
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Complications
- Glycemic Control and Retinal Microvascular Changes in Type 2 Diabetes Mellitus Patients without Clinical Retinopathy
- Kangmin Lee, Ga Hye Lee, Seung Eun Lee, Jee Myung Yang, Kunho Bae
- Received May 15, 2023 Accepted December 15, 2023 Published online March 13, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0149 [Epub ahead of print]

- 638 View
- 34 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate the association of glycemic control and retinal microvascular changes in patients with type 2 diabetes mellitus (T2DM) without diabetic retinopathy (DR).
Methods
This retrospective, observational, cohort study included patients with T2DM without DR. The patients were categorized into intensive control (IC; mean glycosylated hemoglobin [HbA1c] ≤7.0%) and moderate control (MC; mean HbA1c >7.0%) groups. Optical coherence tomography (OCT) and swept-source OCT angiography (OCTA) image parameters were compared between three groups, including healthy controls.
Results
In total, 259 eyes of 259 participants (88 IC, 81 MC, and 90 controls) were included. The foveal avascular zone area was significantly larger in the MC group than IC and control groups (all P<0.05). The IC group had lower vessel density in the superficial retinal layer and deep retinal layer than the controls (all P<0.05). The choriocapillaris (CC) flow deficit (FD) was significantly greater in the MC group than in the IC and control groups (18.2%, 16.7%, and 14.2%, respectively; all P<0.01). In multivariate regression analysis, CC-FD was associated with the mean HbA1c level (P=0.008). There were no significant differences in OCT parameters among the groups.
Conclusion
OCTA revealed that early CC impairment is associated with HbA1c levels; the CC changes precede clinically apparent DR. The OCTA parameters differed among the groups according to the degree of glycemic control. Our results suggest that microvascular changes precede DR and are closely related to glycemic control.
- Drug/Regimen
- Efficacy and Safety of IDegAsp in a Real-World Korean Population with Type 2 Diabetes Mellitus
- Shinae Kang, Yu-Bae Ahn, Tae Keun Oh, Won-Young Lee, Sung Wan Chun, Boram Bae, Amine Dahaoui, Jin Sook Jeong, Sungeun Jung, Hak Chul Jang
- Received August 24, 2023 Accepted November 22, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0297 [Epub ahead of print]
- 672 View
- 47 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
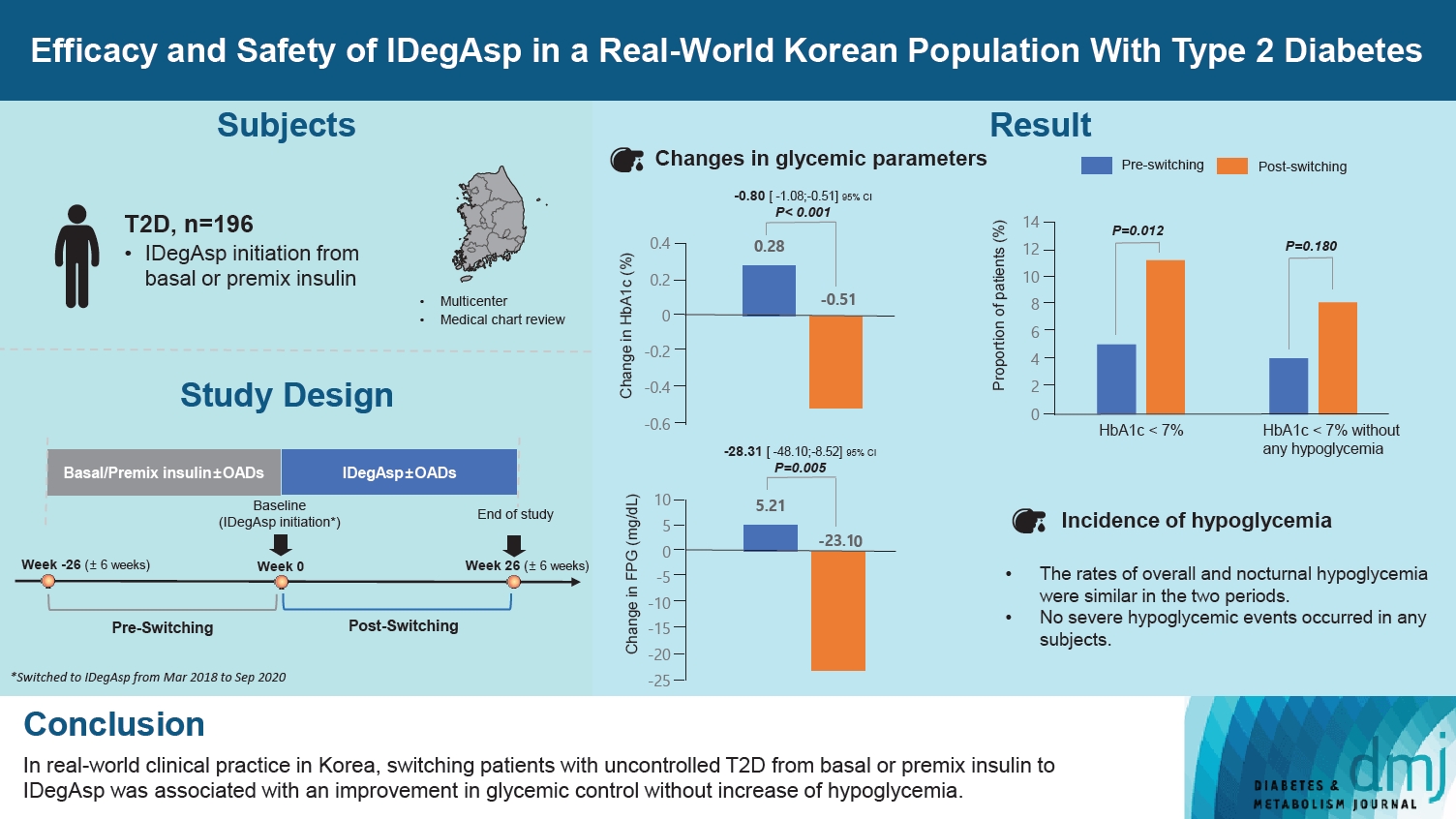
This study investigated the real-world efficacy and safety of insulin degludec/insulin aspart (IDegAsp) in Korean adults with type 2 diabetes mellitus (T2DM), whose insulin treatment was switched to IDegAsp.
Methods
This was a multicenter, retrospective, observational study comprising two 26-week treatment periods, before and after switching to IDegAsp, respectively. Korean adults with uncontrolled T2DM treated with basal or premix insulin (±oral antidiabetic drugs) were enrolled. The primary objective was to compare the degree of glycosylated hemoglobin (HbA1c) change in each 26-week observation period. The analyses included changes in HbA1c, fasting plasma glucose (FPG), body weight, proportion of participants achieving HbA1c <7.0%, hypoglycemic events, and total daily insulin dose (ClinicalTrials.gov, number NCT04656106).
Results
In total, 196 adults (mean age, 65.95 years; mean T2DM duration, 18.99 years) were analyzed. The change in both HbA1c and FPG were significantly different between the pre-switching and the post-switching period (0.28% vs. –0.51%, P<0.001; 5.21 mg/dL vs. –23.10 mg/dL, P=0.005), respectively. After switching, the rate of achieving HbA1c <7.0% was significantly improved (5.10% at baseline vs. 11.22% with IDegAsp, P=0.012). No significant differences (before vs. after switching) were observed in body weight change, and total daily insulin dose. The rates of overall and severe hypoglycemia were similar in the two periods.
Conclusion
In real-world clinical practice in Korea, the change of insulin regimen to IDegAsp was associated with an improvement in glycemic control without increase of hypoglycemia, supporting the use of IDegAsp for patients with T2DM uncontrolled with basal or premix insulin.
- Lifestyle
- Enhancing Diabetes Care through a Mobile Application: A Randomized Clinical Trial on Integrating Physical and Mental Health among Disadvantaged Individuals
- Jae Hyun Bae, Eun Hee Park, Hae Kyung Lee, Kun Ho Yoon, Kyu Chang Won, Hyun Mi Kim, Sin Gon Kim
- Received August 24, 2023 Accepted October 16, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0298 [Epub ahead of print]
- 714 View
- 98 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
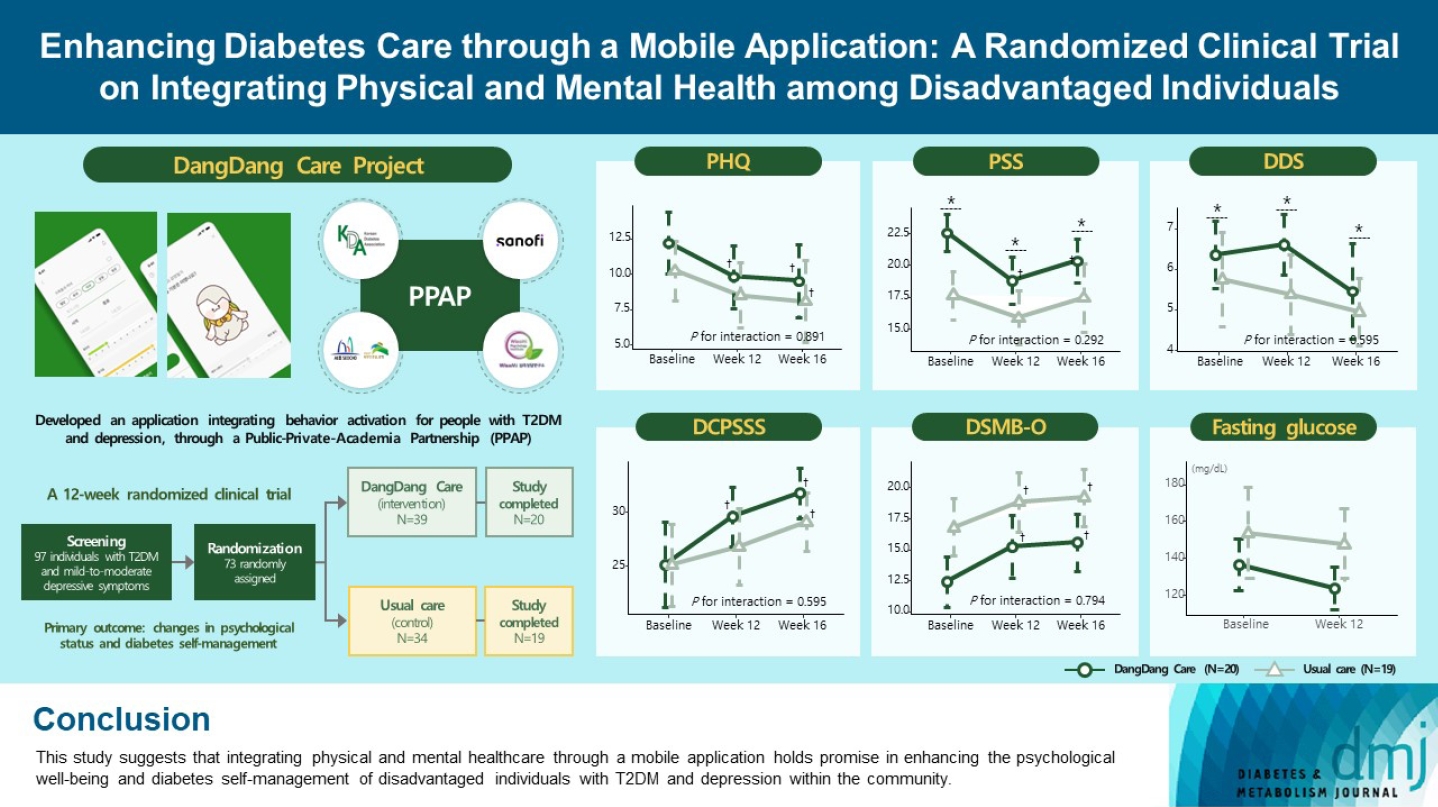
This study examines integrating physical and mental healthcare for disadvantaged persons with type 2 diabetes mellitus and mild-to-moderate depression in the community, using a mobile application within a public-private-academic partnership.
Methods
The Korean Diabetes Association has developed a mobile application combining behavioral activation for psychological well-being and diabetes self-management, with conventional medical therapy. Participants were randomly assigned to receive the application with usual care or only usual care. Primary outcomes measured changes in psychological status and diabetes selfmanagement through questionnaires at week 12 from the baseline. Secondary outcomes assessed glycemic and lipid control, with psychological assessments at week 16.
Results
Thirty-nine of 73 participants completed the study (20 and 19 in the intervention and control groups, respectively) and were included in the analysis. At week 12, the intervention group showed significant reductions in depression severity and perceived stress compared to the control group. Additionally, they reported increased perceived social support and demonstrated improved diabetes self-care behavior. These positive effects persisted through week 16, with the added benefit of reduced anxiety. While fasting glucose levels in the intervention group tended to improve, no other significant differences were observed in laboratory assessments between the groups.
Conclusion
This study provides compelling evidence for the potential efficacy of a mobile application that integrates physical and mental health components to address depressive symptoms and enhance diabetes self-management in disadvantaged individuals with type 2 diabetes mellitus and depression. Further research involving larger and more diverse populations is warranted to validate these findings and solidify their implications.
- Metabolic Risk/Epidemiology
- Glycemic Control Is Associated with Histological Findings of Nonalcoholic Fatty Liver Disease
- Teruki Miyake, Shinya Furukawa, Bunzo Matsuura, Osamu Yoshida, Masumi Miyazaki, Akihito Shiomi, Ayumi Kanamoto, Hironobu Nakaguchi, Yoshiko Nakamura, Yusuke Imai, Mitsuhito Koizumi, Takao Watanabe, Yasunori Yamamoto, Yohei Koizumi, Yoshio Tokumoto, Masashi Hirooka, Teru Kumagi, Eiji Takesita, Yoshio Ikeda, Masanori Abe, Yoichi Hiasa
- Received June 24, 2023 Accepted September 21, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0200 [Epub ahead of print]

- 937 View
- 40 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Poor lifestyle habits may worsen nonalcoholic fatty liver disease (NAFLD), with progression to nonalcoholic steatohepatitis (NASH) and cirrhosis. This study investigated the association between glycemic control status and hepatic histological findings to elucidate the effect of glycemic control on NAFLD.
Methods
This observational study included 331 patients diagnosed with NAFLD by liver biopsy. Effects of the glycemic control status on histological findings of NAFLD were evaluated by comparing the following four glycemic status groups defined by the glycosylated hemoglobin (HbA1c) level at the time of NAFLD diagnosis: ≤5.4%, 5.5%–6.4%, 6.5%–7.4%, and ≥7.5%.
Results
Compared with the lowest HbA1c group (≤5.4%), the higher HbA1c groups (5.5%–6.4%, 6.5%–7.4%, and ≥7.5%) were associated with advanced liver fibrosis and high NAFLD activity score (NAS). On multivariate analysis, an HbA1c level of 6.5%– 7.4% group was significantly associated with advanced fibrosis compared with the lowest HbA1c group after adjusting for age, sex, hemoglobin, alanine aminotransferase, and creatinine levels. When further controlling for body mass index and uric acid, total cholesterol, and triglyceride levels, the higher HbA1c groups were significantly associated with advanced fibrosis compared with the lowest HbA1c group. On the other hand, compared with the lowest HbA1c group, the higher HbA1c groups were also associated with a high NAS in both multivariate analyses.
Conclusion
Glycemic control is associated with NAFLD exacerbation, with even a mild deterioration in glycemic control, especially a HbA1c level of 6.5%–7.4%, contributing to NAFLD progression. -
Citations
Citations to this article as recorded by- Combined effect of histological findings and diabetes mellitus on liver‐related events in patients with metabolic dysfunction‐associated steatotic liver disease
Akihito Shiomi, Teruki Miyake, Shinya Furukawa, Bunzo Matsuura, Osamu Yoshida, Takao Watanabe, Ayumi Kanamoto, Masumi Miyazaki, Hironobu Nakaguchi, Yoshio Tokumoto, Masashi Hirooka, Masanori Abe, Yoichi Hiasa
Hepatology Research.2024;[Epub] CrossRef
- Combined effect of histological findings and diabetes mellitus on liver‐related events in patients with metabolic dysfunction‐associated steatotic liver disease
- Drug Regimen
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Kyung Ah Han, Yong Hyun Kim, Doo Man Kim, Byung Wan Lee, Suk Chon, Tae Seo Sohn, In Kyung Jeong, Eun-Gyoung Hong, Jang Won Son, Jae Jin Nah, Hwa Rang Song, Seong In Cho, Seung-Ah Cho, Kun Ho Yoon
- Diabetes Metab J. 2023;47(6):796-807. Published online February 9, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0315
- 40,099 View
- 576 Download
- 4 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
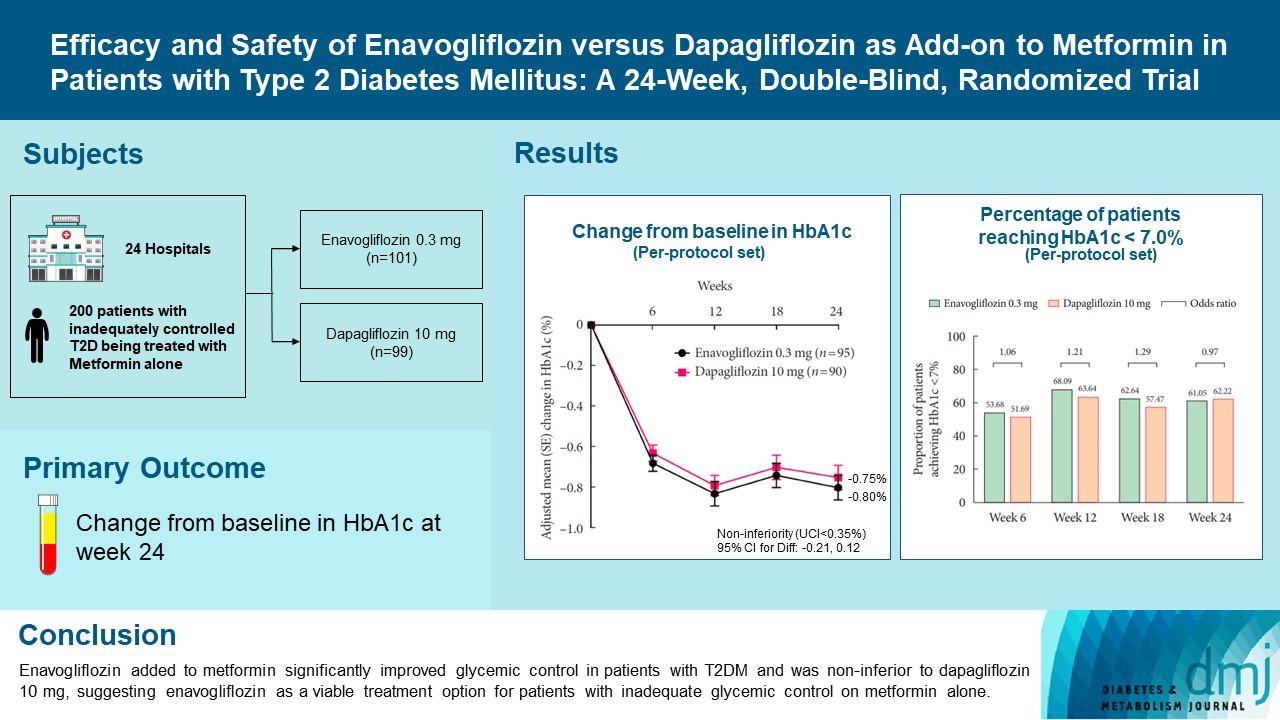
Enavogliflozin is a novel sodium-glucose cotransporter-2 inhibitor currently under clinical development. This study evaluated the efficacy and safety of enavogliflozin as an add-on to metformin in Korean patients with type 2 diabetes mellitus (T2DM) against dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, 200 patients were randomized to receive enavogliflozin 0.3 mg/day (n=101) or dapagliflozin 10 mg/day (n=99) in addition to ongoing metformin therapy for 24 weeks. The primary objective of the study was to prove the non-inferiority of enavogliflozin to dapagliflozin in glycosylated hemoglobin (HbA1c) change at week 24 (non-inferiority margin of 0.35%) (Clinical trial registration number: NCT04634500).
Results
Adjusted mean change of HbA1c at week 24 was –0.80% with enavogliflozin and –0.75% with dapagliflozin (difference, –0.04%; 95% confidence interval, –0.21% to 0.12%). Percentages of patients achieving HbA1c <7.0% were 61% and 62%, respectively. Adjusted mean change of fasting plasma glucose at week 24 was –32.53 and –29.14 mg/dL. An increase in urine glucose-creatinine ratio (60.48 vs. 44.94, P<0.0001) and decrease in homeostasis model assessment of insulin resistance (–1.85 vs. –1.31, P=0.0041) were significantly greater with enavogliflozin than dapagliflozin at week 24. Beneficial effects of enavogliflozin on body weight (–3.77 kg vs. –3.58 kg) and blood pressure (systolic/diastolic, –5.93/–5.41 mm Hg vs. –6.57/–4.26 mm Hg) were comparable with those of dapagliflozin, and both drugs were safe and well-tolerated.
Conclusion
Enavogliflozin added to metformin significantly improved glycemic control in patients with T2DM and was non-inferior to dapagliflozin 10 mg, suggesting enavogliflozin as a viable treatment option for patients with inadequate glycemic control on metformin alone. -
Citations
Citations to this article as recorded by- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Young Sang Lyu, Sangmo Hong, Si Eun Lee, Bo Young Cho, Cheol-Young Park
Cardiovascular Diabetology.2024;[Epub] CrossRef - A 52‐week efficacy and safety study of enavogliflozin versus dapagliflozin as an add‐on to metformin in patients with type 2 diabetes mellitus: ENHANCE‐M extension study
Tae Seo Sohn, Kyung‐Ah Han, Yonghyun Kim, Byung‐Wan Lee, Suk Chon, In‐Kyung Jeong, Eun‐Gyoung Hong, Jang Won Son, JaeJin Na, Jae Min Cho, Seong In Cho, Wan Huh, Kun‐Ho Yoon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - The effect of renal function on the pharmacokinetics and pharmacodynamics of enavogliflozin, a potent and selective sodium‐glucose cotransporter‐2 inhibitor, in type 2 diabetes
Sae Im Jeong, Mu Seong Ban, Jun‐Gi Hwang, Min‐Kyu Park, Soo Lim, Sejoong Kim, Soon Kil Kwon, Yoonjin Kim, Jae Min Cho, Jae Jin Na, Wan Huh, Jae‐Yong Chung
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: A systematic review and meta-analysis
Deep Dutta, B.G. Harish, Beatrice Anne, Lakshmi Nagendra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102816. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - Prospects of using sodium-glucose co-transporter-2 (SGLT-2) inhibitors in patients with metabolic-associated fatty liver disease (MAFLD)
Iryna Kostitska, Nadia Protas, Liliia Petrovska
Diabetes Obesity Metabolic Syndrome.2023; (5): 8. CrossRef - Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
Sang Youl Rhee
Diabetes & Metabolism Journal.2023; 47(6): 769. CrossRef
- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Reviews
- Cardiovascular Risk/Epidemiology
- Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus
- Takayoshi Sasako, Toshimasa Yamauchi, Kohjiro Ueki
- Diabetes Metab J. 2023;47(2):185-197. Published online January 12, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0325
- 5,110 View
- 358 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
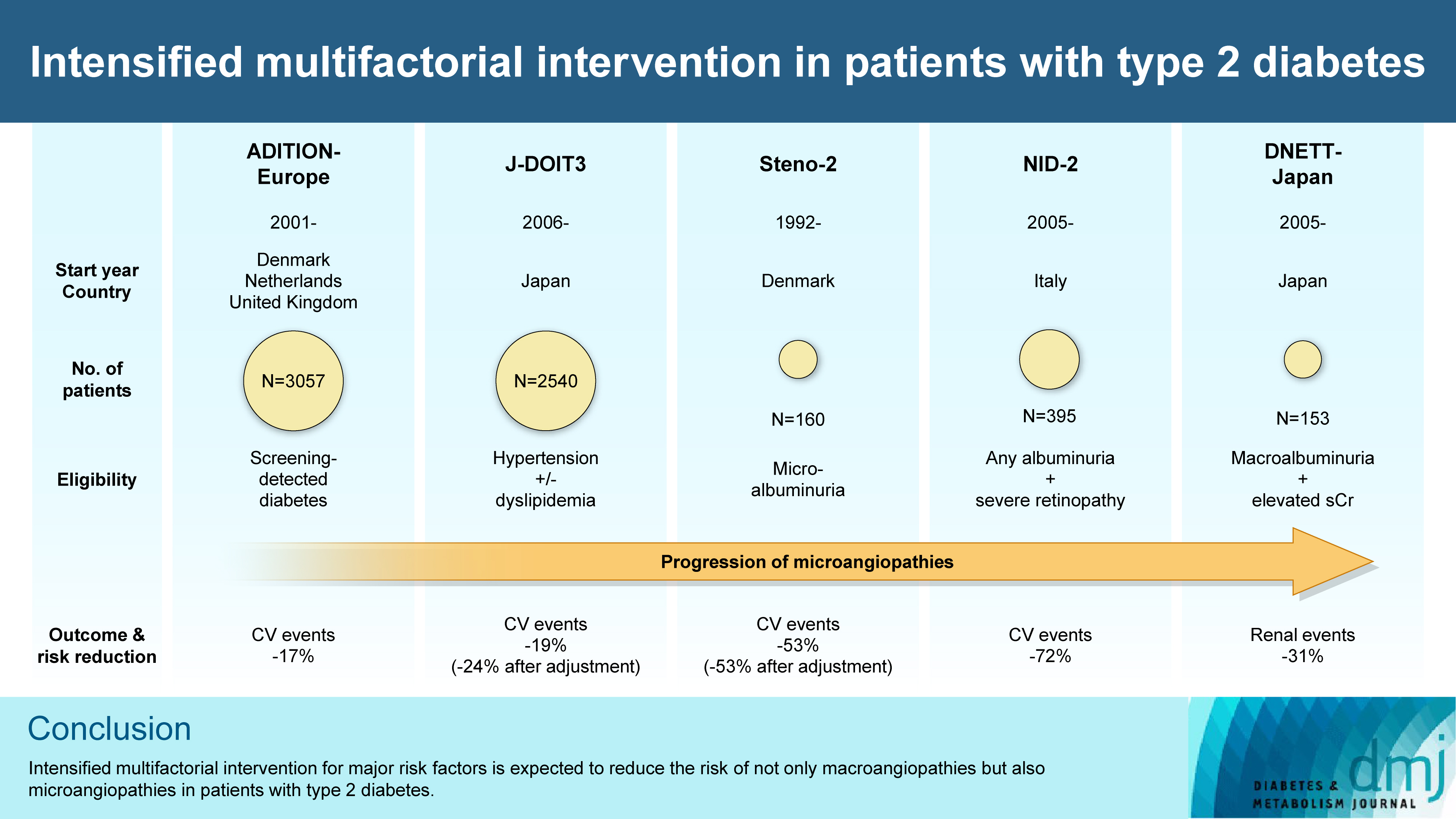
ePub - In the management of diabetes mellitus, one of the most important goals is to prevent its micro- and macrovascular complications, and to that end, multifactorial intervention is widely recommended. Intensified multifactorial intervention with pharmacotherapy for associated risk factors, alongside lifestyle modification, was first shown to be efficacious in patients with microalbuminuria (Steno-2 study), then in those with less advanced microvascular complications (the Anglo-Danish-Dutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care [ADDITION]-Europe and the Japan Diabetes Optimal Treatment study for 3 major risk factors of cardiovascular diseases [J-DOIT3]), and in those with advanced microvascular complications (the Nephropathy In Diabetes-Type 2 [NID-2] study and Diabetic Nephropathy Remission and Regression Team Trial in Japan [DNETT-Japan]). Thus far, multifactorial intervention led to a reduction in cardiovascular and renal events, albeit not necessarily significant. It should be noted that not only baseline characteristics but also the control status of the risk factors and event rates during intervention among the patients widely varied from one trial to the next. Further evidence is needed for the efficacy of multifactorial intervention in a longer duration and in younger or elderly patients. Moreover, now that new classes of antidiabetic drugs are available, it should be addressed whether strict and safe glycemic control, alongside control of other risk factors, could lead to further risk reductions in micro- and macrovascular complications, thereby decreasing all-cause mortality in patients with type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- Exploring mechanisms underlying diabetes comorbidities and strategies to prevent vascular complications
Takayoshi Sasako
Diabetology International.2024; 15(1): 34. CrossRef - Targeting ERS-mitophagy in hippocampal neurons to explore the improvement of memory by tea polyphenols in aged type 2 diabetic rats
Wenjuan Feng, Chenhui Lv, Le Cheng, Xin Song, Xuemin Li, Haoran Xie, Shuangzhi Chen, Xi Wang, Lushan Xue, Cheng Zhang, Jie Kou, Lili Wang, Haifeng Zhao
Free Radical Biology and Medicine.2024; 213: 293. CrossRef - Risk of Dementia Among Patients With Diabetes in a Multidisciplinary, Primary Care Management Program
Kailu Wang, Shi Zhao, Eric Kam-Pui Lee, Susan Zi-May Yau, Yushan Wu, Chi-Tim Hung, Eng-Kiong Yeoh
JAMA Network Open.2024; 7(2): e2355733. CrossRef - Causes of In-Hospital Death and Pharmaceutical Associations with Age of Death during a 10-Year Period (2011–2020) in Individuals with and without Diabetes at a Japanese Community General Hospital
Minae Hosoki, Taiki Hori, Yousuke Kaneko, Kensuke Mori, Saya Yasui, Seijiro Tsuji, Hiroki Yamagami, Saki Kawata, Tomoyo Hara, Shiho Masuda, Yukari Mitsui, Kiyoe Kurahashi, Takeshi Harada, Shingen Nakamura, Toshiki Otoda, Tomoyuki Yuasa, Akio Kuroda, Itsur
Journal of Clinical Medicine.2024; 13(5): 1283. CrossRef - External validation of a minimal-resource model to predict reduced estimated glomerular filtration rate in people with type 2 diabetes without diagnosis of chronic kidney disease in Mexico: a comparison between country-level and regional performance
Camilla Sammut-Powell, Rose Sisk, Ruben Silva-Tinoco, Gustavo de la Pena, Paloma Almeda-Valdes, Sonia Citlali Juarez Comboni, Susana Goncalves, Rory Cameron
Frontiers in Endocrinology.2024;[Epub] CrossRef - Gut Microbiota Targeted Approach by Natural Products in Diabetes Management: An Overview
Priyanka Sati, Praveen Dhyani, Eshita Sharma, Dharam Chand Attri, Arvind Jantwal, Rajni Devi, Daniela Calina, Javad Sharifi-Rad
Current Nutrition Reports.2024;[Epub] CrossRef - Cardiovascular Risk Reduction in Type 2 Diabetes: Further Insights into the Power of Weight Loss and Exercise
Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(3): 302. CrossRef - Sarcopenia: Loss of mighty armor against frailty and aging
Takayoshi Sasako, Kohjiro Ueki
Journal of Diabetes Investigation.2023; 14(10): 1145. CrossRef
- Exploring mechanisms underlying diabetes comorbidities and strategies to prevent vascular complications
- Guideline/Fact Sheet
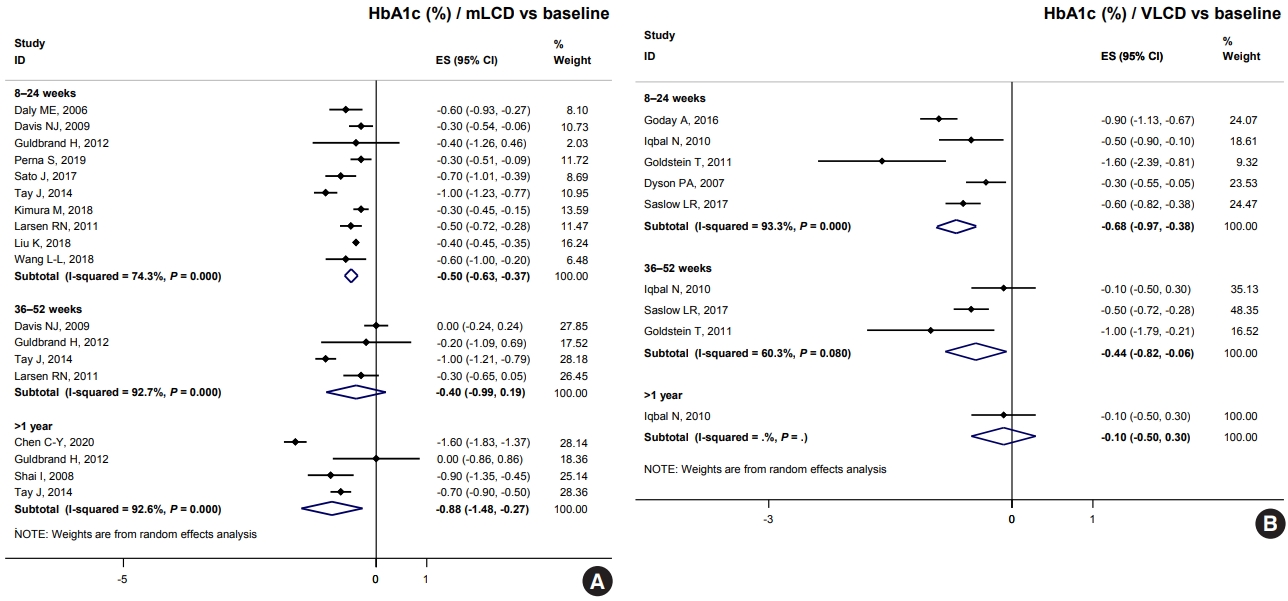
- Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension
- Jong Han Choi, Jee-Hyun Kang, Suk Chon
- Diabetes Metab J. 2022;46(3):377-390. Published online May 25, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0051
- 4,987 View
- 250 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The Joint Committee of the Korean Diabetes Association, the Korean Society for the Study of Obesity, and the Korean Society of Hypertension announced a consensus statement on carbohydrate-restricted diets and intermittent fasting, representing an emerging and popular dietary pattern. In this statement, we recommend moderately-low-carbohydrate or low-carbohydrate diets, not a very-low-carbohydrate diet, for patients with type 2 diabetes mellitus. These diets can be considered a dietary regimen to improve glycemic control and reduce body weight in adults with type 2 diabetes mellitus. This review provides the detailed results of a meta-analysis and systematic literature review on the potential harms and benefits of carbohydrate-restricted diets in patients with diabetes. We expect that this review will help experts and patients by fostering an in-depth understanding and appropriate application of carbohydrate-restricted diets in the comprehensive management of diabetes.
-
Citations
Citations to this article as recorded by- Efficacy of convenience meal-type foods designed for diabetes in the management of metabolic syndrome based on a 3-week trial
Do Gyeong Lee, In Gyeong Kang, Tae Seok Kim, Yun Ahn, Sang Yun Lee, Hye Jin Ahn, Yoo Kyoung Park
Nutrition.2024; 118: 112287. CrossRef - Long-Term Results of a Digital Diabetes Self-Management and Education Support Program Among Adults With Type 2 Diabetes: A Retrospective Cohort Study
Ashley Berthoumieux, Sarah Linke, Melinda Merry, Alison Megliola, Jessie Juusola, Jenna Napoleone
The Science of Diabetes Self-Management and Care.2024; 50(1): 19. CrossRef - Medical nutrition therapy for diabetes mellitus
Suk Chon
Journal of the Korean Medical Association.2023; 66(7): 421. CrossRef
- Efficacy of convenience meal-type foods designed for diabetes in the management of metabolic syndrome based on a 3-week trial
Original Articles
- Drug/Regimen
- Comparison of Efficacy of Glimepiride, Alogliptin, and Alogliptin-Pioglitazone as the Initial Periods of Therapy in Patients with Poorly Controlled Type 2 Diabetes Mellitus: An Open-Label, Multicenter, Randomized, Controlled Study
- Hae Jin Kim, In Kyung Jeong, Kyu Yeon Hur, Soo-Kyung Kim, Jung Hyun Noh, Sung Wan Chun, Eun Seok Kang, Eun-Jung Rhee, Sung Hee Choi
- Diabetes Metab J. 2022;46(5):689-700. Published online March 17, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0183
- 5,691 View
- 377 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The choice of an optimal oral hypoglycemic agent in the initial treatment periods for type 2 diabetes mellitus (T2DM) patients remains difficult and deliberate. We compared the efficacy and safety of glimepiride (GLIM), alogliptin (ALO), and alogliptin-pioglitazone (ALO-PIO) in poorly controlled T2DM patients with drug-naïve or metformin failure.
Methods
In this three-arm, multicenter, open-label, randomized, controlled trial, poorly controlled T2DM patients were randomized to receive GLIM (n=35), ALO (n=31), or ALO-PIO (n=33) therapy for 24 weeks. The primary endpoint was change in the mean glycosylated hemoglobin (HbA1c) levels at week 24 from baseline. Secondary endpoints were changes in HbA1c level at week 12 from baseline, fasting plasma glucose (FPG) levels, lipid profiles at weeks 12 and 24, and parameters of glycemic variability, assessed by continuous glucose monitoring for 24 weeks.
Results
At weeks 12 and 24, the ALO-PIO group showed significant reduction in HbA1c levels compared to the ALO group (–0.96%±0.17% vs. –0.37%±0.17% at week 12; –1.13%±0.19% vs. –0.18%±0.2% at week 24). The ALO-PIO therapy caused greater reduction in FPG levels and significant increase in high-density lipoprotein cholesterol levels at weeks 12 and 24 than the ALO therapy. Compared to low-dose GLIM therapy, ALO-PIO therapy showed greater improvement in glycemic variability. The adverse events were similar among the three arms.
Conclusion
ALO-PIO combination therapy during the early period exerts better glycemic control than ALO monotherapy and excellency in glycemic variability than low-dose sulfonylurea therapy in uncontrolled, drug-naïve or metformin failed T2DM patients. -
Citations
Citations to this article as recorded by- A Comprehensive Review on Weight Loss Associated with Anti-Diabetic Medications
Fatma Haddad, Ghadeer Dokmak, Maryam Bader, Rafik Karaman
Life.2023; 13(4): 1012. CrossRef - Role of Dipeptidyl Peptidase 4 Inhibitors in Antidiabetic Treatment
Ruili Yin, Yongsong Xu, Xin Wang, Longyan Yang, Dong Zhao
Molecules.2022; 27(10): 3055. CrossRef
- A Comprehensive Review on Weight Loss Associated with Anti-Diabetic Medications
- Metabolic Risk/Epidemiology
- Sex Differences in the Effects of CDKAL1 Variants on Glycemic Control in Diabetic Patients: Findings from the Korean Genome and Epidemiology Study
- Hye Ah Lee, Hyesook Park, Young Sun Hong
- Diabetes Metab J. 2022;46(6):879-889. Published online February 8, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0265
- 65,535 View
- 178 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Using long-term data from the Korean Genome and Epidemiology Study, we defined poor glycemic control and investigated possible risk factors, including variants related to type 2 diabetes mellitus (T2DM). In addition, we evaluated interaction effects among risk factors for poor glycemic control.
Methods
Among 436 subjects with newly diagnosed diabetes, poor glycemic control was defined based on glycosylated hemoglobin trajectory patterns by group-based trajectory modeling. For the variants related to T2DM, genetic risk scores (GRSs) were calculated and divided into quartiles. Risk factors for poor glycemic control were assessed using a logistic regression model.
Results
Of the subjects, 43% were in the poor-glycemic-control group. Body mass index (BMI) and triglyceride (TG) were associated with poor glycemic control. The risk for poor glycemic control increased by 11.0% per 1 kg/m2 increase in BMI and by 3.0% per 10 mg/dL increase in TG. The risk for GRS with poor glycemic control was sex-dependent (Pinteraction=0.07), and a relationship by GRS quartiles was found in females but not in males. Moreover, the interaction effect was found to be significant on both additive and multiplicative scales. The interaction effect was evident in the variants of cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like (CDKAL1).
Conclusion
Females with risk alleles of variants in CDKAL1 associated with T2DM had a higher risk for poor glycemic control than males. -
Citations
Citations to this article as recorded by- Hepatic Cdkal1 deletion regulates HDL catabolism and promotes reverse cholesterol transport
Dan Bi An, Soo-jin Ann, Seungmin Seok, Yura Kang, Sang-Hak Lee
Atherosclerosis.2023; 375: 21. CrossRef
- Hepatic Cdkal1 deletion regulates HDL catabolism and promotes reverse cholesterol transport
- Drug/Regimen
- Comparison of Prevailing Insulin Regimens at Different Time Periods in Hospitalized Patients: A Real-World Experience from a Tertiary Hospital
- Sun Joon Moon, Hun Jee Choe, Soo Heon Kwak, Hye Seung Jung, Kyong Soo Park, Young Min Cho
- Diabetes Metab J. 2022;46(3):439-450. Published online October 20, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0065

- 65,535 View
- 270 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Prevailing insulin regimens for glycemic control in hospitalized patients have changed over time. We aimed to determine whether the current basal-bolus insulin (BBI) regimen is superior to the previous insulin regimen, mainly comprising split-mixed insulin therapy.
Methods
This was a single tertiary center, retrospective observational study that included non-critically ill patients with type 2 diabetes mellitus who were treated with split-mixed insulin regimens from 2004 to 2007 (period 1) and with BBI from 2008 to 2018 (period 2). Patients from each period were analyzed after propensity score matching. The mean difference in glucose levels and the achievement of fasting and preprandial glycemic targets by day 6 of admission were assessed. The total daily insulin dose, incidence of hypoglycemia, and length of hospital stay were also evaluated.
Results
Among 244 patients from each period, both fasting glucose (estimated mean±standard error, 147.4±3.1 mg/dL vs. 129.4±3.2 mg/dL, P<0.001, day 6) and preprandial glucose (177.7±2.8 mg/dL vs. 152.8±2.8 mg/dL, P<0.001, day 6) were lower in period 2 than in period 1. By day 6 of hospital admission, 42.6% and 67.2% of patients achieved a preprandial glycemic target of <140 mg/dL in periods 1 and 2, respectively (relative risk, 2.00; 95% confidence interval, 1.54 to 2.59), without an increased incidence of hypoglycemia. Length of stay was shorter in period 2 (10.23±0.26 days vs. 8.70±0.26 days, P<0.001).
Conclusion
BBI improved glycemic control in a more efficacious manner than a split-mixed insulin regimen without increasing the risk of hypoglycemia in a hospital setting.
Short Communication
- Type 1 Diabetes
- Real-World Analysis of Therapeutic Outcome in Type 1 Diabetes Mellitus at a Tertiary Care Center
- Antonia Kietaibl, Michaela Riedl, Latife Bozkurt
- Diabetes Metab J. 2022;46(1):149-153. Published online July 6, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0267

- 4,379 View
- 144 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin replacement in type 1 diabetes mellitus (T1DM) needs intensified treatment, which can either be performed by multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). This retrospective analysis of a real-world scenario aimed to evaluate whether glycaemic and cardiovascular risk factors could be controlled with CSII outclass MDI as suggested by recent evidence. Data from patients with either insulin pump (n=68) or injection (n=224) therapy at an Austrian tertiary care centre were analysed between January 2016 and December 2017. There were no significant differences with regard to the latest glycosylated hemoglobin, cardiovascular risk factor control or diabetes-associated late complications. Hypoglycaemia was less frequent (P<0.001), sensor-augmented therapy was more common (P=0.003) and mean body mass index (BMI) was higher (P=0.002) with CSII treatment. This retrospective analysis of real-world data in T1DM did not demonstrate the superiority of insulin pump treatment with regard to glycaemic control or cardiovascular risk factor control.
Original Article
- Drug/Regimen
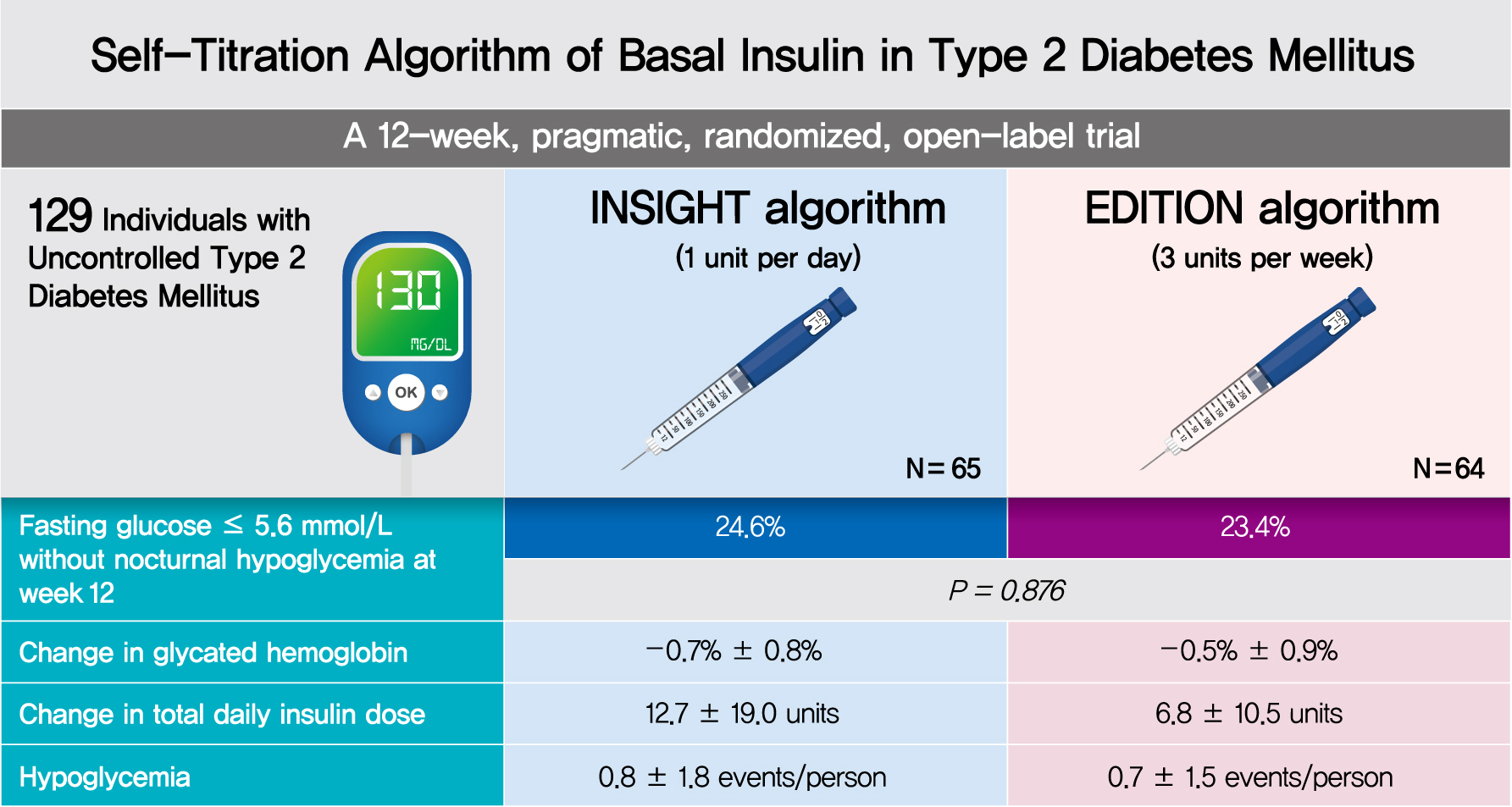
- Efficacy and Safety of Self-Titration Algorithms of Insulin Glargine 300 units/mL in Individuals with Uncontrolled Type 2 Diabetes Mellitus (The Korean TITRATION Study): A Randomized Controlled Trial
- Jae Hyun Bae, Chang Ho Ahn, Ye Seul Yang, Sun Joon Moon, Soo Heon Kwak, Hye Seung Jung, Kyong Soo Park, Young Min Cho
- Diabetes Metab J. 2022;46(1):71-80. Published online June 16, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0274
- 8,034 View
- 434 Download
- 1 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
To compare the efficacy and safety of two insulin self-titration algorithms, Implementing New Strategies with Insulin Glargine for Hyperglycemia Treatment (INSIGHT) and EDITION, for insulin glargine 300 units/mL (Gla-300) in Korean individuals with uncontrolled type 2 diabetes mellitus (T2DM).
Methods
In a 12-week, randomized, open-label trial, individuals with uncontrolled T2DM requiring basal insulin were randomized to either the INSIGHT (adjusted by 1 unit/day) or EDITION (adjusted by 3 units/week) algorithm to achieve a fasting self-monitoring of blood glucose (SMBG) in the range of 4.4 to 5.6 mmol/L. The primary outcome was the proportion of individuals achieving a fasting SMBG ≤5.6 mmol/L without noct urnal hypoglycemia at week 12.
Results
Of 129 individuals (age, 64.1±9.5 years; 66 [51.2%] women), 65 and 64 were randomized to the INSIGHT and EDITION algorithms, respectively. The primary outcome of achievement was comparable between the two groups (24.6% vs. 23.4%, P=0.876). Compared with the EDITION group, the INSIGHT group had a greater reduction in 7-point SMBG but a similar decrease in fasting plasma glucose and glycosylated hemoglobin. The increment of total daily insulin dose was significantly higher in the INSIGHT group than in the EDITION group (between-group difference: 5.8±2.7 units/day, P=0.033). However, body weight was significantly increased only in the EDITION group (0.6±2.4 kg, P=0.038). There was no difference in the occurrence of hypoglycemia between the two groups. Patient satisfaction was significantly increased in the INSIGHT group (P=0.014).
Conclusion
The self-titration of Gla-300 using the INSIGHT algorithm was effective and safe compared with that using the EDITION algorithm in Korean individuals with uncontrolled T2DM (ClinicalTrials.gov number: NCT03406663). -
Citations
Citations to this article as recorded by- Basal insulin titration algorithms in patients with type 2 diabetes: the simplest is the best (?)
V.I. Katerenchuk
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2023; 19(1): 72. CrossRef - Issues of insulin therapy for type 2 diabetes and ways to solve them
V.I. Katerenchuk, A.V. Katerenchuk
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2023; 19(3): 240. CrossRef - Time for Using Machine Learning for Dose Guidance in Titration of People With Type 2 Diabetes? A Systematic Review of Basal Insulin Dose Guidance
Camilla Heisel Nyholm Thomsen, Stine Hangaard, Thomas Kronborg, Peter Vestergaard, Ole Hejlesen, Morten Hasselstrøm Jensen
Journal of Diabetes Science and Technology.2022; : 193229682211459. CrossRef
- Basal insulin titration algorithms in patients with type 2 diabetes: the simplest is the best (?)
Reviews
- Type 1 Diabetes
- Non-Insulin Antidiabetes Treatment in Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- Xiaoling Cai, Chu Lin, Wenjia Yang, Lin Nie, Linong Ji
- Diabetes Metab J. 2021;45(3):312-325. Published online March 15, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0171

- 6,251 View
- 273 Download
- 5 Web of Science
- 5 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- In order to evaluate the efficacy and side effects of the non-insulin antidiabetes medications as an adjunct treatment in type 1 diabetes mellitus (T1DM), we conducted systematic searches in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials for randomized controlled trials published between the date of inception and March 2020 to produce a systematic review and meta-analysis. Overall, 57 studies were included. Compared with placebo, antidiabetes agents in adjunct to insulin treatment resulted in significant reduction in glycosylated hemoglobin (weighted mean difference [WMD], –0.30%; 95% confidence interval [CI], –0.34 to –0.25%; P<0.01) and body weight (WMD, –2.15 kg; 95% CI, –2.77 to –1.53 kg; P<0.01), and required a significantly lower dosage of insulin (WMD, –5.17 unit/day; 95% CI, –6.77 to –3.57 unit/day; P<0.01). Compared with placebo, antidiabetes agents in adjunct to insulin treatment increased the risk of hypoglycemia (relative risk [RR], 1.04; 95% CI, 1.01 to 1.08; P=0.02) and gastrointestinal side effects (RR, 1.99; 95% CI, 1.61 to 2.46; P<0.01) in patients with T1DM. Compared with placebo, the use of non-insulin antidiabetes agents in addition to insulin could lead to glycemic improvement, weight control and lower insulin dosage, while they might be associated with increased risks of hypoglycemia and gastrointestinal side effects in patients with T1DM.
-
Citations
Citations to this article as recorded by- Dioscin: Therapeutic potential for diabetes and complications
Haoyang Gao, Ze Wang, Danlin Zhu, Linlin Zhao, Weihua Xiao
Biomedicine & Pharmacotherapy.2024; 170: 116051. CrossRef - The Impact of Body Mass Index, Residual Beta Cell Function and Estimated Glucose Disposal Rate on the Development of Double Diabetes and Microvascular Complications in Patients With Type 1 Diabetes Mellitus
Rameez Raja Bhagadurshah, Subbiah Eagappan, Raghavan Kasthuri Santharam, Sridhar Subbiah
Cureus.2023;[Epub] CrossRef - Prescribing patterns of adjunctive therapy for the treatment of type 1 diabetes mellitus among Australian endocrinologists
Patrice Forner, Jennifer Snaith, Jerry R. Greenfield
Internal Medicine Journal.2023;[Epub] CrossRef - Type 1 diabetes glycemic management: Insulin therapy, glucose monitoring, and automation
Bruce A. Perkins, Jennifer L. Sherr, Chantal Mathieu
Science.2021; 373(6554): 522. CrossRef - Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
Sun Joon Moon, Inha Jung, Cheol-Young Park
Diabetes & Metabolism Journal.2021; 45(6): 813. CrossRef
- Dioscin: Therapeutic potential for diabetes and complications
- Technology/Device
- Present and Future of Digital Health in Diabetes and Metabolic Disease
- Sang Youl Rhee, Chiweon Kim, Dong Wook Shin, Steven R. Steinhubl
- Diabetes Metab J. 2020;44(6):819-827. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0088

- 9,076 View
- 262 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The use of information and communication technology (ICT) in medical and healthcare services goes beyond everyday life. Expectations of a new medical environment, not previously experienced by ICT, exist in the near future. In particular, chronic metabolic diseases such as diabetes and obesity, have a high prevalence and high social and economic burden. In addition, the continuous evaluation and monitoring of daily life is important for effective treatment and management. Therefore, the wide use of ICTbased digital health systems is required for the treatment and management of these diseases. In this article, we compiled a variety of digital health technologies introduced to date in the field of diabetes and metabolic diseases.
-
Citations
Citations to this article as recorded by- Digital Health in Diabetes and Cardiovascular Disease
Dorothy Avoke, Abdallah Elshafeey, Robert Weinstein, Chang H. Kim, Seth S. Martin
Endocrine Research.2024; : 1. CrossRef - Weight Management Health Note, a Mobile Health Platform for Obesity Management Developed by the Korean Society for the Study of Obesity
Yujung Lee, Hyunji Sang, Sunyoung Kim, Doo Ah Choi, Sang Youl Rhee
Journal of Obesity & Metabolic Syndrome.2024; 33(1): 1. CrossRef - Effectiveness of a Social Networking Site Based Automatic Mobile Message Providing System on Glycemic Control in Patients with Type 2 Diabetes Mellitus
Kyuho Kim, Jae-Seung Yun, Joonyub Lee, Yeoree Yang, Minhan Lee, Yu-Bae Ahn, Jae Hyoung Cho, Seung-Hyun Ko
Endocrinology and Metabolism.2024; 39(2): 344. CrossRef - A data-driven approach to manage type 2 diabetes mellitus through digital health: The Klivo Intervention Program protocol (KIPDM)
Camila Maciel de Oliveira, Luiza Borcony Bolognese, Mercedes Balcells, Davi Casale Aragon, Roberto Luis Zagury, Clemente Nobrega, Chunyu Liu, Dured Dardari
PLOS ONE.2023; 18(2): e0281844. CrossRef - Public Health Framework for Smart Cities within the Comprehensive Approach to Sustainability in Europe: Case Study of Diabetes
Luís Velez Lapão, Jorge César Correia, Marija Jevtic
Sustainability.2023; 15(5): 4269. CrossRef - Lessons for Vietnam on the Use of Digital Technologies to Support Patient-Centered Care in Low- and Middle-Income Countries in the Asia-Pacific Region: Scoping Review
Leona Kosowicz, Kham Tran, Toan Tran Khanh, Thu Ha Dang, Van An Pham, Hue Ta Thi Kim, Hoang Thi Bach Duong, Tran Dong Nguyen, Anh Tuyet Phuong, Trong Hieu Le, Van Anh Ta, Nilmini Wickramasinghe, Penelope Schofield, John Zelcer, Tuan Pham Le, Tuan Anh Nguy
Journal of Medical Internet Research.2023; 25: e43224. CrossRef - Digital health, cardiometabolic disease and ethnicity: an analysis of United Kingdom government policies from 2010 to 2022
Zareen Thorlu-Bangura, Lydia Poole, Harpreet Sood, Nushrat Khan, Fiona Stevenson, Kamlesh Khunti, Paramjit Gill, Madiha Sajid, Wasim Hanif, Neeraj Bhala, Shivali Modha, Kiran Patel, Ann Blandford, Amitava Banerjee, Mel Ramasawmy
Journal of Public Health Policy.2023; 44(2): 179. CrossRef - Digital Behavior Change Interventions to Reduce Sedentary Behavior and Promote Physical Activity in Adults with Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Xiaoyan Zhang, Xue Qiao, Ke Peng, Shan Gao, Yufang Hao
International Journal of Behavioral Medicine.2023;[Epub] CrossRef - Exploring the underlying mechanisms of obesity and diabetes and the potential of Traditional Chinese Medicine: an overview of the literature
Yan-kun Chen, Ting-ting Liu, Farah Khameis Farag Teia, Meng-zhou Xie
Frontiers in Endocrinology.2023;[Epub] CrossRef - Stakeholders’ Perceptions Regarding Digital Therapeutics Reimbursement in South Korea: Qualitative Study
Boram Sim, Jin Han Ju, Byungsoo Kim, Jin Yong Lee
JMIR mHealth and uHealth.2023; 11: e47407. CrossRef - Research Trends in Motivation and Weight Loss: A Bibliometric-Based Review
Uroš Železnik, Peter Kokol, Jasmina Starc, Danica Železnik, Jernej Završnik, Helena Blažun Vošner
Healthcare.2023; 11(23): 3086. CrossRef - Analysis of the management and therapeutic performance of diabetes mellitus employing special target
Hong-Yan Sun, Xiao-Yan Lin
World Journal of Diabetes.2023; 14(12): 1721. CrossRef - Psychoeducational Interventions in Children and Adolescents with Type-1 Diabetes: A Systematic Review
Bárbara Luque, Joaquín Villaécija, Rosario Castillo-Mayén, Esther Cuadrado, Sebastián Rubio, Carmen Tabernero
Clínica y Salud.2022; 33(1): 35. CrossRef - Prevalence of Hyperuricemia Among Chinese Adults: Findings From Two Nationally Representative Cross-Sectional Surveys in 2015–16 and 2018–19
Mei Zhang, Xiaoxia Zhu, Jing Wu, Zhengjing Huang, Zhenping Zhao, Xiao Zhang, Yu Xue, Weiguo Wan, Chun Li, Wenrong Zhang, Linhong Wang, Maigeng Zhou, Hejian Zou, Limin Wang
Frontiers in Immunology.2022;[Epub] CrossRef - Blood Pressure Monitoring as a Digital Health Tool for Improving Diabetes Clinical Outcomes: Retrospective Real-world Study
Yifat Fundoiano-Hershcovitz, Dror Bacher, Marilyn D Ritholz, David L Horwitz, Omar Manejwala, Pavel Goldstein
Journal of Medical Internet Research.2022; 24(2): e32923. CrossRef - The effectiveness of a structured group education programme for people with established type 2 diabetes in a multi-ethnic population in primary care: A cluster randomised trial
Helen Dallosso, Panna Mandalia, Laura J. Gray, Yogini V. Chudasama, Sopna Choudhury, Shahrad Taheri, Naina Patel, Kamlesh Khunti, Melanie J. Davies
Nutrition, Metabolism and Cardiovascular Diseases.2022; 32(6): 1549. CrossRef - Efficacy of Personalized Diabetes Self-care Using an Electronic Medical Record–Integrated Mobile App in Patients With Type 2 Diabetes: 6-Month Randomized Controlled Trial
Eun Young Lee, Seon-Ah Cha, Jae-Seung Yun, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, Min Kyung Hyun, Seung-Hyun Ko
Journal of Medical Internet Research.2022; 24(7): e37430. CrossRef - Qatar Diabetes Mobile Application Trial (QDMAT): an open-label randomised controlled trial to examine the impact of using a mobile application to improve diabetes care in type 2 diabetes mellitus—a study protocol
Noor Suleiman, Meis Alkasem, Zaina Al Amer, Obada Salameh, Noora Al-Thani, Mohammad Khair Hamad, Khaled Baagar, Ibrahem Abdalhakam, Manal Othman, Ragae Dughmosh, Dabia Al-Mohanadi, Ali Al Sanousi, Mohammed Bashir, Odette Chagoury, Shahrad Taheri, Abdul-Ba
Trials.2022;[Epub] CrossRef - Digital environment: An evolutionary component in environmental health
Afiqah Syamimi Masrani, Nik Rosmawati Nik Husain
Journal of Public Health Research.2022; 11(2): 227990362211031. CrossRef - Innovations in Cardio-oncology Resulting from the COVID-19 Pandemic
Lavanya Kondapalli, Garima Arora, Riem Hawi, Efstathia Andrikopoulou, Courtney Estes, Nirav Patel, Carrie G. Lenneman
Current Treatment Options in Oncology.2022; 23(9): 1288. CrossRef - Effects of peer support and mobile application-based walking programme on physical activity and physical function in rural older adults: a cluster randomized controlled trial
Xue Cai, Shanhu Qiu, Dan Luo, Ruxue Li, Chengyu Liu, Yanhui Lu, Cuirong Xu, Mingzi Li
European Geriatric Medicine.2022; 13(5): 1187. CrossRef
- Digital Health in Diabetes and Cardiovascular Disease
Original Article
- Metabolic Risk/Epidemiology
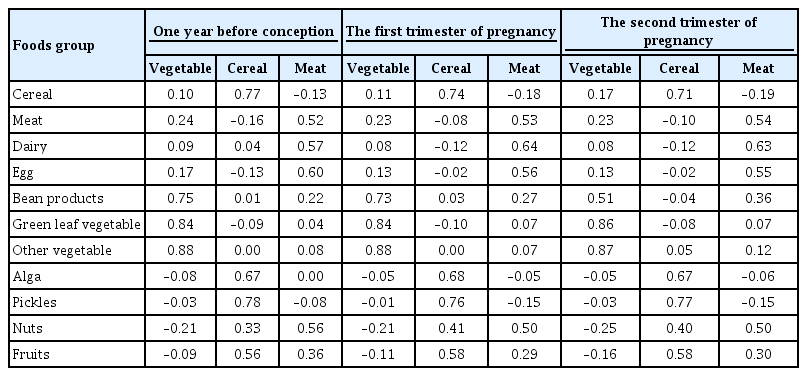
- A Vegetable Dietary Pattern Is Associated with Lowered Risk of Gestational Diabetes Mellitus in Chinese Women
- Qiong Chen, Weiwei Wu, Hailan Yang, Ping Zhang, Yongliang Feng, Keke Wang, Ying Wang, Suping Wang, Yawei Zhang
- Diabetes Metab J. 2020;44(6):887-896. Published online September 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0138
- 6,589 View
- 135 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Identification of modifiable dietary factors, which are involved in the development of gestational diabetes mellitus (GDM), could inform strategies to prevent GDM.
Methods
We examined the dietary patterns in a Chinese population and evaluated their relationship with GDM risk using a case-control study including 1,464 cases and 8,092 control subjects. Propensity score matching was used to reduce the imbalance of covariates between cases and controls. Dietary patterns were identified using factor analysis while their associations with GDM risk were evaluated using logistic regression models.
Results
A “vegetable” dietary pattern was characterized as the consumption of green leafy vegetables (Chinese little greens and bean seedling), other vegetables (cabbages, carrots, tomatoes, eggplants, potatoes, mushrooms, peppers, bamboo shoots, agarics, and garlic), and bean products (soybean milk, tofu, kidney beans, and cowpea). For every quartile increase in the vegetables factor score during 1 year prior to conception, the first trimester, and the second trimester of pregnancy, the GDM risk lowered by 6% (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.89 to 0.99), 7% (OR, 0.94; 95% CI, 0.88 to 0.99), and 9% (OR, 0.91; 95% CI, 0.86 to 0.96).
Conclusion
In conclusion, our study suggests that the vegetable dietary pattern is associated with lower GDM risk; however, the interpretation of the result should with caution due to the limitations in our study, and additional studies are necessary to explore the underlying mechanism of this relationship. -
Citations
Citations to this article as recorded by- Maternal dietary components in the development of gestational diabetes mellitus: a systematic review of observational studies to timely promotion of health
Victoria Lambert, Sonia Edith Muñoz, Carla Gil, María Dolores Román
Nutrition Journal.2023;[Epub] CrossRef - Fruit, vegetable, and fruit juice consumption and risk of gestational diabetes mellitus: a systematic review and meta-analysis
Yan-Ping Liao, Qing-Xiang Zheng, Xiu-Min Jiang, Xiao-Qian Chen, Xiao-Xia Gao, Yu-Qing Pan
Nutrition Journal.2023;[Epub] CrossRef - The effects of plant-based dietary patterns on the risk of developing gestational diabetes mellitus: A systematic review and meta-analysis
Yu Zhu, QingXiang Zheng, Ling Huang, XiuMin Jiang, XiaoXia Gao, JiaNing Li, RuLin Liu, Kent Lai
PLOS ONE.2023; 18(10): e0291732. CrossRef - Molecular pathways and nutrigenomic review of insulin resistance development in gestational diabetes mellitus
Patricia Guevara-Ramírez, Elius Paz-Cruz, Santiago Cadena-Ullauri, Viviana A. Ruiz-Pozo, Rafael Tamayo-Trujillo, Maria L. Felix, Daniel Simancas-Racines, Ana Karina Zambrano
Frontiers in Nutrition.2023;[Epub] CrossRef - Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—a systematic review and meta-analysis of 257,876 pregnancies
Swetha Sampathkumar, Durga Parkhi, Yonas Ghebremichael-Weldeselassie, Nithya Sukumar, Ponnusamy Saravanan
Nutrition & Diabetes.2023;[Epub] CrossRef - Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies
Elaine Luiza Santos Soares de Mendonça, Marilene Brandão Tenório Fragoso, Jerusa Maria de Oliveira, Jadriane Almeida Xavier, Marília Oliveira Fonseca Goulart, Alane Cabral Menezes de Oliveira
Antioxidants.2022; 11(1): 129. CrossRef - Ferulic acid targets ACSL1 to ameliorate lipid metabolic disorders in db/db mice
Jie Gao, Xue Gu, Manqian Zhang, Xingwang Zu, Fukui Shen, Xiaotao Hou, Erwei Hao, Gang Bai
Journal of Functional Foods.2022; 91: 105009. CrossRef - Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance
Jianping Wang, Zuoliang Xie, Peipei Chen, Yuhuan Wang, Baoqing Li, Fen Dai
Open Life Sciences.2022; 17(1): 202. CrossRef - Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women
Weijia Wu, Nu Tang, Jingjing Zeng, Jin Jing, Li Cai
Nutrients.2022; 14(8): 1623. CrossRef - Dietary Acid Load Is Positively Associated With Risk of Gestational Diabetes Mellitus in a Prospective Cohort of Chinese Pregnant Women
Rui Zhao, Leilei Zhou, Gang Lei, Shanshan Wang, Yan Li, Xuefeng Yang, Guoping Xiong, Liping Hao
Frontiers in Nutrition.2022;[Epub] CrossRef
- Maternal dietary components in the development of gestational diabetes mellitus: a systematic review of observational studies to timely promotion of health

 KDA
KDA
 First
First Prev
Prev





