
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Metabolic Risk/Epidemiology
- A Prospective 1-Year Follow-Up of Glycemic Status and C-Peptide Levels of COVID-19 Survivors with Dysglycemia in Acute COVID-19 Infection
- David Tak Wai Lui, Chi Ho Lee, Ying Wong, Carol Ho Yi Fong, Kimberly Hang Tsoi, Yu Cho Woo, Kathryn Choon Beng Tan
- Received June 5, 2023 Accepted October 13, 2023 Published online March 11, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0175 [Epub ahead of print]

- 772 View
- 35 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
We evaluated changes in glycemic status, over 1 year, of coronavirus disease 2019 (COVID-19) survivors with dysglycemia in acute COVID-19.
Methods
COVID-19 survivors who had dysglycemia (defined by glycosylated hemoglobin [HbA1c] 5.7% to 6.4% or random glucose ≥10.0 mmol/L) in acute COVID-19 were recruited from a major COVID-19 treatment center from September to October 2020. Matched non-COVID controls were recruited from community. The 75-g oral glucose tolerance test (OGTT) were performed at baseline (6 weeks after acute COVID-19) and 1 year after acute COVID-19, with HbA1c, insulin and C-peptide measurements. Progression in glycemic status was defined by progression from normoglycemia to prediabetes/diabetes, or prediabetes to diabetes.
Results
Fifty-two COVID-19 survivors were recruited. Compared with non-COVID controls, they had higher C-peptide (P< 0.001) and trend towards higher homeostasis model assessment of insulin resistance (P=0.065). Forty-three COVID-19 survivors attended 1-year reassessment. HbA1c increased from 5.5%±0.3% to 5.7%±0.2% (P<0.001), with increases in glucose on OGTT at fasting (P=0.089), 30-minute (P=0.126), 1-hour (P=0.014), and 2-hour (P=0.165). At baseline, 19 subjects had normoglycemia, 23 had prediabetes, and one had diabetes. Over 1 year, 10 subjects (23.8%; of 42 non-diabetes subjects at baseline) had progression in glycemic status. C-peptide levels remained unchanged (P=0.835). Matsuda index decreased (P=0.007) and there was a trend of body mass index increase from 24.4±2.7 kg/m2 to 25.6±5.2 (P=0.083). Subjects with progression in glycemic status had more severe COVID-19 illness than non-progressors (P=0.030). Reassessment was not performed in the control group.
Conclusion
Subjects who had dysglycemia in acute COVID-19 were characterized by insulin resistance. Over 1 year, a quarter had progression in glycemic status, especially those with more severe COVID-19. Importantly, there was no significant deterioration in insulin secretory capacity.
- Pathophysiology
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Seoil Moon, Hye Seung Jung
- Diabetes Metab J. 2022;46(4):533-542. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0070
- 4,563 View
- 250 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
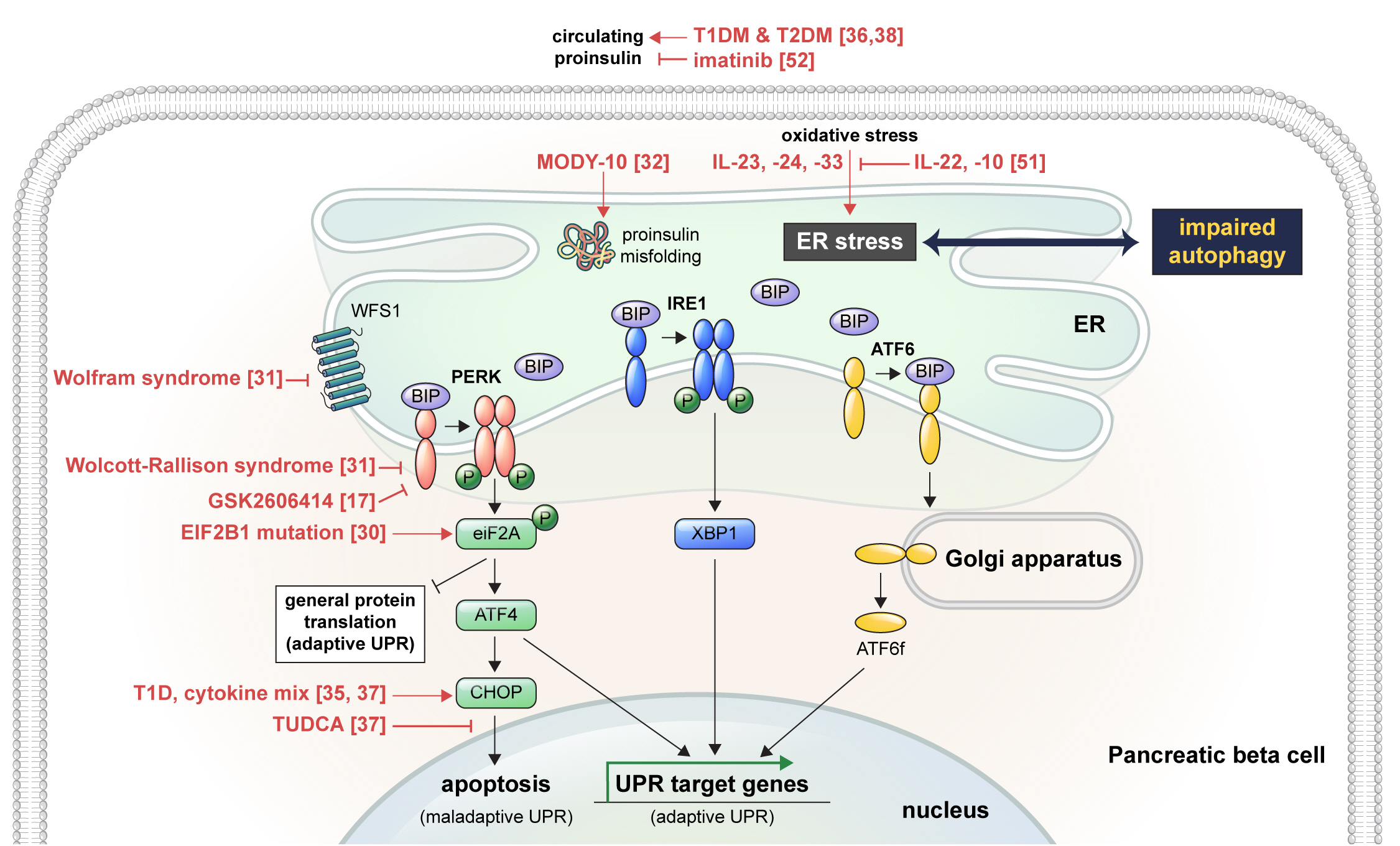
ePub - Pancreatic beta cell homeostasis is crucial for the synthesis and secretion of insulin; disruption of homeostasis causes diabetes, and is a treatment target. Adaptation to endoplasmic reticulum (ER) stress through the unfolded protein response (UPR) and adequate regulation of autophagy, which are closely linked, play essential roles in this homeostasis. In diabetes, the UPR and autophagy are dysregulated, which leads to beta cell failure and death. Various studies have explored methods to preserve pancreatic beta cell function and mass by relieving ER stress and regulating autophagic activity. To promote clinical translation of these research results to potential therapeutics for diabetes, we summarize the current knowledge on ER stress and autophagy in human insulin-secreting cells.
-
Citations
Citations to this article as recorded by- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
Diabetes & Metabolism Journal.2024; 48(2): 231. CrossRef - Endoplasmic reticulum stress: A possible connection between intestinal inflammation and neurodegenerative disorders
Giorgio Vivacqua, Romina Mancinelli, Stefano Leone, Rosa Vaccaro, Ludovica Garro, Simone Carotti, Ludovica Ceci, Paolo Onori, Luigi Pannarale, Antonio Franchitto, Eugenio Gaudio, Arianna Casini
Neurogastroenterology & Motility.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Pancreatic islet remodeling in cotadutide-treated obese mice
Renata Spezani, Thatiany Souza Marinho, Luiz E. Macedo Cardoso, Marcia Barbosa Aguila, Carlos Alberto Mandarim-de-Lacerda
Life Sciences.2023; 327: 121858. CrossRef - Modulation of Unfolded Protein Response Restores Survival and Function of β-Cells Exposed to the Endocrine Disruptor Bisphenol A
Laura Maria Daian, Gabriela Tanko, Andrei Mircea Vacaru, Luiza Ghila, Simona Chera, Ana-Maria Vacaru
International Journal of Molecular Sciences.2023; 24(3): 2023. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Identification and analysis of type 2 diabetes-mellitus-associated autophagy-related genes
Kun Cui, Zhizheng Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between autophagy and insulin resistance: evidence from different tissues
Asie Sadeghi, Maryam Niknam, Mohammad Amin Momeni-Moghaddam, Maryam Shabani, Hamid Aria, Alireza Bastin, Maryam Teimouri, Reza Meshkani, Hamed Akbari
European Journal of Medical Research.2023;[Epub] CrossRef - Beta cell lipotoxicity in the development of type 2 diabetes: the need for species-specific understanding
Patricia Thomas, Meurig T. Gallagher, Gabriela Da Silva Xavier
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Basic Research
- DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice
- Youjin Kim, Si Woo Lee, Hyejin Wang, Ryeong-Hyeon Kim, Hyun Ki Park, Hangkyu Lee, Eun Seok Kang
- Diabetes Metab J. 2022;46(2):337-348. Published online January 21, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0056
- 5,552 View
- 276 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We investigated the antidiabetic effects of DA-1241, a novel G protein-coupled receptor (GPR) 119 agonist, in vitro and in vivo.
Methods
DA-1241 was administrated to high-fat diet (HFD)-fed C57BL/6J mice for 12 weeks after hyperglycaemia developed. Oral/intraperitoneal glucose tolerance test and insulin tolerance test were performed. Serum insulin and glucagon-like peptide-1 (GLP-1) levels were measured during oral glucose tolerance test. Insulinoma cell line (INS-1E) cells and mouse islets were used to find whether DA-1241 directly stimulate insulin secretion in beta cell. HepG2 cells were used to evaluate the gluconeogenesis and autophagic process. Autophagic flux was evaluated by transfecting microtubule-associated protein 1 light chain 3-fused to green fluorescent protein and monomeric red fluorescent (mRFP-GFP-LC3) expression vector to HepG2 cells.
Results
Although DA-1241 treatment did not affect body weight gain and amount of food intake, fasting blood glucose level decreased along with increase in GLP-1 level. DA-1241 improved only oral glucose tolerance test and showed no effect in intraperitoneal glucose tolerance test. No significant effect was observed in insulin tolerance test. DA-1241 did not increase insulin secretion in INS-1E cell and mouse islets. DA-1241 reduced triglyceride content in the liver thereby improved fatty liver. Additionally, DA-1241 reduced gluconeogenic enzyme expression in HepG2 cells and mouse liver. DA-1241 reduced autophagic flow in HepG2 cells.
Conclusion
These findings suggested that DA-1241 augmented glucose-dependent insulin release via stimulation of GLP-1 secretion, and reduced hepatic gluconeogenesis, which might be associated with autophagic blockage, leading to improved glycaemic control. -
Citations
Citations to this article as recorded by- G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype?
Mohan Patil, Ilaria Casari, Leon N. Warne, Marco Falasca
Biomedicine & Pharmacotherapy.2024; 172: 116245. CrossRef - GPR119 agonists for type 2 diabetes: past failures and future hopes for preclinical and early phase candidates
Deanne H Hryciw, Rhiannon K Patten, Raymond J Rodgers, Joseph Proietto, Dana S Hutchinson, Andrew J McAinch
Expert Opinion on Investigational Drugs.2024; 33(3): 183. CrossRef - Immunomodulation through Nutrition Should Be a Key Trend in Type 2 Diabetes Treatment
Katarzyna Napiórkowska-Baran, Paweł Treichel, Marta Czarnowska, Magdalena Drozd, Kinga Koperska, Agata Węglarz, Oskar Schmidt, Samira Darwish, Bartłomiej Szymczak, Zbigniew Bartuzi
International Journal of Molecular Sciences.2024; 25(7): 3769. CrossRef - Discovery of orally active sulfonylphenyl thieno[3,2-d]pyrimidine derivatives as GPR119 agonists
Heecheol Kim, Minjung Kim, Kyujin Oh, Sohee Lee, Sunyoung Lim, Sangdon Lee, Young Hoon Kim, Kwee Hyun Suh, Kyung Hoon Min
European Journal of Medicinal Chemistry.2023; 258: 115584. CrossRef - Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis
Hye Jin Chun, Eun Ran Kim, Minyoung Lee, Da Hyun Choi, Soo Hyun Kim, Eugene Shin, Jin-Hong Kim, Jin Won Cho, Dai Hoon Han, Bong-Soo Cha, Yong-ho Lee
Metabolism.2023; 145: 155612. CrossRef - Human skin stem cell-derived hepatic cells as in vitro drug discovery model for insulin-driven de novo lipogenesis
Karolien Buyl, Martine Vrints, Ruani Fernando, Terry Desmae, Thomas Van Eeckhoutte, Mia Jans, Jan Van Der Schueren, Joost Boeckmans, Robim M. Rodrigues, Veerle De Boe, Vera Rogiers, Joery De Kock, Filip Beirinckx, Tamara Vanhaecke
European Journal of Pharmacology.2023; 957: 175989. CrossRef - GPR119 activation by DA-1241 alleviates hepatic and systemic inflammation in MASH mice through inhibition of NFκB signaling
Seung-Ho Lee, Hansu Park, Eun-Kyoung Yang, Bo Ram Lee, Il-Hoon Jung, Tae-Hyoung Kim, Moon Jung Goo, Yuna Chae, Mi-Kyung Kim
Biomedicine & Pharmacotherapy.2023; 166: 115345. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice
Youjin Kim, Si Woo Lee, Hyejin Wang, Ryeong-Hyeon Kim, Hyun Ki Park, Hangkyu Lee, Eun Seok Kang
Diabetes & Metabolism Journal.2022; 46(2): 337. CrossRef - Autophagy Dysregulation in Metabolic Associated Fatty Liver Disease: A New Therapeutic Target
Chun-Liang Chen, Yu-Cheng Lin
International Journal of Molecular Sciences.2022; 23(17): 10055. CrossRef
- G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype?
- Technology/Device
- Assessment of Insulin Secretion and Insulin Resistance in Human
- So Young Park, Jean-François Gautier, Suk Chon
- Diabetes Metab J. 2021;45(5):641-654. Published online September 30, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0220

- 14,265 View
- 887 Download
- 47 Web of Science
- 23 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- The impaired insulin secretion and increased insulin resistance (or decreased insulin sensitivity) play a major role in the pathogenesis of all types of diabetes mellitus (DM). It is very important to assess the pancreatic β-cell function and insulin resistance/ sensitivity to determine the type of DM and to plan an optimal management and prevention strategy for DM. So far, various methods and indices have been developed to assess the β-cell function and insulin resistance/sensitivity based on static, dynamic test and calculation of their results. In fact, since the metabolism of glucose and insulin is made through a complex process related with various stimuli in several tissues, it is difficult to fully reflect the real physiology. In order to solve the theoretical and practical difficulties, research on new index is still in progress. Also, it is important to select the appropriate method and index for the purpose of use and clinical situation. This review summarized a variety of traditional methods and indices to evaluate pancreatic β-cell function and insulin resistance/sensitivity and introduced novel indices.
-
Citations
Citations to this article as recorded by- Polyphenol-Rich Extract of Fermented Chili Pepper Alleviates Insulin Resistance in HepG2 Cells via Regulating INSR, PTP1B, PPAR-γ, and AMPK Pathways
Tao Wang, Meiqi Li, Shengbao Cai, Linyan Zhou, Xiaosong Hu, Junjie Yi
Fermentation.2023; 9(2): 84. CrossRef - 2,3-Dihydrosorbicillin and chrysopanol stimulate insulin secretion in INS-1 cells
Dahae Lee, Jaekyung Kim, Sungyoul Choi, Jinwon Choi, Jin Woo Lee, Ki Sung Kang, Sang Hee Shim
Bioorganic & Medicinal Chemistry Letters.2023; 83: 129186. CrossRef - Insulin: A connection between pancreatic β cells and the hypothalamus
Brenda De la Cruz Concepción, Yaccil Adilene Flores Cortez, Martha Isela Barragán Bonilla, Juan Miguel Mendoza Bello, Monica Espinoza Rojo
World Journal of Diabetes.2023; 14(2): 76. CrossRef - The Metabolic Score for Insulin Resistance (METS-IR) Predicts Cardiovascular Disease and Its Subtypes in Patients with Hypertension and Obstructive Sleep Apnea
Wenbo Yang, Xintian Cai, Junli Hu, Wen Wen, Heizhati Mulalibieke, Xiaoguang Yao, Ling Yao, Qing Zhu, Jing Hong, Qin Luo, Shasha Liu, Nanfang Li
Clinical Epidemiology.2023; Volume 15: 177. CrossRef - Association between dietary patterns and biomarkers in connection with diabetes mellitus in adolescents: A systematic review
Bernardo Paz Barboza, Camila Tureck, Liliana Paula Bricarello, Mariane de Almeida Alves, Anabelle Retondario, Amanda de Moura Souza, Ricardo Fernandes, Francisco de Assis Guedes de Vasconcelos
Nutrition, Metabolism and Cardiovascular Diseases.2023; 33(4): 685. CrossRef - PNPLA3 rs738409 risk genotype decouples TyG index from HOMA2-IR and intrahepatic lipid content
Ákos Nádasdi, Viktor Gál, Tamás Masszi, Anikó Somogyi, Gábor Firneisz
Cardiovascular Diabetology.2023;[Epub] CrossRef - Ethnic Variability in Glucose and Insulin Response to Rice Among Healthy Overweight Adults: A Randomized Cross-Over Study
Amena Sadiya, Vidya Jakapure, Vijay Kumar
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 993. CrossRef - Familial partial lipodystrophy type 2 and obesity, two adipose tissue pathologies with different inflammatory profiles
Guillaume Treiber, Marie-Paule Gonthier, Alice Guilleux, Samir Medjane, Oriane Bonfanti, Muriel Cogne, Olivier Meilhac, Estelle Nobecourt
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Discriminant Model for Insulin Resistance in Type 2 Diabetic Patients
Erislandis López-Galán, Rafael Barrio-Deler, Manuel Alejandro Fernández-Fernández, Yaquelin Del Toro-Delgado, Isaac Enrique Peñuela-Puente, Miguel Enrique Sánchez-Hechavarría, Mario Eugenio Muñoz-Bustos, Gustavo Alejandro Muñoz-Bustos
Medicina.2023; 59(5): 839. CrossRef - Mechanisms of Oxidative Stress in Metabolic Syndrome
Sepiso K. Masenga, Lombe S. Kabwe, Martin Chakulya, Annet Kirabo
International Journal of Molecular Sciences.2023; 24(9): 7898. CrossRef - Estimated Glucose Disposal Rate Predicts Renal Progression in Type 2 Diabetes Mellitus: A Retrospective Cohort Study
Juan Peng, Aimei Li, Liangqingqing Yin, Qi Yang, Jinting Pan, Bin Yi
Journal of the Endocrine Society.2023;[Epub] CrossRef - Transfer RNA Mutation Associated with Type 2 Diabetes Mellitus
Fanny Rizki Rahmadanthi, Iman Permana Maksum
Biology.2023; 12(6): 871. CrossRef - Honokiol improves endothelial function in type 2 diabetic rats via alleviating oxidative stress and insulin resistance
An He, Huilin Yu, Yu Hu, Huiling Chen, Xiang Li, Jian Shen, Rongjuan Zhuang, Yi Chen, Bryan Richard Sasmita, Minghao Luo, Dingyi Lv
Biochemical and Biophysical Research Communications.2022; 600: 109. CrossRef - Relationship of Vitamin A and Thyroid Function in Individuals With Obesity and After Laparoscopic Sleeve Gastrectomy
Bingwei Ma, Peng Yang, Jingyang Gao, Lei Du, Chunjun Sheng, Taofeek Usman, Xingchun Wang, Shen Qu
Frontiers in Nutrition.2022;[Epub] CrossRef - Efficacy of Personalized Diabetes Self-care Using an Electronic Medical Record–Integrated Mobile App in Patients With Type 2 Diabetes: 6-Month Randomized Controlled Trial
Eun Young Lee, Seon-Ah Cha, Jae-Seung Yun, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, Min Kyung Hyun, Seung-Hyun Ko
Journal of Medical Internet Research.2022; 24(7): e37430. CrossRef - Acyclovir alleviates insulin resistance via activating PKM1 in diabetic mice
Zhuozhou Hu, Jing Zhou, Liang Han, Xiangxiang Li, Chun Li, Tongyu Wu, Jingjing Liu, Wenyang Zhao, Jia Kang, Xinping Chen
Life Sciences.2022; 304: 120725. CrossRef - Identifying Glucose Metabolism Status in Nondiabetic Japanese Adults Using Machine Learning Model with Simple Questionnaire
Tomoki Uchida, Takeshi Kanamori, Takanori Teramoto, Yuji Nonaka, Hiroki Tanaka, Satoshi Nakamura, Norihito Murayama, Rajesh Kaluri
Computational and Mathematical Methods in Medicine.2022; 2022: 1. CrossRef - Elevated triglyceride-glucose (TyG) index predicts impaired islet β-cell function: A hospital-based cross-sectional study
Zi Chen, Jie Wen
Frontiers in Endocrinology.2022;[Epub] CrossRef - Maternal Glycaemic and Insulinemic Status and Newborn DNA Methylation: Findings in Women With Overweight and Obesity
Marion Lecorguillé, Fionnuala M McAuliffe, Patrick J Twomey, Karien Viljoen, John Mehegan, Cecily C Kelleher, Matthew Suderman, Catherine M Phillips
The Journal of Clinical Endocrinology & Metabolism.2022; 108(1): 85. CrossRef - Acanthosis Nigricans: Pointer of Endocrine Entities
Andreea-Maria Radu, Mara Carsote, Mihai Cristian Dumitrascu, Florica Sandru
Diagnostics.2022; 12(10): 2519. CrossRef - Relationship between Insulin Resistance Risk Scales and Non-Alcoholic Fatty Liver Disease and Liver Fibrosis Scales in 219,477 Spanish Workers
José Ignacio Ramírez-Manent, Emilio Martínez-Almoyna, Carlos López, Carla Busquets-Cortés, Hilda González San Miguel, Ángel Arturo López-González
Metabolites.2022; 12(11): 1093. CrossRef - Investigation of Insulin Secretion in Glucose Tolerance Test by the Intake of Novel Imeglimin (Twymeeg)
Hiroshi BANDO, Hiroko OGAWA, Hirohisa URASAKI, Shinji NAGAHIRO, Hiroko URASAKI, Miwako NAKANISHI, Osami WATANABE
Asploro Journal of Biomedical and Clinical Case Reports.2022; 5(3): 113. CrossRef - Evaluation and Screening of Hypoglycemic Activity of Total Ginsenosides GBE-5 Fraction From Panax Ginseng Berry Based on UHPLC–MS Metabolomics
Heyu Wang, Yu Tong, Anqi Wang, Ying Li, Bofan Lu, Hui Li, Lili Jiao, Wei Wu
Frontiers in Nutrition.2022;[Epub] CrossRef
- Polyphenol-Rich Extract of Fermented Chili Pepper Alleviates Insulin Resistance in HepG2 Cells via Regulating INSR, PTP1B, PPAR-γ, and AMPK Pathways
- Basic Research
- Notch1 Has an Important Role in β-Cell Mass Determination and Development of Diabetes
- Young Sil Eom, A-Ryeong Gwon, Kyung Min Kwak, Jin-Young Youn, Heekyoung Park, Kwang-Won Kim, Byung-Joon Kim
- Diabetes Metab J. 2021;45(1):86-96. Published online February 26, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0160
- 6,346 View
- 185 Download
- 7 Web of Science
- 6 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
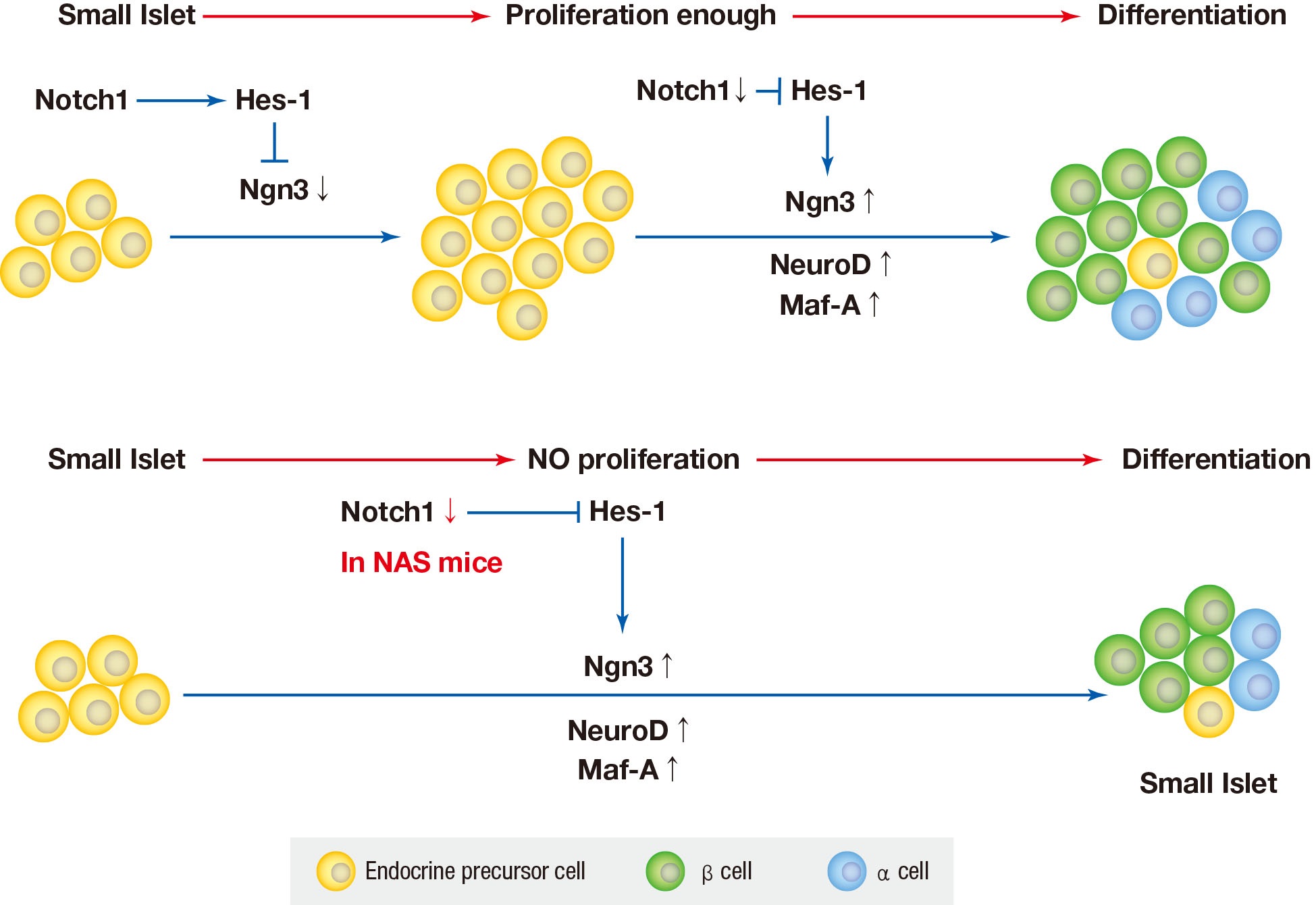
Background Notch signaling pathway plays an important role in regulating pancreatic endocrine and exocrine cell fate during pancreas development. Notch signaling is also expressed in adult pancreas. There are few studies on the effect of Notch on adult pancreas. Here, we investigated the role of Notch in islet mass and glucose homeostasis in adult pancreas using Notch1 antisense transgenic (NAS).
Methods Western blot analysis was performed for the liver of 8-week-old male NAS mice. We also conducted an intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test in 8-week-old male NAS mice and male C57BL/6 mice (control). Morphologic observation of pancreatic islet and β-cell was conducted in two groups. Insulin secretion capacity in islets was measured by glucose-stimulated insulin secretion (GSIS) and perifusion.
Results NAS mice showed higher glucose levels and lower insulin secretion in IPGTT than the control mice. There was no significant difference in insulin resistance. Total islet and β-cell masses were decreased in NAS mice. The number of large islets (≥250 µm) decreased while that of small islets (<250 µm) increased. Reduced insulin secretion was observed in GSIS and perifusion. Neurogenin3, neurogenic differentiation, and MAF bZIP transcription factor A levels increased in NAS mice.
Conclusion Our study provides that Notch1 inhibition decreased insulin secretion and decreased islet and β-cell masses. It is thought that Notch1 inhibition suppresses islet proliferation and induces differentiation of small islets. In conclusion, Notch signaling pathway may play an important role in β-cell mass determination and diabetes.
-
Citations
Citations to this article as recorded by- N6-methylation of RNA-bound adenosine regulator HNRNPC promotes vascular endothelial dysfunction in type 2 diabetes mellitus by activating the PSEN1-mediated Notch pathway
Ying Cai, Tao Chen, Mingzhu Wang, Lihua Deng, Cui Li, Siqian Fu, Kangling Xie
Diabetes Research and Clinical Practice.2023; 197: 110261. CrossRef - Single‐cell RNA sequencing: Inhibited Notch2 signalling underlying the increased lens fibre cells differentiation in high myopia
Yunqian Yao, Ling Wei, Zhenhua Chen, Hao Li, Jiao Qi, Qingfeng Wu, Xingtao Zhou, Yi Lu, Xiangjia Zhu
Cell Proliferation.2023;[Epub] CrossRef - Micro ribonucleic acid‐363 regulates the phosphatidylinositol 3‐kinase/threonine protein kinase axis by targeting NOTCH1 and forkhead box C2, leading to hepatic glucose and lipids metabolism disorder in type 2 diabetes mellitus
Yu‐Huan Peng, Ping Wang, Xiao‐Qun He, Ming‐Zhao Hong, Feng Liu
Journal of Diabetes Investigation.2022; 13(2): 236. CrossRef - Soluble T-cadherin promotes pancreatic β-cell proliferation by upregulating Notch signaling
Tomonori Okita, Shunbun Kita, Shiro Fukuda, Keita Fukuoka, Emi Kawada-Horitani, Masahito Iioka, Yuto Nakamura, Yuya Fujishima, Hitoshi Nishizawa, Dan Kawamori, Taka-aki Matsuoka, Maeda Norikazu, Iichiro Shimomura
iScience.2022; 25(11): 105404. CrossRef - Comparison of islet isolation result and clinical applicability according to GMP‐grade collagenase enzyme blend in adult porcine islet isolation and culture
Kyungmin Kwak, Jae‐kyung Park, Joohyun Shim, Nayoung Ko, Hyoung‐Joo Kim, Yongjin Lee, Jun‐Hyeong Kim, Michael Alexander, Jonathan R. T. Lakey, Hyunil Kim, Kimyung Choi
Xenotransplantation.2021;[Epub] CrossRef - Genome-Wide Meta-analysis Identifies Genetic Variants Associated With Glycemic Response to Sulfonylureas
Adem Y. Dawed, Sook Wah Yee, Kaixin Zhou, Nienke van Leeuwen, Yanfei Zhang, Moneeza K. Siddiqui, Amy Etheridge, Federico Innocenti, Fei Xu, Josephine H. Li, Joline W. Beulens, Amber A. van der Heijden, Roderick C. Slieker, Yu-Chuan Chang, Josep M. Mercade
Diabetes Care.2021; 44(12): 2673. CrossRef
- N6-methylation of RNA-bound adenosine regulator HNRNPC promotes vascular endothelial dysfunction in type 2 diabetes mellitus by activating the PSEN1-mediated Notch pathway
- Clinical Diabetes & Therapeutics
- Early Assessment of the Risk for Gestational Diabetes Mellitus: Can Fasting Parameters of Glucose Metabolism Contribute to Risk Prediction?
- Veronica Falcone, Grammata Kotzaeridi, Melanie Hanne Breil, Ingo Rosicky, Tina Stopp, Gülen Yerlikaya-Schatten, Michael Feichtinger, Wolfgang Eppel, Peter Husslein, Andrea Tura, Christian S. Göbl
- Diabetes Metab J. 2019;43(6):785-793. Published online March 12, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0218
- 8,037 View
- 80 Download
- 20 Web of Science
- 24 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background An early identification of the risk groups might be beneficial in reducing morbidities in patients with gestational diabetes mellitus (GDM). Therefore, this study aimed to assess the biochemical predictors of glycemic conditions, in addition to fasting indices of glucose disposal, to predict the development of GDM in later stage and the need of glucose-lowering medication.
Methods A total of 574 pregnant females (103 with GDM and 471 with normal glucose tolerance [NGT]) were included. A metabolic characterization was performed before 15+6 weeks of gestation by assessing fasting plasma glucose (FPG), fasting insulin (FI), fasting C-peptide (FCP), and glycosylated hemoglobin (HbA1c). Thereafter, the patients were followed-up until the delivery.
Results Females with NGT had lower levels of FPG, FI, FCP, or HbA1c at the early stage of pregnancy, and therefore, showed an improved insulin action as compared to that in females who developed GDM. Higher fasting levels of FPG and FCP were associated with a higher risk of developing GDM. Moreover, the predictive accuracy of this metabolic profiling was also good to distinguish the patients who required glucose-lowering medications. Indices of glucose disposal based on C-peptide improved the predictive accuracy compared to that based on insulin. A modified quantitative insulin sensitivity check index (QUICKIc) showed the best differentiation in terms of predicting GDM (area under the receiver operating characteristics curve [ROC-AUC], 72.1%) or need for pharmacotherapy (ROC-AUC, 83.7%).
Conclusion Fasting measurements of glucose and C-peptide as well as the surrogate indices of glycemic condition could be used for stratifying pregnant females with higher risk of GDM at the beginning of pregnancy.
-
Citations
Citations to this article as recorded by- Maternal fasting serum C-peptide concentrations in the first and second trimesters and subsequent risk of gestational diabetes mellitus: A nested case-control study among Chinese women
Chuanyu Zhao, Haiyan Liu, Yuzhi Deng, Hanbin Wu, Shuo Wang, Xinyi Lyu, Jueming Lei, Haishan Yang, Meina Hu, Yinzhu Zhao, Xu Ma, Xiaoxuan Zou, Ying Yang
Diabetes Research and Clinical Practice.2024; 208: 111111. CrossRef - Future clinical prospects of C-peptide testing in the early diagnosis of gestational diabetes
Charalampos Milionis, Ioannis Ilias, Anastasia Lekkou, Evangelia Venaki, Eftychia Koukkou
World Journal of Experimental Medicine.2024;[Epub] CrossRef - Early prediction of gestational diabetes mellitus using maternal demographic and clinical risk factors
Yanqi Wu, Paul Hamelmann, Myrthe van der Ven, Sima Asvadi, M. Beatrijs van der Hout-van der Jagt, S. Guid Oei, Massimo Mischi, Jan Bergmans, Xi Long
BMC Research Notes.2024;[Epub] CrossRef - Gestationsdiabetes (GDM) (Update 2023)
Alexandra Kautzky-Willer, Yvonne Winhofer, Herbert Kiss, Veronica Falcone, Angelika Berger, Monika Lechleitner, Raimund Weitgasser, Jürgen Harreiter
Wiener klinische Wochenschrift.2023; 135(S1): 115. CrossRef - MIDO GDM: an innovative artificial intelligence-based prediction model for the development of gestational diabetes in Mexican women
Héctor Gallardo-Rincón, María Jesús Ríos-Blancas, Janinne Ortega-Montiel, Alejandra Montoya, Luis Alberto Martinez-Juarez, Julieta Lomelín-Gascón, Rodrigo Saucedo-Martínez, Ricardo Mújica-Rosales, Victoria Galicia-Hernández, Linda Morales-Juárez, Lucía Ma
Scientific Reports.2023;[Epub] CrossRef - Progress in the Prediction of Gestational Diabetes Mellitus by the Combined Detection of Fasting Blood Glucose and Hemoglobin in Early Pregnancy
欢欢 赵
Advances in Clinical Medicine.2023; 13(06): 9980. CrossRef - HOMA‐IR as a risk factor of gestational diabetes mellitus and a novel simple surrogate index in early pregnancy
Shuoning Song, Yuemei Zhang, Xiaolin Qiao, Yanbei Duo, Jiyu Xu, Zhenyao Peng, Jing Zhang, Yan Chen, Xiaorui Nie, Qiujin Sun, Xianchun Yang, Zechun Lu, Shixuan Liu, Tianyi Zhao, Tao Yuan, Yong Fu, Yingyue Dong, Weigang Zhao, Wei Sun, Ailing Wang
International Journal of Gynecology & Obstetrics.2022; 157(3): 694. CrossRef - The diagnostic value of glycosylated hemoglobin for gestational diabetes mellitus in Asian populations: A systematic review and meta‐analysis
Jiani Zhang, Fan Zhou, Tingting Xu, Jinfeng Xu, Yaqian Li, Li Lin, Qi Cao, Xiaodong Wang
Journal of Obstetrics and Gynaecology Research.2022; 48(4): 902. CrossRef - Postprandial Free Fatty Acids at Mid-Pregnancy Increase the Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes Mellitus
So-Yeon Kim, Young Shin Song, Soo-Kyung Kim, Yong-Wook Cho, Kyung-Soo Kim
Diabetes & Metabolism Journal.2022; 46(1): 140. CrossRef - Gestational Diabetes Mellitus in Pregnant Women with Beta-Thalassemia Minor: A Matched Case-Control Study
Veronica Falcone, Florian Heinzl, Bianca Karla Itariu, Theresa Reischer, Stephanie Springer, Dana Anaïs Muin, Petra Pateisky, Philipp Foessleitner, Johannes Ott, Alex Farr, Klara Rosta
Journal of Clinical Medicine.2022; 11(7): 2050. CrossRef - Maternal metabolic factors and the association with gestational diabetes: A systematic review and meta‐analysis
Nahal Habibi, Aya Mousa, Chau Thien Tay, Mahnaz Bahri Khomami, Rhiannon K. Patten, Prabha H. Andraweera, Molla Wassie, Jared Vandersluys, Ali Aflatounian, Tina Bianco‐Miotto, Shao J. Zhou, Jessica A. Grieger
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - Impact Of Prepregnancy Overweight And Obesity On Treatment Modality And Pregnancy Outcome In Women With Gestational Diabetes Mellitus
Tina Linder, Anna Eder, Cécile Monod, Ingo Rosicky, Daniel Eppel, Katharina Redling, Franziska Geissler, Evelyn A. Huhn, Irene Hösli, Christian S. Göbl
Frontiers in Endocrinology.2022;[Epub] CrossRef - Identification of potential gene markers in gestational diabetes mellitus
Weichun Tang, Xiaoyu Wang, Liping Chen, Yiling Lu, Xinyi Kang
Journal of Clinical Laboratory Analysis.2022;[Epub] CrossRef - Association between early-pregnancy serum C-peptide and risk of gestational diabetes mellitus: a nested case–control study among Chinese women
Xue Yang, Yi Ye, Yi Wang, Ping Wu, Qi Lu, Yan Liu, Jiaying Yuan, Xingyue Song, Shijiao Yan, Xiaorong Qi, Yi-Xin Wang, Ying Wen, Gang Liu, Chuanzhu Lv, Chun-Xia Yang, An Pan, Jianli Zhang, Xiong-Fei Pan
Nutrition & Metabolism.2022;[Epub] CrossRef - Early Predictors of Gestational Diabetes Mellitus in IVF-Conceived Pregnancies
Ayla Coussa, Hayder A. Hasan, Thomas M. Barber
Endocrine Practice.2021; 27(6): 579. CrossRef - The Clinical Characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Endocrinology and Metabolism.2021; 36(3): 628. CrossRef - Early Gestational Diabetes Mellitus: Diagnostic Strategies and Clinical Implications
Saptarshi Bhattacharya, Lakshmi Nagendra, Aishwarya Krishnamurthy, Om J. Lakhani, Nitin Kapoor, Bharti Kalra, Sanjay Kalra
Medical Sciences.2021; 9(4): 59. CrossRef - Early markers of gestational diabetes mellitus
Vedrana Ivić, Jasenka Wagner, Andrijana Müller, Lada Zibar, Marta Kadivnik, Barbara Viljetić, Jelena Omazić
Biochemia medica.2021; 31(3): 416. CrossRef - Response: Early Assessment of the Risk for Gestational Diabetes Mellitus: Can Fasting Parameters of Glucose Metabolism Contribute to Risk Prediction? (Diabetes Metab J 2019;43:785–93)
Christian S. Göbl, Andrea Tura
Diabetes & Metabolism Journal.2020; 44(1): 209. CrossRef - First-trimester fasting glycemia as a predictor of gestational diabetes (GDM) and adverse pregnancy outcomes
G. Sesmilo, P. Prats, S. Garcia, I. Rodríguez, A. Rodríguez-Melcón, I. Berges, B. Serra
Acta Diabetologica.2020; 57(6): 697. CrossRef - Letter: Early Assessment of the Risk for Gestational Diabetes Mellitus: Can Fasting Parameters of Glucose Metabolism Contribute to Risk Prediction? (Diabetes Metab J 2019;43:785–93)
Ye Seul Yang, Hye Seung Jung
Diabetes & Metabolism Journal.2020; 44(1): 199. CrossRef - Auch schon im 1. Trimenon ist Nüchternglukose Prädiktor für Gestationsdiabetes
Jens Stupin
Info Diabetologie.2020; 14(2): 14. CrossRef - Comparison of Machine Learning Methods and Conventional Logistic Regressions for Predicting Gestational Diabetes Using Routine Clinical Data: A Retrospective Cohort Study
Yunzhen Ye, Yu Xiong, Qiongjie Zhou, Jiangnan Wu, Xiaotian Li, Xirong Xiao
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Predictive Power of Unconjugated Estriol in Diagnosis of Gestational Diabetes: A Cohort Study
Azam Amirian, Nourossadat Kariman, Mehdi Hedayati, Nasrin Borumandnia, Zohre Sheikhan
Iranian Red Crescent Medical Journal.2019;[Epub] CrossRef
- Maternal fasting serum C-peptide concentrations in the first and second trimesters and subsequent risk of gestational diabetes mellitus: A nested case-control study among Chinese women
- Epidemiology
- Clinical Characteristics of People with Newly Diagnosed Type 2 Diabetes between 2015 and 2016: Difference by Age and Body Mass Index
- Kyoung Hwa Ha, Cheol Young Park, In Kyung Jeong, Hyun Jin Kim, Sang-Yong Kim, Won Jun Kim, Ji Sung Yoon, In Joo Kim, Dae Jung Kim, Sungrae Kim
- Diabetes Metab J. 2018;42(2):137-146. Published online February 14, 2018
- DOI: https://doi.org/10.4093/dmj.2018.42.2.137
- 5,375 View
- 80 Download
- 11 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background We evaluated the clinical characteristics of insulin resistance and β-cell dysfunction in newly diagnosed, drug-naive people with type 2 diabetes by analyzing nationwide cross-sectional data.
Methods We collected the clinical data of 912 participants with newly diagnosed diabetes from 83 primary care clinics and hospitals nationwide from 2015 to 2016. The presence of insulin resistance and β-cell dysfunction was defined as a homeostatic model assessment of insulin resistance (HOMA-IR) value ≥2.5 and fasting C-peptide levels <1.70 ng/mL, respectively.
Results A total of 75.1% and 22.6% of participants had insulin resistance and β-cell dysfunction, respectively. The proportion of participants with insulin resistance but no β-cell dysfunction increased, and the proportion of participants with β-cell dysfunction but no insulin resistance decreased as body mass index (BMI) increased. People diagnosed with diabetes before 40 years of age had significantly higher HOMA-IR and BMI than those diagnosed over 65 years of age (HOMA-IR, 5.0 vs. 3.0; BMI, 28.7 kg/m2 vs. 25.1 kg/m2). However, the β-cell function indices were lower in people diagnosed before 40 years of age than in those diagnosed after 65 years of age (homeostatic model assessment of β-cell function, 39.3 vs. 64.9; insulinogenic index, 10.3 vs. 18.7; disposition index, 0.15 vs. 0.25).
Conclusion We observed that the main pathogenic mechanism of type 2 diabetes is insulin resistance in participants with newly diagnosed type 2 diabetes. In addition, young adults with diabetes are more likely to have higher insulin resistance with obesity and have higher insulin secretory defect with severe hyperglycemia in the early period of diabetes than older populations.
-
Citations
Citations to this article as recorded by- A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - Apparent Insulin Deficiency in an Adult African Population With New-Onset Type 2 Diabetes
Davis Kibirige, Isaac Sekitoleko, Priscilla Balungi, William Lumu, Moffat J. Nyirenda
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Rising Incidence of Diabetes in Young Adults in South Korea: A National Cohort Study
Hyun Ho Choi, Giwoong Choi, Hojun Yoon, Kyoung Hwa Ha, Dae Jung Kim
Diabetes & Metabolism Journal.2022; 46(5): 803. CrossRef - A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Y
Diabetes & Metabolism Journal.2022; 46(6): 855. CrossRef - The Potential Effect of Rhizoma coptidis on Polycystic Ovary Syndrome Based on Network Pharmacology and Molecular Docking
Liyun Duan, De Jin, Xuedong An, Yuehong Zhang, Shenghui Zhao, Rongrong Zhou, Yingying Duan, Yuqing Zhang, Xinmin Liu, Fengmei Lian, Wen yi Kang
Evidence-Based Complementary and Alternative Medicine.2021; 2021: 1. CrossRef - PRKAA2variation and the clinical characteristics of patients newly diagnosed with type 2 diabetes mellitus in Yogyakarta, Indonesia
Dita Maria Virginia, Mae Sri Hartati Wahyuningsih, Dwi Aris Agung Nugrahaningsih
Asian Biomedicine.2021; 15(4): 161. CrossRef - Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study
Jeong Mi Kim, Sang Soo Kim, Jong Ho Kim, Mi Kyung Kim, Tae Nyun Kim, Soon Hee Lee, Chang Won Lee, Ja Young Park, Eun Sook Kim, Kwang Jae Lee, Young Sik Choi, Duk Kyu Kim, In Joo Kim
Diabetes & Metabolism Journal.2020; 44(1): 67. CrossRef - Favorable Glycemic Control with Once-Daily Insulin Degludec/Insulin Aspart after Changing from Basal Insulin in Adults with Type 2 Diabetes
Han Na Jang, Ye Seul Yang, Seong Ok Lee, Tae Jung Oh, Bo Kyung Koo, Hye Seung Jung
Endocrinology and Metabolism.2019; 34(4): 382. CrossRef - Insulin Resistance versus β-Cell Failure: Is It Changing in Koreans?
Mi-kyung Kim
Diabetes & Metabolism Journal.2018; 42(2): 128. CrossRef - Response: Clinical Characteristics of People with Newly Diagnosed Type 2 Diabetes between 2015 and 2016: Difference by Age and Body Mass Index (Diabetes Metab J2018;42:137-46)
Kyoung Hwa Ha, Dae Jung Kim, Sungrae Kim
Diabetes & Metabolism Journal.2018; 42(3): 251. CrossRef - Letter: Clinical Characteristics of People with Newly Diagnosed Type 2 Diabetes between 2015 and 2016: Difference by Age and Body Mass Index (Diabetes Metab J 2018;42:137-46)
Ah Reum Khang
Diabetes & Metabolism Journal.2018; 42(3): 249. CrossRef
- A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
- Others
- Comparison of the Usefulness of the Updated Homeostasis Model Assessment (HOMA2) with the Original HOMA1 in the Prediction of Type 2 Diabetes Mellitus in Koreans
- Young Seok Song, You-Cheol Hwang, Hong-Yup Ahn, Cheol-Young Park
- Diabetes Metab J. 2016;40(4):318-325. Published online May 27, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.4.318
- 4,368 View
- 81 Download
- 39 Web of Science
- 42 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The original homeostasis model assessment (HOMA1) and the updated HOMA model (HOMA2) have been used to evaluate insulin resistance (IR) and β-cell function, but little is known about the usefulness of HOMA2 for the prediction of diabetes in Koreans. The aim of this study was to demonstrate the usefulness of HOMA2 as a predictor of type 2 diabetes mellitus in Koreans without diabetes.
Methods The study population consisted of 104,694 Koreans enrolled at a health checkup program and followed up from 2001 to 2012. Participants were divided into a normal glucose tolerance (NGT) group and a pre-diabetes group according to fasting glucose and glycosylated hemoglobin levels. Anthropometric and laboratory data were measured at the baseline checkup, and HOMA values were calculated at the baseline and follow-up checkups. The hazard ratios (HRs) of the HOMA1 and HOMA2 values and the prevalence of diabetes at follow-up were evaluated using a multivariable Cox proportional hazards model and Kaplan-Meier analysis.
Results After adjusting for several diabetes risk factors, all of the HOMA values except 1/HOMA1-β and 1/HOMA2-β in the NGT group were significant predictors of the progression to diabetes. In the NGT group, there was no significant difference in HOMA1-IR (HR, 1.09; 95% confidence interval [CI], 1.04 to 1.14) and HOMA2-IR (HR, 1.11; 95% CI, 1.04 to 1.19). However, in the pre-diabetes group, 1/HOMA2-β was a more powerful marker (HR, 1.29; 95% CI, 1.26 to 1.31) than HOMA1-IR (HR, 1.23; 95% CI, 1.19 to 1.28) or 1/HOMA1-β (HR, 1.14; 95% CI, 1.12 to 1.16). In the non-diabetic group (NGT+pre-diabetes), 1/HOMA2-β was also a stronger predictor of diabetes (HR, 1.27; 95% CI, 1.25 to 1.29) than HOMA1-IR (HR, 1.14; 95% CI, 1.12 to 1.15) or 1/HOMA1-β (HR, 1.13; 95% CI, 1.11 to 1.14).
Conclusion HOMA2 is more predictive than HOMA1 for the progression to diabetes in pre-diabetes or non-diabetic Koreans.
-
Citations
Citations to this article as recorded by- Non-linear associations of HOMA2-IR with all-cause mortality in general populations: insights from NHANES 1999–2006
Aikai Zhang, Lingchen Huang, Min Tang
BMC Public Health.2024;[Epub] CrossRef - An acute exercise at low to moderate intensity attenuated postprandial lipemia and insulin responses
Lisa L. Ji, Vicki S. Fretwell, Abel Escamilla, Wanxiang Yao, Tianou Zhang, Meizi He, John Q. Zhang
Journal of Exercise Science & Fitness.2024; 22(1): 14. CrossRef - Insufficient compensatory pancreatic β-cells function might be closely associated with hyperuricemia in U.S. adults: evidence from the National Health and Nutrition Examination Survey
Tianran Shen, Qiutong Zheng, Liling Zhong, Xia Zeng, Xiaojing Yuan, Fengxin Mo, Shiheng Zhu, Wenhan Yang, Qingsong Chen
BMC Public Health.2024;[Epub] CrossRef - Glycated haemoglobin, HOMA2‐B, C‐peptide to glucose ratio and type 2 diabetes clusters as predictors for therapy failure in individuals with type 2 diabetes without insulin therapy: A registry analysis
Faisal Aziz, Christoph Sternad, Caren Sourij, Lisa Knoll, Harald Kojzar, Anna Schranz, Alexandra Bürger, Harald Sourij, Felix Aberer
Diabetes, Obesity and Metabolism.2024; 26(3): 1082. CrossRef - Nghiên cứu tương quan giữa chỉ số đề kháng insulin và chỉ số khối cơ thể ở bệnh nhân tiền đái tháo đường có tăng huyết áp
Linh Dương
Journal of Clinical Medicine- Hue Central Hospital.2024;[Epub] CrossRef - Bridelia ferruginea inhibits key carbohydrate digesting enzyme and intestinal glucose absorption and modulates glucose metabolism in diabetic rats
Olajumoke Oyebode, Lindiwe Zuma, Ochuko Lucky Erukainure, Neil Koorbanally, Md. Shahidul Islam
Archives of Physiology and Biochemistry.2023; 129(3): 671. CrossRef - Impact of Pancreatic β-Cell Function on Clopidogrel Responsiveness and Outcomes in Chinese Nondiabetic Patients Undergoing Elective Percutaneous Coronary Intervention
Xiliang Zhao, Jin Wang, Quan Li, Yicong Ye, Yong Zeng
Cardiovascular Drugs and Therapy.2023; 37(3): 487. CrossRef - Development and validation of an insulin resistance model for a population without diabetes mellitus and its clinical implication: a prospective cohort study
Shang-Feng Tsai, Chao-Tung Yang, Wei-Ju Liu, Chia-Lin Lee
eClinicalMedicine.2023; 58: 101934. CrossRef - Association of the triglyceride‐glucose index with subclinical left ventricular dysfunction in type 2 diabetes mellitus patients: A retrospective cross‐sectional study
Qi‐chao Sun, Jie Liu, Ran Meng, Ning Zhang, Jing Yao, Fan Yang, Da‐long Zhu
Journal of Diabetes Investigation.2023; 14(8): 953. CrossRef - Fasting indices of glucose-insulin-metabolism across life span and prediction of glycemic deterioration in children with obesity from new diagnostic cut-offs
Maximiliane Chiara Hammel, Robert Stein, Jürgen Kratzsch, Mandy Vogel, Alexander J. Eckert, Rima Destya Triatin, Marco Colombo, Christof Meigen, Ronny Baber, Juraj Stanik, Ulrike Spielau, Anette Stoltze, Kerstin Wirkner, Anke Tönjes, Harold Snieder, Reinh
The Lancet Regional Health - Europe.2023; 30: 100652. CrossRef - The role of bariatric surgery on beta-cell function and insulin resistance in patients with nonalcoholic fatty liver disease and steatohepatitis
Adisa Poljo, Stephan Kopf, Alba Sulaj, Stephanie Roessler, Thomas Albrecht, Benjamin Goeppert, Sarah Bojko, Beat P. Müller-Stich, Adrian T. Billeter
Surgery for Obesity and Related Diseases.2023; 19(12): 1421. CrossRef - Glutamine Defended the Kidneys Versus Lead Intoxication Via Elevating Endogenous Antioxidants, Reducing Inflammation and Carbonyl Stress, as well as Improving Insulin Resistance and Dyslipidemia
Sina Mahdavifard, Najafzadeh Nowruz
Biological Trace Element Research.2023;[Epub] CrossRef - Sex and genetic background define the metabolic, physiologic, and molecular response to protein restriction
Cara L. Green, Heidi H. Pak, Nicole E. Richardson, Victoria Flores, Deyang Yu, Jay L. Tomasiewicz, Sabrina N. Dumas, Katherine Kredell, Jesse W. Fan, Charlie Kirsh, Krittisak Chaiyakul, Michaela E. Murphy, Reji Babygirija, Gregory A. Barrett-Wilt, Joshua
Cell Metabolism.2022; 34(2): 209. CrossRef - Associations of the HOMA2‐%B and HOMA2‐IR with progression to diabetes and glycaemic deterioration in young and middle‐aged Chinese
Baoqi Fan, Hongjiang Wu, Mai Shi, Aimin Yang, Eric S. H. Lau, Claudia H. T. Tam, Dandan Mao, Cadmon K. P. Lim, Alice P. S. Kong, Ronald C. W. Ma, Elaine Chow, Andrea O. Y. Luk, Juliana C. N. Chan
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Protective effect of acetylcysteine, histidine, and their combination against diabetes vascular complications in type-2 diabetic rats via reducing NF-kβ pathway signaling
Sina Mahdavifard, Manochehr Nakhjavani
Journal of Diabetes & Metabolic Disorders.2022; 21(2): 1233. CrossRef - Visceral fat might impact left ventricular remodeling through changes in arterial stiffness in type 2 diabetes: A cross-sectional study
Haishan Huang, Jing Jin, Yanshan Chen, Lina Wang, Jingyi Zhong, Zhenguo Chen, Lingling Xu
International Journal of Cardiology.2022; 368: 78. CrossRef - The effect of berberine and fenugreek seed co-supplementation on inflammatory factor, lipid and glycemic profile in patients with type 2 diabetes mellitus: a double-blind controlled randomized clinical trial
Shima Nematollahi, Gholam Reza Pishdad, Mehrnoosh Zakerkish, Foroogh Namjoyan, Kambiz Ahmadi Angali, Fatemeh Borazjani
Diabetology & Metabolic Syndrome.2022;[Epub] CrossRef - Association between changes in pancreatic morphology and vascular complications in subjects with type 2 diabetes mellitus: a retrospective study
Yuichiro Iwamoto, Tomohiko Kimura, Fuminori Tatsumi, Toshitomo Sugisaki, Masato Kubo, Erina Nakao, Kazunori Dan, Ryo Wamata, Hideyuki Iwamoto, Kaio Takahashi, Junpei Sanada, Yoshiro Fushimi, Yukino Katakura, Masashi Shimoda, Shuhei Nakanishi, Tomoatsu Mun
Scientific Reports.2022;[Epub] CrossRef - Clustering patterns of metabolic syndrome: A cross-sectional study in children and adolescents in Kyiv
Maiia H. Aliusef, Ganna V. Gnyloskurenko, Alina V. Churylina, Inga O. Mityuryayeva
Frontiers in Pediatrics.2022;[Epub] CrossRef - Circulating spexin levels are influenced by the glycemic status and correlated with pancreatic β-cell function in Chinese subjects
Jiarong Dai, Yunzhi Ni, Di Wu, Yaojing Jiang, Shuoshuo Jin, Shan Zhang, Xuemei Yu, Rui Liu
Acta Diabetologica.2022; 60(2): 305. CrossRef - Association between the triglyceride–glucose index and diabetic nephropathy in patients with type 2 diabetes: A cross‐sectional study
Li Liu, Rui Xia, Xiaoqing Song, Benping Zhang, Wentao He, Xinrong Zhou, Shengzhong Li, Gang Yuan
Journal of Diabetes Investigation.2021; 12(4): 557. CrossRef - The insulin resistance by triglyceride glucose index and risk for dementia: population-based study
Sangmo Hong, Kyungdo Han, Cheol-Young Park
Alzheimer's Research & Therapy.2021;[Epub] CrossRef - Determinants of type 2 diabetes remission after bariatric surgery in obese Japanese patients: a retrospective cohort study
Masahiro Ohira, Yasuhiro Watanabe, Takashi Yamaguchi, Atsuhito Saiki, Shoko Nakamura, Shou Tanaka, Naomi Shimizu, Taiki Nabekura, Takashi Oshiro, Ichiro Tatsuno
Diabetology International.2021; 12(4): 379. CrossRef - Sakarya Eğitim ve Araştırma Hastanesine Başvuran Hastaların HOMA-IR Değerlerinin Yaş ve Cinsiyet Faktörü Açısından Değerlendirilmesi
Mehmet ÖZDİN, Hayrullah YAZAR, Durhasan MUNDAN
Mustafa Kemal Üniversitesi Tıp Dergisi.2021; 12(42): 1. CrossRef - Feeding pattern, biochemical, anthropometric and histological effects of prolonged ad libitum access to sucrose, honey and glucose-fructose solutions in Wistar rats
Carmen Alejandrina Virgen-Carrillo, Alma Gabriela Martínez Moreno, Juan José Rodríguez-Gudiño, Jessica Elizabeth Pineda-Lozano
Nutrition Research and Practice.2021; 15(2): 187. CrossRef - The effect of curcumin and zinc co‐supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo‐controlled trial with a multi‐arm, parallel‐group design
Majid Karandish, Hassan Mozaffari‐khosravi, Seyed Mohammad Mohammadi, Bahman Cheraghian, Maryam Azhdari
Phytotherapy Research.2021; 35(8): 4377. CrossRef - The Association Between Second-Line Oral Antihyperglycemic Medication on Types of Dementia in Type 2 Diabetes: A Nationwide Real-World Longitudinal Study
Won Jun Kim, Jung Hyun Noh, Kyungdo Han, Cheol-Young Park
Journal of Alzheimer's Disease.2021; 81(3): 1263. CrossRef - Personalized Type 2 Diabetes Management Using a Mobile Application Integrated with Electronic Medical Records: An Ongoing Randomized Controlled Trial
Eun-Young Lee, Jae-Seung Yun, Seon-Ah Cha, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, Seung-Hyun Ko
International Journal of Environmental Research and Public Health.2021; 18(10): 5300. CrossRef - Enhancement of Serum Myonectin Levels by Progressive Resistance Training in Rats Fed with High-Fat Diet and Sucrose Solution
Alireza Safarzade, Hadi Safarpour
Zahedan Journal of Research in Medical Sciences.2021;[Epub] CrossRef - Pancreatic β-Cell Dysfunction Is Associated with Nonalcoholic Fatty Liver Disease
Xu Chen, Jinghe Xiao, Juan Pang, Shen Chen, Qing Wang, Wenhua Ling
Nutrients.2021; 13(9): 3139. CrossRef - The correlation between serum resistin and toll-like receptor-4 with insulin resistance in hypertensive subjects with or without type 2 diabetes mellitus
Mustafa Al-Taie, Rayah Baban, Mouayed Hamed
Baghdad Journal of Biochemistry and Applied Biological Sciences.2021; 2(04): 203. CrossRef - Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH
Javier Ampuero, Rocío Aller, Rocío Gallego-Durán, Javier Crespo, José Luis Calleja, Carmelo García-Monzón, Judith Gómez-Camarero, Joan Caballería, Oreste Lo Iacono, Luis Ibañez, Javier García-Samaniego, Agustín Albillos, Rubén Francés, Conrado Fernández-R
Journal of Hepatology.2020; 73(1): 17. CrossRef - Are the Different Diabetes Subgroups Correlated With All-Cause, Cancer-Related, and Cardiovascular-Related Mortality?
Peng-Fei Li, Wei-Liang Chen
The Journal of Clinical Endocrinology & Metabolism.2020; 105(12): e4240. CrossRef - Avoiding holiday seasonal weight gain with nutrient-supported intermittent energy restriction: a pilot study
Steven P. Hirsh, Marianne Pons, Steven V. Joyal, Andrew G. Swick
Journal of Nutritional Science.2019;[Epub] CrossRef - Peripartum Management of Gestational Diabetes Using a Digital Health Care Service: A Pilot, Randomized Controlled Study
Ji-Hee Sung, Da Young Lee, Kyoung Pil Min, Cheol-Young Park
Clinical Therapeutics.2019; 41(11): 2426. CrossRef - Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia
Bernd Richter, Bianca Hemmingsen, Maria-Inti Metzendorf, Yemisi Takwoingi
Cochrane Database of Systematic Reviews.2018;[Epub] CrossRef - The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES)
Bongyoung Kim, Hyun Young Choi, Wonhee Kim, Chiwon Ahn, Juncheol Lee, Jae Guk Kim, Jihoon Kim, Hyungoo Shin, Jae Myung Yu, Shinje Moon, Taulant Muka
PLOS ONE.2018; 13(11): e0206994. CrossRef - Testing for HbA1c, in addition to the oral glucose tolerance test, in screening for abnormal glucose regulation helps to reveal patients with early β-cell function impairment
Yu-Hsuan Li, Wayne Huey-Herng Sheu, Wen-Jane Lee, I-Te Lee, Shih-Yi Lin, Wen-Lieng Lee, Kae-Woei Liang, Jun-Sing Wang
Clinical Chemistry and Laboratory Medicine (CCLM).2018; 56(8): 1345. CrossRef - Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance
Sikandar Hayat Khan, Farah Sobia, Najmusaqib Khan Niazi, Syed Mohsin Manzoor, Nadeem Fazal, Fowad Ahmad
Diabetology & Metabolic Syndrome.2018;[Epub] CrossRef - Pancreatic β-Cell Function and Prognosis of Nondiabetic Patients With Ischemic Stroke
Yuesong Pan, Weiqi Chen, Jing Jing, Huaguang Zheng, Qian Jia, Hao Li, Xingquan Zhao, Liping Liu, Yongjun Wang, Yan He, Yilong Wang
Stroke.2017; 48(11): 2999. CrossRef - Association of serum pancreatic derived factor ( PANDER) with beta-cell dysfunction in type 2 diabetes mellitus
Miral M. Shehata, Mohamed M. Kamal, Mohamed H. El-Hefnawy, Hala O. EL-Mesallamy
Journal of Diabetes and its Complications.2017; 31(4): 748. CrossRef
- Non-linear associations of HOMA2-IR with all-cause mortality in general populations: insights from NHANES 1999–2006
- The Insulin Resistance but Not the Insulin Secretion Parameters Have Changed in the Korean Population during the Last Decade
- Hae Kyung Yang, Jin Hee Lee, In-Young Choi, Hyuk Sang Kwon, Jeong Ah Shin, Seung Hee Jeong, Seung-Hwan Lee, Jae Hyoung Cho, Ho Young Son, Kun Ho Yoon
- Diabetes Metab J. 2015;39(2):117-125. Published online April 20, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.2.117
- 4,565 View
- 46 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background This study aimed to compare the patterns of insulin secretion and resistance between Korean subjects in the 1990s and 2000s.
Methods Insulin secretion and resistance indices were calculated from subjects who underwent 75-g oral glucose tolerance tests in the year 1997 to 1999 and 2007 to 2011 at the Seoul St. Mary's Hospital, Korea.
Results A total of 578 subjects from the 1990s (mean age, 48.5 years) and 504 subjects from the 2000s (mean age, 50.2 years) were enrolled. Compared with the subjects from the 1990s, those from the 2000s exhibited increased insulin resistance (increased homeostatic model assessment for insulin resistance), and reduced insulin sensitivity (reduced Matsuda index and quantitative insulin sensitivity check index), regardless of their glucose tolerance status. However, insulinogenic index did not reveal significant differences between the 2 decades in subjects with or without diabetes. A distinct relationship was confirmed between Matsuda index and total area under the curve (insulin/glucose) in each glucose tolerance group. The mean product of the Matsuda index and the total area under the curve (insulin/glucose) as well as the oral disposition index, was lower in subjects with normal glucose tolerance from the 2000s than in those from the 1990s.
Conclusion After rapid economic growth and changes in lifestyle patterns, insulin resistance has worsened across the glucose tolerance status; however, the insulin secretory function remained unchanged, which resulted in an increase in the susceptibility to the development of type 2 diabetes mellitus among Korean subjects without diabetes. We could not rule out the potential selection bias and therefore, further studies in general Korean population are needed.
-
Citations
Citations to this article as recorded by- Longitudinal Changes in Insulin Resistance, Beta-Cell Function and Glucose Regulation Status in Prediabetes
Chul-Hee Kim, Hong-Kyu Kim, Eun-Hee Kim, Sung-Jin Bae, Jaewon Choe, Joong-Yeol Park
The American Journal of the Medical Sciences.2018; 355(1): 54. CrossRef - Association of serum 25-hydroxyvitamin D and diabetes-related factors in Korean adults without diabetes: The Fifth Korea National Health and Nutrition Examination Survey 2010–2012
Hyunah Kim, Hyunyong Lee, Hyeon Woo Yim, Hun-Sung Kim
Primary Care Diabetes.2018; 12(1): 59. CrossRef - Long‐term effects on glycaemic control and β‐cell preservation of early intensive treatment in patients with newly diagnosed type 2 diabetes: A multicentre randomized trial
Suk Chon, Sang Youl Rhee, Kyu Jeung Ahn, Sei Hyun Baik, Yongsoo Park, Moon Suk Nam, Kwan Woo Lee, Soon Jib Yoo, Gwanpyo Koh, Dae Ho Lee, Young Seol Kim, Jeong‐Taek Woo
Diabetes, Obesity and Metabolism.2018; 20(5): 1121. CrossRef - Four Plasma Glucose and Insulin Responses to a 75 g OGTT in Healthy Young Japanese Women
Kei Takahashi, Hidetaka Nakamura, Hiroshi Sato, Hideto Matsuda, Kazuo Takada, Tomiko Tsuji
Journal of Diabetes Research.2018; 2018: 1. CrossRef - Comparison of insulin intensification strategies with insulin lispro low mixture twice daily versus basal insulin glargine and prandial insulin lispro once daily in East Asian and Caucasian patients with type 2 diabetes mellitus
In‐Kyung Jeong, Choon Hee Chung, Zhiguang Zhou, Jeong Hee Han, Ran Duan, Diana M. Edralin, Angel Rodriguez
Journal of Diabetes.2017; 9(4): 396. CrossRef - Insulin Secretory Capacity and Insulin Resistance in Korean Type 2 Diabetes Mellitus Patients
Jong-Dai Kim, Won-Young Lee
Endocrinology and Metabolism.2016; 31(3): 354. CrossRef - Antisenescence activity of G9a inhibitor BIX01294 on human bone marrow mesenchymal stromal cells
Min-Ji AHN, Sin-Gu JEONG, Goang-Won CHO
TURKISH JOURNAL OF BIOLOGY.2016; 40: 443. CrossRef - Urinary N-acetyl-β-D-glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes
So Ra Kim, Yong-ho Lee, Sang-Guk Lee, Eun Seok Kang, Bong-Soo Cha, Jeong-Ho Kim, Byung-Wan Lee
Medicine.2016; 95(27): e4114. CrossRef
- Longitudinal Changes in Insulin Resistance, Beta-Cell Function and Glucose Regulation Status in Prediabetes
- Hexane Extract of
Orthosiphon stamineus Induces Insulin Expression and Prevents Glucotoxicity in INS-1 Cells - Hae-Jung Lee, Yoon-Jung Choi, So-Young Park, Jong-Yeon Kim, Kyu-Chang Won, Jong-Keun Son, Yong-Woon Kim
- Diabetes Metab J. 2015;39(1):51-58. Published online February 16, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.1.51
- 4,467 View
- 62 Download
- 10 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Hyperglycemia, a characteristic feature of diabetes, induces glucotoxicity in pancreatic β-cells, resulting in further impairment of insulin secretion and worsening glycemic control. Thus, preservation of insulin secretory capacity is essential for the management of type 2 diabetes. In this study, we evaluated the ability of an
Orthosiphon stamineus (OS) extract to prevent glucotoxicity in insulin-producing cells.Methods We measured insulin mRNA expression and glucose-stimulated insulin secretion (GSIS) in OS-treated INS-1 cells after exposure to a high glucose (HG; 30 mM) concentration.
Results The hexane extract of OS elevated mRNA expression of insulin as well as pancreatic and duodenal homeobox-1 of INS-1 cells in a dose-dependent manner. The hexane OS extract also increased the levels of phosphorylated phosphatidylinositol 3-kinase (PI3K) in a concentration-dependent manner. Additionally, Akt phosphorylation was elevated by treatment with 100 and 200 µmol of the hexane OS extract. Three days of HG exposure suppressed insulin mRNA expression and GSIS; these expressions were restored by treatment with the hexane OS extract. HG elevated peroxide levels in the INS-1 cells. These levels were unaffected by OS treatment under both normal and hyperglycemic conditions.
Conclusion Our results suggested that the hexane extract of OS elevates insulin mRNA expression and prevents glucotoxicity induced by a 3-day treatment with HG. This was associated with the activation of PI-3K and Akt.
-
Citations
Citations to this article as recorded by- An Updated Review of Ethnobotany, Ethnopharmacology, Phytochemistry and Pharmacological Activities of Orthosiphon stamineus Benth
Anandarajagopal Kalusalingam, Dania Najiha Hasnu, Abdullah Khan, Ching Siang Tan, Bama Menon, Venkateshan Narayanan, Khang Wen Goh, Asmuni Mohd Ikmal, Noraini Talip, Poonguzhali Subramanian, Long Chiau Ming
Malaysian Applied Biology.2024; 53(1): 1. CrossRef - Scopoletin protects INS-1 pancreatic β cells from glucotoxicity by reducing oxidative stress and apoptosis
Jae Eun Park, Ji Sook Han
Toxicology in Vitro.2023; 93: 105665. CrossRef - A Systematic Review of Orthosiphon stamineus Benth. in the Treatment of Diabetes and Its Complications
Qirou Wang, Jia Wang, Nannan Li, Junyu Liu, Jingna Zhou, Pengwei Zhuang, Haixia Chen
Molecules.2022; 27(2): 444. CrossRef - Comprehensive chemical and metabolic profiling of anti‐hyperglycemic active fraction from Clerodendranthi Spicati Herba
Yun Luo, Yue Liu, Quan Wen, Yulin Feng, Ting Tan
Journal of Separation Science.2021; 44(9): 1805. CrossRef - Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect
Krista Minéia Wartchow, Letícia Rodrigues, Lucas Zingano Suardi, Barbara Carolina Federhen, Nicholas Guerini Selistre, Carlos-Alberto Gonçalves, Patrícia Sesterheim
International Journal of Molecular Sciences.2019; 20(10): 2458. CrossRef - Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants
Chintha Lankatillake, Tien Huynh, Daniel A. Dias
Plant Methods.2019;[Epub] CrossRef - 50% Ethanol extract of Orthosiphon stamineus modulates genotoxicity and clastogenicity induced by mitomycin C
Dhamraa Waleed Al-dualimi, Aman Shah Abdul Majid, Sarah Furqan Faisal Al-Shimary, Amal Aziz Al-Saadi, Raghdaa Al Zarzour, Muhammad Asif, Chern Ein Oon, Amin Malik Shah Abdul Majid
Drug and Chemical Toxicology.2018; 41(1): 82. CrossRef - Can Tea Extracts Exert a Protective Effect Against Diabetes by Reducing Oxidative Stress and Decreasing Glucotoxicity in Pancreatic β-Cells?
Heeyoung Chae, Patrick Gilon
Diabetes & Metabolism Journal.2015; 39(1): 27. CrossRef
- An Updated Review of Ethnobotany, Ethnopharmacology, Phytochemistry and Pharmacological Activities of Orthosiphon stamineus Benth
- Impact of Serum Triglyceride and High Density Lipoprotein Cholesterol Levels on Early-Phase Insulin Secretion in Normoglycemic and Prediabetic Subjects
- Masanori Shimodaira, Tomohiro Niwa, Koji Nakajima, Mutsuhiro Kobayashi, Norinao Hanyu, Tomohiro Nakayama
- Diabetes Metab J. 2014;38(4):294-301. Published online August 20, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.4.294
- 3,228 View
- 28 Download
- 15 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Increased triglycerides (TGs) and decreased high density lipoprotein cholesterol (HDL-C) levels are established as diabetic risks for nondiabetic subjects. The aim of this study was to investigate the relationship among TG, HDL-C, TG/HDL-C ratio, and early-phase insulin secretion in normoglycemic and prediabetic subjects.
Methods We evaluated 663 Japanese subjects who underwent the 75-g oral glucose tolerance test. On the basis of these results, the subjects were divided into four groups: those with normal glucose tolerance (NGT;
n =341), isolated impaired fasting glucose (i-IFG;n =211), isolated impaired glucose tolerance (i-IGT;n =71), and combined IFG and IGT (IFG+IGT;n =40). Insulin secretion was estimated by the insulinogenic index (IGI) (Δinsulin/Δglucose [30 to 0 minutes]) and disposition index (DI) (IGI/homeostasis model assessment of insulin resistance).Results In prediabetic subjects (i-IFG, i-IGT, and IFG+IGT), linear regression analyses revealed that IGI and DI were positively correlated with HDL-C levels. Moreover, in subjects with i-IGT and (IFG+IGT), but not with i-IFG, the indices of insulin secretion were negatively correlated with the log-transformed TG and TG/HDL-C ratio. In both the subjects with i-IGT, multivariate linear regression analyses revealed that DI was positively correlated with HDL-C and negatively with log-transformed TG and TG/HDL-C ratio. On the other hand, in subjects with NGT, there was no association between insulin secretion and lipid profiles.
Conclusion These results revealed that serum TG and HDL-C levels have different impacts on early-phase insulin secretion on the basis of their glucose tolerance status.
-
Citations
Citations to this article as recorded by- The protective effects of lipoxin A4 on type 2 diabetes mellitus: A Chinese prospective cohort study
Sudan Wang, Xiaoyan Qian, Chao Shen, Qian Sun, Yang Jing, Bingyue Liu, Kexin Zhang, Mengyuan Li, Junrong Wang, Hui Zhou, Chen Dong
Frontiers in Endocrinology.2023;[Epub] CrossRef - Interaction between the GCKR rs1260326 variant and serum HDL cholesterol contributes to HOMA-β and ISIMatusda in the middle-aged T2D individuals
Min Shen, Liying Jiang, Hechun Liu, Hao Dai, Hemin Jiang, Yu Qian, Zhixiao Wang, Shuai Zheng, Heng Chen, Tao Yang, Qi Fu, Kuanfeng Xu
Journal of Human Genetics.2023; 68(12): 835. CrossRef - Elevated triglyceride/high-density lipoprotein-cholesterol ratio as a risk factor for progression to prediabetes: a 5-year retrospective cohort study in Japan
Masanori Shimodaira, Yu Minemura, Tomohiro Nakayama
Journal of Diabetes & Metabolic Disorders.2023;[Epub] CrossRef - Association ofTG/HDLCratio trajectory and risk of type 2 diabetes: A retrospective cohort study inChina
Yanyan Zhang, Pei Qin, Yanmei Lou, Ping Zhao, Xue Li, Ranran Qie, Xiaoyan Wu, Minghui Han, Shengbing Huang, Yang Zhao, Dechen Liu, Yuying Wu, Yang Li, Xingjin Yang, Yang Zhao, Yifei Feng, Changyi Wang, Jianping Ma, Xiaolin Peng, Hongen Chen, Dan Zhao, Sha
Journal of Diabetes.2021; 13(5): 402. CrossRef - Triglycerides/high-density lipoprotein cholesterol is a predictor similar to the triglyceride–glucose index for the diagnosis of metabolic syndrome using International Diabetes Federation criteria of insulin resistance in obese adolescents: a cross-sectio
Nazlı Nur Aslan Çin, Hülya Yardımcı, Nevra Koç, Seyit Ahmet Uçaktürk, Mehtap Akçil Ok
Journal of Pediatric Endocrinology and Metabolism.2020; 33(6): 777. CrossRef - Comparison of Serum PCSK9 Levels in Subjects with Normoglycemia, Impaired Fasting Glucose, and Impaired Glucose Tolerance
Eugene Han, Nan Hee Cho, Seong-Su Moon, Hochan Cho
Endocrinology and Metabolism.2020; 35(2): 480. CrossRef - Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents
B Kang, Y Yang, E Y Lee, H K Yang, H-S Kim, S-Y Lim, J-H Lee, S-S Lee, B-K Suh, K-H Yoon
International Journal of Obesity.2017; 41(5): 789. CrossRef - The TyG index may predict the development of cardiovascular events
Laura Sánchez‐Íñigo, David Navarro‐González, Alejandro Fernández‐Montero, Juan Pastrana‐Delgado, Jose Alfredo Martínez
European Journal of Clinical Investigation.2016; 46(2): 189. CrossRef - TyG Index Change Is More Determinant for Forecasting Type 2 Diabetes Onset Than Weight Gain
David Navarro-González, Laura Sánchez-Íñigo, Alejandro Fernández-Montero, Juan Pastrana-Delgado, Jose Alfredo Martinez
Medicine.2016; 95(19): e3646. CrossRef - Comparative analysis of the efficacy of omega-3 fatty acids for hypertriglyceridaemia management in Korea
H.-S. Kim, H. Kim, Y. J. Jeong, S. J. Yang, S. J. Baik, H. Lee, S.-H. Lee, J. H. Cho, I.-Y. Choi, H. W. Yim, K.-H. Yoon
Journal of Clinical Pharmacy and Therapeutics.2016; 41(5): 508. CrossRef - Relationship between insulin sensitivity and insulin secretion rate: not necessarily hyperbolic
S. H. Kim, A. Silvers, J. Viren, G. M. Reaven
Diabetic Medicine.2016; 33(7): 961. CrossRef - The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status
Meicen Zhou, Lixin Zhu, Xiangli Cui, Linbo Feng, Xuefeng Zhao, Shuli He, Fan Ping, Wei Li, Yuxiu Li
Lipids in Health and Disease.2016;[Epub] CrossRef - Interactive effects of C-reactive protein levels on the association between APOE variants and triglyceride levels in a Taiwanese population
Semon Wu, Lung-An Hsu, Ming-Sheng Teng, Jeng-Feng Lin, Hsin-Hua Chou, Ming-Cheng Lee, Yi-Ming Wu, Cheng-Wen Su, Yu-Lin Ko
Lipids in Health and Disease.2016;[Epub] CrossRef - Lipoproteins and β-Cell Functions: From Basic to Clinical Data
Dae Ho Lee
Diabetes & Metabolism Journal.2014; 38(4): 274. CrossRef
- The protective effects of lipoxin A4 on type 2 diabetes mellitus: A Chinese prospective cohort study
- Neonatal Diabetes Caused by Activating Mutations in the Sulphonylurea Receptor
- Peter Proks
- Diabetes Metab J. 2013;37(3):157-164. Published online June 14, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.3.157
- 3,505 View
- 51 Download
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Adenosine triphosphate (ATP)-sensitive potassium (KATP) channels in pancreatic β-cells play a crucial role in insulin secretion and glucose homeostasis. These channels are composed of two subunits: a pore-forming subunit (Kir6.2) and a regulatory subunit (sulphonylurea receptor-1). Recent studies identified large number of gain of function mutations in the regulatory subunit of the channel which cause neonatal diabetes. Majority of mutations cause neonatal diabetes alone, however some lead to a severe form of neonatal diabetes with associated neurological complications. This review focuses on the functional effects of these mutations as well as the implications for treatment.
-
Citations
Citations to this article as recorded by- Long-term Follow-up of Glycemic and Neurological Outcomes in an International Series of Patients With Sulfonylurea-Treated ABCC8 Permanent Neonatal Diabetes
Pamela Bowman, Frances Mathews, Fabrizio Barbetti, Maggie H. Shepherd, Janine Sanchez, Barbara Piccini, Jacques Beltrand, Lisa R. Letourneau-Freiberg, Michel Polak, Siri Atma W. Greeley, Eamon Rawlins, Tarig Babiker, Nicholas J. Thomas, Elisa De Franco, S
Diabetes Care.2021; 44(1): 35. CrossRef - Structure based analysis of KATP channel with a DEND syndrome mutation in murine skeletal muscle
Shoichiro Horita, Tomoyuki Ono, Saul Gonzalez-Resines, Yuko Ono, Megumi Yamachi, Songji Zhao, Carmen Domene, Yuko Maejima, Kenju Shimomura
Scientific Reports.2021;[Epub] CrossRef - Clinical and Genetic Characteristics of ABCC8 Nonneonatal Diabetes Mellitus: A Systematic Review
Meng Li, Xueyao Han, Linong Ji, Karim Gariani
Journal of Diabetes Research.2021; 2021: 1. CrossRef - Spacial models of malfunctioned protein complexes help to elucidate signal transduction critical for insulin release
Katarzyna Walczewska-Szewc, Wieslaw Nowak
Biosystems.2019; 177: 48. CrossRef - Cantu syndrome–associated SUR2 (ABCC9) mutations in distinct structural domains result in KATP channel gain-of-function by differential mechanisms
Conor McClenaghan, Alex Hanson, Monica Sala-Rabanal, Helen I. Roessler, Dragana Josifova, Dorothy K. Grange, Gijs van Haaften, Colin G. Nichols
Journal of Biological Chemistry.2018; 293(6): 2041. CrossRef - Hyperinsulinism-Causing Mutations Cause Multiple Molecular Defects in SUR1 NBD1
Claudia P. Alvarez, Marijana Stagljar, D. Ranjith Muhandiram, Voula Kanelis
Biochemistry.2017; 56(18): 2400. CrossRef - KATP Channel Mutations and Neonatal Diabetes
Kenju Shimomura, Yuko Maejima
Internal Medicine.2017; 56(18): 2387. CrossRef - Molecular action of sulphonylureas on KATP channels: a real partnership between drugs and nucleotides
Heidi de Wet, Peter Proks
Biochemical Society Transactions.2015; 43(5): 901. CrossRef - Successful development and use of a thermodynamic stability screen for optimizing the yield of nucleotide binding domains
Elvin D. de Araujo, Voula Kanelis
Protein Expression and Purification.2014; 103: 38. CrossRef - Reclasificación y transferencia de insulina a sulfonilureas en un paciente con mutación en KCNJ11 tras 15 años de tratamiento con insulina
Magdalena Capponi, Carmen Quirós, Ignacio Conget, Enric Esmatjes, Marga Giménez
Avances en Diabetología.2014; 30(4): 115. CrossRef
- Long-term Follow-up of Glycemic and Neurological Outcomes in an International Series of Patients With Sulfonylurea-Treated ABCC8 Permanent Neonatal Diabetes
- A Systematic Review of Oxidative Stress and Safety of Antioxidants in Diabetes: Focus on Islets and Their Defense
- Udayakumar Karunakaran, Keun-Gyu Park
- Diabetes Metab J. 2013;37(2):106-112. Published online April 16, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.2.106
- 5,194 View
- 69 Download
- 132 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader A growing body of evidence suggests that hyperglycemia-induced oxidative stress plays an important role in diabetic complications, especially β-cell dysfunction and failure. Under physiological conditions, reactive oxygen species serve as second messengers that facilitate signal transduction and gene expression in pancreatic β-cells. However, under pathological conditions, an imbalance in redox homeostasis leads to aberrant tissue damage and β-cell death due to a lack of antioxidant defense systems. Taking into account the vulnerability of islets to oxidative damage, induction of endogenous antioxidant enzymes or exogenous antioxidant administration has been proposed as a way to protect β-cells against diabetic insults. Here, we consider recent insights into how the redox response becomes deregulated under diabetic conditions, as well as the therapeutic benefits of antioxidants, which may provide clues for developing strategies aimed at the treatment or prevention of diabetes associated with β-cell failure.
-
Citations
Citations to this article as recorded by- Association Between Serum Ferritin and the Duration of Type 2 Diabetes Mellitus in a Tertiary Care Hospital in Chennai
Sankar Arumugam, Anbalagan Suyambulingam
Cureus.2024;[Epub] CrossRef - Pancreatic Parenchymal Atrophy and Pancreatic Fat Accumulation Measured by Multidetector Computed Tomography as a Stable Marker of Chronic Progressive Type 2 Diabetes Mellitus—A Cross Sectional Observational Study
Kshipra Devadiga, Khanak K Nandolia, Mahendra Singh, Pankaj Sharma, Udit Chauhan, Ravi Kant
Avicenna Journal of Medicine.2024;[Epub] CrossRef - Toward Ultrasound Molecular Imaging of Endothelial Dysfunction in Diabetes: Targets, Strategies, and Challenges
Jiahan Liu, Chen Wang, Shuo Qiu, Wenqi Sun, Guodong Yang, Lijun Yuan
ACS Applied Bio Materials.2024; 7(3): 1416. CrossRef - Interference of altered plasma trace elements profile with hyperhomocysteinemia and oxidative stress damage to insulin secretion dysfunction in Psammomys obesus: focus on the selenium
Asma Bouazza, Eric Fontaine, Xavier Leverve, Elhadj-Ahmed Koceir
Archives of Physiology and Biochemistry.2023; 129(2): 505. CrossRef - Vanillic acid potentiates insulin secretion and prevents pancreatic β-cells cytotoxicity under H2O2-induced oxidative stress
Chandan Muddahally Naganna, K. Yogendra Prasad, V. P. Mahendra, P. Ganesan, Ravi Kumar
Molecular Biology Reports.2023; 50(2): 1311. CrossRef - Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress
Phiwayinkosi V Dludla, Sihle E Mabhida, Khanyisani Ziqubu, Bongani B Nkambule, Sithandiwe E Mazibuko-Mbeje, Sidney Hanser, Albert Kotze Basson, Carmen Pheiffer, Andre Pascal Kengne
World Journal of Diabetes.2023; 14(3): 130. CrossRef - Redox Balance in Type 2 Diabetes: Therapeutic Potential and the Challenge of Antioxidant-Based Therapy
Lital Argaev-Frenkel, Tovit Rosenzweig
Antioxidants.2023; 12(5): 994. CrossRef - Polygenic Variants Linked to Oxidative Stress and the Antioxidant System Are Associated with Type 2 Diabetes Risk and Interact with Lifestyle Factors
Youngjin Choi, Hyuk-Ku Kwon, Sunmin Park
Antioxidants.2023; 12(6): 1280. CrossRef - Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits
Anna Merecz-Sadowska, Przemysław Sitarek, Tomasz Kowalczyk, Karolina Zajdel, Mariusz Jęcek, Paweł Nowak, Radosław Zajdel
Nutrients.2023; 15(13): 3016. CrossRef - Selenium—More than Just a Fortuitous Sulfur Substitute in Redox Biology
Luisa B. Maia, Biplab K. Maiti, Isabel Moura, José J. G. Moura
Molecules.2023; 29(1): 120. CrossRef - Nitroxyl Radical as a Theranostic Contrast Agent in Magnetic Resonance Redox Imaging
Ken-ichiro Matsumoto, Ikuo Nakanishi, Zhivko Zhelev, Rumiana Bakalova, Ichio Aoki
Antioxidants & Redox Signaling.2022; 36(1-3): 95. CrossRef - Speciation study and biological activity of copper (II) complexes with picolinic and 6-methylpicolinic acid with different components of blood serum of low molecular mass in KNO3 1.0 mol·L−1 at 25 °C
Edgar Del Carpio, María L. Serrano, Lino Hernández, Waleska Madden, Vito Lubes, Vanessa R. Landaeta, Rafael E. Rodríguez-Lugo, Giuseppe Lubes, Anita Stern, Carlos Ciangherotti, Lissette Jiménez
Polyhedron.2022; 211: 115562. CrossRef - Pharmaceutical formulation and polymer chemistry for cell encapsulation applied to the creation of a lab-on-a-chip bio-microsystem
Armin Mooranian, Melissa Jones, Corina Mihaela Ionescu, Daniel Walker, Susbin Raj Wagle, Bozica Kovacevic, Jacqueline Chester, Thomas Foster, Edan Johnston, Momir Mikov, Hani Al-Salami
Therapeutic Delivery.2022; 13(1): 51. CrossRef - Racial Differences in Pain, Nutrition, and Oxidative Stress
Larissa J. Strath, Robert E. Sorge
Pain and Therapy.2022; 11(1): 37. CrossRef - Aerobic training reduces pancreatic fat content and improves β‐cell function: A randomized controlled trial using IDEAL‐IQ magnetic resonance imaging
Min Li, Qidong Zheng, Joshua D. Miller, Panpan Zuo, Xiaodan Yuan, Jitao Feng, Chao Liu, Shan Bao, Qingqing Lou
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - Synthesis, Antioxidant, and Antidiabetic Activities of Ketone Derivatives of Succinimide
Bushra Waheed, Syed Muhammad Mukarram Shah, Fida Hussain, Mohammad Ijaz Khan, Anwar Zeb, Muhammad Saeed Jan, Arpita Roy
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles
Neetu Tripathi, Manoj Kumar Goshisht
ACS Applied Bio Materials.2022; 5(4): 1391. CrossRef - Chemical speciation, antioxidant activity and molecular docking of copper(II) complexes with pyridinedicarboxylic acids and different ligands of low molecular mass
Edgar Del Carpio, María L. Serrano, Lino Hernández, Waleska Madden, Vito Lubes, Vanessa R. Landaeta, Rafael E. Rodríguez-Lugo, Giuseppe Lubes, Anita Stern, Carlos Ciangherotti, Lissette Jiménez
Physics and Chemistry of Liquids.2022; 60(6): 943. CrossRef - Inflammaging and Osteoarthritis
Francesca Motta, Elisa Barone, Antonio Sica, Carlo Selmi
Clinical Reviews in Allergy & Immunology.2022; 64(2): 222. CrossRef - Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation
Thobekile S. Leyane, Sandy W. Jere, Nicolette N. Houreld
International Journal of Molecular Sciences.2022; 23(13): 7273. CrossRef - Validation of the traditional medicinal use of a Mexican endemic orchid (Prosthechea karwinskii) through UPLC-ESI-qTOF-MS/MS characterization of its bioactive compounds
Gabriela Soledad Barragán-Zarate, Luicita Lagunez-Rivera, Rodolfo Solano, Candy Carranza-Álvarez, Diego Manuel Hernández-Benavides, Gerard Vilarem
Heliyon.2022; 8(7): e09867. CrossRef - N-acetyl-L-cysteine ameliorates hepatocyte pyroptosis of dog type 1 diabetes mellitus via suppression of NLRP3/NF-κB pathway
Haihua Huo, Haitong Wu, Feiyang Ma, Xinrun Li, Jianzhao Liao, Lianmei Hu, Qingyue Han, Ying Li, Jiaqiang Pan, Hui Zhang, Zhaoxin Tang, Jianying Guo
Life Sciences.2022; 306: 120802. CrossRef - Vitamin D regulates insulin and ameliorates apoptosis and oxidative stress in pancreatic tissues of rats with streptozotocin-induced diabetes
Fatima El Zahra M. Fathi, Kadry M. Sadek, Asmaa F. Khafaga, Abdel Wahab Al senosy, Hanan A. Ghoniem, Sahar Fayez, Mohamed F. Zeweil
Environmental Science and Pollution Research.2022; 29(60): 90219. CrossRef - Hypoglycemic, Hypolipidemic and Antioxidant Potentials of Ethanolic Stem Bark Extract of Anacardium occidentale in Streptozotocin-Induced Diabetic Rats
F. A Bamisaye, R. A Ibrahim, A.O. Sulyman, A.O. Jubril, Olawale Ajuwon
Nigerian Journal of Physiological Sciences.2022; 37(1): 137. CrossRef - Protective Effects of the Bilobalide on Retinal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats
Qiang Su, Jing Dong, Donglei Zhang, Lu Yang, Rupak Roy
Applied Biochemistry and Biotechnology.2022; 194(12): 6407. CrossRef - Effects of Psychotropic Medication on Somatic Sterol Biosynthesis of Adult Mice
Marta Balog, Allison C Anderson, Marija Heffer, Zeljka Korade, Karoly Mirnics
Biomolecules.2022; 12(10): 1535. CrossRef - Myricitrin and Its Solid Lipid Nanoparticle Increase Insulin Secretion and Content of Isolated Islets from the Pancreas of Male Mice
Akram Ahangarpour, Ali Akbar Oroojan
Brazilian Journal of Pharmaceutical Sciences.2022;[Epub] CrossRef - Diabetes and plant‐derived natural products: From ethnopharmacological approaches to their potential for modern drug discovery and development
Arun K. Jugran, Sandeep Rawat, Hari P. Devkota, Indra D. Bhatt, Ranbeer S. Rawal
Phytotherapy Research.2021; 35(1): 223. CrossRef - Exploring brain insulin resistance in adults with bipolar depression using extracellular vesicles of neuronal origin
Rodrigo B. Mansur, Francheska Delgado-Peraza, Mehala Subramaniapillai, Yena Lee, Michelle Iacobucci, Flora Nasri, Nelson Rodrigues, Joshua D. Rosenblat, Elisa Brietzke, Victoria E. Cosgrove, Nicole E. Kramer, Trisha Suppes, Charles L. Raison, Andrea Fagio
Journal of Psychiatric Research.2021; 133: 82. CrossRef - Protective Effects of Iridoid Glycoside from Corni Fructus on Type 2 Diabetes with Nonalcoholic Fatty Liver in Mice
Dou Niu, Xue Chen, Ting Wang, Fuxing Wang, Qiusheng Zhang, Xiaochang Xue, Jiefang Kang, Dan Qian Chen
BioMed Research International.2021; 2021: 1. CrossRef - Microdose Lithium Protects against Pancreatic Islet Destruction and Renal Impairment in Streptozotocin-Elicited Diabetes
Jiahui Zhang, Fnu Anshul, Deepak K. Malhotra, Juan Jaume, Lance D. Dworkin, Rujun Gong
Antioxidants.2021; 10(1): 138. CrossRef - p-Terphenyls From Aspergillus sp. GZWMJZ-055: Identification, Derivation, Antioxidant and α-Glycosidase Inhibitory Activities
Yanchao Xu, Yong Wang, Dan Wu, Wenwen He, Liping Wang, Weiming Zhu
Frontiers in Microbiology.2021;[Epub] CrossRef - Updated Role of Neuropeptide Y in Nicotine-Induced Endothelial Dysfunction and Atherosclerosis
Yan-li Zheng, Wan-da Wang, Mei-mei Li, Shu Lin, Hui-li Lin
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - IRS-2/Akt/GSK-3β/Nrf2 Pathway Contributes to the Protective Effects of Chikusetsu Saponin IVa against Lipotoxicity
Lei Wang, Jialin Duan, Na Jia, Meiyou Liu, Shanshan Cao, Yan Weng, Wei Zhang, Jinyi Cao, Ruili Li, Jia Cui, Jingwen Wang, Silvana Hrelia
Oxidative Medicine and Cellular Longevity.2021; 2021: 1. CrossRef - In Vivo and In Vitro Assays Evaluating the Biological Activity of Taurine, Glucose and Energetic Beverages
Marcos Mateo-Fernández, Fernando Valenzuela-Gómez, Rafael Font, Mercedes Del Río-Celestino, Tania Merinas-Amo, Ángeles Alonso-Moraga
Molecules.2021; 26(8): 2198. CrossRef - Effect of propolis on glycemic control in patients with type 2 diabetes: an updated systematic review and meta-analysis of randomized controlled trials
Zahra Mosallanezhad, Cain Clark, Fatemeh Bahreini, Zahra Motamed, Abdolhamid Mosallanezhad, Seyedeh Fatemeh Hosseini, Aneseh Shaban-Khalaf, Zahra Sohrabi
Nutrition & Food Science .2021; 51(7): 1124. CrossRef - The Correlation between Selenium Dependent Glutathione Peroxidase Activity and Oxidant/Antioxidant Balance in Sera of Diabetic Patients with Nephropathy
Abdulateef Altuhafi, Muhammed Altun, Mahmoud Hussein Hadwan
Reports of Biochemistry and Molecular Biology.2021; 10(2): 164. CrossRef - Effect of alpha-lipoic acid on oxidative stress parameters: A systematic review and meta-analysis
Sanaz Rezaei Zonooz, Motahareh Hasani, Mojgan Morvaridzadeh, Ana Beatriz Pizarro, Hafez Heydari, Somaye Yosaee, Gholamreza Rezamand, Javad Heshmati
Journal of Functional Foods.2021; 87: 104774. CrossRef - The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease
Kristian Leisegang, Shubhadeep Roychoudhury, Petr Slama, Renata Finelli
Antioxidants.2021; 10(11): 1834. CrossRef - Роль системы врождённого иммунитета и окислительного стресса в развитии сахарного диабета 1-типа. Пероксиредоксин 6 как новый антидиабетический препарат
Е.Г. Новосёлова, О.В. Глушкова, М.О. Хренов, С.М. Лунин, Т.В. Новосёлова, С.Б. Парфенюк
Биохимия.2021; 86(12): 1840. CrossRef - Role of Innate Immunity and Oxidative Stress in the Development of Type 1 Diabetes Mellitus. Peroxiredoxin 6 as a New Anti-Diabetic Agent
Elena G. Novoselova, Olga V. Glushkova, Maxim O. Khrenov, Sergey M. Lunin, Tatyana V. Novoselova, Svetlana B. Parfenuyk
Biochemistry (Moscow).2021; 86(12-13): 1579. CrossRef - Participation of Hsp70 and Hsp90α Heat Shock Proteins in Stress Response in the Course of Type 1 Diabetes Mellitus
E. G. Novoselova, O. V. Glushkova, M. O. Khrenov, S. B. Parfenyuk, S. M. Lunin, T. V. Novoselova, E. E. Fesenko
Doklady Biological Sciences.2020; 493(1): 124. CrossRef - The correlation between blood oxidative stress and sialic acid content in diabetic patients with nephropathy, hypertension, and hyperlipidemia
Sedigheh Shahvali, Armita Shahesmaeili, Mojgan Sanjari, Somayyeh Karami-Mohajeri
Diabetology International.2020; 11(1): 19. CrossRef - Green synthesis of gold nanoparticles from Fritillaria cirrhosa and its anti-diabetic activity on Streptozotocin induced rats
Ying Guo, Nan Jiang, Li Zhang, Min Yin
Arabian Journal of Chemistry.2020; 13(4): 5096. CrossRef - The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases
Andrés García-Sánchez, Alejandra Guillermina Miranda-Díaz, Ernesto Germán Cardona-Muñoz
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef - Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations
Susbin Raj Wagle, Bozica Kovacevic, Daniel Walker, Corina Mihaela Ionescu, Melissa Jones, Goran Stojanovic, Sanja Kojic, Armin Mooranian, Hani Al-Salami
Pharmaceutics.2020; 12(8): 708. CrossRef - Triglyceride is independently correlated with insulin resistance and islet beta cell function: a study in population with different glucose and lipid metabolism states
Minglei Ma, Haibin Liu, Jie Yu, Shuli He, Pingping Li, Chunxiao Ma, Huabing Zhang, Lingling Xu, Fan Ping, Wei Li, Qi Sun, Yuxiu Li
Lipids in Health and Disease.2020;[Epub] CrossRef - Protective Effects of Grape Seed Proanthocyanidins on the Kidneys of Diabetic Rats through the Nrf2 Signalling Pathway
Yusong Ding, Haiyan Li, Yang Li, Dandan Liu, Liyuan Zhang, Tongling Wang, Tao Liu, Long Ma, Rocío De la Puerta
Evidence-Based Complementary and Alternative Medicine.2020; 2020: 1. CrossRef - The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
Diabetes & Metabolism Journal.2020; 44(2): 222. CrossRef - Roles of Autophagy in Oxidative Stress
Hyeong Rok Yun, Yong Hwa Jo, Jieun Kim, Yoonhwa Shin, Sung Soo Kim, Tae Gyu Choi
International Journal of Molecular Sciences.2020; 21(9): 3289. CrossRef - Antioxidant and prebiotic effects of a beverage composed by tropical fruits and yacon in alloxan-induced diabetic rats
Ana Paula DIONISIO, Luciano Bruno de CARVALHO-SILVA, Nara Menezes VIEIRA, Nedio Jair WURLITZER, Ana Carolina da Silva PEREIRA, Maria de Fatima BORGES, Deborah dos Santos GARRUTI, Idila dos Santos ARAÚJO
Food Science and Technology.2020; 40(1): 202. CrossRef - Pro-oxidant–antioxidant balance (PAB) as a prognostic index in assessing the cardiovascular risk factors: A narrative review
Hamideh Ghazizadeh, Maryam Saberi-Karimian, Maliheh Aghasizadeh, Reza Sahebi, Hamed Ghazavi, Hamed Khedmatgozar, Ameneh Timar, Mohadeseh Rohban, Ali Javandoost, Majid Ghayour-Mobarhan
Obesity Medicine.2020; 19: 100272. CrossRef - Rosemary Leaf Extract Inhibits Glycation, Breast Cancer Proliferation, and Diabetes Risks
Yixiao Shen, Jing Han, Xiaoyan Zheng, Binling Ai, Yang Yang, Dao Xiao, Lili Zheng, Zhanwu Sheng
Applied Sciences.2020; 10(7): 2249. CrossRef - Trigonella stellata reduced the deleterious effects of diabetes mellitus through alleviation of oxidative stress, antioxidant- and drug-metabolizing enzymes activities
Salah A. Sheweita, Sara A. ElHady, Hala M. Hammoda
Journal of Ethnopharmacology.2020; 256: 112821. CrossRef - A Shift Toward a Plant-Centered Diet From Young to Middle Adulthood and Subsequent Risk of Type 2 Diabetes and Weight Gain: The Coronary Artery Risk Development in Young Adults (CARDIA) Study
Yuni Choi, Nicole Larson, Daniel D. Gallaher, Andrew O. Odegaard, Jamal S. Rana, James M. Shikany, Lyn M. Steffen, David R. Jacobs
Diabetes Care.2020; 43(11): 2796. CrossRef - Ascorbic acid supplementation in type 2 diabetes mellitus
Lipeng Shi, Xuqin Du, Pei Guo, Lumei Huang, Peng Qi, Qianhui Gong
Medicine.2020; 99(45): e23125. CrossRef - Analysis of the Effect of Fish Bars Made of Bilih Fish (Mystacoleuseus padangensis Blkr) Flour to Reduce Oxidative Stress in a Diabetic Rat Model
Deni ELNOVRIZA, Hadi RIYADI, Rimbawan RIMBAWAN, Evy DAMAYANTHI, Adi WINARTO
Journal of Nutritional Science and Vitaminology.2020; 66(Supplement): S36. CrossRef - Antioxidant activity of crude ethanolic extract and fractions of Ziziphus mauritiana Lam. (Rhamnaceae) leaves from Burkina Faso
Estelle N.H. Youl, Cyrille A.P. Ouédraogo, Moustapha Gambo, Moussa Ouédraogo, Martin Kiendrebéogo, Aristide Traoré, Innocent Pierre Guissou
Journal of Basic and Clinical Physiology and Pharmacology.2019;[Epub] CrossRef - CD36 dependent redoxosomes promotes ceramide-mediated pancreatic β-cell failure via p66Shc activation
Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 134: 505. CrossRef - Comparative proteomic analysis reveals drug resistance of Staphylococcus xylosus ATCC700404 under tylosin stress
Xin Liu, Jinpeng Wang, Mo Chen, Ruixiang Che, Wenya Ding, Fei Yu, Yonghui Zhou, Wenqiang Cui, Xing Xiaoxu, Bello-Onaghise God’spower, Yanhua Li
BMC Veterinary Research.2019;[Epub] CrossRef - The Potential of Bilih Fish (Mystacoleuseus padangensis Blkr) Flour as a Zinc Source to Control Blood Glucose and Impact on Oxidative Stress in a Diabetic Rat Model
Deni Elnovriza, Hadi Riyadi, Rimbawan ., Evy Damayanthi, Adi Winarto
Pakistan Journal of Nutrition.2019; 18(3): 264. CrossRef - Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments
Zuo, Prather, Stetskiv, Garrison, Meade, Peace, Zhou
International Journal of Molecular Sciences.2019; 20(18): 4472. CrossRef - The variations of some salivary parameters as probable indices of the hereditary diabetes
Menicagli Roberto, Marotta Ortensio
International Journal of Preventive Medicine.2019; 10(1): 11. CrossRef - The Interrelationship between Job Stress with the Immune System and Functional Memory of Women Working in Diagnostic Laboratories
Mansoureh Sadeghi-Yarandi, Anahita Khodabakhshi-Koolaee, Mohammad Reza Falsafinejad, Neda Khaletbari
The Neuroscience Journal of Shefaye Khatam.2019; 7(2): 23. CrossRef - Effect of Cinnamon Administration on Fertility of Normal and Diabetic Male Rats
Mahmoud Al-Shawabk, Abdulrahim Al Jamal
Pakistan Journal of Nutrition.2019; 18(5): 491. CrossRef - White Sesame Seed Oil Mitigates Blood Glucose Level, Reduces Oxidative Stress, and Improves Biomarkers of Hepatic and Renal Function in Participants with Type 2 Diabetes Mellitus
Farhan Aslam, Sanaullah Iqbal, Muhammad Nasir, Aftab Ahmad Anjum
Journal of the American College of Nutrition.2019; 38(3): 235. CrossRef - Effect of combination of Gynura procumbens aqueous extract and Trigona spp. honey on fertility and libido of streptozotocin-induced hyperglycaemic male rats
Khaidatul Akmar, MahanemMat Noor
Asian Pacific Journal of Reproduction.2019; 8(2): 56. CrossRef - Serum Antioxidant Parameters are Significantly Increased in Patients with Type 2 Diabetes Mellitus after Consumption of Chinese Propolis: A Randomized Controlled Trial Based on Fasting Serum Glucose Level
Weina Gao, Lingling Pu, Jingyu Wei, Zhanxin Yao, Yawen Wang, Tala Shi, Liting Zhao, Changya Jiao, Changjiang Guo
Diabetes Therapy.2018; 9(1): 101. CrossRef - Baccharis trimera (Carqueja) Improves Metabolic and Redox Status in an Experimental Model of Type 1 Diabetes
Natália Nogueira do Nascimento Kaut, Ana Carolina Silveira Rabelo, Glaucy Rodrigues Araujo, Jason Guy Taylor, Marcelo Eustáquio Silva, Maria Lúcia Pedrosa, Miriam Martins Chaves, Joamyr Victor Rossoni Junior, Daniela Caldeira Costa
Evidence-Based Complementary and Alternative Medicine.2018; 2018: 1. CrossRef - The Potential of South African Herbal Tisanes, Rooibos and Honeybush in the Management of Type 2 Diabetes Mellitus
Olawale Ajuwon, Ademola Ayeleso, Gbenga Adefolaju
Molecules.2018; 23(12): 3207. CrossRef - Clinical variables and ethnicity may influenced by polymorphism of CAT −262C/T and MnSOD 47C/T antioxidant enzymes in Algerian type1 diabetes without complications
A. Eddaikra, H. Amroun, R. Raache, A. Galleze, N. Abdallah-Elhadj, M. Azzouz, F. Meçabih, B. Mechti, M.C. Abbadi, C. Touil-Boukoffa, N. Attal
Gene.2018; 670: 182. CrossRef - Crystal structure and functional characterization of selenocysteine-containing glutathione peroxidase 4 suggests an alternative mechanism of peroxide reduction
Astrid Borchert, Jacqueline Kalms, Sophia R. Roth, Marlena Rademacher, Andrea Schmidt, Hermann-Georg Holzhutter, Hartmut Kuhn, Patrick Scheerer
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2018; 1863(9): 1095. CrossRef - The effects of green cardamom supplementation on blood glucose, lipids profile, oxidative stress, sirtuin-1 and irisin in type 2 diabetic patients: a study protocol for a randomized placebo-controlled clinical trial
Mohadeseh Aghasi, Shohreh Ghazi-Zahedi, Fariba Koohdani, Fereydoun Siassi, Ensieh Nasli-Esfahani, Ali Keshavarz, Mostafa Qorbani, Hoorieh Khoshamal, Asma Salari-Moghaddam, Gity Sotoudeh
BMC Complementary and Alternative Medicine.2018;[Epub] CrossRef - Mice pancreatic islets protection from oxidative stress induced by single-walled carbon nanotubes through naringin
A Ahangarpour, S Alboghobeish, AA Oroojan, MA Dehghani
Human & Experimental Toxicology.2018; 37(12): 1268. CrossRef - Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity
Biplab Giri, Sananda Dey, Tanaya Das, Mrinmoy Sarkar, Jhimli Banerjee, Sandeep Kumar Dash
Biomedicine & Pharmacotherapy.2018; 107: 306. CrossRef - Cognitive dysfunction and metabolic comorbidities in mood disorders: A repurposing opportunity for glucagon-like peptide 1 receptor agonists?
Rodrigo B. Mansur, Yena Lee, Mehala Subramaniapillai, Elisa Brietzke, Roger S. McIntyre
Neuropharmacology.2018; 136: 335. CrossRef - The protective effects of a novel synthetic β-elemene derivative on human umbilical vein endothelial cells against oxidative stress-induced injury: Involvement of antioxidation and PI3k/Akt/eNOS/NO signaling pathways
Khalil Ali Ahmad, Hong Ze, Jichao Chen, Farhan Ullah Khan, Chen Xuezhuo, Jinyi Xu, Ding Qilong
Biomedicine & Pharmacotherapy.2018; 106: 1734. CrossRef - Curcumin alleviates liver oxidative stress in type 1 diabetic rats
Zhenglu Xie, Binbin Wu, Guozhi Shen, Xiaqing Li, Qianying Wu
Molecular Medicine Reports.2017;[Epub] CrossRef - Treatment with a GLP−1R agonist over four weeks promotes weight loss-moderated changes in frontal-striatal brain structures in individuals with mood disorders
Rodrigo B. Mansur, Andre Zugman, Juhie Ahmed, Danielle S. Cha, Mehala Subramaniapillai, Yena Lee, Julie Lovshin, Jung G. Lee, Jae-Hon Lee, Vladislav Drobinin, Jason Newport, Elisa Brietzke, Eva Z. Reininghaus, Kang Sim, Maj Vinberg, Natalie Rasgon, Tomas
European Neuropsychopharmacology.2017; 27(11): 1153. CrossRef - Plasma vascular endothelial growth factor B levels are increased in patients with newly diagnosed type 2 diabetes mellitus and associated with the first phase of glucose-stimulated insulin secretion function of β-cell
J. Wu, H. Wei, H. Qu, Z. Feng, J. Long, Q. Ge, H. Deng
Journal of Endocrinological Investigation.2017; 40(11): 1219. CrossRef - Associations of Dietary Antioxidants and Risk of Type 2 Diabetes: Data from the 2007–2012 Korea National Health and Nutrition Examination Survey
Dan Quansah, Kyungho Ha, Shinyoung Jun, Seong-Ah Kim, Sangah Shin, Gyung-Ah Wie, Hyojee Joung
Molecules.2017; 22(10): 1664. CrossRef - Curcumin attenuates oxidative stress in liver in Type 1 diabetic rats
Zhenglu Xie, Xinqi Zeng, Xiaqing Li, Binbin Wu, Guozhi Shen, Qianying Wu, Changbiao Wu
Open Life Sciences.2017; 12(1): 452. CrossRef - Metabolic control and periodontal treatment decreases elevated oxidative stress in the early phases of type 1 diabetes onset
Cüneyt A. Aral, Özlem Nalbantoğlu, Bilge G. Nur, Mustafa Altunsoy, Kübra Aral
Archives of Oral Biology.2017; 82: 115. CrossRef - Evaluation and Application of a Novel Quantitative Antioxidant Activity Assay Based on Cellular Metabolomics
Jianteng Wei, Qingping Hu, Ningli Wang, Yewei Liu, Dong Pei, Duolong Di
Chromatographia.2017; 80(4): 617. CrossRef - C-X-C motif chemokine ligand 8 promotes endothelial cell homing via the Akt-signal transducer and activator of transcription pathway to accelerate healing of ischemic and hypoxic skin ulcers
Lei Shen, Peng Zhang, Shanqiang Zhang, Liping Xie, Lijie Yao, Weiya Lang, Jie Lian, Wei Qin, Meng Zhang, Liang Ji
Experimental and Therapeutic Medicine.2017; 13(6): 3021. CrossRef - STEAP4: its emerging role in metabolism and homeostasis of cellular iron and copper
Rachel T Scarl, C Martin Lawrence, Hannah M Gordon, Craig S Nunemaker
Journal of Endocrinology.2017; 234(3): R123. CrossRef - Current views on neuropeptide Y and diabetes-related atherosclerosis
Wei-wei Sun, Ping Zhu, Yan-chuan Shi, Chen-liang Zhang, Xu-feng Huang, Shi-yu Liang, Zhi-yuan Song, Shu Lin
Diabetes and Vascular Disease Research.2017; 14(4): 277. CrossRef - Teucrium polium extract reverses symptoms of streptozotocin-induced diabetes in rats via rebalancing the Pdx1 and FoxO1 expressions
Parvaneh Sadat Tabatabaie, Razieh Yazdanparast
Biomedicine & Pharmacotherapy.2017; 93: 1033. CrossRef - Morin activates the Nrf2-ARE pathway and reduces oxidative stress-induced DNA damage in pancreatic beta cells
Pachamuthu Vanitha, Sankareswaran Senthilkumar, Sireesh Dornadula, Sundaramurthy Anandhakumar, Palanisamy Rajaguru, Kunka Mohanram Ramkumar
European Journal of Pharmacology.2017; 801: 9. CrossRef - Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir
Jian Zhang, Xiao Zhao, Yunyun Jiang, Wen Zhao, Ting Guo, Yongqiang Cao, Junwei Teng, Xiaona Hao, Juan Zhao, Zhennai Yang
Journal of Dairy Science.2017; 100(8): 6025. CrossRef - Metformin prevents glucotoxicity by alleviating oxidative and ER stress–induced CD36 expression in pancreatic beta cells
Jun Sung Moon, Udayakumar Karunakaran, Suma Elumalai, In-Kyu Lee, Hyoung Woo Lee, Yong-Woon Kim, Kyu Chang Won
Journal of Diabetes and its Complications.2017; 31(1): 21. CrossRef - Differential dose-dependent effects of zinc oxide nanoparticles on oxidative stress-mediated pancreatic β-cell death
Swati C Asani, Rinku D Umrani, Kishore M Paknikar
Nanomedicine.2017; 12(7): 745. CrossRef - Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy
Yu-Guo Yuan, Qiu-Ling Peng, Sangiliyandi Gurunathan
International Journal of Molecular Sciences.2017; 18(3): 569. CrossRef - Rac1-NADPH oxidase signaling promotes CD36 activation under glucotoxic conditions in pancreatic beta cells
Suma Elumalai, Udayakumar Karunakaran, In Kyu Lee, Jun Sung Moon, Kyu Chang Won
Redox Biology.2017; 11: 126. CrossRef - Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin
Samsulrizal Nurdiana, Yong Meng Goh, Hafandi Ahmad, Sulaiman Md Dom, Nur Syimal’ain Azmi, Noor Syaffinaz Noor Mohamad Zin, Mahdi Ebrahimi
BMC Complementary and Alternative Medicine.2017;[Epub] CrossRef - Murraya paniculata (L.) (Orange Jasmine): Potential Nutraceuticals with Ameliorative Effect in Alloxan‐Induced Diabetic Rats
Cicero Diego Almino Menezes, Francisca Adilfa de Oliveira Garcia, Glauce Socorro de Barros Viana, Patricia Gonçalves Pinheiro, Cícero Francisco Bezerra Felipe, Thaís Rodrigues de Albuquerque, Alisson Cordeiro Moreira, Enaide Soares Santos, Maynara Rodrigu
Phytotherapy Research.2017; 31(11): 1747. CrossRef - Exploiting biological activities of brown seaweed Ishige okamurae Yendo for potential industrial applications: a review
K. K. Asanka Sanjeewa, Won Woo Lee, Jae-Il Kim, You-Jin Jeon
Journal of Applied Phycology.2017; 29(6): 3109. CrossRef - The thioredoxin and glutathione-dependent H2O2 consumption pathways in muscle mitochondria: Involvement in H2O2 metabolism and consequence to H2O2 efflux assays
Daniel Munro, Sheena Banh, Emianka Sotiri, Nahid Tamanna, Jason R. Treberg
Free Radical Biology and Medicine.2016; 96: 334. CrossRef - Effect of crocin extracted from saffron on pro-oxidant–anti-oxidant balance in subjects with metabolic syndrome: A randomized, placebo-controlled clinical trial
I. Nikbakht-Jam, M. Khademi, M. Nosrati, S. Eslami, M. Foroutan-Tanha, A. Sahebkar, S. Tavalaie, M. Ghayour-Mobarhan, G.A.A. Ferns, F. Hadizadeh, S.A.S. Tabassi, S.A. Mohajeri, M. Emamian
European Journal of Integrative Medicine.2016; 8(3): 307. CrossRef - Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic β-cell line
Armin Mooranian, Rebecca Negrulj, Frank Arfuso, Hani Al-Salami
Artificial Cells, Nanomedicine, and Biotechnology.2016; 44(7): 1642. CrossRef - Lipid peroxidation is associated with poor control of type-2 diabetes mellitus
Sameer Hassan Fatani, Abdullatif Taha Babakr, EssamEldin Mohamed NourEldin, Abdalla A. Almarzouki
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2016; 10(2): S64. CrossRef - Increased Electron Paramagnetic Resonance Signal Correlates with Mitochondrial Dysfunction and Oxidative Stress in an Alzheimer’s disease Mouse Brain
Du Fang, Zhihua Zhang, Hang Li, Qing Yu, Justin T. Douglas, Anna Bratasz, Periannan Kuppusamy, Shirley ShiDu Yan, Hemachandra Reddy
Journal of Alzheimer's Disease.2016; 51(2): 571. CrossRef - Coffee silverskin extract improves glucose-stimulated insulin secretion and protects against streptozotocin-induced damage in pancreatic INS-1E beta cells
Beatriz Fernandez-Gomez, Sonia Ramos, Luis Goya, María Dolores Mesa, María Dolores del Castillo, María Ángeles Martín
Food Research International.2016; 89: 1015. CrossRef - Male Subfertility Induced by Heterozygous Expression of Catalytically Inactive Glutathione Peroxidase 4 Is Rescued in Vivo by Systemic Inactivation of the Alox15 Gene
Simone Hanna Brütsch, Marlena Rademacher, Sophia Regina Roth, Karin Müller, Susanne Eder, Dagmar Viertel, Christiane Franz, Hartmut Kuhn, Astrid Borchert
Journal of Biological Chemistry.2016; 291(45): 23578. CrossRef - Assessment of antioxidant activity, lipid profile, general biochemical and immune system responses of Wistar rats fed with dairy dessert containing Lactobacillus acidophilus La-5
C.S. Moura, P.C.B. Lollo, P.N. Morato, E.A. Esmerino, L.P. Margalho, V.A. Santos-Junior, P.T. Coimbra, L.P. Cappato, M.C. Silva, A.S. Garcia-Gomes, D. Granato, H.M. A. Bolini, A.S. Sant'Ana, A.G. Cruz, Jaime Amaya-Farfan
Food Research International.2016; 90: 275. CrossRef - Discovery of biomarkers for oxidative stress based on cellular metabolomics
Ningli Wang, Jianteng Wei, Yewei Liu, Dong Pei, Qingping Hu, Yu Wang, Duolong Di
Biomarkers.2016; 21(5): 449. CrossRef - Diabetic nephropathy and endothelial dysfunction: Current and future therapies, and emerging of vascular imaging for preclinical renal-kinetic study
Wilson KC Leung, L Gao, Parco M Siu, Christopher WK Lai
Life Sciences.2016; 166: 121. CrossRef - Brazilian Green Propolis Improves Antioxidant Function in Patients with Type 2 Diabetes Mellitus
Liting Zhao, Lingling Pu, Jingyu Wei, Jinghua Li, Jianquan Wu, Zhonghao Xin, Weina Gao, Changjiang Guo
International Journal of Environmental Research and Public Health.2016; 13(5): 498. CrossRef - Inhibition of Adenylyl Cyclase Type 5 Increases Longevity and Healthful Aging through Oxidative Stress Protection
Stephen F. Vatner, Ronald E. Pachon, Dorothy E. Vatner
Oxidative Medicine and Cellular Longevity.2015; 2015: 1. CrossRef - Recent Breakthroughs in the Antioxidant and Anti-Inflammatory Effects of Morella and Myrica Species
Bruno Silva, Ana Seca, Maria Barreto, Diana Pinto
International Journal of Molecular Sciences.2015; 16(8): 17160. CrossRef - Oxidative Stress Type Influences the Properties of Antioxidants Containing Polyphenols in RINm5F Beta Cells
Nathalie Auberval, Stéphanie Dal, William Bietiger, Elodie Seyfritz, Jean Peluso, Christian Muller, Minjie Zhao, Eric Marchioni, Michel Pinget, Nathalie Jeandidier, Elisa Maillard, Valérie Schini-Kerth, Séverine Sigrist
Evidence-Based Complementary and Alternative Medicine.2015; 2015: 1. CrossRef - CD36 initiated signaling mediates ceramide-induced TXNIP expression in pancreatic beta-cells
Udayakumar Karunakaran, Jun Sung Moon, Hyoung Woo Lee, Kyu Chang Won
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2015; 1852(11): 2414. CrossRef - Free radicals and related reactive species as mediators of tissue injury and disease: implications for Health
James P. Kehrer, Lars-Oliver Klotz
Critical Reviews in Toxicology.2015; 45(9): 765. CrossRef - Expression of Inactive Glutathione Peroxidase 4 Leads to Embryonic Lethality, and Inactivation of theAlox15Gene Does Not Rescue Such Knock-In Mice
Simone Hanna Brütsch, Chi Chiu Wang, Lu Li, Hannelore Stender, Nilgün Neziroglu, Constanze Richter, Hartmut Kuhn, Astrid Borchert
Antioxidants & Redox Signaling.2015; 22(4): 281. CrossRef - Islet β-cell failure in type 2 diabetes – Within the network of toxic lipids
Justyna Janikiewicz, Katarzyna Hanzelka, Kamil Kozinski, Katarzyna Kolczynska, Agnieszka Dobrzyn
Biochemical and Biophysical Research Communications.2015; 460(3): 491. CrossRef - Endoplasmic reticulum stress and Nrf2 repression in circulating cells of type 2 diabetic patients without the recommended glycemic goals
C. Mozzini, U. Garbin, C. Stranieri, A. Pasini, E. Solani, I. A. Tinelli, L. Cominacini, A.M. Fratta Pasini
Free Radical Research.2015; 49(3): 244. CrossRef - El estrés oxidativo como predictor de longevidad; estudio de casos y controles
Ángel Belenguer Varea, Kheira Mohamed Abdelaziz, Juan Antonio Avellana Zaragoza, Consuelo Borrás Blasco, Paula Sanchis Aguilar, José Viña Ribes
Revista Española de Geriatría y Gerontología.2015; 50(1): 16. CrossRef - Microbial phenolic metabolites improve glucose-stimulated insulin secretion and protect pancreatic beta cells against tert-butyl hydroperoxide-induced toxicity via ERKs and PKC pathways
Elisa Fernández-Millán, Sonia Ramos, Carmen Alvarez, Laura Bravo, Luis Goya, María Ángeles Martín
Food and Chemical Toxicology.2014; 66: 245. CrossRef - Decreased serum CA19‐9 is associated with improvement of insulin resistance and metabolic control in patients with obesity and type 2 diabetes after Roux‐en‐Y gastric bypass
Yinfang Tu, Haoyong Yu, Pin Zhang, Jianzhong Di, Xiaodong Han, Songhua Wu, Yuqian Bao, Weiping Jia
Journal of Diabetes Investigation.2014; 5(6): 694. CrossRef - Oxidative Stress Is Associated with an Increased Antioxidant Defense in Elderly Subjects: A Multilevel Approach
Gemma Flores-Mateo, Roberto Elosua, Teresa Rodriguez-Blanco, Josep Basora-Gallisà, Mònica Bulló, Jordi Salas-Salvadó, Miguel Ángel Martínez-González, Ramon Estruch, Dolores Corella, Montserrat Fitó, Miquel Fiol, Fernando Arós, Enrique Gómez-Gracia, Isaac
PLoS ONE.2014; 9(9): e105881. CrossRef - Inflamed moods: A review of the interactions between inflammation and mood disorders
Joshua D. Rosenblat, Danielle S. Cha, Rodrigo B. Mansur, Roger S. McIntyre
Progress in Neuro-Psychopharmacology and Biological Psychiatry.2014; 53: 23. CrossRef - Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes
Soo Lim, Jae Hyun Bae, Eun Ju Chun, Haeryoung Kim, So Yeon Kim, Kyoung Min Kim, Sung Hee Choi, Kyong Soo Park, Jose C. Florez, Hak Chul Jang
Acta Diabetologica.2014; 51(5): 739. CrossRef - Role of Lipid Peroxidation Products, Plasma Total Antioxidant Status, and Cu-, Zn-Superoxide Dismutase Activity as Biomarkers of Oxidative Stress in Elderly Prediabetics
Sylwia Dzięgielewska-Gęsiak, Ewa Wysocka, Sławomir Michalak, Ewa Nowakowska-Zajdel, Teresa Kokot, Małgorzata Muc-Wierzgoń
Oxidative Medicine and Cellular Longevity.2014; 2014: 1. CrossRef - Inhibitors ofα-glucosidase andα-amylase fromCyperus rotundus
Hong Hanh Thi Tran, Minh Chau Nguyen, Hoang Tram Le, Thi Luyen Nguyen, Thanh Binh Pham, Van Minh Chau, Hoai Nam Nguyen, Tien Dat Nguyen
Pharmaceutical Biology.2014; 52(1): 74. CrossRef - Diabetes Mellitus and Disturbances in Brain Connectivity: A Bidirectional Relationship?
Rodrigo B. Mansur, Danielle S. Cha, Hanna O. Woldeyohannes, Joanna K. Soczynska, Andre Zugman, Elisa Brietzke, Roger S. McIntyre
NeuroMolecular Medicine.2014; 16(4): 658. CrossRef - Pathogenesis of Chronic Hyperglycemia: From Reductive Stress to Oxidative Stress
Liang-Jun Yan
Journal of Diabetes Research.2014; 2014: 1. CrossRef - Vitamin C Protects against INS832/13 ^|^beta;-Cell Death and/or Dysfunction Caused by Glucolipotoxicity or 3T3-L1 Adipocyte Coculture
Ruojun WANG, Jia LIU, Xiaoxuan GUO, Fengyi GAO, Baoping JI, Feng ZHOU
Journal of Nutritional Science and Vitaminology.2014; 60(5): 313. CrossRef - Antioxidant activities of novel small-molecule polysaccharide fractions purified from Portulaca oleracea L.
Yu-Ping Li, Li-Hua Yao, Guan-Jie Wu, Xiao-Fang Pi, Yan-Chun Gong, Ruo-Shong Ye, Chen-Xi Wang
Food Science and Biotechnology.2014; 23(6): 2045. CrossRef - Anti-diabetic properties of a non-conventional radical scavenger, as compared to pioglitazone and exendin-4, in streptozotocin-nicotinamide diabetic mice
Michela Novelli, Donatella Canistro, Manuela Martano, Niccola Funel, Andrea Sapone, Simone Melega, Matilde Masini, Vincenzo De Tata, Anna Pippa, Cecilia Vecoli, Daniela Campani, Rocco De Siena, Antonio Soleti, Moreno Paolini, Pellegrino Masiello
European Journal of Pharmacology.2014; 729: 37. CrossRef - Therapeutic Roles of Heme Oxygenase-1 in Metabolic Diseases: Curcumin and Resveratrol Analogues as Possible Inducers of Heme Oxygenase-1
Yong Son, Ju Hwan Lee, Hun-Taeg Chung, Hyun-Ock Pae
Oxidative Medicine and Cellular Longevity.2013; 2013: 1. CrossRef - Endoplasmic Reticulum Oxidoreductin-1α (Ero1α) Improves Folding and Secretion of Mutant Proinsulin and Limits Mutant Proinsulin-induced Endoplasmic Reticulum Stress
Jordan Wright, Julia Birk, Leena Haataja, Ming Liu, Thomas Ramming, Michael A. Weiss, Christian Appenzeller-Herzog, Peter Arvan
Journal of Biological Chemistry.2013; 288(43): 31010. CrossRef - Moving on from GWAS: Functional Studies on the G6PC2 Gene Implicated in the Regulation of Fasting Blood Glucose
Richard M. O’Brien
Current Diabetes Reports.2013; 13(6): 768. CrossRef
- Association Between Serum Ferritin and the Duration of Type 2 Diabetes Mellitus in a Tertiary Care Hospital in Chennai
- Fuel-Stimulated Insulin Secretion Depends upon Mitochondria Activation and the Integration of Mitochondrial and Cytosolic Substrate Cycles
- Gary W. Cline
- Diabetes Metab J. 2011;35(5):458-465. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.458
- 65,535 View
- 38 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The pancreatic islet β-cell is uniquely specialized to couple its metabolism and rates of insulin secretion with the levels of circulating nutrient fuels, with the mitochondrial playing a central regulatory role in this process. In the β-cell, mitochondrial activation generates an integrated signal reflecting rates of oxidativephosphorylation, Kreb's cycle flux, and anaplerosis that ultimately determines the rate of insulin exocytosis. Mitochondrial activation can be regulated by proton leak and mediated by UCP2, and by alkalinization to utilize the pH gradient to drive substrate and ion transport. Converging lines of evidence support the hypothesis that substrate cycles driven by rates of Kreb's cycle flux and by anaplerosis play an integral role in coupling responsive changes in mitochondrial metabolism with insulin secretion. The components and mechanisms that account for the integrated signal of ATP production, substrate cycling, the regulation of cellular redox state, and the production of other secondary signaling intermediates are operative in both rodent and human islet β-cells.
-
Citations
Citations to this article as recorded by- Nicotinamide Adenine Dinucleotide: The Redox Sensor in Aging-Related Disorders
Giovanna Schiuma, Djidjell Lara, James Clement, Marco Narducci, Roberta Rizzo
Antioxidants & Redox Signaling.2024;[Epub] CrossRef - ROCK1 regulates insulin secretion from β-cells
Byung-Jun Sung, Sung-Bin Lim, Won-Mo Yang, Jae Hyeon Kim, Rohit N. Kulkarni, Young-Bum Kim, Moon-Kyu Lee
Molecular Metabolism.2022; 66: 101625. CrossRef - Hyperglycemic Stress and Carbon Stress in Diabetic Glucotoxicity
Xiaoting Luo, Jinzi Wu, Siqun Jing, Liang-Jun Yan
Aging and disease.2016; 7(1): 90. CrossRef - Characterization of Phospholipids in Insulin Secretory Granules and Mitochondria in Pancreatic Beta Cells and Their Changes with Glucose Stimulation
Michael J. MacDonald, Lacmbouh Ade, James M. Ntambi, Israr-Ul H. Ansari, Scott W. Stoker
Journal of Biological Chemistry.2015; 290(17): 11075. CrossRef - Roles of Pyruvate, NADH, and Mitochondrial Complex I in Redox Balance and Imbalance inβCell Function and Dysfunction
Xiaoting Luo, Rongrong Li, Liang-Jun Yan
Journal of Diabetes Research.2015; 2015: 1. CrossRef - Peroxisome Proliferator-activated Receptor γ (PPARγ) and Its Target Genes Are Downstream Effectors of FoxO1 Protein in Islet β-Cells
Dhananjay Gupta, Averi A. Leahy, Navjot Monga, Mina Peshavaria, Thomas L. Jetton, Jack L. Leahy
Journal of Biological Chemistry.2013; 288(35): 25440. CrossRef - Desnutrin/ATGL Activates PPARδ to Promote Mitochondrial Function for Insulin Secretion in Islet β Cells
Tianyi Tang, Marcia J. Abbott, Maryam Ahmadian, Andressa B. Lopes, Yuhui Wang, Hei Sook Sul
Cell Metabolism.2013; 18(6): 883. CrossRef - Potent humanin analog increases glucose‐stimulated insulin secretion through enhanced metabolism in the β cell
Regina Kuliawat, Laura Klein, Zhenwei Gong, Marianna Nicoletta‐Gentile, Anjana Nemkal, Lingguang Cui, Claire Bastie, Kai Su, Derek Huffman, Manju Surana, Nir Barzilai, Norman Fleischer, Radhika Muzumdar
The FASEB Journal.2013; 27(12): 4890. CrossRef - Proliferation and differentiation of osteoblasts from the mandible of osteoporotic rats
Shu-Juan Yu, Hong-Chen Liu, Ling-Ling E, Dong-Sheng Wang, Guo-Xiong Zhu
Experimental Biology and Medicine.2012; 237(4): 395. CrossRef
- Nicotinamide Adenine Dinucleotide: The Redox Sensor in Aging-Related Disorders
- The Causes and Consequences of Low Levels of High Density Lipoproteins in Patients with Diabetes
- Philip J. Barter
- Diabetes Metab J. 2011;35(2):101-106. Published online April 30, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.2.101
- 4,139 View
- 38 Download
- 42 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Type 2 diabetes is commonly accompanied by a low level of high density lipoprotein cholesterol (HDL-C) that contributes to the increased cardiovascular risk associated with this condition. Given that HDLs have the ability to improve increase the uptake of glucose by skeletal muscle and to stimulate the secretion of insulin from pancreatic beta cells the possibility arises that a low HDL concentration in type 2 diabetes may also contribute to a worsening of diabetic control. Thus, there is a double case for raising the level of HDL-C in patients with type 2 diabetes: to reduce cardiovascular risk and to improve glycemic control. Approaches to raising HDL-C include lifestyle factors such as weight reduction, increased physical activity and stopping smoking. Of currently available drugs, the most effective is niacin. Newer formulations of niacin are reasonably well tolerated and have the ability to increase HDL-C by up to 30%. The effect of niacin on cardiovascular events in type 2 diabetes is currently being tested in a large-scale clinical outcome trial.
-
Citations
Citations to this article as recorded by- Children and Adolescents With Hybrid Diabetes: A Management Conundrum
Charles A. Gagnon, Jessica A. Schmitt, Ambika P. Ashraf
Endocrine Practice.2024; 30(1): 49. CrossRef - Remnant Cholesterol Is an Independent Predictor of Type 2 Diabetes: A Nationwide Population-Based Cohort Study
Ji Hye Huh, Eun Roh, Seong Jin Lee, Sung-Hee Ihm, Kyung-Do Han, Jun Goo Kang
Diabetes Care.2023; 46(2): 305. CrossRef - Ameliorating effect of probiotic on nonalcoholic fatty liver disease and lipolytic gene expression in rabbits
Marina Aziz, Shabaan A. Hemeda, Ghadeer M. Albadrani, Sabreen E. Fadl, Fatma Elgendey
Scientific Reports.2023;[Epub] CrossRef - Searching for new genes associated with the familial hypercholesterolemia phenotype using whole-genome sequencing and machine learning
D. E. Ivanoshchuk, A. B. Kolker, O. V. Timoshchenko, S. E. Semaev, E. V. Shakhtshneider
Vavilov Journal of Genetics and Breeding.2023; 27(5): 522. CrossRef - Non-HDL cholesterol as a predictor for incident type 2 diabetes in community-dwelling adults: longitudinal findings over 12 years
In-Ho Seo, Da-Hye Son, Hye Sun Lee, Yong-Jae Lee
Translational Research.2022; 243: 52. CrossRef - Biological and sociocultural determinants of increased blood pressure among women with a history of gestational diabetes mellitus in rural China: a retrospective cohort study
Jia Guo, Wencong Lv, Shan Jiang, Yujia Tang, Qing Long, Jundi Yang, James Allen Wiley, Monica Parry
BMJ Open.2022; 12(1): e049625. CrossRef - A Nutrigenetic Update on CETP Gene–Diet Interactions on Lipid-Related Outcomes
Ramatu Wuni, Gunter G. C. Kuhnle, Alexandra Azzari Wynn-Jones, Karani Santhanakrishnan Vimaleswaran
Current Atherosclerosis Reports.2022; 24(2): 119. CrossRef - HDL functionality in type 1 and type 2 diabetes: new insights
M. John Chapman
Current Opinion in Endocrinology, Diabetes & Obesity.2022; 29(2): 112. CrossRef - Telomeres do not always shorten over time in individuals with type 1 diabetes
Anna Syreeni, Luke M. Carroll, Stefan Mutter, Andrzej S. Januszewski, Carol Forsblom, Markku Lehto, Per-Henrik Groop, Alicia J. Jenkins
Diabetes Research and Clinical Practice.2022; 188: 109926. CrossRef - Impact of Lipid Genetic Risk Score and Saturated Fatty Acid Intake on Central Obesity in an Asian Indian Population
Ramatu Wuni, Evelyn Adela Nathania, Ashok K. Ayyappa, Nagarajan Lakshmipriya, Kandaswamy Ramya, Rajagopal Gayathri, Gunasekaran Geetha, Ranjit Mohan Anjana, Gunter G. C. Kuhnle, Venkatesan Radha, Viswanathan Mohan, Vasudevan Sudha, Karani Santhanakrishnan
Nutrients.2022; 14(13): 2713. CrossRef - Prevalence and Characteristics of Prediabetes and Metabolic Syndrome in Seemingly Healthy Persons at a Health Check-Up Clinic
Watip Tangjittipokin, Lanraphat Srisawat, Nipaporn Teerawattanapong, Tassanee Narkdontri, Mayuree Homsanit, Nattachet Plengvidhya
Journal of Multidisciplinary Healthcare.2022; Volume 15: 1585. CrossRef - ABCA1 C69T Gene Polymorphism Association with Dysglycemia in Saudi Prediabetic Adults
Ghada M. A. Ajabnoor, Suhad M. Bahijri, Wafa Alrashidi, Sumia Mohammad Enani, Aliaa A. Alamoudi, Lubna Al Sheikh, Basmah Eldakhakhny
Genes.2022; 13(12): 2277. CrossRef - Dysfunctional High-Density Lipoproteins in Type 2 Diabetes Mellitus: Molecular Mechanisms and Therapeutic Implications
Isabella Bonilha, Francesca Zimetti, Ilaria Zanotti, Bianca Papotti, Andrei C. Sposito
Journal of Clinical Medicine.2021; 10(11): 2233. CrossRef - Metabolic Syndrome Associated with Tobacco and Caffeine Products Use Among Refugee Adolescents: Risk of Dyslipidemia
Basma Damiri, Omar Khatib, Zaher Nazzal, Diala Sanduka, Siwar Igbaria, Ammar Thabaleh, Ahmad Farhoud, Lubna Saudi, Souad Belkebir, Rayyan Al Ali, Mohammed Alili, Mahmoud Hamdan, Omar A Safarini, Omar Younis
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 4121. CrossRef - Cysteine is a better predictor of coronary artery disease than conventional homocysteine in high-risk subjects under preventive medication
Ana Lima, Rita Ferin, António Fontes, Emília Santos, Dinis Martins, José Baptista, Maria L. Pavão
Nutrition, Metabolism and Cardiovascular Diseases.2020; 30(8): 1281. CrossRef - Changes in redox and endoplasmic reticulum homeostasis are related to congenital generalized lipodystrophy type 2
Aquiles Sales Craveiro Sarmento, Josivan Gomes Lima, Ana Rafaela de Souza Timoteo, Marcela Abbott Galvão Ururahy, Aurigena Antunes de Araújo, Roseane Carvalho Vasconcelos, Verônica Kristina Cândido Dantas, Lucymara Fassarella Agnez-Lima, Julliane Tamara A
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2020; 1865(4): 158610. CrossRef - Prevalence of Dyslipidaemia among Type 2 Diabetes Mellitus Patients in the Western Cape, South Africa
Elizabeth I. Omodanisi, Yibanathi Tomose, Benjamin I. Okeleye, Seteno K. O. Ntwampe, Yapo G. Aboua
International Journal of Environmental Research and Public Health.2020; 17(23): 8735. CrossRef - Higher High Density Lipoprotein 2 (HDL2) to Total HDL Cholesterol Ratio Is Associated with a Lower Risk for Incident Hypertension
You-Cheol Hwang, Wilfred Y. Fujimoto, Steven E. Kahn, Donna L. Leonetti, Edward J. Boyko
Diabetes & Metabolism Journal.2019; 43(1): 114. CrossRef - Identifying genetic markers associated with susceptibility to cardiovascular diseases
Hitesh Shukla, Jessica Louise Mason, Abdullah Sabyah
Future Science OA.2019;[Epub] CrossRef - Components and risk factors of metabolic syndrome among rural Nigerian workers
RufinaN. B Ayogu, Chikodili Nwajuaku, ElizabethA Udenta
Nigerian Medical Journal.2019; 60(2): 53. CrossRef - Short-Term Consumption of Probiotic Yogurt Improved HDL-C of Type 2 Diabetes Mellitus Patients: A Double-Blind Randomized Controlled Trial
Lily Arsanti Lestari, Dian Ratnasari, Elsa Fairuz Azizah, Ivana Noor Farida, Farah Nuriannisa, Kartika Yuliani, Rio Jati Kusuma, Emy Huriyati, Nyoman Kertia
Romanian Journal of Diabetes Nutrition and Metabolic Diseases.2019; 26(4): 381. CrossRef - Effect of liquid ubiquinol supplementation on glucose, lipids and antioxidant capacity in type 2 diabetes patients: a double-blind, randomised, placebo-controlled trial
Chi-Hua Yen, Ying-Ju Chu, Bor-Jen Lee, Yi-Chin Lin, Ping-Ting Lin
British Journal of Nutrition.2018; 120(1): 57. CrossRef - CETP inhibition, statins and diabetes
Philip J. Barter, Blake J. Cochran, Kerry-Anne Rye
Atherosclerosis.2018; 278: 143. CrossRef - Specialized Pro-resolving Lipid Mediators: Modulation of Diabetes-Associated Cardio-, Reno-, and Retino-Vascular Complications
Monica de Gaetano, Caitriona McEvoy, Darrell Andrews, Antonino Cacace, Jonathan Hunter, Eoin Brennan, Catherine Godson
Frontiers in Pharmacology.2018;[Epub] CrossRef - The Role of High-Density Lipoproteins in Diabetes and Its Vascular Complications
Nathan Wong, Stephen Nicholls, Joanne Tan, Christina Bursill
International Journal of Molecular Sciences.2018; 19(6): 1680. CrossRef - Relationship between the Finnish Diabetes Risk Score (FINDRISC), vitamin D levels, and insulin resistance in obese subjects
Marcos M. Lima-Martínez, Carlos Arrau, Saimar Jerez, Mariela Paoli, Juan P. González-Rivas, Ramfis Nieto-Martínez, Gianluca Iacobellis
Primary Care Diabetes.2017; 11(1): 94. CrossRef - History of Gestational Diabetes Mellitus in Relation to Cardiovascular Disease and Cardiovascular Risk Factors in US Women
Derrick C. V. Shostrom, Yangbo Sun, Jacob J. Oleson, Linda G. Snetselaar, Wei Bao
Frontiers in Endocrinology.2017;[Epub] CrossRef - The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk
Marek Femlak, Anna Gluba-Brzózka, Aleksandra Ciałkowska-Rysz, Jacek Rysz
Lipids in Health and Disease.2017;[Epub] CrossRef - Chinese Olive (Canarium album L.) Fruit Extract Attenuates Metabolic Dysfunction in Diabetic Rats
Yu-Te Yeh, An-Na Chiang, Shu-Chen Hsieh
Nutrients.2017; 9(10): 1123. CrossRef - Low Serum High Density Lipoprotein Cholesterol Concentration is an Independent Predictor for Enhanced Inflammation and Endothelial Activation
Wan Nor Hanis Wan Ahmad, Farah Sakri, Atiqah Mokhsin, Thuhairah Rahman, Nadzimah Mohd Nasir, Suraya Abdul-Razak, Mazapuspavina Md Yasin, Aletza Mohd Ismail, Zaliha Ismail, Hapizah Nawawi, Christina Bursill
PLOS ONE.2015; 10(1): e0116867. CrossRef - Gender-Related Differences in HDL Structure with the Progression of Microalbuminuria in Patients with Type 2 Diabetes
Manouchehr Nakhjavani
Journal of Diabetes, Metabolic Disorders & Control.2015;[Epub] CrossRef - Smoking is associated with impaired glucose regulation and a decrease in insulin sensitivity and the disposition index in first-degree relatives of type 2 diabetes subjects independently of the presence of metabolic syndrome
PierMarco Piatti, Emanuela Setola, Elena Galluccio, Sabrina Costa, Barbara Fontana, Michela Stuccillo, Valentina Crippa, Alberto Cappelletti, Alberto Margonato, Emanuele Bosi, Lucilla D. Monti
Acta Diabetologica.2014; 51(5): 793. CrossRef - Atherogenic dyslipidaemic profiles associated with the development of Type 2 diabetes: a 3.1‐year longitudinal study
Y.‐C. Hwang, H.‐Y. Ahn, S.‐H. Yu, S.‐W. Park, C.‐Y. Park
Diabetic Medicine.2014; 31(1): 24. CrossRef - The effect of insulin resistance on postprandial triglycerides in Korean type 2 diabetic patients
Kyeong Hye Park, Kwang Joon Kim, Byung-Wan Lee, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee
Acta Diabetologica.2014; 51(1): 15. CrossRef - Association of HDL-C and apolipoprotein A-I with the risk of type 2 diabetes in subjects with impaired fasting glucose
You-Cheol Hwang, Hong-Yup Ahn, Sung-Woo Park, Cheol-Young Park
European Journal of Endocrinology.2014; 171(1): 137. CrossRef - Estimation of the disposition index based on components of metabolic syndrome
Jiunn-Diann Lin, Chang Hsun Hseih, Chuan Chieh Liu, Wei-Cheng Lian, Chung-Ze Wu, Chun-Hsien Hsu, Dee Pei, Te-Lin Hsia, Yen-Lin Chen
Endocrine Journal.2014; 61(8): 789. CrossRef - Apolipoprotein B and non-HDL cholesterol are more powerful predictors for incident type 2 diabetes than fasting glucose or glycated hemoglobin in subjects with normal glucose tolerance: a 3.3-year retrospective longitudinal study
You-Cheol Hwang, Hong-Yup Ahn, Sung-Woo Park, Cheol-Young Park
Acta Diabetologica.2014; 51(6): 941. CrossRef - How to control residual cardiovascular risk despite statin treatment: Focusing on HDL–cholesterol
Soo Lim, Yae Min Park, Ichiro Sakuma, Kwang Kon Koh
International Journal of Cardiology.2013; 166(1): 8. CrossRef - Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction
Young Mi Song, Sun-Ok Song, Yong-Keun Jung, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2012; 8(7): 1085. CrossRef - Pentoxifylline Attenuates Methionine- and Choline-Deficient-Diet-Induced Steatohepatitis by Suppressing TNF-αExpression and Endoplasmic Reticulum Stress
Min Kyung Chae, Sang Gyu Park, Sun-Ok Song, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Experimental Diabetes Research.2012; 2012: 1. CrossRef - Dual pathways of p53 mediated glucolipotoxicity-induced apoptosis of rat cardiomyoblast cell: activation of p53 proapoptosis and inhibition of Nrf2-NQO1 antiapoptosis
Hye Jin Wang, Eun Young Lee, Seung Jin Han, Soo Hyun Kim, Byung-Wan Lee, Chul Woo Ahn, Bong Soo Cha, Hyun Chul Lee
Metabolism.2012; 61(4): 496. CrossRef - Association of Lipid and Lipoprotein Profiles with Future Development of Type 2 Diabetes in Nondiabetic Korean Subjects: A 4-Year Retrospective, Longitudinal Study
Mi Hae Seo, Ji Cheol Bae, Se Eun Park, Eun Jung Rhee, Cheol Young Park, Ki Won Oh, Sung Woo Park, Sun Woo Kim, Won-Young Lee
The Journal of Clinical Endocrinology & Metabolism.2011; 96(12): E2050. CrossRef
- Children and Adolescents With Hybrid Diabetes: A Management Conundrum

 KDA
KDA
 First
First Prev
Prev





