- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 48(2); 2024 > Article
-
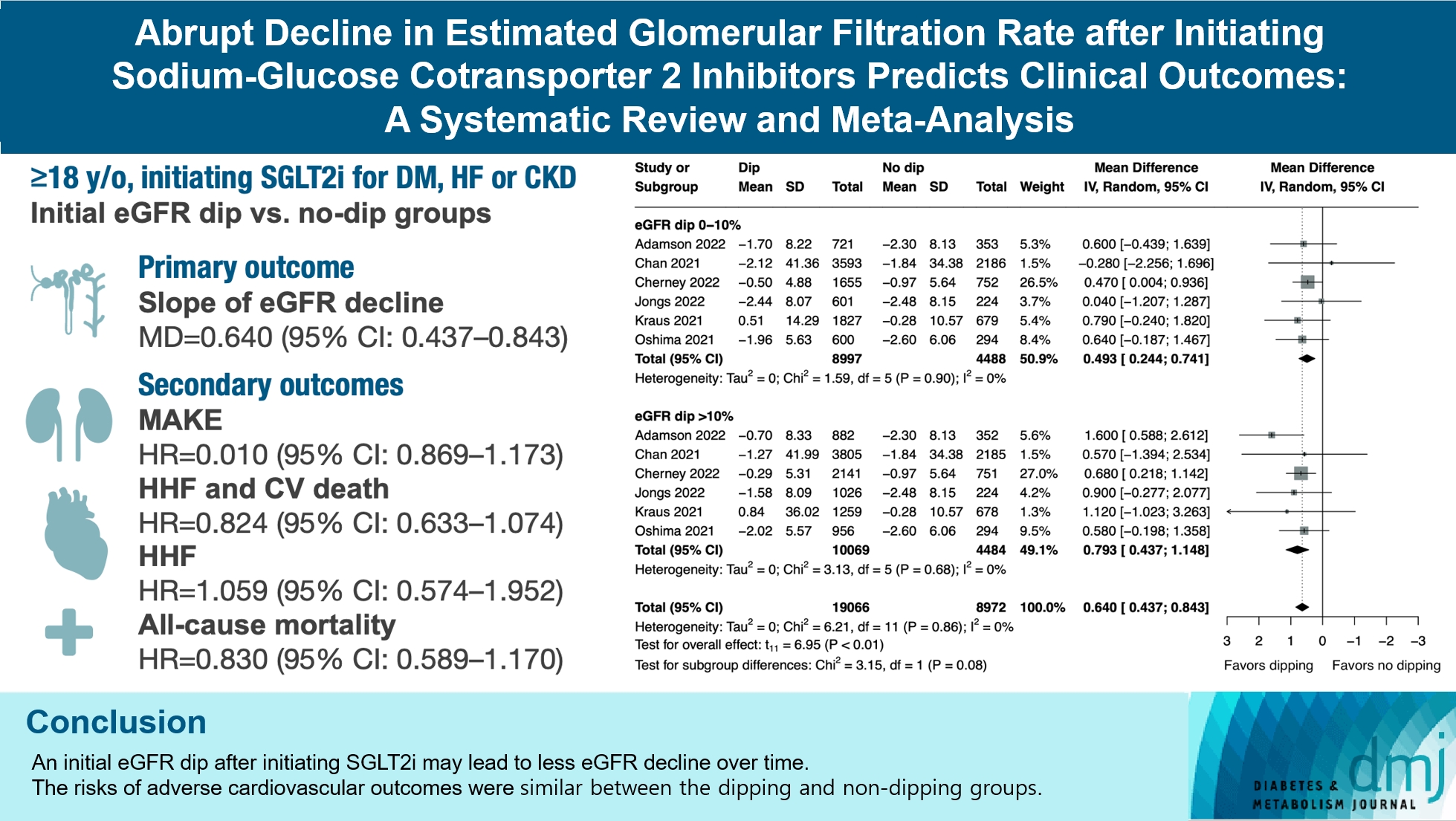
Original ArticleDrug/Regimen Abrupt Decline in Estimated Glomerular Filtration Rate after Initiating Sodium-Glucose Cotransporter 2 Inhibitors Predicts Clinical Outcomes: A Systematic Review and Meta-Analysis
-
Min-Hsiang Chuang1*
 , Yu-Shuo Tang2,3*
, Yu-Shuo Tang2,3* , Jui-Yi Chen4,5, Heng-Chih Pan6, Hung-Wei Liao2,7, Wen-Kai Chu8, Chung-Yi Cheng2,3,7
, Jui-Yi Chen4,5, Heng-Chih Pan6, Hung-Wei Liao2,7, Wen-Kai Chu8, Chung-Yi Cheng2,3,7 , Vin-Cent Wu9, Michael Heung10
, Vin-Cent Wu9, Michael Heung10
-
Diabetes & Metabolism Journal 2024;48(2):242-252.
DOI: https://doi.org/10.4093/dmj.2023.0201
Published online: January 26, 2024
- 1,501 Views
- 202 Download
1Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan
2Division of Nephrology, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
3Taipei Medical University Research Center of Urology and Kidney, Taipei, Taiwan
4Division of Nephrology, Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan
5Department of Health and Nutrition, Chia Nan University of Pharmacy and Science, Tainan, Taiwan
6Division of Nephrology, Department of Internal Medicine, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan
7Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
8Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
9Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
10Division of Nephrology, Department of Medicine, University of Michigan, Ann Arbor, MI, USA
-
Corresponding authors: Chung-Yi Cheng
 Division of Nephrology, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, No. 111, Section 3, Xinglong Rd, Wenshan District, Taipei 116, Taiwan E-mail: 94426@w.tmu.edu.tw
Division of Nephrology, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, No. 111, Section 3, Xinglong Rd, Wenshan District, Taipei 116, Taiwan E-mail: 94426@w.tmu.edu.tw -
Michael Heung
 Division of Nephrology, Department of Medicine, University of Michigan, 500 S State St, Ann Arbor, MI 48109, USA E-mail: mheung@med.umich.edu
Division of Nephrology, Department of Medicine, University of Michigan, 500 S State St, Ann Arbor, MI 48109, USA E-mail: mheung@med.umich.edu - *Min-Hsiang Chuang and Yu-Shuo Tang contributed equally to this study as first authors.
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The initiation of sodium-glucose cotransporter-2 inhibitors (SGLT2i) typically leads to a reversible initial dip in estimated glomerular filtration rate (eGFR). The implications of this phenomenon on clinical outcomes are not well-defined.
-
Methods
- We searched MEDLINE, Embase, and Cochrane Library from inception to March 23, 2023 to identify randomized controlled trials and cohort studies comparing kidney and cardiovascular outcomes in patients with and without initial eGFR dip after initiating SGLT2i. Pooled estimates were calculated using random-effect meta-analysis.
-
Results
- We included seven studies in our analysis, which revealed that an initial eGFR dip following the initiation of SGLT2i was associated with less annual eGFR decline (mean difference, 0.64; 95% confidence interval [CI], 0.437 to 0.843) regardless of baseline eGFR. The risk of major adverse kidney events was similar between the non-dipping and dipping groups but reduced in patients with a ≤10% eGFR dip (hazard ratio [HR], 0.915; 95% CI, 0.865 to 0.967). No significant differences were observed in the composite of hospitalized heart failure and cardiovascular death (HR, 0.824; 95% CI, 0.633 to 1.074), hospitalized heart failure (HR, 1.059; 95% CI, 0.574 to 1.952), or all-cause mortality (HR, 0.83; 95% CI, 0.589 to 1.170). The risk of serious adverse events (AEs), discontinuation of SGLT2i due to AEs, kidney-related AEs, and volume depletion were similar between the two groups. Patients with >10% eGFR dip had increased risk of hyperkalemia compared to the non-dipping group.
-
Conclusion
- Initial eGFR dip after initiating SGLT2i might be associated with less annual eGFR decline. There were no significant disparities in the risks of adverse cardiovascular outcomes between the dipping and non-dipping groups.
Highlights
- Since the emergence of sodium-glucose cotransporter-2 inhibitors (SGLT2i), numerous randomized controlled trials have demonstrated their efficacy in renoprotection and improving annual estimated glomerular filtration rate (eGFR) decline, beyond their initially intended anti-diabetic effects via inhibition of glucose uptake in the proximal convoluted tubule [1-3]. These agents were shown to reduce the incidence of acute kidney injury by 41% [4] and decrease the composite risk of worsening eGFR, end-stage renal disease, or renal death by 20% to 37% [5-7].
- Although SGLT2i are known to have renoprotective effects, their initiation can cause an acute and persistent decline (“dip”) in eGFR sometimes exceeding 10% below baseline, which is typically reversible after discontinuation of SGLT2i [8-11]. Glomerular hyperfiltration, observed in 10%–67% and 6%–73% of patients with type 1 and type 2 diabetes mellitus respectively [12], may involve upregulation of SGLTs and an imbalance of vasoactive humoral factors regulating pre- and postglomerular arterioles [13]. By inhibiting SGLT2, SGLT2i may alleviate glomerular hyperfiltration [14-16], and the reversible nature of SGLT2i-induced dip also suggests a hemodynamic regulation of intraglomerular pressure [15,17].
- The level of eGFR dip can vary widely among different individuals [8-11] and may reflect the effect of SGLT2i in relieving intraglomerular pressure [18], which has implications for clinical outcomes. However, current understanding of the dip phenomenon is limited and previous studies have yielded inconsistent results. While one study reported a larger initial eGFR dip being associated with a slower chronic decline in eGFR [10], others did not find significant differences among patients with different levels of eGFR dip [9,19]; discrepancies also existed regarding the risk of adverse cardiovascular and mortality outcomes [19,20]. Therefore, we conducted this systematic review and meta-analysis to comprehensively evaluate the kidney, cardiovascular, and survival outcomes among patients with different levels of eGFR dip after initiating SGLT2i.
INTRODUCTION
- The current study was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Supplementary Table 1) [21]. The protocol for this systematic review and meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42023411012). The current study was exempt from ethics approval because it was conducted through collection of data from primary studies for which ethical approval had been obtained.
- Data sources and search strategies
- We searched the electronic databases of MEDLINE, the Cochrane Central Register of Controlled Trials, and Embase for eligible studies, with no language restriction, from inception to March 23, 2023. Randomized controlled trials or cohort studies that evaluated differences in kidney or cardiovascular outcomes, risk of death, and adverse events (AEs) among patients with different levels of initial eGFR decline after starting SGLT2i were included. The search terms included various SGLT2i drug names, relevant MeSH or Emtree terms, and related keywords. The syntax of the search strategy was provided in Supplementary Table 2. Manual screening of the references of included articles was also conducted to identify additional potentially eligible studies. Two investigators (M.H.C. and Y.S.T.) searched published reports independently to increase methodological rigor and to prevent ascertainment bias. We then obtained the full text of the selected papers for quality assessment and data synthesis. If necessary, we contacted the authors of the papers to obtain more information.
- Inclusion and exclusion criteria
- The following criteria were applied to screen for eligible studies: (1) population: adults (age ≥18 years) initiating SGLT2i for type 2 diabetes mellitus, heart failure, or chronic kidney disease; (2) exposure: individuals with initial decline of eGFR after initiating SGLT2i. The time periods defining the initial eGFR decline were based on those of individual studies, ranging from 2 weeks to 3 months; (3) comparison: individuals without initial eGFR decline after initiating SGLT2i; and (4) outcomes: the slope of long-term kidney function decline, mortality risk, adverse kidney outcomes, adverse cardiovascular outcomes, and AEs during treatment.
- Criteria for exclusion included: (1) pediatric population (age <18 years); (2) studies without aforementioned exposure and control groups; (2) studies that used SGLT2i for indications other than type 2 diabetes mellitus, heart failure, or chronic kidney disease; (3) those not reporting details for the outcomes of interest; (4) studies on animal models; (5) those published only in abstract format; and (6) those published without peer review.
- Study selection and data extraction
- The titles and abstracts of the retrieved studies were screened by two reviewers (M.H.C. and Y.S.T.) independently for eligibility based on the inclusion and exclusion criteria detailed above. Studies that met the criteria were evaluated in full text by the same two reviewers, and any disagreements were resolved through discussion with a third author. Two reviewers independently extracted data from the included studies, including the first author, year of publication, patient characteristics, study design, sample sizes, therapies administered, initial changes in eGFR after starting SGLT2i, and relevant data on kidney or cardiovascular outcomes, mortality risk, and AEs during SGLT2i treatment. The extracted data were cross-checked for accuracy by the two reviewers.
- Outcomes
- The primary outcome of the present study was the slope of eGFR decline measured in mL/min/1.73 m2 per year. Secondary outcomes included major adverse kidney events (MAKE) including sustained ≥50% decrease in eGFR, eGFR <15 mL/min/1.73 m2, doubling of creatinine level, need for chronic dialysis or renal transplant, or death from renal failure. Other secondary outcomes included hospitalized heart failure, cardiovascular death, all-cause death, and various AE-related outcomes such as discontinuation of SGLT2i due to AEs, serious AEs, volume depletion, hyperkalemia, acute kidney injury, or kidney-related AEs. The outcomes were compared between patients with and without initial eGFR dip, with the non-dipping group set as the reference.
- Quality and certainty assessment
- Methodological quality of the included studies was assessed independently by two reviewers (M.H.C. and Y.S.T.) using the Newcastle-Ottawa Scale for cohort studies [22]. The scale contained eight items in three categories: selection of cohorts (four items), comparability (one item), and evaluation of outcomes (three items). Studies were scored from 0 to 9 points and those scoring ≥7 points were deemed of high quality. Studies were considered of moderate quality with some risk of bias if they scored 4 to 6, and of low quality if they scored 0 to 3 [23]. Two review authors (Y.S.T. and J.Y.C.) independently assessed and graded the certainty of evidence regarding our primary and secondary outcomes as high, moderate, low, or very low according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [24]. Any disagreement between the two reviewers were settled through consultation with a third author (V.C.W.).
- Data analysis
- Data synthesis was conducted with random-effect meta-analysis. Continuous data was reported as mean difference (MD) with 95% confidence interval (CI) and dichotomous data as odds ratio (OR) with 95% CI using the Mantel-Haenszel method, or hazard ratio (HR) with 95% CI using generic inverse variance analysis. The non-dipping group, i.e., patients whose eGFR remained the same or increased after initiating SGLT2i, was used as the control group. Heterogeneity was assessed using the I2 statistic and considered substantial if the I2 exceeded 50% [25]. Publication bias was evaluated through visual inspection of the funnel plot, and we performed sensitivity analysis with the leave-one-out approach to assess the impact of individual trials on the overall outcome estimates. In order to prevent double-counting of the shared group (specifically, the control cohort) in studies with multiple comparative cohorts, the events and total numbers of dichotomous data in the control group were evenly divided if they were included more than once in any particular analysis [26]. Pre-specified subgroup analysis stratified by a cut-off of 10% eGFR dip was conducted to explore differences in primary and secondary outcomes among patients with different levels of initial dip in eGFR. Network meta-analysis was employed for comparison of the annual eGFR decline, MAKE, and cardiovascular outcomes between the subgroups with >10% and ≤10% eGFR. We conducted meta-regression analyses to examine the impact of various study characteristics, such as age, baseline eGFR, proportion of females, and proportion of patients with diabetes mellitus, on kidney outcomes. Trial sequential analysis (TSA) was performed on all-cause mortality and the composite outcome of hospitalized heart failure and cardiovascular death to control type I and type II errors and estimate the required information size since these outcomes had few included studies. The α-spending boundaries were calculated with a two-sided type I error risk of 5%, and the futility boundaries were calculated with a 90% power using the O’Brien-Fleming function. Statistical analyses were conducted using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and TSA software version 0.9.5.10 Beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, The Capital Region, Denmark).
METHODS
- Selection and characteristics of studies
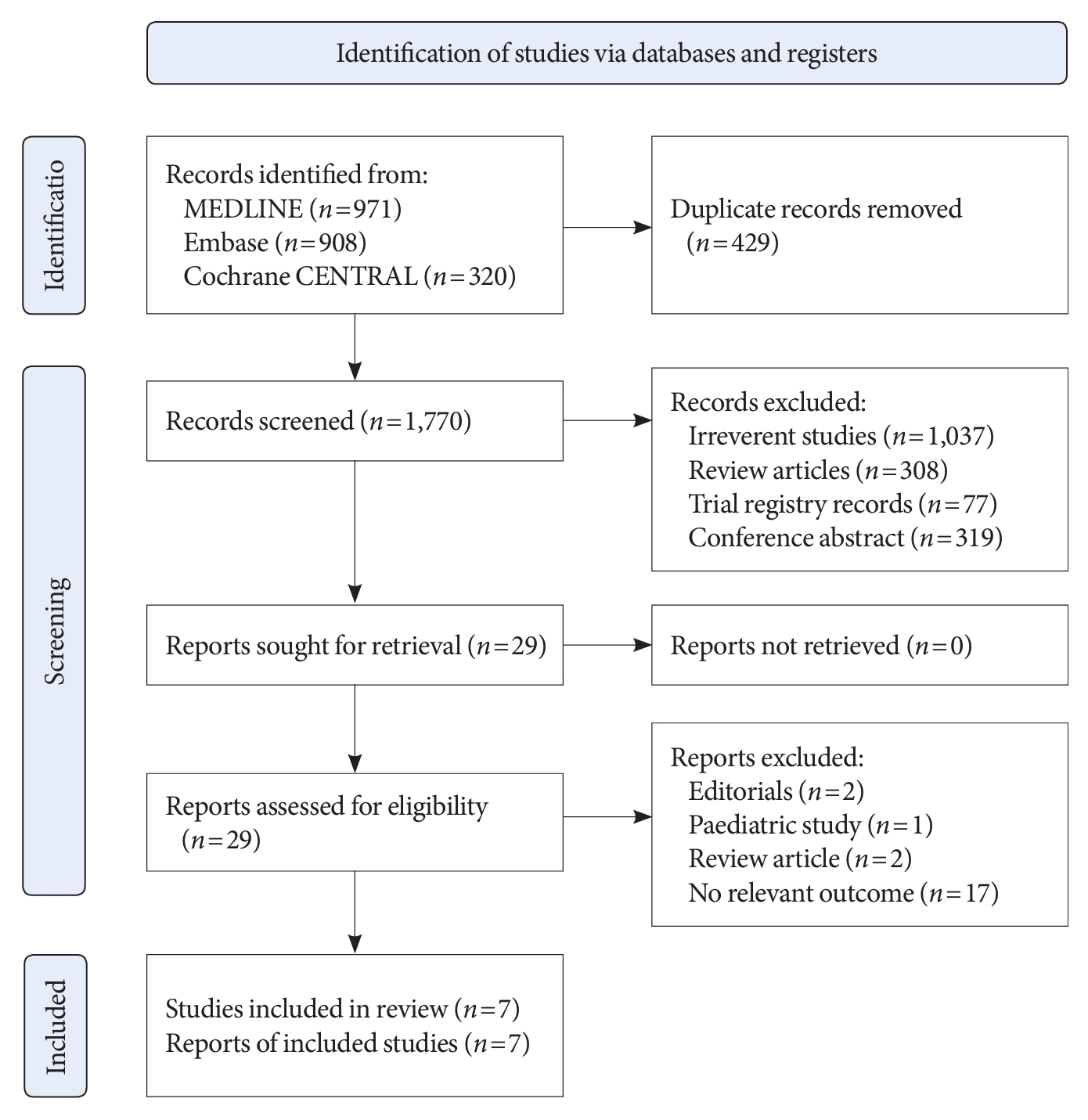
- A total of 2,199 records were identified through our literature search. After removal of duplicates (n=429), 1,770 records were screened and 29 were evaluated in full text. Finally, seven studies published between 2020 and 2022 were included in the current meta-analysis. A flowchart of the selection process was shown in Fig. 1.
- All studies were observational studies. The mean age of participants was between 58.8 and 67.8 years; females comprised 20% to 45% of patients. After initiation of an SGLT2i, 28% to 41% of patients experienced a ≤10% dip in eGFR, while 28% to 49% had a >10% dip. Details regarding characteristics of the included studies were shown in Table 1.
- Quality assessment
- The included studies scored between 6 and 9 points on the Newcastle-Ottawa Scale. Five studies [9,19,20,27,28] were rated as high quality, while two studies [11,29] were considered to be of moderate quality. Two studies [11,29] raised concern regarding the comparability of cohorts, and five studies [11,20,27-29] were considered to be at risk of bias in terms of the adequacy of follow-up (Supplementary Table 3).
- Outcomes
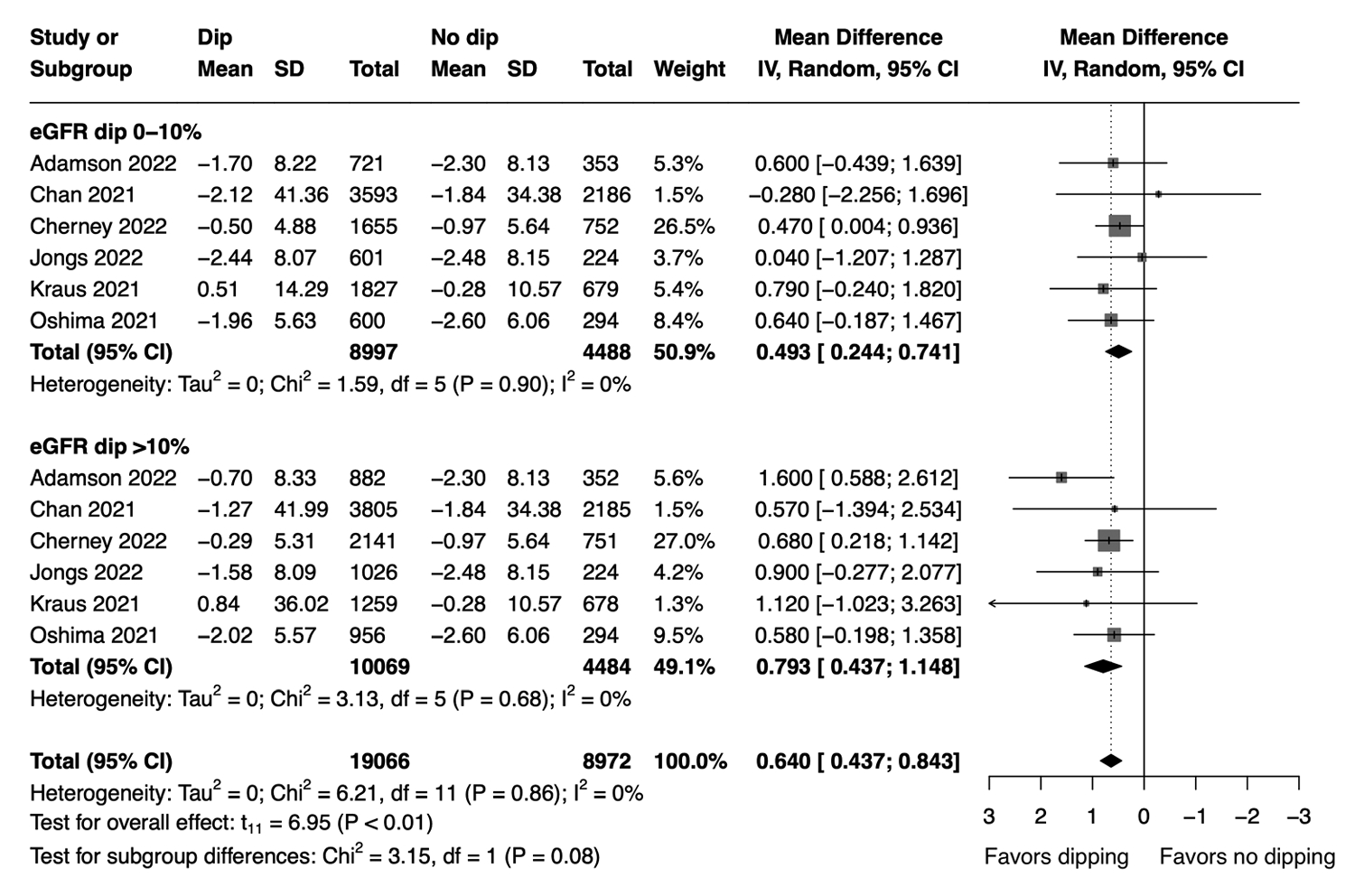
- Our meta-analysis demonstrated that patients with initial eGFR dip after starting SGLT2i had less annual eGFR decline compared to the non-dipping group (MD, 0.64; 95% CI, 0.437 to 0.843 mL/min/1.73 m2 per year; P<0.01; I2=0%; sensitivity analysis, consistent results). Subgroup analysis showed similar findings in patients with ≤10% dip in eGFR (MD, 0.493; 95% CI, 0.244 to 0.741 mL/min/1.73 m2 per year) and those with >10% dip in eGFR (MD, 0.793; 95% CI, 0.437 to 1.148 mL/min/1.73 m2 per year) (Fig. 2). Comparison between the two subgroups showed that patients with >10% eGFR dip had less annual eGFR decline compared to those with ≤10% dip (MD, 0.299; 95% CI, 0.051 to 0.547 mL/min/1.73 m2 per year) (Supplementary Table 4). When subgrouped by baseline eGFR, patients with initial eGFR dip had less annual eGFR decline compared to the non-dipping group regardless of their baseline eGFR level (Supplementary Fig. 1).
- In comparison, no significant difference in annual eGFR decline was found between the dipping and non-dipping groups in patients given placebo (MD, 0.258; 95% CI, –0.031 to 0.547 mL/min/1.73m2 per year) (Supplementary Fig. 2). No apparent publication bias was identified through visual inspection of the funnel plot (Supplementary Fig. 3). Results from meta-regression analysis indicated that age, baseline eGFR, proportion of females, and proportion of patients with diabetes were not significant moderators of annual eGFR decline (Supplementary Table 5).
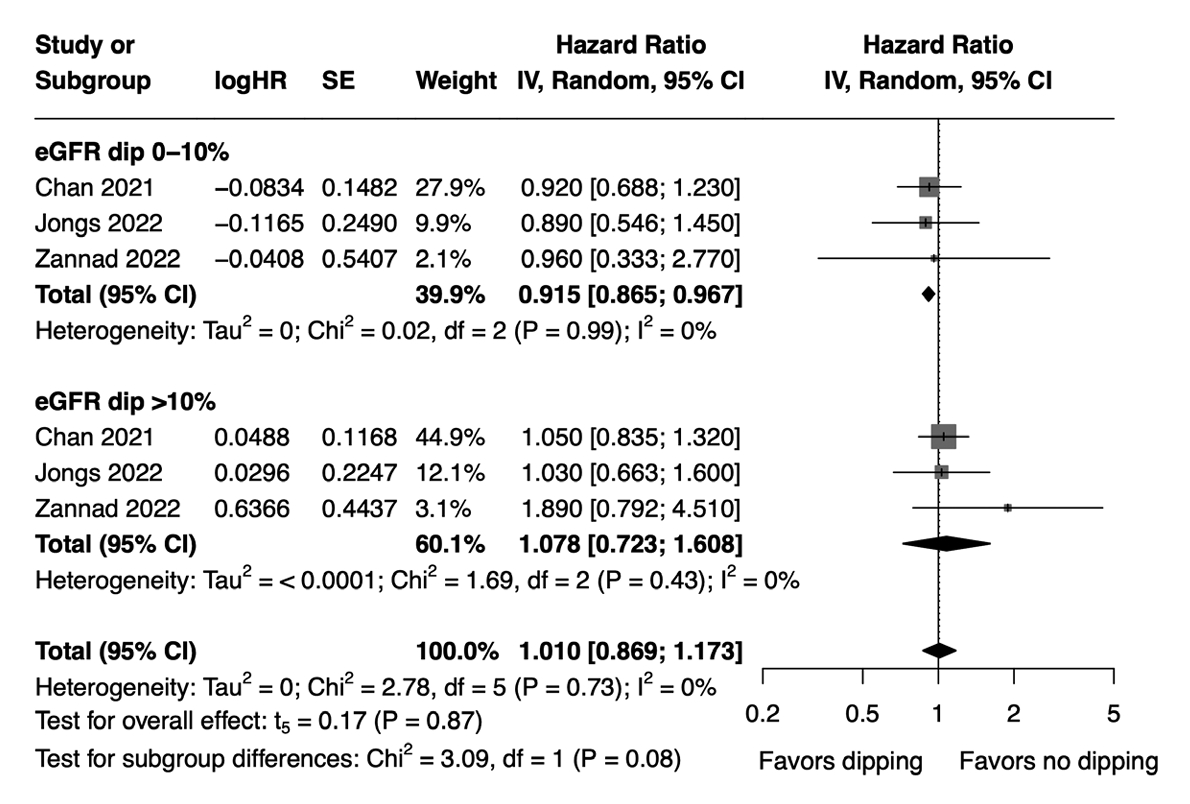
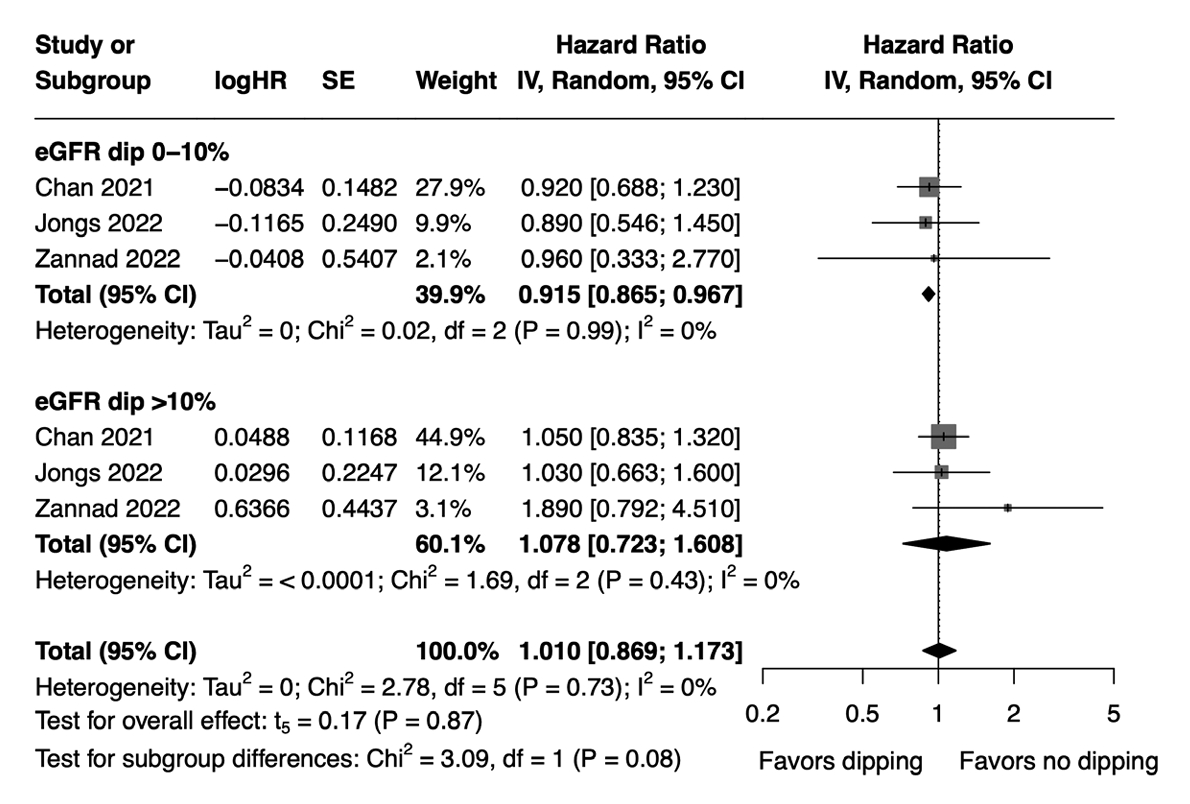
- The hazard of MAKE did not significantly differ between the dipping and non-dipping groups (HR, 1.01; 95% CI, 0.869 to 1.173; I2=0%; sensitivity analysis, consistent results). However, subgroup analysis revealed a marginally lower hazard of MAKE in patients with an initial eGFR dip of ≤10% compared to the non-dipping group (HR, 0.915; 95% CI, 0.865 to 0.967), whereas no significant difference was observed between patients with an initial eGFR dip of >10% and the non-dipping group (HR, 1.078; 95% CI, 0.723 to 1.608) (Fig. 3). The effect sizes were not significantly different between the two subgroups (HR, 1.190; 95% CI, 0.870 to 1.629) (Supplementary Table 4). Meta-regression analysis showed that age, baseline eGFR, proportion of females, and proportion of patients with diabetes mellitus were not significant moderators of MAKE (Supplementary Table 5). Of note, the study by Zannad et al. [20] was not included in our meta-regression analysis for the association between MAKE and proportion of patients with diabetes mellitus due to the unavailability of relevant data in that study.
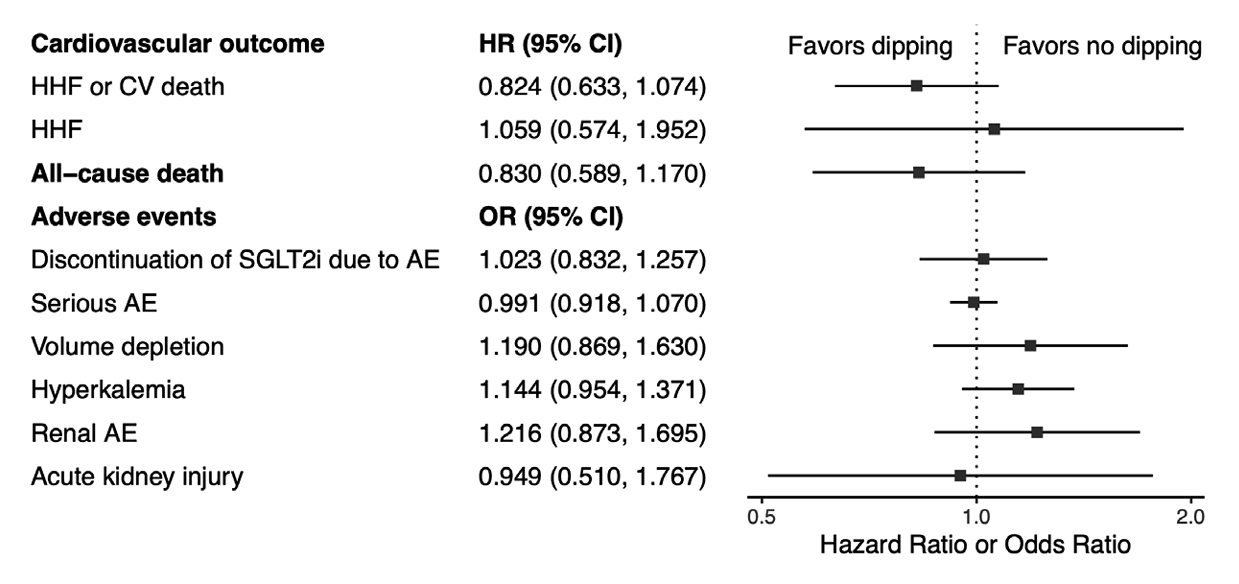
- There was a non-significant reduction in the hazard of the composite outcome of hospitalized heart failure and cardiovascular death in the dipping group, but the difference did not reach statistical significance (HR, 0.824; 95% CI, 0.633 to 1.074; I2=0%; sensitivity analysis, consistent results) (Fig. 4, Supplementary Fig. 4A). Additionally, no significant difference was observed in the hazard of hospitalized heart failure between the two groups (HR, 1.059; 95% CI, 0.574 to 1.952; I2=37%; sensitivity analysis, consistent results) (Fig. 4, Supplementary Fig. 4B). No significant differences were observed regarding the cardiovascular outcomes in patients with ≥10% eGFR dip compared to those with <10% eGFR dip (Supplementary Table 4).
- There was no significant difference in the hazard of all-cause mortality between the two groups (HR, 0.83; 95% CI, 0.589 to 1.170; I2=15%; sensitivity analysis, consistent results) (Fig. 4, Supplementary Fig. 5). The effect sizes were similar in patients with ≥10% and <10% eGFR dip (Supplementary Table 4).
- There were no significant differences between the dipping and non-dipping groups regarding the risk of SGLT2i discontinuation due to AEs (OR, 1.023; 95% CI, 0.832 to 1.257; I2=5%) (Fig. 4, Supplementary Fig. 6), serious AEs (OR, 0.991; 95% CI, 0.918 to 1.070; I2=0%) (Fig. 4, Supplementary Fig. 7), renal AEs (OR, 1.216; 95% CI, 0.873 to 1.695; I2=56%) (Fig. 4, Supplementary Fig. 8), acute kidney injury (OR, 0.949; 95% CI, 0.510 to 1.767; I2=56%) (Fig. 4, Supplementary Fig. 9), and volume depletion (OR, 1.190; 95% CI, 0.869 to 1.630; I2=0%) (Fig. 4, Supplementary Fig. 10). There was no significant difference in the overall risk of hyperkalemia between the two groups (OR, 1.144; 95% CI, 0.954 to 1.371; I2=0%) (Fig. 4, Supplementary Fig. 11). However, subgroup analysis demonstrated that an initial eGFR dip of >10% was associated with a higher risk of hyperkalemia compared to the non-dipping group (OR, 1.240; 95% CI, 1.148 to 1.338) (Supplementary Fig. 11).
- Trial sequential analysis
- The required information size for all-cause mortality was estimated to be 16,198 when the incidence in control group was set at 10.5%, and 75,628 for the composite outcome of hospitalized heart failure and cardiovascular death when the incidence in control group was set at 14.4%. The cumulative Z curve of both outcomes did not cross the trial sequential monitoring boundaries, the futility boundaries, or the required information size, suggesting that the current evidence has yet to reach a solid conclusion (Supplementary Figs. 12 and 13).
- Certainty of evidence
- The certainty of evidence for our primary and secondary outcomes was assessed as low at baseline since the included studies were observational in nature. However, most outcomes were judged to have a low risk of bias, inconsistency, indirectness, and publication bias. Outcomes related to hospitalized heart failure, cardiovascular death, kidney-related AEs, volume depletion, and hyperkalemia were further downgraded due to imprecision of results. More information regarding our certainty of evidence assessment can be found in Supplementary Table 6.
RESULTS
Primary outcome: slope of eGFR decline
Secondary outcomes: major adverse kidney events
Secondary outcomes: hospitalized heart failure and cardiovascular death
Secondary outcomes: all-cause mortality
Secondary outcomes: adverse events
- Our study demonstrated that the initial eGFR dip induced by SGLT2i was associated with a more favorable annual eGFR decline compared to the non-dipping group. No significant differences were observed in all-cause mortality, the composite outcome of hospitalized heart failure and cardiovascular death, discontinuation of SGLT2i due to AEs, serious AEs, volume depletion, kidney-related AEs, and acute kidney injury between the dipping and non-dipping groups. Notably, patients in the subgroup with a ≤10% dip had a significantly reduced risk of MAKE, while patients with >10% dip exhibited a potential risk of hyperkalemia.
- Kidney outcomes
- In the current study, it was found that patients who experienced the dip phenomenon had less annual eGFR decline compared to the non-dipping group, supporting the involvement of hemodynamic-related mechanisms given the reversible nature of such dip phenomenon [9-11,15], as well as a potential association between the dip level and intraglomerular pressure before treatment [18]. In addition, the subgroup of patients with dip >10% was found to have greater improved eGFR slope than those with ≤10% dip. These results should encourage clinicians not to abruptly discontinue SGLT2i upon detecting an initial dip in eGFR. The study by Zannad et al. [20] was not included in our analysis for eGFR slope due to lack of relevant data for the calculation of annual eGFR decline, but the trend of eGFR changes observed in that study was generally in line with our findings.
- While no significant difference in MAKE was found between the dipping and non-dipping groups, our subgroup analysis revealed a reduced risk of MAKE in patients with ≤10% eGFR dip, but not in those with >10% dip. The discrepancy between annual eGFR decline and the occurrence of MAKE might be due to differences in baseline characteristics among the subgroups. The SGLT2i-induced eGFR dip was reportedly more likely to occur in patients who were older, had a longer history of diabetes, a higher body mass index, or were using diuretics [9,27]. Additional factors found to be associated with an eGFR dip >10% included male sex, increased sodium intake, and lower eGFR at baseline [9,30]. Moreover, the relationship between the risk of MAKE and the dip level might not be linear. An observational study in 2021 suggested that an eGFR dip >30% after initiating SGLT2i was associated with an increased risk of serum creatinine doubling and eGFR <15 mL/min/1.73 m2 [28], indicating that “extreme dippers” may be at higher risk of MAKE. However, since few studies have evaluated clinical outcomes of such extreme dippers [20,28], a subgroup analysis for this population was not feasible in the current study.
- Our meta-regression analysis showed that age, sex, baseline eGFR, and diabetes status were not significantly correlated to renal outcomes. The improved annual eGFR slope in patients with initial eGFR dip compared to the non-dipping group was also consistently observed in all subgroups stratified by baseline eGFR. One potential explanation for the lack of significant moderation by these factors in the overall analysis could be the non-linear relationship between baseline eGFR and renal outcomes that could not be detected by the meta-regression model. Additionally, the included studies displayed relatively low heterogeneity concerning participant characteristics such as mean age, the proportion of females, and the prevalence of diabetes mellitus. This low heterogeneity led to clustered data points, which could potentially limit our ability to demonstrate statistically significant associations.
- The exact mechanism of which SGLT2i induce eGFR dip remained an area of active research. Several hypotheses were postulated to explain the renoprotective effect and the dip phenomenon related to SGLT2i. The reversible initial eGFR decline with subsequent improved annual eGFR slope supports the hypotheses that renoprotective effect originate hemodynamically [15,17], which might involve pre-glomerular arteriole constriction and/or post-glomerular arteriole dilatation [31,32].
- Inhibition of SGLT-2 has been shown to increase the sodium concentration in the distal convoluted tubule, which in turn leads to pre-glomerular arteriole constriction through tubuloglomerular feedback [33]. This constriction results in a transient decrease in eGFR and can help alleviate intraglomerular pressure [16]. Additionally, there is evidence to suggest that post-glomerular arteriole dilation, similar to the action of renin-angiotensin blockers, may have also contributed to the transient dip in eGFR [31]. A similar phenomenon was observed in the Systolic Blood Pressure Intervention Trial (SPRINT) study, of which the initial eGFR changes in the intensive blood-pressure control group that stabilized during the subsequent study period [34] and subgroup analysis of urine biomarkers of tubule function and repair [35] also suggested that the changes might be caused by hemodynamic factors rather than intrinsic renal damages.
- In our study, we found that SGLT2i-induced dip was associated with a reduction in annual eGFR decline and MAKE with ≤10% dipper. The acute change in eGFR after initiating SGLT2i could reflect intraglomerular pressure changes and might be used as a marker for predicting the renoprotective effects of SGLT2i. However, further research is still needed to determine the optimal range of dip, particularly in extreme dippers where limited data were available.
- Cardiovascular outcomes
- Our study showed a non-significant reduced risk for the composite outcome of hospitalized heart failure and cardiovascular death in patients with initial eGFR dip, but the finding did not reach statistical significance. Moreover, the risk of hospitalized heart failure was similar between the dipping and non-dipping groups. We included only three studies in our analysis of cardiovascular outcomes (i.e., two [19,20] for evaluation on the composite of hospitalized heart failure and cardiovascular death, and two [20,28] for evaluation of hospitalized heart failure), as the number of eligible studies was limited by high variability in outcome definition and cut-points of dip levels selected to divide the compared groups. Therefore, our findings on cardiovascular outcomes should be interpreted with caution considering their wide CIs and the non-conclusive results from our TSA.
- In a post hoc analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) study [27], patients with initial eGFR dip >10% had lower risks of hospitalized heart failure and cardiovascular death compared to those with dip ≤10% (including the non-dipping individuals). While this finding was generally in line with ours, the difference between the dipping versus non-dipping group was not reported in that study. According to a prior observational study, individuals who experienced an initial eGFR decline of over 30% were at a higher risk of new-onset atrial fibrillation, MAKE, and heart failure [28]. However, it is yet to be determined in future research if the occurrence of these adverse cardiac events ultimately affects cardiovascular survival.
- All-cause mortality and adverse events
- We found no significant differences in all-cause mortality, hyperkalemia, acute kidney injury, volume depletion, serious AEs, and discontinuation of SGLT2i due to AEs between the dipping and non-dipping groups. However, in the subgroup of patients with an eGFR dip >10%, there was an increased risk of hyperkalemia. It is noteworthy that a substantial eGFR decline exceeding 10%, induced by SGLT2 inhibitors, predominantly manifests in patients with a lower baseline eGFR [9,30]. While we did observe a marked improvement in the chronic eGFR slope and no heightened risk of MAKE in this particular group, impaired urinary potassium excretion can transpire when there is a concurrent reduction in glomerular filtration rate, tubular flow, or sodium delivery to the distal nephron [36]. This consideration underscores the nuanced interplay between eGFR dynamics, the use of SGLT2 inhibitors, and the associated risk of hyperkalemia. As a result, patients with a dip level >10% compared to baseline should have their potassium levels monitored carefully.
- Limitation
- There were some limitations to consider in the current study. Firstly, variations existed in the included studies regarding their definition of MAKE and the cut-off points for eGFR dip. Although we excluded studies with significantly different cutoff values and outcome definitions to obtain similar patient groups, these variations could still have influenced the results. Secondly, the relationship between clinical outcomes and the dip level might not be strictly linear; furthermore, this study was unable to evaluate outcomes in patients with initial eGFR dip >30% due to limited data. Future studies are required to determine the acceptable dip range. Thirdly, all included studies were observational, and therefore the findings should be interpreted with caution. Though most studies were evaluated to be of high quality and the remaining two of moderate quality, selection bias, information bias, and confounding factors could still have affected our results. Fourth, it is important to note that our findings regarding cardiovascular outcomes should not be deemed conclusive since the number of patients recruited did not exceed the required sample size. Moreover, although our analyses demonstrated low to moderate statistical heterogeneity in all the outcomes, some clinical heterogeneity still existed in the included studies regarding their designs and clinical scenarios. Despite the limitations, our findings contributed to the current understanding of the role of eGFR dip in predicting kidney outcomes in patients treated with SGLT2i.
- Conclusion
- In summary, our findings suggested that acute eGFR dip induced by SGLT2i was associated with a favorable annual eGFR decline. Patients with a ≤10% dip had a significantly reduced risk of MAKE, while patients with >10% dip had a potential risk of hyperkalemia, emphasizing the importance of monitoring potassium levels in these patients when initiating SGLT2i. However, studies are still needed to determine the optimal threshold for eGFR dip and its impact on clinical outcomes. Our findings should be interpreted with caution given the observational nature of the included studies.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Table 3.
Supplementary Table 4.
Supplementary Table 5.
Supplementary Table 6.
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Fig. 4.
Supplementary Fig. 5.
Supplementary Fig. 6.
Supplementary Fig. 7.
Supplementary Fig. 8.
Supplementary Fig. 9.
Supplementary Fig. 10.
Supplementary Fig. 11.
Supplementary Fig. 12.
Supplementary Fig. 13.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: C.Y.C., V.C.W., M.H.
Acquisition, analysis, or interpretation of data: M.H.C., Y.S.T., J.Y.C., H.C.P., V.C.W., M.H.
Drafting the work or revising: all authors.
Final approval of the manuscript: all authors.
-
FUNDING
None
NOTES
-
Acknowledgements
- None
| Study | SGLT2i | Study design | No. of patienta | Age, yr | Female, % | Follow-up period | Location | Population | DM, % | HF, % | eGFR, mL/min/1.73 m2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oshima et al. (2021) [9] | Canagliflozin | Post hoc analyses of RCT | >10%: 956 | 63.3±9.3 | 33.6 | Median 2.6 yr | 34 Countries | T2DM, CKD with pro-teinuria | 100 (inclusion criteria) | 13.3 | 57.9±18.1 |
| 0%–10%: 600 | 62.7±8.9 | 33.7 | 14.3 | 56.9±18.2 | |||||||
| No dip: 588 | 62.1±9.1 | 36.2 | 18.2 | 53.6±18.1 | |||||||
| Kraus et al. (2021) [11] | Empagliflozin | Post hoc analyses of RCT | >10%: 1,259 | 64.6±8.3 | 30.5 | Median 3.1 yr | 42 Countries | T2DM, CV disease | 100 (inclusion criteria) | 10.9 | 68.4±18.1 |
| 0%–10%: 1,827 | 62.8±8.4 | 28.0 | 8.2 | 79.5±22.9 | |||||||
| No dip: 1,357 | 62.2±8.8 | 27.8 | 10.0 | 72.9±20.6 | |||||||
| Jongs et al. (2022) [19] | Dapagliflozin | Post hoc analyses of RCT | >10%: 1,026 | 62.9±11.5 | 34.0 | Median 2.3 yr | 21 Countries | CKD with pro-teinuria | 72.8 | 10.6 | 43.0±12.3 |
| 0%–10%: 601 | 61.1±12.1 | 30.3 | 62.9 | 9.8 | 43.4±12.3 | ||||||
| No dip: 448 | 61.1±12.7 | 34.2 | 65.2 | 13.4 | 43.9±12.5 | ||||||
| Zannad et al. (2022) [20] | Empagliflozin | Post hoc analyses of RCT | >11.4%: 594 | 67.7±10.5 | 29.0 | Median 16 mo | 20 Countries | HFrEF | NR | 100 (inclusion criteria) | 60.7±21.7 |
| 1%–11.4%: 578 | 67.2±11.0 | 21.6 | 65.3±21.9 | ||||||||
| No dip: 610 | 66.9±10.9 | 20.0 | 59.3±22.8 | ||||||||
| Adamson et al. (2022) [27] | Dapagliflozin | Post hoc analyses of RCT | >10%: 882 | 67.8±10.7 | 25.1 | Median 18.2 mo | 20 Countries | HFrEF | 46.8 | 100 (inclusion criteria) | 62.6±17.9 |
| 0%–10%: 721 | 65.3±11.2 | 20.5 | 38.8 | 69.5±20.9 | |||||||
| No dip: 706 | 65.2±10.8 | 25.4 | 39.2 | 66.6±19.3 | |||||||
| Chan et al. (2021) [28] | Empagliflozin, dapagliflozin, canagliflozin | Retrospective cohort study | >10%: 3,805 | 59.5±12.1 | 44.5 | Mean 13.9 mo | Taiwan | T2DM | 100 (inclusion criteria) | 5.5 | 95.9±32.9 |
| 0%–10%: 3,593 | 58.8±11.6 | 38.7 | 4.0 | 93.0±27.8 | |||||||
| No dip: 4,371 | 58.9±11.5 | 40.4 | 4.7 | 87.2±26.1 | |||||||
| Cherney et al. (2022) [29] | Ertugliflozin | Post hoc anal-yses of RCT | >8%: 2,141 | 64.3±8.1 | 30.7 | Median 3.0 yr | 34 Countries | T2DM | 100 (inclusion criteria) | NR | 81.1±21.5 |
| 0%–8%: 1,655 | 64.9±8.1 | 28.5 | 71.3±20.0 | ||||||||
| No dip: 1,503 | 63.7±8.0 | 29.3 | 74.2±19.2 |
Values are presented as mean±standard deviation.
SGLT2i, sodium-glucose cotransporter 2 inhibitor; DM, diabetes mellitus; HF, heart failure; eGFR, estimated glomerular filtration rate; RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus; CKD, chronic kidney disease; CV, cardiovascular; HFrEF, heart failure with reduced ejection fraction; NR, not reported.
a Participants grouped according to level of eGFR dip after initiation of SGLT2i.
- 1. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436-46.ArticlePubMed
- 2. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-306.ArticlePubMed
- 3. Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose cotransporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788-801.PubMedPMC
- 4. Donnan JR, Grandy CA, Chibrikov E, Marra CA, Aubrey-Bassler K, Johnston K, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open 2019;9:e022577.ArticlePubMedPMC
- 5. Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PA, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med 2020;10:1-10.ArticlePubMedPDF
- 6. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31-9.ArticlePubMed
- 7. Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab 2019;21:1237-50.ArticlePubMedPDF
- 8. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlavek J, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020;323:1353-68.ArticlePubMedPMC
- 9. Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int 2021;99:999-1009.ArticlePubMed
- 10. Cherney DZ, Charbonnel B, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley R, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia 2021;64:1256-67.ArticlePubMedPMCPDF
- 11. Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZ, Sattar N, et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 2021;99:750-62.ArticlePubMed
- 12. Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017;28:1023-39.ArticlePubMedPMC
- 13. Vallon V, Komers R. Pathophysiology of the diabetic kidney. Compr Physiol 2011;1:1175-232.ArticlePubMedPMCPDF
- 14. Thomson SC, Vallon V. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am J Physiol Renal Physiol 2021;320:F761-71.ArticlePubMedPMC
- 15. Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587-97.ArticlePubMed
- 16. Kidokoro K, Cherney DZ, Bozovic A, Nagasu H, Satoh M, Kanda E, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 2019;140:303-15.ArticlePubMed
- 17. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323-34.ArticlePubMed
- 18. Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 2020;16:317-36.ArticlePubMedPMCPDF
- 19. Jongs N, Chertow GM, Greene T, McMurray JJV, Langkilde AM, Correa-Rotter R, et al. Correlates and consequences of an acute change in eGFR in response to the SGLT2 inhibitor dapagliflozin in patients with CKD. J Am Soc Nephrol 2022;33:2094-107.ArticlePubMedPMC
- 20. Zannad F, Ferreira JP, Gregson J, Kraus BJ, Mattheus M, Hauske SJ, et al. Early changes in estimated glomerular filtration rate post-initiation of empagliflozin in EMPEROR-Reduced. Eur J Heart Fail 2022;24:1829-39.PubMed
- 21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.ArticlePubMedPMC
- 22. Ottawa Hospital Research Institute: Newcastle-Ottawa quality assessment scale case control studies. Available from: https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (cited 2023 Oct 17).
- 23. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45.ArticlePubMedPMCPDF
- 24. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.ArticlePubMedPMC
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.ArticlePubMedPMC
- 26. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.3, 2022 (updated February 2022). Available from: https://training.cochrane.org/handbook/archive/v6.3 (cited 2023 Oct 17).
- 27. Adamson C, Docherty KF, Heerspink HJ, de Boer RA, Damman K, Inzucchi SE, et al. Initial decline (Dip) in estimated glomerular filtration rate after initiation of dapagliflozin in patients with heart failure and reduced ejection fraction: insights from DAPA-HF. Circulation 2022;146:438-49.ArticlePubMedPMC
- 28. Chan YH, Chen SW, Chao TF, Kao YW, Huang CY, Chu PH. Impact of the initial decline in estimated glomerular filtration rate on the risk of new-onset atrial fibrillation and adverse cardiovascular and renal events in patients with type 2 diabetes treated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab 2021;23:2077-89.ArticlePubMedPDF
- 29. Cherney DZ, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley RE, Frederich R, et al. Initial eGFR changes with ertugliflozin and associations with clinical parameters: analyses from the VERTIS CV trial. Am J Nephrol 2022;53:516-25.ArticlePubMedPDF
- 30. Sugiyama S, Yoshida A, Hieshima K, Kurinami N, Jinnouchi K, Tanaka M, et al. Initial acute decline in estimated glomerular filtration rate after sodium-glucose cotransporter-2 inhibitor in patients with chronic kidney disease. J Clin Med Res 2020;12:724-33.ArticlePubMedPMC
- 31. van Bommel EJ, Muskiet MH, van Baar MJ, Tonneijck L, Smits MM, Emanuel AL, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 2020;97:202-12.ArticlePubMed
- 32. Skrtic M, Yang GK, Perkins BA, Soleymanlou N, Lytvyn Y, von Eynatten M, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 2014;57:2599-602.ArticlePubMedPDF
- 33. Bailey CJ, Day C, Bellary S. Renal protection with SGLT2 inhibitors: effects in acute and chronic kidney disease. Curr Diab Rep 2022;22:39-52.ArticlePubMedPMCPDF
- 34. Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol 2017;28:2812-23.PubMedPMC
- 35. Malhotra R, Craven T, Ambrosius WT, Killeen AA, Haley WE, Cheung AK, et al. Effects of intensive blood pressure lowering on kidney tubule injury in CKD: a longitudinal subgroup analysis in SPRINT. Am J Kidney Dis 2019;73:21-30.ArticlePubMedPMC
- 36. Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant 2019;34(Suppl 3):iiI2-11.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations


 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite