- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 48(1); 2024 > Article
-
Original ArticleBasic Research DWN12088, A Prolyl-tRNA Synthetase Inhibitor, Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis
-
Dong-Keon Lee1,2*
 , Su Ho Jo1*
, Su Ho Jo1* , Eun Soo Lee1, Kyung Bong Ha1, Na Won Park1, Deok-Hoon Kong2, Sang-In Park2, Joon Seok Park3, Choon Hee Chung1
, Eun Soo Lee1, Kyung Bong Ha1, Na Won Park1, Deok-Hoon Kong2, Sang-In Park2, Joon Seok Park3, Choon Hee Chung1
-
Diabetes & Metabolism Journal 2024;48(1):97-111.
DOI: https://doi.org/10.4093/dmj.2022.0367
Published online: January 3, 2024
- 1,785 Views
- 181 Download
1Department of Internal Medicine and Research Institute of Metabolism and Inflammation, Yonsei University Wonju College of Medicine, Wonju, Korea
2Division of Research Program, Scripps Korea Antibody Institute, Chuncheon, Korea
3Drug Discovery Center, Daewoong Pharmaceutical Co. Ltd., Seoul, Korea
-
Corresponding author: Choon Hee Chung
 Department of Internal Medicine and Research Institute of Metabolism and Inflammation, Yonsei University Wonju College of Medicine, 20 Ilsan-ro, Wonju 26426, Korea E-mail: cchung@yonsei.ac.kr
Department of Internal Medicine and Research Institute of Metabolism and Inflammation, Yonsei University Wonju College of Medicine, 20 Ilsan-ro, Wonju 26426, Korea E-mail: cchung@yonsei.ac.kr - *Dong-Keon Lee and Su Ho Jo contributed equally to this study as first authors.
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Nonalcoholic steatohepatitis (NASH) is a liver disease caused by obesity that leads to hepatic lipoapoptosis, resulting in fibrosis and cirrhosis. However, the mechanism underlying NASH is largely unknown, and there is currently no effective therapeutic agent against it. DWN12088, an agent used for treating idiopathic pulmonary fibrosis, is a selective prolyl-tRNA synthetase (PRS) inhibitor that suppresses the synthesis of collagen. However, the mechanism underlying the hepatoprotective effect of DWN12088 is not clear. Therefore, we investigated the role of DWN12088 in NASH progression.
-
Methods
- Mice were fed a chow diet or methionine-choline deficient (MCD)-diet, which was administered with DWN12088 or saline by oral gavage for 6 weeks. The effects of DWN12088 on NASH were evaluated by pathophysiological examinations, such as real-time quantitative reverse transcription polymerase chain reaction, immunoblotting, biochemical analysis, and immunohistochemistry. Molecular and cellular mechanisms of hepatic injury were assessed by in vitro cell culture.
-
Results
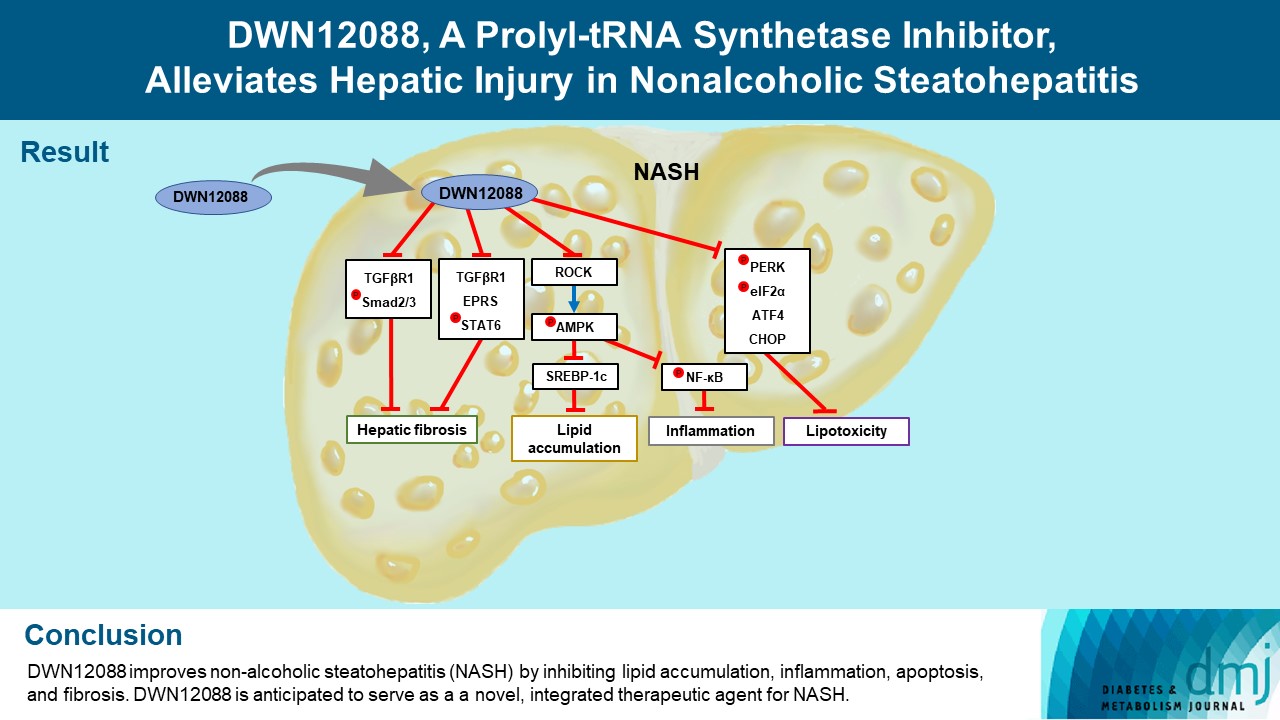
- DWN12088 attenuated palmitic acid (PA)-induced lipid accumulation and lipoapoptosis by downregulating the Rho-kinase (ROCK)/AMP-activated protein kinase (AMPK)/sterol regulatory element-binding protein-1c (SREBP-1c) and protein kinase R-like endoplasmic reticulum kinase (PERK)/α subunit of eukaryotic initiation factor 2 (eIF2α)/activating transcription factor 4 (ATF4)/C/EBP-homologous protein (CHOP) signaling cascades. PA increased but DWN12088 inhibited the phosphorylation of nuclear factor-κB (NF-κB) p65 (Ser536, Ser276) and the expression of proinflammatory genes. Moreover, the DWN12088 inhibited transforming growth factor β (TGFβ)-induced pro-fibrotic gene expression by suppressing TGFβ receptor 1 (TGFβR1)/Smad2/3 and TGFβR1/glutamyl-prolyl-tRNA synthetase (EPRS)/signal transducer and activator of transcription 6 (STAT6) axis signaling. In the case of MCD-diet-induced NASH, DWN12088 reduced hepatic steatosis, inflammation, and lipoapoptosis and prevented the progression of fibrosis.
-
Conclusion
- Our findings provide new insights about DWN12088, namely that it plays an important role in the overall improvement of NASH. Hence, DWN12088 shows great potential to be developed as a new integrated therapeutic agent for NASH.
- Nonalcoholic fatty liver disease (NAFLD) is the most widespread liver disease in the world [1]. It has a prevalence rate of 33% in the general population and 70% to 75% in the obese and diabetic patient population in Western countries [2,3]. Particularly, obesity is a major public health issue because it provokes metabolic syndromes including hypertension, type 2 diabetes mellitus, NAFLD, and insulin resistance [4]. Insulin resistance plays a pivotal role, by increasing the influx of free fatty acids (FFAs) to the liver, in the development of hepatic steatosis [5,6]. While early-stage steatosis is typically considered benign, later-stage nonalcoholic steatohepatitis (NASH) is destructive, resulting in the death of hepatocytes and progression to fibrosis and cirrhosis [7].
- Hepatic steatosis is caused by increased influx of FFAs, such as palmitic acid (PA), in liver tissues. Sterol regulatory element-binding protein-1c (SREBP-1c) is an essential transcription factor that regulates lipid synthesis in insulin-sensitive tissues, such as the liver, muscle, and adipose tissue [8]. Aberrant activation of SREBP-1c leads to excessive lipid accumulation and insulin receptor substrate 1 (IRS-1) suppression, resulting in increased muscular insulin resistance [9]. The activity of SREBP-1c is known to be elevated in insulin and nutrient-rich conditions, whereas its activity is suppressed by AMP-activated protein kinase (AMPK) [10,11]. Recent studies showed that serine/threonine kinase Rho-kinase 1 (ROCK-1), a downstream effector of the small G protein RhoA, was activated by PA. Additionally, ROCK1 knockdown recovered the downregulation of AMPK phosphorylation in PA-stimulated L6 myotubes, indicating that ROCK is related to the effect of insulindependent AMPK action [12]. In summary, PA-mediated ROCK activation inhibits AMPK activity, resulting in increased expression of lipogenesis-related genes, such as Srebp-1c and fatty acid synthase (Fas).
- Increased levels of circulating serum FFA and accumulation of hepatic lipid in non-adipose tissues can lead to hepatocellular dysfunction or cell death, partly because of the diversion of unoxidized FFAs to nonoxidative pathways, resulting in lipoapoptosis [13]. Excess amounts of nonesterified free fatty acids (NEFFAs) that have not been converted to triglyceride (TG) in hepatocytes, promote hepatic lipotoxicity, and its pathologic feature is observed in NASH [14]. The mechanisms involved in FFA-induced lipotoxicity are not clearly known. Nevertheless, recent studies demonstrate that hepatic lipoapoptosis mainly arises from FFA-induced lipotoxic stress of intracellular organelles, such as the endoplasmic reticulum (ER) and mitochondria [15]. Therefore, saturated FFAs, especially PA, have been implicated in ER stress-mediated apoptosis of hepatocytes [16,17]. Protein kinase R-like endoplasmic reticulum kinase (PERK), one of the major transducers of ER stress, phosphorylates α subunit of eukaryotic initiation factor 2 (eIF2α) on Ser 51, and phosphorylated eIF2α suppresses the initiation of general translation to decrease the protein load in the ER [18]. Conversely, activated eIF2α elevates in the translation of the activating transcription factor 4 (ATF4), and activated ATF4 increases the expression of C/EBP-homologous protein (CHOP), and the CHOP in turn inhibits anti-apoptotic B-cell lymphoma 2 (Bcl2) expression [19,20]. Accordingly, the ER stress-mediated PERK/eIF2α/ATF4/CHOP axis signaling pathway is heavily involved in PA-induced lipoapoptosis.
- Liver fibrosis is caused by excessive accumulation of extracellular matrix proteins such as collagen, fibrin, and fibronectin, as seen in most chronic liver diseases. Progression of liver fibrosis results in cirrhosis, hepatic failure, and portal hypertension. Halofuginone (HF) is an analog of alkaloid febrifugine originally isolated from the plant Dichroa febrifuga. In recent years, HF has attracted much attention because of its wide range of beneficial biologic activities, such as in malaria, cancer, and particularly in fibrosis-related diseases. Recently, HF has been reported to be involved in two functional mechanisms, namely inhibition of the transforming growth factor β (TGFβ)/smad3 signaling pathway [21] and inhibition of prolyl-tRNA synthetase (PRS) activity [22]. Treatment with HF decreases TGFβ1-induced collagen synthesis in fibroblasts [23] without affecting TGFβ receptor expression or TGFβ product levels [24], indicating that HF targets TGFβ receptor 1 (TGFβR1)-mediated SMAD3 phosphorylation [25]. Furthermore, PRS has been found to block collagen synthesis and production [26]. HF inhibits the mRNA expression levels of TGFβ1-increased collagen I a1 (Col1a1) and collagen I a2 (Col1a2). However, their levels are recovered by the addition of exogenous proline [26], indicating that HF competitively binds to the proline-binding pocket of the PRS catalytic site [27], which involves the loading of proline to the tRNA during the translational process of proline-rich profibrotic genes, such as Col1a1 and fibronectin (Fn1). Some previous studies reported that proline contents are higher in fibrotic tissues than in non-fibrotic tissues [28]. Therefore, proline accumulation in fibrotic tissues could decrease via the anti-fibrotic effect of HF. Despite its positive effects, several adverse effects of high doses of HF have been reported, such as cytotoxicity, organ failure, and gastrointestinal toxicity [29]. DWN12088 (Daewoong Pharmaceutical, Seoul, Korea), a first-in-class PRS inhibitor, which is a novel therapeutic agent for idiopathic pulmonary fibrosis (IPF), showed anti-fibrotic properties, safety, and tolerability in a phase 2 trial [30]. However, the mechanisms underlying the hepatoprotective effect of DWN12088 have not yet been studied in vitro and in vivo. In this study, we aimed to determine whether DWN12088 alleviates hepatic injury in NASH.
INTRODUCTION
- Chemicals, plasmids, and materials
- DWN12088 was obtained from Daewoong Pharmaceutical Co., Ltd. HF was purchased from (Calbiochem, San Diego, CA, USA). Palmitate was purchased from Sigma-Aldrich (St. Louis, MO, USA), and it was conjugated to fatty acid-free bovine serum albumin (BSA) (Sigma-Aldrich) as previously described [31]. MG132, thiazolyl blue tetrazolium blue (MTT), and Oil red O were purchased from Sigma-Aldrich. Boron-dipyrromethene (BODIPY) was obtained from Invitrogen (Carlsbad, CA, USA).
- Mice experiments
- Male 8-week-old C57BL/6J mice were purchased from Dae Han Bio Link Co. (Eumseong, Korea) and allowed to adapt for 1 week. All mice were housed in a cage with a 12-hour lightdark cycle in a pathogen free animal facility. At 9 weeks old, the mice were fed a chow or methionine-choline deficient (MCD)-diet, administrated with DWN12088 (10 mg/kg, every 2 days apart, n=10 mice per group) or saline (equal volume, every 2 days apart, n=10 mice per group) by oral gavage for 6 weeks. The body weights of the mice were recorded weekly. After 15 weeks, the liver tissues and serum were collected and stored at –80°C for later assessment. All animal experiments were approved by the Institutional Animal Care and Use Committee of Yonsei University at the Wonju Campus (YWC-200401-1). All experimental procedures were performed in accordance with the guidelines of institutional animal care.
- Cell culture and treatments
- Mouse normal hepatocyte cell line alpha mouse liver 12 (AML12) were maintained in 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (Corning Inc., Corning, NY, USA), containing 10% fetal bovine serum, 100 units/mL of penicillin, 100 µg/mL of streptomycin, 40 ng/mL of dexamethasone, and 1× insulin-transferrin-selenium cocktail (Gibco, Grand Island, NY, USA). Human hepatic stellate cells (HSCs) LX-2 were cultured in DMEM supplemented with 10% fetal bovine serum and 100 units/mL of penicillin. Mouse primary peritoneal macrophages were collected from the peritoneal cavity of 6- to 8-week-old mice as previously described [32]. Peritoneal macrophages and RAW264.7 cells of the murine macrophage cell line were cultured in DMEM, containing 5% fetal bovine serum supplied with 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 25 mM of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). The cells were incubated at 37°C in a 5% CO2 humidified incubator. Experiments were performed when the cells had reached 60% to 80% confluence.
- Cell viability
- The details on measurement of cell viability are described in the ‘Supplementary Methods’ section.
- RNA isolation and real-time quantitative reverse transcription polymerase chain reaction
- The details on RNA isolation, real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR), and primer information are provided in the ‘Supplementary Methods and Supplementary Table 1’ section.
- Immunoblotting
- Cell and tissue lysates as well as cytosolic and nuclear extracts were prepared as previously described [33]. Equal amounts of total protein were separated by 8% to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes using a transfer device. The blots were blocked with 3% BSA or 5% skim milk in Tris-buffered saline (T&I, Seoul, Korea) containing 0.1% Tween 20 (Sigma-Aldrich) for 1 hour and immunoblotted with the indicated antibodies. The antibodies information is listed in the ‘Supplementary Methods’ section.
- Luciferase reporter assay
- The details on experimental procedures are explained in the ‘Supplementary Methods’ section.
- Immunocytochemical and histological examination
- The details on experimental procedures and material information are provided in the ‘Supplementary Methods’ section.
- Hematoxylin and eosin, Masson’s trichrome, Oil red O, and BODIPY staining
- The details on experimental procedures are provided in the ‘Supplementary Methods’ section.
- NAS scoring
- Total NAFLD activity score (NAS) was calculated as the sum of steatosis, lobular inflammation, and hepatocyte ballooning scores in the hematoxylin and eosin (H&E)-stained liver section slides. The NAS score range (0–8) was calculated by a sum of steatosis (0–3), lobular inflammation (0–3), and hepatocyte ballooning (0–2). The scores were blindly analyzed in eight fields per slide section.
- TUNEL assay
- Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed on liver tissue sections. The section slides were analyzed using an in situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. All the slides were mounted with mounting medium and observed using a Zeiss LSM710confocal microscope (Carl Zeiss, Berlin, Germany) at ×400 magnification. The percentage of TUNEL-positive cells was determined for at least four fields per section in a randomized manner using the ImageJ software (National Institutes of Health, Bethesda, DM, USA).
- Biochemical analysis
- Levels of TG, cholesterol, FFAs, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured using a colorimetric assay kit (Asan Pharm., Seoul, Korea), according to the manufacturer’s instructions. Hydroxyproline content in the liver tissue was analyzed using a hydroxyproline assay kit (Cell Biolabs, San Diego, CA, USA), according to the manufacturer’s instructions.
- Statistical analysis
- All statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). The number of replicates is listed in the figures and the representative results were obtained from at least three independent experiments. All values are presented as the mean±standard deviation. The statistical significance was evaluated using an unpaired two-tailed t-test between two groups or one-way or two-way analysis of variance (ANOVA) followed by the Holm-Sidak multiple comparison test, depending on the experimental groups analyzed. A P value of <0.05 was considered statistically significant.
METHODS
- High-dose halofuginone reduces cell viability, but not DWN12088
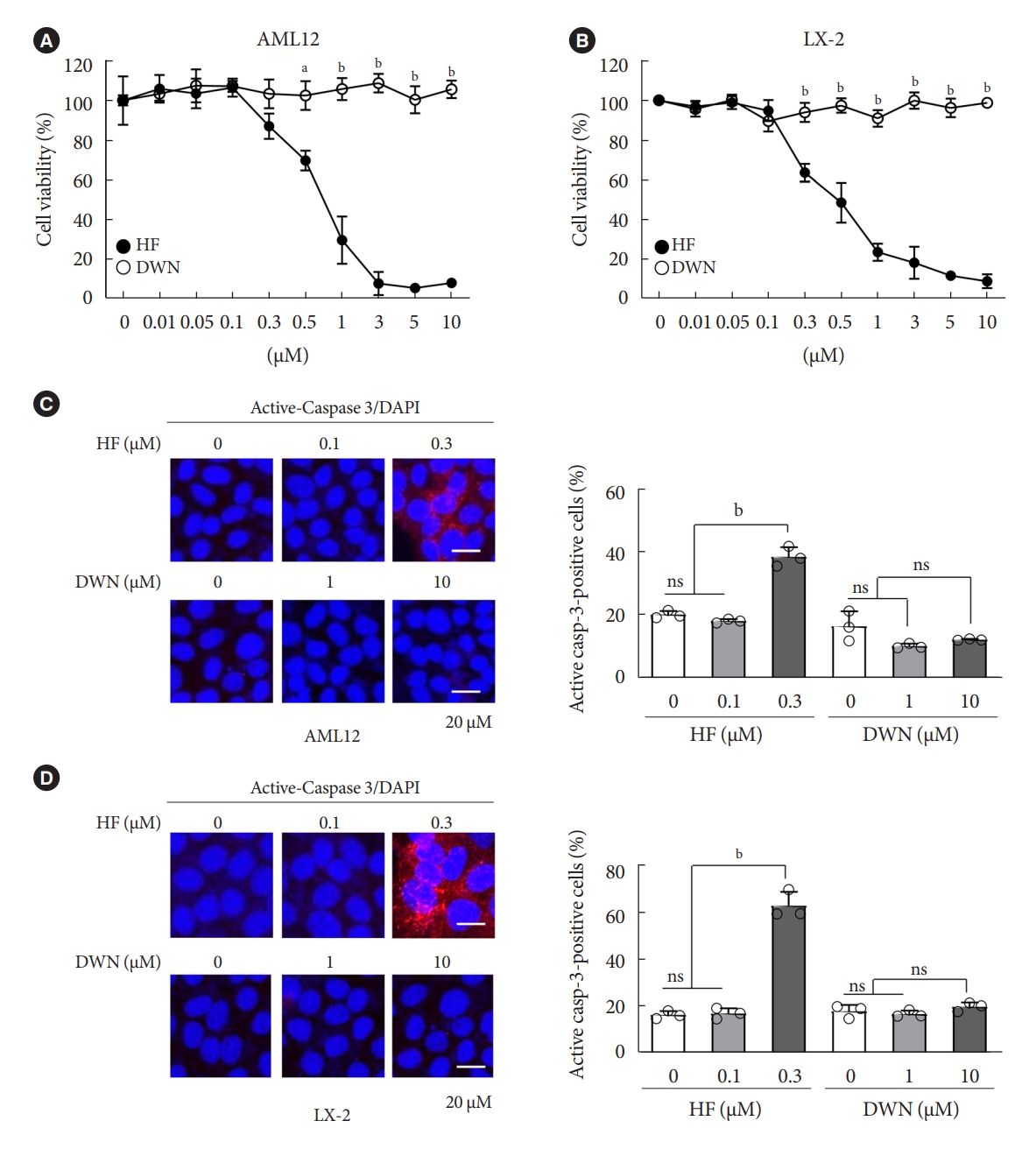
- HF, an analog of alkaloid febrifugine, is an anti-fibrotic agent that suppresses the transcriptional and translational processes of proline-rich profibrotic genes, such as COL1A1, COL1A2, and FN1 [34,35]. However, recently, HF has been reported to cause several adverse effects, such as poor oral bioavailability and gastrointestinal toxicity [29]. Similarly, DWN12088, a therapeutic agent for IPF, is a selective PRS inhibitor that inhibits the synthesis and production of collagen. To compare the cytotoxic effects of HF and DWN12088, mouse normal hepatocytes AML12 and human HSCs LX-2 were treated with indicated dose conditions of HF and DWN12088 for 24 hours. The cell viability was decreased by HF in a dose-dependent manner, but not by DWN12088 (Fig. 1A and B). As expected, active-caspase 3 expression was markedly increased by 0.3 μM HF, but not by the high-dose of DWN12088 (Fig. 1C and D). Of note, high-dose DWN12088 was not as cytotoxic as high-dose HF, indicating the suitability of DWN12088 as a new anti-fibrotic drug.
- DWN12088 decreases profibrotic gene expression and hepatic fibrosis in TGFβ-treated HSCs and liver of MCD-diet mice
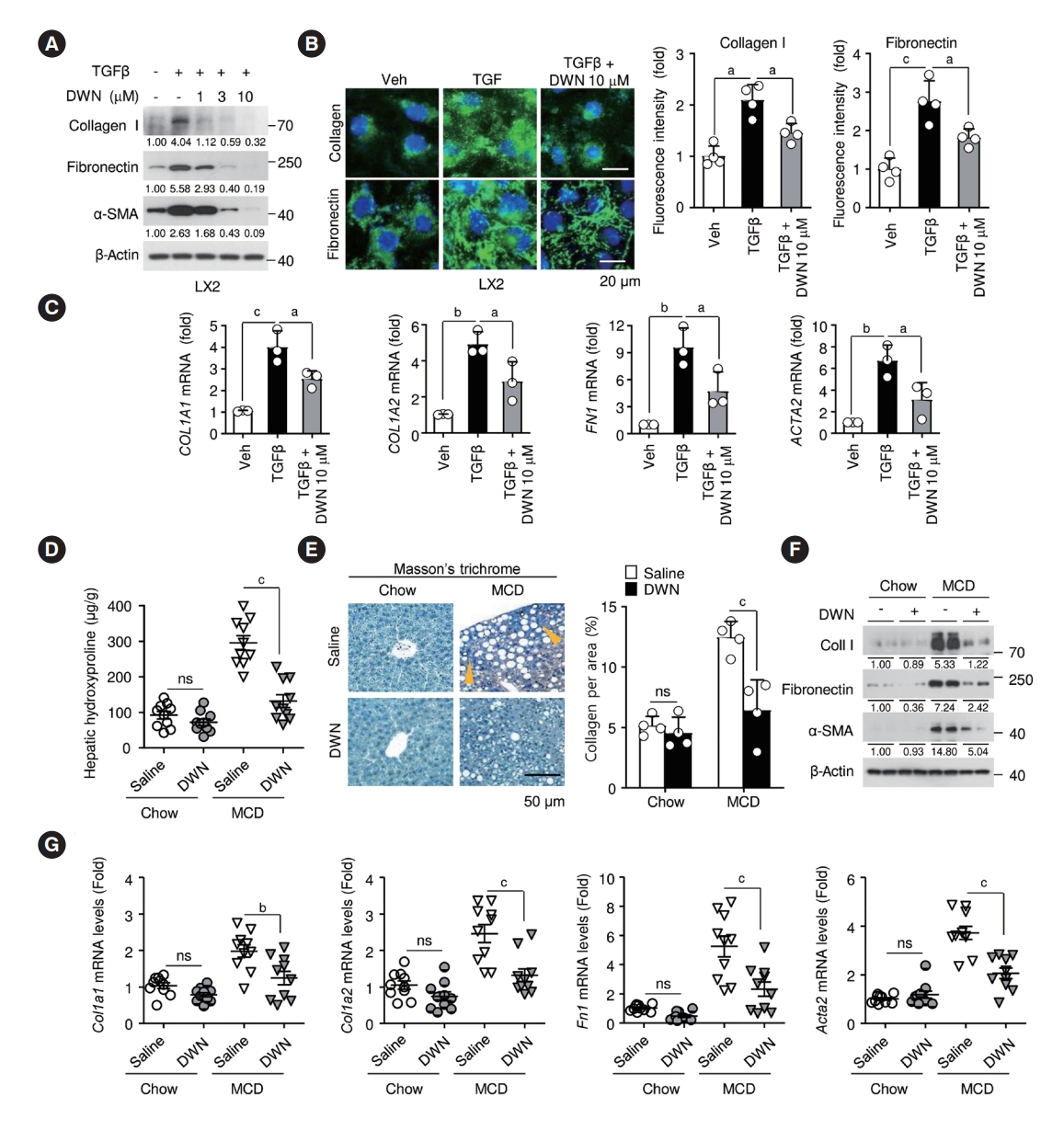
- To assess whether DWN12088 could suppress in vitro liver fibrosis, we first examined the expression levels of profibrotic marker genes, such as collagen I, fibronectin, and α-smooth muscle actin (α-SMA), in LX-2 cells pretreated with or without DWN12088 after TGFβ stimulation. DWN12088 treatment crucially decreased TGFβ-induced profibrotic marker gene expressions, which were confirmed by immunoblotting and immunofluorescence staining (Fig. 2A and B). Similarly, DWN12088 markedly inhibited TGFβ-dependent COL1A1, COL1A2, FN1, and actin alpha 2 (ACTA2) mRNA expressions (Fig. 2C). TGFβ evokes fibrosis by upregulating the TGFβR1/Smad2/3 and TGFβR1/glutamyl-prolyl-tRNA synthetase (EPRS)/signal transducer and activator of transcription 6 (STAT6) signaling cascades [36,37]. Phosphorylated smad2/3 and STAT6 causes transcriptional activations of the promoters of fibrosis-related genes, such as COL1A1, FN1, and ACTA2. We identified the anti-fibrotic effects of DWN12088 involved in the signal modulators. TGFβ treatment increased the phosphorylation of smad2/3 and STAT6 and expression of EPRS, whereas TGFβ and DWN12088 co-treatment downregulated TGFβ signal modulators, such as smad2/3, STAT6, and EPRS in LX-2 cells (Supplementary Fig. 1). Additionally, the similar effects of DWN12088 were also observed in AML12 hepatocytes (Supplementary Fig. 2). Next, we assessed whether DWN12088 treatment decreases MCD-diet-induced liver fibrosis. Hepatic hydroxyproline contents and Masson’s trichrome staining showed that compared to that with saline treatment, DWN12088 significantly attenuated MCD-diet-induced collagen fiber formation in liver tissues (Fig. 2D and E). As expected, DWN12088 also drastically inhibited MCD-diet-induced protein and mRNA levels of Col1a1, Col1a2, Fn1, and Acta2 (Fig. 2F and G). These results suggest that DWN12088 effectively ameliorates hepatic fibrosis by inhibiting the TGFβ signal modulators-mediated upregulations of profibrotic marker gene expressions.
- DWN12088 attenuates lipid accumulation by inhibiting ROCK/AMPK/SREBP-1c signaling cascades in palmitic acid-stimulated hepatocytes and liver of MCD-diet mice
- To explore whether DWN12088 reduces lipid accumulation, AML12 cells were treated with PA alone or with DWN12088 (5 μM, 10 μM) for 24 hours. Oil red O staining and TG contents showed that DWN12088 effectively inhibited PA-induced lipid accumulation in a dose-dependent manner (Fig. 3A and B). SREBP-1c plays a pivotal role in the transcriptional activation of fatty acid synthesis-related genes. PA-activated ROCK blocks AMPK activity, resulting in upregulated SREBP-1c and FAS expressions [12]. Therefore, we examined the inhibitory effects of DWN12088 on PA-activated ROCK/AMPK/SREBP-1c signaling pathways. DWN12088 treatment significantly suppressed the activity of PA-induced signal modulators in a dose-dependent manner. Moreover, PA increased the levels of lipogenesis marker proteins FAS and adipophilin (ADRP) and diminished the levels of lipolysis marker proteins peroxisome proliferatoractivated receptor α (PPARα) and peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α), but these effects were dose-dependently reversed by the DWN12088 (Fig. 3C). Similarly, DWN12088 reversed mRNA expressions of PAregulated lipid synthesis-related genes, such as Srebp-1c, Fas, Adrp, Ppara, and Pgc1a (Supplementary Fig. 3A). Next, we investigated whether DWN12088 treatment attenuates MCD-diet-induced hepatic steatosis. Picture data of liver morphology showed that DWN12088 markedly diminished MCD-diet-induced hepatic steatosis compared to that with saline treatment (Fig. 3D). Compared with that of the chow group, the MCD-diet group lost body and liver weights. However, the body and liver weights did not differ between the saline- and DWN12088-administrated groups (Fig. 3E). There was however no significant difference between the hepatic TG levels of the mice within the same treatment group (Supplementary Table 2). Based on this, five mice were selected as representative samples for each group and Oil red O (Supplementary Table 3) and BODIPY staining was conducted. The Oil red O and BODIPY staining revealed that DWN12088 substantially suppressed MCD-diet-induced lipid accumulation in liver tissues compared to that by saline (Fig. 3F and G). Moreover, similar effects were also confirmed by biochemical analysis of hepatic TG, serum TG, total cholesterol, and serum FFA levels (Fig. 3H). We then confirmed whether DWN12088 attenuated MCD-diet-induced lipid synthesis-related proteins, such as FAS, ADRP, and acetyl-CoA carboxylase α (ACCα) by inhibiting the ROCK/AMPK/SREBP-1c signaling pathway. As expected, compared to that with saline treatment, DWN12088 treatment significantly decreased MCD-diet-induced lipid accumulation-related signal modulators and marker proteins in the liver tissues (Fig. 3I and J). Furthermore, MCD-diet-increased mRNA levels of lipogenesis-related genes, such as Srebp-1c, Fas, and Adrp, and MCD-diet-inhibited mRNA levels of lipolysis-related genes, such as Ppara, Pgc1a, and carnitine palmitoyltransferase 1A (CPT1a) were recovered by DWN12088 (Supplementary Fig. 3B). Taken together, these data suggest that DWN12088 has a protective effect against PA-stimulated lipid accumulation and MCD-diet-induced hepatic steatosis.
- DWN12088 mitigates inflammatory responses by inhibiting NF-κB activation in palmitic acid-stimulated macrophages and liver of MCD-diet mice
- AMPK signaling can inhibit NF-κB-induced inflammatory responses [38]. PA may be suppressed in the phosphorylation of AMPKα at Thr172 via the increase of ROCK expression, leading to NF-κB activation. We assessed whether DWN12088 suppresses PA-induced NF-κB activation by inhibiting ROCK signaling cascades in murine macrophages RAW264.7 cells. As expected, DWN12088 treatments markedly restored PA-inhibited AMPK phosphorylation via inhibition of ROCK expression in a dose-dependent manner (Fig. 4A). Moreover, DWN12088 significantly decreased PA-mediated NF-κB activation by increasing NF-κB p65 nuclear translocation via phosphorylation and degradation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) (Fig. 4B). Additionally, phosphorylation of NF-κB p65 at Ser 536 and Ser 276 in the cytosol and nuclei, NF-κB-driven luciferase activity, and NF-κB p65 nuclear translocation revealed that DWN12088 significantly inhibited PA-induced NF-κB activation (Fig. 4C-E). Furthermore, PA-induced mRNA expressions of proinflammatory genes, such as tumor necrosis factor-α (Tnfa), interleukin 1β (Il1b), and Il6 and PA-inhibited mRNA expressions of anti-inflammatory genes Il10 and arginase 1 (Arg1) were reversed by DWN12088 in the RAW264.7 cells (Fig. 4F). Similar effects of DWN12088 on mRNA expressions of inflammatory genes were also observed in peritoneal macrophages (Supplementary Fig. 4). To evaluate whether DWN12088 inhibited MCD-diet-induced steatohepatitis, we first analyzed the infiltration of M1 proinflammatory macrophages in the liver tissues. Immunofluorescence staining and qRT-PCR analysis have shown that DWN12088 markedly decreased MCD-diet-induced infiltration of M1 proinflammatory macrophages and mRNA levels of M1 macrophage marker F4/80, also known as EGF-like module-containing mucin-like hormone receptor-like 1 (Emr1) (Fig. 4G and H). In addition, DWN12088 treatment attenuated MCD-diet-induced IκBα degradation and p65 NF-κB phosphorylation, resulting in increased NF-κB activation (Fig. 4I and J). As expected, mRNA levels of NF-κBdependent proinflammatory genes, such as Tnfa, Il1b, Il6, and CC motif chemokine ligand 2 (Ccl2) were significantly increased, and expressions of anti-inflammatory genes, such as Il10 and Arg1, were inhibited in the liver tissues of saline-treated MCDdiet group, whereas the expressions were reversed in the DWN12088 group (Fig. 4K). Taken together, these results suggest that DWN12088 substantially improved steatohepatitis by inhibiting NF-κB-mediated inflammatory responses.
- DWN12088 reduces lipoapoptosis and hepatic injury by blocking ER stress-related signaling cascades in palmitic acid-stimulated hepatocytes and liver of MCD-diet mice
- Activation of PA-mediated ER stress signal pathway is implicated in apoptosis via lipotoxicity [39]. We first investigated inhibitory effects of DWN12088 on the PA-mediated ER stress and apoptotic signaling pathways. PA-induced PERK and eIF2α phosphorylation, and ATF4 and CHOP expression were significantly inhibited by DWN12088. In addition, the reduction in the level of anti-apoptotic protein Bcl2 and increase in the levels of proapoptotic proteins, such as BCL2 associated X, apoptosis regulator (Bax) and active-caspase 3 in PA-stimulated cells, were dose-dependently reversed by DWN12088, resulting in the recovery of poly(ADP-ribose) polymerase (PARP) cleavage (Fig. 5A). Furthermore, similar results were confirmed by active-caspase 3-targeted immunofluorescence staining and TUNEL assay (Fig. 5B and C). To evaluate whether DWN12088 inhibited MCD-diet-induced hepatic injury, we first confirmed inhibitory effects of DWN12088 on the MCD-diet-mediated ER stress and proapoptotic signaling cascades in the liver tissues. As expected, compared to that with saline treatment, DWN12088 treatment markedly decreased MCD-diet-induced phosphorylation and expression of ER stress and proapoptotic signal modulators in the liver tissues (Fig. 5D). Immunofluorescence staining also showed similar results (Fig. 5E). Furthermore, MCD-diet-increased circulating ALT/AST levels and percentages of TUNEL-positive hepatocytes were drastically diminished by DWN12088 treatment (Fig. 5F and G). Moreover, H&E staining have shown that saline-treated MCD-diet group had increased the total NAS. In contrast, the NAS scores were markedly diminished in the DWN12088-treated MCD-diet group (Fig. 5H). Hence, it can be said that DWN12088 treatment prevents hepatic injury by inhibiting ER stress-mediated signaling pathway.
RESULTS
- HF is a bioactive agent that blocks the catalytic activity of PRS, which involves the loading of proline to tRNA during the translation of proline-rich profibrotic genes, resulting in the suppression of collagen and fibronectin gene expressions [26]. DWN12088, a first-in-class PRS inhibitor, is known to be an anti-fibrotic agent that improves IPF by inhibiting the synthesis of collagen [40], but to date, no studies have been reported on its efficacy in improving hepatic injury caused by NASH. In this study, we first investigated whether DWN12088 inhibits TGFβ-induced expressions of profibrotic genes, such as collagen I, fibronectin, and α-SMA, in human HSCs LX-2. As expected, the DWN12088 treatments significantly decreased the expressions of profibrotic genes. In addition, DWN12088 treatment downregulated TGFβ-mediated activation of TGFβR1/Smad2/3 and TGFβR1/EPRS/STAT6 axis signaling. Similar results were found with mouse normal hepatocytes AML12. Consistent with our findings, a previous study demonstrated that HF and EPRS-targeting shRNA suppressed TGFβR1/Smad2/3 and TGFβR1/EPRS/STAT6 signaling pathways, leading to the downregulation of collagen 1 and fibronectin expressions in TGFβ-treated LX-2 cells and CCl4-treated mouse liver [37]. In addition, we showed that DWN12088 treatment attenuates MCD-diet-induced expressions of collagen 1, fibronectin, and α-SMA. Taken together, these finding indicate that DWN12088 plays an important role in the inhibition of hepatic fibrosis.
- A recent study reported that the expressions of glucose transporter 1 (GLUT1) and hexokinase-2 were suppressed by the inhibition of AKT/mammalian target of rapamycin complex 1 (mTORC1) signaling pathway in HF-treated colorectal cancer cells, resulting in diminished glucose uptake [41]. The mechanism by which HF inhibits lipogenesis remains unclear. In particular, several side effects, such as organ failure and gastrointestinal toxicity have been reported with the use of high-dose HF, and for this reason, it is difficult to study this agent in vivo [27]. Interestingly, we found that DWN12088 downregulates two distinct signaling cascades, ROCK/AMPK/SREBP-1c and PERK/eIF2α/ATF4/CHOP, resulting in suppressed lipid accumulation and lipoapoptosis in PA-stimulated AML12 cells in the liver of the MCD-diet mice. These findings are supported by the results of previous studies, namely that ROCK knockdown reverses the downregulation of AMPK phosphorylation, leading to inhibitions of SREBP-1c and FAS expressions in PA-stimulated L6 myotube cells [12]. Moreover, PA-activated ER stress signaling cascades, namely the PERK/eIF2α/ATF4/CHOP axis, were decreased by it targeting siRNA, resulting in inhibited anti-apoptotic Bcl2 expression [19]. Our results indicate that DWN12088 markedly inhibits hepatic lipoapoptosis by promoting lipid accumulation via downregulation of ROCK-and ER stress-mediated signaling cascades.
- Since the activation of AMPK signaling suppresses NF-κB-mediated activation of inflammatory responses [38], PA may be inhibited by the phosphorylation of AMPKα on Thr172 via an increase in ROCK expression, resulting in elevated activation of NF-κB. Our data showed that DWN12088 treatment decreased PA-mediated NF-κB activation by inhibiting the downstream ROCK signaling cascades, which is consistent with the results of previous studies [12,38]. The free NF-κB dissociated from the NF-κB/IκBα inactive complex can be phosphorylated at Ser 536 and Ser 276 in the cytosol and nuclei, respectively [42]. Our results showed that PA increased but DWN12088 inhibited the phosphorylation of NF-κB p65 (Ser536, Ser276) and expressions of proinflammatory genes, such as Tnfa, Il1b, Il6, and Ccl2. Similar results were confirmed in DWN12088-treated MCD-diet fed mice. Taken together, these results suggest that DWN12088 significantly improved steatohepatitis by inhibiting NF-κB-mediated inflammatory responses.
- In summary, to our knowledge, our work demonstrates for the first time that DWN12088 was safer at high doses than HF. As expected, DWN12088 suppressed hepatic fibrosis by inhibiting TGFβR1/Smad2/3 and TGFβR1/EPRS/STAT6 axis signaling. Furthermore, DWN12088 markedly attenuated hepatic lipid accumulation and lipotoxicity by inhibiting the ROCK/AMPK/SREBP-1c and PERK/eIF2α/ATF4/CHOP axis signaling pathways. We also showed that DWN12088 markedly reduced steatohepatitis by inhibiting the NF-κB-mediated inflammatory responses. In confirmation, AMPK inhibitor (compound C) treatment blocked the anti-steatosis and anti-inflammatory effects of DWN12088 (Supplementary Fig. 5).
- Steatosis and inflammation are suppressed through general control nonderepressible 2 (GCN2)/mTORC1 signaling through PRS inhibitor [43]. We therefore speculate that the anti-steatosis and anti-inflammatory effects by DWN12088 are through the inhibition of PRS. The effects of DWN12088 on ROCK/AMPK/SREBP-1c and NF-κB signaling were confirmed in relation to inhibition of steatosis and inflammation respectively. Based on the above-mentioned findings, the beneficial effect of DWN12088 goes beyond its specific PRS inhibition. It also prevents fat accumulation and fat toxicity both in vivo and in vitro, thus preventing the onset of liver fibrosis. Therefore, our findings provide substantive evidence that DWN12088 plays a pivotal role in alleviating hepatic fibrosis, and we provide evidence that it shows great potential to be developed as a new integrated therapeutic agent.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Fig. 4.
Supplementary Fig. 5.
-
CONFLICTS OF INTEREST
Drug Discovery Center, Daewoong Pharmaceutical provided us with DWN12088 for this study. No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: D.K.L., J.S.P., C.H.C.
Acquisition, analysis, or interpretation of data: D.K.L., S.H.J., E.S.L., K.B.H., N.W.P., D.H.K., S.I.P.
Drafting the work or revising: D.K.L., S.H.J.
Final approval of the manuscript: all authors.
-
FUNDING
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No, 2021R1A2B5B01002354). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No, 2022R1I1A1A01068782), this work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No, 2020R1A5A8019180).
NOTES
-
Acknowledgements
- DWN12088 used in this study was provided by Drug Discovery Center, Daewoong Pharmaceutical. We thank Dr. CH Lee for the discussions and recommendations for our research. Part of our study was also presented in oral presentation from at the 35th Spring Conference of the Korean Diabetes Association, Gyeongju, Korea, May 12 to 14, 2022.



- 1. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960-7.ArticlePubMed
- 2. Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al. Prevalence of and risk factors for hepatic steatosis and non-alcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2011;34:1139-44.ArticlePubMedPMCPDF
- 3. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016;65:1096-108.ArticlePubMedPMC
- 4. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782-7.ArticlePubMedPDF
- 5. Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci 2013;14:20704-28.ArticlePubMedPMC
- 6. Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52:774-88.ArticlePubMed
- 7. Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 2014;147:765-83.ArticlePubMedPMC
- 8. Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 2007;68:72-82.ArticlePubMedPDF
- 9. Bi Y, Wu W, Shi J, Liang H, Yin W, Chen Y, et al. Role for sterol regulatory element binding protein-1c activation in mediating skeletal muscle insulin resistance via repression of rat insulin receptor substrate-1 transcription. Diabetologia 2014;57:592-602.ArticlePubMedPDF
- 10. Commerford SR, Peng L, Dube JJ, O’Doherty RM. In vivo regulation of SREBP-1c in skeletal muscle: effects of nutritional status, glucose, insulin, and leptin. Am J Physiol Regul Integr Comp Physiol 2004;287:R218-27.ArticlePubMed
- 11. Wu W, Tang S, Shi J, Yin W, Cao S, Bu R, et al. Metformin attenuates palmitic acid-induced insulin resistance in L6 cells through the AMP-activated protein kinase/sterol regulatory element-binding protein-1c pathway. Int J Mol Med 2015;35:1734-40.ArticlePubMed
- 12. Tang S, Wu W, Tang W, Ge Z, Wang H, Hong T, et al. Suppression of Rho-kinase 1 is responsible for insulin regulation of the AMPK/SREBP-1c pathway in skeletal muscle cells exposed to palmitate. Acta Diabetol 2017;54:635-44.ArticlePubMedPDF
- 13. Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta 2002;1585:202-12.ArticlePubMed
- 14. Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003;125:437-43.ArticlePubMed
- 15. Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 2006;47:2726-37.ArticlePubMed
- 16. Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 2006;291:E275-81.ArticlePubMed
- 17. Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem 2007;303:105-13.ArticlePubMedPDF
- 18. Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999;397:271-4.ArticlePubMedPDF
- 19. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 2001;21:1249-59.ArticlePubMedPMCPDF
- 20. Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 2009;20:436-43.ArticlePubMedPMC
- 21. Ziegler S, Pries V, Hedberg C, Waldmann H. Target identification for small bioactive molecules: finding the needle in the haystack. Angew Chem Int Ed Engl 2013;52:2744-92.ArticlePubMed
- 22. Kang YB, Mallikarjuna PR, Fabian DA, Gorajana A, Lim CL, Tan EL. Bioactive molecules: current trends in discovery, synthesis, delivery and testing. IeJSME 2013;7(Suppl 1):S32-46.
- 23. Halevy O, Nagler A, Levi-Schaffer F, Genina O, Pines M. Inhibition of collagen type I synthesis by skin fibroblasts of graft versus host disease and scleroderma patients: effect of halofuginone. Biochem Pharmacol 1996;52:1057-63.ArticlePubMed
- 24. Turgeman T, Hagai Y, Huebner K, Jassal DS, Anderson JE, Genin O, et al. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscul Disord 2008;18:857-68.ArticlePubMed
- 25. Luo Y, Xie X, Luo D, Wang Y, Gao Y. The role of halofuginone in fibrosis: more to be explored? J Leukoc Biol 2017;102:1333-45.ArticlePubMedPDF
- 26. Keller TL, Zocco D, Sundrud MS, Hendrick M, Edenius M, Yum J, et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat Chem Biol 2012;8:311-7.ArticlePubMedPMCPDF
- 27. Pines M, Spector I. Halofuginone: the multifaceted molecule. Molecules 2015;20:573-94.ArticlePubMedPMC
- 28. Kershenobich D, Fierro FJ, Rojkind M. The relationship between the free pool of proline and collagen content in human liver cirrhosis. J Clin Invest 1970;49:2246-9.ArticlePubMedPMC
- 29. Pines M, Snyder D, Yarkoni S, Nagler A. Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biol Blood Marrow Transplant 2003;9:417-25.ArticlePubMed
- 30. ClinicalTrials.gov. Clinical trial to evaluate the safety and efficacy of DWN12088 in patients with IPF. Available from: https://ClinicalTrials.gov/show/NCT05389215 (2023 Apr 26).
- 31. Zeng X, Zhu M, Liu X, Chen X, Yuan Y, Li L, et al. Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr Metab (Lond) 2020;17:11.ArticlePubMedPMCPDF
- 32. Lee DK, Kim JH, Kim J, Choi S, Park M, Park W, et al. REDD-1 aggravates endotoxin-induced inflammation via atypical NFκB activation. FASEB J 2018;32:4585-99.ArticlePubMedPDF
- 33. Kim JH, Na HJ, Kim CK, Kim JY, Ha KS, Lee H, et al. The nonprovitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: role of H(2)O(2) in NF-kappaB activation. Free Radic Biol Med 2008;45:885-96.PubMed
- 34. Nelson EF, Huang CW, Ewel JM, Chang AA, Yuan C. Halofuginone down-regulates Smad3 expression and inhibits the TGFbeta-induced expression of fibrotic markers in human corneal fibroblasts. Mol Vis 2012;18:479-87.PubMedPMC
- 35. McGaha TL, Phelps RG, Spiera H, Bona C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol 2002;118:461-70.PubMed
- 36. Fabregat I, Moreno-Caceres J, Sanchez A, Dooley S, Dewidar B, Giannelli G, et al. TGF-β signalling and liver disease. FEBS J 2016;283:2219-32.PubMed
- 37. Song DG, Kim D, Jung JW, Nam SH, Kim JE, Kim HJ, et al. Glutamyl-prolyl-tRNA synthetase induces fibrotic extracellular matrix via both transcriptional and translational mechanisms. FASEB J 2019;33:4341-54.ArticlePubMedPDF
- 38. Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667-76.ArticlePubMedPMC
- 39. Cho H, Wu M, Zhang L, Thompson R, Nath A, Chan C. Signaling dynamics of palmitate-induced ER stress responses mediated by ATF4 in HepG2 cells. BMC Syst Biol 2013;7:9.ArticlePubMedPMCPDF
- 40. Lee CH, Cho M, Kim JM, Lee JH, Kim DW, Park MY, et al. A first-in-class PRS inhibitor, DWN12088, as a novel therapeutic agent for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2020;202:A2786.Article
- 41. Chen GQ, Tang CF, Shi XK, Lin CY, Fatima S, Pan XH, et al. Halofuginone inhibits colorectal cancer growth through suppression of Akt/mTORC1 signaling and glucose metabolism. Oncotarget 2015;6:24148-62.ArticlePubMedPMC
- 42. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci 2005;30:43-52.PubMed
- 43. Kim Y, Sundrud MS, Zhou C, Edenius M, Zocco D, Powers K, et al. Aminoacyl-tRNA synthetase inhibition activates a pathway that branches from the canonical amino acid response in mammalian cells. Proc Natl Acad Sci U S A 2020;117:8900-11.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations


 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite