- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(2); 2023 > Article
-
ReviewCardiovascular Risk/Epidemiology Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus
-
Takayoshi Sasako1
 , Toshimasa Yamauchi1, Kohjiro Ueki2,3
, Toshimasa Yamauchi1, Kohjiro Ueki2,3
-
Diabetes & Metabolism Journal 2023;47(2):185-197.
DOI: https://doi.org/10.4093/dmj.2022.0325
Published online: January 12, 2023
1Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
2Diabetes Research Center, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan
3Department of Molecular Diabetology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
-
Corresponding author: Kohjiro Ueki
 Diabetes Research Center, Research Institute, National Center for Global Health and Medicine, 1-21-2 Toyama Shinjuku-Ku, Tokyo 162-8655, Japan E-mail: uekik@ri.ncgm.go.jp
Diabetes Research Center, Research Institute, National Center for Global Health and Medicine, 1-21-2 Toyama Shinjuku-Ku, Tokyo 162-8655, Japan E-mail: uekik@ri.ncgm.go.jp
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- In the management of diabetes mellitus, one of the most important goals is to prevent its micro- and macrovascular complications, and to that end, multifactorial intervention is widely recommended. Intensified multifactorial intervention with pharmacotherapy for associated risk factors, alongside lifestyle modification, was first shown to be efficacious in patients with microalbuminuria (Steno-2 study), then in those with less advanced microvascular complications (the Anglo-Danish-Dutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care [ADDITION]-Europe and the Japan Diabetes Optimal Treatment study for 3 major risk factors of cardiovascular diseases [J-DOIT3]), and in those with advanced microvascular complications (the Nephropathy In Diabetes-Type 2 [NID-2] study and Diabetic Nephropathy Remission and Regression Team Trial in Japan [DNETT-Japan]). Thus far, multifactorial intervention led to a reduction in cardiovascular and renal events, albeit not necessarily significant. It should be noted that not only baseline characteristics but also the control status of the risk factors and event rates during intervention among the patients widely varied from one trial to the next. Further evidence is needed for the efficacy of multifactorial intervention in a longer duration and in younger or elderly patients. Moreover, now that new classes of antidiabetic drugs are available, it should be addressed whether strict and safe glycemic control, alongside control of other risk factors, could lead to further risk reductions in micro- and macrovascular complications, thereby decreasing all-cause mortality in patients with type 2 diabetes mellitus.
- Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia due to insufficient insulin action, and it leads to abnormalities in almost the entire metabolic system [1,2]. One of the most important goals of diabetes management is to prevent the development or progression of diabetic complications [3], but it is well known that glycemic control alone is not sufficient, and indeed, multifactorial intervention is widely recommended in order to prevent not only macrovascular but also microvascular complications [3-7].
- Results began to be reported from the late 1970s onwards from clinical trials examining the efficacy of multifactorial intervention, which focused mainly on primary prevention of coronary heart disease through pharmacological or non-pharmacological approaches [8], but not in the field of diabetes. Notably, a meta-analysis of these trials found no significant effect on mortality, suggesting that the benefits of multifactorial intervention could be evident only in patients at high risk, such as those with hypertension [8]. To the best of our knowledge, the Risk Factor Intervention Study is the first trial to examine the effect of multifactorial intervention on diabetes, blood pressure, lipids, and smoking, in patients with treated hypertension and an additional risk factor including diabetes. It was reported in 1994 that multifactorial intervention was associated with no reduction in mortality but with a reduction in the risk of stroke [9].
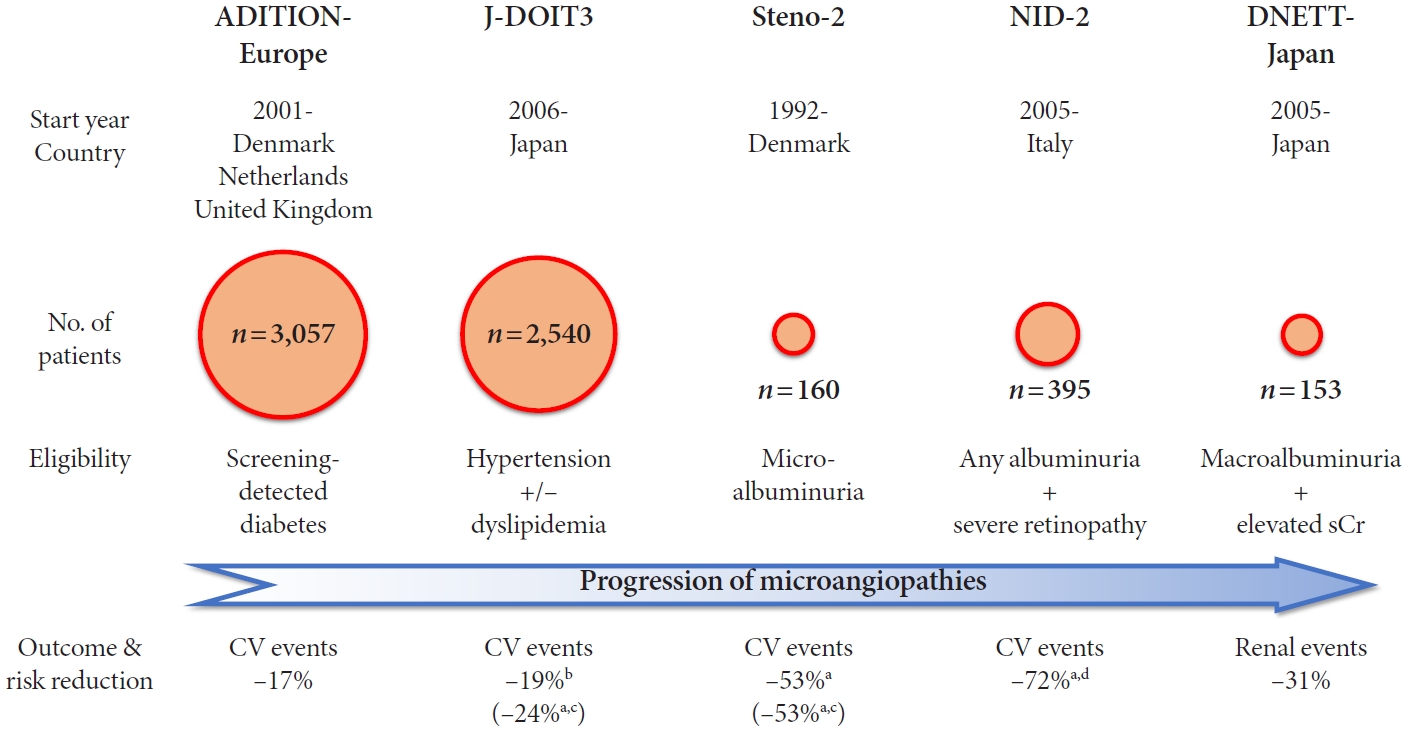
- Two years before this report, the Steno-2 study was launched, the first trial to examine the effects of multifactorial intervention in patients with type 2 diabetes mellitus [10], with microalbuminuria defined as the key inclusion criterion [11,12]. This was a landmark study in the field of diabetes, despite involving only 160 participants.
- Two other trials are of major interest, in that they were conducted to elucidate the role of multifactorial intervention in patients with type 2 diabetes mellitus [13,14]: the Anglo-DanishDutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care (ADDITION)-Europe [15], and the Japan Diabetes Optimal Treatment study for 3 major risk factors of cardiovascular diseases (J-DOIT3) [16], both of which recruited more than 2,000 patients with less advanced microangiopathies.
- Moreover, results of two other trials were recently reported: the Nephropathy In Diabetes-Type 2 (NID-2) study [17], and Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan) [18], both recruiting a smaller number of patients with advanced microangiopathies.
- Fig. 1 gives an overview of these five trials examining the efficacy of multifactorial intervention, polypharmacotherapy for multiple risk factors in combination with lifestyle modification, on ‘hard outcomes,’ i.e., cardiovascular and renal events, in patients with type 2 diabetes mellitus.
INTRODUCTION
- In the Steno-2 study, patients with microalbuminuria were registered and randomly assigned to either conventional therapy or intensive therapy aimed at stricter multifactorial treatment targets (e.g., glycosylated hemoglobin [HbA1c] <6.5%). The primary outcome for microangiopathies during 4 years of intervention was defined as progression to macroalbuminuria, and that for macroangiopathies during 8 years of intervention was defined as a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, amputation, and revascularization (Fig. 1) [11,12].
- The baseline patient characteristics in the Steno-2 study are summarized in Table 1 [11,12]. In the microangiopathy study, during a mean follow-up of 3.8 years, intensive therapy was shown to be associated with a significant risk reduction in progression to macroalbuminuria (odds ratio, 0.27; 95% confidence interval [CI], 0.10 to 0.75) [11].
- In the macroangiopathy study, during a mean follow-up of 7.8 years, and major risk factors were better with statistical significance in the intensive therapy group during the intervention period, such as HbA1c (7.9% in the intensive therapy group vs. 9.0% in the conventional therapy group, the same as below) (Table 1) [12].
- Event rates in the Steno-2 study are summarized in Table 1. Intensive therapy was shown to be associated with a significant risk reduction in the primary outcome (hazard ratio [HR], 0.47; 95% CI, 0.24 to 0.73), even after adjustment for baseline risk factors (HR, 0.47; 95% CI, 0.22 to 0.74) (Fig. 1) [12], while it was later reported that this had not affected morality at this point [19].
- Intensive therapy was associated with a significant risk reduction in nephropathy (HR, 0.39; 95% CI, 0.17 to 0.87), retinopathy (HR, 0.42; 95% CI, 0.21 to 0.86), and autonomic neuropathy (HR, 0.37; 95% CI, 0.18 to 0.79). End-stage renal failure was reported only in the conventional therapy group (3.8%), while estimated glomerular filtration rate (eGFR) was lower by around 30 mL/min/1.73 m2 in both groups compared to baseline (Table 1). Blindness was less frequent in the intensive therapy group (1.3% vs. 8.8%). Among major adverse events, severe hypoglycemia was shown to be also less frequent in the intensive therapy group (6.3% vs. 15.0%) [12].
- In subsequent sub-analyses, intensive therapy was associated with a significant risk reduction in stroke [20]. The largest effect on the cardiovascular risk calculated on the basis of the UK Prospective Diabetes Study (UKPDS) [21] was accounted for by changes in lipids among those in major risk factors [20]. Besides, intensive therapy was shown to be more cost-effective than conventional therapy [22].
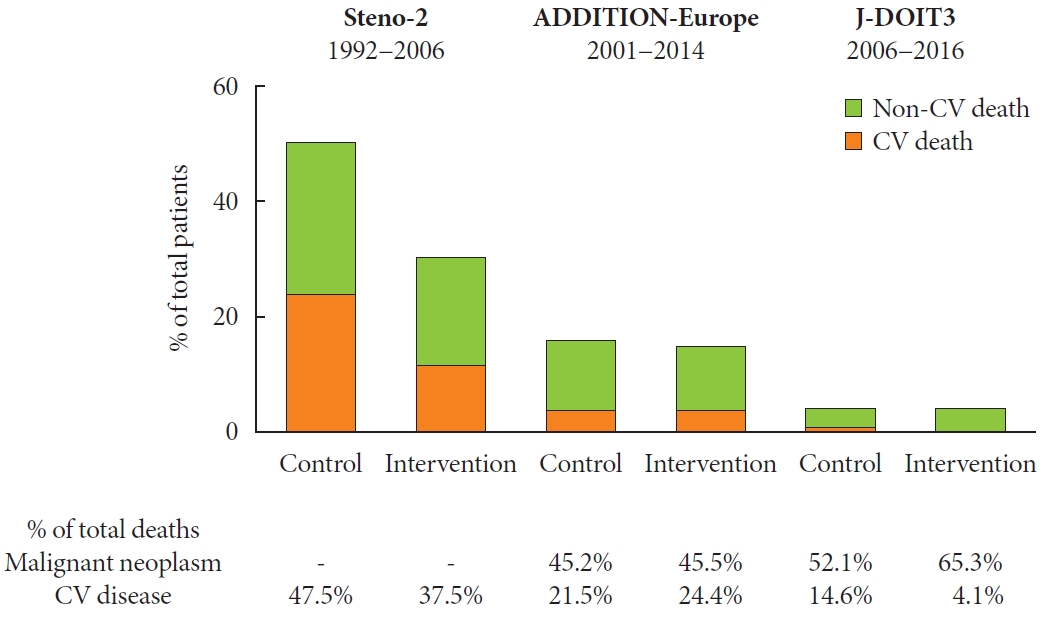
- Moreover, the patients were followed up even after completion of intervention with death from any cause as the primary outcome. During a mean follow-up of 13.3 years, intensive therapy was shown to be associated with a significant risk reduction in mortality (HR, 0.54; 95% CI, 0.32 to 0.89). It was associated with a significant risk reduction in other events, and nearly half of deaths were shown to be due to cardiovascular disease in the conventional therapy group, which was significantly reduced in the intensive therapy group (Fig. 2) [19].
- After a median follow-up of 21.2 years, intensive therapy was shown to be still associated with a significant risk reduction in mortality (HR, 0.55; 95% CI, 0.36 to 0.83), yielding an elongation of a median survival of at least 7.9 years, as well as a significant reduction in other events [23].
- In sub-analyses, intensive therapy was associated with a significant reduction in stroke [24] and heart failure [25], as well as a significant reduction in the combined renal events including mortality, with the annual eGFR decline shown to be less rapid with intensive therapy (3.1 mL/min/1.73 m2/year vs. 4.0 mL/min/1.73 m2/year) [26]. Again, intensified multifactorial intervention was shown to be associated with no increase in total costs [27].
STENO-2 STUDY
- ADDITION-Europe examined the effects of multifactorial intervention on macrovascular complications and mortality in patients with screening-detected type 2 diabetes mellitus (Fig. 1) [15,28-31], who were randomly assigned to either routine care or intensive treatment following the Steno-2 study [12], with the primary outcome defined as a composite of cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke, revascularization, and amputation.
- The baseline patient characteristics are summarized in Table 1 [15,32,33]. Major risk factors were slightly improved without statistical significance in the intensive treatment group during the intervention period, such as HbA1c (6.6% vs. 6.7%) (Table 1) [15], showing the feasibility of risk factor control in patients with screening-detected diabetes [34].
- Event rates during the mean follow-up of 5.3 years are summarized in Table 1 [15]. Intensive treatment was shown to be associated with a non-significant risk reduction in the primary outcome (HR, 0.83; 95% CI, 0.65 to 1.05) (Fig. 1) and in morality (HR, 0.91; 95% CI, 0.69 to 1.21), with no increase reported in adverse events including hypoglycemia [15].
- In subsequent sub-analyses, intensive treatment was associated with a non-significant reduction not only in the first event but also in any events of the primary outcome [35]. It was associated with no reduction in nephropathy, retinopathy, or neuropathy, with the eGFR value shown to be rather elevated compared to baseline in both groups compared to the baseline [32].
- Intensive treatment was associated with an improvement in aortic stiffness [36], but not in peripheral neuropathy [37], cardiac autonomic neuropathy [38], peripheral arterial disease [37], or cognitive function [39]. It was not shown to be cost-effective [40], with only slight changes shown in psychological outcomes [41-43], although glycemic control was associated with diabetes-specific quality of life [44]. It was also reported that screening for diabetes per se was not associated with a reduction in mortality [45].
- Moreover, the patients were followed up even after completion of intervention. During a mean follow-up of 9.61 years, intensive treatment was associated with a risk reduction, albeit non-significant, in the primary outcome (HR, 0.87; 95% CI, 0.73 to 1.04) and in morality (HR, 0.90; 95% CI, 0.76 to 1.07). Nearly half of deaths were due to malignant neoplasms in both groups (Fig. 2) [33]. A subsequent sub-analysis showed that intensive treatment did not affect psychological outcomes [46].
ADDITION-EUROPE
- The J-DOIT3 examined the effects of multifactorial intervention on macrovascular complications and mortality in patients with type 2 diabetes mellitus (Fig. 1) [16,47], as one of the programs comprising the strategic research, Japan Diabetes Outcome Intervention Trial, launched by the Ministry of Health, Labor and Welfare (MHLW) [48]. The J-DOIT3 was aimed at a 30% reduction in diabetic complications, whereas the JDOIT1 was focused on conversion of impaired glucose tolerance to diabetes [49], and the J-DOIT2 was on dropout from diabetes treatment [50], respectively.
- In the J-DOIT3 study, patients with type 2 diabetes mellitus with hypertension and/or dyslipidemia were registered and randomly assigned to either conventional therapy or intensive therapy for stricter multifactorial treatment targets (e.g., HbA1c <6.2%), with the primary outcome defined as a composite of myocardial infarction, stroke, revascularization, and all-cause mortality [16,47].
- The baseline patient characteristics are summarized in Table 1 [16,51]. Major risk factors were significantly improved in the intensive therapy group during the intervention period, such as HbA1c (6.79% vs. 7.20%) (Table 1) [16,51].
- Event rates during the median follow-up of 8.5 years are summarized in Table 1 [16]. Intensive therapy was shown to be associated with a non-significant risk reduction in the primary outcome (HR, 0.81; 95% CI, 0.63 to 1.04), which was statistically significant after adjustment for baseline risk factors (HR, 0.76; 95% CI, 0.59 to 0.99) (Fig. 1). It was associated with a significant risk reduction in cerebrovascular events in a post hoc breakdown (HR, 0.42; 95% CI, 0.24 to 0.74) [16], and also with a significant risk reduction in the secondary outcomes, i.e., nephropathy events (HR, 0.68; 95% CI, 0.56 to 0.82) [16,51], and retinopathy events (HR, 0.86; 95% CI, 0.74 to 1.00) [16], with end-stage renal failure reported only in the conventional therapy group (0.4%) and no blindness reported in either group [16].
- Although the incidence of any hypoglycemia was higher in the intensive therapy group (41% vs. 22%), the incidence of severe hypoglycemia was quite low in both groups (0.6% and 0.3%) during the intervention period. More than half of deaths were due to malignant neoplasms in both groups (Fig. 2) [16].
- In subsequent sub-analyses, glycemic control was associated with onset of nephropathy, and blood pressure control was associated with progression of eGFR decline, with the annual eGFR decline less than 2 mL/min/1.73 m2/year in both groups during the first 3 years [51]. Intensive therapy did not lead to an elevate risk in fractures [52].
J-DOIT3
- In the NID-2 study, patients with type 2 diabetes mellitus with micro- or macroalbuminuria and severe retinopathy were registered and randomly assigned to either standard of care or multifactorial intensive therapy involving the same treatment targets (e.g., HbA1c <7%), with a history of myocardial infarction or stroke as one of the exclusion criteria. The primary outcome was defined as a composite of cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke, amputation, and revascularization [17,53].
- The baseline patient characteristics are summarized in Table 1. Major risk factors were significantly improved in the multifactorial intensive therapy group during the intervention period, e.g., HbA1c (6.9% vs. 7.4%) (Table 1) [17].
- Mortality rates during intervention for a median of 3.40 and 3.84 years in the standard of care group and the multifactorial intensive therapy group, respectively, are shown in Table 1. Multifactorial intensive therapy was shown to be associated with a significant risk reduction in the primary outcome during the intervention period (HR, 0.28; 95% CI, 0.13 to 0.63) (Fig. 1), although it was just an additional secondary outcome because the follow-up for the primary analysis was continued even after intervention was terminated until a sufficient number of events were reported [17].
- eGFR was shown to be mildly declined in both groups (Table 1). Among adverse events, hyperkalemia and eGFR decline by 30% were shown to be more frequent, albeit non-significantly, in the multifactorial intensive therapy group (10% vs. 5% and 16% vs. 9%, respectively), possibly due to more frequent concurrent use of renin-angiotensin system blockers, while end-stage renal failure was reported in both groups (3.4% vs. 2.7%) [17].
- After almost an additional decade of follow-up after the end of intervention, during the median follow-up of 13.0 years in total, multifactorial intensive therapy was shown to be associated with a significant risk reduction in the primary outcome (HR, 0.49; 95% CI, 0.35 to 0.69), and in mortality (HR, 0.58; 95% CI, 0.34 to 0.98), as well as in myocardial infarction and stroke [17].
- Some other analyses were performed before the end of follow-up, and albuminuria and eGFR at baseline were shown to be associated with the risk of cardiovascular events [54], and HbA1c was also shown to be positively associated with the risk after adjustment for aspirin or statin use and other risk factors [55,56].
NID-2
- In the DNETT-Japan, patients with type 2 diabetes mellitus with macroalbuminuria and elevated serum creatinine were registered and randomly assigned to either conventional treatment or multifactorial intensive treatment for stricter multifactorial treatment targets (e.g., HbA1c <6.2%), with a history of recent myocardial infarction or stroke as one of the exclusion criteria. The primary outcome was defined as a composite of end-stage renal failure, doubling of serum creatinine or death [18,57].
- The baseline patient characteristics are summarized in Table 1. Major risk factors showed no significant between-group differences during the intervention period, e.g., HbA1c (6.86% vs. 6.94%) (Table 1) [18].
- Mortality rates during the median follow-up of 3.11 years are shown in Table 1. Multifactorial intensive treatment was shown to be associated with a non-significant risk reduction in the primary outcome (HR, 0.69; 95% CI, 0.43 to 1.11) (Fig. 1). It was not associated with a reduction in cardiovascular events (HR, 0.56; 95% CI, 0.19 to 1.64), end-stage renal failure (HR, 0.77; 95% CI, 0.31 to 1.86), or mortality (HR, 0.54; 95% CI, 0.10 to 2.97) [18].
- eGFR was shown to have declined by around 10 mL/min/1.73 m2 in both groups (Table 1). Severe adverse events were not increased in the multifactorial intensive treatment group [18]. It was also shown that, low-density lipoprotein cholesterol (LDL-C) during the intervention was associated with the risk of the primary outcome after adjustment with baseline characteristics [18].
DNETT-JAPAN
- Intensive multifactorial therapy was shown to be feasible with less frequent hypoglycemia [58], with the improved risk factor profile shown to be sustained even after the end of intervention [59]. It suppressed progression of the intima-media thickness of the carotid artery in patients with short-duration type 2 diabetes mellitus [60-62], and those with type 2 diabetes mellitus and at least two insufficient risk factors [63]. It also delayed onset of microalbuminuria in patients with short-duration type 2 diabetes mellitus [64]. Moreover, intensive multifactorial therapy by structured care including administration of a renin-angiotensin system blocker showed no effect on renal outcomes in patients with elevated serum creatinine, but addressing multiple targets led to a risk reduction in the renal outcome [65]. Intensive multifactorial therapy for stricter targets in the elderly with a mean age over 70 years did not affect fatal or nonfatal events without inter-group difference in risk factors [66].
OTHER TRIALS
- Overall, intensified multifactorial intervention is shown to be associated with risk reductions in micro- and macrovascular complications, which are shown to be significant in some trials and non-significant in others.
- As summarized in Table 1, the background patient characteristics are shown to vary among the trials, with HbA1c and eGFR, for instance, shown to be higher in the Steno-2 study than in the other four trials (Table 1). Thus, the study results might not apply to patients with microalbuminuria, but with better glycemic control and without glomerular hyperfiltration, in a ‘straight forward’ manner, although they could possibly be ‘extrapolated’ to these patients as well.
- Although risk factor targets were not fully achieved in the Steno-2 study, as was pointed out previously [67], those are shown to have been generally well controlled in the trials launched after 2000, with the mean HbA1c shown to be less than 7% in the intervention groups in all trials, as well as in the control groups in the ADDTION-Europe and the DNETT-Japan. The mean systolic blood pressure was less than 130 mm Hg even in the conventional therapy group in the J-DOIT3, and the mean LDL-C was less than 100 mg/dL even in the routine care group in the ADDITION-Europe (Table 1). This could be due to recent advances in diabetes care resulting in low event rates during intervention [68]. The incidence of the primary outcome in the ADDITION-Europe was lower than expected on the basis of the preceding UKPDS, which was initiated in the late 1970s [69]. The incidences of myocardial infarction and stroke in the J-DOIT3 were almost half or less of those observed in the Japan Diabetes Complications Study [70], a preceding clinical trial initiated in the mid 1990s [16]. The mortality rate in the J-DOIT3 is also deemed to be much lower than those in recent surveys in the general population [71].
- Still there remain several issues to be addressed, and first, the long-term effects of multifactorial intervention on vascular complications and mortality should be further evaluated [68,72], given that the potential benefits of intensive multifactorial therapy may become more evident during a long-term follow-up, as in the UKPDS, the Steno-2 study, and the 10-year follow-up of the Veterans Affairs Diabetes Trial (VADT) [19,73,74], while any observed benefits could be seen to fade, as in the 15-year follow-up of the VADT [75].
- Given the mean patient age of around 60 years in these trials, it is also important to examine the effects of intensive multifactorial intervention in younger patients [71], as well as in elderly patients, as they were not included in many of the clinical trials conducted to date, with some exceptions [66], possibly due to low event rates in younger patients and susceptibility to adverse events in elderly patients. Retrospective real-world data may complement prospective clinical trials and provide us with insight into the effects of intensified multifactorial intervention in these patients, although it should also be noted that adverse events are carefully monitored in clinical trials but are not necessarily analyzed in retrospective cohort studies [76,77]. Especially in elderly patients with diabetes, the treatment would be better to be individualized rather than uniformly intensified on the basis of their prognosis and preferences [78].
- Moreover, intensive multifactorial intervention using the so-called new classes of antidiabetic drugs, e.g., dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and sodium-glucose cotransporter 2 (SGLT2) inhibitors, may contribute to better and safer glycemic control [68]. In the J-DOIT3, in which intervention was continued until as recently as March, 2016, DPP-4 inhibitors were administered in over half of its participants at the end of intervention, but the use of GLP-1 receptor agonists or SGLT2 inhibitors remained still less common [16], while the latter two drugs are expected to show beneficial effects on cardiovascular and renal events [79,80].
- Furthermore, while the mortality rate was lower in the J-DOIT3 study than in the others [16,71], it is deemed important to assess the effects of intensive therapy on all-cause mortality. In the Steno-2 study, almost half of deaths were due to cardiovascular disease in the conventional therapy group, but in the ADDITION-Europe, nearly half of deaths were due to malignant neoplasm and less than a quarter of them were due to cardiovascular disease in both groups. Again, in the JDOIT3 study, over half of deaths in both groups were due to malignant neoplasm, whereas 15% of deaths in the conventional therapy group and only 4% of deaths in the intensive therapy group were due to cardiovascular disease, although direct comparisons are difficult between these three trials, due to differences in baseline characteristics, e.g., age, race, sex, and duration of diabetes, and course of treatment (Table 1, Fig. 2). Cardiovascular deaths have also been relatively decreased in the general population with diabetes, accounting for over onethird, around a quarter, and just 15% of all deaths in the United States [81], the United Kingdom [82], and Japan [83], respectively. Indeed, deaths from malignant neoplasm are almost equivalent to those from cardiovascular deaths in the United Kingdom [82], and over twice as frequent as those from cardiovascular deaths in Japan [83]. Thus, it is increasingly important to focus on how best to achieve further risk reductions not only in micro- and macrovascular complications but also in diseases whose risks are increased by diabetes, such as malignant neoplasm, frailty and dementia, thereby decreasing all-cause mortality in patients with type 2 diabetes mellitus.
- Lastly, it is essential to clarify the contribution of each risk factor targeted in multifactorial intervention to events and to what extent it should be controlled. Classically, it was assumed based on epidemiological findings that the contribution of risk factors to events, especially macroangiopathies, vary from one risk factor to the next, e.g., lipids largely affect coronary heart disease, blood pressure affects stroke, and hyperglycemia affects amputation [10,84].
- Although the Steno-2 study suggested the importance of lipid control over glycemic control [20], recently, growing attention has been focused on the impact of glycemic control on cardiovascular events. A meta-analysis of classical five trials showed that intensive glycemic control was associated with a significant reduction in nonfatal myocardial infarction and coronary heart disease, but not in stroke and morality [85]. More patients receiving intensive glycemic control were shown to experience hypoglycemia in these trials [85], thus raising their risk of cardiovascular events and mortality [86]. An analysis of these five and more than 10 additional trials showed that differential glycemic exposure (% HbA1c×years) was negatively correlated with reductions in cardiovascular risk [87], suggesting that it could be effective to maintain good glycemic control for a longer duration to prevent cardiovascular events. Similarly, an analysis of more recent 15 trials treating patients with type 2 diabetes mellitus with DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors, all of which are considered to be associated with a low risk of hypoglycemia, showed that differential glycemic control was significantly correlated with a risk reduction in cardiovascular events and nonfatal stroke, irrespective of the class of drugs, but not in nonfatal myocardial infarction [88]. The 8-year data of the NID-2 study stated above also seem to suggest the importance of glycemic control in the prevention of cardiovascular events [55,56].
- Further analyses are expected to clarify how intensive glycemic control without involving an increase in hypoglycemia, which might have been difficult to achieve and analyze, in combination with multifactorial intervention for other risk factors, could contribute to reductions in risk of cardiovascular events and mortality. The treatment target for HbA1c as well as those for other risk factors should be also explored, as they could differ depending on the event of interest.
PERSPECTIVE
- Intensified multifactorial intervention for major risk factors, e.g., HbA1c, blood pressure, and lipids, is expected to reduce the risk of not only macroangiopathies but also microangiopathies in patients with type 2 diabetes mellitus. Multifactorial intervention needs to be examined for its effects over a longer duration and in younger or elderly patients, and new classes of antidiabetic drugs are expected to augment the treatment benefits. Given the recent relative decrease in cardiovascular deaths, it remains to be addressed whether intensified multifactorial intervention could reduce the risk of all-cause mortality over the long-term. Moreover, multifactorial intervention should be further optimized by clarifying how the risk of individual events may be affected by control of HbA1c and other individual risk factors.
CONCLUSIONS
-
CONFLICTS OF INTEREST
Takayoshi Sasako reports personal fees from AstraZeneca, Daiichi Sankyo, Eli Lilly Japan, Kissei, Kowa, Mitsubishi Tanabe, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk, Ono, Sanofi, Sumitomo Pharma, Takeda, and Teijin Pharma. Toshimasa Yamauchi reports personal fees from Abbott Japan, Astellas, AstraZeneca, Bayer Yakuhin, Covidien Japan, Daiichi Sankyo, DOJINDO LABORATORIES, Eli Lilly Japan, FUJIFILM Toyama Chemical, Kissei, Kowa, Kyorin Pharma, Kyowa Kirin, Mitsubishi Tanabe, MSD, Musashino Co. Ltd., Nippon Becton Dickinson, Nippon Boehringer Ingelheim, Novartis, Novo Nordisk, Ono, Roche DC Japan, Sanofi, Sanwa Kagaku Kenkyusho, Sumitomo Pharma, Taisho Pharma, Takeda, Teijin Pharma, Terumo, and Viatris Japan; grants and endowments from Aero Switch Therapeutics Inc., Astellas, AstraZeneca, Daiichi Sankyo, EA Pharma, Kowa, Kyowa Kirin, Minophagen Pharmaceutical, Mitsubishi Corporation Life Sciences, Mitsubishi Tanabe, MSD, Nippon Boehringer Ingelheim, Nipro, Novartis, Novo Nordisk, Ono, Sanofi, Sanwa Kagaku Kenkyusho, SHIONOGI, Sumitomo Pharma, Takeda, Taisho Pharma, TOSOH, and Asahi Mutual Life Insurance. Kohjiro Ueki reports personal fees from AstraZeneca, Bayer Yakuhin, Daiichi Sankyo, Eli Lilly Japan, Kowa, Mitsubishi Tanabe, Nippon Boehringer Ingelheim, Novo Nordisk, Ono, Sumitomo Pharma, and Taisho Pharmaceutical; grants and endowments from Nippon Boehringer Ingelheim, Kyowa Kirin, Mitsubishi Tanabe, Novo Nordisk, Ono, Sanofi, Sumitomo Pharma, and Takeda; consulting fees from Abbott Japan, AstraZeneca, Bayer Yakuhin, Kyowa Kirin, Mitsubishi Tanabe, Sumitomo Pharma, and Terumo.
-
FUNDING
This study was supported by the MHLW (Comprehensive Research on Life-Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus, 22FA1014).
NOTES
-
Acknowledgements
- We thank Masami Kunii and Kaori Tanabe for secretarial assistance.


|
ADDITION-Europe [15,32,33] |
J-DOIT3 [16,51] |
Steno-2 [11,12] |
NID-2 [17] |
DNETT-Japan [18] |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Intervention | Control Intervention | Control Intervention | Control Intervention | Control Intervention | |||||||
| Outcome & intervention | |||||||||||
| Primary outcome | 3-point MACE, revascularization, amputation | MI, stroke, revascularization, all-cause mortality | 3-point MACE, revascularization, amputation | 3-point MACE, revascularization, amputation | Doubling of sCr, ESRD, all-cause mortality | ||||||
| Glycemic/BP/lipid control | Yes | Yes | Yes | Yes | Yes | ||||||
| Smoking cessation | Yes | Yes | Yes | Yes | Yes | ||||||
| Lifestyle modification | Yes | Yes | Yes | Yes | Yes | ||||||
| Aspirin for primary prevention | Yes | No | Yes (after 1999) | Yes | No | ||||||
| Others | - | Smoking cessation aid (if necessary) | Vitamin-mineral supplement | - | Vitamin supplement | ||||||
| Baseline patient characteristics | |||||||||||
| Mean age, yr | 60.2 | 60.3 | 59.1 | 58.9 | 55.2 | 54.9 | 68.2 | 66.1 | 57.6 | 56.8 | |
| Female sex, % | 42.7 | 41.5 | 37.8 | 38.2 | 30.0 | 21.3 | 55.9 | 50.2 | 45.0 | 41.1 | |
| Mean duration of diabetes, yr | - a | - a | 8.47 | 8.58 | Median 6.0 | Median 5.5 | Median 9 | Median 9 | 15.1 | 15.9 | |
| History of CVD, %b | 8.0 | 9.7 | 11.2 | 11.5 | 27.5 | 27.5 | - | - | - | - | |
| Mean HbA1c, % | 7.0 | 7.0 | 7.98 | 8.01 | 8.8 | 8.4 | 7.3 | 7.5 | 7.1 | 7.1 | |
| Mean systolic BP, mm Hg | 149.8 | 148.5 | 134.1 | 133.5 | 149 | 146 | 134.7 | 133.8 | 139.5 | 138.5 | |
| Mean diastolic BP, mm Hg | 86.5 | 86.1 | 80.0 | 79.3 | 86 | 85 | 78.3 | 80.8 | 76.6 | 76.9 | |
| Mean LDL-C, mg/dL | 135.3 | 131.5 | 125.6 | 125.5 | 131.5 | 127.6 | 73 | 69 | 118.9 | 118 | |
| Mean eGFR, mL/min/1.73 m2 | Median 77.8 | Median 78.0 | 82.2 | 82.3 | 118 | 116 | 62.7 | 65.4 | 39.5 | 40.1 | |
| Median ACR, mg/g | 8 | 8 | 10.8 | 11.1 | 69 | 78 | 57.3 | 120.5 | 1,725 | 1,450 | |
| Control status of risk factors during the intervention period | |||||||||||
| Median duration, yr | Mean 5.3 | 8.5 | Mean 7.8 | 3.40 | 3.84 | 3.11 | |||||
| Mean HbA1c, % | 6.7 | 6.6 | 7.20 | 6.79 | 9.0 | 7.9 | 7.4 | 6.9 | 6.94 | 6.86 | |
| Mean systolic BP, mm Hg | 138.1 | 134.8 | 128.7 | 123.4 | 146 | 132 | 135.1 | 127.3 | 132.4 | 132.2 | |
| Mean diastolic BP, mm Hg | 80.7 | 79.5 | 74.4 | 71.5 | 78 | 73 | 78.8 | 78.1 | 72.0 | 72.2 | |
| Mean LDL-C, mg/dL | 88.9 | 81.2 | 103.7 | 85.5 | 119 | 81 | 122.3 | 100.5 | 104.0 | 98.6 | |
| Mean eGFR, mL/min/1.73 m2 | 84.2 | 82.3 | - | - | 86 | 86 | 60.7 | 60.4 | 29.2 | 31.1 | |
| Event rates during the intervention period | |||||||||||
| Primary outcome, % | 8.5 | 7.2 | 10.5 | 8.6 | 43.8 | 23.8 | 26.6c | 11.6c | - | - | |
| Myocardial infarction, % | 2.3 | 1.7 | 0.9d | 0.4d | 10.0e | 5.0e | - | - | - | - | |
| Stroke, % | 1.4 | 1.3 | 2.9d | 1.2d | 13.8e | 3.8e | - | - | - | - | |
| Death, % | 6.7 | 6.2 | 3.8 | 3.9 | 18.8 | 15.0 | 10.1 | 3.9 | 5.0 | 2.7 | |
| ESRD, % | - | - | 0.4 | 0.0 | 3.8 | 0.0 | 2.7 | 3.4 | - | - | |
Data in the control group or in the multifactorial intervention group are shown.
ADDITION-Europe, the Anglo-Danish-Dutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care; J-DOIT3, the Japan Diabetes Optimal Treatment study for 3 major risk factors of cardiovascular diseases; NID-2, the Nephropathy In Diabetes-Type 2; DNETT-Japan, the Diabetic Nephropathy Remission and Regression Team Trial in Japan; MACE, major adverse cardiovascular events; MI, myocardial infarction; sCr, serum creatinine; ESRD, end-stage renal disease; BP, blood pressure; CVD, cardiovascular disease; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; ACR, urinary albumin-to-creatinine ratio.
a Screening-detected diabetes was the key inclusion criterion,
b Sum of the number of participants with a history of myocardial infarction and that of those with a history of stroke in the ADDITION-Europe in this table; electrocardiographic evidence of ischemia in the Steno-2 study was excluded in this table; patients with a history of myocardial infarction or stroke were excluded in the NID-2 study; patients with a history of recent myocardial infarction or stroke were excluded in the DNETT-Japan,
c Primary outcome during the intervention period was an additional secondary outcome,
d Breakdown of first events,
e Breakdown of first events with fatal events excluded.
- 1. World Health Organization. Report of a WHO consultation: definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus Geneva: World Health Organization, Department of Noncommunicable Disease Surveillance; 1999.
- 2. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010;1:212-28.ArticlePubMedPMC
- 3. Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020;11:165-223.ArticlePubMedPMCPDF
- 4. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461-98.ArticlePubMedPDF
- 5. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J 2021;45:461-81.ArticlePubMedPMCPDF
- 6. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S144-74.
- 7. Chan JC, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021;396:2019-82.ArticlePubMed
- 8. Ebrahim S, Smith GD. Systematic review of randomised controlled trials of multiple risk factor interventions for preventing coronary heart disease. BMJ 1997;314:1666-74.ArticlePubMedPMC
- 9. Agewall S, Wikstrand J, Samuelsson O, Persson B, Andersson OK, Fagerberg B. The efficacy of multiple risk factor intervention in treated hypertensive men during long-term follow up. Risk Factor Intervention Study Group. J Intern Med 1994;236:651-9.ArticlePubMed
- 10. Kempler P. Learning from large cardiovascular clinical trials: classical cardiovascular risk factors. Diabetes Res Clin Pract 2005;68 Suppl 1:S43-7.Article
- 11. Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999;353:617-22.ArticlePubMed
- 12. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383-93.ArticlePubMed
- 13. Tanner M. In screen-detected type 2 diabetes, intensive therapy did not differ from usual care for CV events at 10 years. Ann Intern Med 2020;172:JC41.ArticlePubMed
- 14. Yagyu H, Shimano H. Treatment of diabetes mellitus has borne much fruit in the prevention of cardiovascular disease. J Diabetes Investig 2022;13:1472-88.ArticlePubMedPMCPDF
- 15. Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 2011;378:156-67.ArticlePubMedPMC
- 16. Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (JDOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:951-64.PubMed
- 17. Sasso FC, Pafundi PC, Simeon V, De Nicola L, Chiodini P, Galiero R, et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: a randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc Diabetol 2021;20:145.ArticlePubMedPMCPDF
- 18. Shikata K, Haneda M, Ninomiya T, Koya D, Suzuki Y, Suzuki D, et al. Randomized trial of an intensified, multifactorial intervention in patients with advanced-stage diabetic kidney disease: Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan). J Diabetes Investig 2021;12:207-16.ArticlePubMedPMCPDF
- 19. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580-91.ArticlePubMed
- 20. Gaede P, Pedersen O. Intensive integrated therapy of type 2 diabetes: implications for long-term prognosis. Diabetes 2004;53 Suppl 3:S39-47.PubMed
- 21. Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671-9.ArticlePubMed
- 22. Gaede P, Valentine WJ, Palmer AJ, Tucker DM, Lammert M, Parving HH, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008;31:1510-5.PubMedPMC
- 23. Gaede P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016;59:2298-307.ArticlePubMedPMCPDF
- 24. Gaede P, Oellgaard J, Kruuse C, Rossing P, Parving HH, Pedersen O. Beneficial impact of intensified multifactorial intervention on risk of stroke: outcome of 21 years of follow-up in the randomised Steno-2 Study. Diabetologia 2019;62:1575-80.ArticlePubMedPMCPDF
- 25. Oellgaard J, Gaede P, Rossing P, Rorth R, Kober L, Parving HH, et al. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study. Diabetologia 2018;61:1724-33.ArticlePubMedPMCPDF
- 26. Oellgaard J, Gaede P, Rossing P, Persson F, Parving HH, Pedersen O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int 2017;91:982-8.ArticlePubMed
- 27. Gaede J, Oellgaard J, Ibsen R, Gaede P, Nortoft E, Parving HH, et al. A cost analysis of intensified vs conventional multifactorial therapy in individuals with type 2 diabetes: a post hoc analysis of the Steno-2 study. Diabetologia 2019;62:147-55.ArticlePubMedPMCPDF
- 28. Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G, et al. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with type 2 diabetes detected by screening. Int J Obes Relat Metab Disord 2000;24 Suppl 3:S6-11.ArticlePubMedPDF
- 29. Janssen PG, Gorter KJ, Stolk RP, Rutten GE. Randomised controlled trial of intensive multifactorial treatment for cardiovascular risk in patients with screen-detected type 2 diabetes: 1-year data from the ADDITION Netherlands study. Br J Gen Pract 2009;59:43-8.ArticlePubMedPMC
- 30. Echouffo-Tcheugui JB, Simmons RK, Williams KM, Barling RS, Prevost AT, Kinmonth AL, et al. The ADDITION-Cambridge trial protocol: a cluster: randomised controlled trial of screening for type 2 diabetes and intensive treatment for screen-detected patients. BMC Public Health 2009;9:136.ArticlePubMedPMCPDF
- 31. Webb DR, Khunti K, Srinivasan B, Gray LJ, Taub N, Campbell S, et al. Rationale and design of the ADDITION-Leicester study, a systematic screening programme and randomised controlled trial of multi-factorial cardiovascular risk intervention in people with type 2 diabetes mellitus detected by screening. Trials 2010;11:16.ArticlePubMedPMCPDF
- 32. Sandbæk A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GE, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care 2014;37:2015-23.PubMed
- 33. Griffin SJ, Rutten GE, Khunti K, Witte DR, Lauritzen T, Sharp SJ, et al. Long-term effects of intensive multifactorial therapy in individuals with screen-detected type 2 diabetes in primary care: 10-year follow-up of the ADDITION-Europe cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:925-37.ArticlePubMed
- 34. Preiss D, Sattar N. The case for diabetes screening: ADDITION-Europe. Lancet 2011;378:106-8.ArticlePubMed
- 35. Simmons RK, Sharp SJ, Sandbaek A, Borch-Johnsen K, Davies MJ, Khunti K, et al. Does early intensive multifactorial treatment reduce total cardiovascular burden in individuals with screen-detected diabetes?: findings from the ADDITION-Europe cluster-randomized trial. Diabet Med 2012;29:e409-16.ArticlePubMedPMCPDF
- 36. Johansen NB, Charles M, Vistisen D, Rasmussen SS, Wiinberg N, Borch-Johnsen K, et al. Effect of intensive multifactorial treatment compared with routine care on aortic stiffness and central blood pressure among individuals with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care 2012;35:2207-14.PubMedPMC
- 37. Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screendetected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care 2011;34:2244-9.PubMedPMC
- 38. Charles M, Fleischer J, Witte DR, Ejskjaer N, Borch-Johnsen K, Lauritzen T, et al. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia 2013;56:101-8.ArticlePubMedPDF
- 39. Koekkoek PS, Ruis C, van den Donk M, Biessels GJ, Gorter KJ, Kappelle LJ, et al. Intensive multifactorial treatment and cognitive functioning in screen-detected type 2 diabetes: the ADDITION-Netherlands study: a cluster-randomized trial. J Neurol Sci 2012;314:71-7.ArticlePubMed
- 40. Tao L, Wilson EC, Wareham NJ, Sandbaek A, Rutten GE, Lauritzen T, et al. Cost-effectiveness of intensive multifactorial treatment compared with routine care for individuals with screendetected type 2 diabetes: analysis of the ADDITION-UK cluster-randomized controlled trial. Diabet Med 2015;32:907-19.ArticlePubMedPMCPDF
- 41. Thoolen BJ, de Ridder DT, Bensing JM, Gorter KJ, Rutten GE. Psychological outcomes of patients with screen-detected type 2 diabetes: the influence of time since diagnosis and treatment intensity. Diabetes Care 2006;29:2257-62.PubMed
- 42. van den Donk M, Gorter KJ, Rutten GE. No negative effects of a multi-factorial, intensified treatment on self-reported health status, treatment satisfaction, and diabetes-related distress in screen-detected type 2 diabetes patients: the ADDITIONNetherlands study. Qual Life Res 2010;19:509-13.ArticlePubMedPMCPDF
- 43. Van den Donk M, Griffin SJ, Stellato RK, Simmons RK, Sandbaek A, Lauritzen T, et al. Effect of early intensive multifactorial therapy compared with routine care on self-reported health status, general well-being, diabetes-specific quality of life and treatment satisfaction in screen-detected type 2 diabetes mellitus patients (ADDITION-Europe): a cluster-randomised trial. Diabetologia 2013;56:2367-77.ArticlePubMedPMCPDF
- 44. Kuznetsov L, Griffin SJ, Davies MJ, Lauritzen T, Khunti K, Rutten GE, et al. Diabetes-specific quality of life but not health status is independently associated with glycaemic control among patients with type 2 diabetes: a cross-sectional analysis of the ADDITION-Europe trial cohort. Diabetes Res Clin Pract 2014;104:281-7.ArticlePubMed
- 45. Simmons RK, Echouffo-Tcheugui JB, Sharp SJ, Sargeant LA, Williams KM, Prevost AT, et al. Screening for type 2 diabetes and population mortality over 10 years (ADDITION-Cambridge): a cluster-randomised controlled trial. Lancet 2012;380:1741-8.ArticlePubMedPMC
- 46. Dalsgaard EM, Sandbaek A, Griffin SJ, Rutten GE, Khunti K, Davies MJ, et al. Patient-reported outcomes after 10-year follow-up of intensive, multifactorial treatment in individuals with screen-detected type 2 diabetes: the ADDITION-Europe trial. Diabet Med 2020;37:1509-18.ArticlePubMedPMCPDF
- 47. Ueki K, Sasako T, Kato M, Okazaki Y, Okahata S, Katsuyama H, et al. Design of and rationale for the Japan Diabetes Optimal Integrated Treatment study for 3 major risk factors of cardiovascular diseases (J-DOIT3): a multicenter, open-label, randomized, parallel-group trial. BMJ Open Diabetes Res Care 2016;4:e000123.ArticlePubMedPMC
- 48. Yazaki Y, Kadowaki T. Combating diabetes and obesity in Japan. Nat Med 2006;12:73-4.ArticlePubMedPDF
- 49. Sakane N, Kotani K, Takahashi K, Sano Y, Tsuzaki K, Okazaki K, et al. Effects of telephone-delivered lifestyle support on the development of diabetes in participants at high risk of type 2 diabetes: J-DOIT1, a pragmatic cluster randomised trial. BMJ Open 2015;5:e007316.ArticlePubMedPMC
- 50. Hayashino Y, Suzuki H, Yamazaki K, Goto A, Izumi K, Noda M. A cluster randomized trial on the effect of a multifaceted intervention improved the technical quality of diabetes care by primary care physicians: the Japan Diabetes Outcome Intervention Trial-2 (J-DOIT2). Diabet Med 2016;33:599-608.ArticlePubMedPMCPDF
- 51. Ueki K, Sasako T, Okazaki Y, Miyake K, Nangaku M, Ohashi Y, et al. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int 2021;99:256-66.ArticlePubMed
- 52. Sasako T, Ueki K, Miyake K, Okazaki Y, Takeuchi Y, Ohashi Y, et al. Effect of a multifactorial intervention on fracture in patients with type 2 diabetes: subanalysis of the J-DOIT3 Study. J Clin Endocrinol Metab 2021;106:e2116-28.ArticlePubMedPMCPDF
- 53. Sasso FC, De Nicola L, Carbonara O, Nasti R, Minutolo R, Salvatore T, et al. Cardiovascular risk factors and disease management in type 2 diabetic patients with diabetic nephropathy. Diabetes Care 2006;29:498-503.ArticlePubMedPDF
- 54. Sasso FC, Chiodini P, Carbonara O, De Nicola L, Conte G, Salvatore T, et al. High cardiovascular risk in patients with type 2 diabetic nephropathy: the predictive role of albuminuria and glomerular filtration rate: the NID-2 Prospective Cohort Study. Nephrol Dial Transplant 2012;27:2269-74.ArticlePubMed
- 55. Sasso FC, Marfella R, Pagano A, Porta G, Signoriello G, Lascar N, et al. Lack of effect of aspirin in primary CV prevention in type 2 diabetic patients with nephropathy: results from 8 years follow-up of NID-2 study. Acta Diabetol 2015;52:239-47.ArticlePubMedPDF
- 56. Sasso FC, Lascar N, Ascione A, Carbonara O, De Nicola L, Minutolo R, et al. Moderate-intensity statin therapy seems ineffective in primary cardiovascular prevention in patients with type 2 diabetes complicated by nephropathy: a multicenter prospective 8 years follow up study. Cardiovasc Diabetol 2016;15:147.ArticlePubMedPMCPDF
- 57. Shikata K, Haneda M, Koya D, Suzuki Y, Tomino Y, Yamada K, et al. Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan): rationale and study design. Diabetes Res Clin Pract 2010;87:228-32.ArticlePubMed
- 58. Crasto W, Jarvis J, Khunti K, Skinner TC, Gray LJ, Brela J, et al. Multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: the Microalbuminuria Education and Medication Optimisation (MEMO) study. Diabetes Res Clin Pract 2011;93:328-36.ArticlePubMed
- 59. Crasto W, Morrison AE, Gray LJ, John E, Jarvis J, Brela J, et al. The Microalbuminuria Education Medication and Optimisation (MEMO) study: 4 years follow-up of multifactorial intervention in high-risk individuals with type 2 diabetes. Diabet Med 2020;37:286-97.ArticlePubMedPDF
- 60. Zhao XH, Xu ZR, Zhang Q, Gu HF, Yang YM. Effect of intensive multifactorial treatment on the intima-media thickness of large arteries in patients with new-onset type 2 diabetes mellitus. J Zhejiang Univ Sci B 2012;13:378-85.ArticlePubMedPMCPDF
- 61. Yang Y, Yao JJ, Du JL, Bai R, Sun LP, Sun GH, et al. Primary prevention of macroangiopathy in patients with short-duration type 2 diabetes by intensified multifactorial intervention: seven-year follow-up of diabetes complications in Chinese. Diabetes Care 2013;36:978-84.PubMedPMC
- 62. Shi C, Men L, Yu C, Yao J, Bai R, Yang Y, et al. Atherosclerosis associated with dynamic inflammation changes after multifactorial intervention in short-duration type 2 diabetes: a randomized, controlled, 10-year follow-up trial. J Diabetes Complications 2017;31:1286-92.ArticlePubMed
- 63. Tripolt NJ, Narath SH, Eder M, Pieber TR, Wascher TC, Sourij H. Multiple risk factor intervention reduces carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 2014;13:95.ArticlePubMedPMCPDF
- 64. Shi C, Fang X, Yang Y, Bai R, Yu S, Sun G, et al. Intensive multifactorial intervention improved renal impairment in short-duration type 2 diabetes: a randomized, controlled, 7-year followup trial. J Diabetes Complications 2020;34:107468.ArticlePubMed
- 65. Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care 2009;32:977-82.PubMedPMC
- 66. Araki A, Iimuro S, Sakurai T, Umegaki H, Iijima K, Nakano H, et al. Long-term multiple risk factor interventions in Japanese elderly diabetic patients: the Japanese Elderly Diabetes Intervention Trial: study design, baseline characteristics and effects of intervention. Geriatr Gerontol Int 2012;12 Suppl 1:7-17.ArticlePubMed
- 67. Solomon CG. Reducing cardiovascular risk in type 2 diabetes. N Engl J Med 2003;348:457-9.ArticlePubMed
- 68. Sasako T, Kadowaki T, Ueki K. ADDITION-Europe: the first decade and beyond. Lancet Diabetes Endocrinol 2019;7:891-3.ArticlePubMed
- 69. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.ArticlePubMed
- 70. Sone H, Tanaka S, Iimuro S, Tanaka S, Oida K, Yamasaki Y, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010;53:419-28.ArticlePubMedPMC
- 71. Chan JCN. How can we optimise diabetes care in real-world practice? Lancet Diabetes Endocrinol 2017;5:927-9.ArticlePubMed
- 72. Araki E, Senokuchi T, Furukawa N. Impacts of tight multifactorial intervention in patients with type 2 diabetes: implications from the Japan Diabetes Outcome Intervention Trial 3. J Diabetes Investig 2018;9:1022-4.ArticlePubMedPMCPDF
- 73. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89.ArticlePubMed
- 74. Hayward RA, Reaven PD, Emanuele NV; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:978.ArticlePubMed
- 75. Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, et al. Intensive glucose control in patients with type 2 diabetes: 15-year follow-up. N Engl J Med 2019;380:2215-24.ArticlePubMedPMC
- 76. Sasako T, Yamauchi T. Addressing screams for evidence on renoprotection by GLP-1 receptor agonists. Kidney Int 2022;101:222-4.ArticlePubMed
- 77. Sasako T, Yamauchi T. Clinical trials in participants with type 2 diabetes undertaken in Japan. J Jpn Diabetes Soc 2022;65:518-21.
- 78. Huang ES. Management of diabetes mellitus in older people with comorbidities. BMJ 2016;353:i2200.ArticlePubMedPMC
- 79. Neuen BL, Young T, Heerspink HJ, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845-54.ArticlePubMed
- 80. Zhu J, Yu X, Zheng Y, Li J, Wang Y, Lin Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol 2020;8:192-205.ArticlePubMed
- 81. Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018;391:2430-40.ArticlePubMed
- 82. Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 2021;9:165-73.ArticlePubMedPMC
- 83. Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001- 2010: report of Committee on Causes of Death in Diabetes Mellitus. Diabetol Int 2017;8:117-36.ArticlePubMedPMCPDF
- 84. Laakso M, Lehto S. Epidemiology of risk factors for cardiovascular disease in diabetes and impaired glucose tolerance. Atherosclerosis 1998;137 Suppl:S65-73.ArticlePubMed
- 85. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765-72.ArticlePubMed
- 86. International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol 2019;7:385-96.ArticlePubMed
- 87. Roussel R, Steg PG, Mohammedi K, Marre M, Potier L. Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: a perspective on glucose-lowering interventions. Diabetes Obes Metab 2018;20:238-44.PubMedPDF
- 88. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes: state-of-the-art. Mol Metab 2021;46:101102.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Exploring mechanisms underlying diabetes comorbidities and strategies to prevent vascular complications
Takayoshi Sasako
Diabetology International.2024; 15(1): 34. CrossRef - Targeting ERS-mitophagy in hippocampal neurons to explore the improvement of memory by tea polyphenols in aged type 2 diabetic rats
Wenjuan Feng, Chenhui Lv, Le Cheng, Xin Song, Xuemin Li, Haoran Xie, Shuangzhi Chen, Xi Wang, Lushan Xue, Cheng Zhang, Jie Kou, Lili Wang, Haifeng Zhao
Free Radical Biology and Medicine.2024; 213: 293. CrossRef - Risk of Dementia Among Patients With Diabetes in a Multidisciplinary, Primary Care Management Program
Kailu Wang, Shi Zhao, Eric Kam-Pui Lee, Susan Zi-May Yau, Yushan Wu, Chi-Tim Hung, Eng-Kiong Yeoh
JAMA Network Open.2024; 7(2): e2355733. CrossRef - Causes of In-Hospital Death and Pharmaceutical Associations with Age of Death during a 10-Year Period (2011–2020) in Individuals with and without Diabetes at a Japanese Community General Hospital
Minae Hosoki, Taiki Hori, Yousuke Kaneko, Kensuke Mori, Saya Yasui, Seijiro Tsuji, Hiroki Yamagami, Saki Kawata, Tomoyo Hara, Shiho Masuda, Yukari Mitsui, Kiyoe Kurahashi, Takeshi Harada, Shingen Nakamura, Toshiki Otoda, Tomoyuki Yuasa, Akio Kuroda, Itsur
Journal of Clinical Medicine.2024; 13(5): 1283. CrossRef - External validation of a minimal-resource model to predict reduced estimated glomerular filtration rate in people with type 2 diabetes without diagnosis of chronic kidney disease in Mexico: a comparison between country-level and regional performance
Camilla Sammut-Powell, Rose Sisk, Ruben Silva-Tinoco, Gustavo de la Pena, Paloma Almeda-Valdes, Sonia Citlali Juarez Comboni, Susana Goncalves, Rory Cameron
Frontiers in Endocrinology.2024;[Epub] CrossRef - Gut Microbiota Targeted Approach by Natural Products in Diabetes Management: An Overview
Priyanka Sati, Praveen Dhyani, Eshita Sharma, Dharam Chand Attri, Arvind Jantwal, Rajni Devi, Daniela Calina, Javad Sharifi-Rad
Current Nutrition Reports.2024;[Epub] CrossRef - Cardiovascular Risk Reduction in Type 2 Diabetes: Further Insights into the Power of Weight Loss and Exercise
Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(3): 302. CrossRef - Sarcopenia: Loss of mighty armor against frailty and aging
Takayoshi Sasako, Kohjiro Ueki
Journal of Diabetes Investigation.2023; 14(10): 1145. CrossRef
- Figure
- Related articles
-
- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
- Clinical and Lifestyle Determinants of Continuous Glucose Monitoring Metrics in Insulin-Treated Patients with Type 2 Diabetes Mellitus
- The Beneficial Effect of Glycemic Control against Adverse Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease
- Clinical Effects of a Home Care Pilot Program for Patients with Type 1 Diabetes Mellitus: A Retrospective Cohort Study
- Glycemic Control and Adverse Clinical Outcomes in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: Results from KNOW-CKD

 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite