-analysis to assess the effects of SGLT2 inhibitors on the preservation of estimated glomerular filtration rate (eGFR). We limited the data of SGLT2 inhibitors which can be prescribed in Korea. Both eGFR value and its change from the baseline were significantly more preserved in the SGLT2 inhibitor treatment group compared to the control group after 156 weeks. However, some known adverse events were increased in SGLT2 inhibitor treatment, such as genital infection, diabetic ketoacidosis, and volume depletion. We recommend the long-term use SGLT2 inhibitor in patients with type 2 diabetes mellitus (T2DM) for attenuation of renal function decline. However, we cannot generalize our recommendation due to lack of long-term clinical trials testing reno-protective effects of every SGLT2 inhibitor in a broad range of patients with T2DM. This recommendation can be revised and updated after publication of several large-scale renal outcome trials.
-
Citations
Citations to this article as recorded by- Real-World Treatment Patterns according to Clinical Practice Guidelines in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease in Korea: Multicenter, Retrospective, Observational Study
Ye Seul Yang, Nam Hoon Kim, Jong Ha Baek, Seung-Hyun Ko, Jang Won Son, Seung-Hwan Lee, Sang Youl Rhee, Soo-Kyung Kim, Tae Seo Sohn, Ji Eun Jun, In-Kyung Jeong, Chong Hwa Kim, Keeho Song, Eun-Jung Rhee, Junghyun Noh, Kyu Yeon Hur
Diabetes & Metabolism Journal.2024; 48(2): 279. CrossRef - Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
Nam Hoon Kim, Nan Hee Kim
Diabetes & Metabolism Journal.2022; 46(4): 543. CrossRef - Real-World Prescription Patterns and Barriers Related to the Use of Sodium-Glucose Cotransporter 2 Inhibitors among Korean Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease
Jong Ha Baek, Ye Seul Yang, Seung-Hyun Ko, Kyung Do Han, Jae Hyeon Kim, Min Kyong Moon, Jong Suk Park, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Jong Han Choi, Kyu Yeon Hur
Diabetes & Metabolism Journal.2022; 46(5): 701. CrossRef
- Real-World Treatment Patterns according to Clinical Practice Guidelines in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease in Korea: Multicenter, Retrospective, Observational Study
- Guideline/Fact Sheet
- Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Position Statement of the Fatty Liver Research Group of the Korean Diabetes Association
- Byung-Wan Lee, Yong-ho Lee, Cheol-Young Park, Eun-Jung Rhee, Won-Young Lee, Nan-Hee Kim, Kyung Mook Choi, Keun-Gyu Park, Yeon-Kyung Choi, Bong-Soo Cha, Dae Ho Lee, Korean Diabetes Association (KDA) Fatty Liver Research Group
- Diabetes Metab J. 2020;44(3):382-401. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0010
- 12,338 View
- 337 Download
- 42 Web of Science
- 42 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader This clinical practice position statement, a product of the Fatty Liver Research Group of the Korean Diabetes Association, proposes recommendations for the diagnosis, progression and/or severity assessment, management, and follow-up of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes mellitus (T2DM). Patients with both T2DM and NAFLD have an increased risk of non-alcoholic steatohepatitis (NASH) and fibrosis and a higher risk of cardiovascular diseases and diabetic complications compared to those without NAFLD. With regards to the evaluation of patients with T2DM and NAFLD, ultrasonography-based stepwise approaches using noninvasive biomarker models such as fibrosis-4 or the NAFLD fibrosis score as well as imaging studies such as vibration-controlled transient elastography with controlled attenuation parameter or magnetic resonance imaging-proton density fat fraction are recommended. After the diagnosis of NAFLD, the stage of fibrosis needs to be assessed appropriately. For management, weight reduction achieved by lifestyle modification has proven beneficial and is recommended in combination with antidiabetic agent(s). Evidence that some antidiabetic agents improve NAFLD/NASH with fibrosis in patients with T2DM is emerging. However, there are currently no definite pharmacologic treatments for NAFLD in patients with T2DM. For specific cases, bariatric surgery may be an option if indicated.
-
Citations
Citations to this article as recorded by- A combined extract containing Schisandra chinensis (SCE) reduced hepatic triglyceride accumulation in rats fed a high-sucrose diet
Haneul Lee, Eun Young Kang, Joowon Lee, Yejin Kim, Sumin Kang, Hayoon Kim, Hyun Kyung Kim, Gyoungok Gang, Sang-gil Lee, Cao Lei, Gwang-woong Go
Food Science and Biotechnology.2024; 33(6): 1449. CrossRef - Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
BMJ.2024; : e076388. CrossRef - Risk Scores for Prediction of Major Cardiovascular Events in Non-Alcoholic Fatty Liver Disease: A No Man’s Land?
Liliana Gheorghe, Roxana Nemteanu, Andreea Clim, Gina Eosefina Botnariu, Irina Iuliana Costache, Alina Plesa
Life.2023; 13(4): 857. CrossRef - Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis
Hye Jin Chun, Eun Ran Kim, Minyoung Lee, Da Hyun Choi, Soo Hyun Kim, Eugene Shin, Jin-Hong Kim, Jin Won Cho, Dai Hoon Han, Bong-Soo Cha, Yong-ho Lee
Metabolism.2023; 145: 155612. CrossRef - Association between fatty liver index and risk of end-stage renal disease stratified by kidney function in patients with type 2 diabetes: A nationwide population-based study
Goh Eun Chung, Kyungdo Han, Kyu-Na Lee, Jung Ho Bae, Sun Young Yang, Su-Yeon Choi, Jeong Yoon Yim, Nam Ju Heo
Diabetes & Metabolism.2023; 49(4): 101454. CrossRef - Comparison of glucagon-like peptide-1 receptor agonists and thiazolidinediones on treating nonalcoholic fatty liver disease: A network meta-analysis
Min Jeong Park, Hayeon Kim, Myeong Gyu Kim, Kyungim Kim
Clinical and Molecular Hepatology.2023; 29(3): 693. CrossRef - Histological analysis of hypoglycemic agents on liver fibrosis in patients with non-alcoholic fatty liver disease: a systematic review
Qingxing Xie, Xiaohui Pan, Xinyue Zhang, Jinfang Ma, Ge Peng, Nanwei Tong
Chinese Medical Journal.2023; 136(16): 2014. CrossRef - Hepatotropc effects of glucose-lowering drugs: non-alcoholic fatty liver disease in focus
E. V. Uzhakova, Z. E. Zshanko, E. N. Smirnova
Experimental and Clinical Gastroenterology.2023; (6): 121. CrossRef - Complementary effects of dapagliflozin and lobeglitazone on metabolism in a diet-induced obese mouse model
Yun Kyung Lee, Tae Jung Oh, Ji In Lee, Bo Yoon Choi, Hyen Chung Cho, Hak Chul Jang, Sung Hee Choi
European Journal of Pharmacology.2023; 957: 175946. CrossRef - Hepatic T-cell senescence and exhaustion are implicated in the progression of fatty liver disease in patients with type 2 diabetes and mouse model with nonalcoholic steatohepatitis
Byeong Chang Sim, Yea Eun Kang, Sun Kyoung You, Seong Eun Lee, Ha Thi Nga, Ho Yeop Lee, Thi Linh Nguyen, Ji Sun Moon, Jingwen Tian, Hyo Ju Jang, Jeong Eun Lee, Hyon-Seung Yi
Cell Death & Disease.2023;[Epub] CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Circ_0004535/miR-1827/CASP8 network involved in type 2 diabetes mellitus with nonalcoholic fatty liver disease
Min Li, Ai Zeng, Xinle Tang, Hui Xu, Wei Xiong, Yanying Guo
Scientific Reports.2023;[Epub] CrossRef - Stratification by obesity class, rather than age, can identify a higher percent of children at risk for non‐alcoholic fatty liver disease and metabolic dysfunction
Aurelia Radulescu, Adam J. Dugan, Mary Killian, Suzanna L. Attia, Marialena Mouzaki, George J. Fuchs, Rohit Kohli, Henrietta Bada, Philip A. Kern, Samir Softic
Pediatric Obesity.2022;[Epub] CrossRef - Renal Tubular Damage Marker, Urinary N-acetyl-β-D-Glucosaminidase, as a Predictive Marker of Hepatic Fibrosis in Type 2 Diabetes Mellitus
Hae Kyung Kim, Minyoung Lee, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Diabetes & Metabolism Journal.2022; 46(1): 104. CrossRef - Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway
Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2022; 37(1): 74. CrossRef - State-of-the-Art Overview of the Pharmacological Treatment of Non-Alcoholic Steatohepatitis
Yongin Cho, Yong-ho Lee
Endocrinology and Metabolism.2022; 37(1): 38. CrossRef - Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study)
Yongin Cho, Hyungjin Rhee, Young-eun Kim, Minyoung Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Jin-Young Choi, Yong-ho Lee
BMC Medicine.2022;[Epub] CrossRef - Obesity is an important determinant of severity in newly defined metabolic dysfunction-associated fatty liver disease
Ji Hye Huh, Kwang Joon Kim, Seung Up Kim, Bong-Soo Cha, Byung-Wan Lee
Hepatobiliary & Pancreatic Diseases International.2022; 21(3): 241. CrossRef - Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice
Min Ju Kim, Ramakrishna Chilakala, Hee Geun Jo, Seung-Jae Lee, Dong-Sung Lee, Sun Hee Cheong
International Journal of Molecular Sciences.2022; 23(7): 4015. CrossRef - The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study
Jiyun Park, Gyuri Kim, Bong-Sung Kim, Kyung-Do Han, So Yoon Kwon, So Hee Park, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim
Cardiovascular Diabetology.2022;[Epub] CrossRef - Cumulative Exposure to High γ-Glutamyl Transferase Level and Risk of Diabetes: A Nationwide Population-Based Study
Ji-Yeon Park, Kyungdo Han, Hun-Sung Kim, Jae-Hyoung Cho, Kun-Ho Yoon, Mee Kyoung Kim, Seung-Hwan Lee
Endocrinology and Metabolism.2022; 37(2): 272. CrossRef - SGLT-2 inhibitors and GLP-1 receptor agonists in metabolic dysfunction-associated fatty liver disease
Jun Sung Moon, Jun Hwa Hong, Yong Jin Jung, Ele Ferrannini, Michael A. Nauck, Soo Lim
Trends in Endocrinology & Metabolism.2022; 33(6): 424. CrossRef - Plasma Aldo-Keto Reductase Family 1 Member B10 as a Biomarker Performs Well in the Diagnosis of Nonalcoholic Steatohepatitis and Fibrosis
Aron Park, Seung Joon Choi, Sungjin Park, Seong Min Kim, Hye Eun Lee, Minjae Joo, Kyoung Kon Kim, Doojin Kim, Dong Hae Chung, Jae Been Im, Jaehun Jung, Seung Kak Shin, Byung-Chul Oh, Cheolsoo Choi, Seungyoon Nam, Dae Ho Lee
International Journal of Molecular Sciences.2022; 23(9): 5035. CrossRef - Chinese Herbal Medicine for Type 2 Diabetes Mellitus With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis
Sihan Peng, Lu Liu, Ziyan Xie, Xiyu Zhang, Chunguang Xie, Sha Ye, Xiangeng Zhang, Xiaoli Liang, Hongyan Wang, Ya Liu
Frontiers in Pharmacology.2022;[Epub] CrossRef - Non-alcoholic fatty liver disease and sarcopenia is associated with the risk of albuminuria independent of insulin resistance, and obesity
Eugene Han, Mi Kyung Kim, Seung-Soon Im, Byoung Kuk Jang, Hye Soon Kim
Journal of Diabetes and its Complications.2022; 36(8): 108253. CrossRef - Extra-Glycemic Effects of Anti-Diabetic Medications: Two Birds with One Stone?
Eun-Jung Rhee
Endocrinology and Metabolism.2022; 37(3): 415. CrossRef - Plasma Metabolomics and Machine Learning-Driven Novel Diagnostic Signature for Non-Alcoholic Steatohepatitis
Moongi Ji, Yunju Jo, Seung Joon Choi, Seong Min Kim, Kyoung Kon Kim, Byung-Chul Oh, Dongryeol Ryu, Man-Jeong Paik, Dae Ho Lee
Biomedicines.2022; 10(7): 1669. CrossRef - Association Between DPP4 Inhibitor Use and the Incidence of Cirrhosis, ESRD, and Some Cancers in Patients With Diabetes
Yewon Na, Soo Wan Kim, Ie Byung Park, Soo Jung Choi, Seungyoon Nam, Jaehun Jung, Dae Ho Lee
The Journal of Clinical Endocrinology & Metabolism.2022; 107(11): 3022. CrossRef - Nonalcoholic fatty liver disease is associated with early left ventricular diastolic dysfunction in patients with type 2 diabeteS
Walaa Sheba, Eman Morsy, Salah Altahan, Mona Ayaad, Sameh A. Lashen
Alexandria Journal of Medicine.2022; 58(1): 117. CrossRef - The association between changes in hepatic steatosis and hepatic fibrosis with cardiovascular outcomes and mortality in patients with New-Onset type 2 Diabetes: A nationwide cohort study
Jiyun Park, Gyuri Kim, Bong-Sung Kim, Kyung-Do Han, So Yoon Kwon, So Hee Park, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim
Diabetes Research and Clinical Practice.2022; 194: 110191. CrossRef - Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2022;46:630-9)
Ji Hye Huh
Diabetes & Metabolism Journal.2022; 46(6): 953. CrossRef - The association of fatty liver index and BARD score with all-cause and cause-specific mortality in patients with type 2 diabetes mellitus: a nationwide population-based study
Goh Eun Chung, Su-Min Jeong, Eun Ju Cho, Ji Won Yoon, Jeong-Ju Yoo, Yuri Cho, Kyu-na Lee, Dong Wook Shin, Yoon Jun Kim, Jung-Hwan Yoon, Kyungdo Han, Su Jong Yu
Cardiovascular Diabetology.2022;[Epub] CrossRef - Non-Albumin Proteinuria (NAP) as a Complementary Marker for Diabetic Kidney Disease (DKD)
Jaehyun Bae, Young Jun Won, Byung-Wan Lee
Life.2021; 11(3): 224. CrossRef - Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: Diagnosis and Treatment
Sook Jung Lee, Byung-Wan Lee
The Journal of Korean Diabetes.2021; 22(1): 38. CrossRef - Non-alcoholic fatty liver disease
Elizabeth E Powell, Vincent Wai-Sun Wong, Mary Rinella
The Lancet.2021; 397(10290): 2212. CrossRef - Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway
In-Jin Cho, Da-Hee Oh, Jin Yoo, You-Cheol Hwang, Kyu Jeung Ahn, Ho-Yeon Chung, Soung Won Jeong, Ju-Young Moon, Sang-Ho Lee, Sung-Jig Lim, In-Kyung Jeong
Scientific Reports.2021;[Epub] CrossRef - Patient Management in Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus
A. E. Bagriy, A. D. Zubov, M. V. Khomenko, E. S. Mikhailichenko, E. A. Pylaeva, N. A. Khaustova, E. V. Bryukhovetskaya
Russian Journal of Gastroenterology, Hepatology, Coloproctology.2021; 31(2): 14. CrossRef - Liver Fibrosis Indices for the Prediction of Mortality in Korean Subjects: A 16-Year Prospective Cohort Study
Tae Jung Oh, Kyuho Kim, Jae Hoon Moon, Sung Hee Choi, Nam H Cho, Hak Chul Jang
Journal of the Endocrine Society.2021;[Epub] CrossRef - Reduced Rank Regression-Derived Dietary Patterns Related to the Fatty Liver Index and Associations with Type 2 Diabetes Mellitus among Ghanaian Populations under Transition: The RODAM Study
Tracy Bonsu Osei, Anne-Marieke van Dijk, Sjoerd Dingerink, Felix Patience Chilunga, Erik Beune, Karlijn Anna Catharina Meeks, Silver Bahendeka, Matthias Bernd Schulze, Charles Agyemang, Mary Nicolaou, Adriaan Georgius Holleboom, Ina Danquah
Nutrients.2021; 13(11): 3679. CrossRef - Efficacy and Safety of GLP-1 Receptor Agonists in Patients With Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis
Yuan Zhu, Jiao Xu, Dong Zhang, Xingyu Mu, Yi Shi, Shangtao Chen, Zengxiang Wu, Shuangqing Li
Frontiers in Endocrinology.2021;[Epub] CrossRef - Liver fibrosis indices are related to diabetic peripheral neuropathy in individuals with type 2 diabetes
Kyuho Kim, Tae Jung Oh, Hyen Chung Cho, Yun Kyung Lee, Chang Ho Ahn, Bo Kyung Koo, Jae Hoon Moon, Sung Hee Choi, Hak Chul Jang
Scientific Reports.2021;[Epub] CrossRef - Evaluation of Grapefruit Juice in terms of Interleukin 18 Gene Expression in Rats with Fatty Liver and Healthy Rats
Simin Bahmanpoor, Noosha Zia-Jahromi
journal of ilam university of medical sciences.2021; 29(4): 74. CrossRef
- A combined extract containing Schisandra chinensis (SCE) reduced hepatic triglyceride accumulation in rats fed a high-sucrose diet
- Guideline/Fact Sheet
- Metformin Treatment for Patients with Diabetes and Chronic Kidney Disease: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
- Kyu Yeon Hur, Mee Kyoung Kim, Seung Hyun Ko, Miyeun Han, Dong Won Lee, Hyuk-Sang Kwon
- Diabetes Metab J. 2020;44(1):3-10. Published online February 21, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0004
- 9,452 View
- 332 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader The safety of metformin use for patients with type 2 diabetes mellitus (T2DM) and advanced kidney disease is controversial, and more recent guidelines have suggested that metformin be used cautiously in this group until more definitive evidence concerning its safety is available. The Korean Diabetes Association and the Korean Society of Nephrology have agreed on consensus statements concerning metformin use for patients with T2DM and renal dysfunction, particularly when these patients undergo imaging studies using iodinated contrast media (ICM). Metformin can be used safely when the estimated glomerular filtration rate (eGFR) is ≥45 mL/min/1.73 m2. If the eGFR is between 30 and 44 mL/min/1.73 m2, metformin treatment should not be started. If metformin is already in use, a daily dose of ≤1,000 mg is recommended. Metformin is contraindicated when the eGFR is <30 mL/min/1.73 m2. Renal function should be evaluated prior to any ICM-related procedures. During procedures involving intravenous administration of ICM, metformin should be discontinued starting the day of the procedures and up to 48 hours post-procedures if the eGFR is <60 mL/min/1.73 m2.
-
Citations
Citations to this article as recorded by- Distribution and elimination kinetics of midazolam and metabolites after post-resuscitation care: a prospective observational study
Wonjoon Jeong, Jung Sunwoo, Yeonho You, Jung Soo Park, Jin Hong Min, Yong Nam In, Hong Joon Ahn, So Young Jeon, Jang Hee Hong, Ji Hye Song, Hyein Kang, My Tuyen Thi Nguyen, Jaehan Kim, Changshin Kang
Scientific Reports.2024;[Epub] CrossRef - Prediction of glycosylated hemoglobin level in patients with cardiovascular diseases and type 2 diabetes mellitus with respect to anti-diabetic medication
Alisher Ikramov, Shakhnoza Mukhtarova, Raisa Trigulova, Dilnoza Alimova, Saodat Abdullaeva
Frontiers in Endocrinology.2024;[Epub] CrossRef - Determinants of vitamin B12 deficiency in patients with type-2 diabetes mellitus — A primary-care retrospective cohort study
Andrew Kien Han Wee, Rehena Sultana
BMC Primary Care.2023;[Epub] CrossRef - Risk factors for post-contrast acute kidney injury in patients sequentially administered iodine- and gadolinium-based contrast media on the same visit to the emergency department: a retrospective study
Changshin Kang, Soo Hyun Han, Jung Soo Park, Dae Eun Choi
Kidney Research and Clinical Practice.2023; 42(3): 358. CrossRef - Guideline for the diagnosis and treatment of diabetes mellitus in patients with transfusion-dependent thalassemia
Mohammad E. Khamseh, Mojtaba Malek, Nahid Hashemi-madani, Fariba Ghassemi, Neda Rahimian, Amir Ziaee, Mohammad Reza Foroughi-Gilvaee, Pooya Faranoush, Negin Sadighnia, Ali Elahinia, Mohammad Reza Rezvany, Mohammad Faranoush
Iranian Journal of Blood and Cancer.2023; 15(4): 293. CrossRef - Continuous use of metformin in patients receiving contrast medium: what is the evidence? A systematic review and meta-analysis
Ting-Wan Kao, Kuo-Hua Lee, Wing P. Chan, Kang-Chih Fan, Che-Wei Liu, Yu-Chen Huang
European Radiology.2022; 32(5): 3045. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes Mellitus and Cardiovascular Disease: The Past, Present, and Future
Filipe Ferrari, Rafael S. Scheffel, Vítor M. Martins, Raul D. Santos, Ricardo Stein
American Journal of Cardiovascular Drugs.2022; 22(4): 363. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Treatment Patterns of Type 2 Diabetes Assessed Using a Common Data Model Based on Electronic Health Records of 2000–2019
Kyung Ae Lee, Heung Yong Jin, Yu Ji Kim, Yong-Jin Im, Eun-Young Kim, Tae Sun Park
Journal of Korean Medical Science.2021;[Epub] CrossRef - Albuminuria Is Associated with Steatosis Burden in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2021;45:698-707)
Eugene Han, Hye Soon Kim
Diabetes & Metabolism Journal.2021; 45(6): 972. CrossRef - KRCP's past and future path
Tae-Hyun Yoo
Kidney Research and Clinical Practice.2020; 39(3): 233. CrossRef - Metformin Use and Risk of All-Cause Mortality and Cardiovascular Events in Patients With Chronic Kidney Disease—A Systematic Review and Meta-Analysis
Yao Hu, Min Lei, Guibao Ke, Xin Huang, Xuan Peng, Lihui Zhong, Ping Fu
Frontiers in Endocrinology.2020;[Epub] CrossRef - Pharmacotherapy for patients with diabetes mellitus
Joon Ho Moon, Soo Lim
Journal of the Korean Medical Association.2020; 63(12): 766. CrossRef
- Distribution and elimination kinetics of midazolam and metabolites after post-resuscitation care: a prospective observational study
- Clinical Care/Education
- 2019 Clinical Practice Guidelines for Type 2 Diabetes Mellitus in Korea
- Mee Kyoung Kim, Seung-Hyun Ko, Bo-Yeon Kim, Eun Seok Kang, Junghyun Noh, Soo-Kyung Kim, Seok-O Park, Kyu Yeon Hur, Suk Chon, Min Kyong Moon, Nan-Hee Kim, Sang Yong Kim, Sang Youl Rhee, Kang-Woo Lee, Jae Hyeon Kim, Eun-Jung Rhee, SungWan Chun, Sung Hoon Yu, Dae Jung Kim, Hyuk-Sang Kwon, Kyong Soo Park
- Diabetes Metab J. 2019;43(4):398-406. Published online August 20, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0137

- 16,327 View
- 477 Download
- 160 Web of Science
- 171 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The Committee of Clinical Practice Guidelines of the Korean Diabetes Association revised and updated the 6th Clinical Practice Guidelines in 2019. Targets of glycemic, blood pressure, and lipid control in type 2 diabetes mellitus (T2DM) were updated. The obese and overweight population is increasing steadily in Korea, and half of the Koreans with diabetes are obese. Evidence-based recommendations for weight-loss therapy for obesity management as treatment for hyperglycemia in T2DM were provided. In addition, evidence from large clinical studies assessing cardiovascular outcomes following the use of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide 1 receptor agonists in patients with T2DM were incorporated into the recommendations.
-
Citations
Citations to this article as recorded by- Serum-based ATR-FTIR spectroscopy combined with multivariate analysis for the diagnosis of pre-diabetes and diabetes
Weiyi Pang, Yu Xing, Camilo L. M. Morais, Qiufeng Lao, Shengle Li, Zipeng Qiao, You Li, Maneesh N. Singh, Valério G. Barauna, Francis L. Martin, Zhiyong Zhang
The Analyst.2024; 149(2): 497. CrossRef - Fruits and the Risk of Type 2 Diabetes: The Korean Genome and Epidemiology Study Cohort
Hojun Yu, Cheol Min Lee, Seung-Won Oh
Korean Journal of Family Medicine.2024; 45(1): 44. CrossRef - Effectiveness and Safety of Sodium-Glucose Cotransporter 2 Inhibitors Added to Dual or Triple Treatment in Patients with Type 2 Diabetes Mellitus
Yesol Hong, Yoomin Jeon, Yoona Choi, Tae Kyu Chung, Howard Lee
Diabetes Therapy.2024; 15(2): 487. CrossRef - Risk factors for carotid plaque formation in type 2 diabetes mellitus
Jin Chen, Wenwen Li, Jingzhu Cao, Yuhan Lu, Chaoqun Wang, Jin Lu
Journal of Translational Medicine.2024;[Epub] CrossRef - Cumulative exposure to impaired fasting glucose and gastrointestinal cancer risk: A nationwide cohort study
Byeong Yun Ahn, Bokyung Kim, Sanghyun Park, Sang Gyun Kim, Kyungdo Han, Soo‐Jeong Cho
Cancer.2024;[Epub] CrossRef - Effects of a Health Partnership Program Using Mobile Health Application for Male Workers with Cardiovascular Risk Factors in Small and Medium Enterprises: A Randomized Controlled Trial
Eun Jin Kim, Seon Young Hwang
Journal of Korean Academy of Nursing.2024; 54(1): 44. CrossRef - The clinical relevance of a polygenic risk score for type 2 diabetes mellitus in the Korean population
Na Yeon Kim, Haekyung Lee, Sehee Kim, Ye-Jee Kim, Hyunsuk Lee, Junhyeong Lee, Soo Heon Kwak, Seunggeun Lee
Scientific Reports.2024;[Epub] CrossRef - Real-World Outcomes of Individualized Targeted Therapy with Insulin Glargine 300 Units/mL in Insulin-Naïve Korean People with Type 2 Diabetes: TOBE Study
Eun-Gyoung Hong, Kyung-Wan Min, Jung Soo Lim, Kyu-Jeung Ahn, Chul Woo Ahn, Jae-Myung Yu, Hye Soon Kim, Hyun Jin Kim, Won Kim, Dong Han Kim, Hak Chul Jang
Advances in Therapy.2024;[Epub] CrossRef - The Potential Role of Presepsin in Predicting Severe Infection in Patients with Diabetic Foot Ulcers
Eun Yeong Ha, Il Rae Park, Seung Min Chung, Young Nam Roh, Chul Hyun Park, Tae-Gon Kim, Woong Kim, Jun Sung Moon
Journal of Clinical Medicine.2024; 13(8): 2311. CrossRef - Safety of sodium–glucose cotransporter 2 inhibitors in Asian type 2 diabetes populations
Jaime A Davidson, Norlela Sukor, Fen‐Lee Hew, Mafauzy Mohamed, Zanariah Hussein
Journal of Diabetes Investigation.2023; 14(2): 167. CrossRef - β-hydroxybutyrate as a biomarker of β-cell function in new-onset type 2 diabetes and its association with treatment response at 6 months
Minyoung Lee, Yongin Cho, Yong-ho Lee, Eun Seok Kang, Bong-soo Cha, Byung-Wan Lee
Diabetes & Metabolism.2023; 49(4): 101427. CrossRef - Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Journal of Lipid and Atherosclerosis.2023; 12(1): 12. CrossRef - Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 1. CrossRef - Cardiovascular Outcomes according to Comorbidities and Low-Density Lipoprotein Cholesterol in Korean People with Type 2 Diabetes Mellitus
Min Kyong Moon, Junghyun Noh, Eun-Jung Rhee, Sang Hyun Park, Hyeon Chang Kim, Byung Jin Kim, Hae Jin Kim, Seonghoon Choi, Jin Oh Na, Young Youl Hyun, Bum Joon Kim, Kyung-Do Han, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(1): 45. CrossRef - Impaired ketogenesis is associated with metabolic-associated fatty liver disease in subjects with type 2 diabetes
Sejeong Lee, Jaehyun Bae, Doo Ri Jo, Minyoung Lee, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Frontiers in Endocrinology.2023;[Epub] CrossRef - Identifying the Associations of Nightly Fasting Duration and Meal Timing with Type 2 Diabetes Mellitus Using Data from the 2016–2020 Korea National Health and Nutrition Survey
Junkyung Kwak, Kyeong-A Jang, Haeng-Ran Kim, Min-Sook Kang, Kyung Won Lee, Dayeon Shin
Nutrients.2023; 15(6): 1385. CrossRef - Development and Adaptability of Smartphone-based Dietary Coaching Program for Patients Undergoing Diabetes and Prediabetes with Continuous Glucose Monitoring Device
Myoung Soo Kim, Jung Mi Ryu, Minkyeong Kang, Jiwon Park, Yeh Chan Ahn, Yang Seok Kim
Journal of Health Informatics and Statistics.2023; 48(1): 36. CrossRef - Glucose Control in Korean Patients with Type 2 Diabetes Mellitus according to Body Mass Index
Ye-lim Shin, Heesoh Yoo, Joo Young Hong, Jooeun Kim, Kyung-do Han, Kyu-Na Lee, Yang-Hyun Kim
Journal of Obesity & Metabolic Syndrome.2023; 32(1): 55. CrossRef - Diabetes severity is strongly associated with the risk of active tuberculosis in people with type 2 diabetes: a nationwide cohort study with a 6-year follow-up
Ji Young Kang, Kyungdo Han, Seung-Hwan Lee, Mee Kyoung Kim
Respiratory Research.2023;[Epub] CrossRef - Developing a Classification Algorithm for Prediabetes Risk Detection From Home Care Nursing Notes
Eunjoo Jeon, Aeri Kim, Jisoo Lee, Hyunsook Heo, Hana Lee, Kyungmi Woo
CIN: Computers, Informatics, Nursing.2023; 41(7): 539. CrossRef - Predictive value of the Framingham steatosis index for cardiovascular risk: a nationwide population-based cohort study
Yun Kyung Cho, Myungjin Kim, Ye-Jee Kim, Chang Hee Jung, Woo Je Lee, Joong-Yeol Park
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Association of low muscle strength with metabolic dysfunction-associated fatty liver disease: A nationwide study
Gyu Bae Lee, Youn Huh, Sang Hyun Lee, Byoungduck Han, Yang-Hyun Kim, Do-Hoon Kim, Seon Mee Kim, Youn Seon Choi, Kyung Hwan Cho, Ga Eun Nam
World Journal of Gastroenterology.2023; 29(45): 5962. CrossRef - Low fasting glucose‐to‐estimated average glucose ratio was associated with superior response to insulin degludec/aspart compared with basal insulin in patients with type 2 diabetes
Han Na Jang, Ye Seul Yang, Tae Jung Oh, Bo Kyung Koo, Seong Ok Lee, Kyong Soo Park, Hak Chul Jang, Hye Seung Jung
Journal of Diabetes Investigation.2022; 13(1): 85. CrossRef - Relationship between the early initiation of insulin treatment and diabetic complications in patients newly diagnosed with type 2 diabetes mellitus in Korea: A nationwide cohort study
Ha‐Lim Jeon, Won Kim, Bongseong Kim, Ju‐Young Shin
Journal of Diabetes Investigation.2022; 13(5): 830. CrossRef - Relationship of the Triglyceride-Glucose Index with Subclinical White Matter Hypersensitivities of Presumed Vascular Origin Among Community-Dwelling Koreans
Dong-Hyuk Jung, Byoungjin Park, Yong-Jae Lee
International Journal of General Medicine.2022; Volume 15: 603. CrossRef - Prevalence of vitamin B12 deficiency and its association with metformin-treated type 2 diabetic patients: A cross sectional study
Shaimaa B. Almatrafi, El-Sayed H. Bakr, Asem A. Almatrafi, Manal M. Altayeb
Human Nutrition & Metabolism.2022; 27: 200138. CrossRef - Retinal Vascular Caliber Changes in Early Type 2 Diabetic Patients without Retinopathy
Jeong Woo Park, Jeong Hun Bae, Su Jeong Song, Joon Mo Kim
Journal of the Korean Ophthalmological Society.2022; 63(1): 20. CrossRef - Renal Tubular Damage Marker, Urinary N-acetyl-β-D-Glucosaminidase, as a Predictive Marker of Hepatic Fibrosis in Type 2 Diabetes Mellitus
Hae Kyung Kim, Minyoung Lee, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Diabetes & Metabolism Journal.2022; 46(1): 104. CrossRef - Efficacy and Safety of Self-Titration Algorithms of Insulin Glargine 300 units/mL in Individuals with Uncontrolled Type 2 Diabetes Mellitus (The Korean TITRATION Study): A Randomized Controlled Trial
Jae Hyun Bae, Chang Ho Ahn, Ye Seul Yang, Sun Joon Moon, Soo Heon Kwak, Hye Seung Jung, Kyong Soo Park, Young Min Cho
Diabetes & Metabolism Journal.2022; 46(1): 71. CrossRef - Sodium-Glucose Cotransporter-2 Inhibitor-Related Diabetic Ketoacidosis: Accuracy Verification of Operational Definition
Dong Yoon Kang, Hyunah Kim, SooJeong Ko, HyungMin Kim, Jiwon Shinn, Min-Gyu Kang, Sun-ju Byeon, Jeong-Hee Choi, Soo-Yong Shin, Hun-Sung Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Relationship between the dynamics of non-alcoholic fatty liver disease and incident diabetes mellitus
Ji Eun Han, Han-Bit Shin, Young Hwan Ahn, Hyo Jung Cho, Jae Youn Cheong, Bumhee Park, Soon Sun Kim
Scientific Reports.2022;[Epub] CrossRef - Position of Sulfonylureas in the Current ERA: Review of National and International Guidelines
Viswanathan Mohan, Banshi Saboo, Jabbar Khader, Kirtikumar D Modi, Sushil Jindal, Subhash Kumar Wangnoo, Sugumaran Amarnath
Clinical Medicine Insights: Endocrinology and Diabetes.2022; 15: 117955142210746. CrossRef - Real-world comparison of mono and dual combination therapies of metformin, sulfonylurea, and dipeptidyl peptidase-4 inhibitors using a common data model
Kyung Ae Lee, Heung Yong Jin, Yu Ji Kim, Sang Soo Kim, Eun-Hee Cho, Tae Sun Park
Medicine.2022; 101(8): e28823. CrossRef - Atherogenic Index of Plasma and Its Association with Risk Factors of Coronary Artery Disease and Nutrient Intake in Korean Adult Men: The 2013–2014 KNHANES
Hye Ran Shin, SuJin Song, Jin Ah Cho, Sun Yung Ly
Nutrients.2022; 14(5): 1071. CrossRef - Sodium–glucose cotransporter 2 inhibitors do not increase the risk of fractures in real‐world clinical practice in Korea: A national observational cohort study
Kyoung Hwa Ha, Dae Jung Kim, Yong Jun Choi
Journal of Diabetes Investigation.2022; 13(6): 986. CrossRef - Performance of Diabetes and Kidney Disease Screening Scores in Contemporary United States and Korean Populations
Liela Meng, Keun-Sang Kwon, Dae Jung Kim, Yong-ho Lee, Jeehyoung Kim, Abhijit V. Kshirsagar, Heejung Bang
Diabetes & Metabolism Journal.2022; 46(2): 273. CrossRef - Integrated metagenomics and metabolomics analysis illustrates the systemic impact of the gut microbiota on host metabolism after bariatric surgery
Yeyoung Han, Gihyeon Kim, Eunyong Ahn, Sunhee Jung, Youngae Jung, Yunjae Kim, Eunyoung Ha, Yoonseok Heo, Do Hyun Ryu, Hansoo Park, Geum‐Sook Hwang
Diabetes, Obesity and Metabolism.2022; 24(7): 1224. CrossRef - The use of complex marketing analysis and QSPR methodology for the necessity of a drug development grounding for the treatment of type 2 diabetes mellitus with increased bioavailability
Inna Kovalevska, Olena Ruban, Alina Volkova, Alla Kotvitska, Alina Cherkashyna
Pharmacia.2022; 69(2): 303. CrossRef - Comparative Study of Ex Vivo Antiplatelet Activity of Aspirin and Cilostazol in Patients with Diabetes and High Risk of Cardiovascular Disease
Sangmo Hong, Woo Je Lee, Cheol-Young Park
Endocrinology and Metabolism.2022; 37(2): 233. CrossRef - Long-term effect of the eradication of Helicobacter pylori on the hemoglobin A1c in type 2 diabetes or prediabetes patients
Won Seok Kim, Yonghoon Choi, Nayoung Kim, Seon Hee Lim, Gitark Noh, Ki Wook Kim, Jaehyung Park, Hyeongho Jo, Hyuk Yoon, Cheol Min Shin, Young Soo Park, Dong Ho Lee
The Korean Journal of Internal Medicine.2022; 37(3): 579. CrossRef - Novel Glycemic Index Based on Continuous Glucose Monitoring to Predict Poor Clinical Outcomes in Critically Ill Patients: A Pilot Study
Eun Yeong Ha, Seung Min Chung, Il Rae Park, Yin Young Lee, Eun Young Choi, Jun Sung Moon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Understanding and Utilizing Claim Data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) Database for Research
Dae-Sung Kyoung, Hun-Sung Kim
Journal of Lipid and Atherosclerosis.2022; 11(2): 103. CrossRef - Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study
Si Hyoung Kim, Yun Kyung Cho, Ye-Jee Kim, Chang Hee Jung, Woo Je Lee, Joong-Yeol Park, Ji Hye Huh, Jun Goo Kang, Seong Jin Lee, Sung-Hee Ihm
Cardiovascular Diabetology.2022;[Epub] CrossRef - Current Status of Low-Density Lipoprotein Cholesterol Target Achievement in Patients with Type 2 Diabetes Mellitus in Korea Compared with Recent Guidelines
Soo Jin Yun, In-Kyung Jeong, Jin-Hye Cha, Juneyoung Lee, Ho Chan Cho, Sung Hee Choi, SungWan Chun, Hyun Jeong Jeon, Ho-Cheol Kang, Sang Soo Kim, Seung-Hyun Ko, Gwanpyo Koh, Su Kyoung Kwon, Jae Hyuk Lee, Min Kyong Moon, Junghyun Noh, Cheol-Young Park, Sung
Diabetes & Metabolism Journal.2022; 46(3): 464. CrossRef - Sodium Glucose Cotransporter-2 Inhibitors as an Add-on Therapy to Metformin Plus Dipeptidyl Peptidase-4 Inhibitor in Patients with Type 2 Diabetes
Jaehyun Bae, Young-eun Kim, Minyoung Lee, Yong-ho Lee, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
Yonsei Medical Journal.2022; 63(6): 539. CrossRef - Predictors for successful weight reduction during treatment with Dapagliflozin among patients with type 2 diabetes mellitus in primary care
Youn Huh, Young Sik Kim
BMC Primary Care.2022;[Epub] CrossRef - Implications of the heterogeneity between guideline recommendations for the use of low dose aspirin in primary prevention of cardiovascular disease
Xiao-Ying Li, Li Li, Sang-Hoon Na, Francesca Santilli, Zhongwei Shi, Michael Blaha
American Journal of Preventive Cardiology.2022; 11: 100363. CrossRef - Factors Influencing the Utilization of Diabetes Complication Tests Under the COVID-19 Pandemic: Machine Learning Approach
Haewon Byeon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effects of exercise initiation and smoking cessation after new-onset type 2 diabetes mellitus on risk of mortality and cardiovascular outcomes
Mee Kyoung Kim, Kyungdo Han, Bongsung Kim, Jinyoung Kim, Hyuk-Sang Kwon
Scientific Reports.2022;[Epub] CrossRef - Improvement in Age at Mortality and Changes in Causes of Death in the Population with Diabetes: An Analysis of Data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
Eugene Han, Sun Ok Song, Hye Soon Kim, Kang Ju Son, Sun Ha Jee, Bong-Soo Cha, Byung-Wan Lee
Endocrinology and Metabolism.2022; 37(3): 466. CrossRef - Analysis of Continuity of Care and Its Related Factors in Diabetic Patients: A Cross-Sectional Study
Ji Yeh Shin, Ha Jin Kim, BeLong Cho, Yun Jun Yang, Jae Moon Yun
Korean Journal of Family Medicine.2022; 43(4): 246. CrossRef - Development of Various Diabetes Prediction Models Using Machine Learning Techniques
Juyoung Shin, Jaewon Kim, Chanjung Lee, Joon Young Yoon, Seyeon Kim, Seungjae Song, Hun-Sung Kim
Diabetes & Metabolism Journal.2022; 46(4): 650. CrossRef - A causal relationship between alcohol intake and type 2 diabetes mellitus: A two-sample Mendelian randomization study
Meiling Liu, Sunmin Park
Nutrition, Metabolism and Cardiovascular Diseases.2022; 32(12): 2865. CrossRef - Early glycaemic variability increases 28-day mortality and prolongs intensive care unit stay in critically ill patients with pneumonia
Seong Ho Kim, Ji Young Kim, Eun Song Kim, Il Rae Park, Eun Yeong Ha, Seung Min Chung, Jun Sung Moon, Ji Sung Yoon, Kyu Chang Won, Hyoung Woo Lee
Annals of Medicine.2022; 54(1): 2724. CrossRef - Association of the Estimated Pulse Wave Velocity with Cardio-Vascular Disease Outcomes among Men and Women Aged 40–69 Years in the Korean Population: An 18-Year Follow-Up Report on the Ansung–Ansan Cohort in the Korean Genome Environment Study
Byung Sik Kim, Yonggu Lee, Jin-Kyu Park, Young-Hyo Lim, Jeong-Hun Shin
Journal of Personalized Medicine.2022; 12(10): 1611. CrossRef - Perfluorinated compounds in adults and their association with fasting glucose and incident diabetes: a prospective cohort study
Seung Min Chung, Dong-Gyu Heo, Ju-Hyun Kim, Ji Sung Yoon, Hyoung Woo Lee, Jong-Yeon Kim, Jun Sung Moon, Kyu Chang Won
Environmental Health.2022;[Epub] CrossRef - Differences in health behavior and nutrient intake status between diabetes-aware and unaware Korean adults based on the Korea national health and nutrition examination survey 2016–18 data: A cross-sectional study
Anshul Sharma, Chen Lulu, Kee-Ho Song, Hae-Jeung Lee
Frontiers in Public Health.2022;[Epub] CrossRef - Improving Machine Learning Diabetes Prediction Models for the Utmost Clinical Effectiveness
Juyoung Shin, Joonyub Lee, Taehoon Ko, Kanghyuck Lee, Yera Choi, Hun-Sung Kim
Journal of Personalized Medicine.2022; 12(11): 1899. CrossRef - Does Pitavastatin Therapy for Patients with Type 2 Diabetes and Dyslipidemia Affect Serum Adiponectin Levels and Insulin Sensitivity?
Jeongmin Lee, Min-Hee Kim, Jung-Min Lee, Sang-Ah Chang
Journal of Clinical Medicine.2022; 11(22): 6756. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Efficacy and Safety of Once Weekly Dulaglutide in East Asian Patients with Type 2 Diabetes: Subgroup Analysis by Potential Influential Factors
Jianhua Ma, Bin Zhang, Jianing Hou, Yongde Peng
Diabetes Therapy.2021; 12(1): 211. CrossRef - Early combination versus initial metformin monotherapy in the management of newly diagnosed type 2 diabetes: AnEast Asianperspective
Linong Ji, Juliana C. N. Chan, Miao Yu, Kun Ho Yoon, Sin Gon Kim, Sung Hee Choi, Chien‐Ning Huang, Shih Te Tu, Chih‐Yuan Wang, Päivi Maria Paldánius, Wayne H. H. Sheu
Diabetes, Obesity and Metabolism.2021; 23(1): 3. CrossRef - Effectiveness and safety of sodium‐glucose co‐transporter‐2 inhibitors compared with dipeptidyl peptidase‐4 inhibitors in older adults with type 2 diabetes: A nationwide population‐based study
Seung Jin Han, Kyoung Hwa Ha, Nami Lee, Dae Jung Kim
Diabetes, Obesity and Metabolism.2021; 23(3): 682. CrossRef - Glucodynamics and glucocracy in type 2 diabetes mellitus: clinical evidence and practice-based opinion on modern sulfonylurea use, from an International Expert Group (South Asia, Middle East & Africa) via modified Delphi method
Sanjay Kalra, Das A. K., Fariduddin Md., Shaikh K., Shah P., Rehim A. A., John M., Shaikh S., Orabi A., Saraswati M. R., Shahjada Selim, Baruah M. P., K. K. Gangopadhyay, Langi Y. A., Nair T., Dhanwal D., Thapa S. D., Deshmukh V., D. Dutta, Khalfan H., Ma
Current Medical Research and Opinion.2021; 37(3): 403. CrossRef - The association between lipoprotein (a) and carotid atherosclerosis in patients with type 2 diabetes without pre-existing cardiovascular disease: A cross-sectional study
Ji Eun Jun, Hongsun Kang, You-Cheol Hwang, Kyu Jeung Ahn, Ho-Yeon Chung, In-Kyung Jeong
Diabetes Research and Clinical Practice.2021; 171: 108622. CrossRef - Glycaemic control with add‐on thiazolidinedione or a sodium‐glucose co‐transporter‐2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: A 24‐week, randomized controlled trial
Jaehyun Bae, Ji Hye Huh, Minyoung Lee, Yong‐Ho Lee, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2021; 23(2): 609. CrossRef - Effect of Tai Chi on Quality of Life, Body Mass Index, and Waist-Hip Ratio in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Jiawei Qin, Yannan Chen, Shuai Guo, Yue You, Ying Xu, Jingsong Wu, Zhizhen Liu, Jia Huang, Lidian Chen, Jing Tao
Frontiers in Endocrinology.2021;[Epub] CrossRef - Severe hypoglycemia as a preventable risk factor for cardiovascular disease in patients with type 2 diabetes mellitus
Soo-Yeon Choi, Seung-Hyun Ko
The Korean Journal of Internal Medicine.2021; 36(2): 263. CrossRef - Diabetes Fact Sheets in Korea, 2020: An Appraisal of Current Status
Chan-Hee Jung, Jang Won Son, Shinae Kang, Won Jun Kim, Hun-Sung Kim, Hae Soon Kim, Mihae Seo, Hye-Jung Shin, Seong-Su Lee, Su Jin Jeong, Yongin Cho, Seung Jin Han, Hyang Mi Jang, Mira Rho, Shinbi Lee, Mihyun Koo, Been Yoo, Jung-Wha Moon, Hye Young Lee, Ja
Diabetes & Metabolism Journal.2021; 45(1): 1. CrossRef - Estimation of sodium‐glucose cotransporter 2 inhibitor–related genital and urinary tract infections via electronic medical record–based common data model
SooJeong Ko, HyungMin Kim, Jiwon Shinn, Sun‐ju Byeon, Jeong‐Hee Choi, Hun‐Sung Kim
Journal of Clinical Pharmacy and Therapeutics.2021; 46(4): 975. CrossRef - Pharmacokinetic and Pharmacodynamic Comparison of Two Formulations of a Fixed-Dose Combination of Gemigliptin/Rosuvastatin 50/20 mg: A Randomized, Open-Label, Single-Dose, Two-Way Crossover Study
Eunsol Yang, Hyounggyoon Yoo, In-Jin Jang, Kyung-Sang Yu, SeungHwan Lee
Drug Design, Development and Therapy.2021; Volume 15: 651. CrossRef - Severe hypoglycemia and the risk of end stage renal disease in type 2 diabetes
Jae-Seung Yun, Yong-Moon Park, Kyungdo Han, Hyung-Wook Kim, Seon-Ah Cha, Yu-Bae Ahn, Seung-Hyun Ko
Scientific Reports.2021;[Epub] CrossRef - Long-term HbA1c variability and the development and progression of diabetic retinopathy in subjects with type 2 diabetes
Han Ul Kim, Sung Pyo Park, Yong-Kyu Kim
Scientific Reports.2021;[Epub] CrossRef - Impaired fasting glucose levels in overweight or obese subjects for screening of type 2 diabetes in Korea
Jin-Hee Lee, Suk Chon, Seon-Ah Cha, Sun-Young Lim, Kook-Rye Kim, Jae-Seung Yun, Sang Youl Rhee, Kun-Ho Yoon, Yu-Bae Ahn, Jeong-Taek Woo, Seung-Hyun Ko
The Korean Journal of Internal Medicine.2021; 36(2): 382. CrossRef - Simple Sugar Intake in Diabetics and Non-Diabetic Patients Who Visit Primary Care Clinic
Seo Young Park, Geun Bae Moon, Young Sik Kim, Seung Hee Kim
Korean Journal of Family Practice.2021; 11(1): 29. CrossRef - Effect of Teneligliptin versus Sulfonylurea on Major Adverse Cardiovascular Outcomes in People with Type 2 Diabetes Mellitus: A Real-World Study in Korea
Da Hea Seo, Kyoung Hwa Ha, So Hun Kim, Dae Jung Kim
Endocrinology and Metabolism.2021; 36(1): 70. CrossRef - Best Achievements in Clinical Medicine in Diabetes and Dyslipidemia in 2020
Eun-Jung Rhee, Mee-Kyung Kim, Won-Young Lee
Endocrinology and Metabolism.2021; 36(1): 41. CrossRef - Factors Associated with Poor Glycemic Control Amongst Rural Residents with Diabetes in Korea
Junhee Ahn, Youngran Yang
Healthcare.2021; 9(4): 391. CrossRef - Diabetes and Stroke
Junghyun Noh
The Journal of Korean Diabetes.2021; 22(1): 26. CrossRef - Epidemiology of cardiovascular disease and its risk factors in Korea
Hyeon Chang Kim
Global Health & Medicine.2021; 3(3): 134. CrossRef - Cumulative exposure to impaired fasting glucose and future risk of type 2 diabetes mellitus
Mee Kyoung Kim, Kyungdo Han, Eun Sil Koh, Oak-Kee Hong, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
Diabetes Research and Clinical Practice.2021; 175: 108799. CrossRef - No Relevant Pharmacokinetic Drug–Drug Interaction Between the Sodium-Glucose Co-Transporter-2 Inhibitor Empagliflozin and Lobeglitazone, a Peroxisome Proliferator-Activated Receptor-γ Agonist, in Healthy Subjects
Yu Kyong Kim, Jun Gi Hwang, Min Kyu Park
Drug Design, Development and Therapy.2021; Volume 15: 1725. CrossRef - Comparative Renal Effects of Dipeptidyl Peptidase-4 Inhibitors and Sodium-Glucose Cotransporter 2 Inhibitors on Individual Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis
Jae Hyun Bae, Eun-Gee Park, Sunhee Kim, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Endocrinology and Metabolism.2021; 36(2): 388. CrossRef - The Association Between Second-Line Oral Antihyperglycemic Medication on Types of Dementia in Type 2 Diabetes: A Nationwide Real-World Longitudinal Study
Won Jun Kim, Jung Hyun Noh, Kyungdo Han, Cheol-Young Park
Journal of Alzheimer's Disease.2021; 81(3): 1263. CrossRef - Personalized Type 2 Diabetes Management Using a Mobile Application Integrated with Electronic Medical Records: An Ongoing Randomized Controlled Trial
Eun-Young Lee, Jae-Seung Yun, Seon-Ah Cha, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, Seung-Hyun Ko
International Journal of Environmental Research and Public Health.2021; 18(10): 5300. CrossRef - Cerium Oxide Nanoparticle-Containing Colorimetric Contact Lenses for Noninvasively Monitoring Human Tear Glucose
Sijin Park, Juil Hwang, Hee-Jae Jeon, Woo Ri Bae, In-Kyung Jeong, Tae Gi Kim, Jaheon Kang, Young-Geun Han, Euiheon Chung, Dong Yun Lee
ACS Applied Nano Materials.2021; 4(5): 5198. CrossRef - Low nutritional status links to the prevalence of pre-metabolic syndrome and its cluster in metabolically high-risk Korean adults
Jieun Kim, Kyoungsik Jeong, Siwoo Lee, Bok-Nam Seo, Younghwa Baek
Medicine.2021; 100(20): e25905. CrossRef - Dynamics of Serum Retinol and Alpha-Tocopherol Levels According to Non-Alcoholic Fatty Liver Disease Status
Dongsub Jeon, Minkook Son, Juhyun Shim
Nutrients.2021; 13(5): 1720. CrossRef - Classification and Prediction on the Effects of Nutritional Intake on Overweight/Obesity, Dyslipidemia, Hypertension and Type 2 Diabetes Mellitus Using Deep Learning Model: 4–7th Korea National Health and Nutrition Examination Survey
Hyerim Kim, Dong Hoon Lim, Yoona Kim
International Journal of Environmental Research and Public Health.2021; 18(11): 5597. CrossRef - Comparison of insulin glargine 300 U/mL versus glargine 100 U/mL on glycemic control and hypoglycemic events in East Asian patients with type 2 diabetes: A Patient-level meta-analysis of phase 3 studies
Linong Ji, Yan Bi, Shandong Ye, Yun Huang, Xia Zhang, Shuhua Shang, Nan Cui, Huiqiu Yin, Minlu Zhang
Diabetes Research and Clinical Practice.2021; 176: 108848. CrossRef - Modifiable Risk Factors for Cardiovascular Disease in Korea and Japan
Ahmed Arafa, Hyeok-Hee Lee, Ehab S. Eshak, Kokoro Shirai, Keyang Liu, Jiaqi Li, Naharin Sultana Anni, Sun Young Shim, Hyeon Chang Kim, Hiroyasu Iso
Korean Circulation Journal.2021; 51(8): 643. CrossRef - Sodium-Glucose Cotransporter-2 Inhibitors Ameliorate Liver Enzyme Abnormalities in Korean Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease
Won Euh, Soo Lim, Jin-Wook Kim
Frontiers in Endocrinology.2021;[Epub] CrossRef - Effect of serum isoniazid level on treatment outcomes among tuberculosis patients with slow response – A retrospective cohort study
Hyung Woo Kim, Ah Young Shin, Jick Hwan Ha, Joong Hyun Ahn, Hye Seon Kang, Ju Sang Kim
Journal of Infection and Chemotherapy.2021; 27(11): 1555. CrossRef - Recent Perspective of Metformin
Sangmo Hong
The Journal of Korean Diabetes.2021; 22(2): 85. CrossRef - Diabetes Monotherapies versus Metformin-Based Combination Therapy for the Treatment of Type 2 Diabetes
Awadhesh K Singh, Ritu Singh, Partha Pratim Chakraborty
International Journal of General Medicine.2021; Volume 14: 3833. CrossRef - The Metabolic Score for Insulin Resistance (METS-IR) as a Predictor of Incident Ischemic Heart Disease: A Longitudinal Study among Korean without Diabetes
Jihyun Yoon, Donghyuk Jung, Yongjae Lee, Byoungjin Park
Journal of Personalized Medicine.2021; 11(8): 742. CrossRef - Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study (Diabetes Metab J 2021;45:368-78)
Ja Young Jeon
Diabetes & Metabolism Journal.2021; 45(4): 613. CrossRef - 2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Kyu Yeon Hur, Min Kyong Moon, Jong Suk Park, Soo-Kyung Kim, Seung-Hwan Lee, Jae-Seung Yun, Jong Ha Baek, Junghyun Noh, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Ye Seul Yang, Jang Won Son, Jong Han Choi, Kee Ho Song, Nam Hoon Kim, Sang Yong Kim, Jin Wha Kim,
Diabetes & Metabolism Journal.2021; 45(4): 461. CrossRef - Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study (Diabetes Metab J 2021;45:368-78)
Kyoung Jin Kim, Jimi Choi, Jae Hyun Bae, Kyeong Jin Kim, Hye Jin Yoo, Ji A Seo, Nan Hee Kim, Kyung Mook Choi, Sei Hyun Baik, Sin Gon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2021; 45(4): 617. CrossRef - Cardiovascular Safety of SGLT2 Inhibitors Compared to DPP4 Inhibitors and Sulfonylureas as the Second-Line of Therapy in T2DM Using Large, Real-World Clinical Data in Korea
Kyuho Kim, Sung Hee Choi
Diabetes & Metabolism Journal.2021; 45(4): 502. CrossRef - The Effects of Dietary Education Interventions on Individuals with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Juri Kim, Myung-Haeng Hur
International Journal of Environmental Research and Public Health.2021; 18(16): 8439. CrossRef - Treatment Patterns of Type 2 Diabetes Assessed Using a Common Data Model Based on Electronic Health Records of 2000–2019
Kyung Ae Lee, Heung Yong Jin, Yu Ji Kim, Yong-Jin Im, Eun-Young Kim, Tae Sun Park
Journal of Korean Medical Science.2021;[Epub] CrossRef - Nonalcoholic fatty liver disease and the risk of insulin-requiring gestational diabetes
Sang Youn You, Kyungdo Han, Seung-Hawn Lee, Mee Kyoung Kim
Diabetology & Metabolic Syndrome.2021;[Epub] CrossRef - Comparison of the Effects of Various Antidiabetic Medication on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus
Jeonghoon Ha, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Seung Hyun Ko, Moo Il Kang, Sung Dae Moon, Ki-Hyun Baek
Endocrinology and Metabolism.2021; 36(4): 895. CrossRef - Non-Laboratory-Based Simple Screening Model for Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Developed Using Multi-Center Cohorts
Jiwon Kim, Minyoung Lee, Soo Yeon Kim, Ji-Hye Kim, Ji Sun Nam, Sung Wan Chun, Se Eun Park, Kwang Joon Kim, Yong-ho Lee, Joo Young Nam, Eun Seok Kang
Endocrinology and Metabolism.2021; 36(4): 823. CrossRef - High Fibrosis-4 Index Is Related with Worse Clinical Outcome in Patients with Coronavirus Disease 2019 and Diabetes Mellitus: A Multicenter Observational Study
Sung-Woo Kim, Jae-Han Jeon, Jun Sung Moon, Mi Kyung Kim
Endocrinology and Metabolism.2021; 36(4): 800. CrossRef - Predictive Value of the Atherogenic Index of Plasma (AIP) for the Risk of Incident Ischemic Heart Disease among Non-Diabetic Koreans
Julie J. Kim, Jihyun Yoon, Yong-Jae Lee, Byoungjin Park, Dong-Hyuk Jung
Nutrients.2021; 13(9): 3231. CrossRef - Quantity and quality of complementary and alternative medicine recommendations in clinical practice guidelines for type 2 diabetes mellitus: A systematic review
Jeremy Y. Ng, Kiran D. Verma, Kevin Gilotra
Nutrition, Metabolism and Cardiovascular Diseases.2021; 31(11): 3004. CrossRef - Framingham Risk Score Assessment in Subjects with Pre-diabetes and Diabetes: A Cross-Sectional Study in Korea
Hyuk Sang Kwon, Kee Ho Song, Jae Myung Yu, Dong Sun Kim, Ho Sang Shon, Kyu Jeung Ahn, Sung Hee Choi, Seung Hyun Ko, Won Kim, Kyoung Hwa Lee, Il Seong Nam-Goong, Tae Sun Park
Journal of Obesity & Metabolic Syndrome.2021; 30(3): 261. CrossRef - Frequency of Exposure to Impaired Fasting Glucose and Risk of Mortality and Cardiovascular Outcomes
Seung-Hwan Lee, Kyungdo Han, Hyuk-Sang Kwon, Mee Kyoung Kim
Endocrinology and Metabolism.2021; 36(5): 1007. CrossRef - How Can We Adopt the Glucose Tolerance Test to Facilitate Predicting Pregnancy Outcome in Gestational Diabetes Mellitus?
Kyeong Jin Kim, Nam Hoon Kim, Jimi Choi, Sin Gon Kim, Kyung Ju Lee
Endocrinology and Metabolism.2021; 36(5): 988. CrossRef - Symptom Clusters and Quality of Life in Patients with Type 2 Diabetes Mellitus
Su-Yeon Hong, Yang-Sook Yoo
Korean Journal of Adult Nursing.2021; 33(5): 498. CrossRef - Long-term effectiveness and safety of quadruple combination therapy with empagliflozin versus dapagliflozin in patients with type 2 diabetes: 3-year prospective observational study
Eu Jeong Ku, Dong-Hwa Lee, Hyun Jeong Jeon, Tae Keun Oh
Diabetes Research and Clinical Practice.2021; 182: 109123. CrossRef - Long-term effects of the mean hemoglobin A1c levels after percutaneous coronary intervention in patients with diabetes
Jaekyung Bae, Ji-Hyung Yoon, Jung-Hee Lee, Jong-Ho Nam, Chan-Hee Lee, Jang-Won Son, Ung Kim, Jong-Seon Park, Dong-Gu Shin
The Korean Journal of Internal Medicine.2021; 36(6): 1365. CrossRef - Role of Leptin with hypothyroidism in Iraqi diabetic type 2 patients
Sulaiman M. Hasan
Bionatura.2021; 6(4): 2274. CrossRef - Impacts of statin and metformin on neuropathy in patients with type 2 diabetes mellitus: Korean Health Insurance data
Hong Ki Min, Se Hee Kim, Jong Han Choi, Kyomin Choi, Hae-Rim Kim, Sang-Heon Lee
World Journal of Clinical Cases.2021; 9(33): 10198. CrossRef - Muscle strength, an independent determinant of glycemic control in older adults with long-standing type 2 diabetes: a prospective cohort study
Bo Kyung Koo, Seoil Moon, Min Kyong Moon
BMC Geriatrics.2021;[Epub] CrossRef - Comparisons of Neuropsychological Characteristics of Elderly Subjects With Versus Without History of Agent Orange Exposure
Seunggyu Han, Jinhee Choi, Hyung Seok So, Hayun Choi, Hong Jin Jeon, Jinseob Kim, Kiwon Kim
Journal of Korean Neuropsychiatric Association.2021; 60(4): 346. CrossRef - Uric Acid Variability as a Predictive Marker of Newly Developed Cardiovascular Events in Type 2 Diabetes
Hae Kyung Kim, Minyoung Lee, Yong-ho Lee, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Sleep Duration and Its Associations with Mortality and Quality of Life in Chronic Obstructive Pulmonary Disease: Results from the 2007–2015 KNAHNES
So Jeong Kim, Nakwon Kwak, Sun Mi Choi, Jinwoo Lee, Young Sik Park, Chang-Hoon Lee, Sang-Min Lee, Chul-Gyu Yoo, Jaeyoung Cho
Respiration.2021; 100(11): 1043. CrossRef - Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Position Statement of the Fatty Liver Research Group of the Korean Diabetes Association
Byung-Wan Lee, Yong-ho Lee, Cheol-Young Park, Eun-Jung Rhee, Won-Young Lee, Nan-Hee Kim, Kyung Mook Choi, Keun-Gyu Park, Yeon-Kyung Choi, Bong-Soo Cha, Dae Ho Lee
Diabetes & Metabolism Journal.2020; 44(3): 382. CrossRef - Effects of Co‐administration of Sulfonylureas and Antimicrobial Drugs on Hypoglycemia in Patients with Type 2 Diabetes Using a Case‐Crossover Design
Sera Lee, Miyoung Ock, Hun‐Sung Kim, Hyunah Kim
Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy.2020; 40(9): 902. CrossRef - Diabetic kidney disease: seven questions
Dong Ho Yang, So-Young Lee
Journal of the Korean Medical Association.2020; 63(1): 6. CrossRef -
Diabetes and Metabolism Journal in 2020: Good to Great
In-Kyung Jeong
Diabetes & Metabolism Journal.2020; 44(1): 1. CrossRef - The Use of Mobile Personal Health Records for Hemoglobin A1c Regulation in Patients With Diabetes: Retrospective Observational Study
Dongjin Seo, Yu Rang Park, Yura Lee, Ji Young Kim, Joong-Yeol Park, Jae-Ho Lee
Journal of Medical Internet Research.2020; 22(6): e15372. CrossRef - Non-glucose risk factors in the pathogenesis of diabetic peripheral neuropathy
Kyung Ae Lee, Tae Sun Park, Heung Yong Jin
Endocrine.2020; 70(3): 465. CrossRef - An Assessment of Pharmacokinetic Interaction Between Lobeglitazone and Sitagliptin After Multiple Oral Administrations in Healthy Men
Seol Ju Moon, Kyung-Sang Yu, Min-Gul Kim
Clinical Therapeutics.2020; 42(6): 1047. CrossRef - The Clinical Characteristics and Outcomes of Patients with Moderate-to-Severe Coronavirus Disease 2019 Infection and Diabetes in Daegu, South Korea
Mi Kyung Kim, Jae-Han Jeon, Sung-Woo Kim, Jun Sung Moon, Nan Hee Cho, Eugene Han, Ji Hong You, Ji Yeon Lee, Miri Hyun, Jae Seok Park, Yong Shik Kwon, Yeon-Kyung Choi, Ki Tae Kwon, Shin Yup Lee, Eon Ju Jeon, Jin-Woo Kim, Hyo-Lim Hong, Hyun Hee Kwon, Chi Yo
Diabetes & Metabolism Journal.2020; 44(4): 602. CrossRef - The Risk of Diabetes on Clinical Outcomes in Patients with Coronavirus Disease 2019: A Retrospective Cohort Study
Seung Min Chung, Yin Young Lee, Eunyeong Ha, Ji Sung Yoon, Kyu Chang Won, Hyoung Woo Lee, Jian Hur, Kyung Soo Hong, Jong Geol Jang, Hyun Jung Jin, Eun Young Choi, Kyeong-Cheol Shin, Jin Hong Chung, Kwan Ho Lee, June Hong Ahn, Jun Sung Moon
Diabetes & Metabolism Journal.2020; 44(3): 405. CrossRef - A model to predict risk of stroke in middle-aged adults with type 2 diabetes generated from a nationwide population-based cohort study in Korea
Mee-Kyoung Kim, Kyungdo Han, Jae-Hyoung Cho, Hyuk-Sang Kwon, Kun-Ho Yoon, Seung-Hwan Lee
Diabetes Research and Clinical Practice.2020; 163: 108157. CrossRef - Effects of Cardiovascular Risk Factor Variability on Health Outcomes
Seung-Hwan Lee, Mee Kyoung Kim, Eun-Jung Rhee
Endocrinology and Metabolism.2020; 35(2): 217. CrossRef - Sodium-Glucose Cotransporter-2 Inhibitor for Renal Function Preservation in Patients with Type 2 Diabetes Mellitus: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
Tae Jung Oh, Ju-Young Moon, Kyu Yeon Hur, Seung Hyun Ko, Hyun Jung Kim, Taehee Kim, Dong Won Lee, Min Kyong Moon
Diabetes & Metabolism Journal.2020; 44(4): 489. CrossRef - Metformin Treatment for Patients with Diabetes and Chronic Kidney Disease: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
Kyu Yeon Hur, Mee Kyoung Kim, Seung Hyun Ko, Miyeun Han, Dong Won Lee, Hyuk-Sang Kwon
Diabetes & Metabolism Journal.2020; 44(1): 3. CrossRef - Prevalence and Current Management of Cardiovascular Risk Factors in Korean Adults Based on Fact Sheets
Eun-Jung Rhee
Endocrinology and Metabolism.2020; 35(1): 85. CrossRef - Using adult stem cells to monitor endothelial dysfunction in diabetes mellitus
Rohit Jain, Hassan Awal, Sabyasachi Sen
Journal of Diabetes and its Complications.2020; 34(7): 107588. CrossRef - Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction
Nam Hoon Kim, Sin Gon Kim
Diabetes & Metabolism Journal.2020; 44(2): 213. CrossRef - Metformin treatment for patients with diabetes and chronic kidney disease: A Korean Diabetes Association and Korean Society of Nephrology consensus statement
Kyu Yeon Hur, Mee Kyoung Kim, Seung Hyun Ko, Miyeun Han, Dong Won Lee, Hyuk-Sang Kwon
Kidney Research and Clinical Practice.2020; 39(1): 32. CrossRef - Generation of iPSC-derived insulin-producing cells from patients with type 1 and type 2 diabetes compared with healthy control
Min Jung Kim, Eun Young Lee, Young-Hye You, Hae Kyung Yang, Kun-Ho Yoon, Ji-Won Kim
Stem Cell Research.2020; 48: 101958. CrossRef - Glucose-Lowering Effect of Home-Delivered Therapeutic Meals in Patients with Type 2 Diabetes
Jong Han Choi, Se Hee Min, Kyeong Hye Lim, Uoon Jeong Shin, Min-Seon Kim
The Journal of Korean Diabetes.2020; 21(1): 46. CrossRef - Subclinical thyroid dysfunction in the first trimester of pregnancy: ‘Disease’ versus physiological (pulsatile) variation in TSH concentrations
Krzysztof C. Lewandowski, Karolina Garnysz, Wojciech Horzelski, Joanna Kawalec, Karolina Budzen, Mariusz Grzesiak, Andrzej Lewinski
Clinical Endocrinology.2020; 93(6): 739. CrossRef - Usefulness of carotid ultrasonography and treatment of carotid disease
Seung Min Kim, Yeon Jung Kim, Keonwoo Kim, Bum Joon Kim
Journal of the Korean Medical Association.2020; 63(6): 342. CrossRef - Clinical Characteristics and Prevalence of Comorbidities according to Metformin Use in Korean Patients with Type 2 Diabetes
Sang Ouk Chin, In Gyoon Ha, Sang Youl Rhee, Su Jin Jeong, Suk Chon, Sung Hoon Kim, Kyu Jeung Ahn, Sei Hyun Baik, Yongsoo Park, Moon Suk Nam, Kwan Woo Lee, Jeong Taek Woo
International Journal of Endocrinology.2020; 2020: 1. CrossRef - Prepregnancy smoking and the risk of gestational diabetes requiring insulin therapy
Mee Kyoung Kim, Kyungdo Han, Sang Youn You, Hyuk-Sang Kwon, Kun-Ho Yoon, Seung-Hwan Lee
Scientific Reports.2020;[Epub] CrossRef - Use of SGLT-2 Inhibitors in Patients with Type 2 Diabetes Mellitus and Abdominal Obesity: An Asian Perspective and Expert Recommendations
Wayne Huey-Herng Sheu, Siew Pheng Chan, Bien J. Matawaran, Chaicharn Deerochanawong, Ambrish Mithal, Juliana Chan, Ketut Suastika, Chin Meng Khoo, Huu Man Nguyen, Ji Linong, Andrea Luk, Kun-Ho Yoon
Diabetes & Metabolism Journal.2020; 44(1): 11. CrossRef - Response: The Risk of Diabetes on Clinical Outcomes in Patients with Coronavirus Disease 2019: A Retrospective Cohort Study (Diabetes Metab J 2020;44:405–13)
Seung Min Chung, June Hong Ahn, Jun Sung Moon
Diabetes & Metabolism Journal.2020; 44(4): 625. CrossRef - Effect of roux-en Y gastric bypass surgery on patients with type 2 diabetes mellitus
Ke-Yan Chen, Ying-Li Liu, Jin-Cai Shang, De-Wang Su, Rong-Rong Yao, De-Zhi Ke, Hao Tian
Medicine.2020; 99(23): e20382. CrossRef - Comparison and Implication of the Contemporary Blood Pressure Guidelines on Korean Population
So Mi Jemma Cho, Hokyou Lee, Hyeon Chang Kim
Korean Circulation Journal.2020; 50(6): 485. CrossRef - Efficacy and safety of evogliptin treatment in patients with type 2 diabetes: A multicentre, active‐controlled, randomized, double‐blind study with open‐label extension (the EVERGREEN study)
Gyuri Kim, Soo Lim, Hyuk‐Sang Kwon, Ie B. Park, Kyu J. Ahn, Cheol‐Young Park, Su K. Kwon, Hye S. Kim, Seok W. Park, Sin G. Kim, Min K. Moon, Eun S. Kim, Choon H. Chung, Kang S. Park, Mikyung Kim, Dong J. Chung, Chang B. Lee, Tae H. Kim, Moon‐Kyu Lee
Diabetes, Obesity and Metabolism.2020; 22(9): 1527. CrossRef - Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease
Kyung-Soo Kim, Byung-Wan Lee
Clinical and Molecular Hepatology.2020; 26(4): 430. CrossRef - Predicting the Development of Myocardial Infarction in Middle-Aged Adults with Type 2 Diabetes: A Risk Model Generated from a Nationwide Population-Based Cohort Study in Korea
Seung-Hwan Lee, Kyungdo Han, Hun-Sung Kim, Jae-Hyoung Cho, Kun-Ho Yoon, Mee Kyoung Kim
Endocrinology and Metabolism.2020; 35(3): 636. CrossRef - Fasting Plasma Glucose Level Independently Predicts the Mortality of Patients with Coronavirus Disease 2019 Infection: A Multicenter, Retrospective Cohort Study
Min Cheol Chang, Jong-Moon Hwang, Jae-Han Jeon, Sang Gyu Kwak, Donghwi Park, Jun Sung Moon
Endocrinology and Metabolism.2020; 35(3): 595. CrossRef - Sodium-glucose cotransporter-2 inhibitor for renal function preservation in patients with type 2 diabetes mellitus: A Korean Diabetes Association and Korean Society of Nephrology consensus statement
Tae Jung Oh, Ju-Young Moon, Kyu Yeon Hur, Seung Hyun Ko, Hyun Jung Kim, Taehee Kim, Dong Won Lee, Min Kyong Moon
Kidney Research and Clinical Practice.2020; 39(3): 269. CrossRef - Dynamic changes in nocturnal blood glucose levels are associated with sleep-related features in patients with obstructive sleep apnea
Jung-Ick Byun, Kwang Su Cha, Ji Eun Jun, Tae-Joon Kim, Ki-Young Jung, In-Kyung Jeong, Won Chul Shin
Scientific Reports.2020;[Epub] CrossRef Effect of Switching from Linagliptin to Teneligliptin Dipeptidyl Peptidase-4 Inhibitors in Older Patients with Type 2 Diabetes Mellitus
Eugene Han, Minyoung Lee, Yong-ho Lee, Hye Soon Kim, Byung-wan Lee, Bong-Soo Cha, Eun Seok Kang
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 4113. CrossRef- A prospective cohort study on effects of gemigliptin on cardiovascular outcomes in patients with type 2 diabetes (OPTIMUS study)
Eun Heui Kim, Sang Soo Kim, Dong Jun Kim, Young Sik Choi, Chang Won Lee, Bon Jeong Ku, Kwang Soo Cha, Kee Ho Song, Dae Kyeong Kim, In Joo Kim
Scientific Reports.2020;[Epub] CrossRef - Effect of Variability in Blood Pressure, Glucose and Cholesterol Concentrations, and Body Weight on Emergency Hospitalization and 30‐Day Mortality in the General Population
Seung‐Hwan Lee, Kyungdo Han, Hyuk‐Sang Kwon, Kun‐Ho Yoon, Mee Kyoung Kim
Journal of the American Heart Association.2020;[Epub] CrossRef - Systematic review of clinical practice guidelines to identify recommendations for sleep in type 2 diabetes mellitus management
Aisling Smyth, Mark Jenkins, Melissa Dunham, Yvonne Kutzer, Shahrad Taheri, Lisa Whitehead
Diabetes Research and Clinical Practice.2020; 170: 108532. CrossRef - The risk factors for treatment-related mortality within first three months after kidney transplantation
Ye Na Kim, Do Hyoung Kim, Ho Sik Shin, Sangjin Lee, Nuri Lee, Min-Jeong Park, Wonkeun Song, Seri Jeong, Robert Jeenchen Chen
PLOS ONE.2020; 15(12): e0243586. CrossRef - The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study
Sangmo Hong, Kyungdo Han, Cheol-Young Park
BMC Medicine.2020;[Epub] CrossRef - The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data
Byoungjin Park, Yong-Jae Lee, Hye Sun Lee, Dong-Hyuk Jung
Cardiovascular Diabetology.2020;[Epub] CrossRef - Increased Age of Death and Change in Causes of Death Among Persons With Diabetes Mellitus From the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
Eugene Han, Sun Ok Song, Hye Soon Kim, Kang Ju Son, Sun Ha Jee, Bong-Soo Cha, Byung-Wan Lee
SSRN Electronic Journal .2020;[Epub] CrossRef - Letter: Dipeptidyl Peptidase-4 Inhibitors versus Other Antidiabetic Drugs Added to Metformin Monotherapy in Diabetic Retinopathy Progression: A Real World-Based Cohort Study (Diabetes Metab J 2019;43:640–8)
Jun Sung Moon
Diabetes & Metabolism Journal.2019; 43(6): 911. CrossRef - Differences in prevalence of hypertension subtypes according to the 2018 Korean Society of Hypertension and 2017 American College of Cardiology/American Heart Association guidelines: The Korean National Health and Nutrition Examination Survey, 2007–2017 (
So Mi Jemma Cho, Hokyou Lee, Hyeon Chang Kim
Clinical Hypertension.2019;[Epub] CrossRef - Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
Sabyasachi Sen
Diabetes & Metabolism Journal.2019; 43(6): 744. CrossRef - Cholesterol levels and development of cardiovascular disease in Koreans with type 2 diabetes mellitus and without pre-existing cardiovascular disease
Mee Kyoung Kim, Kyungdo Han, Han Na Joung, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
Cardiovascular Diabetology.2019;[Epub] CrossRef - Management of Cardiovascular Risk Factors in Elderly Diabetes Mellitus Patients
Sung Hoon Yu
The Journal of Korean Diabetes.2019; 20(4): 233. CrossRef - Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J2019;43:582–9)
Tae Seo Sohn
Diabetes & Metabolism Journal.2019; 43(6): 909. CrossRef - An Age of Sodium-Glucose Cotransporter-2 Inhibitor Priority: Are We Ready?
Ji A Seo
Diabetes & Metabolism Journal.2019; 43(5): 578. CrossRef - Association between Diabetes and the Use of Removable Dental Prostheses among the Korean Population
Jae-Hyun Lee, Jung-Suk Han, Kyungdo Han, Su-Young Lee
Journal of Korean Medical Science.2019;[Epub] CrossRef - Metabolic Surgery and Diabetes Mellitus: Its Effects and Side Effects
Mee Kyoung Kim
The Journal of Korean Diabetes.2019; 20(4): 205. CrossRef
- Serum-based ATR-FTIR spectroscopy combined with multivariate analysis for the diagnosis of pre-diabetes and diabetes
- Clinical Diabetes & Therapeutics
- Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
- Hyun Jin Kim, Seok O Park, Seung-Hyun Ko, Sang Youl Rhee, Kyu-Yeon Hur, Nan-Hee Kim, Min Kyong Moon, Byung-Wan Lee, Jin Hwa Kim, Kyung Mook Choi
- Diabetes Metab J. 2017;41(6):423-429. Published online December 19, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.6.423

- 5,794 View
- 71 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The glucagon-like peptide-1 receptor agonists (GLP-1RAs) were recommended as a monotherapy or combination therapy with oral hypoglycemic agents or basal insulin in the position statement of the Korean Diabetes Association 2017 for pharmacological therapy. Many randomized clinical trials and systematic reviews report that GLP-1RAs have considerable glucose-lowering effect and lead to weight reduction and low risk of hypoglycemia when used as a monotherapy or combination therapy. The cardiovascular safety of GLP-1RAs has been assessed in several randomized clinical trials and systematic reviews. The results of cardiovascular outcome trials of long-acting GLP-1RAs (liraglutide, semaglutide) demonstrated cardiovascular benefits in subjects with type 2 diabetes mellitus and a high risk of cardiovascular disease. The GLP-1RA may be a choice of therapy when weight control and avoidance of hypoglycemia are important, and patients with high risk of cardiovascular disease might also favor choosing GLP-1RA.
-
Citations
Citations to this article as recorded by- Anti-inflammatory effect of glucagon-like Peptide-1 receptor agonist on the neurosensory retina in an acute optic nerve injury rat model
Yeon Woong Chung, Ji Young Lee, Hyun Hee Ju, Jin A. Choi
European Journal of Pharmacology.2022; 933: 175269. CrossRef - Diabetes Risk Data Mining Method Based on Electronic Medical Record Analysis
Yang Liu, Zhaoxiang Yu, Yunlong Yang, Zhihan Lv
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef - Paradigm Shift for the Treatment of Type 2 Diabetes Mellitus in Patients with Cardiovascular Disease: Cardiologist's Perspective
Doo Soo Jeon
Cardiovascular Prevention and Pharmacotherapy.2020; 2(1): 11. CrossRef - The Role of Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes in Asia
Ju-Ming Lu
Advances in Therapy.2019; 36(4): 798. CrossRef - A Review of Practical Issues on the Use of Glucagon-Like Peptide-1 Receptor Agonists for the Management of Type 2 Diabetes
Irene Romera, Ana Cebrián-Cuenca, Fernando Álvarez-Guisasola, Fernando Gomez-Peralta, Jesús Reviriego
Diabetes Therapy.2019; 10(1): 5. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
Hyun Jin Kim
The Journal of Korean Diabetes.2018; 19(1): 35. CrossRef
- Anti-inflammatory effect of glucagon-like Peptide-1 receptor agonist on the neurosensory retina in an acute optic nerve injury rat model
- Clinical Diabetes & Therapeutics
- Insulin Therapy for Adult Patients with Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association, 2017
- Byung-Wan Lee, Jin Hwa Kim, Seung-Hyun Ko, Kyu-Yeon Hur, Nan-Hee Kim, Sang Youl Rhee, Hyun Jin Kim, Min Kyong Moon, Seok-O Park, Kyung Mook Choi
- Diabetes Metab J. 2017;41(5):367-373. Published online October 24, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.5.367

- 7,420 View
- 220 Download
- 7 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The Korean Diabetes Association (KDA) has regularly updated its Clinical Practice Guidelines. In 2017, the KDA published a position statement on the use of antihyperglycemic agents for patients with type 2 diabetes mellitus (T2DM). Growing evidence from new multinational clinical trials using novel and traditional insulin analogues has also been accumulated. Following global trends, many results of clinical trials, especially concerning the clinical efficacy and safety of insulin therapy, have been published about Korean patients with T2DM. After a systematic search of recent evidence, the KDA updated and modified its clinical practice recommendations regarding the initiation, choice, and intensification of insulin and created an insulin treatment algorithm for the first time to guide physicians caring for adult Korean patients with T2DM.
-
Citations
Citations to this article as recorded by- Real-World Outcomes of Individualized Targeted Therapy with Insulin Glargine 300 Units/mL in Insulin-Naïve Korean People with Type 2 Diabetes: TOBE Study
Eun-Gyoung Hong, Kyung-Wan Min, Jung Soo Lim, Kyu-Jeung Ahn, Chul Woo Ahn, Jae-Myung Yu, Hye Soon Kim, Hyun Jin Kim, Won Kim, Dong Han Kim, Hak Chul Jang
Advances in Therapy.2024;[Epub] CrossRef - Tolerability and Effectiveness of Switching to Dulaglutide in Patients With Type 2 Diabetes Inadequately Controlled With Insulin Therapy
Youngsook Kim, Ji Hye Huh, Minyoung Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Frontiers in Endocrinology.2022;[Epub] CrossRef - Increasing Individual Target Glucose Levels to Prevent Hypoglycemia in Patients with Diabetes
Juyoung Shin, Hyunah Kim, Hun-Sung Kim, Churlmin Kim, Whan-Seok Choi
Korean Journal of Family Medicine.2021; 42(4): 269. CrossRef - Effects of an actual insulin injection demonstration on insulin acceptance among patients with T2DM: a pragmatic randomized controlled trial
Atthayaporn Choomai, Apichai Wattanapisit, Orathai Tiangtam
Romanian Journal of Internal Medicine.2021; 59(2): 151. CrossRef - Favorable Glycemic Control with Once-Daily Insulin Degludec/Insulin Aspart after Changing from Basal Insulin in Adults with Type 2 Diabetes
Han Na Jang, Ye Seul Yang, Seong Ok Lee, Tae Jung Oh, Bo Kyung Koo, Hye Seung Jung
Endocrinology and Metabolism.2019; 34(4): 382. CrossRef - Capacity and confidence building for general practitioners on optimum insulin use
Sanjay Kalra, Prasun Deb, KalyanK Gangopadhyay, Sunil Gupta, Abhay Ahluwalia
Journal of Family Medicine and Primary Care.2019; 8(10): 3096. CrossRef - Educational Strategies for Insulin Injection Therapy in Elderly Diabetic Patients
Eun Chong Shin
The Journal of Korean Diabetes.2018; 19(2): 101. CrossRef - Summary of Insulin Therapy for Adult Patients with Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association, 2017
Byung-Wan Lee
The Journal of Korean Diabetes.2018; 19(1): 31. CrossRef - New anti-diabetic agents
Doo-Man Kim
Journal of the Korean Medical Association.2017; 60(12): 992. CrossRef
- Real-World Outcomes of Individualized Targeted Therapy with Insulin Glargine 300 Units/mL in Insulin-Naïve Korean People with Type 2 Diabetes: TOBE Study

 KDA
KDA
 First
First Prev
Prev





