Enhancing Diabetes Care through a Mobile Application: A Randomized Clinical Trial on Integrating Physical and Mental Health among Disadvantaged Individuals
Article information
Abstract
Background
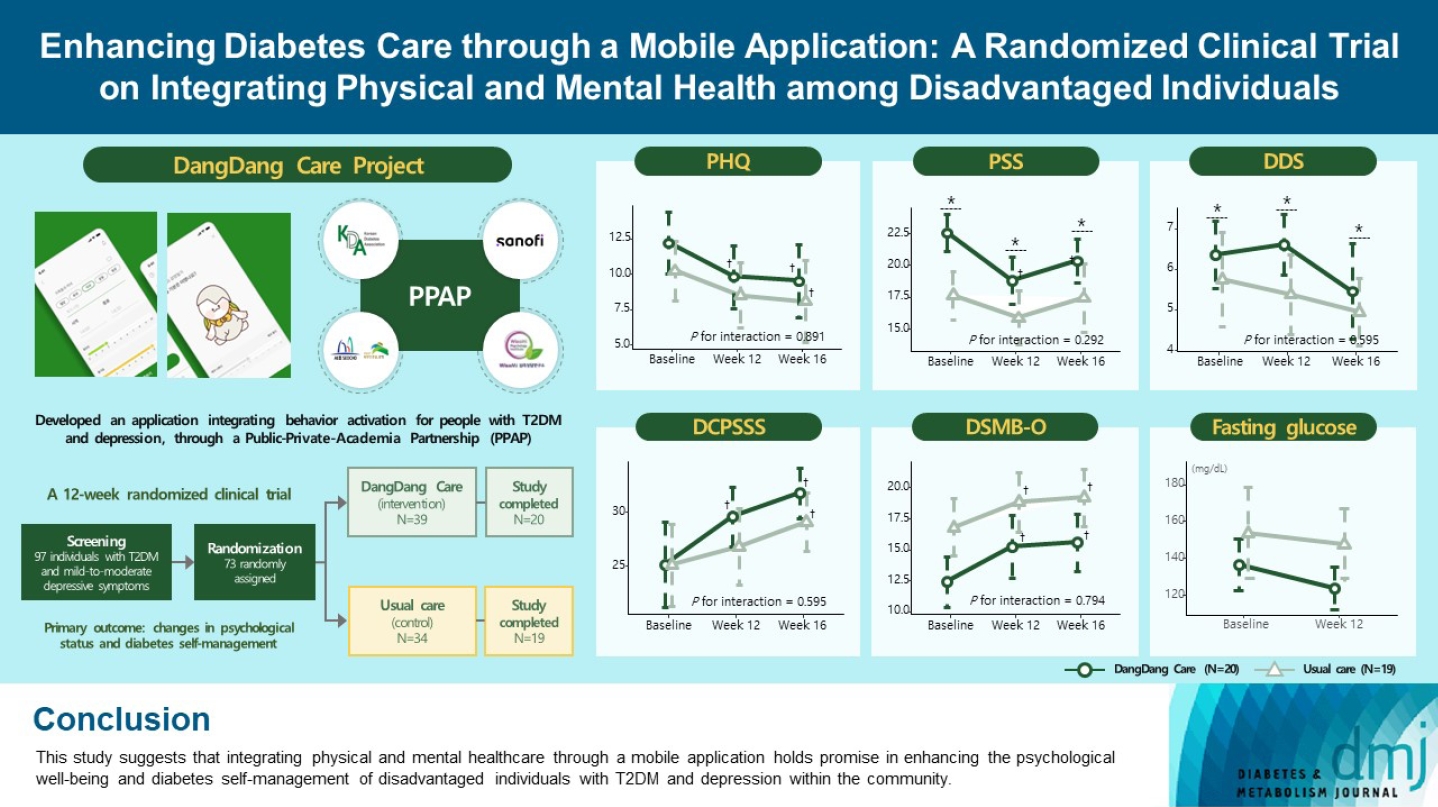
This study examines integrating physical and mental healthcare for disadvantaged persons with type 2 diabetes mellitus and mild-to-moderate depression in the community, using a mobile application within a public-private-academic partnership.
Methods
The Korean Diabetes Association has developed a mobile application combining behavioral activation for psychological well-being and diabetes self-management, with conventional medical therapy. Participants were randomly assigned to receive the application with usual care or only usual care. Primary outcomes measured changes in psychological status and diabetes self-management through questionnaires at week 12 from the baseline. Secondary outcomes assessed glycemic and lipid control, with psychological assessments at week 16.
Results
Thirty-nine of 73 participants completed the study (20 and 19 in the intervention and control groups, respectively) and were included in the analysis. At week 12, the intervention group showed significant reductions in depression severity and perceived stress compared to the control group. Additionally, they reported increased perceived social support and demonstrated improved diabetes self-care behavior. These positive effects persisted through week 16, with the added benefit of reduced anxiety. While fasting glucose levels in the intervention group tended to improve, no other significant differences were observed in laboratory assessments between the groups.
Conclusion
This study provides compelling evidence for the potential efficacy of a mobile application that integrates physical and mental health components to address depressive symptoms and enhance diabetes self-management in disadvantaged individuals with type 2 diabetes mellitus and depression. Further research involving larger and more diverse populations is warranted to validate these findings and solidify their implications.
Highlights
• This study suggests the efficacy of a mobile app integrating physical and mental health.
• The app targeted depressive symptoms and self-diabetes management in disadvantaged T2DM.
• It benefited perceived stress, diabetes distress, and depression severity.
• It improved perceived social support and diabetes management, even in older adults.
• Collaborative PPAP efforts underscore multifaceted healthcare for vulnerable groups.
INTRODUCTION
As of 2020, there were approximately 6 million adults aged 30 years or older living with diabetes in the Republic of Korea [1]. This figure reached a remarkable 30 years ahead of the projected estimate made in 2012. The global diabetes epidemic is primarily attributed to factors such as the increasing prevalence of obesity and lifestyle changes [2]. However, severe mental illness (SMI) also plays a significant role in the rising prevalence of diabetes [3]. Individuals with SMI are about two to three times more likely to have diabetes compared to the general population [3]. Furthermore, those with SMI experience more frequent and severe diabetic complications and have higher mortality compared to individuals without such conditions. A meta-analysis of cohort studies, including major depressive disorder and other SMIs, revealed that individuals with SMIs had a median loss of 10 years in life expectancy compared to the general population or control groups [4]. These outcomes are predominantly due to cardiovascular diseases, for which diabetes is a major risk factor [5-8].
In addition to these challenges, people with SMI often confront health inequities stemming from differences in social, economic, environmental, or healthcare resources, leading to health disparities. In the Republic of Korea, the prevalence of diabetes exhibits fluctuations tied to income levels, with a higher occurrence observed among individuals with lower incomes, irrespective of gender [9]. Additionally, an analysis using data from the National Health Insurance Service revealed that medication adherence among people diagnosed with diabetes was lowest (around 70%) among those belonging to the lowest income group or receiving medical aid, with adherence improving as income levels rose [9]. Hospital visit rates during the initial 6 months following a diagnosis of diabetes were notably elevated at 47.0% for medical aid recipients, while the rates ranged from 33.9% to 38.4% for other income groups [9]. This highlights that vulnerable populations encounter barriers in accessing pharmacotherapy for diabetes, potentially leading to challenges in managing their condition. Moreover, these groups undergo fewer health screenings, resulting in a larger proportion of undiagnosed diabetes cases and ultimately culminating in higher mortality rates [10].
In light of these discoveries, it becomes evident that effectively addressing the mental well-being of individuals managing both diabetes and depression is pivotal to mitigating the repercussions of these health discrepancies. Tending to mental health concerns and furnishing support to those grappling with depression alongside their diabetes care is imperative in narrowing the healthcare divide and fostering enhanced health outcomes for all individuals, regardless of their socioeconomic status. Embracing a holistic strategy that acknowledges the interconnectedness of physical and mental health is crucial in alleviating the burden of diabetes among vulnerable populations and nurturing a more equitable healthcare system.
Despite the presence of several initiatives, including the 2030 Diabetes Camp organized by the Korean Diabetes Association (KDA) [11], only one program has truly focused on a comprehensive approach to managing the mental and physical health of people with diabetes. Recognizing the significance of establishing an integrated mental health care service that not only facilitates connections between individuals but also tailors content to their specific needs, the KDA embarked on a mission to bridge this gap. However, many of these individuals encounter obstacles when attempting to access such support through conventional medical facilities, a situation that has been further compounded by challenges brought on by the coronavirus disease 2019 (COVID-19) pandemic [12]. Consequently, we investigated the viability of employing a mobile application to provide comprehensive physical and mental healthcare services to economically disadvantaged people with type 2 diabetes mellitus (T2DM) and mild-to-moderate depression.
METHODS
Overview
We have developed a mobile application named ‘DangDang Care,’ signifying the adept and steady management of diabetes through psychological well-being. This application is meticulously designed to provide psychological support and enhance diabetes self-management for persons with T2DM while concurrently experiencing depressive symptoms. The application integrates a behavioral activation program. To assess its effectiveness and feasibility in fostering consistent diabetes management complemented by psychological support, we conducted a 12-week randomized, parallel-group, open-label trial. This initiative was carried out in partnership with public health centers and the private sector, targeting individuals diagnosed with T2DM and exhibiting mild-to-moderate depression.
Development of a mobile application
We integrated behavior activation techniques into our mobile application, ‘DangDang Care,’ aiming to enhance the emotional well-being and self-management of disadvantaged individuals with T2DM and depressive symptoms. Behavioral activation is a psychological strategy that encourages individuals to engage in positive and rewarding activities to address depression and related mood disorders [13]. The fundamental idea is that participating in fulfilling or health-promoting activities can trigger positive emotions and motivate continued engagement in such activities. Behavioral activation is an approach that begins externally, while depression often stems from an internal lack of motivation or willingness. Behavioral activation involves setting and working towards goals in various aspects of daily life, including those deemed beneficial for managing diabetes. It provides individuals with an opportunity to participate in activities that serve as positive reinforcements, assisting them in planning and gradually increasing their activity levels regardless of their mood. Therefore, for individuals with diabetes who require daily lifestyle interventions, behavioral activation techniques can be a valuable therapeutic approach for improving both diabetes management and concurrent depression [14].
DangDang Care is a 12-week psychological intervention encompassing seven modules (Supplementary Table 1). To cater the application to the specific requirements of Korean individuals with T2DM, we adapted and supplemented content from previous studies [15,16]. In order to motivate participants and instill a sense of accomplishment, we visualized the completion of each module with the growth journey of ‘Dangbuki,’ a character that symbolizes awakening from an egg and growing based on the user’s activities (Supplementary Fig. 1). The development of the application was a collaborative effort involving two clinical psychologists with doctoral qualifications, two endocrinologists, and a skilled application development team.
Study population
We recruited economically disadvantaged individuals with T2DM and mild-to-moderate depressive symptoms, with the valuable assistance of Eunpyeong-gu and Seocho-gu public health centers. Seocho-gu, one of the 25 administrative districts in Seoul, ranks fourth in gross regional domestic product (GRDP). It is in the southeastern part of Seoul and has the most advanced medical facilities, though with fewer public healthcare institutions. In contrast, Eunpyeong-gu, positioned 22nd in GRDP among the 25 districts and located in the northwestern region, has fewer high-level medical facilities but compensates with a higher concentration of public healthcare institutions, making it a region relatively lacking in medical resources. The inclusion criteria were as follows: (1) adults aged 20 to 80 years with T2DM; (2) individuals belonging to disadvantaged populations, such as medical aid beneficiaries or those classified within low-income groups (earning 30% or less of the median income); (3) mild-to-moderate depressive symptoms, defined as a Patient Health Questionnaire-9 (PHQ-9) score ranging between 5 and 19; (4) proficiency in reading and comprehending Korean, as well as competence in using a mobile application; and (5) a clear grasp of the study’s purpose and providing written consent. Exclusion criteria encompassed: (1) type 1 diabetes mellitus or gestational diabetes; (2) diabetes complications that hindered diabetes self-management; (3) severe depressive symptoms impacting application usage and completion of self-report questionnaires; (4) SMIs beyond depressive symptoms (such as schizophrenia, bipolar disorders, or neurodegenerative disorders); (5) severe life-threatening medical conditions; (6) physical conditions impeding mobility; (7) a history of traumatic brain injury; and (8) unwillingness to complete the questionnaire and share personal information.
Procedures
Individuals who volunteered to participate in the study visited the public health center after an 8-hour fasting period. Initially, the study’s objectives and procedures were thoroughly explained to the potential participants, and they voluntarily provided their informed consent. Subsequently, the participants were randomly assigned (1:1) to either the intervention group receiving DangDang Care in addition to usual diabetes care or the control group receiving only usual care. Both groups underwent baseline assessments, including venous blood sampling for glucose and lipid analysis, as well as completing questionnaires pertaining to their mental health and diabetes self-management status.
For those assigned to the intervention group, assistance was provided in installing the application, along with instructions on its utilization. After 12 weeks, all study participants returned to the public health center for follow-up venous blood sampling and completion of the questionnaires. During the study period, no changes were made to the participants’ standard diabetes care, except for the integration of the mobile application in the intervention group. We considered participants as dropouts if they had not used DangDang Care for more than 2 weeks. At the conclusion of the study (at week 16), the participants were invited to return to the public health center and to complete the questionnaires again, without any specific intervention related to the application usage. Following the 16-week assessment, both groups were informed that they could continue using the application if they so desired. An overview of the study procedures is presented in Fig. 1.
Outcomes and measures
Primary outcomes were changes in the psychological status and diabetes self-management of participants assessed through questionnaires at week 12 compared to baseline. Secondary outcomes encompassed changes in glycemic and lipid control. Follow-up assessments of psychological status were conducted at week 16. The metrics utilized comprised a range of mental health variables, including PHQ-9, Generalized Anxiety Disorder-7 (GAD-7), Korean version of the Perceived Stress Scale (K-PSS), Brief Diabetes Distress Screening Instrument (DDS-2), and European Quality of Life-5 Dimensions 3 Level (EQ-5D-3L). Diabetes self-management was evaluated through variables such as Diabetes Self-Efficacy Scale (DSES), Diabetes Care Profile-Social Support Scale (DCP-SSS), and Diabetes Self-Management Behaviors for Older Koreans (DSMB-O). Laboratory tests included measurements of fasting plasma glucose, glycosylated hemoglobin (HbA1c), and lipid profile. More detailed information on the measures can be found in Supplementary Table 2.
Statistical analysis
Given the pilot nature of this study, an exact sample size calculation was not performed. Instead, estimations were made based on a presumed effect size of 0.085, α value of 0.05, and power of 0.80. Consequently, we aimed to enroll 31 participants per group, resulting in a total target enrollment of 124 participants. In our recruitment strategy, we accounted for potential dropouts (40%) and incomplete outcome data (10%).
Continuous variables were presented as either mean±standard deviation or median with interquartile range, while categorical variables were expressed as numbers and percentages. Baseline characteristics and laboratory assessments were analyzed using various statistical tests, such as Student’s t-test or Mann-Whitney U test for continuous variables, and the chi-square test or Fisher’s exact test for categorical variables. For laboratory assessments, changes from baseline within to week 12 within each group were evaluated using a paired t-test. Additionally, an analysis of covariance (ANCOVA) was executed to compare changes between the groups. The ANCOVA model integrated baseline laboratory values and adjustment variables, including age, sex, diabetes duration, presence of diabetic complications, and visual impairment or hearing disorders. These adjustment variables were chosen based on baseline factors with a P value below 0.1 resulting from intergroup comparisons. To examine alterations in questionnaire results over time between the groups, we employed a mixed effects model with repeat measurement (MMRM) using compound symmetry as a variance-covariance structure. This method considered within-subject correlation due to repeated evaluation of values. The MMRM incorporated a fixed effect for the group, visit, and the interaction between the group and visit, along with the same covariates employed in the ANCOVA. All reported P values were two-sided, and statistical significance was defined at P<0.05. Statistical analyses were performed using the SAS Enterprise Guide version 7.1 (SAS Institute Inc., Gary, NC, USA) and R software version 4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical statement and protocol registration
This study adhered to the principles of the Declaration of Helsinki. The study protocol received approval from the Public Institutional Review Board designated by the Ministry of Health and Welfare (IRB No. P01-202110-12-002). Additionally, the study is registered on ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT05992545). Our reporting of trial outcomes is consistent with the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement and the CONSORT-Outcomes 2022 extension (Supplementary Table 3) [17].
RESULTS
Study participants
The original plan aimed to enroll 124 participants; however, due to the complications presented by the COVID-19 pandemic, a total of 97 participants were ultimately recruited. Among these, 39 were assigned to the intervention group, while the control group comprised 34 participants. Over the course of the study, dropouts and incomplete data resulted in the exclusion of 19 individuals from the intervention group and 15 from the control group. Consequently, the final analysis was conducted on a cohort of 20 participants in the intervention group and 19 participants in the control group (Fig. 1). The baseline characteristics of the persistent and dropout groups are presented in Supplementary Table 4.
Baseline characteristics
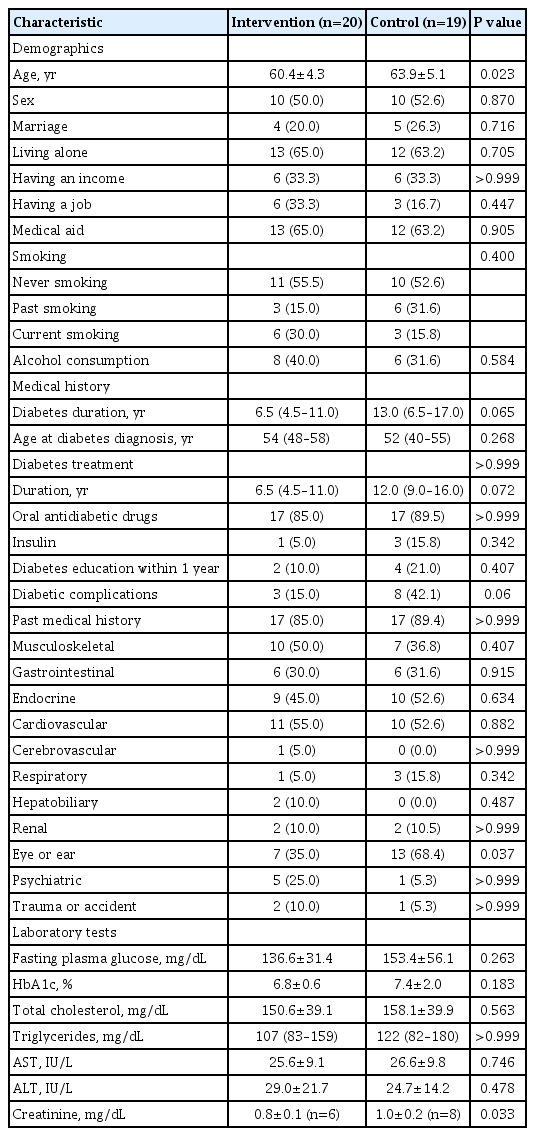
The intervention and control groups demonstrated similar median ages (intervention group 54 years, control group 52 years) and median duration of diabetes (intervention group 6.5 years, control group 13 years), with no significant distinctions. The mean fasting blood glucose levels were 136.6 mg/dL in the intervention group and 153.4 mg/dL in the control group. Correspondingly, HbA1c levels were 6.8% for the intervention group and 7.4% for the control group, showing no substantial difference. Most participants in both groups were undergoing diabetes pharmacotherapy. Notably, the control group exhibited higher rates of pharmacotherapy duration, insulin treatment, prevalence of diabetic complications, and a greater history of visual impairment or hearing disorders. However, except for the history of visual impairment or hearing disorders, no statistically significant differences were found between the two groups for these parameters (Table 1).
Changes in psychological status
The intervention group showed significant improvements in the K-PSS by week 12 compared to the control group, indicating a reduction in perceived stress (Fig. 2A and B). This positive effect persisted at week 16. Concerning the DDS-2, an indicator of diabetes distress, there was a significant increase in the intervention group at week 12 compared to the control group, followed by a decrease at week 16, showing an overall positive impact (Fig. 2C and D). The PHQ-9, which measures depression severity, notably decreased in the intervention group by week 12 and in both groups by week 16 in comparison to baseline, reflecting an improvement in depression severity (Fig. 2E and F). By week 16, the intervention group demonstrated a substantial reduction in the GAD-7 from baseline, despite no significant difference at week 12 (Supplementary Fig. 2A and B). The EQ-5D-3L index displayed a tendency to decrease more in the intervention group compared to the control group (Supplementary Fig. 2C and D). However, no significant interaction effects between the groups and visits were observed for the assessed outcomes (Fig. 2, Supplementary Table 5, Supplementary Fig. 2).

Changes in psychologic status questionnaire scores. (A) Korean version of the Perceived Stress Scale (K-PSS) change from baseline. (B) K-PSS. (C) Brief Diabetes Distress Screening Instrument (DDS-2) change from baseline. (D) DDS-2. (E) Patient Health Questionnaire-9 (PHQ-9) change from baseline. (F) PHQ-9. Data are means (with error bars showing standard errors). aP<0.05 for the difference to baseline within each group, bP<0.05 for the difference between the groups within each visit.
Changes in diabetes self-management
Fig. 3 and Supplementary Table 6 illustrate alterations in diabetes self-management questionnaire scores. There was no significant difference in these scores between the intervention and control groups at both weeks 12 and 16. However, the DCP-SSS, indicating perceived social support, exhibited a noteworthy increase in the intervention group by week 12 and in both groups by week 16 (Fig. 3A and B). Intriguingly, the DSMB-O, reflecting diabetes self-management behavior in older adults with T2DM, demonstrated significant enhancements in both the intervention and control groups throughout the study duration (Fig. 3C and 3D). On the other hand, the DSES did not yield significant results (Fig. 3E and F). In line with the findings in psychological status, no significant interaction effects were observed between the groups and visits for the measured outcomes.
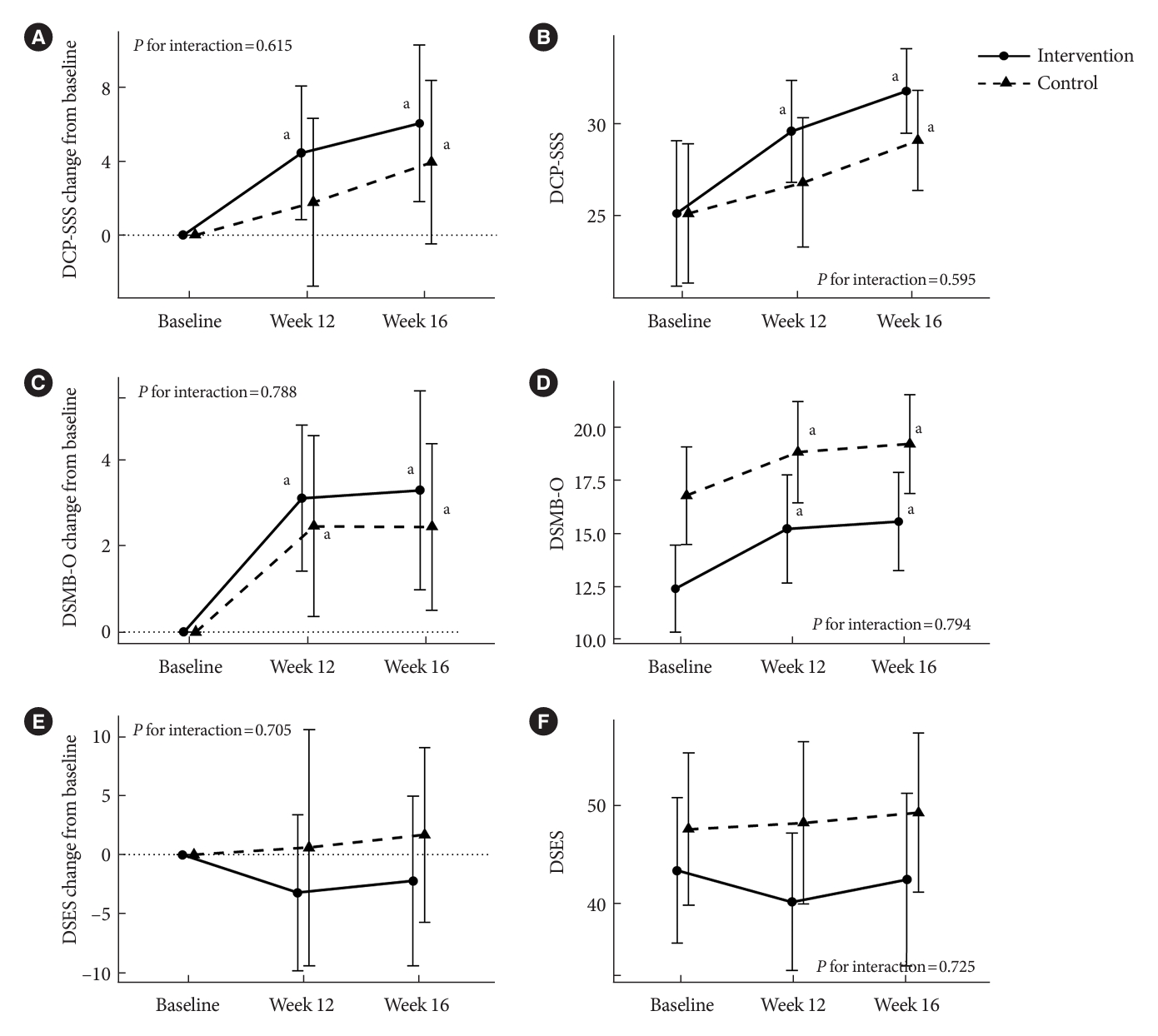
Changes in diabetes self-management questionnaire scores. (A) Diabetes Care Profile-Social Support Scale (DCP-SSS) change from baseline. (B) DCP-SSS. (C) Diabetes Self-Management Behaviors for Older Koreans (DSMB-O) change from baseline. (D) DSMB-O. (E) Diabetes Self-Efficacy Scale (DSES) change from baseline. (F) DSES. Data are means (with error bars showing standard errors). aP<0.05 for the difference to baseline within each group.
Changes in laboratory assessments
In terms of secondary outcomes, at week 12, there was no significant difference in HbA1c between the intervention and control groups in comparison to baseline. Nevertheless, there was a trend towards a greater decrease in fasting plasma glucose levels in the intervention group compared to the control group by week 12. The results of other laboratory tests, encompassing the lipid profile, remained consistent with no significant changes observed throughout the study period (Table 2).
Safety and adverse events
During the study period, no treatment-emergent adverse events were observed in either of the two groups. While comprehensive investigations into medication alterations were not conducted, no occurrences requiring early hospital visits or admissions due to acute or chronic diabetic complications were recorded. Furthermore, no adverse events related to the use of the mobile application were reported.
DISCUSSION
This study provides encouraging evidence for the potential effectiveness of a mobile application that integrates physical and mental health components. It aims to address depressive symptoms and enhance diabetes self-management among disadvantaged individuals living with T2DM and depression in the community. The results demonstrated the positive impacts on perceived stress, diabetes distress, and depression severity, as well as improvements in perceived social support and diabetes self-management behavior, even among older adults. The collaborative efforts involving various stakeholders, including the government, private sector, and academia, highlight the significance of a multifaceted approach in effectively meeting the healthcare needs of vulnerable populations.
DangDang Care stands as the first mobile application in the Republic of Korea dedicated to assisting disadvantaged individuals living with T2DM. Its innovation lies in the amalgamation of physical and mental healthcare. What sets this application apart is its behavioral activation program, which focuses on enhancing users’ psychological well-being through the cultivation of gratifying and meaningful experiences in their daily lives. This program complements the management of depression, aligning seamlessly with the needs of individuals with T2DM, where lifestyle modifications are pivotal for effective self-management [18,19]. An important aspect to consider when interpreting the results of this study is that, despite the randomization used to determine the intervention and control groups, there were differences in baseline questionnaire scores between the two groups. While we did observe changes within each group compared to their baseline scores, we did not find significant interactions between the group and the visit. It is worth noting that although we achieved the primary outcome, caution is warranted when interpreting these results solely as effects attributed to the intervention. Nevertheless, this application effortlessly facilitates diabetes self-management education and support by integrating indispensable diabetes management behaviors such as blood glucose monitoring, medication adherence, sleep patterns, dietary habits, and exercise routines, all integrated into daily activities. By combining these components, the application offers a promising and suitable solution for proficiently addressing both the physical and mental dimensions of diabetes care.
Effectively addressing non-communicable diseases (NCDs) within disadvantaged or vulnerable populations demands collaborative efforts that extend beyond the roles of individuals or healthcare professionals. It necessitates the involvement of multiple stakeholders, including government entities. This study underscores the significance of public-private-academic partnerships (PPAP) [20] in tackling NCDs, with a specific emphasis on T2DM and depression [21,22]. The PPAP can manifest in diverse forms, with each party contributing to varying extents and shouldering different degrees of risk. In the context of this study, academia played a vital role by providing expertise in application development, research execution, and analysis. The private sector offered comprehensive support throughout the application development process, while the public sector actively participated in patient recruitment and the implementation of applications for these individuals. The incorporation of such partnerships in the development and implementation of the mobile application serves as an exemplary model for future initiatives, emphasizing the significance of collective endeavors in enhancing healthcare outcomes for disadvantaged or vulnerable groups.
In recent years, there has been an increasing recognition of the paramount importance of social determinants of health in relation to NCDs [23]. The COVID-19 era has further underscored the significance of these factors [24]. Throughout the COVID-19 pandemic, individuals with T2DM were found to experience higher rates of depression and lower resilience compared to the period before COVID-19, particularly in comparison to those without diabetes [25,26]. This highlights a heightened vulnerability among this population. Furthermore, an interesting observation during the COVID-19 pandemic suggested that changes in behaviors associated with anxiety and depressive symptoms seemed to positively impact on diabetes self-care, at least in the short term [27]. Given the intricate interplay between diabetes, depression, and comorbidities [28], especially during periods of limited face-to-face interactions, mobile applications like the one developed in this study can offer valuable remote access to integrated physical and mental healthcare. The availability of such an application during these challenging times may provide essential support to individuals managing chronic diseases, including T2DM and depression. This underlines the potential advantages of using technology-based approaches to address healthcare needs during difficult circumstances.
While this study did not demonstrate significant improvements in HbA1c levels, it is important to note that the participants had relatively well-controlled baseline glycemic status, with levels ranging from 6.8% to 7.4%. Considering that only approximately half of Korean adults with diabetes achieved HbA1c levels below 7.0% during 2019–2020 [1], the limited changes in HbA1c among these individuals can be understood in the context of the unchanged standard care for diabetes. Furthermore, it’s worth noting that randomized controlled trials using mobile applications have typically shown modest reductions in HbA1c levels [29]. Therefore, observing a significant change in HbA1c over a brief 12-week period without any medication intervention would likely have been challenging. Despite the absence of significant improvements in glycemic control, the study demonstrated positive effects in other aspects, including reductions in depression severity and perceived stress, as well as improvements in diabetes self-care behavior. These findings suggest that the intervention may offer broader benefits beyond glycemic control, positively enhancing the overall health status of the participants.
In this study, a high dropout rate of 46.6% was observed. It was expected that a higher dropout rate might occur due to economic disadvantages, depression factors, and the pilot nature of the study. However, these challenges were further exacerbated by the COVID-19 pandemic. During crises like the pandemic, vulnerable individuals become even more susceptible to health-related difficulties. Furthermore, since DangDang Care was not considered essential healthcare, a lack of understanding regarding study participation may have impacted application usage and retention rates. Initial difficulties in grasping the concept of behavior activation and technical issues with the mobile application likely also contributed to the higher dropout rates. These factors highlight the importance of future improvements in the application’s user experience and user interface.
This study has limitations and strengths. It was conducted on a relatively small scale, and unfortunately, the initial target for participant recruitment could not met due to the influence of the COVID-19 pandemic. Additionally, the duration of the study was limited to 12 weeks, making it challenging to ascertain the long-term sustainability of the observed effects. The expected low retention rate raised concerns about sustaining participant engagement, especially within the disadvantaged population, prompting the necessity to explore more effective engagement strategies. One significant aspect not addressed was medication adjustments, a factor that should be accounted for when assessing the enduring effects on glycemic control. The study also required a considerable investment of human resources to support engagement among vulnerable populations. This involved tasks such as guiding participants through application installation, promoting behavioral activation, and facilitating usage. While approximately 80% of the intervention group reported overall satisfaction with the application and provided a neutral or positive response about its assistance in diabetes management, they also stressed the need for a more user-friendly experience and a lighter version of the application.
Despite these limitations and areas for improvement, the value of this study is substantial as it represents a pioneering endeavor that validates the effectiveness and feasibility of the application as a fundamental step. Moreover, the focus on addressing concerns and offering support to vulnerable populations in managing diabetes deserves commendation. It is noteworthy that we provided blood glucose meters to all participants at the commencement, a factor that could have contributed to the improvements in HbA1c levels observed in the control group. This underscored the significance of not only acknowledging but also implementing comprehensive interventions to enhance diabetes outcomes in such vulnerable populations.
In conclusion, this study suggests that integrating physical and mental healthcare through a mobile application holds promise in enhancing the psychological well-being and diabetes self-management of disadvantaged individuals with T2DM and mild-to-moderate depression within the community. While further research involving larger and more diverse populations is required to validate these findings, the results provide valuable evidence for the potential efficacy of utilizing such mobile applications to enhance the overall health and well-being of people living with T2DM and depression.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2023.0298.
Seven modules of a behavioral activation program incorporated into DangDang Care
Detailed information on the study measures
CONSORT-Outcomes checklist (combined CONSORT 2010 and CONSORT-Outcomes 2022 items)
Baseline characteristics of the persistent and dropout groups
Comparison of psychological status questionnaire scores between the intervention and control groups
Comparison of diabetes self-management questionnaire scores between the intervention and control groups
The 12-week growth journey of Dangbuki with the step-by-step process of the 7 modules of the behavioral activation program.
Changes in the Generalized Anxiety Disorder-7 (GAD-7) and the European Quality of Life-5 Dimensions 3 Level (EQ-5D-3L) index. (A) GAD-7 changes from baseline. (B) GAD-7. (C) EQ-5D-3L index change from baseline. (D) EQ5D-3L index. Data are means (with error bars showing standard errors). aP<0.05 for the difference to baseline within each group.
Notes
CONFLICTS OF INTEREST
Hae Kyung Lee is an employee of Sanofi-Aventis Korea. Hyun Mi Kim is the founder of WiseMi Psychology Institute. Kyu Chang Won has been honorary editor of the Diabetes & Metabolism Journal since 2020. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: J.H.B., E.H.P., H.K.L., H.M.K., S.G.K.
Acquisition, analysis, or interpretation of data: J.H.B., E.H.P., H.M.K., S.G.K.
Drafting the work or revising: J.H.B., E.H.P.
Final approval of the manuscript: J.H.B., E.H.P., H.K.L., K.H.Y., K.C.W., H.M.K., S.G.K.
FUNDING
This study was supported by the Korean Diabetes Association and Sanofi-Aventis Korea.
Acknowledgements
We would like to express our sincere gratitude to Eunpyeonggu and Seocho-gu for their invaluable support in recruiting the study participants. Additionally, we want to express special thanks to Jimi Choi for providing valuable assistance in the statistical analysis.