
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(1); 2023 > Article
-
ReviewTechnology/Device Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
-
Jee Hee Yoo1,2
 , Jae Hyeon Kim2,3
, Jae Hyeon Kim2,3
-
Diabetes & Metabolism Journal 2023;47(1):27-41.
DOI: https://doi.org/10.4093/dmj.2022.0271
Published online: January 12, 2023
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
2Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
3Department of Clinical Research Design and Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Korea
- Corresponding author: Jae Hyeon Kim https://orcid.org/0000-0001-5001-963X Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea E-mail: jaehyeon@skku.edu
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Continuous glucose monitoring (CGM) technology has evolved over the past decade with the integration of various devices including insulin pumps, connected insulin pens (CIPs), automated insulin delivery (AID) systems, and virtual platforms. CGM has shown consistent benefits in glycemic outcomes in type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) treated with insulin. Moreover, the combined effect of CGM and education have been shown to improve glycemic outcomes more than CGM alone. Now a CIP is the expected future technology that does not need to be worn all day like insulin pumps and helps to calculate insulin doses with a built-in bolus calculator. Although only a few clinical trials have assessed the effectiveness of CIPs, they consistently show benefits in glycemic outcomes by reducing missed doses of insulin and improving problematic adherence. AID systems and virtual platforms made it possible to achieve target glycosylated hemoglobin in diabetes while minimizing hypoglycemia, which has always been challenging in T1DM. Now fully automatic AID systems and tools for diabetes decisions based on artificial intelligence are in development. These advances in technology could reduce the burden associated with insulin treatment for diabetes.
- Achieving target glycosylated hemoglobin (HbA1c) while minimizing hypoglycemia has always been a challenge for diabetes treated with insulin [1]. However, technological advances including continuous glucose monitoring (CGM), connected insulin pens (CIPs), insulin pumps, and automated insulin delivery (AID) algorithms combined with education have made it possible to achieve the optimal control of diabetes using insulin (Fig. 1) [2,3]. In addition, cloud-based data collection programs can support diabetes and healthcare providers (HCPs) to make the optimal decision easily by offering various data from various devices such as glucose trend data, carbohydrate intake, insulin on board (IOB), and a bolus calculator in one platform [4]. These are of great importance in improving the glycemic status. These programs can even help to support decision-making by artificial intelligence (AI) algorithms [5].
- We review the detailed information on present and future expectations for CGM, CIPs, AID systems, and virtual platforms. In addition, we report how they affect glycemic outcomes while minimizing the burden of diabetes management and demonstrate that the greatest benefit occurs when coupled with structured education. We especially focused on CGMs and the new emergence of CIPs. Only the CGM and devices integrated with CGM are outlined here.
INTRODUCTION
- Present CGM systems
- The first CGM system was approved by the U.S. Food and Drug Administration (FDA) in 1999. The glucose data in this system was blinded to patients or HCP [6]. As the glucose levels were not available in real-time, the data were analyzed retrospectively after the use of CGM. Further, the data was only collected for 3 days with poor accuracy. Because of this limited accuracy, the early CGM was approved as an adjuvant to self-monitoring of blood glucose (SMBG) to reduce the number of fingerstick measurements.
- However, the Dexcom G5 (Dexcom, San Diego, CA, USA) reached an accuracy of 9.0% mean absolute relative difference (MARD) similar to the glucose meter devices of MARD below 10%. Thus, in 2016, the FDA approved this as a non-adjunctive device that could replace fingerstick glucose monitoring when making treatment decisions [7,8]. This approval has led to other CGMs with similar accuracy. Nowadays, some CGM devices (e.g., Dexcom G6, G7, FreeStyle Libre 1,2,3 [Abbott Diabetes Care, Alameda, CA, USA], and Guardian Sensor 4) are precalibrated in the factory, and therefore do not require calibrations to adjust sensor accuracy. In 2021, the definition of professional CGM has changed to devices that are owned and applied in the clinic, regardless of whether the data provided are blinded or unblended [9].
- With the steady improvement in sensor accuracy, duration of wear, and smaller size, the use of CGM is expanding widely. There are several CGM devices in current use, which are summarized in Table 1. The Dexcom G6 specified for 10-day wear is the first interoperable device with an AID system. It offers accurate readings during euglycemia and even in hypoglycemia without calibration (overall MARD: 10%, in hypoglycemia with glucose level <54 mg/dL and mean absolute differences: 10.8 mg/dL) [10]. However, the size (45×30×15 mm) of the device is a bit larger than FreeStyle Libre, and the transmitter and sensor are not combined but separated.
- Abbott FreeStyle Libre is an intermittent scanning CGM (isCGM). It is also known as flash glucose monitoring (FGM) which requires scanning for the storage of the glucose value measured and does not provide hypo/hyperglycemia alarms. However, with third-party transmitter devices (e.g., Miao Miao and Bubble Mini), the measured glucose value can be transferred to a smartphone and could be used like a real-time CGM (rtCGM). It is comfortable with a small size sensor (35×5 mm), could be worn up to 14-day long, and does not need calibration. The MARD was estimated to be 11.4%, slightly higher than 10.0% [11]. Fortunately, an updated algorithm applied to FreeStyle Libre 2 has been used since 2021 in it, and the MARD achieved 9.2% in adults and 9.7% in pediatrics when the sensor is applied to the upper arm [12].
- FreeStyle Libre 2 became available in 2020, it has optional alarms for hypo/hyperglycemia compared to FreeStyle Libre. With the advantage of optional alarms, users can choose to turn the alarm on or off. Another advantage is that the alarm is not consistent when once it is confirmed. Thus, it is suitable for subjects who suffer from alarm fatigue. The sensor accuracy of the MARD was improved as we mentioned above [12]. In addition, as with Dexcom G6, the device was approved as an integrated (or interoperable) CGM in August 2021. However, it is still FGM.
- The Medtronic Guardian 3 sensor (Medtronic, Northridge, CA, USA) was approved by the FDA in February 2018. However, the device still needs calibration twice a day, and can only be worn for up to 7 days [13]. The device integrates with pumps in Medtronics (Minimed 640G, and 670G) but not with others. In May 2021, Medtronics developed the Guardian 4 sensor with the same 7-day duration of wear but requiring no calibrations. The device integrates with newly developed technology, the advanced hybrid closed-loop system (HCLS) MiniMed 780G, and a CIP called InPen [14,15].
- patients who have skin reactions to adhesive [9]. With Eversense, skin eruption can be reduced by changing the siliconebased adhesive every day.
- Future CGM systems
- There are potential options for the use of CGM in the management of diabetes in the near future. Dexcom G7 received a CE mark in Europe in March 2022. Compared to the Dexcom G6, Dexcom G7 has several advantages. Dexcom G7 not only has improved in accuracy but has also improved in terms of features and size. It provides more accurate glucose readings than Dexcom G6, in that the overall MARDs for the arm and abdomen were 8.2% and 9.1%, respectively [17]. The G7 combined sensor and transmitter in a single body (all-in-one wearable) and is 60% smaller than the Dexcom G6. Skin reaction to an adhesive patch might be reduced by the smaller size of the patch area. The warm-up period was shortened to 27 minutes compared to 2 hours in G6. G7 also has advantages for those who suffer from alarm fatigue because the audible glucose alerts can be disabled for up to 6 hours. The CGM wear time was extended to 10.5 days from 10 days before. Dexcom announced that the FDA submission of the G7 CGM system may expand from a 10- to a 15-day sensor, and this might benefit the cost per device [18].
- Following the technological trend of rtCGM, Abbott launched FreeStyle Libre 3, which transitioned from isCGM to rtCGM, in March 2022. It was cleared for a CE mark in September 2020, and FDA approval in May 2022. The device is now only available in the UK at the timing of writing. Compared to FreeStyle Libre2, the size has been reduced by more than 70%. It has the same accuracy and the same length of wear time (14 days) as FreeStyle Libre 2 [12].
- Eversense E3 could be implanted for 180 days and received a CE mark in June 2020. In February 2022, the FDA also approved it and now it is available in the USA. Although the sensor time is increased up to 180 days (6 months), a disadvantage is that calibration is still required [19]. Fortunately, the number of calibrations reduces from 2 times/day for 21 days, to 1 time/day after day 21.
- We anticipate that future diabetes technology with non-invasive CGM could be worn on the wrist. Recently, HGR GWave, non-invasive radiofrequency sensing, showed accurate results in five subjects with type 2 diabetes mellitus (T2DM). The HGR readings had a high correlation with glucose measurements at R2=0.924 (P<0.0001) [20]. Further clinical studies are needed to facilitate the use of non-invasive radiofrequency sensing for diabetes in actual practice.
CURRENT AND FUTURE CGM TECHNOLOGIES
- CGM has emerged as a useful tool to assess glycemic status and is consistently proved to be effective compared to SMBG alone in patients with type 1 diabetes mellitus (T1DM) and T2DM who are receiving insulin-based therapy [21-24]. Thus, we have reviewed the efficacy of different CGM systems (rtCGM vs. isCGM) with populations of different glycemic statuses to assist clinicians more comprehensively and provide guidance in the selection of an appropriate CGM. Two types of CGM are available: rtCGM and isCGM. In general, CGM refers to rtCGM that allows individuals to visualize real-time data in 1 to 5 minutes. IsCGM is referred to as FGM because the glucose value can only be seen by scanning the sensor; thus, it is regarded as a separate entity from CGM. As the glucose monitoring methods are different, they also have different glycemic effects.
- The effectiveness of rtCGM has been confirmed in various randomized controlled trials (RCTs) with different types of diabetes and insulin regimens (Table 2). The glycemic outcome was different based on the glycemic status of the study population included. Studies showed that rtCGM had a beneficial effect on reducing HbA1c, particularly in patients with markedly high HbA1c, and on reducing hypoglycemia, especially in patients with a history of severe hypoglycemia. In studies with T1DM and T2DM both were poorly controlled using multiple daily injections (MDI; DIAMOND and GOLD), regardless of the type of diabetes, the rtCGM had the advantage of reducing HbA1c by 0.3% to 0.6% more than non-CGM users [21-23]. Specifically, in a study evaluating the HbA1c reduction in T1DM using MDI with a mean HbA1c of 8.6%, the mean differences of time in range (TIR, 70 to 180 mg/dL) were 5.4% (77 minutes/day) between the rtCGM and SMBG groups (P=0.005) [21]. Also, the time below range (TBR, <70 mg/dL) decreased from 4.5% to 3.0% at week 24 in the rtCGM users. Unfortunately, the T2DM trials did not show differences in hypoglycemia between the groups [23].
- A notable finding is that there were no between-group differences in HbA1c or TIR in the study evaluating the incidence of hypoglycemia, in contrast to trials evaluating HbA1c reductions in individuals with high HbA1c. These results are from the included participants, who were patients with a high risk for hypoglycemia and with relatively lower portion of high HbA1c (with HbA1c lower than 9.0%). Thus, it is natural that hypoglycemic events were significantly reduced with the relatively high portion of hypoglycemia, and it is also natural that there’re were no differences in hyperglycemia between the groups because of the relatively lower portion of individuals with high HbA1c [25].
- Trials were conducted on the effectiveness of rtCGM for treating T2DM with basal insulin and not MDI [24]. The study population included HbA1c between 7.8% to 11.5%, with a mean HbA1c of 9.0% for both the rtCGM and SMBG groups. The mean HbA1c level decreased to 8.0% in the rtCGM group with a mean adjusted difference of –0.4% (P=0.02) between the CGM and the SMBG group. The results were similar to those of the study of populations using MDI. Interestingly, they also explored the effect of discontinuing rtCGM after 8 months in the same population of T2DM using basal insulin [26]. In the study, TIR increased from 38% to 62% after 8 months of CGM use but decreased after discontinuing CGM to 50% at 14 months (mean change from 8 to 14 months –12%, P=0.01), emphasizing the importance of consistent CGM use.
- The glycemic outcomes from isCGM and rtCGM are different. The RCT outcomes of isCGM are listed in Table 3. There are not many studies for isCGM, isCGM did not show an HbA1c reduction even in a study with poorly controlled diabetes (HbA1c range, 7.5% to 12.0%) [27]. Although the isCGM does not have the ability to reduce HbA1c, it still can be used to avoid hypoglycemia [28,29].
- Several meta-analysis studies have compared the effects of rtCGM and isCGM on glycemic outcomes [30,31]. In a meta-analysis identifying 15 RCTs involving 2,461 patients, rtCGM led to higher improvements in mean HbA1c (0.23%) and TIR (5.8%), whereas isCGM was associated with a greater decline in TBR [31]. However, this meta-analysis was performed without considering the purpose and baseline characteristics of the study. In studies aimed at improving HbA1c or TIR, poorly controlled diabetes with high HbA1c were enrolled, but in the case of hypoglycemia prevention, subjects with a high risk of hypoglycemia were enrolled rather than subjects with a high level of HbA1c. It is relatively difficult to show a decrease in HbA1c in studies seeking to prevent hypoglycemia in a well-controlled population. Therefore, considering that the study differs HbA1c at entry, and differs primary outcomes, meta-analyses should be divided into the studies with HbA1c near target with the primary outcome of reduction in hypoglycemia, and high HbA1c with primary outcome of HbA1c reduction, separately [32].
GLYCEMIC EFFICACY OF CGM ITSELF
- The use of rtCGM reduces HbA1c by about 0.3% to 0.6% [33]. Beck et al. [21] revealed that the HbA1c was 0.6% lower, and TIR was 5.3% (1.28 hour/day) higher in the rtCGM group than in the control group with poorly controlled T1DM. However, despite the benefits of rtCGM itself, subjects in the rtCGM group did not achieve the target of HbA1c <7.0% and TIR >70%. HbA1c and TIR only achieved 7.7% and 51.1% (12.3 hours/day), respectively. The use of rtCGM without education limits better glycemic outcomes.
- Today, structured education specifically for CGM has been recognized as an essential part of diabetes therapy. By increasing the ability of the patient to adjust the insulin dose rather than using fixed-dose insulin, an additional glycemic improvement can be achieved. Several studies have noted the importance and effectiveness of education [34-38]. Yoo et al. [38] emphasized personalized education in a study comparing the efficacy of rtCGM combined with structured education or without education. In an RCT, participants with 3 months of structured education had a 15.3% (3 hours 42 min/day) increase in TIR compared with the control group. These results showed remarkable increases in TIR than in a previous study that used rtCGM without education. The group with education had a more HbA1c decrease of 0.5% compared to those without education. The HbA1c changed from 8.4% to 7.2%, nearly achieving an HbA1c of 7.0%. Studies with very young children and with children had similar results [34,36].
- RCT studies showed that isCGM has high efficacy in reducing hypoglycemia whereas it does not improve HbA1c. However, users educated in isCGM improve glycemic outcomes. Hermanns et al. [35] showed a significant difference of –0.17% reduction in the HbA1c of an isCGM with education group compared to the isCGM without education. In addition, Yaron et al. [37] reported a 0.82% reduction of HbA1c for 10 weeks in isCGM users who had attended education for insulin dose adjustment.
EFFECT OF EDUCATION ON CGM USE
- Currently available and future CIPs with CGM
- A missed insulin injection, inappropriate timing of injection, and problematic adherence in patients with insulin-based therapy are common and have always been challenging [39]. These problems are related to an increase in HbA1c and eventually lead to increases in diabetes complications [40].
- The CIP is a new technology with a function that displays the last dose of insulin and timing and has reminder alerts that integrate with a smart app. Today, with greater advances in the technology of the CIP, it integrates with CGM and even integrates with mobile apps to help users to calculate bolus doses easily with a bolus calculator, and finally, all the data are submitted to a virtual platform. Several studies have shown that the emergence of new technologies overcome barriers and report better glycemic outcomes than pens without the function [41-43]. Currently, a few clinical trials are collecting data on the effectiveness of these CIPs.
- An overview of the characteristics of CIPs and smart cap integrating with CGM is summarized in Table 4. In 2017, the Medtronic InPen (Companion Medical, San Diego, CA, USA) became the first FDA-approved CIP [44]. The InPen, a Bluetooth-enabled wireless insulin pen, has functions that not only memorize the number of doses and timing of insulin injections in rapid acting insulin but it also has a bolus calculator and IOB display like insulin pumps. The display of injection timing with IOB helps to prevent insulin stacking. With the bolus calculator, the users can calculate the appropriate doses easier, like pump users. Thus, a device with the bolus calculator function, not limited to InPen, can be a good choice for patients with diabetes who want to optimize bolus doses with correction factors (CF), insulin-carbohydrate ratio (ICR), and duration of insulin actions, but are hesitant about always attaching the devices. The bolus calculator in the mobile app of InPen also has a simple version like ‘meal size estimation (small, medium, or large)’ for individuals with diabetes who are unfamiliar with carbohydrate counting. It also has alerts for injection reminders to help avoid injection omission.
- The benefit of the CIP is not only in diabetes, but it also helps HCPs to make better decisions in diabetes management by engaging diabetes with correct insulin use information [45]. With the CIP, there is no need to make decisions about insulin doses, timing, or the number of insulin injections based on a presumption that the patient is following the insulin prescription. Data from the device integrate with CGM in one platform. Thus, with a CIP, the glucose values and the amount of carbohydrate intake, and the amount and time of the insulin dose can be seen at a glance. The importance of the device is the benefits in glycemic outcomes, but the number of studies is limited. Vigersky et al. [46] reported an increase in TIR of 2.3% (0.6 hour/day) and a decrease in time above range (TAR >180 mg/dL) of 2.4%, with no change in TBR in the InPen users compared to pre-InPen use in poorly controlled T1DM (glucose management indicator >8.0%) when combined with CGM.
- NovoPen 6 and NovoPen Echo Plus by Novo Nordisk (Bagsværd, Denmark) are CIPs similar to InPen; they automatically memorize insulin doses and timing and further integrate with CGM. The devices have not yet been cleared by the FDA [42]. Compared to InPen, which works with Bluetooth, the Novopen 6/Echo Plus works with near-field communication technology. In addition, the battery life of the Novopen6/Echo Plus (5 years) is much longer than those of InPen (1 year) without recharging. In collaboration with Glooko/Diasend (Gothenburg, Sweden) digital diabetes management platforms, the information obtained by NovePen6/Echo Plus can be integrated with various CGMs available in Glooko. However, it does not offer injection reminders or IOB display. Two observation studies have evaluated the efficacy of NovoPen 6 integrating with CGM, one in adults and the other in pediatric patients [42,43]. Adolfsson et al. [42] revealed significant changes during the 12-month follow-up period, with an 8.5% (1.9 hour/day) increase of TIR, 6.2% decrease of TAR (>180 mg/dL, 1.8 hour/day), and 1.5% TBR (<54 mg/dL, 0.3 hour/day) in 94 patients with T1DM. They also found that missed bolus dose (MBD) injections decreased by 43% over the study (P=0.0002). In another study, they found that the TBR (<54 mg/dL) reduces by 0.64% from 2.82% to 2.18% in at least 12 months of follow-up in 39 pediatric patients with T1DM. However, there were no changes in MBD meals, TIR, and TAR (>180 mg/dL) [43].
- Bigfoot Biomedical received FDA approval as a diabetes management system featuring connected insulin caps for an insulin pen in 2021 [47]. It is the only smart cap solution that works with disposable insulin pens integrated with FreeStyle Libre 2, and it will also be available with FreesStyle Libre 3 soon. It has similar features to the InPen. It offers a bolus calculator, IOB display, missed dose reminder, and hypoglycemia alarm and combines this with the Bigfoot Unity mobile app. Caps are available for both basal and rapid-acting insulins compared to the InPen, which only has rapid-acting insulins. There are two smart pen caps, a white one for rapid-acting (bolus) insulin and a black one for long-acting (basal) insulin. Data for both rapid-acting insulins and long-acting insulins are presented in one place in the Bigfoot Unity mobile app. Lilly launched the Tempo pen and a small smart button device that attaches to a prefilled tempo pen [41]. A study has shown the benefits of the smart button integrated with rtCGM (Dexcom G5) in TIR, TAR, and TBR, and also in MBD [41]. It was a 12-week, single-arm, exploratory, two-period study for subjects with T1DM (n=38) or T2DM (n=26). MBD significantly decreased from 0.74 to 0.62 MBD/day (P=0.008). In addition, TAR, TBR, and mean HbA1c decreased from 53.6% to 48.1% (P=0.004), from 4.49% to 2.93% (P<0.001), and from 8.8% to 8.4% (P<0.001), respectively. TIR also increased significantly from 41.9% to 49.0% (P<0.001).
- We have reviewed the CIPs and caps that are currently on the market, and several are expected to be on the market soon. Diaconn (Seoul, Korea) P8 of the CIP by G2E, and SoloSmart Mini [48] of smart caps by Sanofi (Paris, France) are also in development. SoloSmart Mini is a reusable insulin pen cap that enables the recording of insulin doses and the timing of injections. The SoloSmart Mini will be launched in Korea at the end of 2022. Diaconn P8 allows 0.1-unit increments of dosing and a bolus maximum of 300 units with various insulin cartridges and CGMs. The device has an in-built bolus calculator, IOB, and last doses of insulin injections on display. As with the CIP by Bigfoot Biomedical, they have separate pens for basal and rapid-acting insulins. Both CIP and insulin pump of G2E can be linked with and can be shown in just one App, thus users can switch the two devices flexibly whenever the user wants.
- With the growing evidence on CIP efficacy, in 2022, the American Association of Clinical Endocrinology 2021 [49] and the American Diabetes Association (ADA) [9] announced that the insulin pen can be helpful for diabetes management and may be used in patients using injectable therapy.
- Currently available and future insulin pumps with CGM
- Increasing evidence demonstrates the effectiveness of insulin pumps when used in combination with a CGM and closedloop algorithm controller to automate basal insulin delivery, these are known as HCLS [50-52]. The outcomes are well summarized in the review article by Moon et al. [53]. Thus, this review only includes updated clinical trials beyond the previous review article (Table 5).
- AID systems are becoming more advanced. Of these devices, the clinical benefits of advanced HCLS (AHCLS) are among the highlights. AHCLS differs from HCLS, which modulates basal insulin delivery but does not administer automated correction boluses. AHCLS has an additional function with automated bolus correction. Medtronic MiniMed 780G is the first AHCLS which includes a target set point of 100 or 120 mg/dL and an automated correction bolus feature up to every 5 minutes. The RCT showed 12.5% higher TIR in AHCLS compared to the sensor augmented pump (SAP) therapy with predictive low-glucose suspend (PLGS, 70.4% vs. 57.9%, P<0.001) in 59 patients with T1DM previously using insulin pump therapy [54]. Recently in a single-arm prospective study involving 157 adolescents and adults with T1DM, the study revealed an additional increase in TIR of 5.7% (increased from 68.8% to 74.5%, P<0.001) as a result of conversion from SAP with PLGS to AHCLS for 3 months [55]. Compared with the run-in period, AHCLS reduced HbA1c from 7.5% to 7.0%, a decrease of 0.5%, and TBR (<70 mg/dL) decreased from 3.3% to 2.3% (all P<0.001). In addition, when the target was set to 100 mg/dL, TIR increased to 75.4%. The results were similar in other prospective single-arm study with 34 children and adolescents for 12 weeks of treatment [56]. Glycemic efficacy in the study was different because the study population was consisted of T1DM with MDI therapy, not insulin pumps before the study entry. Furthermore, 10 days of education was added. Remarkably, TIR increased from 42.1% at baseline to 78.8% in the study phase (P<0.001). HbA1c decreased from 8.6% at baseline to 6.5% at the end of the study (P=0.001). Thus, with the advance in technology and structured education, the glycemic outcomes could be remarkably improved even when the patients have less burden with automated bolus correction. We await the results of the Advanced Hybrid Closed-Loop in an Adult Population with Type 1 Diabetes (ADAPT) study comparing AHCLS to CGM with MDI [57].
- Although not as sophisticated as the MiniMed 780G, the Tandem T:slim X2 also performs bolus correction every hour, so it is sometimes classified as AHCLP. Existing RCTs also consistently showed an 11% TIR increase compared to SAP [51]. For the entire 12-month observation of a real-world study of 9,451 users of the t:slim X2 insulin pump with Control-IQ technology, TIR increased from 63.6% to 73.6%, exceeding the target value of TIR 70% with no increase in hypoglycemia (TBR <70 mg/dL: <1%) [58].
- The Insulet Omnipod 5 (Insulet, Acton, MA, USA) is in the spotlight as the first tubeless patch pump with an algorithm in the pod that has FDA approval, although it is still HCLS, which does not have bolus auto-correction. The tubeless patch pump, as the name suggests, has several advantages, for example, it does not have a long tube line and it is in the form of a small patch, giving the convenience of wearing comfort. A multicenter prospective study involving 235 patients with T1DM resulted in a 0.71% HbA1c reduction in children and a 0.38% reduction in adults [59]. In addition, TIR was improved from standard therapy by 15.6% (3.7 hours/day) in children and 9.3% (2.2 hours/day) in adults (both P<0.0001). A study for very young children also showed similar results [60].
- The insulin-only iLet Bionic Pancreas (Beta Bionics, Irvine, CA, USA) with greater automation can significantly reduce the burden of T1DM and also showed good results in the ADA conference of 2022 [61]. It differs from currently marketed artificial pancreas systems (APSs) in that users do not need exact carbohydrate counting, just simple input such as “typical, larger, or smaller meals than usual.” In addition, users only need to enter their body weight to initialize the system with no run-in period before automation. In that the ultimate goal of the AID system is to reduce the burden of T1DM through full automation, this device is closer to the future. The study comparing iLet Bionic Pancreas (integrated with Dexcom G6) to a standard of care involving currently available APS or SAP integrated with CGM or MDI with CGMs, showed the benefits for 440 adults and children with higher HbA1c levels during 13 weeks of treatment. In the iLet group, the HbA1c changed from 7.6% to 7.1%, and the mean difference between the iLet and control group was 0.5% (P<0.001). The TIR was 11% (2.6 hours/day) higher than the control group (P<0.001). There was no difference in TBR <54 mg/dL in both groups, but all groups achieved the target below 1%. However, even in patients with poorly controlled diabetes, at the study end, TIR was only 69% in adults, so caution is needed in selecting this iLet Bionic Pancreas for those with well-controlled diabetes.
- Do-it-yourself closed-loop systems with CGM
- Do-it-yourself closed-loop (DIY) systems are open-source algorithms designed to automate insulin delivery that link to existing CGMs and insulin pumps. These systems were created before the first commercial AID systems received FDA approval in 2016 by an online community (non-profit organization) who were directly related to diabetes. Thus, are freely available on the internet by app and are easily accessible. However, since they have not received FDA approval, they cannot be prescribed by the HCP and the patient has to take full responsibility for the failure of DIY. There are three types of DIY available: Open APS, Android APS, and Loop. Android APS does not require additional hardware except with Medtronic pumps or Omnipod; however, Open APS and Loop require additional hardware called “Rig” and “Riley Link,” respectively.
- The evidence of safety and effectiveness from real-world studies is growing [62]. In these studies, the glycemic efficacy is similar to commercially available systems [63-65]. A prospective real-world study for the safety and effectiveness of the Tidepool Loop (Tidepool, Palo Alto, CA, USA) open-source AID system was published [64]. The TIR increased from 67% to 73% over 6 months and decreased severe hypoglycemia (TBR <54 mg/dL) by 0.05% (All P<0.01). Mean HbA1c decreased with a mean difference of 0.33%, even in subjects with a mean HbA1c of 6.8%. With this growing evidence, Tidepool has submitted the Loop algorithms to the FDA to build a commercial version. We hope that these DIYs will be FDA-approved and used more safely. Recently, the first RCT comparing Android APS to SAP was shown at the ADA 2022 conference. The mean TIR was 74.5% using Android APS achieving the TIR of 70%, but only 56.4% was shown in the SAP group [66].
EFFECTS OF CGM INTEGRATION WITH OTHER DEVICES
- Factors that have greatly improved glycemic status include CGM, an AID system, CIPs, and structured education programs but the area of particular importance is a glycemic monitoring platform [67]. It collects the data from all of the above and allows the HCP and patients to see various and accurate information at a glance. This helps both the patients and HCPs to make better decisions for glycemic improvement by recommending the optimal basal rate, CF, and ICR [68]. These cloud-based data collection programs include Tidepool, Glooko/Diasend, Jade Diabetes (Victoria, Australia), and MySugar (Vienna, Austria) available worldwide, and Diaconn web only available in Korea.
- These free online platforms allow the user to integrate with various types of CGM, glucose meters, insulin pumps, and CIPs. Large companies such as Glooko/Diasend and Tidepool have similar abilities to provide glucose trends, amount of carbohydrate intake, basal and bolus dosages, exercise, and other biometric factors. However, the device compatibility is different. Both Glooko/Diasend and Tidepool integrate with CGMs by Dexcom and Abbott and insulin pumps by Insulet, Tandem, and Medtronics, and CIPs from Medtronics InPen. The Glooko/Diasend does not support CGMs by Medtronics (e.g., Guardian connect, Guardian 3 sensor), on the other hand, Tidepool does not support CIPs from Novo Nordisk (Novopen 6/Echo plus). Diaconn web is organized by G2E and only available in South Korea. The platform has similar abilities to Glooko in reporting data. However, they only support their own insulin pump (Diaconn G8) and CIP (Diaconn P8), and other CGMs of Dexcom G6, and FreeStyle Libre with a thirdparty transmitter. Jade Diabetes recently launched the voice-based insulin dosing system, Alexa.
GLYCEMIC MONITORING PLATFORMS
- The advancement of CGM has enabled a complete glucose profile to be obtained, thus making it possible to diagnose the glycemic status of an individual precisely. The device is getting smaller, more comfortable to wear, accurate, and even non-invasive glucose monitoring technologies are in development [69]. In addition, the advancement of the AID system and CIP integrated with CGM has reduced the burden of T1DM by overcoming the time consumed on insulin management. The development of diabetes technology made it possible to avoid hyperglycemia without increasing hypoglycemia with a more comfortable fit and less effort, which could not previously be shown in diabetes with insulin therapy [70].
- In the future, we expect that a fully automatic AID system will emerge, just as Bionic Pancreas has developed no carbohydrate counting pumps, which was previously burdensome for users. However, most of the HCLS still need to modify a variety of parameters including carbohydrate counting, and these require expertise and experience from the HCP. Thus, as there is a platform where you can see various technologies in one place, it is necessary to develop a decision support tool that can help with complex insulin dose calculating over a short time through that platform for optimal management. Indeed, Glooko has made a digital decision support system called DreaMed Advisor Pro (DreaMed Diabetes Ltd., Petah Tikva, Israel) which automatically provides the exact insulin dosing recommendations and other treatment tips through an AI-based algorithm [71]. In a study comparing physicians to DreaMed Advisor Pro in insulin dose adjustment, the changes in insulin dosing were similar to those given by the physicians.
THE FUTURE OF DIABETES THERAPY INTEGRATED WITH CGM
- It has become possible to fully diagnose an individual glycemic status through rapidly evolving CGM technology. CGM use is growing exponentially with valuable benefits on clinical outcomes, furthermore, various technologies can be integrated with it. These complex bunch of data including glucose trend, amount of carbohydrate intake, IOB, and physical activity are summarized in a virtual platform, which helps the HCP and patients with diabetes easily analyze and helps diagnose the glycemic status. This has resulted in a dramatic improvement in glycemic control. There is no doubt that evolving diabetes technology has changed the treatment paradigm for patients with diabetes using insulin treatment and reduced the burden of diabetes self-management such as the adjustment of insulin dosing. However, the burden of the disease is significant. Despite the technological advances, accessing new technology with complexity and rapid changes could be a burden to some patients if it is not fully automated. Thus, in the future, we expect the CGM to be smaller, more comfortable, last longer, and, importantly, be more accurate. In addition, we expect the advancement of the AID system with a fully automatic algorithm according to the meaning of the term “artificial pancreas system,” which no longer needs carbohydrate counting or meal estimation. Currently, all the information from the CGM and integrated devices are connected to the virtual platform, but a tool for decision support is in development, so the vast information may feel rather complicated. Thus, constant efforts for developing AI-based personalized decision support such as DreaMed advisor, which recommends adjustments to the CF, ICR, and basal rate automatically, are needed to optimize insulin use in diabetes aiming to accomplish the greatest TIR with no hypoglycemia.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None
NOTES
-
Acknowledgements
- None

| Dexcom G6 | Dexcom G7 | Guardian Sensor 3 | Guardian Sensor 4 | FreeStyle Libre | FreeStyle Libre 2 | FreeStyle Libre 3 | Eversense | Eversense E3 | |
|---|---|---|---|---|---|---|---|---|---|
| Company | Dexcom | Dexcom | Medtronic | Medtronic | Abbott | Abbott | Abbott | Senseonics | Senseonics |
| Size, mm | 45×30×15 | 27.3×24×4.6 | 35×28×9.5 | 35×28×9.5 | 35×5 | 35×5 | 21×2.9 | 18.3×3a | 18.3×3a |
| FDA approved year | 2018 | Awaiting | 2018 | Awaiting | 2017 | 2020 | 2022 | 2018 | 2022 |
| Approved age | ≥2 years | ≥2 years | ≥2 years | ≥14 years | ≥18 years | ≥4 years | ≥4 years | ≥18 years | ≥18 years |
| Sensor type | Real-time | Real-time | Real-time | Real-time | Intermittent scanning | Intermittent scanning | Real-time | Real-time (implantable) | Real-time (implantable) |
| Wear time | 10 days | 10.5 days | 7 days | 7 days | 14 days | 14 days | 14 days | 90 days | 180 days |
| Warm-up period | 2 hours | 27 minutes | 2 hours | 2 hours | 1 hour | 1 hour | 1 hour | 24 hours | 24 hours |
| Calibration required | No | No | Yes (2 times/day) | No | No | No | No | Yes (2 times/day) | Yes (2 times/day for 21 days, 1 time/day after day 21) |
| Alarms for hypo/hyperglycemia | Yes | Yes (can be delayed up to 6 hours) | Yes | Yes | No | Yes (optional) | Yes (optional) | Yes (on-body vibration) | Yes (on-body vibration) |
| Integrity with insulin pumps | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No |
| Platform | Dexcom clarity | Dexcom clarity | CareLink Personal | CareLink Personal | Libreview | Libreview | Libreview | ||
| LibreLinkUp | Eversense DMS | Eversense DMS | |||||||
| Accuracy (MARD) | 10.0% | 8.2% (arm), 9.1% (abdomen) | 8.7% (arm), 9.6% (abdomen) | 10.6% | 11.4% (arm) | 9.2% (adults), 9.7% (pediatrics) | 9.2% (adults), 9.7% (pediatrics) | 8.8% | 9.1% |
| Accuracy in low glucose range (MAD) | 10.9 mg/dL (glucose level <54 mg/dL) | 8.5 mg/dL (arm), 10.3 mg/dL (abdomen) (glucose level 40–60 mg/dL) | NA | NA | 11.3 mg/dL (glucose level <100 mg/dL) | 9.1 mg/dL (adults), 8.8 mg/dL (pediatrics) | 9.1 mg/dL (adults), 8.8 mg/dL (pediatrics) | NA | 7.5 mg/dL (glucose level ≤60 mg/dL) |
| Bluetooth free range | 6 m | 6 m | 6 m | 6 m | NA | NA | 10 m | 7.6 m | 7.6 m |
| Study | Study design | Study population | Diabetes type | Insulin regimen | Baseline glycemic status (CGM vs. SMBG) | Primary outcomes | Results (CGM vs. SMBG) |
|---|---|---|---|---|---|---|---|
| Lind et al. (2017) [22] | Crossover, 26 weeks | 161 adults | T1DM | MDI | HbA1c ≥7.5% | Difference in HbA1c at week 26 | Adjusted between-group differences: −0.4% (P<0.001) |
| Mean HbA1c: 8.5% vs. 8.5% | 7.9% vs. 8.4% | ||||||
| Beck et al. (2017) [21] | Parallel, 24 weeks | 158 adults and children | T1DM | MDI | HbA1c 7.5%–9.9% | Difference in change in HbA1c from baseline to 24 weeks | Adjusted between-group differences: −0.6% (P<0.001) |
| Mean HbA1c: 8.6% vs. 8.6% | 7.7% vs. 8.2% | ||||||
| Heinemann et al. (2018) [25] | Parallel, 26 weeks | 149 adults | T1DMa with severe hypoglycemia | MDI | HbA1c ≤9.0% | Baseline-adjusted number of hypoglycemic events (defined as glucose ≤54 mg/dL for ≥20 min) | Adjusted between group HR: 0.28 (P<0.001) |
| Mean HbA1c: 7.3% vs. 7.6% | 10.8 events to 3.5 events vs. 14.4 events to 13.7 events | ||||||
| Beck et al. (2017) [23] | Parallel, 24 weeks | 158 adults | T2DM | MDI | HbA1c 7.5%–10.0% | HbA1c reduction at 24 weeks | Adjusted between-group differences: −0.3% (P<0.001) |
| Mean HbA1c: 8.5% vs. 8.5% | 7.7% vs. 8.0% | ||||||
| Martens et al. (2021) [24] | Parallel, 8 months | 175 adults | T2DM | Basal insulin | HbA1c 7.8%–11.5% | HbA1c reduction at 8 months | Adjusted between-group differences: −0.4% (P=0.02) |
| Mean HbA1c: 9.1% vs. 9.0% | 8.0% vs. 8.4% |
rtCGM, real-time continuous glucose monitoring; SMBG, self-monitoring blood glucose monitoring; T1DM, type 1 diabetes mellitus; MDI, multiple daily insulin injection; HbA1c, glycosylated hemoglobin; HR, hazard ratio; T2DM, type 2 diabetes mellitus.
a With a history of impaired hypoglycemia awareness or severe hypoglycemia.
| Study | Study design | Study population | Diabetes type | Insulin regimen | Baseline glycemic status (CGM vs. SMBG) | Primary outcomes | Results (CGM vs. SMBG) |
|---|---|---|---|---|---|---|---|
| Bolinder et al. (2016) [28] | Parallel, 6 months | 241 adults | T1DM | CSII, MDI | HbA1c ≤7.5% | Change in time in hypoglycemia (<70 mg/dL) between baseline and 6 months | Adjusted between-group differences: −1.24 hour (P<0.001) |
| Mean HbA1c: 6.7% vs. 6.7% | 3.38–2.03 hours vs. 3.44–3.27 hours | ||||||
| Oskarsson et al. (2018) [29] | Parallel, 6 months | 167 adults | T1DM | MDI | HbA1c ≤7.5% | Change in time in hypoglycemia (<70 mg/dL) between baseline and 6 months | Adjusted between-group differences: −1.65 hour (P<0.001) |
| Mean HbA1c: 6.7% vs. 6.7% | 3.44–1.86 hours vs. 3.73–3.66 hours | ||||||
| Haak et al. (2017) [27] | Parallel, 24 weeks | 224 adults | T2DM | CSII, MDI | HbA1c 7.5%–12.0% | Difference in HbA1c at 6 months | Adjusted between-group differences: −0.29% (P=0.8222) |
| Mean HbA1c: 8.7% vs. 8.9% | 8.37% vs. 8.34% |
|
Pen |
Caps |
|||
|---|---|---|---|---|
| InPen | NovoPen6/Echo Plus | DIA:CONN P8 | Bigfoot Unity | |
| Company | Medtronics | NovoNordisk | G2E | Bigfoot Biomedical |
| FDA approved | 2017 | Not yet | Not yet | 2021 |
| Approval age | ≥7 years | Not yet | Not yet | ≥12 years |
| Integrated CGM | Guardian Connect, Guardian 4 sensor | Not directly (CGM those available with Glooko/Diasend) | Dexcom, FreeStyle Libre, i-sense | FreeStyle Libre 2 (later with FreeStyle Libre 3) |
| Available insulinsa | Humalog, Novolog, Fiasp | Cartridges by Novo Nordisk (Novolog, Fiasp, Levemir, Degludec) | Long-acting insulin, rapid acting insulinb | Black for rapid-acting insulin (Humalog, Novolog); White for long-acting insulin (Lantus, Tresiba) |
| Maximum insulin dose | 30 units | 60/30 units | 300 units | NA |
| Dose increments | 0.5 unit | 1/0.5 unit | 0.1 unit | NA |
| Insulin injection reminder | Yes (rapid and long-acting insulin) | No | Yes | Yes |
| Last injection time and dose | Yes | Yes | Yes | Yes |
| Bolus calculator | Yes | No | Yes | Yes |
| IOB display | Yes | No | Yes | Yes |
| Alarms | Yes (2 hours after rapid-acting insulin, cartridge replaced after 28 days) | No | Yes | <70 mg/dL (optional) |
| <55 mg/dL (Mandatory) | ||||
| Missing basal dose more than 24 hours | ||||
| Battery life | 1 year | 5 years | Not yet | 1 year |
| Software | Bluetooth | NFC | Bluetooth | Bluetooth |
- 1. Yoo JH, Kim JH. Time in range from continuous glucose monitoring: a novel metric for glycemic control. Diabetes Metab J 2020;44:828-39.ArticlePubMedPMCPDF
- 2. Lin R, Brown F, James S, Jones J, Ekinci E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med 2021;38:e14528.ArticlePubMedPDF
- 3. Klonoff DC, Kerr D. Smart pens will improve insulin therapy. J Diabetes Sci Technol 2018;12:551-3.ArticlePubMedPMCPDF
- 4. Fleming GA, Petrie JR, Bergenstal RM, Holl RW, Peters AL, Heinemann L. Diabetes digital app technology: benefits, challenges, and recommendations: a consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) diabetes technology working group. Diabetes Care 2020;43:250-60.ArticlePubMedPDF
- 5. Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet 2019;394:1265-73.ArticlePubMed
- 6. Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci 2012;69:83-93.ArticlePubMed
- 7. Edelman SV. Regulation catches up to reality. J Diabetes Sci Technol 2017;11:160-4.ArticlePubMedPMCPDF
- 8. Aleppo G, Ruedy KJ, Riddlesworth TD, Kruger DF, Peters AL, Hirsch I, et al. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 2017;40:538-45.ArticlePubMedPMCPDF
- 9. American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 7. Diabetes technology: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S97-112.ArticlePubMedPDF
- 10. Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther 2018;20:395-402.ArticlePubMedPMC
- 11. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther 2015;17:787-94.ArticlePubMedPMC
- 12. Alva S, Bailey T, Brazg R, Budiman ES, Castorino K, Christiansen MP, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol 2022;16:70-7.ArticlePubMedPMCPDF
- 13. Medtronic. GUARDIAN(TM) SENSOR 3. Available from: https://www.medtronic.com/us-en/healthcare-professionals/products/diabetes/continuous-glucose-monitoring-systems/guardian-sensor-3.html (cited 2022 Oct 31).
- 14. Medtronic. InPen. Available from: https://www.medtronicdiabetes.com/products/inpen-smart-insulin-pen-system (cited 2022 Oct 31).
- 15. Medtronic. MiniMed(TM) 780G system with Guardian(TM) 4 sensor. Available from: https://www.medtronic-diabetes.com/en-gb/insulin-pump-therapy/minimed-780g-system (cited 2022 Oct 31).
- 16. Christiansen MP, Klaff LJ, Brazg R, Chang AR, Levy CJ, Lam D, et al. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol Ther 2018;20:197-206.ArticlePubMedPMC
- 17. Garg SK, Kipnes M, Castorino K, Bailey TS, Akturk HK, Welsh JB, et al. Accuracy and safety of Dexcom G7 continuous glucose monitoring in adults with diabetes. Diabetes Technol Ther 2022;24:373-80.ArticlePubMedPMC
- 18. Zipp R. Dexcom CEO Sayer on G7 FDA submission, developing a 15-day sensor, M&A plans. Available from: https://www.medtechdive.com/news/dexcom-ceo-g7-fda-15-day-sensorma/625013/ (cited 2022 Oct 31).
- 19. Garg SK, Liljenquist D, Bode B, Christiansen MP, Bailey TS, Brazg RL, et al. Evaluation of accuracy and safety of the nextgeneration up to 180-day long-term implantable eversense continuous glucose monitoring system: the promise study. Diabetes Technol Ther 2022;24:84-92.ArticlePubMedPMC
- 20. Schwarz Y, Konvalina N, Tirosh A. A pilot trial to evaluate the accuracy of a novel non-invasive glucose meter. Sensors (Basel) 2021;21:6704.ArticlePubMedPMC
- 21. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the diamond randomized clinical trial. JAMA 2017;317:371-8.ArticlePubMed
- 22. Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the gold randomized clinical trial. JAMA 2017;317:379-87.ArticlePubMed
- 23. Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365-74.ArticlePubMed
- 24. Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA 2021;325:2262-72.PubMed
- 25. Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 2018;391:1367-77.ArticlePubMed
- 26. Aleppo G, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, et al. The effect of discontinuing continuous glucose monitoring in adults with type 2 diabetes treated with basal insulin. Diabetes Care 2021;44:2729-37.PubMedPMC
- 27. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulintreated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55-73.ArticlePubMedPMCPDF
- 28. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254-63.ArticlePubMed
- 29. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia 2018;61:539-50.ArticlePubMedPMCPDF
- 30. Park C, Le QA. The effectiveness of continuous glucose monitoring in patients with type 2 diabetes: a systematic review of literature and meta-analysis. Diabetes Technol Ther 2018;20:613-21.ArticlePubMed
- 31. Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care 2020;43:1146-56.ArticlePubMedPDF
- 32. American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S83-96.
- 33. Seyed Ahmadi S, Westman K, Pivodic A, Olafsdottir AF, Dahlqvist S, Hirsch IB, et al. The association between HbA1c and time in hypoglycemia during CGM and self-monitoring of blood glucose in people with type 1 diabetes and multiple daily insulin injections: a randomized clinical trial (GOLD-4). Diabetes Care 2020;43:2017-24.ArticlePubMedPMCPDF
- 34. Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group. A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care 2021;44:464-72.PubMed
- 35. Hermanns N, Ehrmann D, Schipfer M, Kroger J, Haak T, Kulzer B. The impact of a structured education and treatment programme (FLASH) for people with diabetes using a flash sensor-based glucose monitoring system: results of a randomized controlled trial. Diabetes Res Clin Pract 2019;150:111-21.ArticlePubMed
- 36. Pemberton JS, Kershaw M, Dias R, Idkowiak J, Mohamed Z, Saraff V, et al. DYNAMIC: dynamic glucose management strategies delivered through a structured education program improves time in range in a socioeconomically deprived cohort of children and young people with type 1 diabetes with a history of hypoglycemia. Pediatr Diabetes 2021;22:249-60.ArticlePubMedPDF
- 37. Yaron M, Roitman E, Aharon-Hananel G, Landau Z, Ganz T, Yanuv I, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 2019;42:1178-84.ArticlePubMedPDF
- 38. Yoo JH, Kim G, Lee HJ, Sim KH, Jin SM, Kim JH. Effect of structured individualized education on continuous glucose monitoring use in poorly controlled patients with type 1 diabetes: a randomized controlled trial. Diabetes Res Clin Pract 2022;184:109209.ArticlePubMed
- 39. Davies MJ, Gagliardino JJ, Gray LJ, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with type 1 or type 2 diabetes mellitus: a systematic review. Diabet Med 2013;30:512-24.ArticlePubMed
- 40. Munshi MN, Slyne C, Greenberg JM, Greaves T, Lee A, Carl S, et al. Nonadherence to insulin therapy detected by Bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care 2019;42:1129-31.ArticlePubMedPDF
- 41. Edwards S, He X, Wang W, Poon JL, Meadows E, Price D, et al. Use of connected pen as a diagnostic tool to evaluate missed bolus dosing behavior in people with type 1 and type 2 diabetes. Diabetes Technol Ther 2022;24:61-6.ArticlePubMedPMC
- 42. Adolfsson P, Hartvig NV, Kaas A, Moller JB, Hellman J. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther 2020;22:709-18.ArticlePubMedPMC
- 43. Adolfsson P, Bjornsson V, Hartvig NV, Kaas A, Moller JB, Ogionwo Lange E. Improved glycemic control observed in children with type 1 diabetes following the introduction of smart insulin pens: a real-world study. Diabetes Ther 2022;13:43-56.ArticlePubMedPMCPDF
- 44. Gildon BW. InPen smart insulin pen system: product review and user experience. Diabetes Spectr 2018;31:354-8.ArticlePubMedPMCPDF
- 45. Sy SL, Munshi MM, Toschi E. Can smart pens help improve diabetes management? J Diabetes Sci Technol 2022;16:628-34.ArticlePubMedPMCPDF
- 46. Vigersky R, Smith M, Thanasekaran S, Gaetano A, Im GH, Cordero TL, et al. Impact of inpen smart insulin pen use on real-world glycemic and insulin dosing outcomes in individuals with poorly controlled diabetes. Diabetes 2021;70(Supplement_1):219-OR.
- 47. Bigfoot, BIOMEDICAL. The first FDA CLEARED DOSE DECISION SUPPORT SYSTEM INTEGRATED with iCGM. Available from: https://www.bigfootbiomedical.com/ (cited 2022 Oct 31).
- 48. Sanofi. Sanofi and Abbott partner to integrate glucose sensing and insulin delivery technologies to help change the way diabetes is managed. Available from: https://www.sanofi.com/en/media-room/press-releases/2019/2019-09-16-05-00-00-1915647 (cited 2022 Oct 31).
- 49. Grunberger G, Sherr J, Allende M, Blevins T, Bode B, Handelsman Y, et al. American Association of Clinical Endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract 2021;27:505-37.ArticlePubMed
- 50. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707-17.ArticlePubMedPMC
- 51. Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836-45.ArticlePubMedPMC
- 52. Ware J, Allen JM, Boughton CK, Wilinska ME, Hartnell S, Thankamony A, et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med 2022;386:209-19.ArticlePubMed
- 53. Moon SJ, Jung I, Park CY. Current advances of artificial pancreas systems: a comprehensive review of the clinical evidence. Diabetes Metab J 2021;45:813-39.ArticlePubMedPMCPDF
- 54. Collyns OJ, Meier RA, Betts ZL, Chan DS, Frampton C, Frewen CM, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care 2021;44:969-75.ArticlePubMedPDF
- 55. Carlson AL, Sherr JL, Shulman DI, Garg SK, Pop-Busui R, Bode BW, et al. Safety and glycemic outcomes during the MiniMed advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24:178-89.ArticlePubMedPMC
- 56. Petrovski G, Al Khalaf F, Campbell J, Day E, Almajaly D, Hussain K, et al. Glycemic outcomes of advanced hybrid closed loop system in children and adolescents with type 1 diabetes, previously treated with multiple daily injections (MiniMed 780G system in T1D individuals, previously treated with MDI). BMC Endocr Disord 2022;22:80.ArticlePubMedPMCPDF
- 57. de Portu S, Vorrink L, Re R, Shin J, Castaneda J, Habteab A, et al. Randomised controlled trial of advanced hybrid closed loop in an adult population with type 1 diabetes (ADAPT): study protocol and rationale. BMJ Open 2022;12:e050635.ArticlePubMedPMC
- 58. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 2021;23:601-8.ArticlePubMedPMC
- 59. Brown SA, Forlenza GP, Bode BW, Pinsker JE, Levy CJ, Criego AB, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44:1630-40.ArticlePubMedPMC
- 60. Sherr JL, Bode BW, Forlenza GP, Laffel LM, Schoelwer MJ, Buckingham BA, et al. Safety and glycemic outcomes with a tubeless automated insulin delivery system in very young children with type 1 diabetes: a single-arm multicenter clinical trial. Diabetes Care 2022;45:1907-10.ArticlePubMedPMC
- 61. American Diabetes Association 82nd scientificsessions. Results from insulin-only bionic pancreas trial to be reported. Available from: https://www.adameetingnews.org/live-updates/session-coverage/results-from-insulin-only-bionic-pancreastrial-to-be-reported/ (cited 2022 Oct 31).
- 62. Braune K, Lal RA, Petruzelkova L, Scheiner G, Winterdijk P, Schmidt S, et al. Open-source automated insulin delivery: international consensus statement and practical guidance for health-care professionals. Lancet Diabetes Endocrinol 2022;10:58-74.ArticlePubMed
- 63. Choi MS, Lee S, Kim J, Kim G, Park SM, Kim JH. Do-it-yourself open artificial pancreas system in children and adolescents with type 1 diabetes mellitus: real-world data. Diabetes Metab J 2022;46:154-9.ArticlePubMedPMCPDF
- 64. Lum JW, Bailey RJ, Barnes-Lomen V, Naranjo D, Hood KK, Lal RA, et al. A real-world prospective study of the safety and effectiveness of the loop open source automated insulin delivery system. Diabetes Technol Ther 2021;23:367-75.ArticlePubMedPMC
- 65. Petruzelkova L, Soupal J, Plasova V, Jiranova P, Neuman V, Plachy L, et al. Excellent glycemic control maintained by opensource hybrid closed-loop AndroidAPS during and after sustained physical activity. Diabetes Technol Ther 2018;20:744-50.ArticlePubMed
- 66. Burside MJ, Lewis DM, Crocket H, Meier R, Williman J, Sanders OJ, et al. The CREATE trial: randomized clinical trial comparing open-source automated insulin delivery with sensor augmented pump therapy in type 1 diabetes. Diabetes 2022;71(Supplement_1):286-OR.
- 67. Crossen S, Raymond J, Neinstein A. Top 10 tips for successfully implementing a diabetes telehealth program. Diabetes Technol Ther 2020;22:920-8.ArticlePubMedPMC
- 68. Ramakrishnan P, Yan K, Balijepalli C, Druyts E. Changing face of healthcare: digital therapeutics in the management of diabetes. Curr Med Res Opin 2021;37:2089-91.ArticlePubMed
- 69. Kesavadev J, Saboo B, Krishna MB, Krishnan G. Evolution of insulin delivery devices: from syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther 2020;11:1251-69.ArticlePubMedPMCPDF
- 70. Gerhardsson P, Schwandt A, Witsch M, Kordonouri O, Svensson J, Forsander G, et al. The SWEET project 10-year benchmarking in 19 countries worldwide is associated with improved HbA1c and increased use of diabetes technology in youth with type 1 diabetes. Diabetes Technol Ther 2021;23:491-9.ArticlePubMed
- 71. Nimri R, Oron T, Muller I, Kraljevic I, Alonso MM, Keskinen P, et al. Adjustment of insulin pump settings in type 1 diabetes management: Advisor Pro device compared to physicians’ recommendations. J Diabetes Sci Technol 2022;16:364-72.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System
Kyung-Soo Kim, Seung-Hwan Lee, Won Sang Yoo, Cheol-Young Park
Diabetes Technology & Therapeutics.2024; 26(4): 222. CrossRef - Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Recent advances in the precision control strategy of artificial pancreas
Wuyi Ming, Xudong Guo, Guojun Zhang, Yinxia Liu, Yongxin Wang, Hongmei Zhang, Haofang Liang, Yuan Yang
Medical & Biological Engineering & Computing.2024;[Epub] CrossRef - Digital Health in Diabetes and Cardiovascular Disease
Dorothy Avoke, Abdallah Elshafeey, Robert Weinstein, Chang H. Kim, Seth S. Martin
Endocrine Research.2024; : 1. CrossRef - Continuous glucose monitoring with structured education in adults with type 2 diabetes managed by multiple daily insulin injections: a multicentre randomised controlled trial
Ji Yoon Kim, Sang-Man Jin, Kang Hee Sim, Bo-Yeon Kim, Jae Hyoung Cho, Jun Sung Moon, Soo Lim, Eun Seok Kang, Cheol-Young Park, Sin Gon Kim, Jae Hyeon Kim
Diabetologia.2024;[Epub] CrossRef - Glycemic Outcomes During Early Use of the MiniMed™ 780G Advanced Hybrid Closed-Loop System with Guardian™ 4 Sensor
Toni L. Cordero, Zheng Dai, Arcelia Arrieta, Fang Niu, Melissa Vella, John Shin, Andrew S. Rhinehart, Jennifer McVean, Scott W. Lee, Robert H. Slover, Gregory P. Forlenza, Dorothy I. Shulman, Rodica Pop-Busui, James R. Thrasher, Mark S. Kipnes, Mark P. Ch
Diabetes Technology & Therapeutics.2023; 25(9): 652. CrossRef - Navigating the Seas of Glycemic Control: The Role of Continuous Glucose Monitoring in Type 1 Diabetes Mellitus
Jun Sung Moon
Diabetes & Metabolism Journal.2023; 47(3): 345. CrossRef - APSec1.0: Innovative Security Protocol Design with Formal Security Analysis for the Artificial Pancreas System
Jiyoon Kim, Jongmin Oh, Daehyeon Son, Hoseok Kwon, Philip Virgil Astillo, Ilsun You
Sensors.2023; 23(12): 5501. CrossRef - Advances and Development of Electronic Neural Interfaces
Xue Jiaxiang, Liu Zhixin
Journal of Computing and Natural Science.2023; : 147. CrossRef - Continuous Glucose Monitoring (CGM) and Metabolic Control in a Cohort of Patients with Type 1 Diabetes and Coeliac Disease
Flavia Amaro, Maria Alessandra Saltarelli, Marina Primavera, Marina Cerruto, Stefano Tumini
Endocrines.2023; 4(3): 595. CrossRef - Comparison of Glycemia Risk Index with Time in Range for Assessing Glycemic Quality
Ji Yoon Kim, Jee Hee Yoo, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2023; 25(12): 883. CrossRef - The Benefits Of Continuous Glucose Monitoring In Pregnancy
Jee Hee Yoo, Jae Hyeon Kim
Endocrinology and Metabolism.2023; 38(5): 472. CrossRef - The Growing Challenge of Diabetes Management in an Aging Society
Seung-Hwan Lee
Diabetes & Metabolism Journal.2023; 47(5): 630. CrossRef - Recent advances in artificial intelligence-assisted endocrinology and diabetes
Ioannis T. Oikonomakos, Ranjit M. Anjana, Viswanathan Mohan, Charlotte Steenblock, Stefan R. Bornstein
Exploration of Endocrine and Metabolic Disease.2023; 1(1): 16. CrossRef - An Observational Pilot Study of a Tailored Environmental Monitoring and Alert System for Improved Management of Chronic Respiratory Diseases
Mohammed Alotaibi, Fady Alnajjar, Badr A Alsayed, Tareq Alhmiedat, Ashraf M Marei, Anas Bushnag, Luqman Ali
Journal of Multidisciplinary Healthcare.2023; Volume 16: 3799. CrossRef - Smart Insulin Pen: Managing Insulin Therapy for People with Diabetes in the Digital Era
Jee Hee Yoo, Jae Hyeon Kim
The Journal of Korean Diabetes.2023; 24(4): 190. CrossRef

 KDA
KDA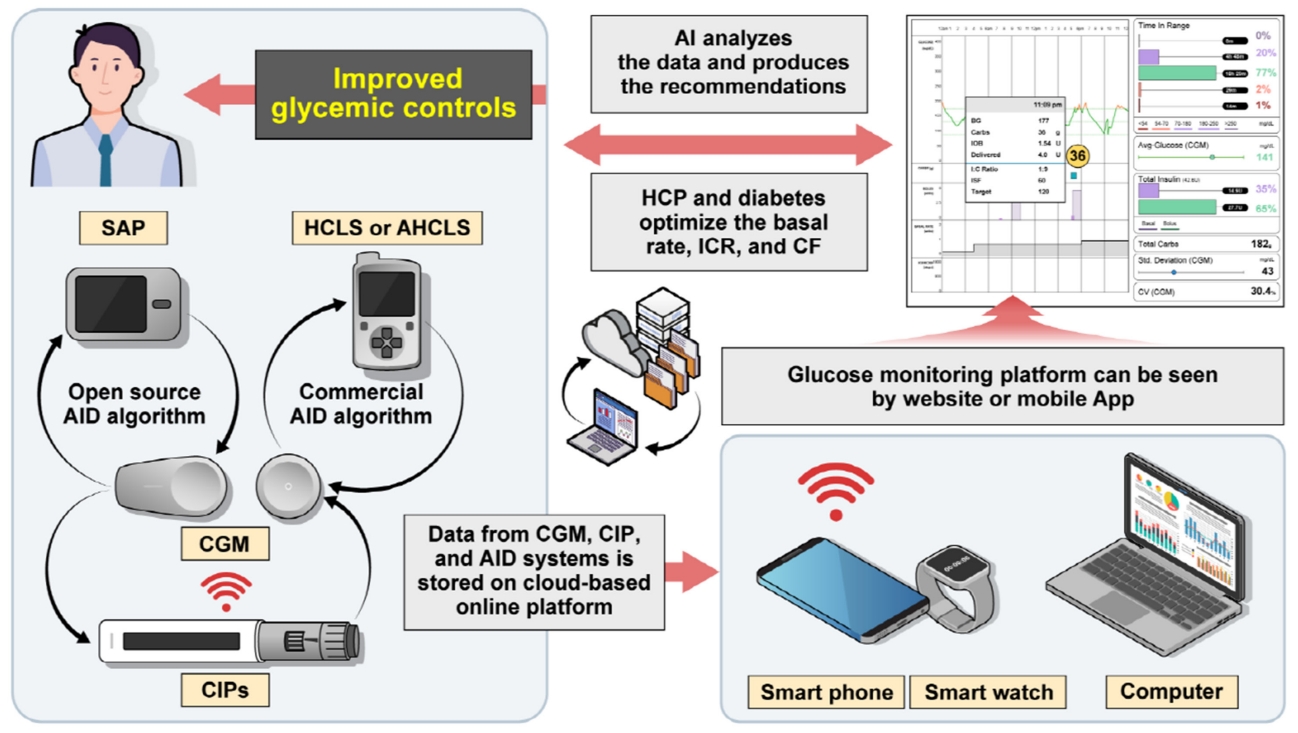
 PubReader
PubReader ePub Link
ePub Link Cite
Cite





