
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Type 1 Diabetes
- Optimal Coefficient of Variance Threshold to Minimize Hypoglycemia Risk in Individuals with Well-Controlled Type 1 Diabetes Mellitus
- Jee Hee Yoo, Seung Hee Yang, Sang-Man Jin, Jae Hyeon Kim
- Received March 14, 2023 Accepted August 12, 2023 Published online March 4, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0083 [Epub ahead of print]

- 516 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the optimal coefficient of variance (%CV) for preventing hypoglycemia based on real-time continuous glucose monitoring (rt-CGM) data in people with type 1 diabetes mellitus (T1DM) already achieving their mean glucose (MG) target.
Methods
Data from 172 subjects who underwent rt-CGM for at least 90 days and for whom 439 90-day glycemic profiles were available were analyzed. Receiver operator characteristic analysis was conducted to determine the cut-off value of %CV to achieve time below range (%TBR)<54 mg/dL <1 and =0.
Results
Overall mean glycosylated hemoglobin was 6.8% and median %TBR<54 mg/dL was 0.2%. MG was significantly higher and %CV significantly lower in profiles achieving %TBR<54 mg/dL <1 compared to %TBR<54 mg/dL ≥1 (all P<0.001). The cut-off value of %CV for achieving %TBR<54 mg/dL <1 was 37.5%, 37.3%, and 31.0%, in the whole population, MG >135 mg/dL, and ≤135 mg/dL, respectively. The cut-off value for %TBR<54 mg/dL=0% was 29.2% in MG ≤135 mg/dL. In profiles with MG ≤135 mg/dL, 94.2% of profiles with a %CV <31 achieved the target of %TBR<54 mg/dL <1, and 97.3% with a %CV <29.2 achieved the target of %TBR<54 mg/ dL=0%. When MG was >135 mg/dL, 99.4% of profiles with a %CV <37.3 achieved %TBR<54 mg/dL <1.
Conclusion
In well-controlled T1DM with MG ≤135 mg/dL, we suggest a %CV <31% to achieve the %TBR<54 mg/dL <1 target. Furthermore, we suggest a %CV <29.2% to achieve the target of %TBR<54 mg/dL =0 for people at high risk of hypoglycemia.
- Drug/Regimen
- Pioglitazone as Add-on THERAPY in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Ji Hye Heo, Kyung Ah Han, Jun Hwa Hong, Hyun-Ae Seo, Eun-Gyoung Hong, Jae Myung Yu, Hye Seung Jung, Bong-Soo Cha
- Received September 1, 2023 Accepted October 25, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0314 [Epub ahead of print]
- 1,212 View
- 115 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
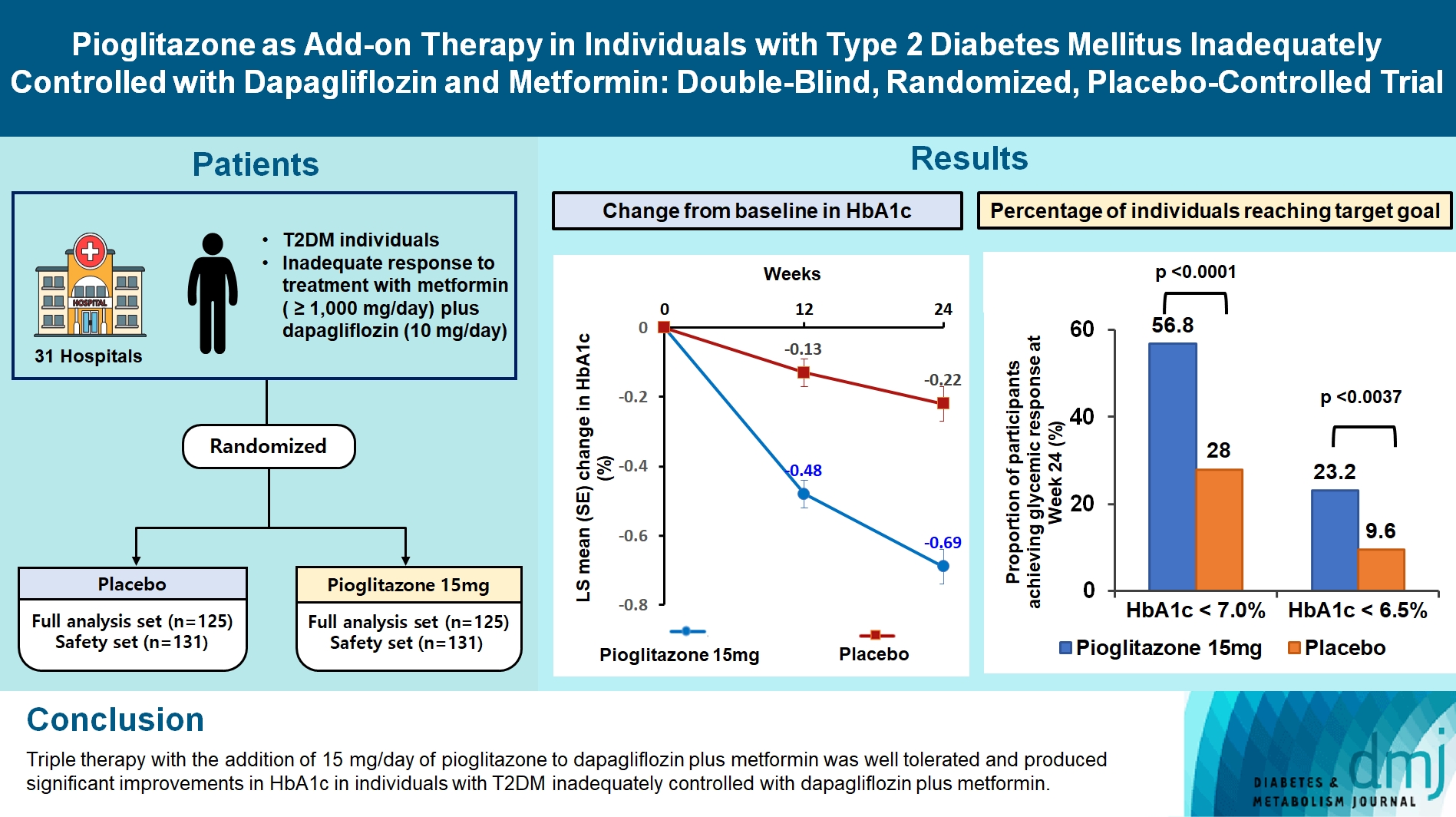
This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
Results
At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
Conclusion
Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
- Drug/Regimen
- Abrupt Decline in Estimated Glomerular Filtration Rate after Initiating Sodium-Glucose Cotransporter 2 Inhibitors Predicts Clinical Outcomes: A Systematic Review and Meta-Analysis
- Min-Hsiang Chuang, Yu-Shuo Tang, Jui-Yi Chen, Heng-Chih Pan, Hung-Wei Liao, Wen-Kai Chu, Chung-Yi Cheng, Vin-Cent Wu, Michael Heung
- Diabetes Metab J. 2024;48(2):242-252. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0201
- 1,546 View
- 206 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
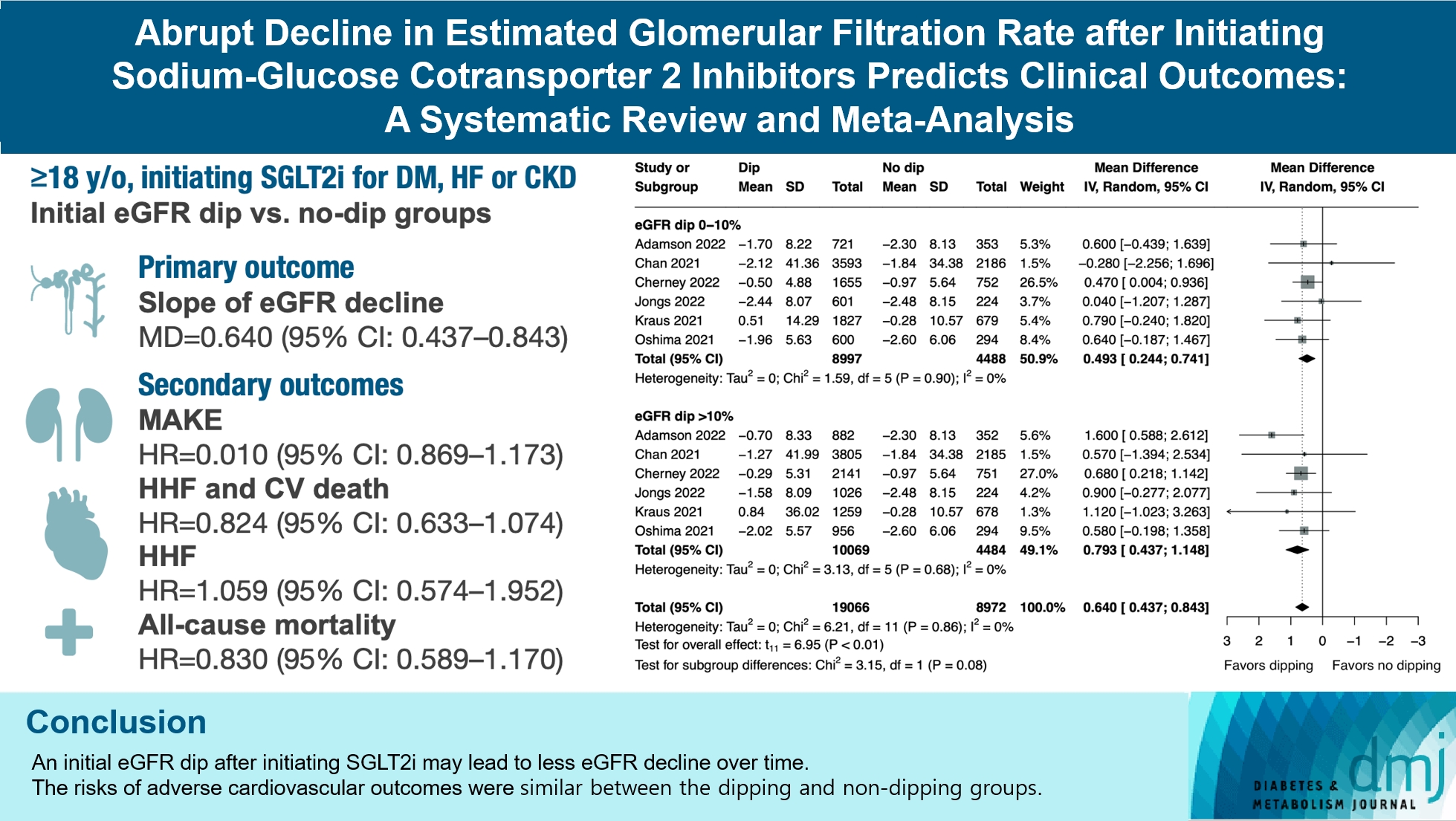
The initiation of sodium-glucose cotransporter-2 inhibitors (SGLT2i) typically leads to a reversible initial dip in estimated glomerular filtration rate (eGFR). The implications of this phenomenon on clinical outcomes are not well-defined.
Methods
We searched MEDLINE, Embase, and Cochrane Library from inception to March 23, 2023 to identify randomized controlled trials and cohort studies comparing kidney and cardiovascular outcomes in patients with and without initial eGFR dip after initiating SGLT2i. Pooled estimates were calculated using random-effect meta-analysis.
Results
We included seven studies in our analysis, which revealed that an initial eGFR dip following the initiation of SGLT2i was associated with less annual eGFR decline (mean difference, 0.64; 95% confidence interval [CI], 0.437 to 0.843) regardless of baseline eGFR. The risk of major adverse kidney events was similar between the non-dipping and dipping groups but reduced in patients with a ≤10% eGFR dip (hazard ratio [HR], 0.915; 95% CI, 0.865 to 0.967). No significant differences were observed in the composite of hospitalized heart failure and cardiovascular death (HR, 0.824; 95% CI, 0.633 to 1.074), hospitalized heart failure (HR, 1.059; 95% CI, 0.574 to 1.952), or all-cause mortality (HR, 0.83; 95% CI, 0.589 to 1.170). The risk of serious adverse events (AEs), discontinuation of SGLT2i due to AEs, kidney-related AEs, and volume depletion were similar between the two groups. Patients with >10% eGFR dip had increased risk of hyperkalemia compared to the non-dipping group.
Conclusion
Initial eGFR dip after initiating SGLT2i might be associated with less annual eGFR decline. There were no significant disparities in the risks of adverse cardiovascular outcomes between the dipping and non-dipping groups.
- Drug/Regimen
- Two-Year Therapeutic Efficacy and Safety of Initial Triple Combination of Metformin, Sitagliptin, and Empagliflozin in Drug-Naïve Type 2 Diabetes Mellitus Patients
- Young-Hwan Park, Minji Sohn, So Yeon Lee, Soo Lim
- Diabetes Metab J. 2024;48(2):253-264. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0128
- 1,741 View
- 278 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
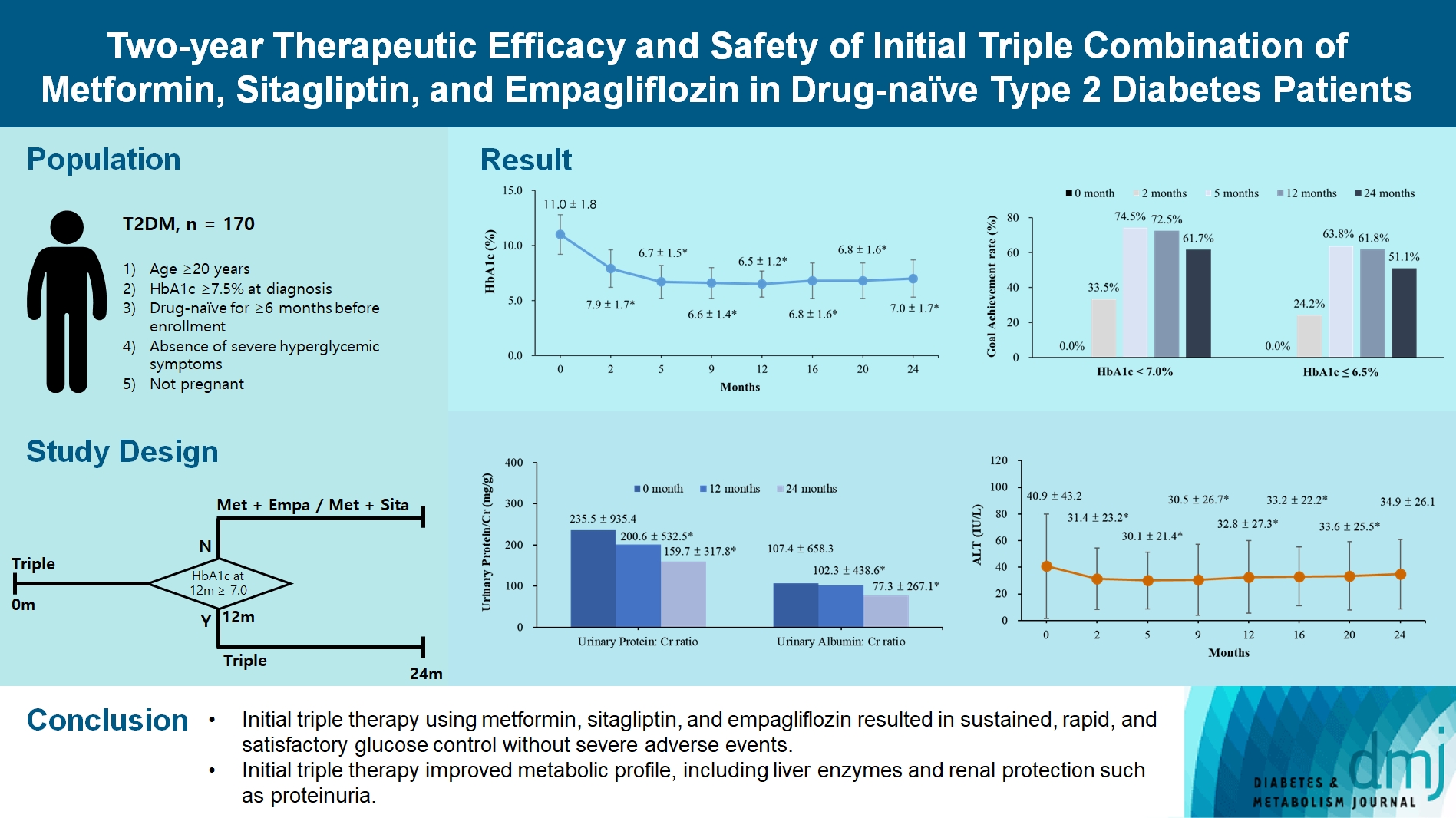
We investigated the long-term efficacy and safety of initial triple therapy using metformin, a dipeptidyl peptidase-4 inhibitor, and a sodium-glucose cotransporter-2 inhibitor, in patients with type 2 diabetes mellitus.
Methods
We enrolled 170 drug-naïve patients with glycosylated hemoglobin (HbA1c) level >7.5% who had started triple therapy (metformin, sitagliptin, and empagliflozin). Glycemic, metabolic, and urinary parameters were measured for 24 months.
Results
After 24 months, HbA1c level decreased significantly from 11.0%±1.8% to 7.0%±1.7%. At 12 and 24 months, the rates of achievement of the glycemic target goal (HbA1c <7.0%) were 72.5% and 61.7%, respectively, and homeostasis model assessment of β-cell function and insulin resistance indices improved. Whole-body fat percentage decreased by 1.08%, and whole-body muscle percentage increased by 0.97% after 24 months. Fatty liver indices and albuminuria improved significantly. The concentration of ketone bodies was elevated at the baseline but decreased after 24 months. There were no serious adverse events, including ketoacidosis.
Conclusion
Initial triple combination therapy with metformin, sitagliptin, and empagliflozin led to achievement of the glycemic target goal, which was maintained for 24 months without severe hypoglycemia but with improved metabolic function and albuminuria. This combination therapy may be a good strategy for drug-naïve patients with type 2 diabetes mellitus.
- Metabolic Risk/Epidemiology
- Association of Measures of Glucose Metabolism with Colorectal Cancer Risk in Older Chinese: A 13-Year Follow-up of the Guangzhou Biobank Cohort Study-Cardiovascular Disease Substudy and Meta-Analysis
- Shu Yi Wang, Wei Sen Zhang, Chao Qiang Jiang, Ya Li Jin, Tong Zhu, Feng Zhu, Lin Xu
- Diabetes Metab J. 2024;48(1):134-145. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0383

- 1,128 View
- 140 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Abnormal glucose metabolism is a risk factor for colorectal cancer (CRC). However, association of glycosylated hemoglobin (HbA1c) with CRC risk remains under-reported. We examined the association between glycemic indicators (HbA1c, fasting plasma glucose, fasting insulin, 2-hour glucose, 2-hour insulin, and homeostasis model of risk assessment-insulin resistance index) and CRC risk using prospective analysis and meta-analysis.
Methods
Participants (n=1,915) from the Guangzhou Biobank Cohort Study-Cardiovascular Disease Substudy were included. CRC events were identified through record linkage. Cox regression was used to assess the associations of glycemic indicators with CRC risk. A meta-analysis was performed to investigate the association between HbA1c and CRC risk.
Results
During an average of 12.9 years follow-up (standard deviation, 2.8), 42 incident CRC cases occurred. After adjusting for potential confounders, the hazard ratio (95% confidence interval [CI]) of CRC for per % increment in HbA1c was 1.28 (95% CI, 1.01 to 1.63) in overall population, 1.51 (95% CI, 1.13 to 2.02) in women and 1.06 (95% CI, 0.68 to 1.68) in men. No significant association of other measures of glycemic indicators and baseline diabetes with CRC risk was found. Meta-analyses of 523,857 participants including our results showed that per % increment of HbA1c was associated with 13% higher risk of CRC, with the pooled risk ratio being 1.13 (95% CI, 1.01 to 1.27). Subgroupanalyses found stronger associations in women, colon cancer, Asians, and case-control studies.
Conclusion
Higher HbA1c was a significant predictor of CRC in the general population. Our findings shed light on the pathology of glucose metabolism and CRC, which warrants more in-depth investigation.
- Basic Research
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Ji-Hae Park, Soyeon Kwon, Young Mi Park
- Diabetes Metab J. 2024;48(2):215-230. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0332
- 2,305 View
- 192 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
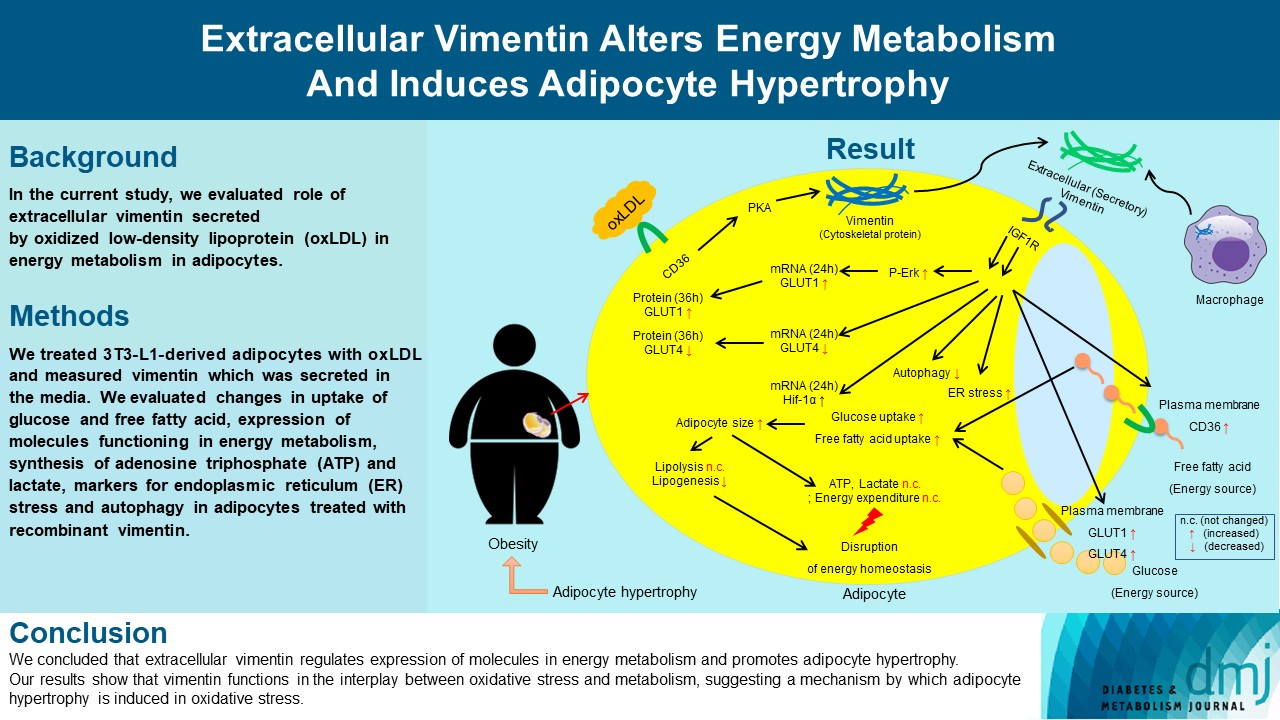
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
- Technology/Device
- Clinical and Lifestyle Determinants of Continuous Glucose Monitoring Metrics in Insulin-Treated Patients with Type 2 Diabetes Mellitus
- Da Young Lee, Namho Kim, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Jihee Kim, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Sung-Min Park, Nan Hee Kim
- Diabetes Metab J. 2023;47(6):826-836. Published online August 24, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0273

- 1,802 View
- 191 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There was limited evidence to evaluate the association between lifestyle habits and continuous glucose monitoring (CGM) metrics. Thus, we aimed to depict the behavioral and metabolic determinants of CGM metrics in insulin-treated patients with type 2 diabetes mellitus (T2DM).
Methods
This is a prospective observational study. We analyzed data from 122 insulin-treated patients with T2DM. Participants wore Dexcom G6 and Fitbit, and diet information was identified for 10 days. Multivariate-adjusted logistic regression analysis was performed for the simultaneous achievement of CGM-based targets, defined by the percentage of time in terms of hyper, hypoglycemia and glycemic variability (GV). Intake of macronutrients and fiber, step counts, sleep, postprandial C-peptide-to-glucose ratio (PCGR), information about glucose lowering medications and metabolic factors were added to the analyses. Additionally, we evaluated the impact of the distribution of energy and macronutrient during a day, and snack consumption on CGM metrics.
Results
Logistic regression analysis revealed that female, participants with high PCGR, low glycosylated hemoglobin (HbA1c) and daytime step count had a higher probability of achieving all targets based on CGM (odds ratios [95% confidence intervals] which were 0.24 [0.09 to 0.65], 1.34 [1.03 to 1.25], 0.95 [0.9 to 0.99], and 1.15 [1.03 to 1.29], respectively). And participants who ate snacks showed a shorter period of hyperglycemia and less GV compared to those without.
Conclusion
We confirmed that residual insulin secretion, daytime step count, HbA1c, and women were the most relevant determinants of adequate glycemic control in insulin-treated patients with T2DM. In addition, individuals with snack consumption were exposed to lower times of hyperglycemia and GV. -
Citations
Citations to this article as recorded by- Explanatory variables of objectively measured 24-h movement behaviors in people with prediabetes and type 2 diabetes: A systematic review
Lotte Bogaert, Iris Willems, Patrick Calders, Eveline Dirinck, Manon Kinaupenne, Marga Decraene, Bruno Lapauw, Boyd Strumane, Margot Van Daele, Vera Verbestel, Marieke De Craemer
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2024; 18(4): 102995. CrossRef
- Explanatory variables of objectively measured 24-h movement behaviors in people with prediabetes and type 2 diabetes: A systematic review
- Drug/Regimen
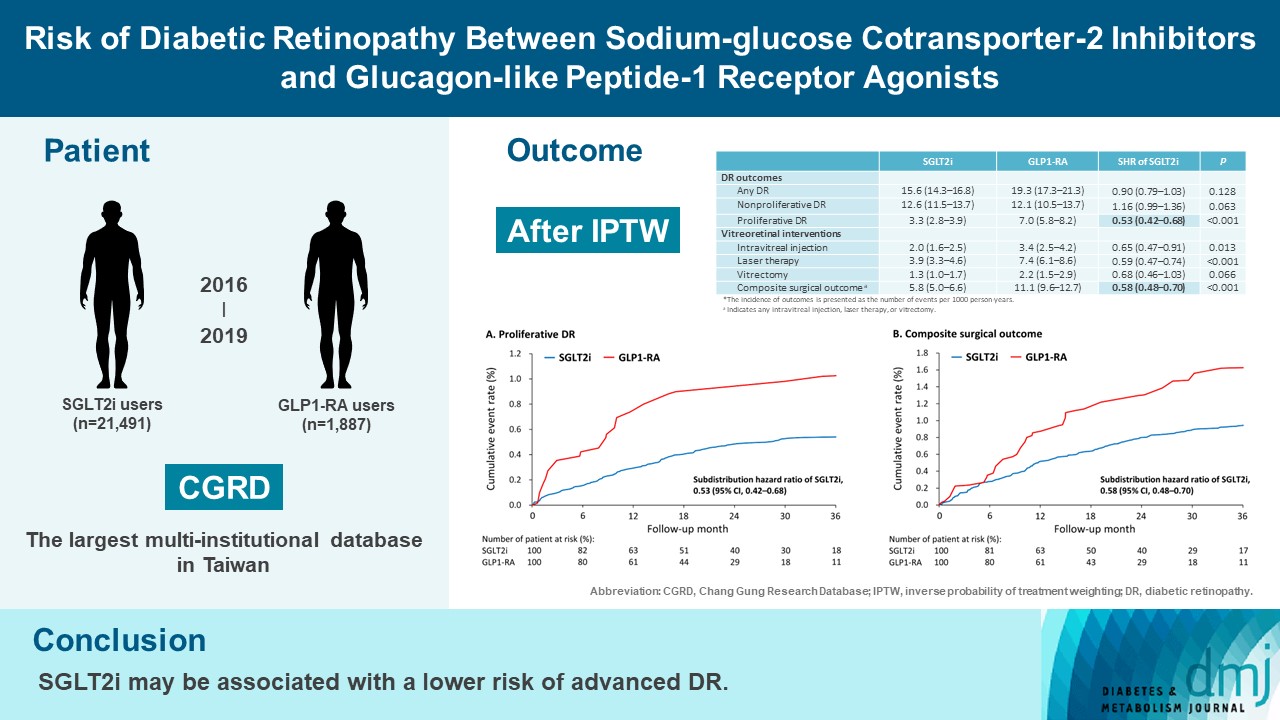
- Risk of Diabetic Retinopathy between Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists
- Tzu-Yi Lin, Eugene Yu-Chuan Kang, Shih-Chieh Shao, Edward Chia-Cheng Lai, Sunir J. Garg, Kuan-Jen Chen, Je-Ho Kang, Wei-Chi Wu, Chi-Chun Lai, Yih-Shiou Hwang
- Diabetes Metab J. 2023;47(3):394-404. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0221
- 6,567 View
- 271 Download
- 7 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To compare risk of diabetic retinopathy (DR) between patients taking sodium-glucose cotransporter-2 inhibitors (SGLT2is) and those taking glucagon-like peptide-1 receptor agonists (GLP1-RAs) in routine care.
Methods
This retrospective cohort study emulating a target trial included patient data from the multi-institutional Chang Gung Research Database in Taiwan. Totally, 33,021 patients with type 2 diabetes mellitus using SGLT2is and GLP1-RAs between 2016 and 2019 were identified. 3,249 patients were excluded due to missing demographics, age <40 years, prior use of any study drug, a diagnosis of retinal disorders, a history of receiving vitreoretinal procedure, no baseline glycosylated hemoglobin, or no follow-up data. Baseline characteristics were balanced using inverse probability of treatment weighting with propensity scores. DR diagnoses and vitreoretinal interventions served as the primary outcomes. Occurrence of proliferative DR and DR receiving vitreoretinal interventions were regarded as vision-threatening DR.
Results
There were 21,491 SGLT2i and 1,887 GLP1-RA users included for the analysis. Patients receiving SGLT2is and GLP-1 RAs exhibited comparable rate of any DR (subdistribution hazard ratio [SHR], 0.90; 95% confidence interval [CI], 0.79 to 1.03), whereas the rate of proliferative DR (SHR, 0.53; 95% CI, 0.42 to 0.68) was significantly lower in the SGLT2i group. Also, SGLT2i users showed significantly reduced risk of composite surgical outcome (SHR, 0.58; 95% CI, 0.48 to 0.70).
Conclusion
Compared to those taking GLP1-RAs, patients receiving SGLT2is had a lower risk of proliferative DR and vitreoretinal interventions, although the rate of any DR was comparable between the SGLT2i and GLP1-RA groups. Thus, SGLT2is may be associated with a lower risk of vision-threatening DR but not DR development. -
Citations
Citations to this article as recorded by- Incretin‐based drugs and the risk of diabetic retinopathy among individuals with type 2 diabetes: A systematic review and meta‐analysis of observational studies
Samuel Igweokpala, Naheemot Olaoluwa Sule, Antonios Douros, Oriana H. Y. Yu, Kristian B. Filion
Diabetes, Obesity and Metabolism.2024; 26(2): 721. CrossRef - Association of sodium–glucose cotransporter‐2 inhibitors and the risk of retinal vascular occlusion: A real‐world retrospective cohort study in Taiwan
Tzu‐Yi Lin, Eugene Yu‐Chuan Kang, Shih‐Chieh Shao, Edward Chia‐Cheng Lai, Nan‐Kai Wang, Sunir J. Garg, Kuan‐Jen Chen, Je‐Ho Kang, Wei‐Chi Wu, Chi‐Chun Lai, Yih‐Shiou Hwang
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - Risk of rotator cuff tear and rotator cuff repair surgery comparison between sodium-glucose cotransporter 2 inhibitors and glucagon like peptide-1 receptor agonists: A real-world study
Yu-Chi Su, Pei-Chun Hsieh, Edward Chia-Cheng Lai, Yu-Ching Lin
Diabetes & Metabolism.2024; 50(2): 101522. CrossRef - Optimising renal risk parameters in type 2 diabetes mellitus: Perspectives from a retinal viewpoint
Sarita Jacob, George I. Varughese
Clinical Medicine.2024; 24(2): 100031. CrossRef - Risk of diabetic retinopathy and diabetic macular oedema with sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in type 2 diabetes: a real-world data study from a global federated database
Aikaterini Eleftheriadou, David Riley, Sizheng S. Zhao, Philip Austin, Gema Hernández, Gregory Y. H. Lip, Timothy L. Jackson, John P. H. Wilding, Uazman Alam
Diabetologia.2024;[Epub] CrossRef - Impact of GLP-1 Agonists and SGLT-2 Inhibitors on Diabetic Retinopathy Progression: An Aggregated Electronic Health Record Data Study
Karen M. Wai, Kapil Mishra, Euna Koo, Cassie Ann Ludwig, Ravi Parikh, Prithvi Mruthyunjaya, Ehsan Rahimy
American Journal of Ophthalmology.2024;[Epub] CrossRef - Risk of Diabetic Retinopathy between Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists (Diabetes Metab J 2023;47:394-404)
Tzu-Yi Lin, Eugene Yu-Chuan Kang, Shih-Chieh Shao, Edward Chia-Cheng Lai, Yih-Shiou Hwang
Diabetes & Metabolism Journal.2023; 47(4): 573. CrossRef - Risk of Diabetic Retinopathy between Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists (Diabetes Metab J 2023;47:394-404)
Jihee Ko, Sun Joon Moon
Diabetes & Metabolism Journal.2023; 47(4): 571. CrossRef - Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Retinopathy in Patients With Type 2 Diabetes
Fu-Shun Yen, James Cheng-Chung Wei, Teng-Shun Yu, Yu-Tung Hung, Chih-Cheng Hsu, Chii-Min Hwu
JAMA Network Open.2023; 6(12): e2348431. CrossRef
- Incretin‐based drugs and the risk of diabetic retinopathy among individuals with type 2 diabetes: A systematic review and meta‐analysis of observational studies
- Technology/Device
- Glycemia according to the Use of Continuous Glucose Monitoring among Adults with Type 1 Diabetes Mellitus in Korea: A Real-World Study
- You-Bin Lee, Minjee Kim, Jae Hyeon Kim
- Diabetes Metab J. 2023;47(3):405-414. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0032

- 3,274 View
- 122 Download
- 2 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We explored the association between continuous glucose monitoring (CGM) use and glycemia among adults with type 1 diabetes mellitus (T1DM) and determined the status of CGM metrics among adults with T1DM using CGM in the real-world.
Methods
For this propensity-matched cross-sectional study, individuals with T1DM who visited the outpatient clinic of the Endocrinology Department of Samsung Medical Center between March 2018 and February 2020 were screened. Among them, 111 CGM users (for ≥9 months) were matched based on propensity score considering age, sex, and diabetes duration in a 1:2 ratio with 203 CGM never-users. The association between CGM use and glycemic measures was explored. In a subpopulation of CGM users who had been using official applications (not “do-it-yourself” software) such that Ambulatory Glucose Profile data for ≥1 month were available (n=87), standardized CGM metrics were summarized.
Results
Linear regression analyses identified CGM use as a determining factor for log-transformed glycosylated hemoglobin. The fully-adjusted odds ratio (OR) and 95% confidence interval (CI) for uncontrolled glycosylated hemoglobin (>8%) were 0.365 (95% CI, 0.190 to 0.703) in CGM users compared to never-users. The fully-adjusted OR for controlled glycosylated hemoglobin (<7%) was 1.861 (95% CI, 1.119 to 3.096) in CGM users compared to never-users. Among individuals who had been using official applications for CGM, time in range (TIR) values within recent 30- and 90-day periods were 62.45%±16.63% and 63.08%±15.32%, respectively.
Conclusion
CGM use was associated with glycemic control status among Korean adults with T1DM in the real-world, although CGM metrics including TIR might require further improvement among CGM users. -
Citations
Citations to this article as recorded by- Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System
Kyung-Soo Kim, Seung-Hwan Lee, Won Sang Yoo, Cheol-Young Park
Diabetes Technology & Therapeutics.2024; 26(4): 222. CrossRef - Navigating the Seas of Glycemic Control: The Role of Continuous Glucose Monitoring in Type 1 Diabetes Mellitus
Jun Sung Moon
Diabetes & Metabolism Journal.2023; 47(3): 345. CrossRef - Smart Insulin Pen: Managing Insulin Therapy for People with Diabetes in the Digital Era
Jee Hee Yoo, Jae Hyeon Kim
The Journal of Korean Diabetes.2023; 24(4): 190. CrossRef
- Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
- Drug Regimen
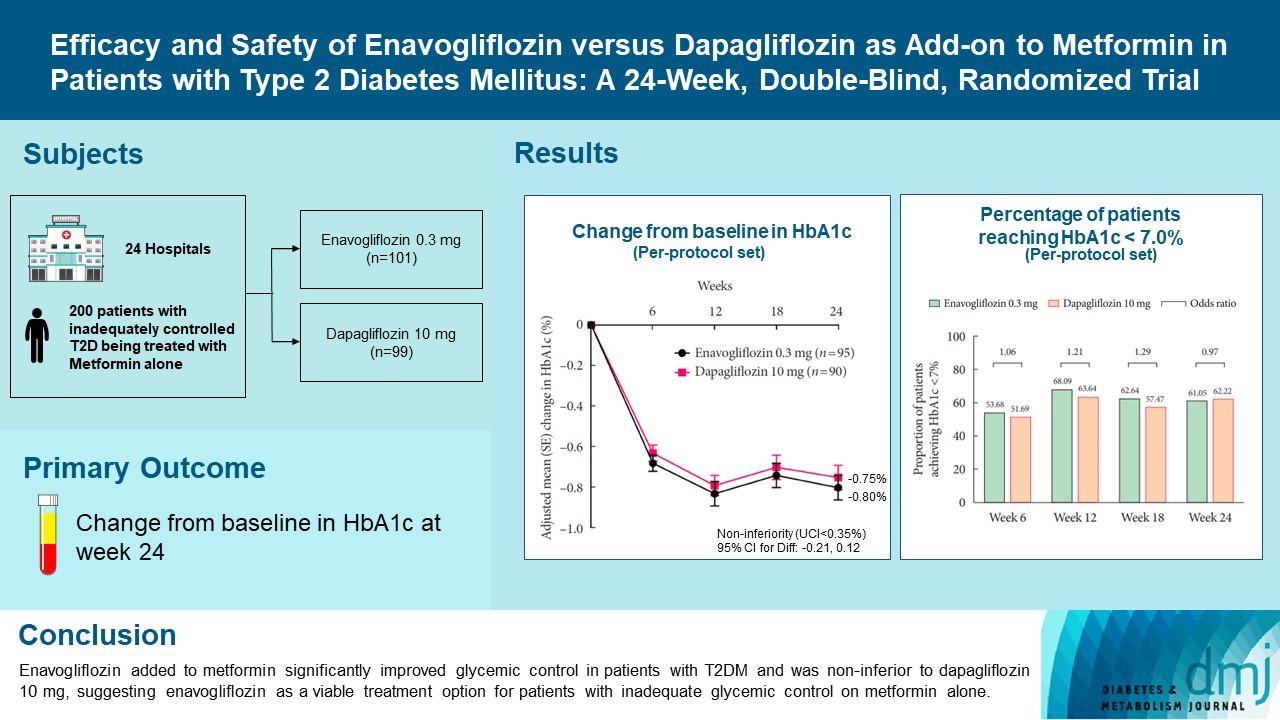
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Kyung Ah Han, Yong Hyun Kim, Doo Man Kim, Byung Wan Lee, Suk Chon, Tae Seo Sohn, In Kyung Jeong, Eun-Gyoung Hong, Jang Won Son, Jae Jin Nah, Hwa Rang Song, Seong In Cho, Seung-Ah Cho, Kun Ho Yoon
- Diabetes Metab J. 2023;47(6):796-807. Published online February 9, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0315
- 40,064 View
- 572 Download
- 4 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Enavogliflozin is a novel sodium-glucose cotransporter-2 inhibitor currently under clinical development. This study evaluated the efficacy and safety of enavogliflozin as an add-on to metformin in Korean patients with type 2 diabetes mellitus (T2DM) against dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, 200 patients were randomized to receive enavogliflozin 0.3 mg/day (n=101) or dapagliflozin 10 mg/day (n=99) in addition to ongoing metformin therapy for 24 weeks. The primary objective of the study was to prove the non-inferiority of enavogliflozin to dapagliflozin in glycosylated hemoglobin (HbA1c) change at week 24 (non-inferiority margin of 0.35%) (Clinical trial registration number: NCT04634500).
Results
Adjusted mean change of HbA1c at week 24 was –0.80% with enavogliflozin and –0.75% with dapagliflozin (difference, –0.04%; 95% confidence interval, –0.21% to 0.12%). Percentages of patients achieving HbA1c <7.0% were 61% and 62%, respectively. Adjusted mean change of fasting plasma glucose at week 24 was –32.53 and –29.14 mg/dL. An increase in urine glucose-creatinine ratio (60.48 vs. 44.94, P<0.0001) and decrease in homeostasis model assessment of insulin resistance (–1.85 vs. –1.31, P=0.0041) were significantly greater with enavogliflozin than dapagliflozin at week 24. Beneficial effects of enavogliflozin on body weight (–3.77 kg vs. –3.58 kg) and blood pressure (systolic/diastolic, –5.93/–5.41 mm Hg vs. –6.57/–4.26 mm Hg) were comparable with those of dapagliflozin, and both drugs were safe and well-tolerated.
Conclusion
Enavogliflozin added to metformin significantly improved glycemic control in patients with T2DM and was non-inferior to dapagliflozin 10 mg, suggesting enavogliflozin as a viable treatment option for patients with inadequate glycemic control on metformin alone. -
Citations
Citations to this article as recorded by- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Young Sang Lyu, Sangmo Hong, Si Eun Lee, Bo Young Cho, Cheol-Young Park
Cardiovascular Diabetology.2024;[Epub] CrossRef - A 52‐week efficacy and safety study of enavogliflozin versus dapagliflozin as an add‐on to metformin in patients with type 2 diabetes mellitus: ENHANCE‐M extension study
Tae Seo Sohn, Kyung‐Ah Han, Yonghyun Kim, Byung‐Wan Lee, Suk Chon, In‐Kyung Jeong, Eun‐Gyoung Hong, Jang Won Son, JaeJin Na, Jae Min Cho, Seong In Cho, Wan Huh, Kun‐Ho Yoon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - The effect of renal function on the pharmacokinetics and pharmacodynamics of enavogliflozin, a potent and selective sodium‐glucose cotransporter‐2 inhibitor, in type 2 diabetes
Sae Im Jeong, Mu Seong Ban, Jun‐Gi Hwang, Min‐Kyu Park, Soo Lim, Sejoong Kim, Soon Kil Kwon, Yoonjin Kim, Jae Min Cho, Jae Jin Na, Wan Huh, Jae‐Yong Chung
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: A systematic review and meta-analysis
Deep Dutta, B.G. Harish, Beatrice Anne, Lakshmi Nagendra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102816. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - Prospects of using sodium-glucose co-transporter-2 (SGLT-2) inhibitors in patients with metabolic-associated fatty liver disease (MAFLD)
Iryna Kostitska, Nadia Protas, Liliia Petrovska
Diabetes Obesity Metabolic Syndrome.2023; (5): 8. CrossRef - Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
Sang Youl Rhee
Diabetes & Metabolism Journal.2023; 47(6): 769. CrossRef
- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Reviews
- Technology/Device
- Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
- Jee Hee Yoo, Jae Hyeon Kim
- Diabetes Metab J. 2023;47(1):27-41. Published online January 12, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0271

- 6,249 View
- 385 Download
- 11 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Continuous glucose monitoring (CGM) technology has evolved over the past decade with the integration of various devices including insulin pumps, connected insulin pens (CIPs), automated insulin delivery (AID) systems, and virtual platforms. CGM has shown consistent benefits in glycemic outcomes in type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) treated with insulin. Moreover, the combined effect of CGM and education have been shown to improve glycemic outcomes more than CGM alone. Now a CIP is the expected future technology that does not need to be worn all day like insulin pumps and helps to calculate insulin doses with a built-in bolus calculator. Although only a few clinical trials have assessed the effectiveness of CIPs, they consistently show benefits in glycemic outcomes by reducing missed doses of insulin and improving problematic adherence. AID systems and virtual platforms made it possible to achieve target glycosylated hemoglobin in diabetes while minimizing hypoglycemia, which has always been challenging in T1DM. Now fully automatic AID systems and tools for diabetes decisions based on artificial intelligence are in development. These advances in technology could reduce the burden associated with insulin treatment for diabetes.
-
Citations
Citations to this article as recorded by- Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System
Kyung-Soo Kim, Seung-Hwan Lee, Won Sang Yoo, Cheol-Young Park
Diabetes Technology & Therapeutics.2024; 26(4): 222. CrossRef - Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Recent advances in the precision control strategy of artificial pancreas
Wuyi Ming, Xudong Guo, Guojun Zhang, Yinxia Liu, Yongxin Wang, Hongmei Zhang, Haofang Liang, Yuan Yang
Medical & Biological Engineering & Computing.2024;[Epub] CrossRef - Digital Health in Diabetes and Cardiovascular Disease
Dorothy Avoke, Abdallah Elshafeey, Robert Weinstein, Chang H. Kim, Seth S. Martin
Endocrine Research.2024; : 1. CrossRef - Continuous glucose monitoring with structured education in adults with type 2 diabetes managed by multiple daily insulin injections: a multicentre randomised controlled trial
Ji Yoon Kim, Sang-Man Jin, Kang Hee Sim, Bo-Yeon Kim, Jae Hyoung Cho, Jun Sung Moon, Soo Lim, Eun Seok Kang, Cheol-Young Park, Sin Gon Kim, Jae Hyeon Kim
Diabetologia.2024;[Epub] CrossRef - Glycemic Outcomes During Early Use of the MiniMed™ 780G Advanced Hybrid Closed-Loop System with Guardian™ 4 Sensor
Toni L. Cordero, Zheng Dai, Arcelia Arrieta, Fang Niu, Melissa Vella, John Shin, Andrew S. Rhinehart, Jennifer McVean, Scott W. Lee, Robert H. Slover, Gregory P. Forlenza, Dorothy I. Shulman, Rodica Pop-Busui, James R. Thrasher, Mark S. Kipnes, Mark P. Ch
Diabetes Technology & Therapeutics.2023; 25(9): 652. CrossRef - Navigating the Seas of Glycemic Control: The Role of Continuous Glucose Monitoring in Type 1 Diabetes Mellitus
Jun Sung Moon
Diabetes & Metabolism Journal.2023; 47(3): 345. CrossRef - APSec1.0: Innovative Security Protocol Design with Formal Security Analysis for the Artificial Pancreas System
Jiyoon Kim, Jongmin Oh, Daehyeon Son, Hoseok Kwon, Philip Virgil Astillo, Ilsun You
Sensors.2023; 23(12): 5501. CrossRef - Advances and Development of Electronic Neural Interfaces
Xue Jiaxiang, Liu Zhixin
Journal of Computing and Natural Science.2023; : 147. CrossRef - Continuous Glucose Monitoring (CGM) and Metabolic Control in a Cohort of Patients with Type 1 Diabetes and Coeliac Disease
Flavia Amaro, Maria Alessandra Saltarelli, Marina Primavera, Marina Cerruto, Stefano Tumini
Endocrines.2023; 4(3): 595. CrossRef - Comparison of Glycemia Risk Index with Time in Range for Assessing Glycemic Quality
Ji Yoon Kim, Jee Hee Yoo, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2023; 25(12): 883. CrossRef - The Benefits Of Continuous Glucose Monitoring In Pregnancy
Jee Hee Yoo, Jae Hyeon Kim
Endocrinology and Metabolism.2023; 38(5): 472. CrossRef - The Growing Challenge of Diabetes Management in an Aging Society
Seung-Hwan Lee
Diabetes & Metabolism Journal.2023; 47(5): 630. CrossRef - Recent advances in artificial intelligence-assisted endocrinology and diabetes
Ioannis T. Oikonomakos, Ranjit M. Anjana, Viswanathan Mohan, Charlotte Steenblock, Stefan R. Bornstein
Exploration of Endocrine and Metabolic Disease.2023; 1(1): 16. CrossRef - An Observational Pilot Study of a Tailored Environmental Monitoring and Alert System for Improved Management of Chronic Respiratory Diseases
Mohammed Alotaibi, Fady Alnajjar, Badr A Alsayed, Tareq Alhmiedat, Ashraf M Marei, Anas Bushnag, Luqman Ali
Journal of Multidisciplinary Healthcare.2023; Volume 16: 3799. CrossRef - Smart Insulin Pen: Managing Insulin Therapy for People with Diabetes in the Digital Era
Jee Hee Yoo, Jae Hyeon Kim
The Journal of Korean Diabetes.2023; 24(4): 190. CrossRef
- Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System
- Pathophysiology
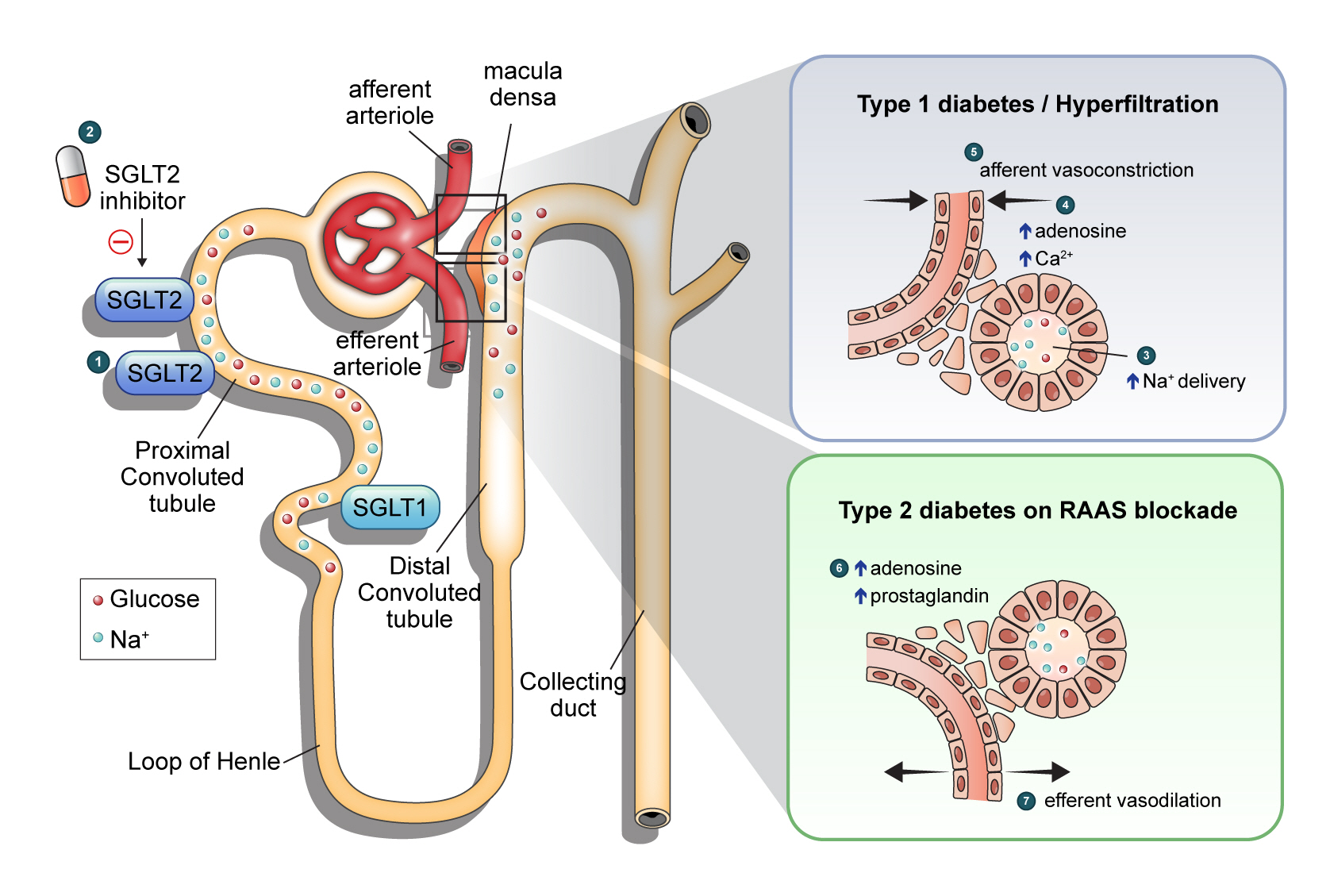
- Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
- Nam Hoon Kim, Nan Hee Kim
- Diabetes Metab J. 2022;46(4):543-551. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0209
- 6,399 View
- 675 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Diabetic kidney disease (DKD) is a prevalent renal complication of diabetes mellitus that ultimately develops into end-stage kidney disease (ESKD) when not managed appropriately. Substantial risk of ESKD remains even with intensive management of hyperglycemia and risk factors of DKD and timely use of renin-angiotensin-aldosterone inhibitors. Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce hyperglycemia primarily by inhibiting glucose and sodium reabsorption in the renal proximal tubule. Currently, their effects expand to prevent or delay cardiovascular and renal adverse events, even in those without diabetes. In dedicated renal outcome trials, SGLT2 inhibitors significantly reduced the risk of composite renal adverse events, including the development of ESKD or renal replacement therapy, which led to the positioning of SGLT2 inhibitors as the mainstay of chronic kidney disease management. Multiple mechanisms of action of SGLT2 inhibitors, including hemodynamic, metabolic, and anti-inflammatory effects, have been proposed. Restoration of tubuloglomerular feedback is a plausible explanation for the alteration in renal hemodynamics induced by SGLT2 inhibition and for the associated renal benefit. This review discusses the clinical rationale and mechanism related to the protection SGLT2 inhibitors exert on the kidney, focusing on renal hemodynamic effects.
-
Citations
Citations to this article as recorded by- Using intravoxel incoherent motion imaging to evaluate uric acid-induced renal injury and efficacy after treatment
Zhong-Yuan Cheng, Shang-Ao Gong, Ping-Kang Chen, Zong-Chao Yu, Chen Qiu, Ji-Xin Lin, Jia-Bin Mo, Long Qian, You-Zhen Feng, Xiang-Ran Cai
British Journal of Radiology.2024; 97(1153): 274. CrossRef - Rethinking eGFR Comparisons in SGLT2 Inhibitor Research
Yuzuru Ohshiro
Journal of the American College of Cardiology.2024; 83(9): e87. CrossRef - SGLT2 Inhibitors and Diabetes: Where Does It Come from and Where Does It Go?
Ji Yoon Kim, Sin Gon Kim
The Journal of Korean Diabetes.2024; 25(1): 9. CrossRef - Cardiorenal outcomes and mortality after sodium‐glucose cotransporter‐2 inhibitor initiation in type 2 diabetes patients with percutaneous coronary intervention history
Jin Hwa Kim, Young Sang Lyu, BongSeong Kim, Mee Kyung Kim, Sang Yong Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Baseline eGFR, albuminuria and renal outcomes in patients with SGLT2 inhibitor treatment: an updated meta-analysis
Yunke Ma, Chu Lin, Xiaoling Cai, Suiyuan Hu, Xingyun Zhu, Fang Lv, Wenjia Yang, Linong Ji
Acta Diabetologica.2023; 60(3): 435. CrossRef - Effects of sodium-glucose cotransporter 2 inhibitors on renal risk factors in patients with abnormal glucose metabolism: a meta-analysis of randomized controlled trials
Mengnan Li, Jian Zhang, Guimei Yang, Jiaxin Zhang, Minmin Han, Yi Zhang, Yunfeng Liu
European Journal of Clinical Pharmacology.2023; 79(6): 859. CrossRef - Age at Mortality in Patients with Type 2 Diabetes Who Underwent Kidney Transplantation: An Analysis of Data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
Sun Ok Song, Eugene Han, Kang Ju Son, Bong-Soo Cha, Byung-Wan Lee
Journal of Clinical Medicine.2023; 12(9): 3160. CrossRef - Exposure–Response Analysis of the Sodium–Glucose Cotransporter-2 Inhibitors Dapagliflozin and Empagliflozin on Kidney Hemodynamics in Patients with Type 2 Diabetes
Sjoukje van der Hoek, Jeroen V. Koomen, Erik J. M. van Bommel, Charlotte M. Mosterd, Rosalie A. Scholtes, Anne C. Hesp, Jasper Stevens, Daniel H. van Raalte, Hiddo J. L. Heerspink
Journal of Personalized Medicine.2023; 13(5): 747. CrossRef - Osteopontin as a Biomarker in Chronic Kidney Disease
Satyesh K. Sinha, Michael Mellody, Maria Beatriz Carpio, Robert Damoiseaux, Susanne B. Nicholas
Biomedicines.2023; 11(5): 1356. CrossRef - Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis
Hye Jin Chun, Eun Ran Kim, Minyoung Lee, Da Hyun Choi, Soo Hyun Kim, Eugene Shin, Jin-Hong Kim, Jin Won Cho, Dai Hoon Han, Bong-Soo Cha, Yong-ho Lee
Metabolism.2023; 145: 155612. CrossRef - Synthesis and biological profile of benzoxazolone derivatives
Parteek Prasher, Tanisqa Mall, Mousmee Sharma
Archiv der Pharmazie.2023;[Epub] CrossRef - SGLT2 inhibitors prevent LPS-induced M1 macrophage polarization and alleviate inflammatory bowel disease by downregulating NHE1 expression
Ye Jin Kim, Jonghwa Jin, Dong-Ho Kim, Daehoon Kim, You Mie Lee, Jun-Kyu Byun, Yeon-Kyung Choi, Keun-Gyu Park
Inflammation Research.2023; 72(10-11): 1981. CrossRef
- Using intravoxel incoherent motion imaging to evaluate uric acid-induced renal injury and efficacy after treatment
Original Articles
- Drug/Regimen
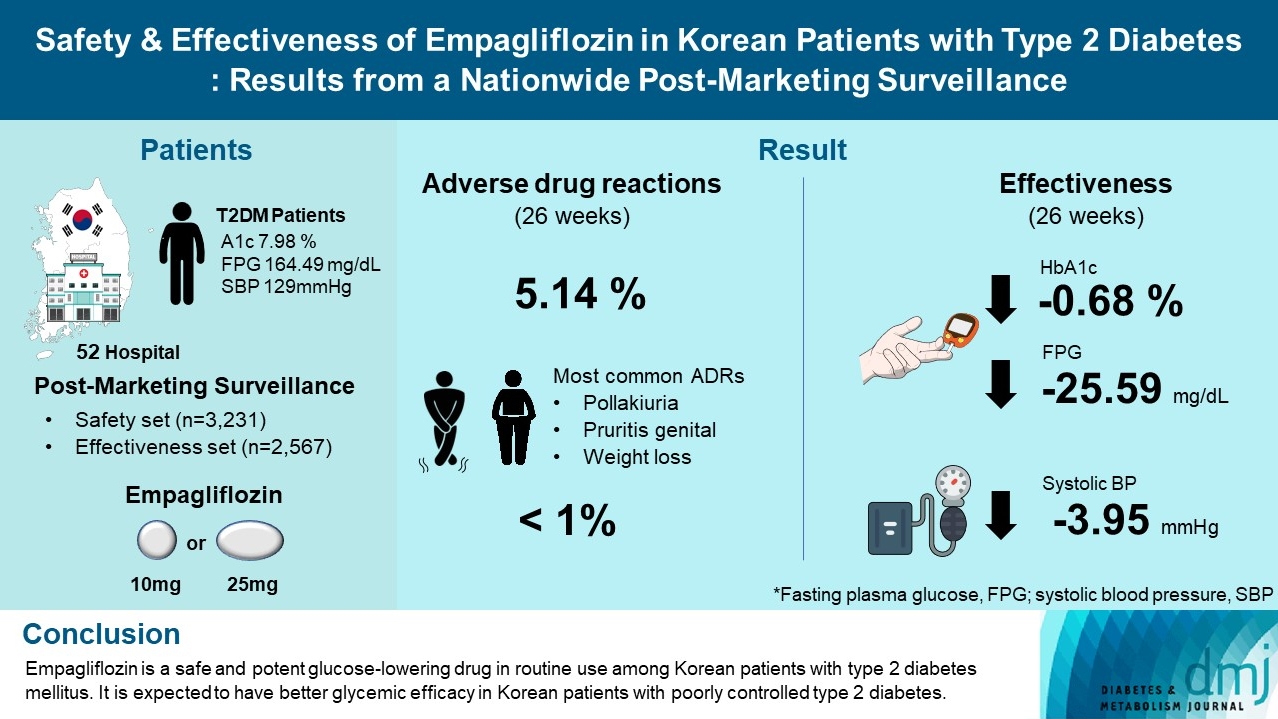
- Safety and Effectiveness of Empagliflozin in Korean Patients with Type 2 Diabetes Mellitus: Results from a Nationwide Post-Marketing Surveillance
- Jun Sung Moon, Nam Hoon Kim, Jin Oh Na, Jae Hyoung Cho, In-Kyung Jeong, Soon Hee Lee, Ji-Oh Mok, Nan Hee Kim, Dong Jin Chung, Jinhong Cho, Dong Woo Lee, Sun Woo Lee, Kyu Chang Won
- Diabetes Metab J. 2023;47(1):82-91. Published online June 20, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0356
- 5,976 View
- 295 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To evaluate the safety and effectiveness of empagliflozin in routine clinical settings, we collected and assessed the clinical profiles of Korean patients with type 2 diabetes mellitus.
Methods
This was a post-marketing surveillance study of empagliflozin 10 and 25 mg. Information on adverse events and adverse drug reactions (ADRs) was collected as safety data sets. Available effectiveness outcomes, including glycosylated hemoglobin (HbA1c) level, fasting plasma glucose, body weight, and blood pressure, were assessed.
Results
The incidence rate of ADRs was 5.14% in the safety dataset (n=3,231). Pollakiuria, pruritis genital, and weight loss were the most common ADRs. ADRs of special interest accounted for only 1.18%, and there were no serious events that led to mortality or hospitalization. In the effectiveness data set (n=2,567), empagliflozin significantly reduced the mean HbA1c level and body weight during the study period by –0.68%±1.39% and –1.91±3.37 kg (both P<0.0001), respectively. In addition, shorter disease duration, absence of dyslipidemia, and higher baseline HbA1c levels were identified as the clinical features characteristic of a “responder” to empagliflozin therapy.
Conclusion
Empagliflozin is a safe and potent glucose-lowering drug in routine use among Korean patients with type 2 diabetes mellitus. It is expected to have better glycemic efficacy in Korean patients with poorly controlled type 2 diabetes mellitus. -
Citations
Citations to this article as recorded by- Evaluation of Efficacy and Safety of Empagliflozin in Bangladeshi Patients with Type 2 Diabetes Mellitus (EFFISAEM Study)
Mohammad Saifuddin, Ajit Kumar Paul, Sultana Marufa Shefin, Md. Jahangir Alam, Shahjada Selim, Sunjida Islam, Tanjina Hossain, Sadiqa Tuqan, Nusrat Sultana, Marufa Mustari, Ramen Chandra Basak, Kazi Ali Aftab, Indrajit Prasad, Mohammad Rafiq Uddin, Shoma
Indian Journal of Endocrinology and Metabolism.2024;[Epub] CrossRef - Comparison of the Pharmacokinetics, Safety, and Tolerability of Two Empagliflozin Formulations in Healthy Korean Subjects
Xu Jiang, Sungyeun Bae, Deok Yong Yoon, Shin Jung Park, Jaeseong Oh, Joo-Youn Cho, Kyung-Sang Yu
Drug Design, Development and Therapy.2023; Volume 17: 2137. CrossRef - Comparative safety of different sodium-glucose transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials
Chun Xing Li, Li Yan Liu, Chen Xiao Zhang, Xu Hua Geng, Si Meng Gu, Yu Qiao Wang, Hua Liu, Qing Xie, Shuo Liang
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Evaluation of Efficacy and Safety of Empagliflozin in Bangladeshi Patients with Type 2 Diabetes Mellitus (EFFISAEM Study)
- Drug/Regimen
- Real-World Prescription Patterns and Barriers Related to the Use of Sodium-Glucose Cotransporter 2 Inhibitors among Korean Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease
- Jong Ha Baek, Ye Seul Yang, Seung-Hyun Ko, Kyung Do Han, Jae Hyeon Kim, Min Kyong Moon, Jong Suk Park, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Jong Han Choi, Kyu Yeon Hur, Committee of Clinical Practice Guidelines, Korean Diabetes Association
- Diabetes Metab J. 2022;46(5):701-712. Published online June 3, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0002

- 4,929 View
- 319 Download
- 6 Web of Science
- 8 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
To evaluate prescription trends and clinical factors of the sodium-glucose cotransporter 2 inhibitors (SGLT2i) use according to the presence of atherosclerotic cardiovascular disease (ASCVD) or heart failure (HF) in Korean patients with type 2 diabetes mellitus (T2DM).
Methods
Prescription patterns of SGLT2i use between 2015 and 2019 were determined using the Korean National Health Insurance Service database of claims.
Results
Of all patients with T2DM (n=4,736,493), the annual prescription rate of SGLT2i increased every year in patients with ASCVD (from 2.2% to 10.7%) or HF (from 2.0% to 11.1%). After the first hospitalization for ASCVD (n=518,572), 13.7% (n=71,259) of patients initiated SGLT2i with a median of 10.6 months. After hospitalization for HF (n=372,853), 11.2% (n=41,717) of patients initiated SGLT2i after a median of 8.8 months. In multivariate regression for hospitalization, older age (per 10 years, odds ratio [OR], 0.57; 95% confidence interval [CI], 0.56 to 0.57), lower household income (OR, 0.93; 95% CI, 0.92 to 0.95), rural residents (OR, 0.95; 95% CI, 0.93 to 0.97), and dipeptidyl peptidase-4 inhibitor (DPP-4i) users (OR, 0.82; 95% CI, 0.81 to 0.84) were associated with lesser initiation of SGLT2i in ASCVD. Additionally, female gender (OR, 0.97; 95% CI, 0.95 to 0.99) was associated with lesser initiation of SGLT2i in HF.
Conclusion
The prescription rate of SGLT2i increased gradually up to 2019 but was suboptimal in patients with ASCVD or HF. After the first hospitalization for ASCVD or HF, older age, female gender, low household income, rural residents, and DPP-4i users were less likely to initiate SGLT2i. -
Citations
Citations to this article as recorded by- Effectiveness and safety of sodium–glucose cotransporter 2 inhibitors in Asian populations
Kyoung Hwa Ha, Dae Jung Kim
Journal of Diabetes Investigation.2024; 15(3): 285. CrossRef - Real-World Treatment Patterns according to Clinical Practice Guidelines in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease in Korea: Multicenter, Retrospective, Observational Study
Ye Seul Yang, Nam Hoon Kim, Jong Ha Baek, Seung-Hyun Ko, Jang Won Son, Seung-Hwan Lee, Sang Youl Rhee, Soo-Kyung Kim, Tae Seo Sohn, Ji Eun Jun, In-Kyung Jeong, Chong Hwa Kim, Keeho Song, Eun-Jung Rhee, Junghyun Noh, Kyu Yeon Hur
Diabetes & Metabolism Journal.2024; 48(2): 279. CrossRef - Hospital Readmissions for Fluid Overload among Individuals with Diabetes and Diabetic Kidney Disease: Risk Factors and Multivariable Prediction Models
Jiashen Cai, Dorothy Huang, Hanis Binte Abdul Kadir, Zhihua Huang, Li Choo Ng, Andrew Ang, Ngiap Chuan Tan, Yong Mong Bee, Wei Yi Tay, Chieh Suai Tan, Cynthia C. Lim
Nephron.2024; : 1. CrossRef - Prescribing patterns of SGLT-2 inhibitors for patients with heart failure: A two-center analysis
Teja Chakrala, Roshni O. Prakash, Justin Kim, Hanzhi Gao, Umar Ghaffar, Jaymin Patel, Alex Parker, Bhagwan Dass
American Heart Journal Plus: Cardiology Research and Practice.2023; 28: 100286. CrossRef - Risk of developing chronic kidney disease in young-onset Type 2 diabetes in Korea
Joonyub Lee, Seung-Hwan Lee, Kun-Ho Yoon, Jae Hyoung Cho, Kyungdo Han, Yeoree Yang
Scientific Reports.2023;[Epub] CrossRef - Comparison of SGLT2 inhibitors with DPP-4 inhibitors combined with metformin in patients with acute myocardial infarction and diabetes mellitus
Young Sang Lyu, Seok Oh, Jin Hwa Kim, Sang Yong Kim, Myung Ho Jeong
Cardiovascular Diabetology.2023;[Epub] CrossRef - Severe hypoglycemia as a risk factor for cardiovascular outcomes in patients with type 2 diabetes: is it preventable?
Seung-Hyun Ko
Cardiovascular Prevention and Pharmacotherapy.2022; 4(3): 106. CrossRef - Association between the Diabetes Drug Cost and Cardiovascular Events and Death in Korea: A National Health Insurance Service Database Analysis
Seung Min Chung, Ji-In Lee, Eugene Han, Hyun-Ae Seo, Eonju Jeon, Hye Soon Kim, Ji Sung Yoon
Endocrinology and Metabolism.2022; 37(5): 759. CrossRef
- Effectiveness and safety of sodium–glucose cotransporter 2 inhibitors in Asian populations
Review
- Guideline/Fact Sheet
- Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension
- Jong Han Choi, Jee-Hyun Kang, Suk Chon
- Diabetes Metab J. 2022;46(3):377-390. Published online May 25, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0051
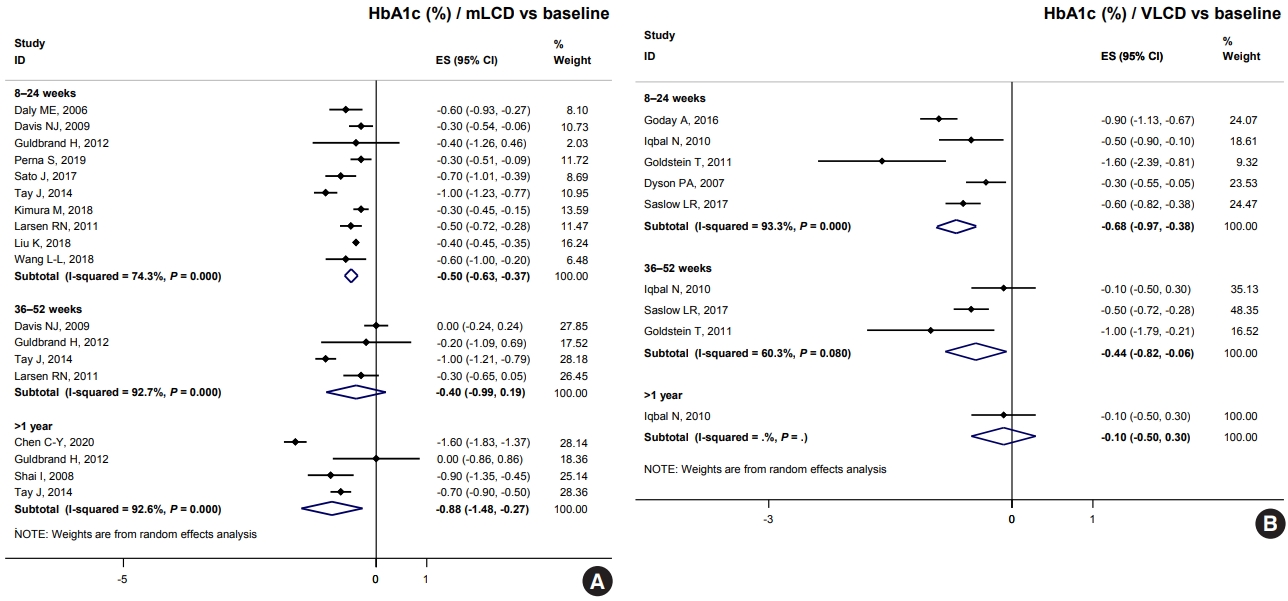
- 4,955 View
- 249 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The Joint Committee of the Korean Diabetes Association, the Korean Society for the Study of Obesity, and the Korean Society of Hypertension announced a consensus statement on carbohydrate-restricted diets and intermittent fasting, representing an emerging and popular dietary pattern. In this statement, we recommend moderately-low-carbohydrate or low-carbohydrate diets, not a very-low-carbohydrate diet, for patients with type 2 diabetes mellitus. These diets can be considered a dietary regimen to improve glycemic control and reduce body weight in adults with type 2 diabetes mellitus. This review provides the detailed results of a meta-analysis and systematic literature review on the potential harms and benefits of carbohydrate-restricted diets in patients with diabetes. We expect that this review will help experts and patients by fostering an in-depth understanding and appropriate application of carbohydrate-restricted diets in the comprehensive management of diabetes.
-
Citations
Citations to this article as recorded by- Efficacy of convenience meal-type foods designed for diabetes in the management of metabolic syndrome based on a 3-week trial
Do Gyeong Lee, In Gyeong Kang, Tae Seok Kim, Yun Ahn, Sang Yun Lee, Hye Jin Ahn, Yoo Kyoung Park
Nutrition.2024; 118: 112287. CrossRef - Long-Term Results of a Digital Diabetes Self-Management and Education Support Program Among Adults With Type 2 Diabetes: A Retrospective Cohort Study
Ashley Berthoumieux, Sarah Linke, Melinda Merry, Alison Megliola, Jessie Juusola, Jenna Napoleone
The Science of Diabetes Self-Management and Care.2024; 50(1): 19. CrossRef - Medical nutrition therapy for diabetes mellitus
Suk Chon
Journal of the Korean Medical Association.2023; 66(7): 421. CrossRef
- Efficacy of convenience meal-type foods designed for diabetes in the management of metabolic syndrome based on a 3-week trial

 KDA
KDA
 First
First Prev
Prev





