
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Review
- Page Path
- HOME > Article category > Review
Reviews
- Metabolic Risk/Epidemiology
- Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism
- Tong Bu, Ziyan Sun, Yi Pan, Xia Deng, Guoyue Yuan
- Received August 14, 2023 Accepted January 1, 2024 Published online April 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0277 [Epub ahead of print]
- 458 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
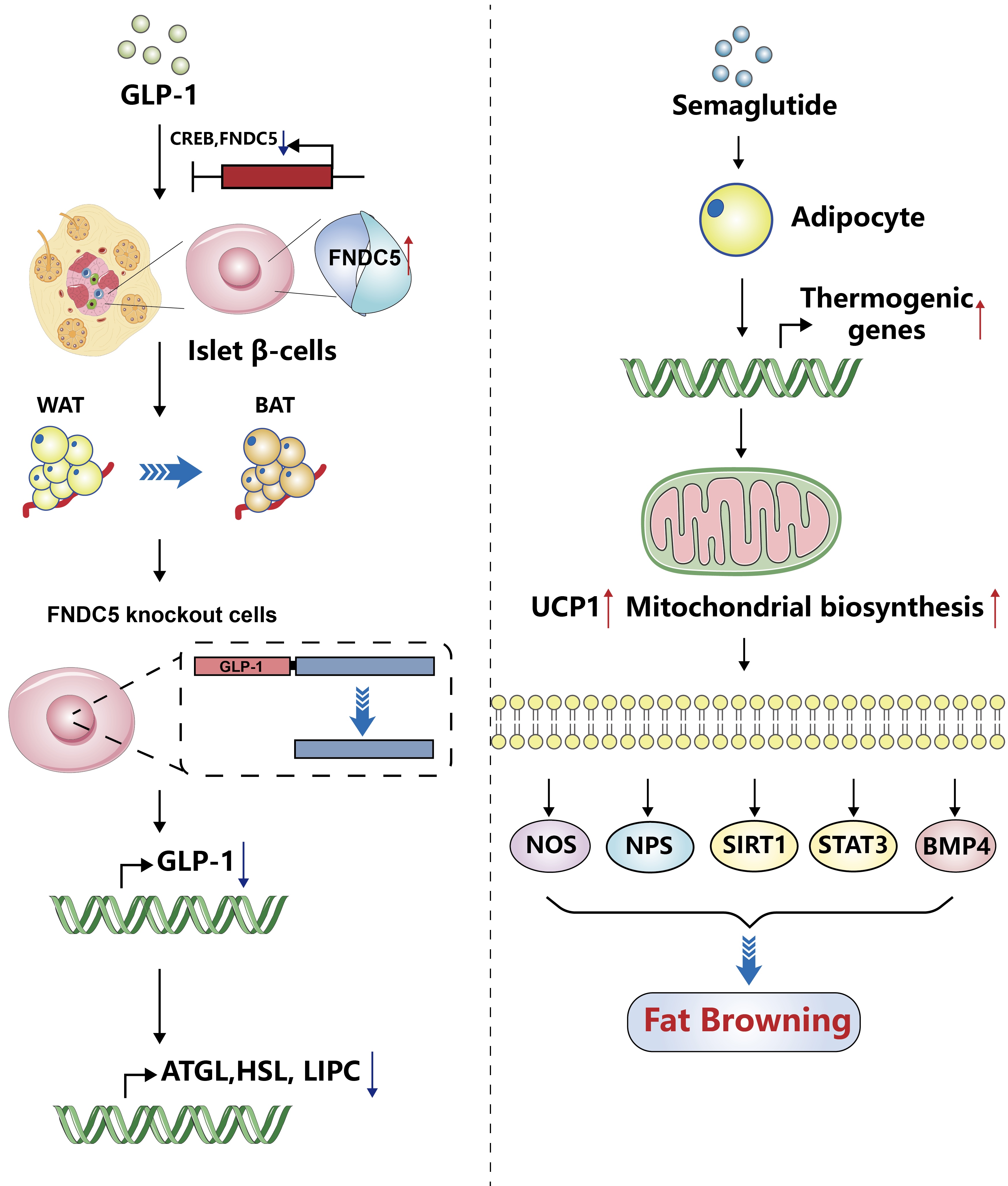
ePub - Glucagon-like peptide-1 (GLP-1) is a 30-amino acid peptide hormone that is mainly expressed in the intestine and hypothalamus. In recent years, basic and clinical studies have shown that GLP-1 is closely related to lipid metabolism, and it can participate in lipid metabolism by inhibiting fat synthesis, promoting fat differentiation, enhancing cholesterol metabolism, and promoting adipose browning. GLP-1 plays a key role in the occurrence and development of metabolic diseases such as obesity, nonalcoholic fatty liver disease, and atherosclerosis by regulating lipid metabolism. It is expected to become a new target for the treatment of metabolic disorders. The effects of GLP-1 and dual agonists on lipid metabolism also provide a more complete treatment plan for metabolic diseases. This article reviews the recent research progress of GLP-1 in lipid metabolism.
- Basic Research
- Roles of Histone Deacetylase 4 in the Inflammatory and Metabolic Processes
- Hyunju Kang, Young-Ki Park, Ji-Young Lee, Minkyung Bae
- Received June 5, 2023 Accepted February 7, 2024 Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0174 [Epub ahead of print]

- 630 View
- 33 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Histone deacetylase 4 (HDAC4), a class IIa HDAC, has gained attention as a potential therapeutic target in treating inflammatory and metabolic processes based on its essential role in various biological pathways by deacetylating non-histone proteins, including transcription factors. The activity of HDAC4 is regulated at the transcriptional, post-transcriptional, and post-translational levels. The functions of HDAC4 are tissue-dependent in response to endogenous and exogenous factors and their substrates. In particular, the association of HDAC4 with non-histone targets, including transcription factors, such as myocyte enhancer factor 2, hypoxia-inducible factor, signal transducer and activator of transcription 1, and forkhead box proteins, play a crucial role in regulating inflammatory and metabolic processes. This review summarizes the regulatory modes of HDAC4 activity and its functions in inflammation, insulin signaling and glucose metabolism, and cardiac muscle development.
- Metabolic Risk/Epidemiology
- One-Carbon Metabolism Nutrients, Genetic Variation, and Diabetes Mellitus
- Jie Zhu, Gunjana Saikia, Xiaotao Zhang, Xiaoxi Shen, Ka Kahe
- Diabetes Metab J. 2024;48(2):170-183. Published online March 12, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0272
- 1,121 View
- 153 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
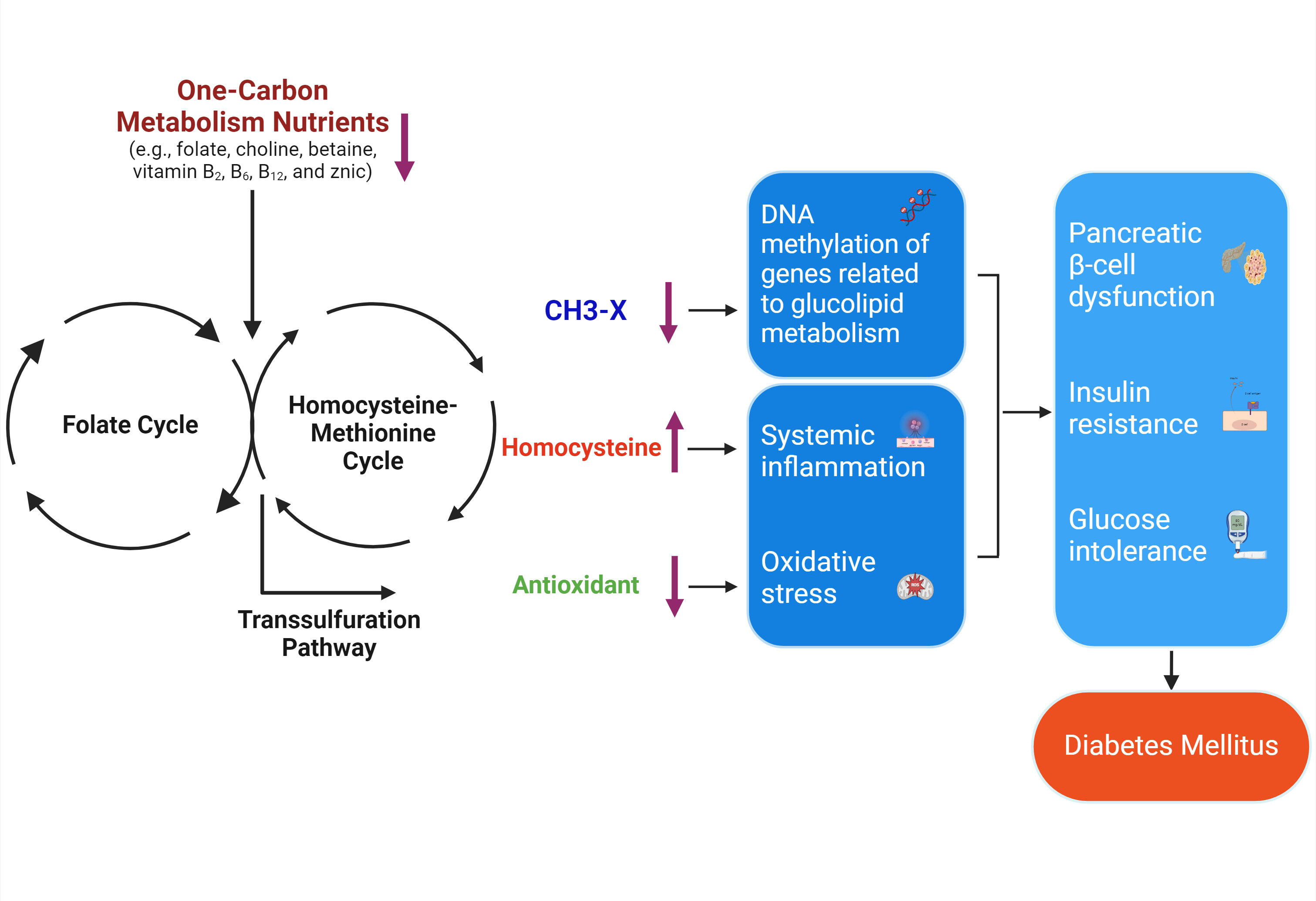
ePub - Diabetes mellitus (DM) affects about 9.3% of the population globally. Hyperhomocysteinemia (HHcy) has been implicated in the pathogenesis of DM, owing to its promotion of oxidative stress, β-cell dysfunction, and insulin resistance. HHcy can result from low status of one-carbon metabolism (OCM) nutrients (e.g., folate, choline, betaine, vitamin B6, B12), which work together to degrade homocysteine by methylation. The etiology of HHcy may also involve genetic variation encoding key enzymes in OCM. This review aimed to provide an overview of the existing literature assessing the link between OCM nutrients status, related genetic factors, and incident DM. We also discussed possible mechanisms underlying the role of OCM in DM development and provided recommendations for future research and practice. Even though the available evidence remains inconsistent, some studies support the potential beneficial effects of intakes or blood levels of OCM nutrients on DM development. Moreover, certain variants in OCM-related genes may influence metabolic handling of methyl-donors and presumably incidental DM. Future studies are warranted to establish the causal inference between OCM and DM and examine the interaction of OCM nutrients and genetic factors with DM development, which will inform the personalized recommendations for OCM nutrients intakes on DM prevention.
- Pathophysiology
- Dysfunctional Mitochondria Clearance in Situ: Mitophagy in Obesity and Diabetes-Associated Cardiometabolic Diseases
- Songling Tang, Di Hao, Wen Ma, Lian Liu, Jiuyu Gao, Peng Yao, Haifang Yu, Lu Gan, Yu Cao
- Received July 4, 2023 Accepted October 29, 2023 Published online February 15, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0213 [Epub ahead of print]
- 981 View
- 61 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
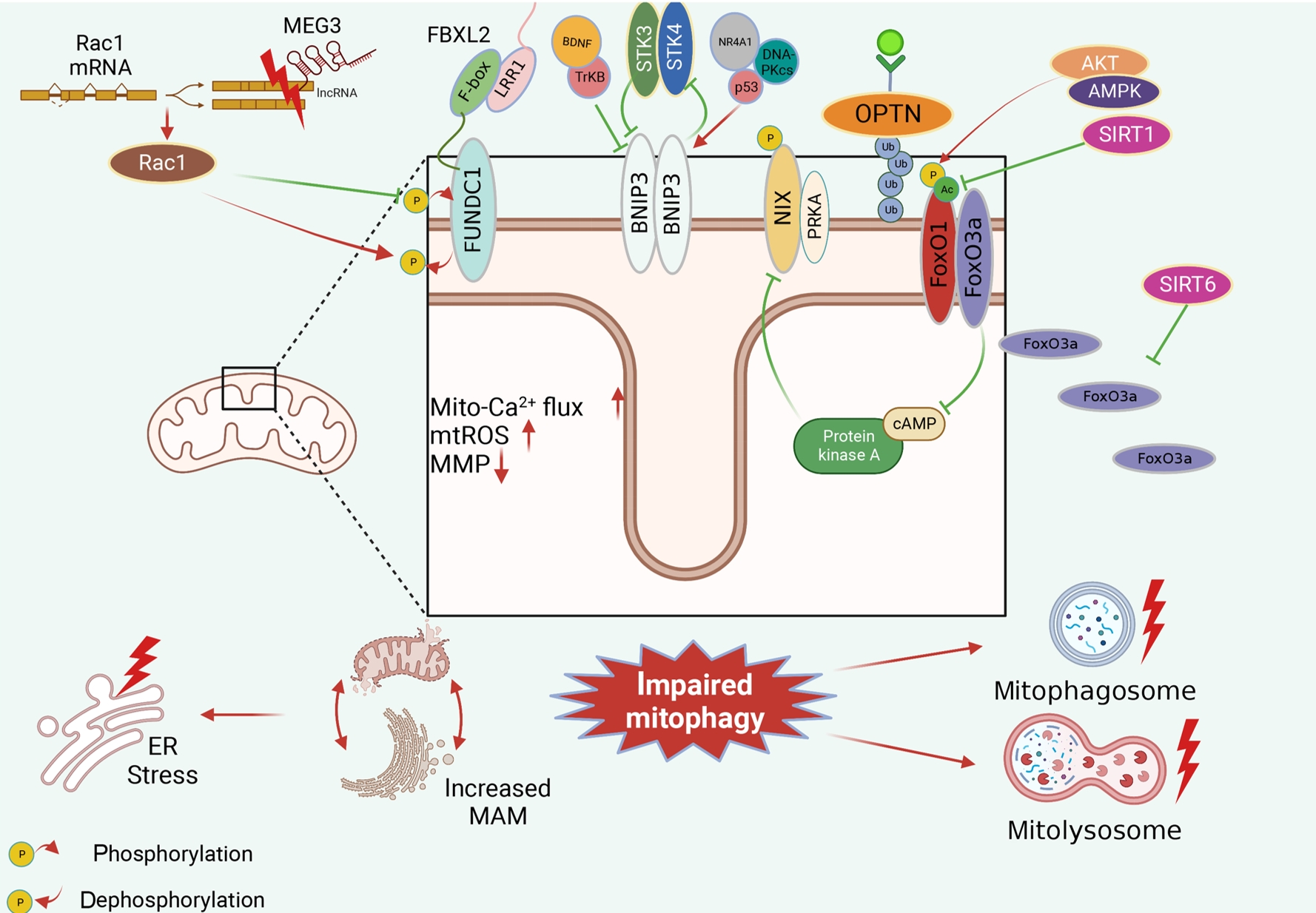
ePub - Several mitochondrial dysfunctions in obesity and diabetes include impaired mitochondrial membrane potential, excessive mitochondrial reactive oxygen species generation, reduced mitochondrial DNA, increased mitochondrial Ca2+ flux, and mitochondrial dynamics disorders. Mitophagy, specialized autophagy, is responsible for clearing dysfunctional mitochondria in physiological and pathological conditions. As a paradox, inhibition and activation of mitophagy have been observed in obesity and diabetes-related heart disorders, with both exerting bidirectional effects. Suppressed mitophagy is beneficial to mitochondrial homeostasis, also known as benign mitophagy. On the contrary, in most cases, excessive mitophagy is harmful to dysfunctional mitochondria elimination and thus is defined as detrimental mitophagy. In obesity and diabetes, two classical pathways appear to regulate mitophagy, including PTEN-induced putative kinase 1 (PINK1)/Parkin-dependent mitophagy and receptors/adapters-dependent mitophagy. After the pharmacologic interventions of mitophagy, mitochondrial morphology and function have been restored, and cell viability has been further improved. Herein, we summarize the mitochondrial dysfunction and mitophagy alterations in obesity and diabetes, as well as the underlying upstream mechanisms, in order to provide novel therapeutic strategies for the obesity and diabetes-related heart disorders.
- Pathophysiology
- Epicardial Adipose Tissue and Heart Failure, Friend or Foe?
- Dong-Hyuk Cho, Seong-Mi Park
- Received June 20, 2023 Accepted December 11, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0190 [Epub ahead of print]
- 863 View
- 72 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Heart failure (HF) management guidelines recommend individualized assessments based on HF phenotypes. Adiposity is a known risk factor for HF. Recently, there has been an increased interest in organ-specific adiposity, specifically the role of the epicardial adipose tissue (EAT), in HF risk. EAT is easily assessable through various imaging modalities and is anatomically and functionally connected to the myocardium. In pathological conditions, EAT secretes inflammatory cytokines, releases excessive fatty acids, and increases mechanical load on the myocardium, resulting in myocardial remodeling. EAT plays a pathophysiological role in characterizing both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). In HFrEF, EAT volume is reduced, reflecting an impaired metabolic reservoir, whereas in HFpEF, the amount of EAT is associated with worse biomarker and hemodynamic profiles, indicating increased EAT activity. Studies have examined the possibility of therapeutically targeting EAT, and recent studies using sodium glucose cotransporter 2 inhibitors have shown potential in reducing EAT volume. However, further research is required to determine the clinical implications of reducing EAT activity in patients with HF.
-
Citations
Citations to this article as recorded by- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review
Jorge E. Jalil, Luigi Gabrielli, María Paz Ocaranza, Paul MacNab, Rodrigo Fernández, Bruno Grassi, Paulina Jofré, Hugo Verdejo, Monica Acevedo, Samuel Cordova, Luis Sanhueza, Douglas Greig
International Journal of Molecular Sciences.2024; 25(8): 4407. CrossRef
- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review
- Others
- Risk Prediction and Management of Chronic Kidney Disease in People Living with Type 2 Diabetes Mellitus
- Ying-Guat Ooi, Tharsini Sarvanandan, Nicholas Ken Yoong Hee, Quan-Hziung Lim, Sharmila S. Paramasivam, Jeyakantha Ratnasingam, Shireene R. Vethakkan, Soo-Kun Lim, Lee-Ling Lim
- Diabetes Metab J. 2024;48(2):196-207. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0244
- 1,840 View
- 350 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
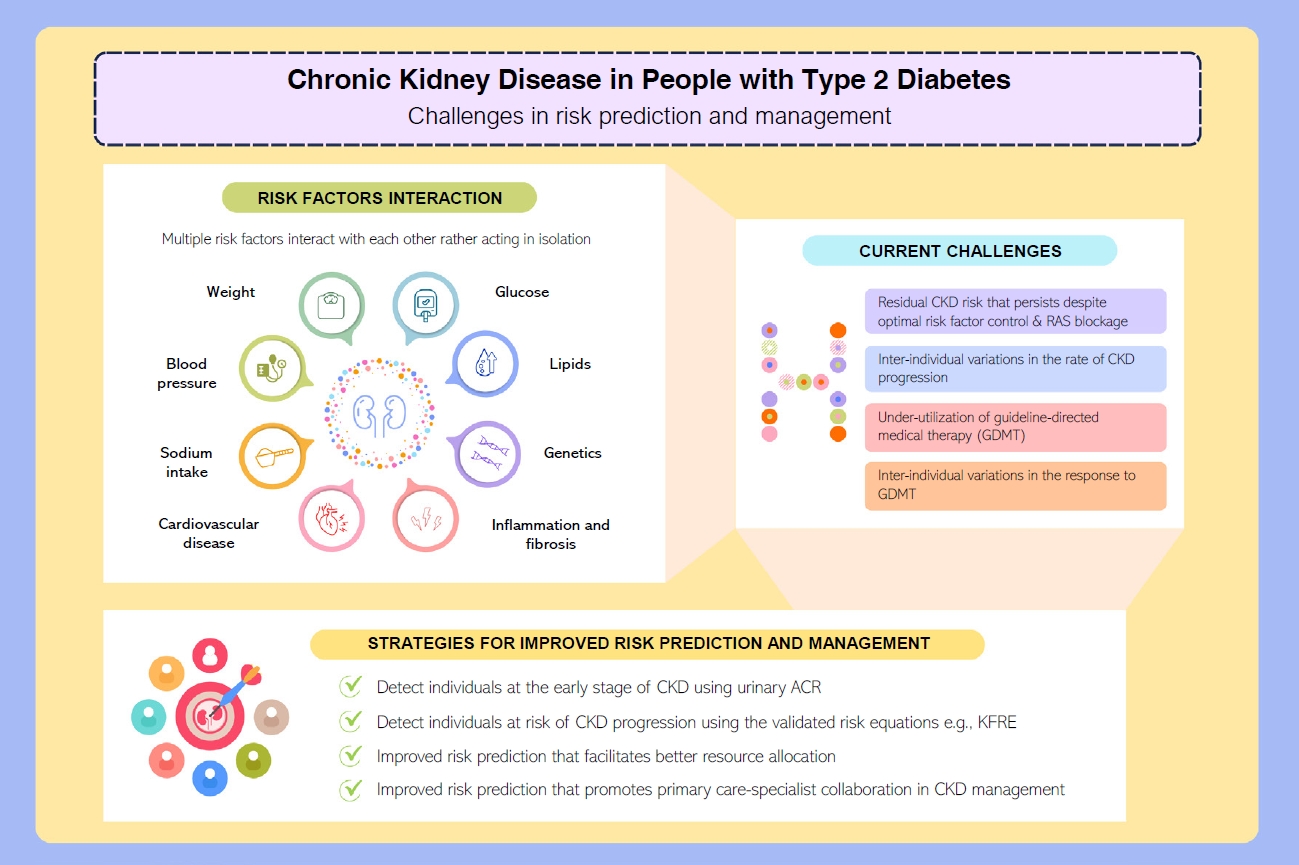
ePub - People with type 2 diabetes mellitus have increased risk of chronic kidney disease and atherosclerotic cardiovascular disease. Improved care delivery and implementation of guideline-directed medical therapy have contributed to the declining incidence of atherosclerotic cardiovascular disease in high-income countries. By contrast, the global incidence of chronic kidney disease and associated mortality is either plateaued or increased, leading to escalating direct and indirect medical costs. Given limited resources, better risk stratification approaches to identify people at risk of rapid progression to end-stage kidney disease can reduce therapeutic inertia, facilitate timely interventions and identify the need for early nephrologist referral. Among people with chronic kidney disease G3a and beyond, the kidney failure risk equations (KFRE) have been externally validated and outperformed other risk prediction models. The KFRE can also guide the timing of preparation for kidney replacement therapy with improved healthcare resources planning and may prevent multiple complications and premature mortality among people with chronic kidney disease with and without type 2 diabetes mellitus. The present review summarizes the evidence of KFRE to date and call for future research to validate and evaluate its impact on cardiovascular and mortality outcomes, as well as healthcare resource utilization in multiethnic populations and different healthcare settings.
- Metabolic Risk/Epidemiology
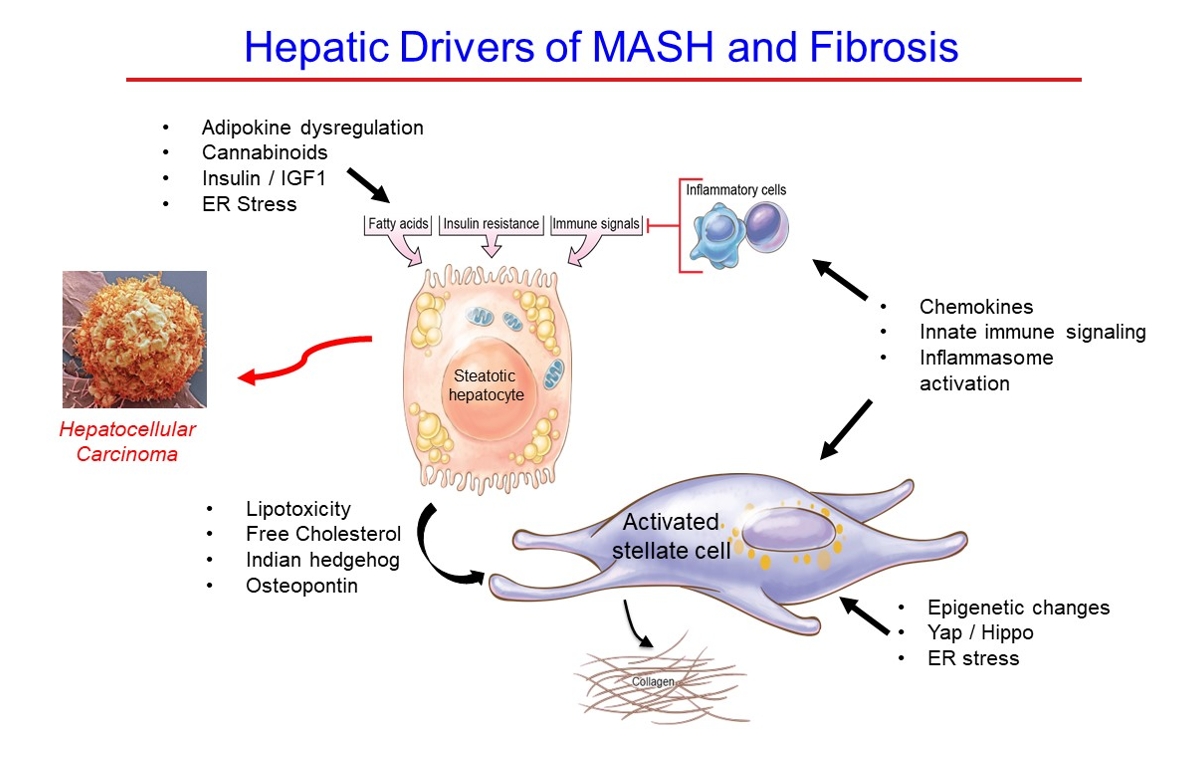
- Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
- Scott L. Friedman
- Diabetes Metab J. 2024;48(2):161-169. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0240
- 2,239 View
- 273 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolic dysfunction-associated steatotic (fatty) liver disease (MASLD), previously termed non-alcoholic fatty liver disease, is a worldwide epidemic that can lead to hepatic inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). The disease is typically a component of the metabolic syndrome that accompanies obesity, and is often overlooked because the liver manifestations are clinically silent until late-stage disease is present (i.e., cirrhosis). Moreover, Asian populations, including Koreans, have a higher fraction of patients who are lean, yet their illness has the same prognosis or worse than those who are obese. Nonetheless, ongoing injury can lead to hepatic inflammation and ballooning of hepatocytes as classic features. Over time, fibrosis develops following activation of hepatic stellate cells, the liver’s main fibrogenic cell type. The disease is usually more advanced in patients with type 2 diabetes mellitus, indicating that all diabetic patients should be screened for liver disease. Although there has been substantial progress in clarifying pathways of injury and fibrosis, there no approved therapies yet, but current research seeks to uncover the pathways driving hepatic inflammation and fibrosis, in hopes of identifying new therapeutic targets. Emerging molecular methods, especially single cell sequencing technologies, are revolutionizing our ability to clarify mechanisms underlying MASLD-associated fibrosis and HCC.
- Metabolic Risk/Epidemiology
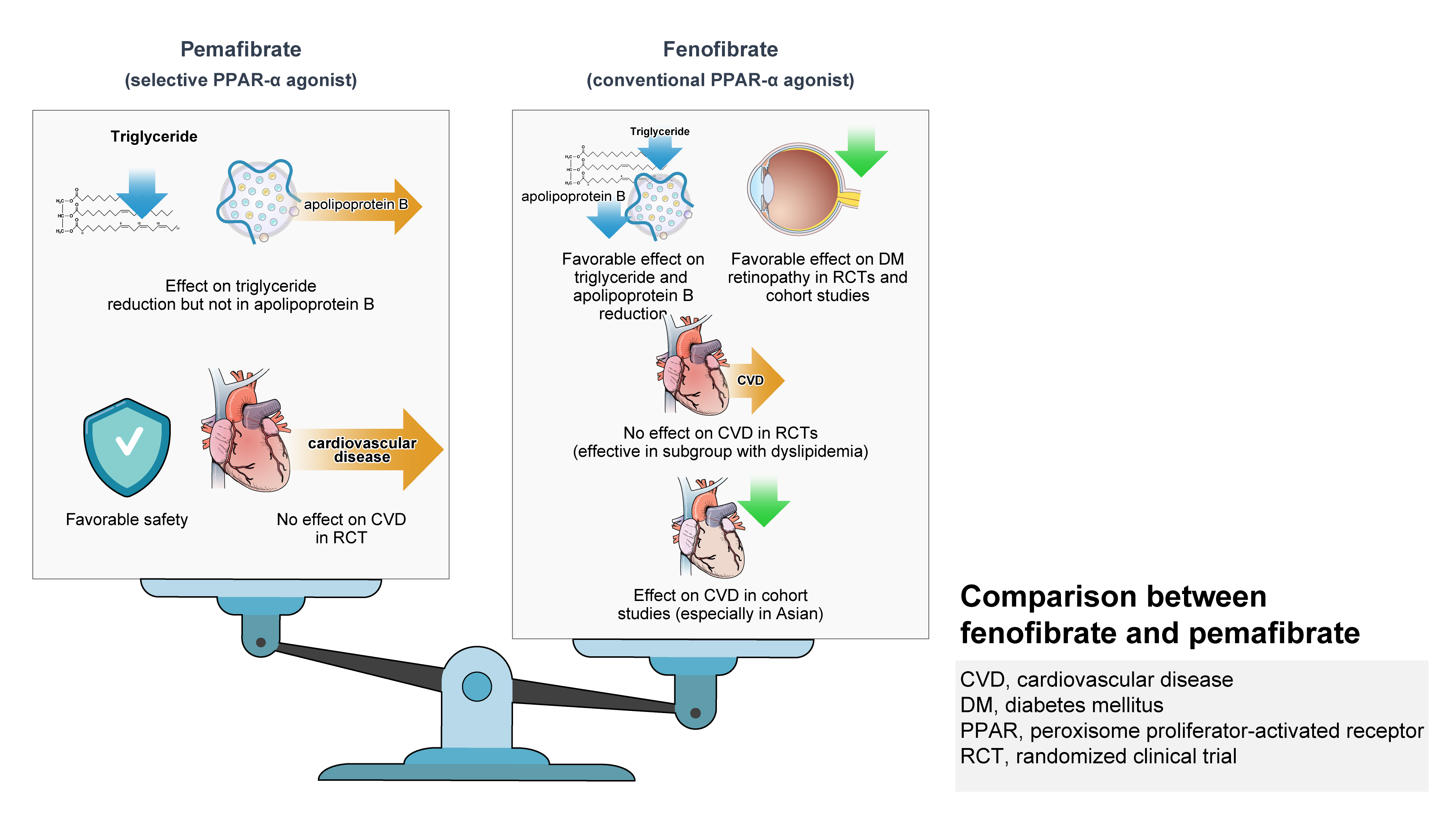
- Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
- Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
- Diabetes Metab J. 2024;48(2):184-195. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0168
- 2,213 View
- 328 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Hypertriglyceridemia and decreased high-density lipoprotein cholesterol (HDL-C) persist despite statin therapy, contributing to residual atherosclerotic cardiovascular disease (ASCVD) risk. Asian subjects are metabolically more susceptible to hypertriglyceridemia than other ethnicities. Fenofibrate regulates hypertriglyceridemia, raises HDL-C levels, and is a recommended treatment for dyslipidemia. However, data on fenofibrate use across different Asian regions are limited. This narrative review summarizes the efficacy and safety data of fenofibrate in Asian subjects with dyslipidemia and related comorbidities (diabetes, metabolic syndrome, diabetic retinopathy, and diabetic nephropathy). Long-term fenofibrate use resulted in fewer cardiovascular (CV) events and reduced the composite of heart failure hospitalizations or CV mortality in type 2 diabetes mellitus. Fenofibrate plays a significant role in improving irisin resistance and microalbuminuria, inhibiting inflammatory responses, and reducing retinopathy incidence. Fenofibrate plus statin combination significantly reduced composite CV events risk in patients with metabolic syndrome and demonstrated decreased triglyceride and increased HDL-C levels with an acceptable safety profile in those with high CV or ASCVD risk. Nevertheless, care is necessary with fenofibrate use due to possible hepatic and renal toxicities in vulnerable individuals. Long-term trials and real-world studies are needed to confirm the clinical benefits of fenofibrate in the heterogeneous Asian population with dyslipidemia.
- Pathophysiology
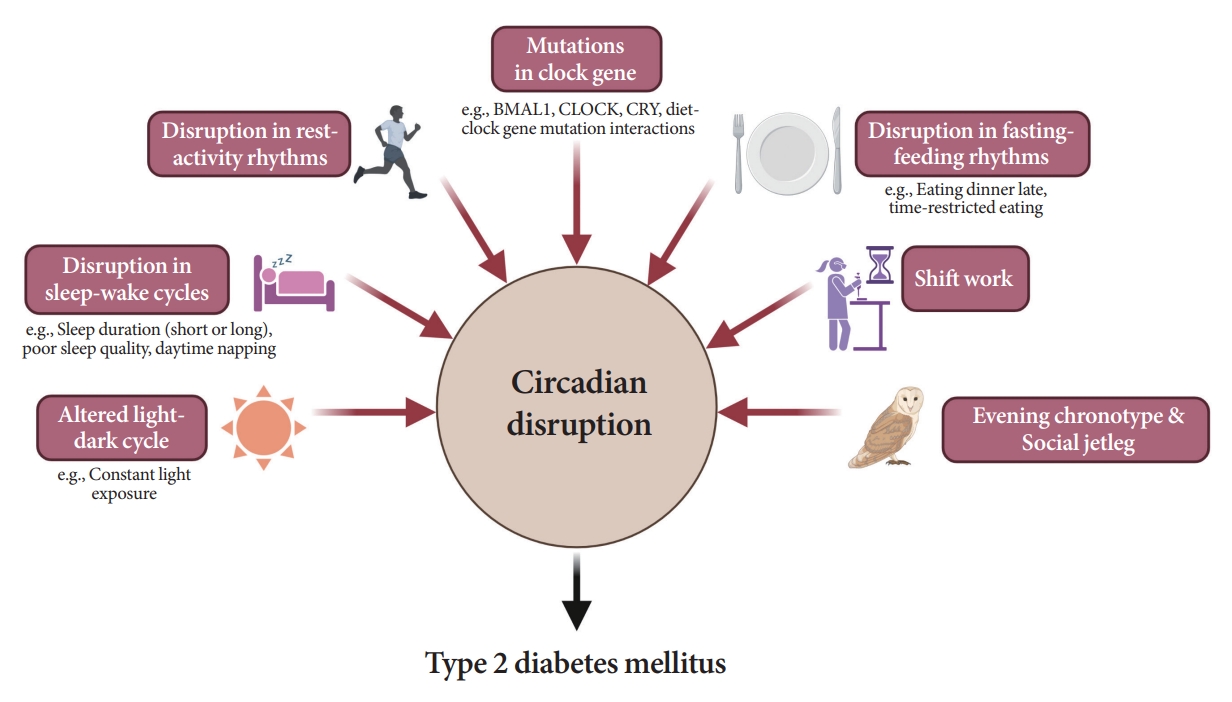
- Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
- Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
- Diabetes Metab J. 2024;48(1):37-52. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0193
- 2,157 View
- 219 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Novel strategies are required to reduce the risk of developing diabetes and/or clinical outcomes and complications of diabetes. In this regard, the role of the circadian system may be a potential candidate for the prevention of diabetes. We reviewed evidence from animal, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of diabetes. The circadian clock governs genetic, metabolic, hormonal, and behavioral signals in anticipation of cyclic 24-hour events through interactions between a “central clock” in the suprachiasmatic nucleus and “peripheral clocks” in the whole body. Currently, circadian rhythmicity in humans can be subjectively or objectively assessed by measuring melatonin and glucocorticoid levels, core body temperature, peripheral blood, oral mucosa, hair follicles, rest-activity cycles, sleep diaries, and circadian chronotypes. In this review, we summarized various circadian misalignments, such as altered light-dark, sleep-wake, rest-activity, fasting-feeding, shift work, evening chronotype, and social jetlag, as well as mutations in clock genes that could contribute to the development of diabetes and poor glycemic status in patients with diabetes. Targeting critical components of the circadian system could deliver potential candidates for the treatment and prevention of type 2 diabetes mellitus in the future.
- Basic Research
- Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases
- Benyuan Zhang, Joon Young Chang, Min Hee Lee, Sang-Hyeon Ju, Hyon-Seung Yi, Minho Shong
- Diabetes Metab J. 2024;48(1):1-18. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0115

- 2,030 View
- 255 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Mitochondrial stress and the dysregulated mitochondrial unfolded protein response (UPRmt) are linked to various diseases, including metabolic disorders, neurodegenerative diseases, and cancer. Mitokines, signaling molecules released by mitochondrial stress response and UPRmt, are crucial mediators of inter-organ communication and influence systemic metabolic and physiological processes. In this review, we provide a comprehensive overview of mitokines, including their regulation by exercise and lifestyle interventions and their implications for various diseases. The endocrine actions of mitokines related to mitochondrial stress and adaptations are highlighted, specifically the broad functions of fibroblast growth factor 21 and growth differentiation factor 15, as well as their specific actions in regulating inter-tissue communication and metabolic homeostasis. Finally, we discuss the potential of physiological and genetic interventions to reduce the hazards associated with dysregulated mitokine signaling and preserve an equilibrium in mitochondrial stress-induced responses. This review provides valuable insights into the mechanisms underlying mitochondrial regulation of health and disease by exploring mitokine interactions and their regulation, which will facilitate the development of targeted therapies and personalized interventions to improve health outcomes and quality of life.
- Pathophysiology
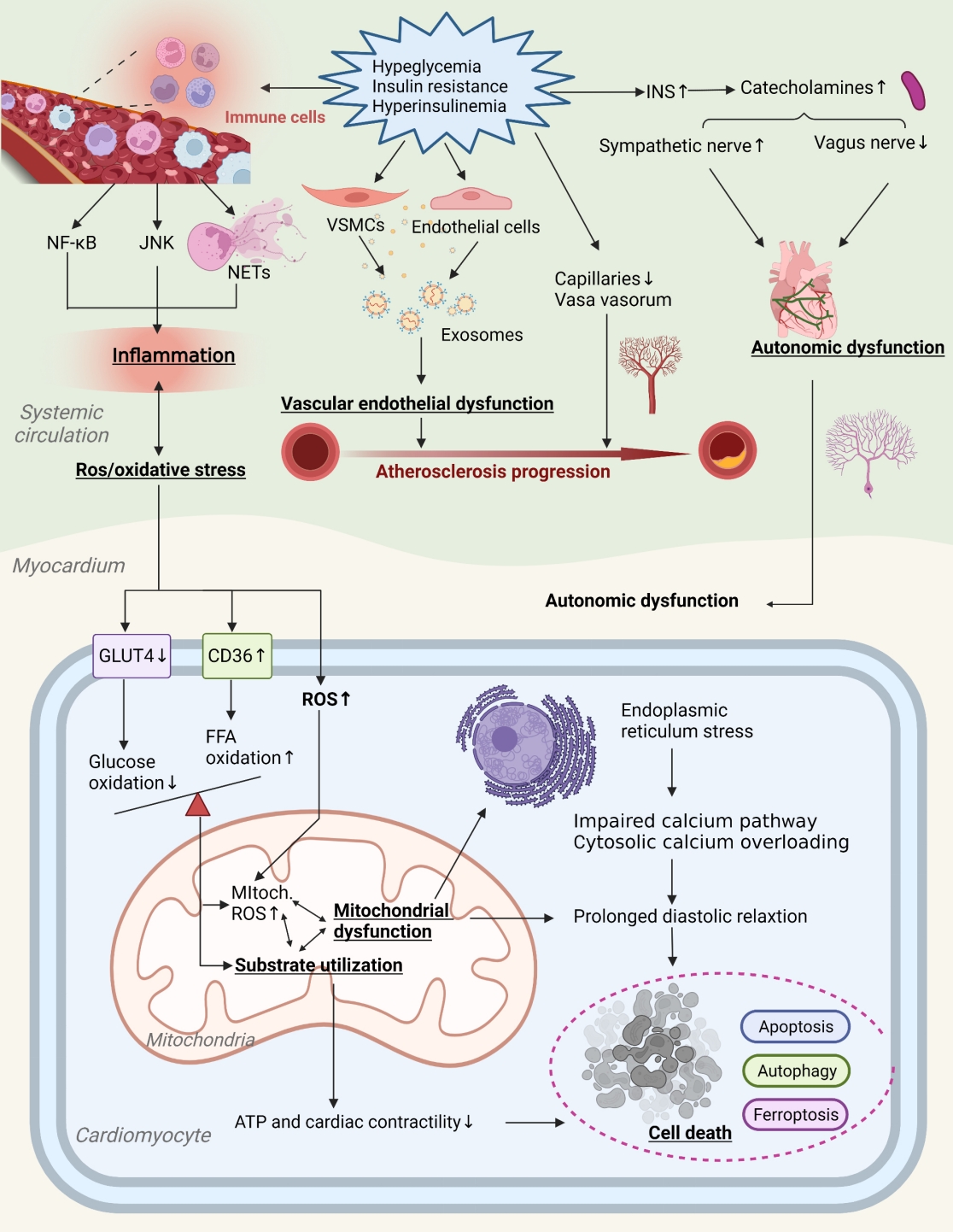
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- 2,156 View
- 182 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Guideline/Fact Sheet
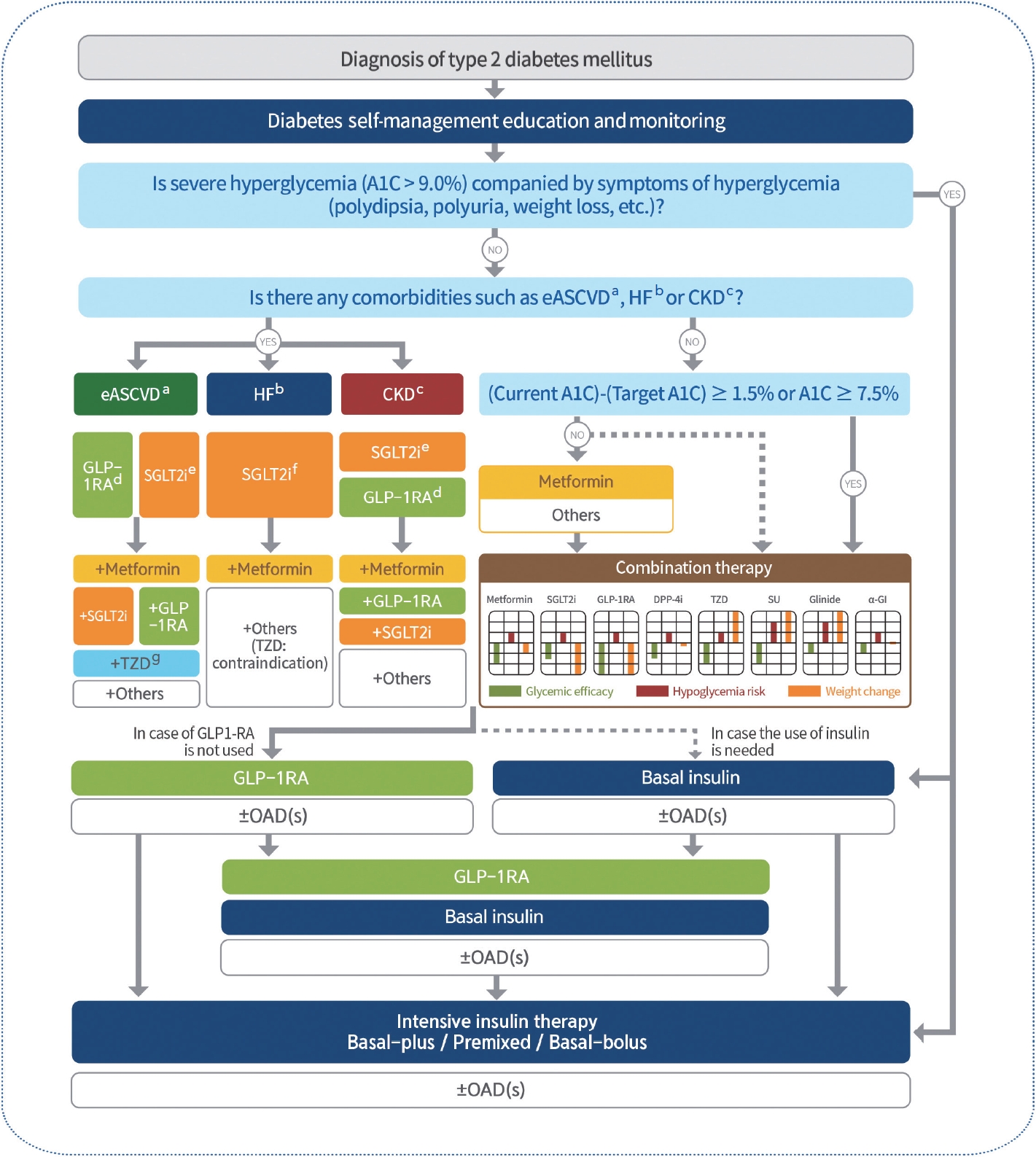
- 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
- Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae Jin Kim, Hyun Min Kim, Jung Hae Ko, Nam Hoon Kim, Chong Hwa Kim, Jeeyun Ahn, Tae Jung Oh, Soo-Kyung Kim, Jaehyun Kim, Eugene Han, Sang-Man Jin, Won Suk Choi, Min Kyong Moon, Committee of Clinical Practice Guidelines, Korean Diabetes Association
- Diabetes Metab J. 2023;47(5):575-594. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0282
- 5,017 View
- 628 Download
- 7 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - In May 2023, the Committee of Clinical Practice Guidelines of the Korean Diabetes Association published the revised clinical practice guidelines for Korean adults with diabetes and prediabetes. We incorporated the latest clinical research findings through a comprehensive systematic literature review and applied them in a manner suitable for the Korean population. These guidelines are designed for all healthcare providers nationwide, including physicians, diabetes experts, and certified diabetes educators who manage patients with diabetes or individuals at risk of developing diabetes. Based on recent changes in international guidelines and the results of a Korean epidemiological study, the recommended age for diabetes screening has been lowered. In collaboration with the relevant Korean medical societies, recently revised guidelines for managing hypertension and dyslipidemia in patients with diabetes have been incorporated into this guideline. An abridgment containing practical information on patient education and systematic management in the clinic was published separately.
-
Citations
Citations to this article as recorded by- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
Eugene Han, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Sang Hoon Ahn, Yong-ho Lee, Seung Up Kim
Metabolism.2024; 152: 155789. CrossRef - Letter by In-Kyung Jeong Regarding Article, Trends in Prevalence of Hypertriglyceridemia and Related Factors in Korean Adults: A Serial Cross-Sectional Study
In-Kyung Jeong
Journal of Lipid and Atherosclerosis.2024; 13(1): 80. CrossRef - Association between cardiovascular disease risk and incident type 2 diabetes mellitus in individuals with prediabetes: A retrospective cohort study
Myung Jin Kim, Yun Kyung Cho, Chang Hee Jung, Woo Je Lee
Diabetes Research and Clinical Practice.2024; 208: 111125. CrossRef - Korea Hypertension Fact Sheet 2023: analysis of nationwide population-based data with a particular focus on hypertension in special populations
Hyeon Chang Kim, Hokyou Lee, Hyeok-Hee Lee, Dasom Son, Minsung Cho, Sojung Shin, Yeeun Seo, Eun-Jin kim, Song Vogue Ahn, Sun Ha Jee, Sungha Park, Hae-Young Lee, Min Ho Shin, Sang-Hyun Ihm, Seung Won Lee, Jong Ku Park, Il Suh, Tae-Yong Lee
Clinical Hypertension.2024;[Epub] CrossRef - Diabetes Duration, Cholesterol Levels, and Risk of Cardiovascular Diseases in Individuals With Type 2 Diabetes
Mee Kyoung Kim, Kyu Na Lee, Kyungdo Han, Seung-Hwan Lee
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Effect of Adding Apolipoprotein B Testing on the Prevalence of Dyslipidemia and Risk of Cardiovascular Disease in the Korean Adult Population
Rihwa Choi, Sang Gon Lee, Eun Hee Lee
Metabolites.2024; 14(3): 169. CrossRef - A self-powered and supercapacitive microneedle continuous glucose monitoring system with a wide range of glucose detection capabilities
Hye-Jun Kil, Jang Hyeon Kim, Kanghae Lee, Tae-Uk Kang, Ju-Hyun Yoo, Yong-ho Lee, Jin-Woo Park
Biosensors and Bioelectronics.2024; 257: 116297. CrossRef - Cardiorenal outcomes and mortality after sodium‐glucose cotransporter‐2 inhibitor initiation in type 2 diabetes patients with percutaneous coronary intervention history
Jin Hwa Kim, Young Sang Lyu, BongSeong Kim, Mee Kyung Kim, Sang Yong Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Recommendations for Pharmacological Treatment of Type 2 Diabetes
Junghyun Noh
The Journal of Korean Diabetes.2023; 24(3): 127. CrossRef - 2023 Clinical Practice Guidelines for Diabetes
Min Kyong Moon
The Journal of Korean Diabetes.2023; 24(3): 120. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk Factors
Ye Seul Yang
The Journal of Korean Diabetes.2023; 24(3): 135. CrossRef - A 33-Year-Old Man Presented with Abdominal Pain and Vomiting Starting a Day Ago
Jong Han Choi
The Korean Journal of Medicine.2023; 98(6): 289. CrossRef - Comorbidity Patterns and Management in Inpatients with Endocrine Diseases by Age Groups in South Korea: Nationwide Data
Sung-Soo Kim, Hun-Sung Kim
Journal of Personalized Medicine.2023; 14(1): 42. CrossRef
- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
- Lifestyle
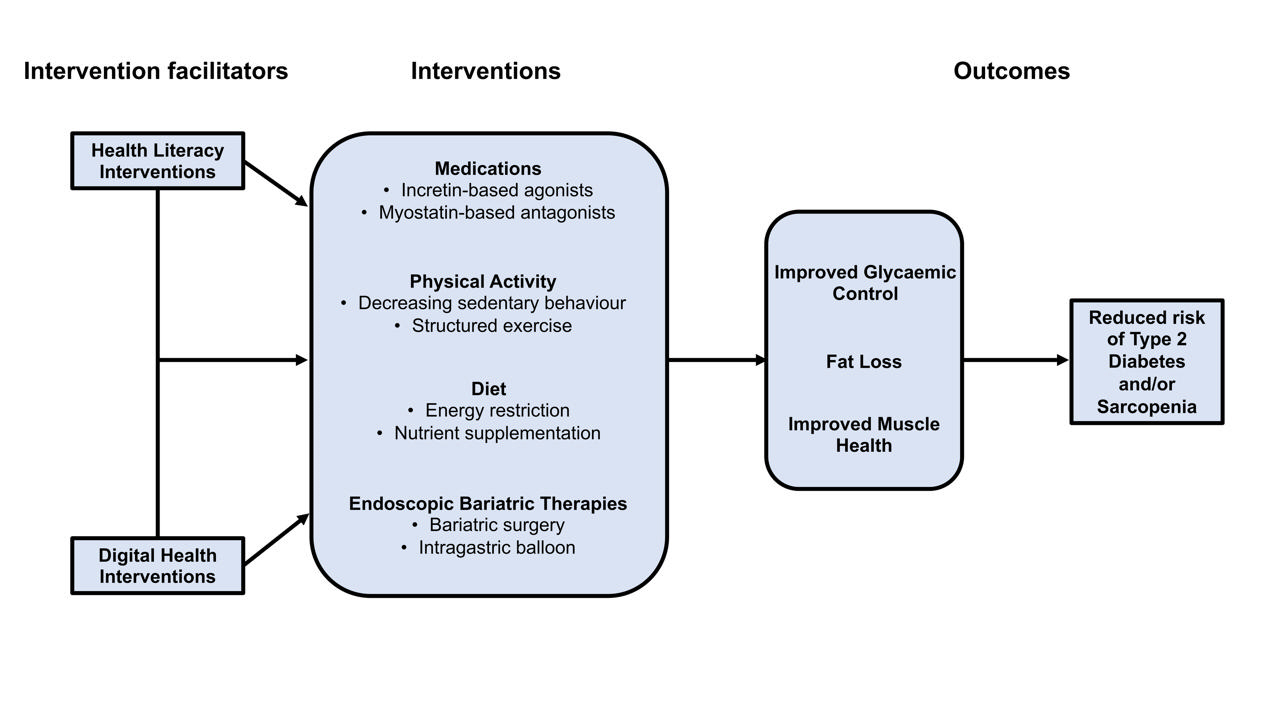
- Type 2 Diabetes Mellitus and Sarcopenia as Comorbid Chronic Diseases in Older Adults: Established and Emerging Treatments and Therapies
- Jakub Mesinovic, Jackson J. Fyfe, Jason Talevski, Michael J. Wheeler, Gloria K.W. Leung, Elena S. George, Melkamu T. Hunegnaw, Costas Glavas, Paul Jansons, Robin M. Daly, David Scott
- Diabetes Metab J. 2023;47(6):719-742. Published online September 14, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0112
- 4,547 View
- 438 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Type 2 diabetes mellitus (T2DM) and sarcopenia (low skeletal muscle mass and function) share a bidirectional relationship. The prevalence of these diseases increases with age and they share common risk factors. Skeletal muscle fat infiltration, commonly referred to as myosteatosis, may be a major contributor to both T2DM and sarcopenia in older adults via independent effects on insulin resistance and muscle health. Many strategies to manage T2DM result in energy restriction and subsequent weight loss, and this can lead to significant declines in muscle mass in the absence of resistance exercise, which is also a first-line treatment for sarcopenia. In this review, we highlight recent evidence on established treatments and emerging therapies targeting weight loss and muscle mass and function improvements in older adults with, or at risk of, T2DM and/or sarcopenia. This includes dietary, physical activity and exercise interventions, new generation incretin-based agonists and myostatin-based antagonists, and endoscopic bariatric therapies. We also highlight how digital health technologies and health literacy interventions can increase uptake of, and adherence to, established and emerging treatments and therapies in older adults with T2DM and/or sarcopenia.
-
Citations
Citations to this article as recorded by- Fucoidan ameliorates diabetic skeletal muscle atrophy through PI3K/Akt pathway
Caixia Li, Yaping Liu, Mingzhi Yang, Haoyue Huang, Lulu Tang, Yufan Miao, Wenjie Li, Xing Li
Journal of Functional Foods.2024; 114: 106076. CrossRef
- Fucoidan ameliorates diabetic skeletal muscle atrophy through PI3K/Akt pathway
- Complications
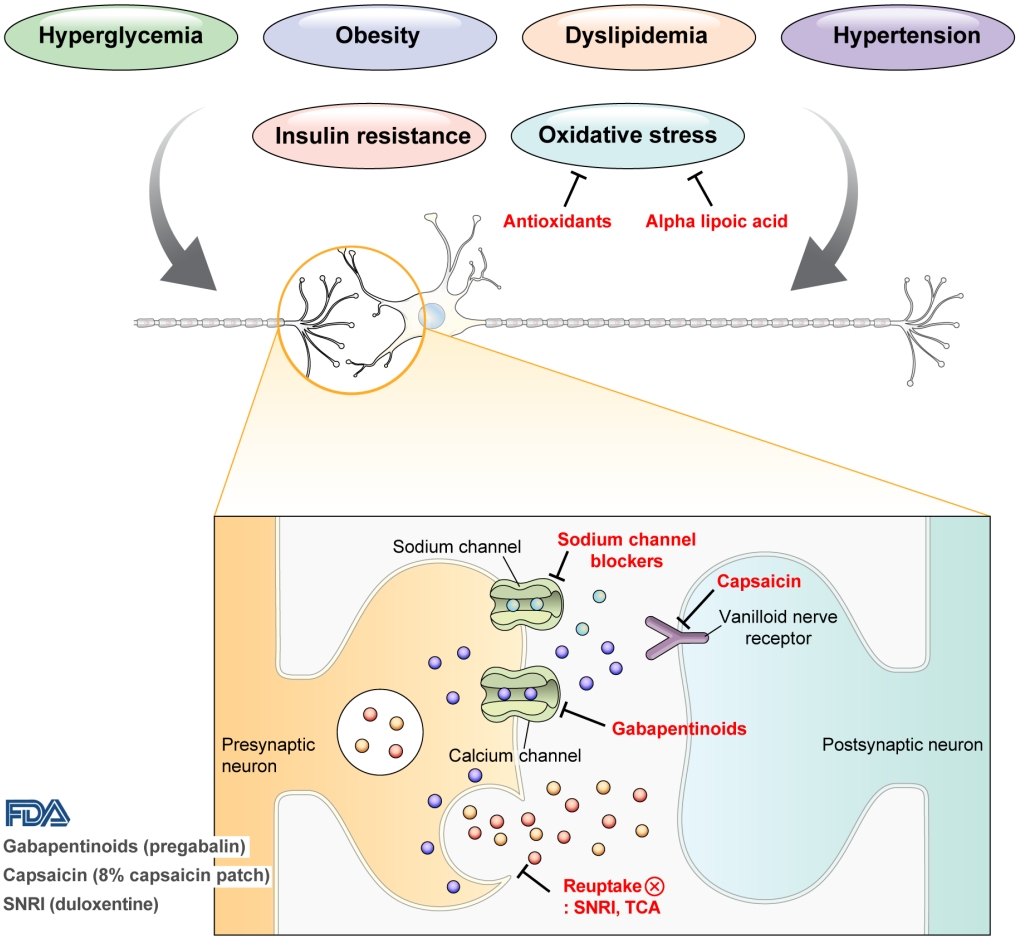
- Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy
- Han Na Jang, Tae Jung Oh
- Diabetes Metab J. 2023;47(6):743-756. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0018
- 3,907 View
- 546 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes. The lifetime prevalence of DPN is thought to be >50%, and 15%–25% of patients with diabetes experience neuropathic pain, referred to as “painful DPN.” Appropriate treatment of painful DPN is important because this pain contributes to a poor quality of life by causing sleep disturbance, anxiety, and depression. The basic principle for the management of painful DPN is to control hyperglycemia and other modifiable risk factors, but these may be insufficient for preventing or improving DPN. Because there is no promising diseasemodifying medication for DPN, the pain itself needs to be managed when treating painful DPN. Drugs for neuropathic pain, such as gabapentinoids, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, alpha-lipoic acid, sodium channel blockers, and topical capsaicin, are used for the management of painful DPN. The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, tapentadol, and the 8% capsaicin patch as drugs for the treatment of painful DPN. Recently, spinal cord stimulation using electrical stimulation is approved by the FDA for the treatment for painful DPN. This review describes the currently available pharmacological and nonpharmacological treatments for painful DPN.
-
Citations
Citations to this article as recorded by- J-2156, a small molecule somatostatin type 4 receptor agonist, alleviated hindpaw hypersensitivity in the streptozotocin-induced rat model of painful diabetic neuropathy but with a 2-fold decrease in potency at an advanced stage in the model, mimicking mo
A. Kuo, M. Z. Imam, R. Li, L. Lin, A. Raboczyj, A. E. Bohmer, J. R. Nicholson, L. Corradini, M. T. Smith
Frontiers in Pharmacology.2024;[Epub] CrossRef - The Chronic Wound–Related Pain Model
Kevin Woo
Clinics in Geriatric Medicine.2024;[Epub] CrossRef
- J-2156, a small molecule somatostatin type 4 receptor agonist, alleviated hindpaw hypersensitivity in the streptozotocin-induced rat model of painful diabetic neuropathy but with a 2-fold decrease in potency at an advanced stage in the model, mimicking mo
- Cardiovascular Risk/Epidemiology
- The Role of Echocardiography in Evaluating Cardiovascular Diseases in Patients with Diabetes Mellitus
- Sun Hwa Lee, Jae-Hyeong Park
- Diabetes Metab J. 2023;47(4):470-483. Published online July 27, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0036

- 2,721 View
- 293 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Patients with diabetes mellitus are highly susceptible to cardiovascular complications, which are directly correlated with cardiovascular morbidity and mortality. In addition to coronary artery disease, there is growing awareness of the risk and prevalence of heart failure (HF) in patients with diabetes. Echocardiography is an essential diagnostic modality commonly performed in patients with symptoms suggestive of cardiovascular diseases (CVD), such as dyspnea or chest pain, to establish or rule out the cause of symptoms. Conventional echocardiographic parameters, such as left ventricular ejection fraction, are helpful not only for diagnosing CVD but also for determining severity, treatment strategy, prognosis, and response to treatment. Echocardiographic myocardial strain, a novel echocardiographic technique, enables the detection of early changes in ventricular dysfunction before HF symptoms develop. This article aims to review the role of echocardiography in evaluating CVD in patients with diabetes mellitus and how to use it in patients with suspected cardiac diseases.
-
Citations
Citations to this article as recorded by- Increased Blood Pressure Variability Over a 16-Year Period Is Associated With Left Ventricular Diastolic Dysfunction in a Population-Based Cohort
Jae-Hyeong Park, Soon-Ki Ahn, Goo-Yeong Cho, Ki-Chul Sung, Seung Ku Lee, Seong Hwan Kim, Chol Shin
American Journal of Hypertension.2024; 37(3): 168. CrossRef - Biomarkers and subclinical left ventricular dysfunction in patients with type 2 diabetes without clinical manifestations of cardiovascular diseases
T. G. Utina, D. U. Akasheva, D. V. Korsunsky, O. N. Dzhioeva, O. M. Drapkina
Cardiovascular Therapy and Prevention.2024; 23(1): 3914. CrossRef - Cardiovascular risk assessment in inflammatory bowel disease with coronary calcium score
Waqar Arif Rasool Chaudhry, Muhammad Ashfaq, Parvinder Kaur, Mahendra Kumar, Maria Faraz, Jahanzeb Malik, Amin Mehmoodi
Annals of Medicine & Surgery.2024; 86(3): 1496. CrossRef
- Increased Blood Pressure Variability Over a 16-Year Period Is Associated With Left Ventricular Diastolic Dysfunction in a Population-Based Cohort

 KDA
KDA
 First
First Prev
Prev





