
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Ji-Hae Park, Soyeon Kwon, Young Mi Park
- Diabetes Metab J. 2024;48(2):215-230. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0332
- 2,319 View
- 193 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
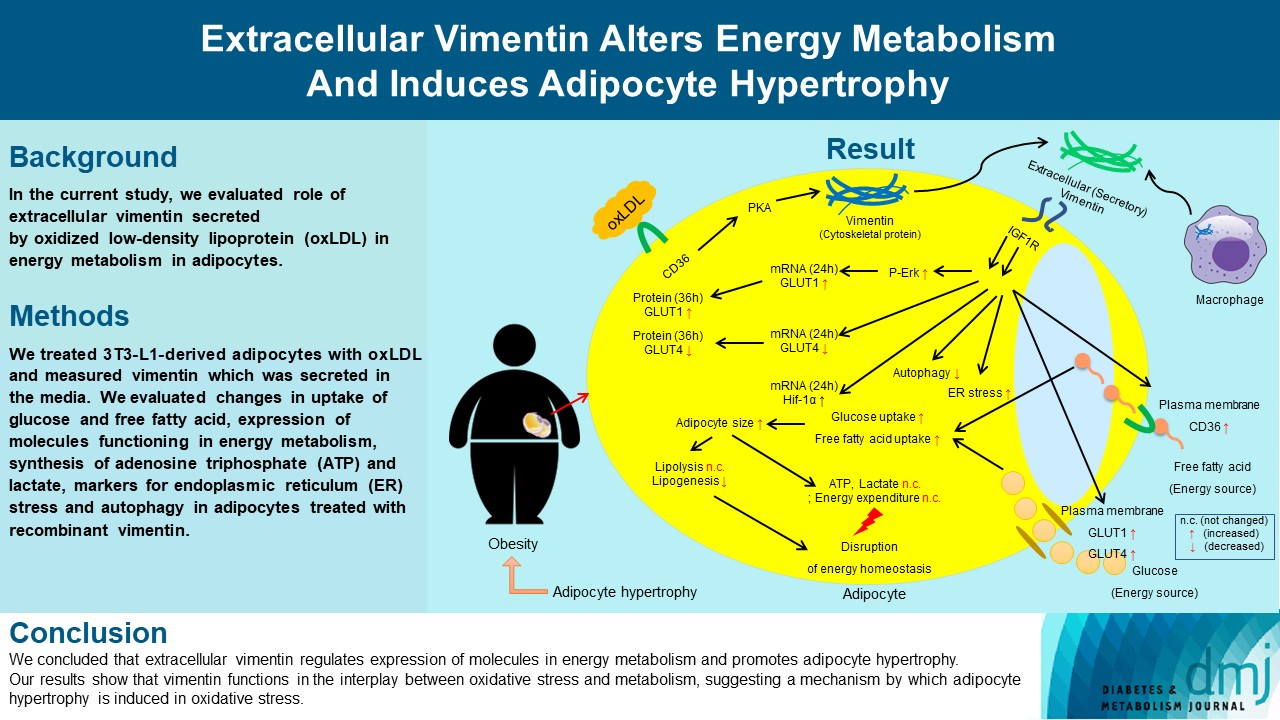
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
- Basic Research
- Role of SUMO-Specific Protease 2 in Leptin-Induced Fatty Acid Metabolism in White Adipocytes
- Praise Chanmee Kim, Ji Seon Lee, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2023;47(3):382-393. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0156
- 3,203 View
- 158 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
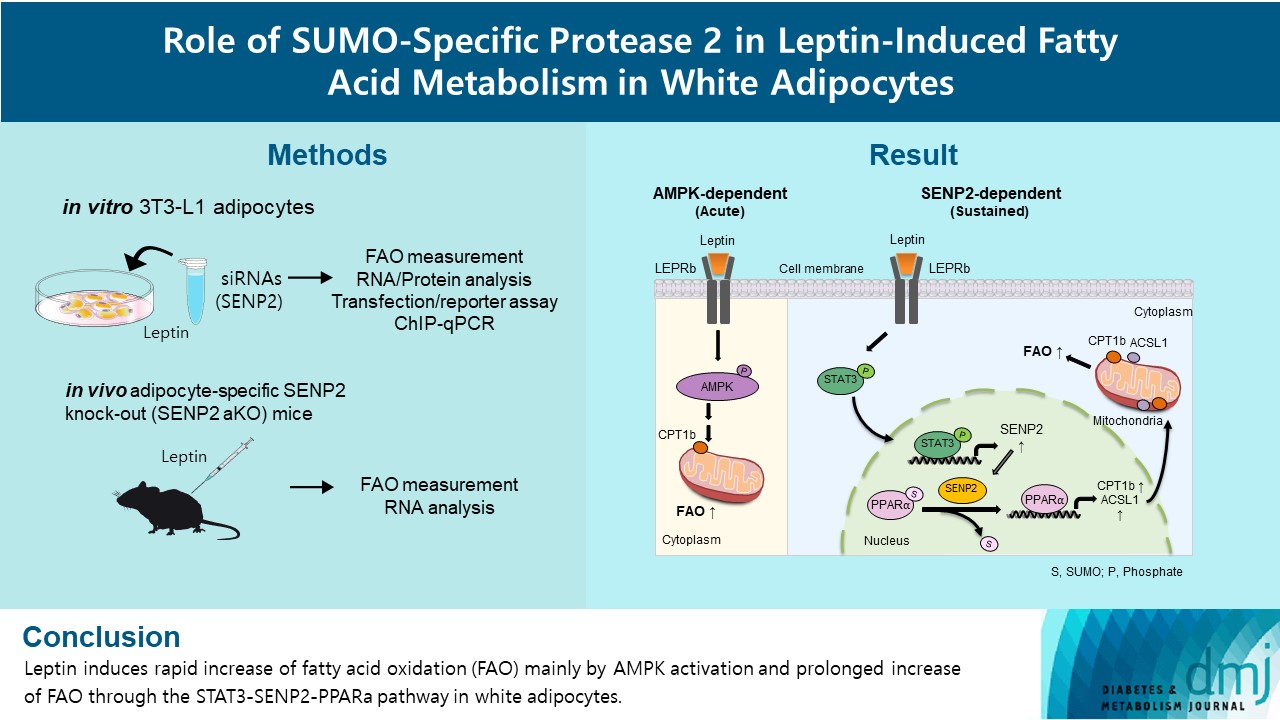
Leptin is a 16-kDa fat-derived hormone with a primary role in controlling adipose tissue levels. Leptin increases fatty acid oxidation (FAO) acutely through adenosine monophosphate-activated protein kinase (AMPK) and on delay through the SUMO-specific protease 2 (SENP2)–peroxisome proliferator-activated receptor δ/γ (PPARδ/γ) pathway in skeletal muscle. Leptin also directly increases FAO and decreases lipogenesis in adipocytes; however, the mechanism behind these effects remains unknown. Here, we investigated the role of SENP2 in the regulation of fatty acid metabolism by leptin in adipocytes and white adipose tissues.
Methods
The effects of leptin mediated by SENP2 on fatty acid metabolism were tested by siRNA-mediated knockdown in 3T3-L1 adipocytes. The role of SENP2 was confirmed in vivo using adipocyte-specific Senp2 knockout (Senp2-aKO) mice. We revealed the molecular mechanism involved in the leptin-induced transcriptional regulation of carnitine palmitoyl transferase 1b (Cpt1b) and long-chain acyl-coenzyme A synthetase 1 (Acsl1) using transfection/reporter assays and chromatin immunoprecipitation.
Results
SENP2 mediated the increased expression of FAO-associated enzymes, CPT1b and ACSL1, which peaked 24 hours after leptin treatment in adipocytes. In contrast, leptin stimulated FAO through AMPK during the initial several hours after treatment. In white adipose tissues, FAO and mRNA levels of Cpt1b and Acsl1 were increased by 2-fold 24 hours after leptin injection in control mice but not in Senp2-aKO mice. Leptin increased PPARα binding to the Cpt1b and Acsl1 promoters in adipocytes through SENP2.
Conclusion
These results suggest that the SENP2-PPARα pathway plays an important role in leptin-induced FAO in white adipocytes. -
Citations
Citations to this article as recorded by- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
Tingting Li, Haidong Wei, Shijie Zhang, Xiaotao Liu, Lu Xing, Yuanyuan Liu, Rixin Gong, Jianhong Li
Poultry Science.2024; 103(1): 103190. CrossRef
- Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart
- Metabolic Risk/Epidemiology
- Postprandial Free Fatty Acids at Mid-Pregnancy Increase the Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes Mellitus
- So-Yeon Kim, Young Shin Song, Soo-Kyung Kim, Yong-Wook Cho, Kyung-Soo Kim
- Diabetes Metab J. 2022;46(1):140-148. Published online August 9, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0023
- 4,997 View
- 160 Download
- 3 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
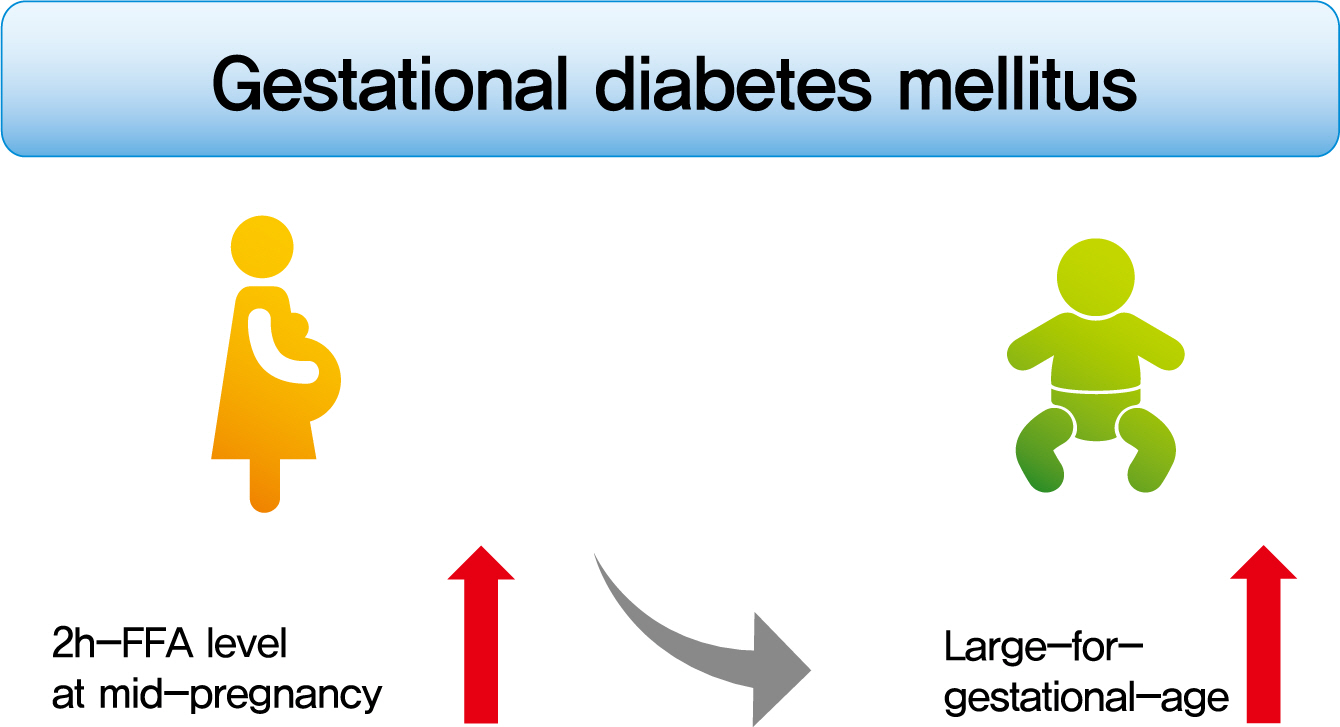
To investigate the association between free fatty acid (FFA) level at mid-pregnancy and large-for-gestational-age (LGA) newborns in women with gestational diabetes mellitus (GDM).
Methods
We enrolled 710 pregnant women diagnosed with GDM from February 2009 to October 2016. GDM was diagnosed by a ‘two-step’ approach with Carpenter and Coustan criteria. We measured plasma lipid profiles including fasting and 2-hour postprandial FFA (2h-FFA) levels at mid-pregnancy. LGA was defined if birthweights of newborns were above the 90th percentile for their gestational age.
Results
Mean age of pregnant women in this study was 33.1 years. Mean pre-pregnancy body mass index (BMI) was 22.4 kg/m2. The prevalence of LGA was 8.3% (n=59). Levels of 2h-FFA were higher in women who delivered LGA newborns than in those who delivered non-LGA newborns (416.7 μEq/L vs. 352.5 μEq/L, P=0.006). However, fasting FFA was not significantly different between the two groups. The prevalence of delivering LGA newborns was increased with increasing tertile of 2h-FFA (T1, 4.3%; T2, 9.8%; T3, 10.7%; P for trend <0.05). After adjustment for maternal age, pre-pregnancy BMI, and fasting plasma glucose, the highest tertile of 2h-FFA was 2.38 times (95% confidence interval, 1.11 to 5.13) more likely to have LGA newborns than the lowest tertile. However, there was no significant difference between groups according to fasting FFA tertiles.
Conclusion
In women with GDM, a high 2h-FFA level (but not fasting FFA) at mid-pregnancy is associated with an increasing risk of delivering LGA newborns. -
Citations
Citations to this article as recorded by- Advances in free fatty acid profiles in gestational diabetes mellitus
Haoyi Du, Danyang Li, Laura Monjowa Molive, Na Wu
Journal of Translational Medicine.2024;[Epub] CrossRef - Modulation of gut microbiota and lipid metabolism in rats fed high-fat diets by Ganoderma lucidum triterpenoids
Aijun Tong, Weihao Wu, Zhengxin Chen, Jiahui Wen, Ruibo Jia, Bin Liu, Hui Cao, Chao Zhao
Current Research in Food Science.2023; 6: 100427. CrossRef - Fetal Abdominal Obesity Detected at 24 to 28 Weeks of Gestation Persists until Delivery Despite Management of Gestational Diabetes Mellitus (Diabetes Metab J 2021;45:547-57)
Wonjin Kim, Soo Kyung Park, Yoo Lee Kim
Diabetes & Metabolism Journal.2021; 45(6): 970. CrossRef
- Advances in free fatty acid profiles in gestational diabetes mellitus
- Pathophysiology
- Distinct Dose-Dependent Association of Free Fatty Acids with Diabetes Development in Nonalcoholic Fatty Liver Disease Patients
- Fuxi Li, Junzhao Ye, Yanhong Sun, Yansong Lin, Tingfeng Wu, Congxiang Shao, Qianqian Ma, Xianhua Liao, Shiting Feng, Bihui Zhong
- Diabetes Metab J. 2021;45(3):417-429. Published online March 15, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0039
- 5,696 View
- 154 Download
- 8 Web of Science
- 7 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
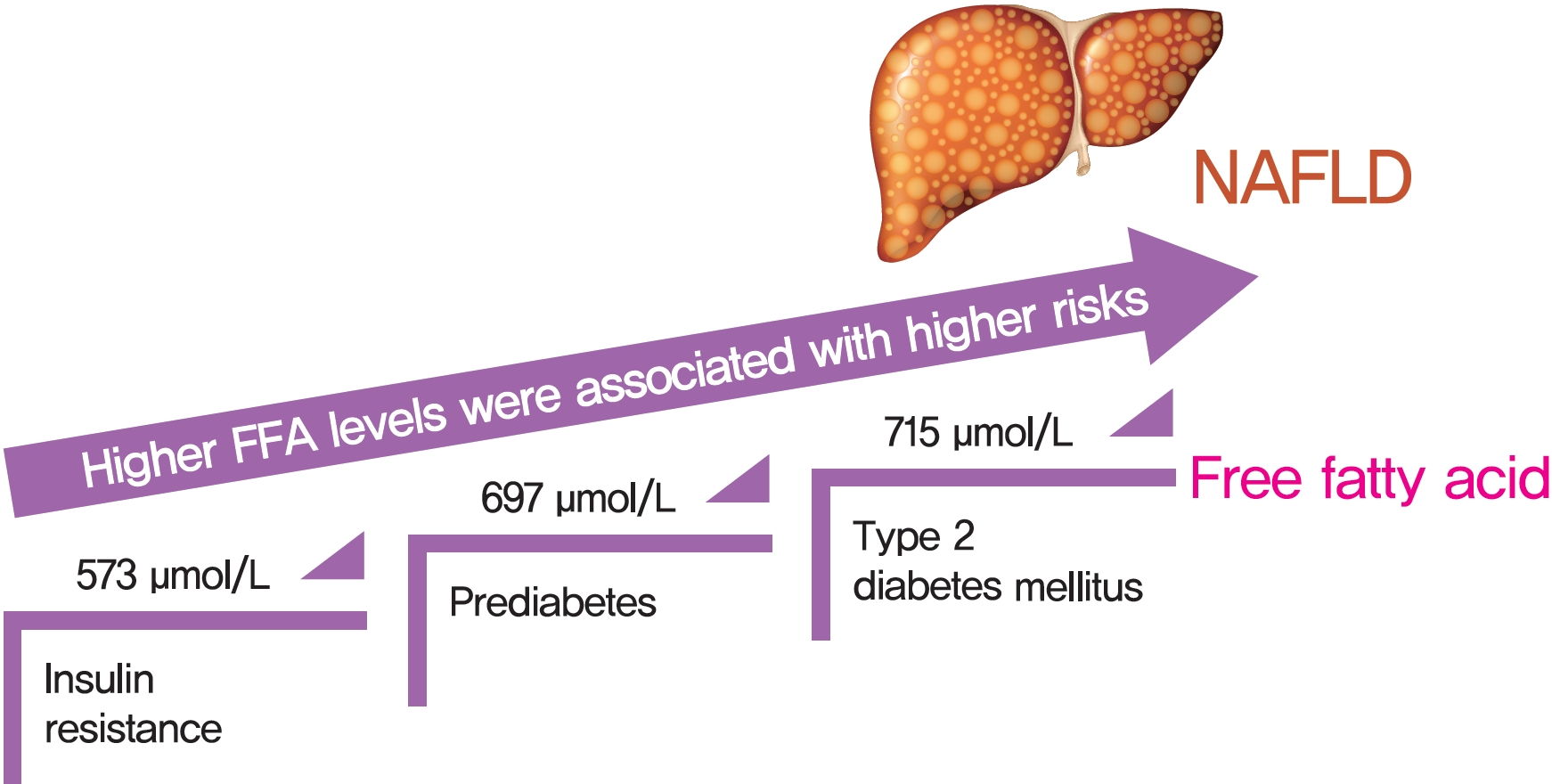
Excessive delivery of free fatty acids (FFAs) to the liver promotes steatosis and insulin resistance (IR), with IR defined as reduced glucose uptake, glycogen synthesis and anti-lipolysis stimulated by normal insulin levels. Whether the associations between FFAs and diabetes development differ between patients with and without nonalcoholic fatty liver disease (NAFLD) remains unclear.
Methods
Consecutive subjects (2,220 NAFLD subjects and 1,790 non-NAFLD subjects according to ultrasound imaging) were enrolled from the First Affiliated Hospital of Sun Yat-sen University between 2009 and 2019. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.
Results
There was an approximate J-shaped relationship between FFA levels and HOMA-IR in the NAFLD group. Higher FFA concentration quartiles were associated with higher risks of IR (odds ratio [OR], 9.24; 95% confidence interval [CI], 6.43 to 13.36), prediabetes (OR, 10.48; 95% CI, 5.66 to 19.39), and type 2 diabetes mellitus (T2DM; OR, 19.43; 95% CI, 12.75 to 29.81) in the NAFLD group but not in the non-NAFLD group. The cut-off points for the FFA levels increased in a stepwise manner in discriminating IR, prediabetes and T2DM (573, 697, and 715 μmol/L) in the NAFLD group but not in non-NAFLD individuals.
Conclusion
A distinct dose-dependent relationship of FFA levels was found with IR, prediabetes and T2DM in NAFLD patients. Screening serum FFA levels in NAFLD patients would be valuable in preventing diabetes development. -
Citations
Citations to this article as recorded by- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
Eugene Han, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Sang Hoon Ahn, Yong-ho Lee, Seung Up Kim
Metabolism.2024; 152: 155789. CrossRef - Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: a systematic review and meta-analysis
Limin Cao, Yu An, Huiyuan Liu, Jinguo Jiang, Wenqi Liu, Yuhan Zhou, Mengyuan Shi, Wei Dai, Yanling Lv, Yuhong Zhao, Yanhui Lu, Liangkai Chen, Yang Xia
BMC Medicine.2024;[Epub] CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
Yujin Jin, Kyung-Sun Heo
The Korean Journal of Physiology & Pharmacology.2023; 27(4): 299. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Mongolian medicine in treating type 2 diabetes mellitus combined with nonalcoholic fatty liver disease via FXR/LXR-mediated P2X7R/NLRP3/NF-κB pathway activation
Shuyin Bao, Xiuzhi Wang, Qianqian Ma, Chengxi Wei, Jixing Nan, Wuliji Ao
Chinese Herbal Medicines.2022; 14(3): 367. CrossRef - Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects
Hwi Seung Kim, Yun Kyung Cho, Eun Hee Kim, Min Jung Lee, Chang Hee Jung, Joong-Yeol Park, Hong-Kyu Kim, Woo Je Lee
Journal of Clinical Medicine.2021; 11(1): 41. CrossRef
- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
- Basic Research
- The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
- Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
- Diabetes Metab J. 2020;44(2):222-233. Published online April 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0053
- 7,528 View
- 169 Download
- 17 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Impaired β-cell function is the key pathophysiology of type 2 diabetes mellitus, and chronic exposure of nutrient excess could lead to this tragedy. For preserving β-cell function, it is essential to understand the cause and mechanisms about the progression of β-cells failure. Glucotoxicity, lipotoxicity, and glucolipotoxicity have been suggested to be a major cause of β-cell dysfunction for decades, but not yet fully understood. Fatty acid translocase cluster determinant 36 (CD36), which is part of the free fatty acid (FFA) transporter system, has been identified in several tissues such as muscle, liver, and insulin-producing cells. Several studies have reported that induction of CD36 increases uptake of FFA in several cells, suggesting the functional interplay between glucose and FFA in terms of insulin secretion and oxidative metabolism. However, we do not currently know the regulating mechanism and physiological role of CD36 on glucolipotoxicity in pancreatic β-cells. Also, the downstream and upstream targets of CD36 related signaling have not been defined. In the present review, we will focus on the expression and function of CD36 related signaling in the pancreatic β-cells in response to hyperglycemia and hyperlipidemia (ceramide) along with the clinical studies on the association between CD36 and metabolic disorders.
-
Citations
Citations to this article as recorded by- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
Alpana Mukhuty, Samanwita Mandal, Chandrani Fouzder, Snehasis Das, Dipanjan Chattopadhyay, Tanmay Majumdar, Rakesh Kundu
Molecular and Cellular Endocrinology.2024; 581: 112112. CrossRef - CD36 gene variant rs1761667(G/A) as a biomarker in obese type 2 diabetes mellitus cases
Ashwin Kumar Shukla, Amreen Shamsad, Atar Singh Kushwah, Shalini Singh, Kauser Usman, Monisha Banerjee
Egyptian Journal of Medical Human Genetics.2024;[Epub] CrossRef - CD36 regulates macrophage and endothelial cell activation and multinucleate giant cell formation in anti neutrophil cytoplasm antibody vasculitis
Xiang Zhang, Catherine King, Alexander Dowell, Paul Moss, Lorraine Harper, Dimitrios Chanouzas, Xiong-zhong Ruan, Alan David Salama
Clinical Immunology.2024; 260: 109914. CrossRef - The association of soluble cluster of differentiation 36 with metabolic diseases: A potential biomarker and therapeutic target
Yun Li, Yaxi Chen, Xiong Z. Ruan
Pediatric Discovery.2023;[Epub] CrossRef - The role of candidate transport proteins in β‐cell long‐chain fatty acid uptake: Where are we now?
Christina Clavelo‐Farrow, Patricia Thomas
Diabetic Medicine.2023;[Epub] CrossRef - SARS-CoV-2 in the pancreas and the impaired islet function in COVID-19 patients
Ningfei Ji, Mingshun Zhang, Liang Ren, Yunyun Wang, Bicheng Hu, Jie Xiang, Yingyun Gong, Chaojie Wu, Guoqiang Qu, Wenqiu Ding, Zhiqiang Yin, Shan Li, Zhengxia Wang, Lianzheng Zhou, Xueqin Chen, Yuan Ma, Jinhai Tang, Yun Liu, Liang Liu, Mao Huang
Emerging Microbes & Infections.2022; 11(1): 1115. CrossRef - Is imaging-based muscle quantity associated with risk of diabetes? A meta-analysis of cohort studies
Shanhu Qiu, Xue Cai, Yang Yuan, Bo Xie, Zilin Sun, Tongzhi Wu
Diabetes Research and Clinical Practice.2022; 189: 109939. CrossRef - Lipotoxicity in a Vicious Cycle of Pancreatic Beta Cell Exhaustion
Vladimir Grubelnik, Jan Zmazek, Matej Završnik, Marko Marhl
Biomedicines.2022; 10(7): 1627. CrossRef - Association of cluster determinant 36, scavenger receptor class B type 1, and major facilitator superfamily domain containing the 2a genetic polymorphism with serum lipid profile in aging population with type 2 diabetes mellitus
Xixiang Wang, Xiaojun Ma, Jingjing Xu, Yujie Guo, Shaobo Zhou, Huiyan Yu, Linhong Yuan
Frontiers in Nutrition.2022;[Epub] CrossRef - CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages
Noorzaileen Eileena Zaidi, Nur Aima Hafiza Shazali, Thean-Chor Leow, Mohd Azuraidi Osman, Kamariah Ibrahim, Wan-Hee Cheng, Kok-Song Lai, Nik Mohd Afizan Nik Abd Rahman
Cells.2022; 11(22): 3556. CrossRef - The Past and Present Lives of the Intraocular Transmembrane Protein CD36
Rucui Yang, Qingping Liu, Mingzhi Zhang
Cells.2022; 12(1): 171. CrossRef - Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus
Sachin Kumar, Tapan Behl, Monika Sachdeva, Aayush Sehgal, Shilpa Kumari, Arun Kumar, Gagandeep Kaur, Harlokesh Narayan Yadav, Simona Bungau
Life Sciences.2021; 264: 118661. CrossRef - Contribution of rs3211938 polymorphism at CD36 to glucose levels, oxidized low-density lipoproteins, insulin resistance, and body mass index in Mexican mestizos with type-2 diabetes from western Mexico
Beatriz Teresita Martín-Márquez, Flavio Sandoval-Garcia, Mónica Vazquez-Del Mercado, Erika-Aurora Martínez-García, Fernanda-Isadora Corona-Meraz, Ana-Lilia Fletes-Rayas, Soraya-Amalí Zavaleta-Muñiz
Nutrición Hospitalaria.2021;[Epub] CrossRef - Investigating the association of CD36 gene polymorphisms (rs1761667 and rs1527483) with T2DM and dyslipidemia: Statistical analysis, machine learning based prediction, and meta-analysis
Ma’mon M. Hatmal, Walhan Alshaer, Ismail S. Mahmoud, Mohammad A. I. Al-Hatamleh, Hamzeh J. Al-Ameer, Omar Abuyaman, Malek Zihlif, Rohimah Mohamud, Mais Darras, Mohammad Al Shhab, Rand Abu-Raideh, Hilweh Ismail, Ali Al-Hamadi, Ali Abdelhay, Kanhaiya Singh
PLOS ONE.2021; 16(10): e0257857. CrossRef - Misregulation of Wnt Signaling Pathways at the Plasma Membrane in Brain and Metabolic Diseases
Mustafa Karabicici, Yagmur Azbazdar, Evin Iscan, Gunes Ozhan
Membranes.2021; 11(11): 844. CrossRef
- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
- Obesity and Metabolic Syndrome
-
- PF-04620110, a Potent Antidiabetic Agent, Suppresses Fatty Acid-Induced NLRP3 Inflammasome Activation in Macrophages
- Seung Il Jo, Jung Hwan Bae, Seong Jin Kim, Jong Min Lee, Ji Hun Jeong, Jong-Seok Moon
- Diabetes Metab J. 2019;43(5):683-699. Published online October 24, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0112
- 5,420 View
- 66 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Chronic inflammation has been linked to insulin resistance and type 2 diabetes mellitus (T2DM). High-fat diet (HFD)-derived fatty acid is associated with the activation of chronic inflammation in T2DM. PF-04620110, which is currently in phase 1 clinical trials as a selective acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) inhibitor, is a potent anti-diabetic agent that may be important for the regulation of chronic inflammation in T2DM. However, the mechanisms by which PF-04620110 regulates fatty acid-induced chronic inflammation remain unclear.
Methods PF-04620110 was used
in vitro andin vivo . DGAT1-targeting gRNAs were used for deletion of mouse DGAT1 via CRISPR ribonucleoprotein (RNP) system. The activation of NLRP3 inflammasome was measured by immunoblot or cytokine analysisin vitro andin vivo .Results Here we show that PF-04620110 suppressed fatty acid-induced nucleotide-binding domain, leucine-rich-repeat-containing receptor (NLR), pyrin-domain-containing 3 (NLRP3) inflammasome activation in macrophages. In contrast, PF-04620110 did not change the activation of the NLR family, CARD-domain-containing 4 (NLRC4), or the absent in melanoma 2 (AIM2) inflammasomes. Moreover, PF-04620110 inhibited K+ efflux and the NLRP3 inflammasome complex formation, which are required for NLRP3 inflammasome activation. PF-04620110 reduced the production of interleukin 1β (IL-1β) and IL-18 and blood glucose levels in the plasma of mice fed HFD. Furthermore, genetic inhibition of DGAT1 suppressed fatty acid-induced NLRP3 inflammasome activation.
Conclusion Our results suggest that PF-04620110 suppresses fatty acid-induced NLRP3 inflammasome activation.
-
Citations
Citations to this article as recorded by- Drug Targeting of Acyltransferases in the Triacylglyceride and 1-O-AcylCeramide Biosynthetic Pathways
Maria Hernandez-Corbacho, Daniel Canals
Molecular Pharmacology.2024; 105(3): 166. CrossRef - Possible therapeutic targets for NLRP3 inflammasome-induced breast cancer
Xixi Wang, Junyi Lin, Zhe Wang, Zhi Li, Minghua Wang
Discover Oncology.2023;[Epub] CrossRef
- Drug Targeting of Acyltransferases in the Triacylglyceride and 1-O-AcylCeramide Biosynthetic Pathways
- Drug/Regimen
- Efficacy and Safety of Omega-3 Fatty Acids in Patients Treated with Statins for Residual Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- Ji Eun Jun, In-Kyung Jeong, Jae Myung Yu, Sung Rae Kim, In Kye Lee, Kyung-Ah Han, Sung Hee Choi, Soo-Kyung Kim, Hyeong Kyu Park, Ji-Oh Mok, Yong-ho Lee, Hyuk-Sang Kwon, So Hun Kim, Ho-Cheol Kang, Sang Ah Lee, Chang Beom Lee, Kyung Mook Choi, Sung-Ho Her, Won Yong Shin, Mi-Seung Shin, Hyo-Suk Ahn, Seung Ho Kang, Jin-Man Cho, Sang-Ho Jo, Tae-Joon Cha, Seok Yeon Kim, Kyung Heon Won, Dong-Bin Kim, Jae Hyuk Lee, Moon-Kyu Lee
- Diabetes Metab J. 2020;44(1):78-90. Published online June 20, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0265
- 9,306 View
- 190 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Cardiovascular risk remains increased despite optimal low density lipoprotein cholesterol (LDL-C) level induced by intensive statin therapy. Therefore, recent guidelines recommend non-high density lipoprotein cholesterol (non-HDL-C) as a secondary target for preventing cardiovascular events. The aim of this study was to assess the efficacy and tolerability of omega-3 fatty acids (OM3-FAs) in combination with atorvastatin compared to atorvastatin alone in patients with mixed dyslipidemia.
Methods This randomized, double-blind, placebo-controlled, parallel-group, and phase III multicenter study included adults with fasting triglyceride (TG) levels ≥200 and <500 mg/dL and LDL-C levels <110 mg/dL. Eligible subjects were randomized to ATOMEGA (OM3-FAs 4,000 mg plus atorvastatin calcium 20 mg) or atorvastatin 20 mg plus placebo groups. The primary efficacy endpoints were the percent changes in TG and non-HDL-C levels from baseline at the end of treatment.
Results After 8 weeks of treatment, the percent changes from baseline in TG (−29.8% vs. 3.6%,
P <0.001) and non-HDL-C (−10.1% vs. 4.9%,P <0.001) levels were significantly greater in the ATOMEGA group (n =97) than in the atorvastatin group (n =103). Moreover, the proportion of total subjects reaching TG target of <200 mg/dL in the ATOMEGA group was significantly higher than that in the atorvastatin group (62.9% vs. 22.3%,P <0.001). The incidence of adverse events did not differ between the two groups.Conclusion The addition of OM3-FAs to atorvastatin improved TG and non-HDL-C levels to a significant extent compared to atorvastatin alone in subjects with residual hypertriglyceridemia.
-
Citations
Citations to this article as recorded by- Association Between Omega‐3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose–Response Meta‐Analysis of Randomized Controlled Trials
Tianjiao Wang, Xin Zhang, Na Zhou, Yuxuan Shen, Biao Li, Bingshu E. Chen, Xinzhi Li
Journal of the American Heart Association.2023;[Epub] CrossRef - Nutraceutical support in the prevention and treatment of cardiovascular diseases
E. V. Gracheva, E. A. Starovoytova, E. S. Kulikov, N. A. Kirillova, S. V. Fedosenko, M. A. Balaganskaya, D. V. Kromka
Rational Pharmacotherapy in Cardiology.2023; 19(3): 298. CrossRef - Effect of coadministration of omega-3 fatty acids with glimepiride on glycemic control, lipid profile, irisin, and sirtuin-1 in type 2 diabetes mellitus patients: a randomized controlled trial
Rehab H. Werida, Aalaa Ramzy, Youssri Nassief Ebrahim, Maged Wasfy Helmy
BMC Endocrine Disorders.2023;[Epub] CrossRef - The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence
Karla Paulina Luna-Castillo, Xochitl Citlalli Olivares-Ochoa, Rocío Guadalupe Hernández-Ruiz, Iris Monserrat Llamas-Covarrubias, Saraí Citlalic Rodríguez-Reyes, Alejandra Betancourt-Núñez, Barbara Vizmanos, Erika Martínez-López, José Francisco Muñoz-Valle
Nutrients.2022; 14(5): 1104. CrossRef - The effect of omega-3 fatty acids and its combination with statins on lipid profile in patients with hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials
Yunjiao Yang, Wen Deng, Yanmei Wang, Tongyi Li, Yiding Chen, Cong Long, Qing Wen, Yue Wu, Qiu Chen
Frontiers in Nutrition.2022;[Epub] CrossRef - Comparison of the Efficacy and Safety of Atorvastatin 40 mg/ω-3 Fatty Acids 4 g Fixed-dose Combination and Atorvastatin 40 mg Monotherapy in Hypertriglyceridemic Patients who Poorly Respond to Atorvastatin 40 mg Monotherapy: An 8-week, Multicenter, Random
Jong Shin Woo, Soon Jun Hong, Dong Hoon Cha, Kee Sik Kim, Moo Hyun Kim, Jun-Won Lee, Myung Ho Jeong, Jin-Ok Jeong, Jun-Hee Lee, Doo Soo Jeon, Eun Joo Cho, Soon Kil Kim, Jun Kwan, Chang Gyu Park, Hae Young Lee, Taek Jong Hong, Jinho Shin, Ho Joong Youn, Do
Clinical Therapeutics.2021; 43(8): 1419. CrossRef - All-Cause Mortality and Cardiovascular Death between Statins and Omega-3 Supplementation: A Meta-Analysis and Network Meta-Analysis from 55 Randomized Controlled Trials
Jeongseon Kim, Tung Hoang, Ji-Myung Kim, So Young Bu, Jeong-Hwa Choi, Eunju Park, Seung-Min Lee, Eunmi Park, Ji Yeon Min, In Seok Lee, So Young Youn, Jee-Young Yeon
Nutrients.2020; 12(10): 3203. CrossRef
- Association Between Omega‐3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose–Response Meta‐Analysis of Randomized Controlled Trials
- Clinical Diabetes & Therapeutics
- Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus
- You-Cheol Hwang, Ji Eun Jun, In-Kyung Jeong, Kyu Jeung Ahn, Ho Yeon Chung
- Diabetes Metab J. 2019;43(5):582-589. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0124
- 6,589 View
- 184 Download
- 14 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The apolipoprotein B/A1 (apoB/A1) ratio is a stronger predictor of future cardiovascular disease than is the level of conventional lipids. Statin and ezetimibe combination therapy have shown additional cardioprotective effects over statin monotherapy.
Methods This was a single-center, randomized, open-label, active-controlled study in Korea. A total of 36 patients with type 2 diabetes mellitus were randomized to either rosuvastatin monotherapy (20 mg/day,
n =20) or rosuvastatin/ezetimibe (5 mg/10 mg/day,n =16) combination therapy for 6 weeks.Results After the 6-week treatment, low density lipoprotein cholesterol (LDL-C) and apoB reduction were comparable between the two groups (−94.3±15.4 and −62.0±20.9 mg/dL in the rosuvastatin group, −89.9±22.7 and −66.8±21.6 mg/dL in the rosuvastatin/ezetimibe group,
P =0.54 andP =0.86, respectively). In addition, change in apoB/A1 ratio (−0.44±0.16 in the rosuvastatin group and −0.47±0.25 in the rosuvastatin/ezetimibe group,P =0.58) did not differ between the two groups. On the other hand, triglyceride and free fatty acid (FFA) reductions were greater in the rosuvastatin/ezetimibe group than in the rosuvastatin group (−10.5 mg/dL [interquartile range (IQR), −37.5 to 29.5] and 0.0 µEq/L [IQR, −136.8 to 146.0] in the rosuvastatin group, −49.5 mg/dL [IQR, −108.5 to −27.5] and −170.5 µEq/L [IQR, −353.0 to 0.8] in the rosuvastatin/ezetimibe group,P =0.010 andP =0.049, respectively). Both treatments were generally well tolerated, and there were no differences in muscle or liver enzyme elevation.Conclusion A 6-week combination therapy of low-dose rosuvastatin and ezetimibe showed LDL-C, apoB, and apoB/A1 ratio reduction comparable to that of high-dose rosuvastatin monotherapy in patients with type 2 diabetes mellitus. Triglyceride and FFA reductions were greater with the combination therapy than with rosuvastatin monotherapy.
-
Citations
Citations to this article as recorded by- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review
Yura Kang, Jung Mi Park, Sang-Hak Lee
Yonsei Medical Journal.2024; 65(1): 19. CrossRef - Combination Therapy of Ezetimibe and Rosuvastatin for Dyslipidemia: Current Insights
Maya R Chilbert, Dylan VanDuyn, Sara Salah, Collin M Clark, Qing Ma
Drug Design, Development and Therapy.2022; Volume 16: 2177. CrossRef - Ezetimibe and diabetes mellitus:a new strategy for lowering cholesterol
V.A. Serhiyenko, A.A. Serhiyenko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2022; 18(5): 302. CrossRef - The Effect of Rosuvastatin on Plasma/Serum Levels of High-Sensitivity C-Reactive Protein, Interleukin-6, and D-Dimer in People Living with Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis
Akililu Alemu Ashuro, Yin-Guang Fan, Yuan-Sheng Fu, Dong-Sheng Di, Napoleon Bellua Sam, Hai-Feng Pan, Dong-Qing Ye
AIDS Research and Human Retroviruses.2021; 37(11): 821. CrossRef - Comparison of the Efficacy and Safety of Rosuvastatin/Ezetimibe Combination Therapy and Rosuvastatin Monotherapy on Lipoprotein in Patients With Type 2 Diabetes: Multicenter Randomized Controlled Study
Jiwoo Lee, You-Cheol Hwang, Woo Je Lee, Jong Chul Won, Kee-Ho Song, Cheol-Young Park, Kyu Jeung Ahn, Joong-Yeol Park
Diabetes Therapy.2020; 11(4): 859. CrossRef - Comparison of Renal Effects of Ezetimibe–Statin Combination versus Statin Monotherapy: A Propensity-Score-Matched Analysis
Jaehyun Bae, Namki Hong, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Yong-ho Lee
Journal of Clinical Medicine.2020; 9(3): 798. CrossRef - Combined use of rosuvastatin and ezetimibe improves hepatic steatosis in patients with dyslipidemia
Won Dong Lee, Beom Kyung Kim, Jun Yong Park, Do Young Kim, Sang Hoon Ahn, Kwang-Hyub Han, Seung Up Kim
European Journal of Gastroenterology & Hepatology.2020; 32(12): 1538. CrossRef - Influence of rosuvastatin dose on total fatty acids and free fatty acids in plasma
Cristian I. Ciucanu, Sonia Olariu, Daliborca C. Vlad, Victor Dumitraşcu
Medicine.2020; 99(48): e23356. CrossRef - The effect of switching from statin-monotherapy to statin/ezetimibe combination therapy on lipid profiles in patients with type 2 diabetes and dyslipidemia: a multicenter open-label study (EUCLID)
Mitsuhide Takeshita, Atsushi Tanaka, Atsushi Kawaguchi, Keiko Sato, Shigeru Toyoda, Teruo Inoue, Koichi Node
Vascular Failure.2020; 4(1): 22. CrossRef - Response: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
You-Cheol Hwang
Diabetes & Metabolism Journal.2019; 43(6): 915. CrossRef - Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J2019;43:582–9)
Tae Seo Sohn
Diabetes & Metabolism Journal.2019; 43(6): 909. CrossRef - Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes
Amélie I. S. Sobczak, Claudia A. Blindauer, Alan J. Stewart
Nutrients.2019; 11(9): 2022. CrossRef
- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review
- Clinical Diabetes & Therapeutics
- Effects of Omega-3 Supplementation on Adipocytokines in Prediabetes and Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Tarik Becic, Christian Studenik
- Diabetes Metab J. 2018;42(2):101-116. Published online April 19, 2018
- DOI: https://doi.org/10.4093/dmj.2018.42.2.101
- 7,862 View
- 60 Download
- 16 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The objective of this systematic review and meta-analysis was to determine the effects of omega-3 supplementation on adipocytokine levels in adult prediabetic and diabetic individuals.
Methods We searched PubMed, Medline, EMBASE, Scopus, Web of Science, Google Scholar, Cochrane Trial Register, World Health Organization Clinical Trial Registry Platform, and Clinicaltrial.gov Registry from inception to August 1, 2017 for randomized controlled trials. Pooled effects of interventions were assessed as mean difference using random effects model. We conducted a sensitivity, publication bias and subgroup analysis.
Results Fourteen studies individuals (
n =685) were included in the meta-analysis. Omega-3 supplementation increased levels of adiponectin (0.48 µg/mL; 95% confidence interval [CI], 0.27 to 0.68;P <0.00001,n =10 trials), but effects disappeared after sensitivity analysis. Tumor necrosis factor α (TNF-α) levels were reduced (−1.71; 95% CI, −3.38 to −0.14;P =0.03,n =8 trials). Treatment duration shorter than 12 weeks was associated with greater reduction than longer treatment duration. Levels of other adipocytokines were not significantly affected. Publication bias could generally not be excluded.Conclusion Eicosapentaenoic acid and docosahexaenoic acid supplementation may increase adiponectin and reduce TNF-α levels in this population group. However, due to overall study heterogeneity and potential publication bias, a cautious interpretation is needed.
-
Citations
Citations to this article as recorded by- The Effects of Omega-3 Fatty Acid Supplementation
on the Lipid Profile and Cardiovascular Markers
Following Downhill Running in Long-Distance Runners
Marzena Jaworska, Szymon Siatkowski, Aleksandra Żebrowska
Journal of Human Kinetics.2023; 89: 123. CrossRef - Omega-3 supplementation in the treatment of polycystic ovary syndrome (PCOS) – a review of clinical trials and cohort
Vitoria Melo, Thomas Silva, Thaissa Silva, Juliana Freitas, Joselita Sacramento, Mirian Vazquez, Edilene Araujo
Endocrine Regulations.2022; 56(1): 66. CrossRef - The effects of omega-3 fatty acids in type 2 diabetes: A systematic review and meta-analysis
Yanan Xiao, Qifang Zhang, Xueling Liao, Ulf Elbelt, Karsten H. Weylandt
Prostaglandins, Leukotrienes and Essential Fatty Acids.2022; 182: 102456. CrossRef - Anti-inflammatory and antioxidant activity of astragalus polysaccharide in ulcerative colitis: A systematic review and meta-analysis of animal studies
Heng-Chang Hu, Wei Zhang, Pei-Yu Xiong, Li Song, Bo Jia, Xing-Long Liu
Frontiers in Pharmacology.2022;[Epub] CrossRef - Eicosopenthaenoic acid: Gnawing at the perivascular adipose tissue
Paolo Raggi, Arthur E. Stillman
Atherosclerosis.2021; 316: 69. CrossRef - The Effect of Omega-3 Fatty Acid Supplementation on Serum Adipocytokines, Lipid Profile and Biochemical Markers of Inflammation in Recreational Runners
Aleksandra Żebrowska, Barbara Hall, Anna Stolecka-Warzecha, Arkadiusz Stanula, Ewa Sadowska-Krępa
Nutrients.2021; 13(2): 456. CrossRef - The Influence of Nutrition on Adiponectin—A Narrative Review
Justyna Janiszewska, Joanna Ostrowska, Dorota Szostak-Węgierek
Nutrients.2021; 13(5): 1394. CrossRef - Adiponectin’s roles in lipid and glucose metabolism modulation in fish: Mechanisms and perspectives
Renlei Ji, Xiang Xu, Giovanni M. Turchini, Kangsen Mai, Qinghui Ai
Reviews in Aquaculture.2021; 13(4): 2305. CrossRef - Omega-3 Fatty Acids and Vulnerability to Addiction: Reviewing Preclinical and Clinical Evidence
Valerie L. Darcey, Katherine M. Serafine
Current Pharmaceutical Design.2020; 26(20): 2385. CrossRef - Therapeutic effects of different doses of prebiotic (isolated from Saccharomyces cerevisiae) in comparison to n-3 supplement on glycemic control, lipid profiles and immunological response in diabetic rats
Janina de Sales Guilarducci, Breno Augusto Ribeiro Marcelino, Isaac Filipe Moreira Konig, Tamira Maria Orlando, Mary Suzan Varaschin, Luciano José Pereira
Diabetology & Metabolic Syndrome.2020;[Epub] CrossRef - Endothelial dysfunction in neuroprogressive disorders—causes and suggested treatments
Gerwyn Morris, Basant K. Puri, Lisa Olive, Andre Carvalho, Michael Berk, Ken Walder, Lise Tuset Gustad, Michael Maes
BMC Medicine.2020;[Epub] CrossRef - Evaluation of walking exercise on glycemic control in patients with type 2 diabetes mellitus
Hengchang Hu, Yuanhong Lei, Liping Yin, Xiaoqiong Luo
Medicine.2020; 99(47): e22735. CrossRef - Omega-3 polyunsaturated fatty acids in supporting pregnancy and fetal development: dosing considerations
Olga A. Gromova, Ivan Iu. Torshin, Tatiana R. Grishina, Svetlana I. Maliavskaia
Gynecology.2020; 22(5): 61. CrossRef - A Significant Association Between Rhein and Diabetic Nephropathy in Animals: A Systematic Review and Meta-Analysis
Heng-Chang Hu, Liu-Tao Zheng, Hai-Yan Yin, Yuan Tao, Xiao-Qiong Luo, Kai-Shan Wei, Li-Ping Yin
Frontiers in Pharmacology.2019;[Epub] CrossRef - Dietary n-3 polyunsaturated fatty acids, fish intake and healthy ageing
Esther García-Esquinas, Rosario Ortolá, Jose Ramón Banegas, Esther Lopez-García, Fernando Rodríguez-Artalejo
International Journal of Epidemiology.2019; 48(6): 1914. CrossRef - Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives
Hidekatsu Yanai, Hiroshi Yoshida
International Journal of Molecular Sciences.2019; 20(5): 1190. CrossRef - Examining the Potential of Developing and Implementing Use of Adiponectin-Targeted Therapeutics for Metabolic and Cardiovascular Diseases
Ying Liu, Vivian Vu, Gary Sweeney
Frontiers in Endocrinology.2019;[Epub] CrossRef
- The Effects of Omega-3 Fatty Acid Supplementation
on the Lipid Profile and Cardiovascular Markers
Following Downhill Running in Long-Distance Runners
- Obesity and Metabolic Syndrome
In Vitro Effect of Fatty Acids Identified in the Plasma of Obese Adolescents on the Function of Pancreatic β-Cells- Claudia Velasquez, Juan Sebastian Vasquez, Norman Balcazar
- Diabetes Metab J. 2017;41(4):303-315. Published online May 24, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.4.303
- 3,786 View
- 38 Download
- 8 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The increase in circulating free fatty acid (FFA) levels is a major factor that induces malfunction in pancreatic β-cells. We evaluated the effect of FFAs reconstituted according to the profile of circulating fatty acids found in obese adolescents on the viability and function of the murine insulinoma cell line (mouse insulinoma [MIN6]).
Methods From fatty acids obtained commercially, plasma-FFA profiles of three different youth populations were reconstituted: obese with metabolic syndrome; obese without metabolic syndrome; and normal weight without metabolic syndrome. MIN6 cells were treated for 24 or 48 hours with the three FFA profiles, and glucose-stimulated insulin secretion, cell viability, mitochondrial function and antioxidant activity were evaluated.
Results The high FFA content and high polyunsaturated ω6/ω3 ratio, present in plasma of obese adolescents with metabolic syndrome had a toxic effect on MIN6 cell viability and function, increasing oxidative stress and decreasing glucose-dependent insulin secretion.
Conclusion These results could help to guide nutritional management of obese young individuals, encouraging the increase of ω-3-rich food consumption in order to reduce the likelihood of deterioration of β-cells and the possible development of type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- The reversible effects of free fatty acids on sulfonylurea-stimulated insulin secretion are related to the expression and dynamin-mediated endocytosis of KATP channels in pancreatic β cells
Chenmin Wei, Zichen Zhang, Qi Fu, Yunqiang He, Tao Yang, Min Sun
Endocrine Connections.2023;[Epub] CrossRef - Preparation of fatty acid solutions exerts significant impact on experimental outcomes in cell culture models of lipotoxicity
Axel Römer, Divya Rawat, Thomas Linn, Sebastian F Petry
Biology Methods and Protocols.2022;[Epub] CrossRef - Lipotoxic Impairment of Mitochondrial Function in β-Cells: A Review
Axel Römer, Thomas Linn, Sebastian F. Petry
Antioxidants.2021; 10(2): 293. CrossRef - Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes
Petr Ježek, Martin Jabůrek, Lydie Plecitá-Hlavatá
Antioxidants & Redox Signaling.2019; 31(10): 722. CrossRef - The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action
Habtamu Wondifraw Baynes, Seifu Mideksa, Sintayehu Ambachew
Adipocyte.2018; : 1. CrossRef - Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity
Petr Ježek, Martin Jabůrek, Blanka Holendová, Lydie Plecitá-Hlavatá
Molecules.2018; 23(6): 1483. CrossRef
- The reversible effects of free fatty acids on sulfonylurea-stimulated insulin secretion are related to the expression and dynamin-mediated endocytosis of KATP channels in pancreatic β cells
- Beneficial Effects of Omega-3 Fatty Acids on Low Density Lipoprotein Particle Size in Patients with Type 2 Diabetes Already under Statin Therapy
- Myung Won Lee, Jeong Kyung Park, Jae Won Hong, Kwang Joon Kim, Dong Yeob Shin, Chul Woo Ahn, Young Duk Song, Hong Keun Cho, Seok Won Park, Eun Jig Lee
- Diabetes Metab J. 2013;37(3):207-211. Published online June 14, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.3.207
- 4,171 View
- 46 Download
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Beyond statin therapy for reducing low density lipoprotein cholesterol (LDL-C), additional therapeutic strategies are required to achieve more optimal reduction in cardiovascular risk among diabetic patients with dyslipidemia. To evaluate the effects and the safety of combined treatment with omega-3 fatty acids and statin in dyslipidemic patients with type 2 diabetes, we conducted a randomized, open-label study in Korea. Patients with persistent hypertriglyceridemia (≥200 mg/dL) while taking statin for at least 6 weeks were eligible. Fifty-one patients were randomized to receive either omega-3 fatty acid 4, 2 g, or no drug for 8 weeks while continuing statin therapy. After 8 weeks of treatment, the mean percentage change of low density lipoprotein (LDL) particle size and triglyceride (TG) level was greater in patients who were prescribed 4 g of omega-3 fatty acid with statin than in patients receiving statin monotherapy (2.8%±3.1% vs. 2.3%±3.6%,
P =0.024; -41.0%±24.1% vs. -24.2%±31.9%,P =0.049). Coadministration of omega-3 fatty acids with statin increased LDL particle size and decreased TG level in dyslipidemic patients with type 2 diabetes. The therapy was well tolerated without significant adverse effects.-
Citations
Citations to this article as recorded by- Diabetic cardiac autonomic neuropathy: insulin resistance, lipid profile, and omega-3 polyunsaturated fatty acids
Martin-Yurii Markevich, Volodymyr Segin, Victoria Serhiyenko, Alexandr Serhiyenko
InterConf.2023; (35(163)): 213. CrossRef - Atherogenic features of the fatty acid profile of erythrocyte membranes of patients with fatty liver disease of mixed genesis
M. V. Kruchinina, A. V. Belkovets, M. V. Parulikova, A. A. Gromov
Ateroscleroz.2023; 19(4): 350. CrossRef - Omega-3 supplementation in the treatment of polycystic ovary syndrome (PCOS) – a review of clinical trials and cohort
Vitoria Melo, Thomas Silva, Thaissa Silva, Juliana Freitas, Joselita Sacramento, Mirian Vazquez, Edilene Araujo
Endocrine Regulations.2022; 56(1): 66. CrossRef - Nutrigenetics, omega-3 and plasma lipids/lipoproteins/apolipoproteins with evidence evaluation using the GRADE approach: a systematic review
Justine Keathley, Véronique Garneau, Valérie Marcil, David M Mutch, Julie Robitaille, Iwona Rudkowska, Gabriela Magdalena Sofian, Sophie Desroches, Marie-Claude Vohl
BMJ Open.2022; 12(2): e054417. CrossRef - N-3 fatty acid supplementation mediates lipid profile, including small dense LDL, when combined with statins: a randomized double blind placebo controlled trial
Gediz Dogay Us, Sohail Mushtaq
Lipids in Health and Disease.2022;[Epub] CrossRef - The effect of omega-3 fatty acids and its combination with statins on lipid profile in patients with hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials
Yunjiao Yang, Wen Deng, Yanmei Wang, Tongyi Li, Yiding Chen, Cong Long, Qing Wen, Yue Wu, Qiu Chen
Frontiers in Nutrition.2022;[Epub] CrossRef - Study of the Healthy Effects of Different Fat Ratios Mixtures of Omega-3 to Omega-6 in Male Mice with Alloxan-Induced Diabetes
Ali. M. Atallah, Faryal. F. Hussein
Tikrit journal for agricultural sciences.2021; 21(4): 129. CrossRef - Omega-3 Fatty Acids as Druggable Therapeutics for Neurodegenerative Disorders
Neha M. Chitre, Nader H. Moniri, Kevin S. Murnane
CNS & Neurological Disorders - Drug Targets.2020; 18(10): 735. CrossRef - Efficacy and Safety of Omega-3 Fatty Acids in Patients Treated with Statins for Residual Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Ji Eun Jun, In-Kyung Jeong, Jae Myung Yu, Sung Rae Kim, In Kye Lee, Kyung-Ah Han, Sung Hee Choi, Soo-Kyung Kim, Hyeong Kyu Park, Ji-Oh Mok, Yong-ho Lee, Hyuk-Sang Kwon, So Hun Kim, Ho-Cheol Kang, Sang Ah Lee, Chang Beom Lee, Kyung Mook Choi, Sung-Ho Her,
Diabetes & Metabolism Journal.2020; 44(1): 78. CrossRef - The combination of canagliflozin and omega-3 fatty acid ameliorates insulin resistance and cardiac biomarkersviamodulation of inflammatory cytokines in type 2 diabetic rats
Mohammed Mohsen Safhi, Tarique Anwer, Gyas Khan, Rahimullah Siddiqui, Sivagurunathan Moni Sivakumar, Mohammad Firoz Alam
The Korean Journal of Physiology & Pharmacology.2018; 22(5): 493. CrossRef - Effect of diets rich in either saturated fat or n-6 polyunsaturated fatty acids and supplemented with long-chain n-3 polyunsaturated fatty acids on plasma lipoprotein profiles
C B Dias, N Amigo, L G Wood, X Correig, M L Garg
European Journal of Clinical Nutrition.2017; 71(11): 1297. CrossRef - Effects of 12-week supplementation of marine Omega-3 PUFA-based formulation Omega3Q10 in older adults with prehypertension and/or elevated blood cholesterol
Tian Shen, Guoqiang Xing, Jingfen Zhu, Shuxian Zhang, Yong Cai, Donghua Li, Gang Xu, Evan Xing, Jianyu Rao, Rong Shi
Lipids in Health and Disease.2017;[Epub] CrossRef - Effects of dietary saturated and n-6 polyunsaturated fatty acids on the incorporation of long-chain n-3 polyunsaturated fatty acids into blood lipids
C B Dias, L G Wood, M L Garg
European Journal of Clinical Nutrition.2016; 70(7): 812. CrossRef - Comparative analysis of the efficacy of omega-3 fatty acids for hypertriglyceridaemia management in Korea
H.-S. Kim, H. Kim, Y. J. Jeong, S. J. Yang, S. J. Baik, H. Lee, S.-H. Lee, J. H. Cho, I.-Y. Choi, H. W. Yim, K.-H. Yoon
Journal of Clinical Pharmacy and Therapeutics.2016; 41(5): 508. CrossRef - Effects of Omega-3 Fatty Acid Supplementation on Diabetic Nephropathy Progression in Patients with Diabetes and Hypertriglyceridemia
Eugene Han, Yujung Yun, Gyuri Kim, Yong-ho Lee, Hye Jin Wang, Byung-Wan Lee, Bong Soo Cha, Beom Seok Kim, Eun Seok Kang, Wolf-Hagen Schunck
PLOS ONE.2016; 11(5): e0154683. CrossRef - The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia
James Backes, Deborah Anzalone, Daniel Hilleman, Julia Catini
Lipids in Health and Disease.2016;[Epub] CrossRef - Supercritical fluid extraction of grape seeds: extract chemical composition, antioxidant activity and inhibition of nitrite production in LPS-stimulated Raw 264.7 cells
Concepción Pérez, María Luisa Ruiz del Castillo, Carmen Gil, Gracia Patricia Blanch, Gema Flores
Food & Function.2015; 6(8): 2607. CrossRef - Omega-3 Polyunsaturated Fatty Acids May Attenuate Streptozotocin-Induced Pancreatic β-Cell Death via Autophagy Activation in Fat1 Transgenic Mice
Won-Min Hwang, Dong-Ho Bak, Dong Ho Kim, Ju Young Hong, Seung-Yun Han, Keun-Young Park, Kyu Lim, Dong-Mee Lim, Jae Gu Kang
Endocrinology and Metabolism.2015; 30(4): 569. CrossRef - Long-chain omega-3 fatty acids, fibrates and niacin as therapeutic options in the treatment of hypertriglyceridemia: A review of the literature
Matthew K. Ito
Atherosclerosis.2015; 242(2): 647. CrossRef - Nutraceuticals and dyslipidaemia: Beyond the common therapeutics
Pietro Scicchitano, Matteo Cameli, Maria Maiello, Pietro Amedeo Modesti, Maria Lorenza Muiesan, Salvatore Novo, Pasquale Palmiero, Pier Sergio Saba, Roberto Pedrinelli, Marco Matteo Ciccone
Journal of Functional Foods.2014; 6: 11. CrossRef - The effect of dietary omega-3 polyunsaturated fatty acids on plasma lipids and lipoproteins of C57BL/6 mice is age and sex specific
K.A. Balogun, R.S. Randunu, S.K. Cheema
Prostaglandins, Leukotrienes and Essential Fatty Acids.2014; 91(1-2): 39. CrossRef - Gene-diet interactions with polymorphisms of the MGLL gene on plasma low-density lipoprotein cholesterol and size following an omega-3 polyunsaturated fatty acid supplementation: a clinical trial
Catherine Ouellette, Iwona Rudkowska, Simone Lemieux, Benoit Lamarche, Patrick Couture, Marie-Claude Vohl
Lipids in Health and Disease.2014;[Epub] CrossRef - Saturated fat consumption may not be the main cause of increased blood lipid levels
C.B. Dias, R. Garg, L.G. Wood, M.L. Garg
Medical Hypotheses.2014; 82(2): 187. CrossRef
- Diabetic cardiac autonomic neuropathy: insulin resistance, lipid profile, and omega-3 polyunsaturated fatty acids
- Dietary Oleate Has Beneficial Effects on Every Step of Non-Alcoholic Fatty Liver Disease Progression in a Methionine- and Choline-Deficient Diet-Fed Animal Model
- Ji Young Lee, Jae Hoon Moon, Jong Suk Park, Byung-Wan Lee, Eun Seok Kang, Chul Woo Ahn, Hyun Chul Lee, Bong Soo Cha
- Diabetes Metab J. 2011;35(5):489-496. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.489
- 30,198 View
- 34 Download
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a major cause of liver-related morbidity and mortality. The underlying mechanisms of disease progression remain poorly understood, and primary therapy of NAFLD is not yet established. We investigated the effects of dietary oleate on the development and progression of NAFLD in a methionine- and choline-deficient (MCD) diet-fed animal model.
Methods A total of 30 C57BL/6J mice were randomly divided into three groups (
n =10 in each group) and fed various experimental diets for four weeks: chow, MCD diet, or OMCD (MCD diet with oleate, 0.5 mg/g/day). Liver samples were examined for steatohepatitis and fibrosis parameters and associated genes.Results Additional dietary oleate dramatically reduced MCD diet-induced hepatic steatosis. Hepatic carbohydrate responsive element-binding protein was overexpressed in MCD diet-fed mice, and dietary oleate prevented this overexpression (
P <0.001). Dietary oleate partially prevented MCD diet-induced serum level increases in aspartate aminotransferase and alanine aminotransferase (P <0.001, respectively). The mRNA expressions of hepatic monocyte chemoattractant protein 1, tumor necrosis factor-α and matrix metalloproteinase-9 were increased in MCD diet-fed mice, and this overexpression of inflammatory molecules was prevented by dietary oleate (P <0.001). Hepatic pericellular fibrosis was observed in MCD diet-fed mice, and dietary oleate prevented this fibrosis. Altogether, dietary oleate prevented MCD diet-induced hepatic steatosis, inflammation and fibrosis.Conclusion Dietary oleate has beneficial effects in every step of NAFLD development and progression and could be a nutritional option for NAFLD prevention and treatment.
-
Citations
Citations to this article as recorded by- Bidirectional association between NAFLD and gallstone disease: a systematic review and meta-analysis of observational studies
Shengying Gu, Shanshan Hu, Shuowen Wang, Chendong Qi, Chenyang Shi, Guorong Fan
Expert Review of Gastroenterology & Hepatology.2023; 17(3): 283. CrossRef - The Effect of Bioactive Aliment Compounds and Micronutrients on Non-Alcoholic Fatty Liver Disease
Camelia Munteanu, Betty Schwartz
Antioxidants.2023; 12(4): 903. CrossRef - Single‐cell transcriptomics stratifies organoid models of metabolic dysfunction‐associated steatotic liver disease
Anja Hess, Stefan D Gentile, Amel Ben Saad, Raza‐Ur Rahman, Tim Habboub, Daniel S Pratt, Alan C Mullen
The EMBO Journal.2023;[Epub] CrossRef - Histopathological Examination of the Effects of Tocilizumab and Dexamethasone on the Liver in Rats of Oleic Acid induced Acute Lung Injury
Funda TERZİ, Hüseyin Serkan EROL
Balıkesır Health Sciences Journal.2022;[Epub] CrossRef - Identifying Lipid Metabolites Influenced by Oleic Acid Administration Using High-Performance Liquid Chromatography–Mass Spectrometry-Based Lipidomics
Chao Xu, Dan Song, Askild L. Holck, Youyou Zhou, Rong Liu
ACS Omega.2020; 5(20): 11314. CrossRef - Causative and Sanative dynamicity of ChREBP in Hepato-Metabolic disorders
P. Vineeth Daniel, Prosenjit Mondal
European Journal of Cell Biology.2020; 99(8): 151128. CrossRef - PPARδ attenuates hepatic steatosis through autophagy-mediated fatty acid oxidation
Lei Tong, Long Wang, Shuangshuang Yao, Lina Jin, Jian Yang, Yifei Zhang, Guang Ning, Zhiguo Zhang
Cell Death & Disease.2019;[Epub] CrossRef - Butyrate Protects Mice Against Methionine–Choline-Deficient Diet-Induced Non-alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuating Inflammation and Reducing Endotoxin Levels
Jianzhong Ye, Longxian Lv, Wenrui Wu, Yating Li, Ding Shi, Daiqiong Fang, Feifei Guo, Huiyong Jiang, Ren Yan, Wanchun Ye, Lanjuan Li
Frontiers in Microbiology.2018;[Epub] CrossRef - Olive oil combined with Lycium barbarum polysaccharides attenuates liver apoptosis and inflammation induced by carbon tetrachloride in rats
Yun-Yun Chiang, Jane C.-J. Chao
Journal of Functional Foods.2018; 48: 329. CrossRef - Dietary oleic acid regulates hepatic lipogenesis through a liver X receptor-dependent signaling
Simon Ducheix, Alexandra Montagner, Arnaud Polizzi, Frédéric Lasserre, Marion Régnier, Alice Marmugi, Fadila Benhamed, Justine Bertrand-Michel, Laila Mselli-Lakhal, Nicolas Loiseau, Pascal G. Martin, Jean-Marc Lobaccaro, Laurent Ferrier, Catherine Postic,
PLOS ONE.2017; 12(7): e0181393. CrossRef - Is hepatic lipogenesis fundamental for NAFLD/NASH? A focus on the nuclear receptor coactivator PGC-1β
Simon Ducheix, Maria Carmela Vegliante, Gaetano Villani, Nicola Napoli, Carlo Sabbà, Antonio Moschetta
Cellular and Molecular Life Sciences.2016; 73(20): 3809. CrossRef - Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway
Young Mi Song, Yong-ho Lee, Ji-Won Kim, Dong-Sik Ham, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2015; 11(1): 46. CrossRef - Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease
Sanja Stojsavljević
World Journal of Gastroenterology.2014; 20(48): 18070. CrossRef - Microglial Cell Activation Increases Saturated and Decreases Monounsaturated Fatty Acid Content, but Both Lipid Species are Proinflammatory
Emily B. Button, Andrew S. Mitchell, Marcia M. Domingos, Jessica H.‐J. Chung, Ryan M. Bradley, Ashkan Hashemi, Phillip M. Marvyn, Ashley C. Patterson, Ken D. Stark, Joe Quadrilatero, Robin E. Duncan
Lipids.2014; 49(4): 305. CrossRef - Modeling progressive non-alcoholic fatty liver disease in the laboratory mouse
Jesse D. Riordan, Joseph H. Nadeau
Mammalian Genome.2014; 25(9-10): 473. CrossRef - Rapid chromatographic method to decipher distinct alterations in lipid classes in NAFLD/NASH
Stephan Laggai, Yvette Simon, Theo Ranssweiler, Alexandra K Kiemer, Sonja M Kessler
World Journal of Hepatology.2013; 5(10): 558. CrossRef - Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction
Young Mi Song, Sun-Ok Song, Yong-Keun Jung, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2012; 8(7): 1085. CrossRef
- Bidirectional association between NAFLD and gallstone disease: a systematic review and meta-analysis of observational studies
- Effects of Adding omega-3 Fatty Acids to Simvastatin on Lipids, Lipoprotein Size and Subspecies in Type 2 Diabetes Mellitus with Hypertriglyceridemia.
- Won Jun Kim, Chang Beom Lee, Cheol Young Park, Se Eun Park, Eun Jung Rhee, Won Young Lee, Ki Won Oh, Sung Woo Park, Dae Jung Kim, Hae Jin Kim, Seung Jin Han, Hong Keum Cho
- Korean Diabetes J. 2009;33(6):494-502. Published online December 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.6.494
- 2,552 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
omega-3 fatty acids are known to improve lipid profiles, the distribution of lipoprotein subclasses, and secondary prevention against post-myocardial infarction. Rare reports have emerged of synergistic results of omega-3 fatty acids with simvastatin in cases of type 2 diabetes mellitus with hypertriglyceridemia. The purpose of this study was to determine the combined relationship of omega-3 fatty acids plus simvastatin on lipid, lipoprotein size and the types of subspecies. METHODS: This randomized, multi-center, comparison study evaluated eight weeks of combination therapy (omega-3 fatty acids (Omacor) 4 g/day plus simvastatin 20 mg/day) or monotherapy (simvastatin 20 mg/day) for at least six weeks in 62 diabetic patients. Subjects with a triglyceride concentration of more than 200 mg/dL were eligible for inclusion. RESULTS: No significant differences for omega-3 fatty acids + simvastatin versus simvastatin alone were observed for triglycerides (-22.7% vs. -14.3%, P = 0.292), HDL peak particle size (+2.8% vs. -0.4%, P = 0.076), LDL mean particle size (+0.4% vs -0.1%, P = 0.376) or LDL subspecies types, although the combination therapy showed a tendency toward lower triglycerides, larger HDL, and LDL particle sizes than did the monotherapy. There were no significant differences between the two groups in regard to HDL-C, LDL-C, or HbA1c levels. There were no serious adverse events and no abnormalities in the laboratory values associated with this study. CONCLUSION: omega-3 fatty acids were a safeform of treatment in hypertriglyceridemic patients with type 2 diabetes mellitus. But, regarding efficacy, a much larger sample size and longer-term follow-up may be needed to distinguish between the effects of combination therapy and monotherapy.
- Impairment of Insulin Secretion by Fat Overload in Rat Pancreatic Islets and Effects of Antioxidants.
- Chul Hee Kim, Chan Hee Kim, Hyeong Kyu Park, Kyo Il Suh, Ki Up Lee
- Korean Diabetes J. 2002;26(5):347-356. Published online October 1, 2002
- 736 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
It has recently been suggested that fat overload on pancreatic beta cells is responsible for the abnormal pattern of insulin secretion in type 2 diabetes mellitus. Antioxidant treatment was reported to preserve beta cell function in animal models of diabetes. This study was undertaken to examine the effects of various free fatty acids and triglyceride on insulin secretion in isolated rat pancreatic islets. In addition, we examined the effects of antioxidants. METHODS: Pancreatic islets of normal Sprague-Dawley rats were isolated by intraductal injection of collagenase and Ficoll-gradient centrifugation. The islets were treated with palmitat0e (C16:0), oleate (C18:1), linoleate (C18:2), and triglyceride emulsions (intralipid) for 72hours. Basal and glucose-stimulated insulin secretions were measured. The effects of the antioxidants, vitamin E, alpha-lipoic acid, and N-acetyl cysteine, were examined on the fat-induced change of insulin secretion. RESULTS: All of the free fatty acids and the triglyceride increased the basal insulin secretion. In contrast, insulin secretion stimulated by 27 mM glucose was significantly decreased after the treatment with free fatty acids or triglycerides. The antioxidant could not prevent the fat-induced inhibition of insulin secretion. CONCLUSION: These results show that various free fatty acids and triglyceride commonly cause defects in insulin secretion. However, we could not confirm the the hypothesis that increased oxidative stress may be involved in the pathogenesis of insulin secretory defect associated with fat overload.
- Effects of Free Fatty Acids on Glutathione Redox Status in Cultured Endothelial Cells.
- Joong Yeol Park, Chul Hee Kim, Yun Ey Chung, Hong Kyu Kim, Young Il Kim, Sung Kwan Hong, Jae Dam Lee, Ki Up Lee
- Korean Diabetes J. 1998;22(3):262-270. Published online January 1, 2001
- 854 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Although plasma free fatty acids (FFA) are frequently elevated in diabetes mellitus, its role in the pathogenesis of diabetic vascular complications has not been well investigated. Recent stuclies reported that FFA may cause endothelial dysfunction through an enhancement of oxidative damage by decreasing glutathione redox cycle, an important anti-oxidant defense system in endothelial cells. In this study, we examined the effects of increased availability of FFA on intracellular glutathione redox cycle. METHODS: Bovine pulonary endothelial cells were exposed to 90 umol/L linoleic acid with or without 0.1 mM 2-bromopalmitate, an inhibitor of mitochondrial fatty acid oxidation, for 6hr. Components of the glutathione redox cycle such as total glutathione, reduced glutathione(GSH) and oxidized glutathione(GSSG) concentrations were measured by HPLC. RESULTS: Total glutathione concentration in cultured endothelial cells exposed to linoleic acid was significantly lower than that in control cells (10.8+ 0.5 vs 14.1+0.8 umol/g protein, P<0.05). Linoleic acid significantly decreased GSH concentrations (10.5+0.4 vs. 13.8+0.5 pmol/g protein, P<0.05) and the ratio of GSH/GSSG(26.3+1.3 vs. 47.0+2,1, P<0.05). Compared to cells exposed linoleic acid alone, total glutathione(13.5+0.5umol/g protein, P<0.05) and GSH concentration(13.2+0.4 pmol/g protein, P<0.05) significantly increased in cells treated with 2-bromopalmitate and linoleic acid. The ratio of GSH/GSSG in cells treated with 2-bromopalmitate and linoleic acid was higher th.an that in cells exposed to linoleic acid alone(44.1+1.3, P<0.05). CONCLUSION: Increased provision of FFA resulted in a derangement of glutathione redox cycle in cultured endothelial cells, which appears to be related to an increase in mitochondrial FFA oxidation. These results suggested that FFA can increase the risk of diabetic vascular complications.

 KDA
KDA
 First
First Prev
Prev





