
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Article
- Basic Research
- DGAT2 Plays a Crucial Role to Control ESRRAPROX1 Transcriptional Network to Maintain Hepatic Mitochondrial Sustainability
- Yoseob Lee, Yeseong Hwang, Minki Kim, Hyeonuk Jeon, Seyeon Joo, Sungsoon Fang, Jae-Woo Kim
- Received October 13, 2023 Accepted December 11, 2023 Published online April 22, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0368 [Epub ahead of print]
- 403 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - Background
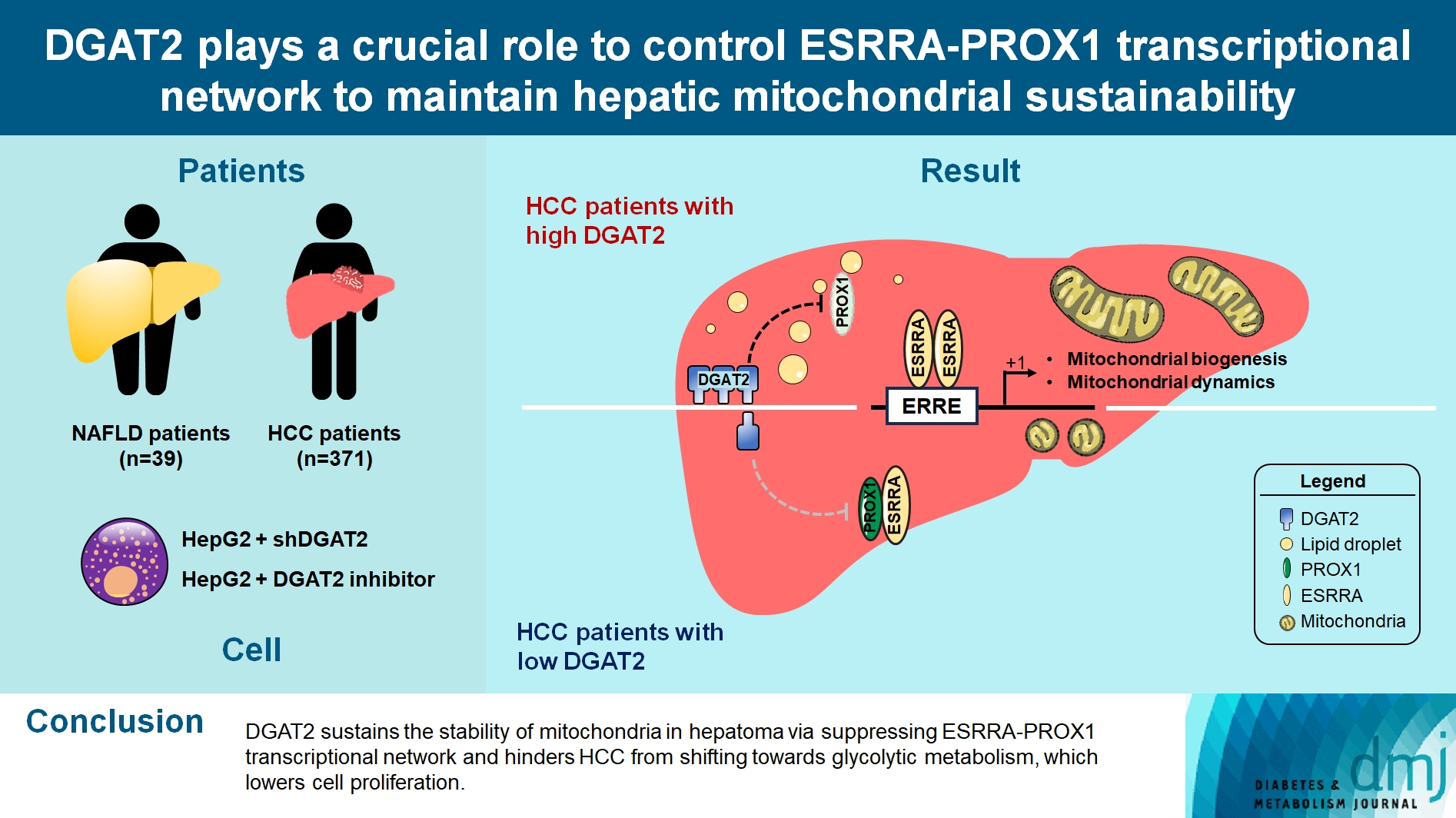
Diacylglycerol O-acyltransferase 2 (DGAT2) synthesizes triacylglycerol (TG) from diacylglycerol; therefore, DGAT2 is considered as a therapeutic target for steatosis. However, the consequence of inhibiting DGAT2 is not fully investigated due to side effects including lethality and lipotoxicity. In this article, we observed the role of DGAT2 in hepatocarcinoma.
Methods
The role of DGAT2 is analyzed via loss-of-function assay. DGAT2 knockdown (KD) and inhibitor treatment on HepG2 cell line was analyzed. Cumulative analysis of cell metabolism with bioinformatic data were assessed, and further compared with different cohorts of liver cancer patients and non-alcoholic fatty liver disease (NAFLD) patients to elucidate how DGAT2 is regulating cancer metabolism.
Results
Mitochondrial function is suppressed in DGAT2 KD HepG2 cell along with the decreased lipid droplets. In the aspect of the cancer, DGAT2 KD upregulates cell proliferation. Analyzing transcriptome of NAFLD and hepatocellular carcinoma (HCC) patients highlights negatively correlating expression patterns of 73 lipid-associated genes including DGAT2. Cancer patients with the lower DGAT2 expression face lower survival rate. DGAT2 KD cell and patients’ transcriptome show downregulation in estrogen- related receptor alpha (ESRRA) via integrated system for motif activity response analysis (ISMARA), with increased dimerization with corepressor prospero homeobox 1 (PROX1).
Conclusion
DGAT2 sustains the stability of mitochondria in hepatoma via suppressing ESRRA-PROX1 transcriptional network and hinders HCC from shifting towards glycolytic metabolism, which lowers cell proliferation.
Review
- Metabolic Risk/Epidemiology
- Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism
- Tong Bu, Ziyan Sun, Yi Pan, Xia Deng, Guoyue Yuan
- Received August 14, 2023 Accepted January 1, 2024 Published online April 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0277 [Epub ahead of print]
- 630 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
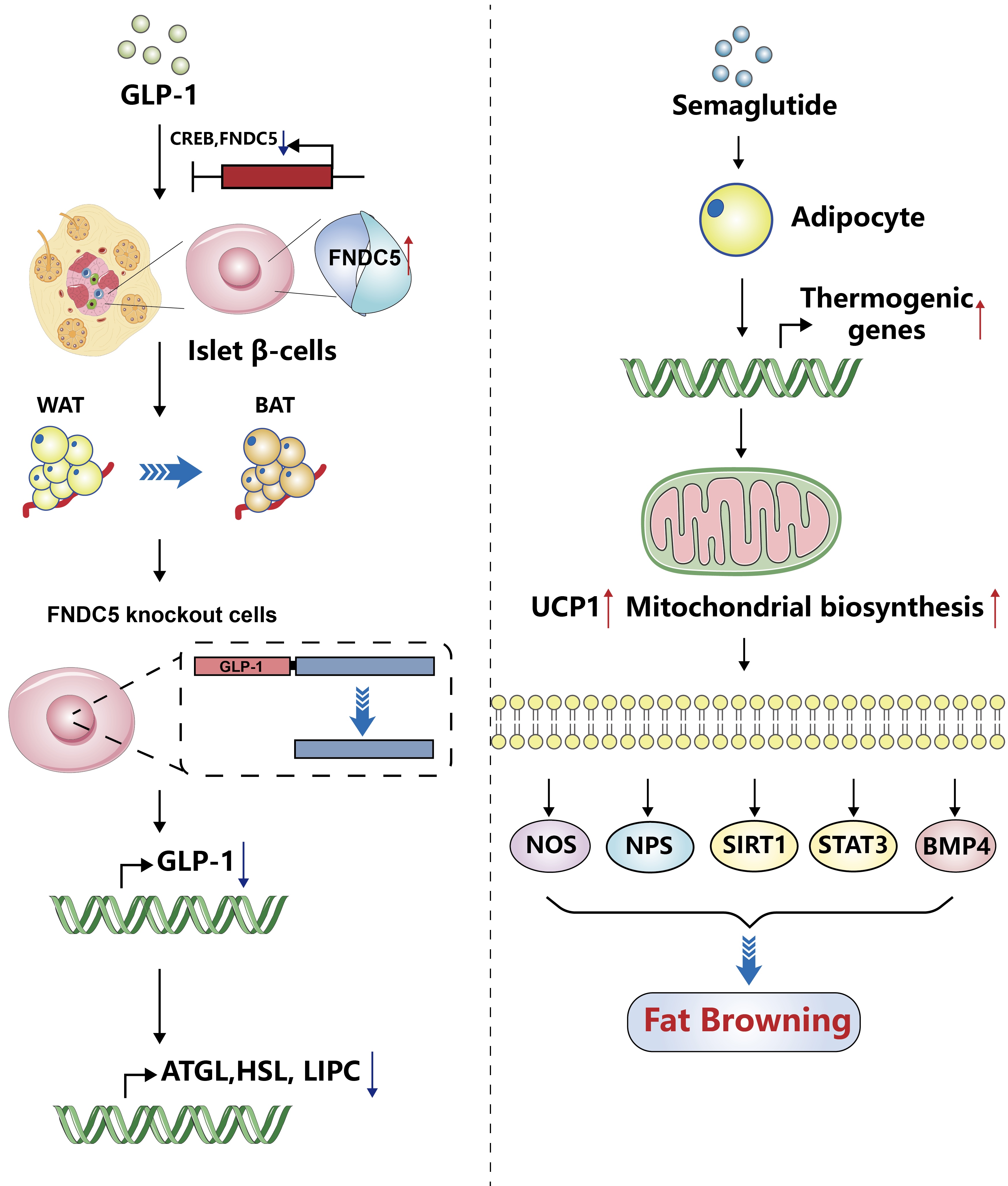
ePub - Glucagon-like peptide-1 (GLP-1) is a 30-amino acid peptide hormone that is mainly expressed in the intestine and hypothalamus. In recent years, basic and clinical studies have shown that GLP-1 is closely related to lipid metabolism, and it can participate in lipid metabolism by inhibiting fat synthesis, promoting fat differentiation, enhancing cholesterol metabolism, and promoting adipose browning. GLP-1 plays a key role in the occurrence and development of metabolic diseases such as obesity, nonalcoholic fatty liver disease, and atherosclerosis by regulating lipid metabolism. It is expected to become a new target for the treatment of metabolic disorders. The effects of GLP-1 and dual agonists on lipid metabolism also provide a more complete treatment plan for metabolic diseases. This article reviews the recent research progress of GLP-1 in lipid metabolism.
Original Article
- Metabolic Risk/Epidemiology
- Healthy Lifestyle and the Risk of Metabolic Dysfunction-Associated Fatty Liver Disease: A Large Prospective Cohort Study
- Qing Chang, Yixiao Zhang, Tingjing Zhang, Zuyun Liu, Limin Cao, Qing Zhang, Li Liu, Shaomei Sun, Xing Wang, Ming Zhou, Qiyu Jia, Kun Song, Yang Ding, Yuhong Zhao, Kaijun Niu, Yang Xia
- Received April 27, 2023 Accepted November 30, 2023 Published online March 19, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0133 [Epub ahead of print]
- 612 View
- 43 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
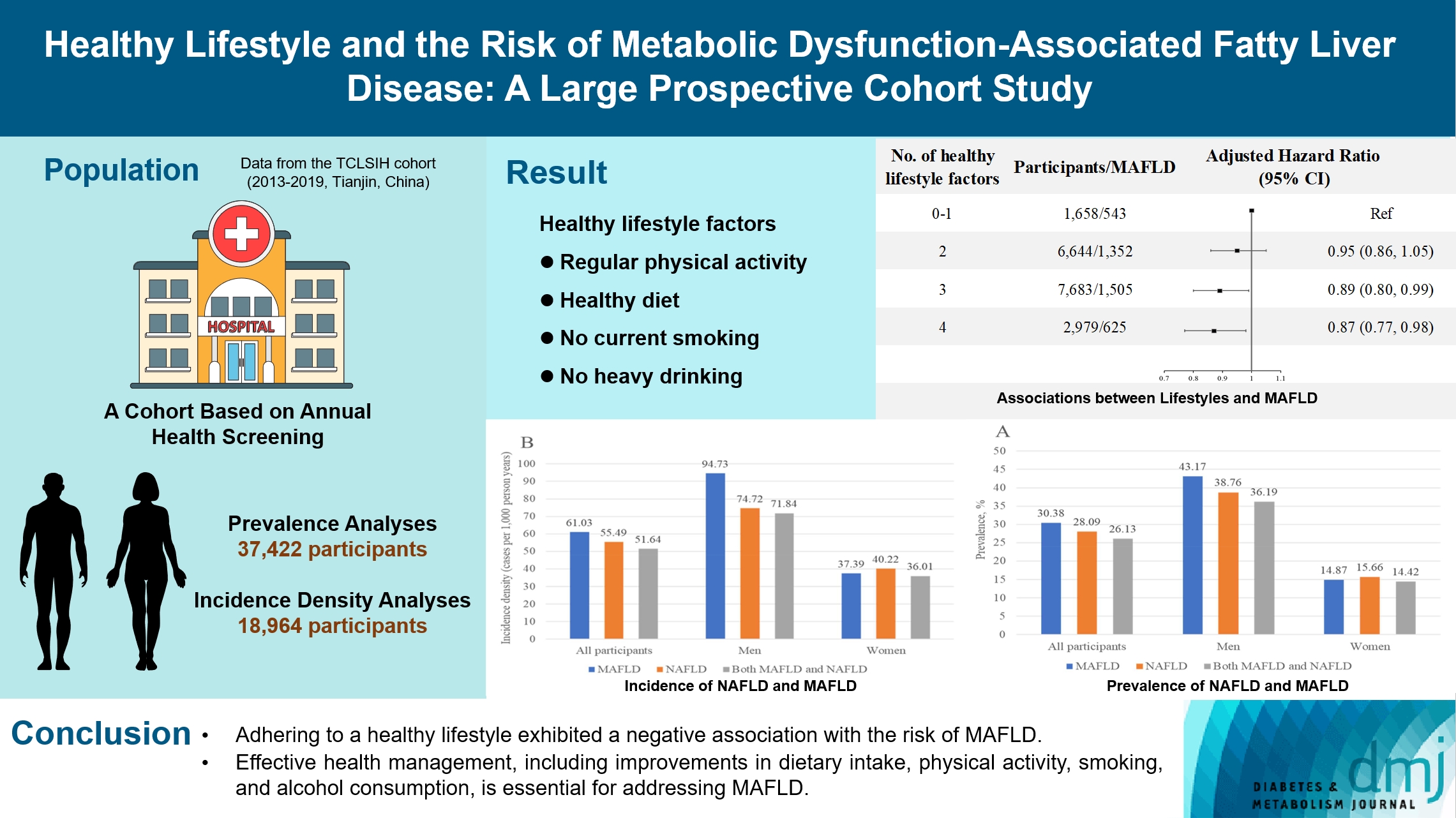
The incidence density of metabolic dysfunction-associated fatty liver disease (MAFLD) and the effect of a healthy lifestyle on the risk of MAFLD remain unknown. We evaluated the prevalence and incidence density of MAFLD and investigated the association between healthy lifestyle and the risk of MAFLD.
Methods
A cross-sectional analysis was conducted on 37,422 participants to explore the prevalence of MAFLD. A cohort analysis of 18,964 individuals was conducted to identify the incidence of MAFLD, as well as the association between healthy lifestyle and MAFLD. Cox proportional hazards regression was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) with adjustments for confounding factors.
Results
The prevalence of MAFLD, non-alcoholic fatty liver disease, and their comorbidities were 30.38%, 28.09%, and 26.13%, respectively. After approximately 70 thousand person-years of follow-up, the incidence densities of the three conditions were 61.03, 55.49, and 51.64 per 1,000 person-years, respectively. Adherence to an overall healthy lifestyle was associated with a 19% decreased risk of MAFLD (HR, 0.81; 95% CI, 0.72 to 0.92), and the effects were modified by baseline age, sex, and body mass index (BMI). Subgroup analyses revealed that younger participants, men, and those with a lower BMI experienced more significant beneficial effects from healthy lifestyle.
Conclusion
Our results highlight the beneficial effect of adherence to a healthy lifestyle on the prevention of MAFLD. Health management for improving dietary intake, physical activity, and smoking and drinking habits are critical to improving MAFLD.
Sulwon Lecture 2023
- Metabolic Risk/Epidemiology
- Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
- Inha Jung, Dae-Jeong Koo, Won-Young Lee
- Received October 4, 2023 Accepted December 26, 2024 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0350 [Epub ahead of print]
- 1,013 View
- 60 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
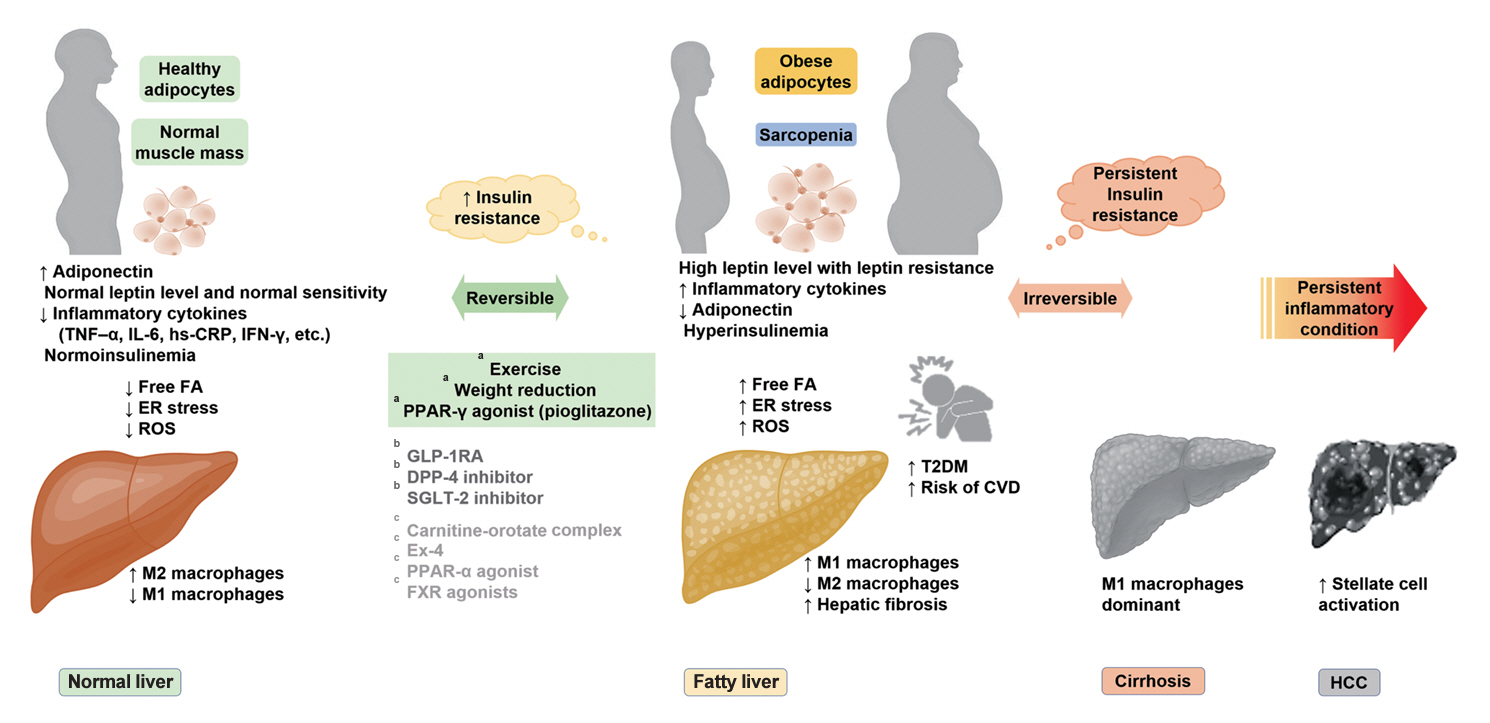
ePub - It has been generally accepted that insulin resistance (IR) and reduced insulin secretory capacity are the basic pathogenesis of type 2 diabetes mellitus (T2DM). In addition to genetic factors, the persistence of systemic inflammation caused by obesity and the associated threat of lipotoxicity increase the risk of T2DM. In particular, the main cause of IR is obesity and subjects with T2DM have a higher body mass index (BMI) than normal subjects according to recent studies. The prevalence of T2DM with IR has increased with increasing BMI during the past three decades. According to recent studies, homeostatic model assessment of IR was increased compared to that of the 1990s. Rising prevalence of obesity in Korea have contributed to the development of IR, non-alcoholic fatty liver disease and T2DM and cutting this vicious cycle is important. My colleagues and I have investigated this pathogenic mechanism on this theme through clinical and experimental studies over 20 years and herein, I would like to summarize some of our studies with deep gratitude for receiving the prestigious 2023 Sulwon Award.
Original Articles
- Metabolic Risk/Epidemiology
- Glycemic Control Is Associated with Histological Findings of Nonalcoholic Fatty Liver Disease
- Teruki Miyake, Shinya Furukawa, Bunzo Matsuura, Osamu Yoshida, Masumi Miyazaki, Akihito Shiomi, Ayumi Kanamoto, Hironobu Nakaguchi, Yoshiko Nakamura, Yusuke Imai, Mitsuhito Koizumi, Takao Watanabe, Yasunori Yamamoto, Yohei Koizumi, Yoshio Tokumoto, Masashi Hirooka, Teru Kumagi, Eiji Takesita, Yoshio Ikeda, Masanori Abe, Yoichi Hiasa
- Received June 24, 2023 Accepted September 21, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0200 [Epub ahead of print]

- 938 View
- 40 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Poor lifestyle habits may worsen nonalcoholic fatty liver disease (NAFLD), with progression to nonalcoholic steatohepatitis (NASH) and cirrhosis. This study investigated the association between glycemic control status and hepatic histological findings to elucidate the effect of glycemic control on NAFLD.
Methods
This observational study included 331 patients diagnosed with NAFLD by liver biopsy. Effects of the glycemic control status on histological findings of NAFLD were evaluated by comparing the following four glycemic status groups defined by the glycosylated hemoglobin (HbA1c) level at the time of NAFLD diagnosis: ≤5.4%, 5.5%–6.4%, 6.5%–7.4%, and ≥7.5%.
Results
Compared with the lowest HbA1c group (≤5.4%), the higher HbA1c groups (5.5%–6.4%, 6.5%–7.4%, and ≥7.5%) were associated with advanced liver fibrosis and high NAFLD activity score (NAS). On multivariate analysis, an HbA1c level of 6.5%– 7.4% group was significantly associated with advanced fibrosis compared with the lowest HbA1c group after adjusting for age, sex, hemoglobin, alanine aminotransferase, and creatinine levels. When further controlling for body mass index and uric acid, total cholesterol, and triglyceride levels, the higher HbA1c groups were significantly associated with advanced fibrosis compared with the lowest HbA1c group. On the other hand, compared with the lowest HbA1c group, the higher HbA1c groups were also associated with a high NAS in both multivariate analyses.
Conclusion
Glycemic control is associated with NAFLD exacerbation, with even a mild deterioration in glycemic control, especially a HbA1c level of 6.5%–7.4%, contributing to NAFLD progression. -
Citations
Citations to this article as recorded by- Combined effect of histological findings and diabetes mellitus on liver‐related events in patients with metabolic dysfunction‐associated steatotic liver disease
Akihito Shiomi, Teruki Miyake, Shinya Furukawa, Bunzo Matsuura, Osamu Yoshida, Takao Watanabe, Ayumi Kanamoto, Masumi Miyazaki, Hironobu Nakaguchi, Yoshio Tokumoto, Masashi Hirooka, Masanori Abe, Yoichi Hiasa
Hepatology Research.2024;[Epub] CrossRef
- Combined effect of histological findings and diabetes mellitus on liver‐related events in patients with metabolic dysfunction‐associated steatotic liver disease
- Metabolic Risk/Epidemiology
- Harnessing Metabolic Indices as a Predictive Tool for Cardiovascular Disease in a Korean Population without Known Major Cardiovascular Event
- Hyun-Jin Kim, Byung Sik Kim, Yonggu Lee, Sang Bong Ahn, Dong Wook Kim, Jeong-Hun Shin
- Received June 22, 2023 Accepted August 18, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0197 [Epub ahead of print]

- 964 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study evaluated the usefulness of indices for metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), and insulin resistance (IR), as predictive tools for cardiovascular disease in middle-aged Korean adults.
Methods
The prospective data obtained from the Ansan-Ansung cohort database, excluding patients with major adverse cardiac and cerebrovascular events (MACCE). The primary outcome was the incidence of MACCE during the follow-up period.
Results
A total of 9,337 patients were included in the analysis, of whom 1,130 (12.1%) experienced MACCE during a median follow-up period of 15.5 years. The metabolic syndrome severity Z-score, metabolic syndrome severity score, hepatic steatosis index, and NAFLD liver fat score were found to significantly predict MACCE at values above the cut-off point and in the second and third tertiles. Among these indices, the hazard ratios of the metabolic syndrome severity score and metabolic syndrome severity Z-score were the highest after adjusting for confounding factors. The area under the receiver operating characteristic curve (AUC) of the 10-year atherosclerotic cardiovascular disease (ASCVD) score for predicting MACCE was 0.716, and the metabolic syndrome severity Z-score had an AUC of 0.619.
Conclusion
The metabolic syndrome severity score is a highly reliable indicator and was closely associated with the 10-year ASCVD risk score in predicting MACCE in the general population. Given the specific characteristics and limitations of metabolic syndrome severity scores as well as the indices of NAFLD and IR, a more practical scoring system that considers these factors is essential to achieve greater accuracy in forecasting cardiovascular outcomes.
- Metabolic Risk/Epidemiology
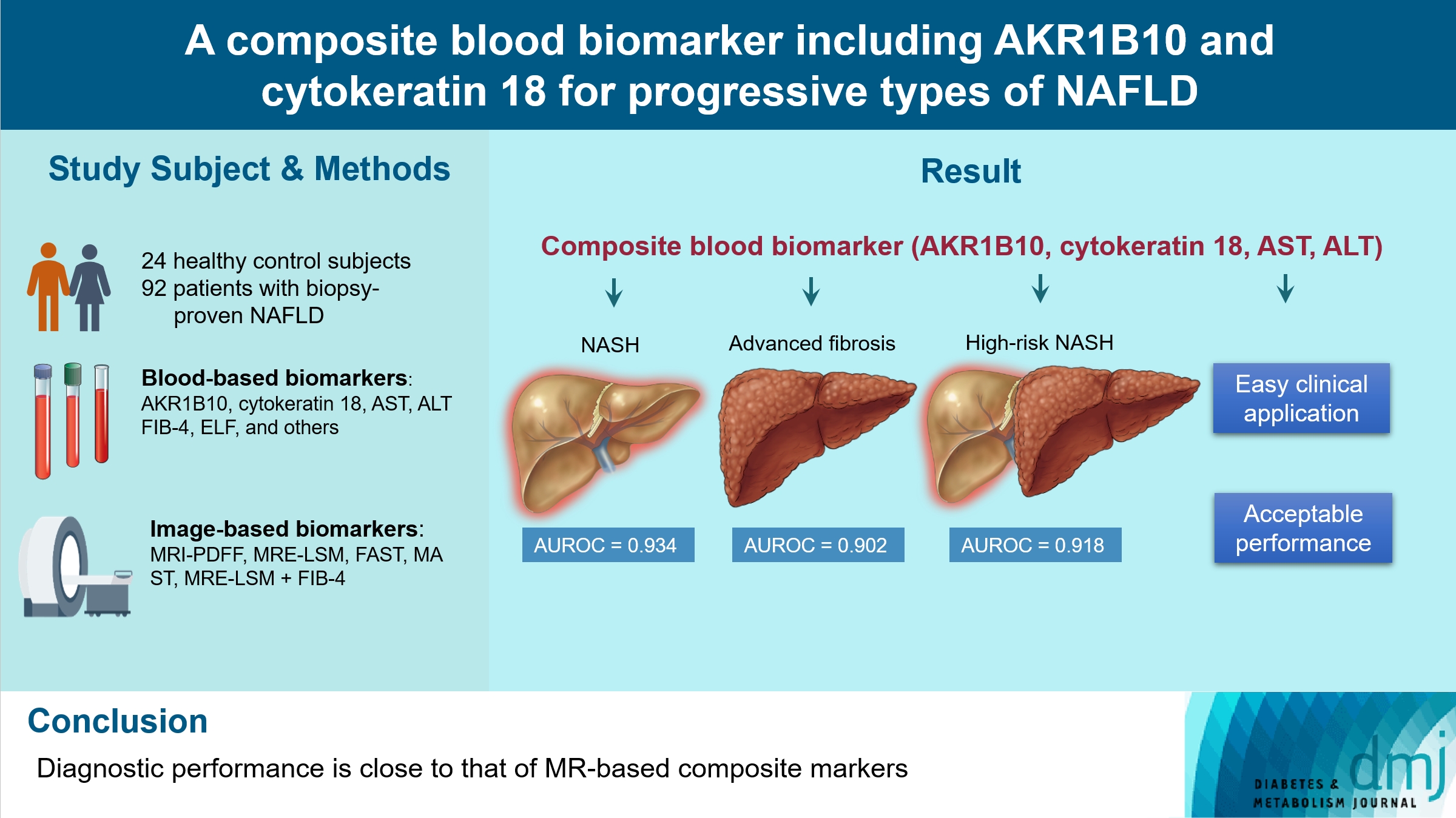
- A Composite Blood Biomarker Including AKR1B10 and Cytokeratin 18 for Progressive Types of Nonalcoholic Fatty Liver Disease
- Seung Joon Choi, Sungjin Yoon, Kyoung-Kon Kim, Doojin Kim, Hye Eun Lee, Kwang Gi Kim, Seung Kak Shin, Ie Byung Park, Seong Min Kim, Dae Ho Lee
- Received June 18, 2023 Accepted August 16, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0189 [Epub ahead of print]
- 791 View
- 50 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We aimed to evaluate whether composite blood biomarkers including aldo-keto reductase family 1 member B10 (AKR1B10) and cytokeratin 18 (CK-18; a nonalcoholic steatohepatitis [NASH] marker) have clinically applicable performance for the diagnosis of NASH, advanced liver fibrosis, and high-risk NASH (NASH+significant fibrosis).
Methods
A total of 116 subjects including healthy control subjects and patients with biopsy-proven nonalcoholic fatty liver disease (NAFLD) were analyzed to assess composite blood-based and imaging-based biomarkers either singly or in combination.
Results
A composite blood biomarker comprised of AKR1B10, CK-18, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) showed excellent performance for the diagnosis of, NASH, advanced fibrosis, and high-risk NASH, with area under the receiver operating characteristic curve values of 0.934 (95% confidence interval [CI], 0.888 to 0.981), 0.902 (95% CI, 0.832 to 0.971), and 0.918 (95% CI, 0.862 to 0.974), respectively. However, the performance of this blood composite biomarker was inferior to that various magnetic resonance (MR)-based composite biomarkers, such as proton density fat fraction/MR elastography- liver stiffness measurement (MRE-LSM)/ALT/AST for NASH, MRE-LSM+fibrosis-4 index for advanced fibrosis, and the known MR imaging-AST (MAST) score for high-risk NASH.
Conclusion
Our blood composite biomarker can be useful to distinguish progressive forms of NAFLD as an initial noninvasive test when MR-based tools are not available.
- Pathophysiology
- Deficiency of ASGR1 Alleviates Diet-Induced Systemic Insulin Resistance via Improved Hepatic Insulin Sensitivity
- Xiaorui Yu, Jiawang Tao, Yuhang Wu, Yan Chen, Penghui Li, Fan Yang, Miaoxiu Tang, Abdul Sammad, Yu Tao, Yingying Xu, Yin-Xiong Li
- Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0124 [Epub ahead of print]

- 901 View
- 61 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Insulin resistance (IR) is the key pathological basis of many metabolic disorders. Lack of asialoglycoprotein receptor 1 (ASGR1) decreased the serum lipid levels and reduced the risk of coronary artery disease. However, whether ASGR1 also participates in the regulatory network of insulin sensitivity and glucose metabolism remains unknown.
Methods
The constructed ASGR1 knockout mice and ASGR1-/- HepG2 cell lines were used to establish the animal model of metabolic syndrome and the IR cell model by high-fat diet (HFD) or drug induction, respectively. Then we evaluated the glucose metabolism and insulin signaling in vivo and in vitro.
Results
ASGR1 deficiency ameliorated systemic IR in mice fed with HFD, evidenced by improved insulin intolerance, serum insulin, and homeostasis model assessment of IR index, mainly contributed from increased insulin signaling in the liver, but not in muscle or adipose tissues. Meanwhile, the insulin signal transduction was significantly enhanced in ASGR1-/- HepG2 cells. By transcriptome analyses and comparison, those differentially expressed genes between ASGR1 null and wild type were enriched in the insulin signal pathway, particularly in phosphoinositide 3-kinase-AKT signaling. Notably, ASGR1 deficiency significantly reduced hepatic gluconeogenesis and glycogenolysis.
Conclusion
The ASGR1 deficiency was consequentially linked with improved hepatic insulin sensitivity under metabolic stress, hepatic IR was the core factor of systemic IR, and overcoming hepatic IR significantly relieved the systemic IR. It suggests that ASGR1 is a potential intervention target for improving systemic IR in metabolic disorders.
Review
- Metabolic Risk/Epidemiology
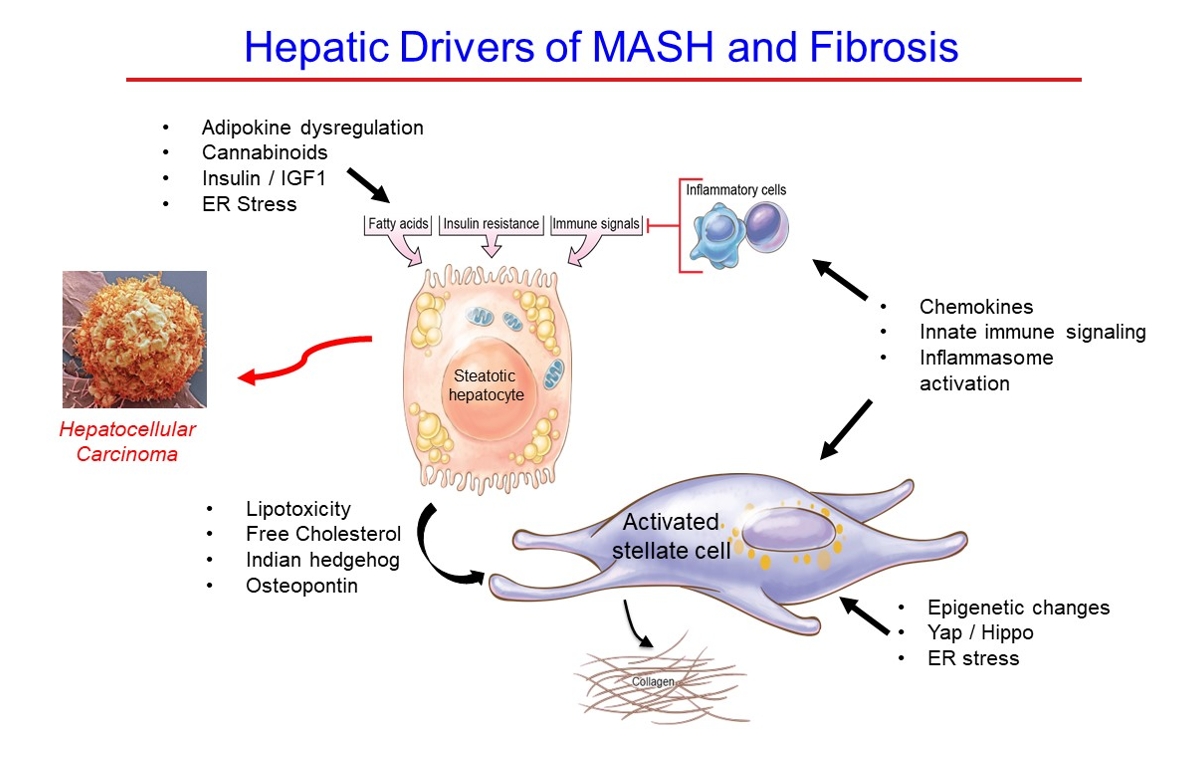
- Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
- Scott L. Friedman
- Diabetes Metab J. 2024;48(2):161-169. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0240
- 2,470 View
- 296 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolic dysfunction-associated steatotic (fatty) liver disease (MASLD), previously termed non-alcoholic fatty liver disease, is a worldwide epidemic that can lead to hepatic inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). The disease is typically a component of the metabolic syndrome that accompanies obesity, and is often overlooked because the liver manifestations are clinically silent until late-stage disease is present (i.e., cirrhosis). Moreover, Asian populations, including Koreans, have a higher fraction of patients who are lean, yet their illness has the same prognosis or worse than those who are obese. Nonetheless, ongoing injury can lead to hepatic inflammation and ballooning of hepatocytes as classic features. Over time, fibrosis develops following activation of hepatic stellate cells, the liver’s main fibrogenic cell type. The disease is usually more advanced in patients with type 2 diabetes mellitus, indicating that all diabetic patients should be screened for liver disease. Although there has been substantial progress in clarifying pathways of injury and fibrosis, there no approved therapies yet, but current research seeks to uncover the pathways driving hepatic inflammation and fibrosis, in hopes of identifying new therapeutic targets. Emerging molecular methods, especially single cell sequencing technologies, are revolutionizing our ability to clarify mechanisms underlying MASLD-associated fibrosis and HCC.
Original Articles
- Basic Research
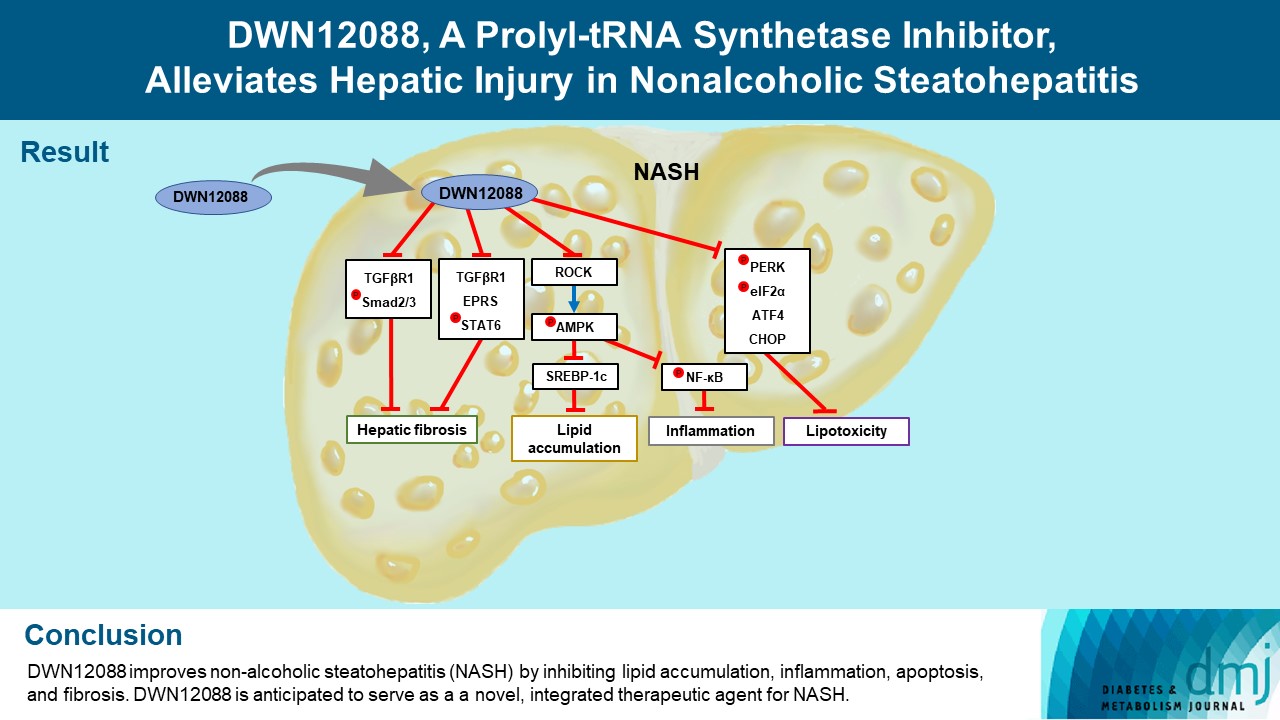
- DWN12088, A Prolyl-tRNA Synthetase Inhibitor, Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis
- Dong-Keon Lee, Su Ho Jo, Eun Soo Lee, Kyung Bong Ha, Na Won Park, Deok-Hoon Kong, Sang-In Park, Joon Seok Park, Choon Hee Chung
- Diabetes Metab J. 2024;48(1):97-111. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0367
- 1,812 View
- 181 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Nonalcoholic steatohepatitis (NASH) is a liver disease caused by obesity that leads to hepatic lipoapoptosis, resulting in fibrosis and cirrhosis. However, the mechanism underlying NASH is largely unknown, and there is currently no effective therapeutic agent against it. DWN12088, an agent used for treating idiopathic pulmonary fibrosis, is a selective prolyl-tRNA synthetase (PRS) inhibitor that suppresses the synthesis of collagen. However, the mechanism underlying the hepatoprotective effect of DWN12088 is not clear. Therefore, we investigated the role of DWN12088 in NASH progression.
Methods
Mice were fed a chow diet or methionine-choline deficient (MCD)-diet, which was administered with DWN12088 or saline by oral gavage for 6 weeks. The effects of DWN12088 on NASH were evaluated by pathophysiological examinations, such as real-time quantitative reverse transcription polymerase chain reaction, immunoblotting, biochemical analysis, and immunohistochemistry. Molecular and cellular mechanisms of hepatic injury were assessed by in vitro cell culture.
Results
DWN12088 attenuated palmitic acid (PA)-induced lipid accumulation and lipoapoptosis by downregulating the Rho-kinase (ROCK)/AMP-activated protein kinase (AMPK)/sterol regulatory element-binding protein-1c (SREBP-1c) and protein kinase R-like endoplasmic reticulum kinase (PERK)/α subunit of eukaryotic initiation factor 2 (eIF2α)/activating transcription factor 4 (ATF4)/C/EBP-homologous protein (CHOP) signaling cascades. PA increased but DWN12088 inhibited the phosphorylation of nuclear factor-κB (NF-κB) p65 (Ser536, Ser276) and the expression of proinflammatory genes. Moreover, the DWN12088 inhibited transforming growth factor β (TGFβ)-induced pro-fibrotic gene expression by suppressing TGFβ receptor 1 (TGFβR1)/Smad2/3 and TGFβR1/glutamyl-prolyl-tRNA synthetase (EPRS)/signal transducer and activator of transcription 6 (STAT6) axis signaling. In the case of MCD-diet-induced NASH, DWN12088 reduced hepatic steatosis, inflammation, and lipoapoptosis and prevented the progression of fibrosis.
Conclusion
Our findings provide new insights about DWN12088, namely that it plays an important role in the overall improvement of NASH. Hence, DWN12088 shows great potential to be developed as a new integrated therapeutic agent for NASH.
- Complications
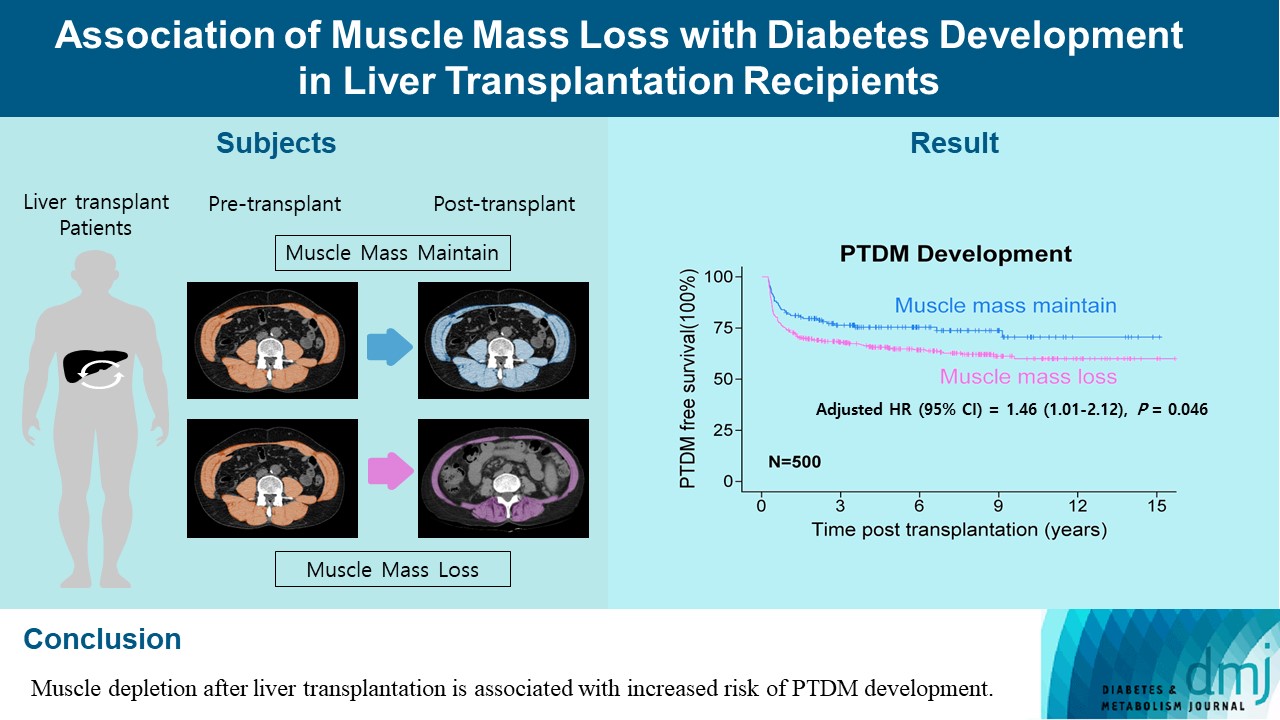
- Association of Muscle Mass Loss with Diabetes Development in Liver Transplantation Recipients
- Sejeong Lee, Minyoung Lee, Young-Eun Kim, Hae Kyung Kim, Sook Jung Lee, Jiwon Kim, Yurim Yang, Chul Hoon Kim, Hyangkyu Lee, Dong Jin Joo, Myoung Soo Kim, Eun Seok Kang
- Diabetes Metab J. 2024;48(1):146-156. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0100
- 975 View
- 121 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Post-transplant diabetes mellitus (PTDM) is one of the most significant complications after transplantation. Patients with end-stage liver diseases requiring transplantation are prone to sarcopenia, but the association between sarcopenia and PTDM remains to be elucidated. We aimed to investigate the effect of postoperative muscle mass loss on PTDM development.
Methods
A total of 500 patients who underwent liver transplantation at a tertiary care hospital between 2005 and 2020 were included. Skeletal muscle area at the level of the L3–L5 vertebrae was measured using computed tomography scans performed before and 1 year after the transplantation. The associations between the change in the muscle area after the transplantation and the incidence of PTDM was investigated using a Cox proportional hazard model.
Results
During the follow-up period (median, 4.9 years), PTDM occurred in 165 patients (33%). The muscle mass loss was greater in patients who developed PTDM than in those without PTDM. Muscle depletion significantly increased risk of developing PTDM after adjustment for other confounding factors (hazard ratio, 1.50; 95% confidence interval, 1.23 to 1.84; P=0.001). Of the 357 subjects who had muscle mass loss, 124 (34.7%) developed PTDM, whereas of the 143 patients in the muscle mass maintenance group, 41 (28.7%) developed PTDM. The cumulative incidence of PTDM was significantly higher in patients with muscle loss than in patients without muscle loss (P=0.034).
Conclusion
Muscle depletion after liver transplantation is associated with increased risk of PTDM development.
- Basic Research
- Effects Of Exercise Training And Chlorogenic Acid Supplementation On Hepatic Lipid Metabolism In Prediabetes Mice
- Samaneh Shirkhani, Sayyed Mohammad Marandi, Mohammad Hossein Nasr-Esfahani, Seung Kyum Kim
- Diabetes Metab J. 2023;47(6):771-783. Published online September 8, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0265
- 2,315 View
- 169 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
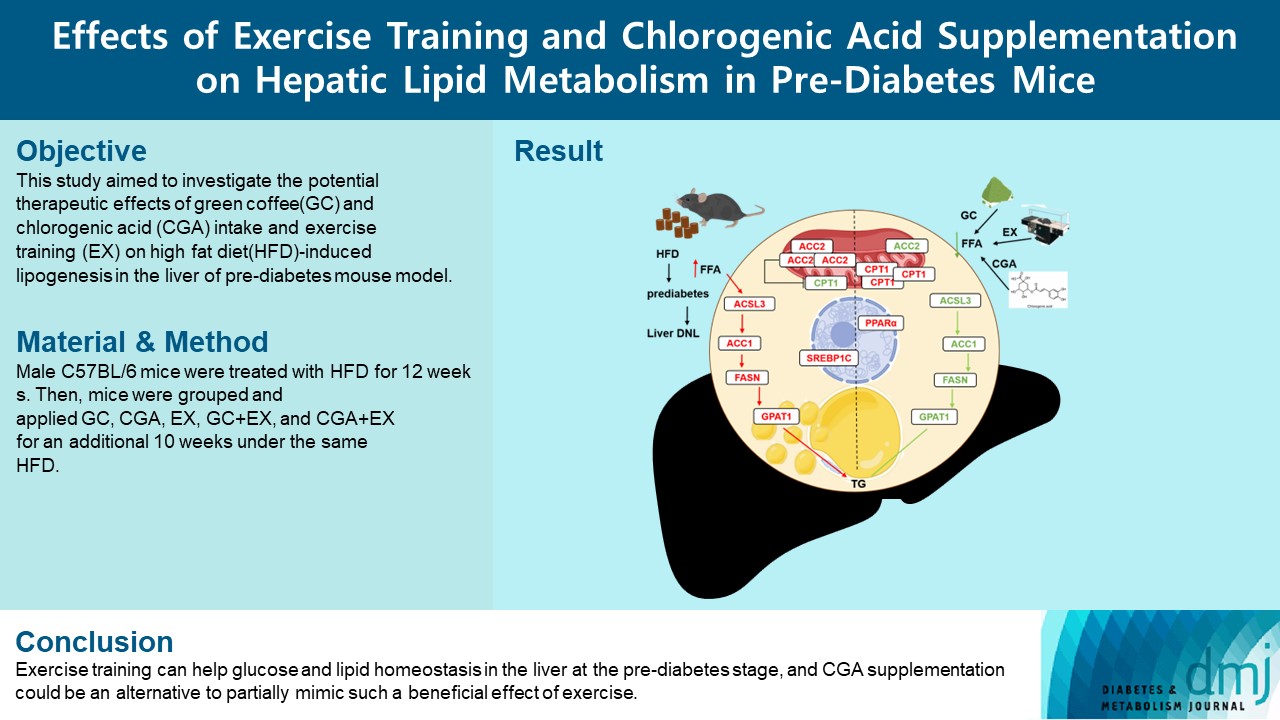
Since prediabetes is a risk factor for metabolic syndromes, it is important to promote a healthy lifestyle to prevent prediabetes. This study aimed to determine the effects of green coffee (GC), chlorogenic acid (CGA) intake, and exercise training (EX) on hepatic lipid metabolism in prediabetes male C57BL/6 mice.
Methods
Forty-nine mice were randomly divided into two groups feeding with a normal diet (n=7) or a high-fat diet (HFD, n=42) for 12 weeks. Then, HFD mice were further divided into six groups (n=7/group): control (pre-D), GC, CGA, EX, GC+EX, and CGA+EX. After additional 10 weeks under the same diet, plasma, and liver samples were obtained.
Results
HFD-induced prediabetes conditions with increases in body weight, glucose, insulin, insulin resistance, and lipid profiles were alleviated in all treatment groups. Acsl3, a candidate gene identified through an in silico approach, was lowered in the pre-D group, while treatments partly restored it. HFD induced adverse alterations of de novo lipogenesis- and β oxidation-associated molecules in the liver. However, GC and CGA supplementation and EX reversed or ameliorated these changes. In most cases, GC or CGA supplementation combined with EX has no synergistic effect and the GC group had similar results to the CGA group.
Conclusion
These findings suggest that regular exercise is an effective non-therapeutic approach for prediabetes, and CGA supplementation could be an alternative to partially mimic the beneficial effects of exercise on prediabetes. -
Citations
Citations to this article as recorded by- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients
Perioperative Precision Medicine.2023;[Epub] CrossRef
- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients
- Basic Research
- Beneficial Effects of a Curcumin Derivative and Transforming Growth Factor-β Receptor I Inhibitor Combination on Nonalcoholic Steatohepatitis
- Kyung Bong Ha, Eun Soo Lee, Na Won Park, Su Ho Jo, Soyeon Shim, Dae-Kee Kim, Chan Mug Ahn, Choon Hee Chung
- Diabetes Metab J. 2023;47(4):500-513. Published online April 25, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0110
- 2,224 View
- 145 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
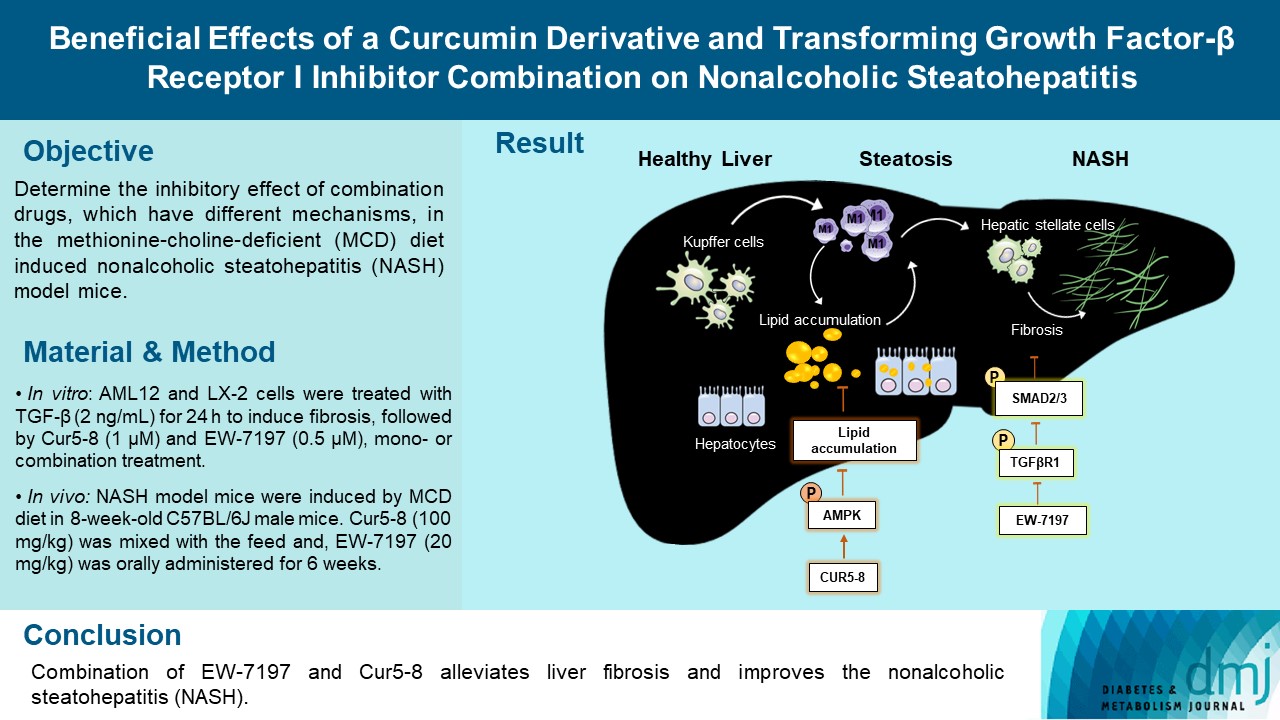
Curcumin 2005-8 (Cur5-8), a derivative of curcumin, improves fatty liver disease via AMP-activated protein kinase activation and autophagy regulation. EW-7197 (vactosertib) is a small molecule inhibitor of transforming growth factor β (TGF-β) receptor I and may scavenge reactive oxygen species and ameliorate fibrosis through the SMAD2/3 canonical pathway. This study aimed to determine whether co-administering these two drugs having different mechanisms is beneficial.
Methods
Hepatocellular fibrosis was induced in mouse hepatocytes (alpha mouse liver 12 [AML12]) and human hepatic stellate cells (LX-2) using TGF-β (2 ng/mL). The cells were then treated with Cur5-8 (1 μM), EW-7197 (0.5 μM), or both. In animal experiments were also conducted during which, methionine-choline deficient diet, Cur5-8 (100 mg/kg), and EW-7197 (20 mg/kg) were administered orally to 8-week-old C57BL/6J mice for 6 weeks.
Results
TGF-β-induced cell morphological changes were improved by EW-7197, and lipid accumulation was restored on the administration of EW-7197 in combination with Cur5-8. In a nonalcoholic steatohepatitis (NASH)-induced mouse model, 6 weeks of EW-7197 and Cur5-8 co-administration alleviated liver fibrosis and improved the nonalcoholic fatty liver disease (NAFLD) activity score.
Conclusion
Co-administering Cur5-8 and EW-7197 to NASH-induced mice and fibrotic hepatocytes reduced liver fibrosis and steatohepatitis while maintaining the advantages of both drugs. This is the first study to show the effect of the drug combination against NASH and NAFLD. Similar effects in other animal models will confirm its potential as a new therapeutic agent.
- Guideline/Fact Sheet
- Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017
- Eugene Han, Kyung-Do Han, Yong-ho Lee, Kyung-Soo Kim, Sangmo Hong, Jung Hwan Park, Cheol-Young Park, on Behalf of Fatty Liver Research Group of the Korean Diabetes Association
- Diabetes Metab J. 2023;47(3):347-355. Published online March 29, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0444
- 3,591 View
- 208 Download
- 6 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
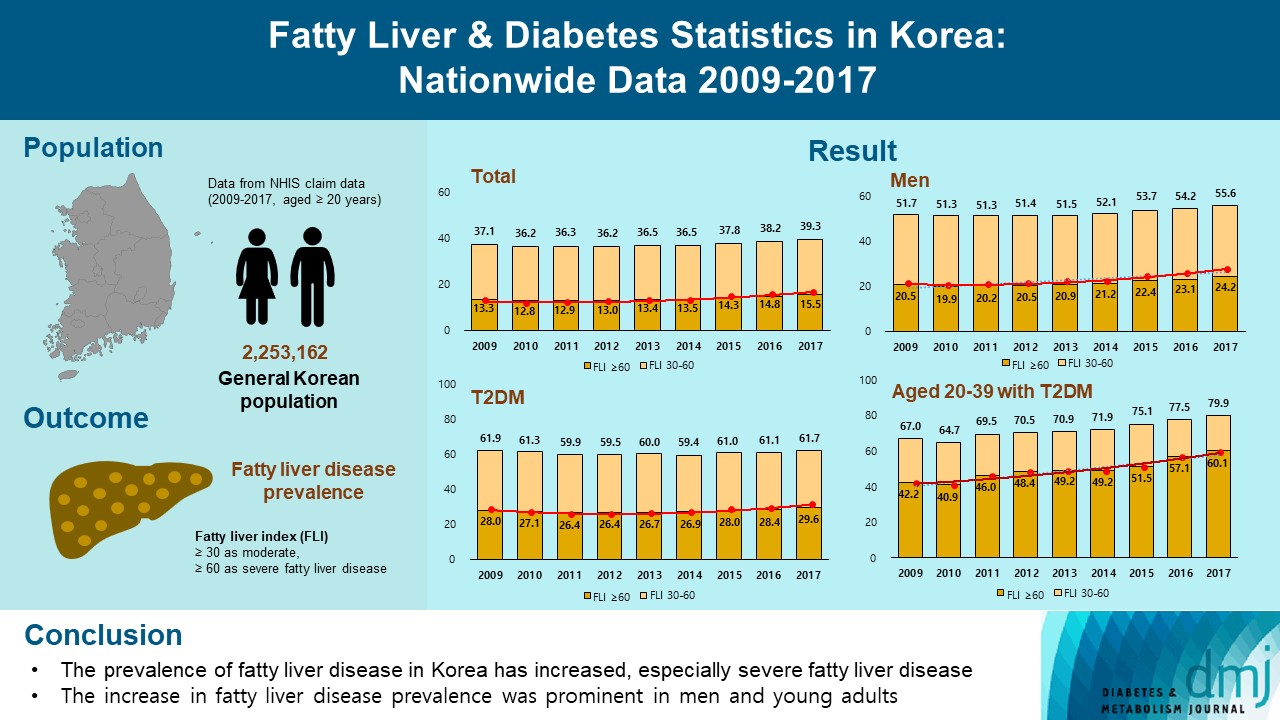
This study investigated the changes of fatty liver disease prevalence in general Korean population.
Methods
This study analyzed data from the Korean National Health Insurance Service from 2009 to 2017 that included individuals aged 20 years or older who had undergone a medical health examination. Fatty liver disease was assessed using the fatty liver index (FLI). The disease severity was defined by FLI cutoff, ≥30 as moderate, and ≥60 as severe fatty liver disease.
Results
The prevalence of Korean adults aged 20 years or over with fatty liver disease (FLI ≥60) increased from 13.3% in 2009 to 15.5% in 2017 (P for trend <0.001). The increase in fatty liver disease prevalence was prominent in men (from 20.5% to 24.2%) and the young age (20 to 39 years) group (from 12.8% to 16.4%) (P for interaction <0.001). The prevalence of fatty liver disease was the highest in type 2 diabetes mellitus (T2DM, 29.6%) population compared to that of prediabetes or normoglycemia (10.0% and 21.8%) in 2017. The prevalence of fatty liver disease had statistically increased in individuals with T2DM and prediabetes (P for trend <0.001). Its prevalence increased more steeply in the young-aged population with T2DM, from 42.2% in 2009 to 60.1% in 2017. When applying a lower FLI cutoff (≥30) similar results were observed.
Conclusion
The prevalence of fatty liver disease in the Korean population has increased. Individuals who are young, male, and have T2DM are vulnerable to fatty liver disease. -
Citations
Citations to this article as recorded by- Longitudinal changes in fatty liver index are associated with risk of hepatocellular carcinoma: A nationwide cohort study in Korea
Min Gu Kang, Chang Hun Lee, Chen Shen, Jong Seung Kim, Ji Hyun Park
Journal of Hepatology.2024; 80(5): e216. CrossRef - Repeated detection of non‐alcoholic fatty liver disease increases the incidence risk of type 2 diabetes in young adults
Jin Hwa Kim, Young Sang Lyu, Mee Kyoung Kim, Sang Yong Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024; 26(1): 180. CrossRef - Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
Eugene Han, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Sang Hoon Ahn, Yong-ho Lee, Seung Up Kim
Metabolism.2024; 152: 155789. CrossRef - Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
BMJ.2024; : e076388. CrossRef - Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
Scott L. Friedman
Diabetes & Metabolism Journal.2024; 48(2): 161. CrossRef - Reply to G. Wang et al
Joo-Hyun Park, Jung Yong Hong, Kyungdo Han
Journal of Clinical Oncology.2023; 41(32): 5070. CrossRef - The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review
Teodora Biciusca, Sorina Ionelia Stan, Mara Amalia Balteanu, Ramona Cioboata, Alice Elena Ghenea, Suzana Danoiu, Ana-Maria Bumbea, Viorel Biciusca
Diagnostics.2023; 13(21): 3316. CrossRef - Lean or Non-obese Nonalcoholic Fatty Liver Disease Patients: Are They Really Lean?
Eugene Han, Yong-ho Lee
Clinical and Molecular Hepatology.2023; 29(4): 980. CrossRef - Approach to Fatty Liver Disease in Patients with Type 2 Diabetes
Ji Cheol Bae
The Journal of Korean Diabetes.2023; 24(3): 107. CrossRef
- Longitudinal changes in fatty liver index are associated with risk of hepatocellular carcinoma: A nationwide cohort study in Korea
- Metabolic Risk/Epidemiology
- Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography
- Hwi Seung Kim, Jiwoo Lee, Eun Hee Kim, Min Jung Lee, In Young Bae, Woo Je Lee, Joong-Yeol Park, Hong-Kyu Kim, Chang Hee Jung
- Diabetes Metab J. 2023;47(1):104-117. Published online January 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0081
- 3,272 View
- 176 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The association of myosteatosis measured using visual muscular quality map in computed tomography (CT) with nonalcoholic fatty liver disease (NAFLD), its severity, and fibrosis was analyzed in a large population.
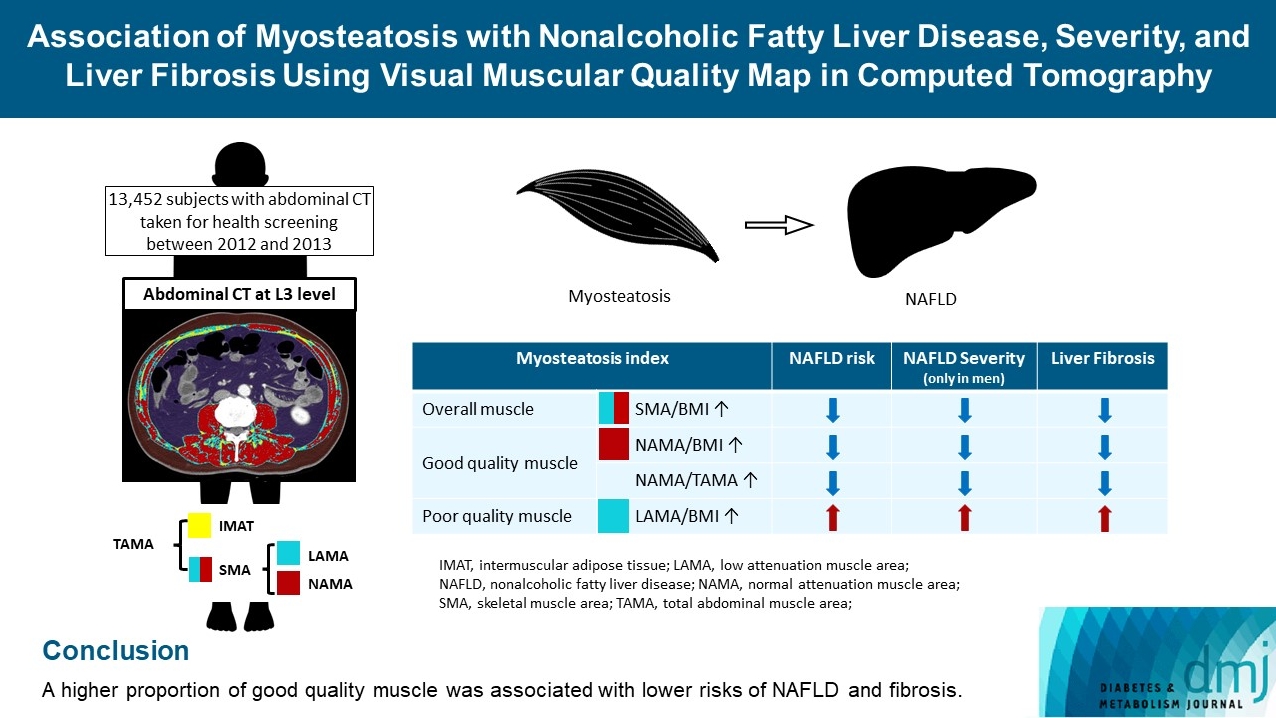
Methods
Subjects (n=13,452) with abdominal CT between 2012 and 2013 were measured total abdominal muscle area (TAMA) at L3 level. TAMA was segmented into intramuscular adipose tissue and skeletal muscle area (SMA), which was further classified into normal attenuation muscle area (NAMA) and low attenuation muscle area (LAMA). The following variables were adopted as indicators of myosteatosis: SMA/body mass index (BMI), NAMA/BMI, NAMA/TAMA, and LAMA/BMI. NAFLD and its severity were assessed by ultrasonography, and liver fibrosis was measured by calculating the NAFLD fibrosis score (NFS) and fibrosis-4 index (FIB-4) scores.
Results
According to multiple logistic regression analyses, as quartiles of SMA/BMI, NAMA/BMI, and NAMA/TAMA increased, the odds ratios (ORs) for NAFLD decreased in each sex (P for trend <0.001 for all). The ORs of moderate/severe NAFLD were significantly higher in the Q1 group than in the Q4 group for SMA/BMI, NAMA/BMI, and NAMA/TAMA in men. The ORs of intermediate/high liver fibrosis scores assessed by NFS and FIB-4 scores increased linearly with decreasing quartiles for SMA/BMI, NAMA/BMI, and NAMA/TAMA in each sex (P for trend <0.001 for all). Conversely, the risk for NAFLD and fibrosis were positively associated with LAMA/BMI quartiles in each sex (P for trend <0.001 for all).
Conclusion
A higher proportion of good quality muscle was associated with lower risks of NAFLD and fibrosis. -
Citations
Citations to this article as recorded by- Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography (Diabetes Metab J 2023;47:104-17)
Hwi Seung Kim, Hong-Kyu Kim, Chang Hee Jung
Diabetes & Metabolism Journal.2023; 47(2): 304. CrossRef - Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography (Diabetes Metab J 2023;47:104-17)
Eun Roh
Diabetes & Metabolism Journal.2023; 47(2): 301. CrossRef - Sarcopenia, a condition shared by various diseases: can we alleviate or delay the progression?
Giovanni Tarantino, Gaia Sinatti, Vincenzo Citro, Silvano Santini, Clara Balsano
Internal and Emergency Medicine.2023; 18(7): 1887. CrossRef - Association of Visceral Fat Obesity, Sarcopenia, and Myosteatosis with Non-Alcoholic Fatty Liver Disease without Obesity
Hong-Kyu Kim, Sung-Jin Bae, Min Jung Lee, Eun Hee Kim, Hana Park, Hwi Seung Kim, Yun Kyung Cho, Chang Hee Jung, Woo Je Lee, Jaewon Choe
Clinical and Molecular Hepatology.2023; 29(4): 987. CrossRef - Current view of the surgical anatomy of the anterolateral abdominal wall muscles and their aponeuroses
A.V. Pavlov, A.S. Baranova, A.V. Fedoseyev, A.I. Vvedensky, G.S. Lazutina, N.V. Ovchinnikova, I.V. Bakharev
Operativnaya khirurgiya i klinicheskaya anatomiya (Pirogovskii nauchnyi zhurnal).2023; 7(3): 44. CrossRef - Muscle Fat Content Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in Chinese Adults
W. Guo, X. Zhao, D. Cheng, X. Liang, M. Miao, X. Li, J. Lu, N. Xu, Shuang Hu, Qun Zhang
The Journal of nutrition, health and aging.2023; 27(11): 960. CrossRef
- Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography (Diabetes Metab J 2023;47:104-17)

 KDA
KDA
 First
First Prev
Prev





