
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
- Comparative Efficacy of Rosuvastatin Monotherapy and Rosuvastatin/Ezetimibe Combination Therapy on Insulin Sensitivity and Vascular Inflammatory Response in Patients with Type 2 Diabetes Mellitus
- Ji Hye Han, Kyong Hye Joung, Jun Choul Lee, Ok Soon Kim, Sorim Choung, Ji Min Kim, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Bon Jeong Ku, Hyun Jin Kim
- Diabetes Metab J. 2024;48(1):112-121. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0402
- 2,121 View
- 225 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
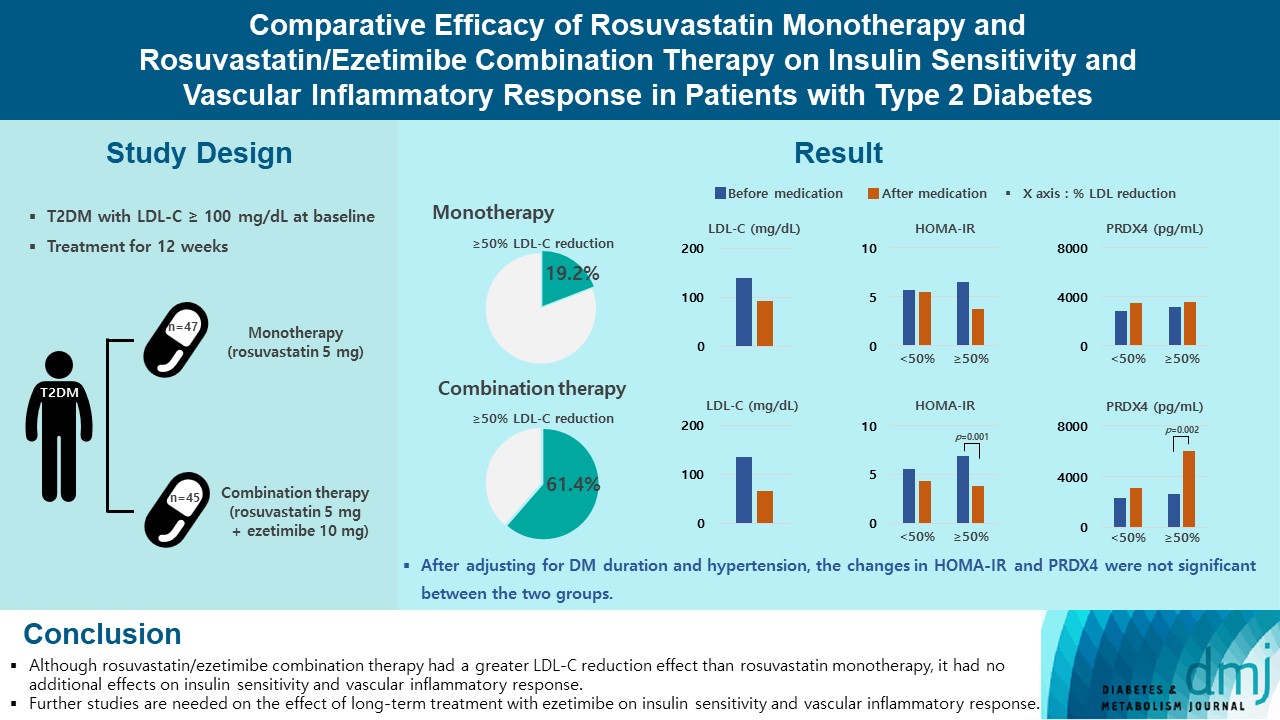
Type 2 diabetes mellitus (T2DM) induces endothelial dysfunction and inflammation, which are the main factors for atherosclerosis and cardiovascular disease. The present study aimed to compare the effects of rosuvastatin monotherapy and rosuvastatin/ezetimibe combination therapy on lipid profile, insulin sensitivity, and vascular inflammatory response in patients with T2DM.
Methods
A total of 101 patients with T2DM and dyslipidemia were randomized to either rosuvastatin monotherapy (5 mg/day, n=47) or rosuvastatin/ezetimibe combination therapy (5 mg/10 mg/day, n=45) and treated for 12 weeks. Serum lipids, glucose, insulin, soluble intercellular adhesion molecule-1 (sICAM-1), and peroxiredoxin 4 (PRDX4) levels were determined before and after 12 weeks of treatment.
Results
The reduction in low density lipoprotein cholesterol (LDL-C) by more than 50% from baseline after treatment was more in the combination therapy group. The serum sICAM-1 levels increased significantly in both groups, but there was no difference between the two groups. The significant changes in homeostasis model assessment of insulin resistance (HOMA-IR) and PRDX4 were confirmed only in the subgroup in which LDL-C was reduced by 50% or more in the combination therapy group. However, after adjusting for diabetes mellitus duration and hypertension, the changes in HOMA-IR and PRDX4 were not significant between the two groups.
Conclusion
Although rosuvastatin/ezetimibe combination therapy had a greater LDL-C reduction effect than rosuvastatin monotherapy, it had no additional effects on insulin sensitivity and vascular inflammatory response. Further studies are needed on the effect of long-term treatment with ezetimibe on insulin sensitivity and vascular inflammatory response. -
Citations
Citations to this article as recorded by- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
Eun Roh
Diabetes & Metabolism Journal.2024; 48(1): 55. CrossRef
- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
- Drug Regimen
- The Efficacy and Safety of Moderate-Intensity Rosuvastatin with Ezetimibe versus High-Intensity Rosuvastatin in High Atherosclerotic Cardiovascular Disease Risk Patients with Type 2 Diabetes Mellitus: A Randomized, Multicenter, Open, Parallel, Phase 4 Study
- Jun Sung Moon, Il Rae Park, Sang Soo Kim, Hye Soon Kim, Nam Hoon Kim, Sin Gon Kim, Seung Hyun Ko, Ji Hyun Lee, Inkyu Lee, Bo Kyeong Lee, Kyu Chang Won
- Diabetes Metab J. 2023;47(6):818-825. Published online November 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0171

- 2,421 View
- 245 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate the efficacy and safety of moderate-intensity rosuvastatin/ezetimibe combination compared to highintensity rosuvastatin in high atherosclerotic cardiovascular disease (ASCVD) risk patients with type 2 diabetes mellitus (T2DM).
Methods
This study was a randomized, multicenter, open, parallel phase 4 study, and enrolled T2DM subjects with an estimated 10-year ASCVD risk ≥7.5%. The primary endpoint was the low-density lipoprotein cholesterol (LDL-C) change rate after 24-week rosuvastatin 10 mg/ezetimibe 10 mg treatment was non-inferior to that of rosuvastatin 20 mg. The achievement proportion of 10-year ASCVD risk <7.5% or comprehensive lipid target (LDL-C <70 mg/dL, non-high-density lipoprotein cholesterol <100 mg/dL, and apolipoprotein B <80 mg/dL) without discontinuation, and several metabolic parameters were explored as secondary endpoints.
Results
A hundred and six participants were assigned to each group. Both groups showed significant reduction in % change of LDL-C from baseline at week 24 (–63.90±6.89 vs. –55.44±6.85, combination vs. monotherapy, p=0.0378; respectively), but the combination treatment was superior to high-intensity monotherapy in LDL-C change (%) from baseline (least square [LS] mean difference, –8.47; 95% confidence interval, –16.44 to –0.49; p=0.0378). The combination treatment showed a higher proportion of achieved comprehensive lipid targets rather than monotherapy (85.36% vs. 62.22% in monotherapy, p=0.015). The ezetimibe combination significantly improved homeostasis model assessment of β-cell function even without A1c changes (LS mean difference, 17.13; p=0.0185).
Conclusion
In high ASCVD risk patients with T2DM, the combination of moderate-intensity rosuvastatin and ezetimibe was not only non-inferior but also superior to improving dyslipidemia with additional benefits compared to high-intensity rosuvastatin monotherapy.
- Basic Research
- Mitochondrial TFAM as a Signaling Regulator between Cellular Organelles: A Perspective on Metabolic Diseases
- Jin-Ho Koh, Yong-Woon Kim, Dae-Yun Seo, Tae-Seo Sohn
- Diabetes Metab J. 2021;45(6):853-865. Published online November 22, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0138
- 6,696 View
- 275 Download
- 14 Web of Science
- 15 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
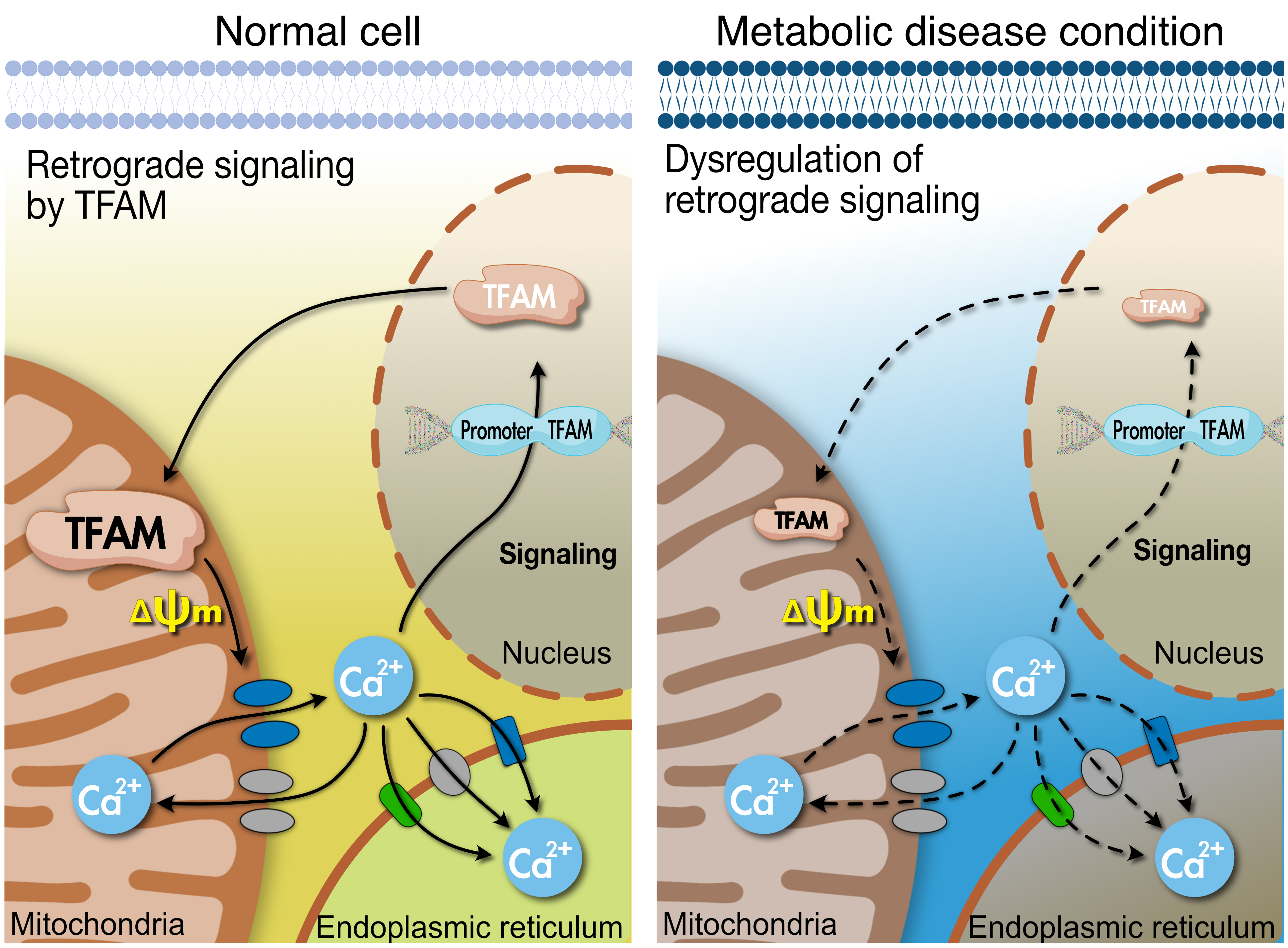
- Tissues actively involved in energy metabolism are more likely to face metabolic challenges from bioenergetic substrates and are susceptible to mitochondrial dysfunction, leading to metabolic diseases. The mitochondria receive signals regarding the metabolic states in cells and transmit them to the nucleus or endoplasmic reticulum (ER) using calcium (Ca2+) for appropriate responses. Overflux of Ca2+ in the mitochondria or dysregulation of the signaling to the nucleus and ER could increase the incidence of metabolic diseases including insulin resistance and type 2 diabetes mellitus. Mitochondrial transcription factor A (Tfam) may regulate Ca2+ flux via changing the mitochondrial membrane potential and signals to other organelles such as the nucleus and ER. Since Tfam is involved in metabolic function in the mitochondria, here, we discuss the contribution of Tfam in coordinating mitochondria-ER activities for Ca2+ flux and describe the mechanisms by which Tfam affects mitochondrial Ca2+ flux in response to metabolic challenges.
-
Citations
Citations to this article as recorded by- Targeted metabolomics reveals the aberrant energy status in diabetic peripheral neuropathy and the neuroprotective mechanism of traditional Chinese medicine JinMaiTong
Bingjia Zhao, Qian Zhang, Yiqian He, Weifang Cao, Wei Song, Xiaochun Liang
Journal of Pharmaceutical Analysis.2024; 14(2): 225. CrossRef - Mitochondrial damage‐associated molecular patterns: A new insight into metabolic inflammation in type 2 diabetes mellitus
Yan Wang, Jingwu Wang, Si‐Yu Tao, Zhengting Liang, Rong xie, Nan‐nan Liu, Ruxue Deng, Yuelin Zhang, Deqiang Deng, Guangjian Jiang
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - Altered Energy Metabolism, Mitochondrial Dysfunction, and Redox Imbalance Influencing Reproductive Performance in Granulosa Cells and Oocyte During Aging
Hiroshi Kobayashi, Chiharu Yoshimoto, Sho Matsubara, Hiroshi Shigetomi, Shogo Imanaka
Reproductive Sciences.2024; 31(4): 906. CrossRef - When Our Best Friend Becomes Our Worst Enemy: The Mitochondrion in Trauma, Surgery, and Critical Illness
May-Kristin Torp, Kåre-Olav Stensløkken, Jarle Vaage
Journal of Intensive Care Medicine.2024;[Epub] CrossRef - Attenuating mitochondrial dysfunction and morphological disruption with PT320 delays dopamine degeneration in MitoPark mice
Vicki Wang, Kuan-Yin Tseng, Tung-Tai Kuo, Eagle Yi-Kung Huang, Kuo-Lun Lan, Zi-Rong Chen, Kuo-Hsing Ma, Nigel H. Greig, Jin Jung, Ho-II Choi, Lars Olson, Barry J. Hoffer, Yuan-Hao Chen
Journal of Biomedical Science.2024;[Epub] CrossRef - Effects of the anti-inflammatory drug celecoxib on cell death signaling in human colon cancer
Ryuto Maruyama, Yuki Kiyohara, Yasuhiro Kudo, Tomoyasu Sugiyama
Naunyn-Schmiedeberg's Archives of Pharmacology.2023; 396(6): 1171. CrossRef - gp130 Activates Mitochondrial Dynamics for Hepatocyte Survival in a Model of Steatohepatitis
Daria Shunkina, Anastasia Dakhnevich, Egor Shunkin, Olga Khaziakhmatova, Valeria Shupletsova, Maria Vulf, Alexandra Komar, Elena Kirienkova, Larisa Litvinova
Biomedicines.2023; 11(2): 396. CrossRef - Pharmacological Activation of Rev-erbα Attenuates Doxorubicin-Induced Cardiotoxicity by PGC-1α Signaling Pathway
Runmei Zou, Shuo Wang, Hong Cai, Yuwen Wang, Cheng Wang, Vivek Pandey
Cardiovascular Therapeutics.2023; 2023: 1. CrossRef - Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells
Damien Meng-Kiat Leow, Irwin Kee-Mun Cheah, Zachary Wei-Jie Fong, Barry Halliwell, Wei-Yi Ong
International Journal of Molecular Sciences.2023; 24(6): 5498. CrossRef - Effect of PPARγ on oxidative stress in diabetes-related dry eye
Jing Wang, Shuangping Chen, Xiuxiu Zhao, Qian Guo, Ruibo Yang, Chen Zhang, Yue Huang, Lechong Ma, Shaozhen Zhao
Experimental Eye Research.2023; 231: 109498. CrossRef - Chiisanoside Mediates the Parkin/ZNF746/PGC-1α Axis by Downregulating MiR-181a to Improve Mitochondrial Biogenesis in 6-OHDA-Caused Neurotoxicity Models In Vitro and In Vivo: Suggestions for Prevention of Parkinson’s Disease
Yu-Ling Hsu, Hui-Jye Chen, Jia-Xin Gao, Ming-Yang Yang, Ru-Huei Fu
Antioxidants.2023; 12(9): 1782. CrossRef - TBBPA causes apoptosis in grass carp hepatocytes involving destroyed ER-mitochondrial function
Dongxu Han, Naixi Yang, Huanyi Liu, Yujie Yao, Shiwen Xu
Chemosphere.2023; 341: 139974. CrossRef - The Protective Mechanism of TFAM on Mitochondrial DNA and its Role in Neurodegenerative Diseases
Ying Song, Wenjun Wang, Beibei Wang, Qiwen Shi
Molecular Neurobiology.2023;[Epub] CrossRef - Impact of Roux-en-Y Gastric Bypass on Mitochondrial Biogenesis and Dynamics in Leukocytes of Obese Women
Zaida Abad-Jiménez, Teresa Vezza, Sandra López-Domènech, Meylin Fernández-Reyes, Francisco Canet, Carlos Morillas, Segundo Ángel Gómez-Abril, Celia Bañuls, Víctor M. Víctor, Milagros Rocha
Antioxidants.2022; 11(7): 1302. CrossRef - The Effects of Galgunhwanggumhwangryun-tang on Glucose and Energy Metabolism in C2C12 Myotubes
Jihong Oh, Song-Yi Han, Soo Kyoung Lim, Hojun Kim
Journal of Korean Medicine for Obesity Research.2022; 22(2): 93. CrossRef
- Targeted metabolomics reveals the aberrant energy status in diabetic peripheral neuropathy and the neuroprotective mechanism of traditional Chinese medicine JinMaiTong
- Clinical Diabetes & Therapeutics
- Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus
- You-Cheol Hwang, Ji Eun Jun, In-Kyung Jeong, Kyu Jeung Ahn, Ho Yeon Chung
- Diabetes Metab J. 2019;43(5):582-589. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0124
- 6,612 View
- 185 Download
- 14 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The apolipoprotein B/A1 (apoB/A1) ratio is a stronger predictor of future cardiovascular disease than is the level of conventional lipids. Statin and ezetimibe combination therapy have shown additional cardioprotective effects over statin monotherapy.
Methods This was a single-center, randomized, open-label, active-controlled study in Korea. A total of 36 patients with type 2 diabetes mellitus were randomized to either rosuvastatin monotherapy (20 mg/day,
n =20) or rosuvastatin/ezetimibe (5 mg/10 mg/day,n =16) combination therapy for 6 weeks.Results After the 6-week treatment, low density lipoprotein cholesterol (LDL-C) and apoB reduction were comparable between the two groups (−94.3±15.4 and −62.0±20.9 mg/dL in the rosuvastatin group, −89.9±22.7 and −66.8±21.6 mg/dL in the rosuvastatin/ezetimibe group,
P =0.54 andP =0.86, respectively). In addition, change in apoB/A1 ratio (−0.44±0.16 in the rosuvastatin group and −0.47±0.25 in the rosuvastatin/ezetimibe group,P =0.58) did not differ between the two groups. On the other hand, triglyceride and free fatty acid (FFA) reductions were greater in the rosuvastatin/ezetimibe group than in the rosuvastatin group (−10.5 mg/dL [interquartile range (IQR), −37.5 to 29.5] and 0.0 µEq/L [IQR, −136.8 to 146.0] in the rosuvastatin group, −49.5 mg/dL [IQR, −108.5 to −27.5] and −170.5 µEq/L [IQR, −353.0 to 0.8] in the rosuvastatin/ezetimibe group,P =0.010 andP =0.049, respectively). Both treatments were generally well tolerated, and there were no differences in muscle or liver enzyme elevation.Conclusion A 6-week combination therapy of low-dose rosuvastatin and ezetimibe showed LDL-C, apoB, and apoB/A1 ratio reduction comparable to that of high-dose rosuvastatin monotherapy in patients with type 2 diabetes mellitus. Triglyceride and FFA reductions were greater with the combination therapy than with rosuvastatin monotherapy.
-
Citations
Citations to this article as recorded by- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review
Yura Kang, Jung Mi Park, Sang-Hak Lee
Yonsei Medical Journal.2024; 65(1): 19. CrossRef - Combination Therapy of Ezetimibe and Rosuvastatin for Dyslipidemia: Current Insights
Maya R Chilbert, Dylan VanDuyn, Sara Salah, Collin M Clark, Qing Ma
Drug Design, Development and Therapy.2022; Volume 16: 2177. CrossRef - Ezetimibe and diabetes mellitus:a new strategy for lowering cholesterol
V.A. Serhiyenko, A.A. Serhiyenko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2022; 18(5): 302. CrossRef - The Effect of Rosuvastatin on Plasma/Serum Levels of High-Sensitivity C-Reactive Protein, Interleukin-6, and D-Dimer in People Living with Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis
Akililu Alemu Ashuro, Yin-Guang Fan, Yuan-Sheng Fu, Dong-Sheng Di, Napoleon Bellua Sam, Hai-Feng Pan, Dong-Qing Ye
AIDS Research and Human Retroviruses.2021; 37(11): 821. CrossRef - Comparison of the Efficacy and Safety of Rosuvastatin/Ezetimibe Combination Therapy and Rosuvastatin Monotherapy on Lipoprotein in Patients With Type 2 Diabetes: Multicenter Randomized Controlled Study
Jiwoo Lee, You-Cheol Hwang, Woo Je Lee, Jong Chul Won, Kee-Ho Song, Cheol-Young Park, Kyu Jeung Ahn, Joong-Yeol Park
Diabetes Therapy.2020; 11(4): 859. CrossRef - Comparison of Renal Effects of Ezetimibe–Statin Combination versus Statin Monotherapy: A Propensity-Score-Matched Analysis
Jaehyun Bae, Namki Hong, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Yong-ho Lee
Journal of Clinical Medicine.2020; 9(3): 798. CrossRef - Combined use of rosuvastatin and ezetimibe improves hepatic steatosis in patients with dyslipidemia
Won Dong Lee, Beom Kyung Kim, Jun Yong Park, Do Young Kim, Sang Hoon Ahn, Kwang-Hyub Han, Seung Up Kim
European Journal of Gastroenterology & Hepatology.2020; 32(12): 1538. CrossRef - Influence of rosuvastatin dose on total fatty acids and free fatty acids in plasma
Cristian I. Ciucanu, Sonia Olariu, Daliborca C. Vlad, Victor Dumitraşcu
Medicine.2020; 99(48): e23356. CrossRef - The effect of switching from statin-monotherapy to statin/ezetimibe combination therapy on lipid profiles in patients with type 2 diabetes and dyslipidemia: a multicenter open-label study (EUCLID)
Mitsuhide Takeshita, Atsushi Tanaka, Atsushi Kawaguchi, Keiko Sato, Shigeru Toyoda, Teruo Inoue, Koichi Node
Vascular Failure.2020; 4(1): 22. CrossRef - Response: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
You-Cheol Hwang
Diabetes & Metabolism Journal.2019; 43(6): 915. CrossRef - Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J2019;43:582–9)
Tae Seo Sohn
Diabetes & Metabolism Journal.2019; 43(6): 909. CrossRef - Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes
Amélie I. S. Sobczak, Claudia A. Blindauer, Alan J. Stewart
Nutrients.2019; 11(9): 2022. CrossRef
- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review
- Obesity and Metabolic Syndrome
- Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism
- Muthu Periasamy, Jose Luis Herrera, Felipe C. G. Reis
- Diabetes Metab J. 2017;41(5):327-336. Published online October 24, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.5.327
- 8,941 View
- 208 Download
- 112 Web of Science
- 119 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Obesity and diabetes has become a major epidemic across the globe. Controlling obesity has been a challenge since this would require either increased physical activity or reduced caloric intake; both are difficult to enforce. There has been renewed interest in exploiting pathways such as uncoupling protein 1 (UCP1)-mediated uncoupling in brown adipose tissue (BAT) and white adipose tissue to increase energy expenditure to control weight gain. However, relying on UCP1-based thermogenesis alone may not be sufficient to control obesity in humans. On the other hand, skeletal muscle is the largest organ and a major contributor to basal metabolic rate and increasing energy expenditure in muscle through nonshivering thermogenic mechanisms, which can substantially affect whole body metabolism and weight gain. In this review we will describe the role of Sarcolipin-mediated uncoupling of Sarcoplasmic Reticulum Calcium ATPase (SERCA) as a potential mechanism for increased energy expenditure both during cold and diet-induced thermogenesis.
-
Citations
Citations to this article as recorded by- Nutrition as the foundation for successful aging: a focus on dietary protein and omega-3 polyunsaturated fatty acids
Aubree L Hawley, Jamie I Baum
Nutrition Reviews.2024; 82(3): 389. CrossRef - Seasonal adaptation of Mangalica pigs in terms of muscle morphology and metabolism
Sangwoo Kim, Chisato Nakayama, Daisuke Kondoh, Tatsuki Okazaki, Erina Yoneda, Kisaki Tomita, Motoki Sasaki, Yuki Muranishi
Anatomia, Histologia, Embryologia.2024;[Epub] CrossRef - In vivo heat production dynamics during a contraction-relaxation cycle in rat single skeletal muscle fibers
Ayaka Tabuchi, Yoshinori Tanaka, Hiroshi Horikawa, Takuto Tazawa, David C. Poole, Yutaka Kano
Journal of Thermal Biology.2024; 119: 103760. CrossRef - Echinacoside stimulates myogenesis and ATP-dependent thermogenesis in the skeletal muscle via the activation of D1-like dopaminergic receptors
Kiros Haddish, Jong Won Yun
Archives of Biochemistry and Biophysics.2024; 752: 109886. CrossRef - Ectodysplasin A2 receptor signaling in skeletal muscle pathophysiology
Sevgi Döndü Özen, Serkan Kir
Trends in Molecular Medicine.2024;[Epub] CrossRef - Chronic melatonin treatment improves obesity by inducing uncoupling of skeletal muscle SERCA-SLN mediated by CaMKII/AMPK/PGC1α pathway and mitochondrial biogenesis in female and male Zücker diabetic fatty rats
D. Salagre, M. Navarro-Alarcón, M. Villalón-Mir, B. Alcázar-Navarrete, G. Gómez-Moreno, F. Tamimi, A. Agil
Biomedicine & Pharmacotherapy.2024; 172: 116314. CrossRef - Homotaurine exhibits contrasting effects of DRD1-mediated thermogenesis-related regulators in C2C12 myoblasts and 3T3−L1 white adipocytes
Kiros Haddish, Jong Won Yun
Biotechnology and Bioprocess Engineering.2024;[Epub] CrossRef - Hairless (Hr) Deficiency Mitigates High‐Fat Diet‐Induced Obesity and Insulin Resistance in Mice
Hongwei Wang, Haoyu Guo, Kuicheng Zhu, Long He, Jian‐jun Yang
Advanced Biology.2024;[Epub] CrossRef - Prediction of resting energy expenditure for adolescents with severe obesity: A multi‐centre analysis
Amy A. Rydin, Cameron Severn, Laura Pyle, Nazeen Morelli, Ashley H. Shoemaker, Stephanie T. Chung, Jack A. Yanovski, Joan C. Han, Janine A. Higgins, Kristen J. Nadeau, Claudia Fox, Aaron S. Kelly, Melanie G. Cree
Pediatric Obesity.2024;[Epub] CrossRef - Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets
Joshua S. Fleishman, Sunil Kumar
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - The effect of high fat diet and endurance training on newly discovery of nonshivering-thermogenic factors under thermoneutrality in mice
S. Daneshyar, A. Ghasemnian, Z. Mirakhori, S.J. Daneshyar
Science & Sports.2023; 38(3): 293. CrossRef - Redox‐metabolic reprogramming of skin in mice lacking functional Nrf2 under basal conditions and cold acclimation
Tamara Zakic, Sara Stojanovic, Aleksandra Jankovic, Aleksandra Korac, Vanja Pekovic‐Vaughan, Bato Korac
BioFactors.2023; 49(3): 600. CrossRef - Rats lackingUcp1present a novel translational tool for the investigation of thermogenic adaptation during cold challenge
Jaycob D. Warfel, Carrie M. Elks, David S. Bayless, Bolormaa Vandanmagsar, Allison C. Stone, Samuel E. Velasquez, Paola Olivares‐Nazar, Robert C. Noland, Sujoy Ghosh, Jingying Zhang, Randall L. Mynatt
Acta Physiologica.2023;[Epub] CrossRef - SENP2 knockdown in human adipocytes reduces glucose metabolism and lipid accumulation, while increases lipid oxidation
Solveig A. Krapf, Jenny Lund, Hege G. Bakke, Tuula A. Nyman, Stefano Bartesaghi, Xiao-Rong Peng, Arild C. Rustan, G. Hege Thoresen, Eili T. Kase
Metabolism Open.2023; 18: 100234. CrossRef - Physiological and molecular mechanisms of cold-induced improvements in glucose homeostasis in humans beyond brown adipose tissue
Sten van Beek, Dzhansel Hashim, Tore Bengtsson, Joris Hoeks
International Journal of Obesity.2023; 47(5): 338. CrossRef - Distinct Transcriptional Responses of Skeletal Muscle to Short-Term Cold Exposure in Tibetan Pigs and Bama Pigs
Chunhuai Yang, Chunwei Cao, Jiali Liu, Ying Zhao, Jianfei Pan, Cong Tao, Yanfang Wang
International Journal of Molecular Sciences.2023; 24(8): 7431. CrossRef - Distinct and shared endothermic strategies in the heat producing tissues of tuna and other teleosts
Baosheng Wu, Xueli Gao, Mingling Hu, Jing Hu, Tianming Lan, Tingfeng Xue, Wenjie Xu, Chenglong Zhu, Yuan Yuan, Jiangmin Zheng, Tao Qin, Peidong Xin, Ye Li, Li Gong, Chenguang Feng, Shunping He, Huan Liu, Haimeng Li, Qing Wang, Zhenhua Ma, Qiang Qiu, Kun W
Science China Life Sciences.2023; 66(11): 2629. CrossRef - Macrophage Involvement in Aging-Associated Skeletal Muscle Regeneration
Chang-Yi Cui, Luigi Ferrucci, Myriam Gorospe
Cells.2023; 12(9): 1214. CrossRef - Anti-nucleolin aptamer, iSN04, inhibits the inflammatory responses in C2C12 myoblasts by modulating the β-catenin/NF-κB signaling pathway
Machi Yamamoto, Mana Miyoshi, Kamino Morioka, Takakazu Mitani, Tomohide Takaya
Biochemical and Biophysical Research Communications.2023; 664: 1. CrossRef - Building Cetacean Locomotor Muscles throughout Ontogeny to Support High-Performance Swimming into Adulthood
S R Noren
Integrative And Comparative Biology.2023; 63(3): 785. CrossRef - Filbertone, (2E)-5-methyl-2-hepten-4-one, regulates thermogenesis and lipid metabolism in skeletal muscle of a high-fat diet fed mice
Hyemee Kim, Byungyong Ahn
Applied Biological Chemistry.2023;[Epub] CrossRef - Silencing of dopamine receptor D5 inhibits the browning of 3T3-L1 adipocytes and ATP-consuming futile cycles in C2C12 muscle cells
Kiros Haddish, Jong Won Yun
Archives of Physiology and Biochemistry.2023; : 1. CrossRef - Dynamic of irisin secretion change after moderate-intensity chronic physical exercise on obese female
Desiana Merawati, Sugiharto, Hendra Susanto, Ahmad Taufiq, Adi Pranoto, Dessy Amelia, Purwo Sri Rejeki
Journal of Basic and Clinical Physiology and Pharmacology.2023; 34(4): 539. CrossRef - Polyamines and Physical Activity in Musculoskeletal Diseases: A Potential Therapeutic Challenge
Letizia Galasso, Annalisa Cappella, Antonino Mulè, Lucia Castelli, Andrea Ciorciari, Alessandra Stacchiotti, Angela Montaruli
International Journal of Molecular Sciences.2023; 24(12): 9798. CrossRef - Phosphate toxicity and SERCA2a dysfunction in sudden cardiac arrest
Ronald B. Brown
The FASEB Journal.2023;[Epub] CrossRef - Hepatic protein kinase Cbeta deficiency mitigates late-onset obesity
Yaoling Shu, Nikhil Gumma, Faizule Hassan, Daniel A. Branch, Lisa A. Baer, Michael C. Ostrowski, Kristin I. Stanford, Kedryn K. Baskin, Kamal D. Mehta
Journal of Biological Chemistry.2023; 299(8): 104917. CrossRef - Functional expression of the thermally activated transient receptor potential channels TRPA1 and TRPM8 in human myotubes
Christine Skagen, Nils Gunnar Løvsletten, Lucia Asoawe, Zeineb Al-Karbawi, Arild C. Rustan, G. Hege Thoresen, Fred Haugen
Journal of Thermal Biology.2023; 116: 103623. CrossRef - Natural products as novel anti-obesity agents: insights into mechanisms of action and potential for therapeutic management
Ummul Fathima Shaik Mohamed Sayed, Said Moshawih, Hui Poh Goh, Nurolaini Kifli, Gaurav Gupta, Sachin Kumar Singh, Dinesh Kumar Chellappan, Kamal Dua, Andi Hermansyah, Hooi Leng Ser, Long Chiau Ming, Bey Hing Goh
Frontiers in Pharmacology.2023;[Epub] CrossRef - Recent advancements in pharmacological strategies to modulate energy balance for combating obesity
Benudhara Pati, Satyabrata Sendh, Bijayashree Sahu, Sunil Pani, Nivedita Jena, Naresh Chandra Bal
RSC Medicinal Chemistry.2023; 14(8): 1429. CrossRef - CCE and EODF as two distinct non-shivering thermogenesis models inducing weight loss
Tianyi Xu, Juan Wang, Hongwei Shi, Xiaofang Wei, Huiling Zhang, Yunyan Ji, Shiting Lu, Yi Yan, Xiuju Yu, Xiaomao Luo, Haidong Wang
Pflügers Archiv - European Journal of Physiology.2023; 475(8): 961. CrossRef - Birth and the Pathway to Adulthood: Integration across Development, Environment, and Evolution
Christopher J Mayerl, Terry R Dial, Mark C Mainwaring, Ashley M Heers, Rebecca Z German
Integrative And Comparative Biology.2023; 63(3): 548. CrossRef - Adipose Tissue and Metabolic Health
Sung-Min An, Seung-Hee Cho, John C. Yoon
Diabetes & Metabolism Journal.2023; 47(5): 595. CrossRef - The RANK-RANK-L-OPG pathway: trait d’union between bone and muscle
Giovanni Iolascon, Sara Liguori, Marco Paoletta, Federica Tomaino, Antimo Moretti
International Journal of Bone Fragility.2023; 3(2): 56. CrossRef - Warm Cells, Hot Mitochondria: Achievements and Problems of Ultralocal Thermometry
Alexey G. Kruglov, Alexey M. Romshin, Anna B. Nikiforova, Arina Plotnikova, Igor I. Vlasov
International Journal of Molecular Sciences.2023; 24(23): 16955. CrossRef - Plant extracts in prevention of obesity
Han-Ning Wang, Jin-Zhu Xiang, Zhi Qi, Min Du
Critical Reviews in Food Science and Nutrition.2022; 62(8): 2221. CrossRef - Transcription factor EB enhances autophagy and ameliorates palmitate‐induced insulin resistance at least partly via upregulating AMPK activity in skeletal muscle cells
Ping Wang, Chun Guang Li, Xian Zhou, Shuzhe Ding
Clinical and Experimental Pharmacology and Physiology.2022; 49(2): 302. CrossRef - ATP-consuming futile cycles as energy dissipating mechanisms to counteract obesity
Alexandra J. Brownstein, Michaela Veliova, Rebeca Acin-Perez, Marc Liesa, Orian S. Shirihai
Reviews in Endocrine and Metabolic Disorders.2022; 23(1): 121. CrossRef - Skeletal muscle plasticity and thermogenesis: Insights from sea otters
Traver Wright, Melinda Sheffield-Moore
Temperature.2022; 9(2): 119. CrossRef - Augmented CCL5/CCR5 signaling in brown adipose tissue inhibits adaptive thermogenesis and worsens insulin resistance in obesity
Pei-Chi Chan, Li-Man Hung, Jiung-Pang Huang, Yuan-Ji Day, Chao-Lan Yu, Feng-Chih Kuo, Chieh-Hua Lu, Yu-Feng Tian, Po-Shiuan Hsieh
Clinical Science.2022; 136(1): 121. CrossRef - Altered muscle mitochondrial, inflammatory and trophic markers, and reduced exercise training adaptations in type 1 diabetes
Dean Minnock, Giosuè Annibalini, Giacomo Valli, Roberta Saltarelli, Mauricio Krause, Elena Barbieri, Giuseppe De Vito
The Journal of Physiology.2022; 600(6): 1405. CrossRef - Maternal exercise intergenerationally drives muscle-based thermogenesis via activation of apelin-AMPK signaling
Jun Seok Son, Song Ah Chae, Liang Zhao, Hongyang Wang, Jeanene M. de Avila, Mei-Jun Zhu, Zhihua Jiang, Min Du
eBioMedicine.2022; 76: 103842. CrossRef -
Ca
2+
leak through ryanodine receptor 1 regulates thermogenesis in resting skeletal muscle
Aldo Meizoso-Huesca, Luke Pearce, Christopher J. Barclay, Bradley S. Launikonis
Proceedings of the National Academy of Sciences.2022;[Epub] CrossRef - Deep transcranial magnetic stimulation in combination with skin thermography in obesity: a window on sympathetic nervous system
Anna Ferrulli, Sara Gandini, Giulio Cammarata, Veronica Redaelli, Stefano Massarini, Concetta Macrì, Ileana Terruzzi, Daniele Cannavaro, Fabio Luzi, Livio Luzi
Acta Diabetologica.2022; 59(5): 729. CrossRef - Knockdown of sarcolipin (SLN) impairs substrate utilization in human skeletal muscle cells
Abel M. Mengeste, Parmeshwar Katare, Andrea Dalmao Fernandez, Jenny Lund, Hege G. Bakke, David Baker, Stefano Bartesaghi, Xiao-Rong Peng, Arild C. Rustan, G. Hege Thoresen, Eili Tranheim Kase
Molecular Biology Reports.2022; 49(7): 6005. CrossRef - The Role of Thyroid Hormones on Skeletal Muscle Thermogenesis
Nadia Sawicka-Gutaj, Abikasinee Erampamoorthy, Ariadna Zybek-Kocik, Angelos Kyriacou, Małgorzata Zgorzalewicz-Stachowiak, Agata Czarnywojtek, Marek Ruchała
Metabolites.2022; 12(4): 336. CrossRef - SS‐31 does not prevent or reduce muscle atrophy 7 days after a 65 kdyne contusion spinal cord injury in young male mice
Zachary A. Graham, Jennifer J. DeBerry, Christopher P. Cardozo, Marcas M. Bamman
Physiological Reports.2022;[Epub] CrossRef - Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c)
Tae Kwan Yoon, Chan Hee Lee, Obin Kwon, Min-Seon Kim
Diabetes & Metabolism Journal.2022; 46(3): 402. CrossRef - Insight Into the Metabolic Adaptations of Electrically Pulse-Stimulated Human Myotubes Using Global Analysis of the Transcriptome and Proteome
Abel M. Mengeste, Nataša Nikolić, Andrea Dalmao Fernandez, Yuan Z. Feng, Tuula A. Nyman, Sander Kersten, Fred Haugen, Eili Tranheim Kase, Vigdis Aas, Arild C. Rustan, G. Hege Thoresen
Frontiers in Physiology.2022;[Epub] CrossRef - The Role of Thermogenic Fat Tissue in Energy Consumption
Masato Horino, Kenji Ikeda, Tetsuya Yamada
Current Issues in Molecular Biology.2022; 44(7): 3166. CrossRef - Investigation of obesogenic effects of hexachlorobenzene, DDT and DDE in male rats
Zeyad Ayad Fadhil Al-Obaidi, Cihan Süleyman Erdogan, Engin Sümer, Hüseyin Bugra Özgün, Burcu Gemici, Süleyman Sandal, Bayram Yilmaz
General and Comparative Endocrinology.2022; 327: 114098. CrossRef - Avian adjustments to cold and non‐shivering thermogenesis: whats, wheres and hows
Punyadhara Pani, Naresh C. Bal
Biological Reviews.2022; 97(6): 2106. CrossRef - Thirty Obesity Myths, Misunderstandings, and/or Oversimplifications: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022
Harold Edward Bays, Angela Golden, Justin Tondt
Obesity Pillars.2022; 3: 100034. CrossRef - Myogenetic Oligodeoxynucleotide Restores Differentiation and Reverses Inflammation of Myoblasts Aggravated by Cancer-Conditioned Medium
Yuma Nihashi, Machi Yamamoto, Takeshi Shimosato, Tomohide Takaya
Muscles.2022; 1(2): 111. CrossRef - Altered skeletal muscle sarco-endoplasmic reticulum Ca2+-ATPase calcium transport efficiency after a thermogenic stimulus
Lydia A. Heemstra, Lauren G. Koch, Steven L. Britton, Colleen M. Novak
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2022; 323(5): R628. CrossRef - Animal Welfare Compromises Associated with Causes of Death in Neonatal Piglets
Kirsty L. Chidgey, Nutnapong Udomteerasuwat, Patrick C. H. Morel, Fernanda Castillo-Alcala
Animals.2022; 12(21): 2933. CrossRef - Brown to White Fat Transition Overlap With Skeletal Muscle During Development of Larger Mammals: Is it a Coincidence?
Sunil Pani, Suchanda Dey, Benudhara Pati, Unmod Senapati, Naresh C Bal
Journal of the Endocrine Society.2022;[Epub] CrossRef - Anti‐adiposity and lipid‐lowering effects of schisandrol A in diet‐induced obese mice
Sang Ryong Kim, Hyo Jin Park, Un Ju Jung
Journal of Food Biochemistry.2022;[Epub] CrossRef - Changes in spike protein antibody titer over 90 days after the second dose of SARS-CoV-2 vaccine in Japanese dialysis patients
Haruki Wakai, Natsumi Abe, Touno Tokuda, Rika Yamanaka, Satoshi Ebihara, Kensuke Izumaru, Daisuke Ishii, Toru Hyodo, Kazunari Yoshida
BMC Infectious Diseases.2022;[Epub] CrossRef - Organotypic cultures as aging associated disease models
Martina M. Sanchez, Isabella A. Bagdasarian, William Darch, Joshua T. Morgan
Aging.2022; 14(22): 9338. CrossRef - Divergent remodeling of the skeletal muscle metabolome over 24 h between young, healthy men and older, metabolically compromised men
Jan-Frieder Harmsen, Michel van Weeghel, Rex Parsons, Georges E. Janssens, Jakob Wefers, Dirk van Moorsel, Jan Hansen, Joris Hoeks, Matthijs K.C. Hesselink, Riekelt H. Houtkooper, Patrick Schrauwen
Cell Reports.2022; 41(11): 111786. CrossRef - Structural functionality of skeletal muscle mitochondria and its correlation with metabolic diseases
Gourabamani Swalsingh, Punyadhara Pani, Naresh C. Bal
Clinical Science.2022; 136(24): 1851. CrossRef - Trans-anethole Induces Thermogenesis via Activating SERCA/SLN Axis in C2C12 Muscle Cells
Sulagna Mukherjee, Minji Choi, Jong Won Yun
Biotechnology and Bioprocess Engineering.2022; 27(6): 938. CrossRef - Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
Kyung-Soo Kim, Yeon Kyung Choi, Mi Jin Kim, Jung Wook Hwang, Kyunghoon Min, Sang Youn Jung, Soo-Kyung Kim, Yong-Soo Choi, Yong-Wook Cho
Diabetes & Metabolism Journal.2021; 45(2): 260. CrossRef - Nutmeg extract potentially alters characteristics of white adipose tissue in rats
Ronny Lesmana, Melisa Siannoto, Gaga I. Nugraha, Hanna Goenawan, Astrid K. Feinisa, Yuni S. Pratiwi, Fifi Veronica, Vita M. Tarawan, Susianti Susianti, Unang Supratman
Veterinary Medicine and Science.2021; 7(2): 512. CrossRef - Contribution of thermogenic mechanisms by male and female mice lacking pituitary adenylate cyclase-activating polypeptide in response to cold acclimation
Ekaterina Filatov, Landon I. Short, Maeghan A. M. Forster, Simon S. Harris, Erik N. Schien, Malcolm C. Hughes, Daemon L. Cline, Colin J. Appleby, Sarah L. Gray
American Journal of Physiology-Endocrinology and Metabolism.2021; 320(3): E475. CrossRef - Skeletal muscle non-shivering thermogenesis as an attractive strategy to combat obesity
Hanbing Li, Can Wang, Linghuan Li, Lingqiao Li
Life Sciences.2021; 269: 119024. CrossRef - Restriction of an intron size en route to endothermy
Jana Královičová, Ivana Borovská, Reuben Pengelly, Eunice Lee, Pavel Abaffy, Radek Šindelka, Frank Grutzner, Igor Vořechovský
Nucleic Acids Research.2021; 49(5): 2460. CrossRef - Skeletal muscle specific mitochondrial dysfunction and altered energy metabolism in a murine model (oim/oim) of severe osteogenesis imperfecta
Victoria L. Gremminger, Emily N. Harrelson, Tara K. Crawford, Adrienne Ohler, Laura C. Schulz, R. Scott Rector, Charlotte L. Phillips
Molecular Genetics and Metabolism.2021; 132(4): 244. CrossRef - Body Protein Sparing in Hibernators: A Source for Biomedical Innovation
Fabrice Bertile, Caroline Habold, Yvon Le Maho, Sylvain Giroud
Frontiers in Physiology.2021;[Epub] CrossRef - The Genomes of Two Billfishes Provide Insights into the Evolution of Endothermy in Teleosts
Baosheng Wu, Chenguang Feng, Chenglong Zhu, Wenjie Xu, Yuan Yuan, Mingliang Hu, Ke Yuan, Yongxin Li, Yandong Ren, Yang Zhou, Haifeng Jiang, Qiang Qiu, Wen Wang, Shunping He, Kun Wang, Guang Yang
Molecular Biology and Evolution.2021; 38(6): 2413. CrossRef - Is Upregulation of Sarcolipin Beneficial or Detrimental to Muscle Function?
Naresh C. Bal, Subash C. Gupta, Meghna Pant, Danesh H. Sopariwala, Geoffrey Gonzalez-Escobedo, Joanne Turner, John S. Gunn, Christopher R. Pierson, Scott Q. Harper, Jill A. Rafael-Fortney, Muthu Periasamy
Frontiers in Physiology.2021;[Epub] CrossRef - Real‐Time Assessment of Mitochondrial Toxicity in HepG2 Cells Using the Seahorse Extracellular Flux Analyzer
Jether Amos Espinosa, Grace Pohan, Michelle R. Arkin, Sarine Markossian
Current Protocols.2021;[Epub] CrossRef - Effects of Ecklonia stolonifera extract on the obesity and skeletal muscle regeneration in high-fat diet-fed mice
Heegu Jin, Hyun-Ji Oh, Junghee Kim, Kang-Pyo Lee, Xionggao Han, Ok-Hwan Lee, Boo-Yong Lee
Journal of Functional Foods.2021; 82: 104511. CrossRef - The Effect of Dietary Intake and Nutritional Status on Anthropometric Development and Systemic Inflammation: An Observational Study
Roxana Maria Martin-Hadmaș, Ștefan Adrian Martin, Adela Romonți, Cristina Oana Mărginean
International Journal of Environmental Research and Public Health.2021; 18(11): 5635. CrossRef - Thermographic imaging of mouse across circadian time reveals body surface temperature elevation associated with non-locomotor body movements
Hiroyuki Shimatani, Yuichi Inoue, Yota Maekawa, Takahito Miyake, Yoshiaki Yamaguchi, Masao Doi, Nicolas Cermakian
PLOS ONE.2021; 16(5): e0252447. CrossRef - Human umbilical cord mesenchymal stem cells in type 2 diabetes mellitus: the emerging therapeutic approach
Andreia Gomes, Pedro Coelho, Raquel Soares, Raquel Costa
Cell and Tissue Research.2021; 385(3): 497. CrossRef - Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic
Yanis Zekri, Frédéric Flamant, Karine Gauthier
Cells.2021; 10(6): 1327. CrossRef - Myogenetic Oligodeoxynucleotide (myoDN) Recovers the Differentiation of Skeletal Muscle Myoblasts Deteriorated by Diabetes Mellitus
Shunichi Nakamura, Shinichi Yonekura, Takeshi Shimosato, Tomohide Takaya
Frontiers in Physiology.2021;[Epub] CrossRef - Lipophorin receptor 1 (LpR1) in Drosophila muscle influences life span by regulating mitochondrial aging
Ae-kyeong Kim, Dae-Woo Kwon, Eunbyul Yeom, Kwang-Pyo Lee, Ki-Sun Kwon, Kweon Yu, Kyu-Sun Lee
Biochemical and Biophysical Research Communications.2021; 568: 95. CrossRef - Skeletal muscle thermogenesis enables aquatic life in the smallest marine mammal
Traver Wright, Randall W. Davis, Heidi C. Pearson, Michael Murray, Melinda Sheffield-Moore
Science.2021; 373(6551): 223. CrossRef - Myoglobin, expressed in brown adipose tissue of mice, regulates the content and activity of mitochondria and lipid droplets
Mostafa A. Aboouf, Julia Armbruster, Markus Thiersch, Max Gassmann, Axel Gödecke, Erich Gnaiger, Glen Kristiansen, Anne Bicker, Thomas Hankeln, Hao Zhu, Thomas A. Gorr
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2021; 1866(12): 159026. CrossRef - Skeletal muscle energy metabolism in obesity
Abel M. Mengeste, Arild C. Rustan, Jenny Lund
Obesity.2021; 29(10): 1582. CrossRef - Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration
Mei Wan, Elise F. Gray-Gaillard, Jennifer H. Elisseeff
Bone Research.2021;[Epub] CrossRef - The small molecule SERCA activator CDN1163 increases energy metabolism in human skeletal muscle cells
Abel M. Mengeste, Jenny Lund, Parmeshwar Katare, Roya Ghobadi, Hege G. Bakke, Per Kristian Lunde, Lars Eide, Gavin O’ Mahony, Sven Göpel, Xiao-Rong Peng, Eili Tranheim Kase, G. Hege Thoresen, Arild C. Rustan
Current Research in Pharmacology and Drug Discovery.2021; 2: 100060. CrossRef - Antioxidant Properties and Cytoprotective Effect of Pistacia lentiscus L. Seed Oil against 7β-Hydroxycholesterol-Induced Toxicity in C2C12 Myoblasts: Reduction in Oxidative Stress, Mitochondrial and Peroxisomal Dysfunctions and Attenuation of Cell Death
Imen Ghzaiel, Amira Zarrouk, Thomas Nury, Michela Libergoli, Francesca Florio, Souha Hammouda, Franck Ménétrier, Laure Avoscan, Aline Yammine, Mohammad Samadi, Norbert Latruffe, Stefano Biressi, Débora Levy, Sérgio Paulo Bydlowski, Sonia Hammami, Anne Vej
Antioxidants.2021; 10(11): 1772. CrossRef - Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions
Faiz-ul Hassan, Asif Nadeem, Zhipeng Li, Maryam Javed, Qingyou Liu, Jahanzaib Azhar, Muhammad Saif-ur Rehman, Kuiqing Cui, Saif ur Rehman
International Journal of Molecular Sciences.2021; 22(22): 12463. CrossRef - Differential Effects of 25-Hydroxyvitamin D3 versus 1α 25-Dihydroxyvitamin D3 on Adipose Tissue Browning in CKD-Associated Cachexia
Robert H. Mak, Uwe Querfeld, Alex Gonzalez, Sujana Gunta, Wai W. Cheung
Cells.2021; 10(12): 3382. CrossRef - Effects of Starvation on Antioxidant-Related Signaling Molecules, Oxidative Stress, and Autophagy in Juvenile Chinese Perch Skeletal Muscle
Ping Wu, Aimin Wang, Jia Cheng, Lin Chen, Yaxiong Pan, Honghui Li, Qi Zhang, Jiaqi Zhang, Wuying Chu, Jianshe Zhang
Marine Biotechnology.2020; 22(1): 81. CrossRef - Thermogenic adipocytes: lineage, function and therapeutic potential
Alice E. Pollard, David Carling
Biochemical Journal.2020; 477(11): 2071. CrossRef - Hypoglycaemic effect of catalpol in a mouse model of high-fat diet-induced prediabetes
Dengqiu Xu, Xiaofei Huang, Hozeifa M. Hassan, Lu Wang, Sijia Li, Zhenzhou Jiang, Luyong Zhang, Tao Wang
Applied Physiology, Nutrition, and Metabolism.2020; 45(10): 1127. CrossRef - The evolution of mechanisms involved in vertebrate endothermy
Lucas J. Legendre, Donald Davesne
Philosophical Transactions of the Royal Society B: Biological Sciences.2020; 375(1793): 20190136. CrossRef - Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis
Naresh C. Bal, Muthu Periasamy
Philosophical Transactions of the Royal Society B: Biological Sciences.2020; 375(1793): 20190135. CrossRef - Prediction of muscle mass in arms and legs based on 3D laser-based photonic body scans’ standard dimensions in a homogenous sample of young men
Cristine Cavegn, Frank Rühli, Nicole Bender, Kaspar Staub
Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization.2020; 8(5): 562. CrossRef - Thermogenesis in Adipose Tissue Activated by Thyroid Hormone
Winifred W. Yau, Paul M. Yen
International Journal of Molecular Sciences.2020; 21(8): 3020. CrossRef - Interaction of a Sarcolipin Pentamer and Monomer with the Sarcoplasmic Reticulum Calcium Pump, SERCA
John Paul Glaves, Joseph O. Primeau, Przemek A. Gorski, L. Michel Espinoza-Fonseca, M. Joanne Lemieux, Howard S. Young
Biophysical Journal.2020; 118(2): 518. CrossRef - Automated CT-derived skeletal muscle mass determination in lower hind limbs of mice using a 3D U-Net deep learning network
Brent van der Heyden, Wouter R. P. H. van de Worp, Ardy van Helvoort, Jan Theys, Annemie M. W. J. Schols, Ramon C. J. Langen, Frank Verhaegen
Journal of Applied Physiology.2020; 128(1): 42. CrossRef - Modified creatinine index and risk for long-term infection-related mortality in hemodialysis patients: ten-year outcomes of the Q-Cohort Study
Hokuto Arase, Shunsuke Yamada, Hiroto Hiyamuta, Masatomo Taniguchi, Masanori Tokumoto, Kazuhiko Tsuruya, Toshiaki Nakano, Takanari Kitazono
Scientific Reports.2020;[Epub] CrossRef - Ticking for Metabolic Health: The Skeletal‐Muscle Clocks
Miguel A. Gutierrez‐Monreal, Jan‐Frieder Harmsen, Patrick Schrauwen, Karyn A. Esser
Obesity.2020;[Epub] CrossRef - Gut microbiota and regulation of myokine-adipokine function
Francesco Suriano, Matthias Van Hul, Patrice D Cani
Current Opinion in Pharmacology.2020; 52: 9. CrossRef - P2X7 Receptor in the Management of Energy Homeostasis: Implications for Obesity, Dyslipidemia, and Insulin Resistance
Roberto Coccurello, Cinzia Volonté
Frontiers in Endocrinology.2020;[Epub] CrossRef - Primary Active Ca2+ Transport Systems in Health and Disease
Jialin Chen, Aljona Sitsel, Veronick Benoy, M. Rosario Sepúlveda, Peter Vangheluwe
Cold Spring Harbor Perspectives in Biology.2020; 12(2): a035113. CrossRef - Sex‐specific alterations in whole body energetics and voluntary activity in heterozygous R163C malignant hyperthermia‐susceptible mice
Jennifer M. Rutkowsky, Trina A. Knotts, Paul D. Allen, Isaac N. Pessah, Jon J. Ramsey
The FASEB Journal.2020; 34(6): 8721. CrossRef - The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review
Adrian M. Gonzalez-Gil, Leticia Elizondo-Montemayor
Nutrients.2020; 12(6): 1899. CrossRef - Emergent Coordination of the CHKB and CPT1B Genes in Eutherian Mammals: Implications for the Origin of Brown Adipose Tissue
Bhavin V. Patel, Fanrong Yao, Aidan Howenstine, Risa Takenaka, Jacob A. Hyatt, Karen E. Sears, Brian M. Shewchuk
Journal of Molecular Biology.2020; 432(23): 6127. CrossRef - Chronic cold exposure induces mitochondrial plasticity in deer mice native to high altitudes
Sajeni Mahalingam, Zachary A. Cheviron, Jay F. Storz, Grant B. McClelland, Graham R. Scott
The Journal of Physiology.2020; 598(23): 5411. CrossRef - Adaptive thermogenesis enhances the life-threatening response to heat in mice with an Ryr1 mutation
Hui J. Wang, Chang Seok Lee, Rachel Sue Zhen Yee, Linda Groom, Inbar Friedman, Lyle Babcock, Dimitra K. Georgiou, Jin Hong, Amy D. Hanna, Joseph Recio, Jong Min Choi, Ting Chang, Nadia H. Agha, Jonathan Romero, Poonam Sarkar, Nicol Voermans, M. Waleed Gab
Nature Communications.2020;[Epub] CrossRef - Sclerostin Influences Exercise-Induced Adaptations in Body Composition and White Adipose Tissue Morphology in Male Mice
Nigel Kurgan, Joshua Stoikos, Bradley J. Baranowski, Jenalyn Yumol, Roopan Dhaliwal, Jake B. Sweezey-Munroe, Val A. Fajardo, William Gittings, Rebecca E.K. Macpherson, Panagiota Klentrou
Journal of Bone and Mineral Research.2020; 38(4): 541. CrossRef - Mitochondrial dysfunction and inhibition of myoblast differentiation in mice with high‐fat‐diet‐induced pre‐diabetes
Dengqiu Xu, Zhenzhou Jiang, Zeren Sun, Lu Wang, Guolin Zhao, Hozeifa M. Hassan, Sisi Fan, Wang Zhou, Shuangshuang Han, Luyong Zhang, Tao Wang
Journal of Cellular Physiology.2019; 234(5): 7510. CrossRef - Noninvasive and in vivo assessment of upper and lower limb skeletal muscle oxidative metabolism activity and microvascular responses to glucose ingestion in humans
Rogério Nogueira Soares, Alessandro L. Colosio, Juan Manuel Murias, Silvia Pogliaghi
Applied Physiology, Nutrition, and Metabolism.2019; 44(10): 1105. CrossRef - Fpr2 Deficiency Alleviates Diet-Induced Insulin Resistance Through Reducing Body Weight Gain and Inhibiting Inflammation Mediated by Macrophage Chemotaxis and M1 Polarization
Xiaofang Chen, Shu Zhuo, Tengfei Zhu, Pengle Yao, Mengmei Yang, Hong Mei, Na Li, Fengguang Ma, Ji Ming Wang, Shiting Chen, Richard D. Ye, Yu Li, Yingying Le
Diabetes.2019; 68(6): 1130. CrossRef - Taurine protects against arsenic trioxide-induced insulin resistance via ROS-Autophagy pathway in skeletal muscle
Lei Yang, Tianming Qiu, Xiaofeng Yao, Liping Jiang, Sen Wei, Pei Pei, Zhidong Wang, Jie Bai, Xiaofang Liu, Guang Yang, Shuang Liu, Xiance Sun
The International Journal of Biochemistry & Cell Biology.2019; 112: 50. CrossRef - Increased triacylglycerol - Fatty acid substrate cycling in human skeletal muscle cells exposed to eicosapentaenoic acid
Nils G. Løvsletten, Siril S. Bakke, Eili T. Kase, D. Margriet Ouwens, G. Hege Thoresen, Arild C. Rustan, Juan J. Loor
PLOS ONE.2018; 13(11): e0208048. CrossRef - Transient receptor potential (TRP) channels: a metabolic TR(i)P to obesity prevention and therapy
M. Bishnoi, P. Khare, L. Brown, S. K. Panchal
Obesity Reviews.2018; 19(9): 1269. CrossRef - Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis
Shreekrishna Lamichane, Babita Dahal Lamichane, Sang-Mo Kwon
International Journal of Molecular Sciences.2018; 19(4): 949. CrossRef - Antiobesity Effect ofAstilbe chinensisFranch. et Savet. Extract through Regulation of Adipogenesis and AMP-Activated Protein Kinase Pathways in 3T3-L1 Adipocyte and High-Fat Diet-Induced C57BL/6N Obese Mice
Xian Hua Zhang, Zhiqiang Wang, Bueom-Goo Kang, Seung Hwan Hwang, Jae-Young Lee, Soon Sung Lim, Bo Huang
Evidence-Based Complementary and Alternative Medicine.2018; 2018: 1. CrossRef - Loss of P2X7 receptor function dampens whole body energy expenditure and fatty acid oxidation
Giacomo Giacovazzo, Savina Apolloni, Roberto Coccurello
Purinergic Signalling.2018; 14(3): 299. CrossRef - Chromatin and Metabolism
Tamaki Suganuma, Jerry L. Workman
Annual Review of Biochemistry.2018; 87(1): 27. CrossRef - Zebrafish as a Model for Obesity and Diabetes
Liqing Zang, Lisette A. Maddison, Wenbiao Chen
Frontiers in Cell and Developmental Biology.2018;[Epub] CrossRef - Comparison between Dual-Energy X-ray Absorptiometry and Bioelectrical Impedance Analyses for Accuracy in Measuring Whole Body Muscle Mass and Appendicular Skeletal Muscle Mass
Seo Lee, Soyeon Ahn, Young Kim, Myoung Ji, Kyoung Kim, Sung Choi, Hak Jang, Soo Lim
Nutrients.2018; 10(6): 738. CrossRef
- Nutrition as the foundation for successful aging: a focus on dietary protein and omega-3 polyunsaturated fatty acids
- Obesity and Metabolic Syndrome
- Serum Calcium and the Risk of Incident Metabolic Syndrome: A 4.3-Year Retrospective Longitudinal Study
- Jong Ha Baek, Sang-Man Jin, Ji Cheol Bae, Jae Hwan Jee, Tae Yang Yu, Soo Kyoung Kim, Kyu Yeon Hur, Moon-Kyu Lee, Jae Hyeon Kim
- Diabetes Metab J. 2017;41(1):60-68. Published online December 26, 2016
- DOI: https://doi.org/10.4093/dmj.2017.41.1.60
- 4,086 View
- 32 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background An association between serum calcium level and risk of metabolic syndrome (MetS) has been suggested in cross-sectional studies. This study aimed to evaluate the association between baseline serum calcium level and risk of incident MetS in a longitudinal study.
Methods We conducted a retrospective longitudinal study of 12,706 participants without MetS who participated in a health screening program, had normal range serum calcium level at baseline (mean age, 51 years), and were followed up for 4.3 years (18,925 person-years). The risk of developing MetS was analyzed according to the baseline serum calcium levels.
Results A total of 3,448 incident cases (27.1%) of MetS developed during the follow-up period. The hazard ratio (HR) for incident MetS did not increase with increasing tertile of serum calcium level in an age- and sex-matched model (
P for trend=0.915). The HRs (95% confidence interval [CI]) for incident MetS comparing the second and the third tertiles to the first tertile of baseline serum calcium level were 0.91 (95% CI, 0.84 to 0.99) and 0.85 (95% CI, 0.78 to 0.92) in a fully adjusted model, respectively (P for trend=0.001). A decreased risk of incident MetS in higher tertiles of serum calcium level was observed in subjects with central obesity and/or a metabolically unhealthy state at baseline.Conclusion There was no positive correlation between baseline serum calcium levels and incident risk of MetS in this longitudinal study. There was an association between higher serum calcium levels and decreased incident MetS in individuals with central obesity or two components of MetS at baseline.
-
Citations
Citations to this article as recorded by- Independent associations of serum calcium with or without albumin adjustment and serum phosphorus with nonalcoholic fatty liver disease: results from NHANES 1999-2018
Haolong Qi, Bin Wang, Lei Zhu
Frontiers in Endocrinology.2024;[Epub] CrossRef - Association of the serum calcium level with metabolic syndrome and its components among adults in Taiwan
Jer-min Chen, Tai-yin Wu, Yi-fan Wu, Kuan-liang Kuo
Archives of Endocrinology and Metabolism.2023;[Epub] CrossRef - Elevated Chinese visceral adiposity index increases the risk of stroke in Chinese patients with metabolic syndrome
Zeyu Liu, Qin Huang, Bi Deng, Minping Wei, Xianjing Feng, Fang Yu, Jie Feng, Yang Du, Jian Xia
Frontiers in Endocrinology.2023;[Epub] CrossRef - Metformin: Expanding the Scope of Application—Starting Earlier than Yesterday, Canceling Later
Yulia A. Kononova, Nikolai P. Likhonosov, Alina Yu. Babenko
International Journal of Molecular Sciences.2022; 23(4): 2363. CrossRef - Metformin in prediabetes: key mechanisms for the prevention of diabetes and cardiometabolic risks
A. Yu. Babenko
Meditsinskiy sovet = Medical Council.2022; (10): 96. CrossRef Calcium and Phosphate Levels are Among Other Factors Associated with Metabolic Syndrome in Patients with Normal Weight
Kamila Osadnik, Tadeusz Osadnik, Marcin Delijewski, Mateusz Lejawa, Martyna Fronczek, Rafał Reguła, Mariusz Gąsior, Natalia Pawlas
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 1281. CrossRef- Association between selected trace elements and body mass index and waist circumference: A cross sectional study
Mahnaz Zohal, Saeedeh Jam-Ashkezari, Nasim Namiranian, Amin Moosavi, Akram Ghadiri-Anari
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(2): 1293. CrossRef - Letter: Increased Serum Angiopoietin-Like 6 Ahead of Metabolic Syndrome in a Prospective Cohort Study (Diabetes Metab J 2019;43:521-9)
Jin Hwa Kim
Diabetes & Metabolism Journal.2019; 43(5): 727. CrossRef - Genotype effects of glucokinase regulator on lipid profiles and glycemic status are modified by circulating calcium levels: results from the Korean Genome and Epidemiology Study
Oh Yoen Kim, So-Young Kwak, Hyunjung Lim, Min-Jeong Shin
Nutrition Research.2018; 60: 96. CrossRef
- Independent associations of serum calcium with or without albumin adjustment and serum phosphorus with nonalcoholic fatty liver disease: results from NHANES 1999-2018
- The Role of the Sweet Taste Receptor in Enteroendocrine Cells and Pancreatic β-Cells
- Itaru Kojima, Yuko Nakagawa
- Diabetes Metab J. 2011;35(5):451-457. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.451
- 4,353 View
- 86 Download
- 45 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The sweet taste receptor is expressed in taste cells located in taste buds of the tongue. This receptor senses sweet substances in the oral cavity, activates taste cells, and transmits the taste signals to adjacent neurons. The sweet taste receptor is a heterodimer of two G protein-coupled receptors, T1R2 and T1R3. Recent studies have shown that this receptor is also expressed in the extragustatory system, including the gastrointestinal tract, pancreatic β-cells, and glucose-responsive neurons in the brain. In the intestine, the sweet taste receptor regulates secretion of incretin hormones and glucose uptake from the lumen. In β-cells, activation of the sweet taste receptor leads to stimulation of insulin secretion. Collectively, the sweet taste receptor plays an important role in recognition and metabolism of energy sources in the body.
-
Citations
Citations to this article as recorded by- Targeting T2Rs, a feasible approach for natural bitter agents from traditional Chinese medicine modulate ABC transporters to treat respiratory diseases
Qi Liang, Ruo-Lan Li, Dan-Dan Tang, Ting Zhang, Lian Zhong, Chun-Jie Wu, Wei Peng
Arabian Journal of Chemistry.2024; 17(1): 105377. CrossRef - Impact of dietary sucralose and sucrose-sweetened water intake on lipid and glucose metabolism in male mice
Xinyi Wu, Le Cui, Haoquan Wang, Jinhong Xu, Zhaozhao Zhong, Xibei Jia, Jiaqi Wang, Huahua Zhang, Yanteng Shi, Yuhang Tang, Qianhui Yang, Qiongdan Liang, Yujing Zhang, Jing Li, Xiaohong Jiang
European Journal of Nutrition.2023; 62(1): 199. CrossRef - How dietary amino acids and high protein diets influence insulin secretion
Yuuki Yanagisawa
Physiological Reports.2023;[Epub] CrossRef - The elusive cephalic phase insulin response: triggers, mechanisms, and functions
Wolfgang Langhans, Alan G. Watts, Alan C. Spector
Physiological Reviews.2023; 103(2): 1423. CrossRef - Trace Amine-Associated Receptors and Monoamine-Mediated Regulation of Insulin Secretion in Pancreatic Islets
Anastasia N. Vaganova, Taisiia S. Shemyakova, Karina V. Lenskaia, Roman N. Rodionov, Charlotte Steenblock, Raul R. Gainetdinov
Biomolecules.2023; 13(11): 1618. CrossRef - Gene expression analyses of TAS1R taste receptors relevant to the treatment of cardiometabolic disease
Mariah R Stavrou, Sean Souchiart So, Angela M Finch, Sara Ballouz, Nicola J Smith
Chemical Senses.2023;[Epub] CrossRef - Dysgeusia
Davis C. Thomas, Deepti Chablani, Srishti Parekh, Reshmy Chellam Pichammal, Karpagavalli Shanmugasundaram, Priyanka Kodaganallur Pitchumani
The Journal of the American Dental Association.2022; 153(3): 251. CrossRef - Oral Microbiota-Host Interaction Mediated by Taste Receptors
Hao Dong, Jiaxin Liu, Jianhui Zhu, Zhiyan Zhou, Marco Tizzano, Xian Peng, Xuedong Zhou, Xin Xu, Xin Zheng
Frontiers in Cellular and Infection Microbiology.2022;[Epub] CrossRef - New frontiers in the hunger management involving GLP‐1, taste and oestrogen
Maja Baretić
Diabetic Medicine.2022;[Epub] CrossRef - The Impact of Artificial Sweeteners on Body Weight Control and Glucose Homeostasis
Michelle D. Pang, Gijs H. Goossens, Ellen E. Blaak
Frontiers in Nutrition.2021;[Epub] CrossRef - TAS1R2 sweet taste receptor genetic variation and dietary intake in Korean females
Jeong-Hwa Choi
Appetite.2021; 164: 105281. CrossRef - Sweet Taste Is Complex: Signaling Cascades and Circuits Involved in Sweet Sensation
Elena von Molitor, Katja Riedel, Michael Krohn, Mathias Hafner, Rüdiger Rudolf, Tiziana Cesetti
Frontiers in Human Neuroscience.2021;[Epub] CrossRef - Hazardous Hyperglisemic Effect of Facial Ischemia Following Subarachnoid Hemorrhage: An Experimental Study
Mehmet Dumlu Aydin, Ozgur Caglar, Mehmet Nuri Kocak, Erdem Karadeniz, Nazan Aydin, Irem Ates, Sevilay Ozmen
Archives of Neuroscience.2020;[Epub] CrossRef - Determinants of Sweetness Preference: A Scoping Review of Human Studies
Carolina Venditti, Kathy Musa-Veloso, Han Youl Lee, Theresa Poon, Alastair Mak, Maryse Darch, Justine Juana, Dylan Fronda, Daniel Noori, Erika Pateman, Maia Jack
Nutrients.2020; 12(3): 718. CrossRef - Allelic variation of the Tas1r3 taste receptor gene affects sweet taste responsiveness and metabolism of glucose in F1 mouse hybrids
Vladimir O. Murovets, Ekaterina A. Lukina, Egor A. Sozontov, Julia V. Andreeva, Raisa P. Khropycheva, Vasiliy A. Zolotarev, Keiko Abe
PLOS ONE.2020; 15(7): e0235913. CrossRef - Effect of sucralose and aspartame on glucose metabolism and gut hormones
Samar Y Ahmad, James K Friel, Dylan S Mackay
Nutrition Reviews.2020; 78(9): 725. CrossRef - Maternal low protein exposure alters glucose tolerance and intestinal nutrient-responsive receptors and transporters expression of rat offspring
Nan Wang, Bo Lv, Limin Guan, Hu Qiao, Bo Sun, Xiao Luo, Ru Jia, Ke Chen, Jianqun Yan
Life Sciences.2020; 243: 117216. CrossRef - Sugar Reduction in Dairy Food: An Overview with Flavoured Milk as an Example
Dipendra Kumar Mahato, Russell Keast, Djin Gie Liem, Catherine Georgina Russell, Sara Cicerale, Shirani Gamlath
Foods.2020; 9(10): 1400. CrossRef - Electrophysiology of the pancreatic islet β-cell sweet taste receptor TIR3
Juan V. Sanchez-Andres, Willy J. Malaisse, Itaru Kojima
Pflügers Archiv - European Journal of Physiology.2019; 471(4): 647. CrossRef - A Single 48 mg Sucralose Sip Unbalances Monocyte Subpopulations and Stimulates Insulin Secretion in Healthy Young Adults
Angélica Y. Gómez-Arauz, Nallely Bueno-Hernández, Leon F. Palomera, Raúl Alcántara-Suárez, Karen L. De León, Lucía A. Méndez-García, Miguel Carrero-Aguirre, Aaron N. Manjarrez-Reyna, Camilo P. Martínez-Reyes, Marcela Esquivel-Velázquez, Alejandra Ruiz-Bar
Journal of Immunology Research.2019; 2019: 1. CrossRef - Sugar reduction without compromising sensory perception. An impossible dream?
Scott C. Hutchings, Julia Y. Q. Low, Russell S. J. Keast
Critical Reviews in Food Science and Nutrition.2019; 59(14): 2287. CrossRef - Oral and Post‐Oral Actions of Low‐Calorie Sweeteners: A Tale of Contradictions and Controversies
John I. Glendinning
Obesity.2018;[Epub] CrossRef - Olfactory, Taste, and Photo Sensory Receptors in Non-sensory Organs: It Just Makes Sense
Nicholas M. Dalesio, Sebastian F. Barreto Ortiz, Jennifer L. Pluznick, Dan E. Berkowitz
Frontiers in Physiology.2018;[Epub] CrossRef - Emerging Concepts in Brain Glucose Metabolic Functions: From Glucose Sensing to How the Sweet Taste of Glucose Regulates Its Own Metabolism in Astrocytes and Neurons
Menizibeya O. Welcome, Nikos E. Mastorakis
NeuroMolecular Medicine.2018; 20(3): 281. CrossRef - Anti-diabetic effects of natural products an overview of therapeutic strategies
Jiyoung Park, Hyeung-Jin Jang
Molecular & Cellular Toxicology.2017; 13(1): 1. CrossRef - Natural sweetener agave inhibits gastric emptying in rats by a cholecystokinin-2- and glucagon like peptide-1 receptor-dependent mechanism
E. Bihter Gürler, Dilek Özbeyli, Hülya Buzcu, Sezin Bayraktar, İrem Carus, Beyza Dağ, Yasemin Geriş, Seda Jeral, Berrak Ç. Yeğen
Food & Function.2017; 8(2): 741. CrossRef - T1R3 homomeric sweet taste receptor regulates adipogenesis through Gαs-mediated microtubules disassembly and Rho activation in 3T3-L1 cells
Yosuke Masubuchi, Yuko Nakagawa, Johan Medina, Masahiro Nagasawa, Itaru Kojima, Mark M. Rasenick, Takeshi Inagaki, Hiroshi Shibata, Hiroaki Matsunami
PLOS ONE.2017; 12(5): e0176841. CrossRef - Hormonal responses to non-nutritive sweeteners in water and diet soda
Allison C. Sylvetsky, Rebecca J. Brown, Jenny E. Blau, Mary Walter, Kristina I. Rother
Nutrition & Metabolism.2016;[Epub] CrossRef - Gastrointestinal defense mechanisms
Hyder Said, Jonathan D. Kaunitz
Current Opinion in Gastroenterology.2016; 32(6): 461. CrossRef - Glucose-Sensing Receptor T1R3: A New Signaling Receptor Activated by Glucose in Pancreatic β-Cells
Itaru Kojima, Yuko Nakagawa, Kunihisa Hamano, Johan Medina, Longfei Li, Masahiro Nagasawa
Biological & Pharmaceutical Bulletin.2015; 38(5): 674. CrossRef - Impaired Glucose Metabolism in Mice Lacking the Tas1r3 Taste Receptor Gene
Vladimir O. Murovets, Alexander A. Bachmanov, Vasiliy A. Zolotarev, Hiroaki Matsunami
PLOS ONE.2015; 10(6): e0130997. CrossRef - Sweet Taste Receptor Signaling Network: Possible Implication for Cognitive Functioning
Menizibeya O. Welcome, Nikos E. Mastorakis, Vladimir A. Pereverzev
Neurology Research International.2015; 2015: 1. CrossRef - Expression of the glucose-sensing receptor T1R3 in pancreatic islet: changes in the expression levels in various nutritional and metabolic states
Anya Medina, Yuko Nakagawa, Jinhui Ma, Longfei Li, Kunihisa Hamano, Toshio Akimoto, Yuzo Ninomiya, Itaru Kojima
Endocrine Journal.2014; 61(8): 797. CrossRef - Normal Roles for Dietary Fructose in Carbohydrate Metabolism
Maren Laughlin
Nutrients.2014; 6(8): 3117. CrossRef - Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3
Yuko Nakagawa, Yoshiaki Ohtsu, Masahiro Nagasawa, Hiroshi Shibata, Itaru Kojima
Endocrine Journal.2014; 61(2): 119. CrossRef - Diverse signaling systems activated by the sweet taste receptor in human GLP-1-secreting cells
Yoshiaki Ohtsu, Yuko Nakagawa, Masahiro Nagasawa, Shigeki Takeda, Hirokazu Arakawa, Itaru Kojima
Molecular and Cellular Endocrinology.2014; 394(1-2): 70. CrossRef - Sweet Taste-Sensing Receptors Expressed in Pancreatic β-Cells: Sweet Molecules Act as Biased Agonists
Itaru Kojima, Yuko Nakagawa, Yoshiaki Ohtsu, Anya Medina, Masahiro Nagasawa
Endocrinology and Metabolism.2014; 29(1): 12. CrossRef - The Role of Sweet Taste in Satiation and Satiety
Yu Low, Kathleen Lacy, Russell Keast
Nutrients.2014; 6(9): 3431. CrossRef - Insulin release: the receptor hypothesis
Willy J. Malaisse
Diabetologia.2014; 57(7): 1287. CrossRef - The involvement of the T1R3 receptor protein in the control of glucose metabolism in mice at different levels of glycemia
V. O. Murovets, A. A. Bachmanov, S. V. Travnikov, A. A. Churikova, V. A. Zolotarev
Journal of Evolutionary Biochemistry and Physiology.2014; 50(4): 334. CrossRef - GKAs for diabetes therapy: why no clinically useful drug after two decades of trying?
Franz M. Matschinsky
Trends in Pharmacological Sciences.2013; 34(2): 90. CrossRef - A Novel Regulatory Function of Sweet Taste-Sensing Receptor in Adipogenic Differentiation of 3T3-L1 Cells
Yosuke Masubuchi, Yuko Nakagawa, Jinhui Ma, Tsutomu Sasaki, Tadahiro Kitamura, Yoritsuna Yamamoto, Hitoshi Kurose, Itaru Kojima, Hiroshi Shibata, Xing-Ming Shi
PLoS ONE.2013; 8(1): e54500. CrossRef - Goût des aliments et comportement alimentaire
M. Fantino
Médecine des Maladies Métaboliques.2012; 6(5): 409. CrossRef - The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice
John I. Glendinning, Jennifer Gillman, Haley Zamer, Robert F. Margolskee, Anthony Sclafani
Physiology & Behavior.2012; 107(1): 50. CrossRef - Repair of diverse diabetic defects of β‐cells in man and mouse by pharmacological glucokinase activation
Nicolai M. Doliba, Deborah Fenner, Bogumil Zelent, Joseph Bass, Ramakanth Sarabu, Franz M. Matschinsky
Diabetes, Obesity and Metabolism.2012; 14(s3): 109. CrossRef
- Targeting T2Rs, a feasible approach for natural bitter agents from traditional Chinese medicine modulate ABC transporters to treat respiratory diseases
- Effects of Vitamin D and Calcium Intervention on the Improvement of Resistance in Patients with Type 2 Diabetes Mellitus.
- Young Mee Choi, Jun Ho Lee, Ji Sook Han
- Korean Diabetes J. 2009;33(4):324-334. Published online August 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.4.324
- 2,523 View
- 46 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Recent reports suggest that the intake of vitamin D and calcium may influence insulin resistance. The aim of this study was to assess the effects of vitamin D and calcium intervention on the improvement of blood glucose and insulin resistance in patients with type 2 diabetes mellitus (DM). METHODS: Fasting blood glucose, glycosylated hemoglobin A1c (HbA1C), serum 25(OH)D3, serum lipid levels, insulin secretion, and activity and dietary surveys were analyzed in type 2 DM patients both before and after a 12-week vitamin D and calcium intake intervention. RESULTS: The serum 25(OH)D3 level was found to be negatively correlated with insulin resistance and fasting blood glucose. Calcium intake level was also negatively correlated with insulin resistance. Fasting blood glucose, HbA1C, and HOMA-IR decreased significantly (P <0.05) following vitamin D and calcium intake intervention in the medical nutrition therapy (MNT) group, while there was no such change observed in the control group. Dietary calcium and vitamin D intakes were significantly (P <0.05) higher in the MNT group than in the control group. The concentrations of serum 25(OH)D3 and insulin secretion increased slightly in the MNT group following the 12-week intervention; however, these results did not reach statistical significance. CONCLUSION: The results of the present study indicate that calcium and vitamin D intervention may be helpful in improving fasting blood glucose, HbA1C, serum 25(OH)D3 and HOMA-IR in patients with type 2 DM who have insufficient serum 25(OH)D3 concentrations. -
Citations
Citations to this article as recorded by- Comparison of Biological Markers and Lifestyle Factors on the Presence of Diabetes Mellitus in Middle-aged adults
Hye-Sun Keum, Soon-Rim Suh
Journal of the Korea Academia-Industrial cooperation Society.2016; 17(2): 104. CrossRef - A prospective randomized controlled trial of the effects of vitamin D supplementation on long-term glycemic control in type 2 diabetes mellitus of Korea
Ohk-Hyun Ryu, Sungwha Lee, Jaemyung Yu, Moon-Gi Choi, Hyung Joon Yoo, Franco Mantero
Endocrine Journal.2014; 61(2): 167. CrossRef - A Study of Snack Consumption, Night-Eating Habits, and Nutrient Intake in Gestational Diabetes Mellitus
Hee-jin Park, JinJu Lee, Ji-Myung Kim, Hyun Ah Lee, Sung-Hoon Kim, Yuri Kim
Clinical Nutrition Research.2013; 2(1): 42. CrossRef - Vitamin D and Diabetes
Dallae Ju
Journal of Korean Diabetes.2011; 12(2): 104. CrossRef - Nutrients and Dish Intake by Fasting Blood Glucose Level
Jihyun Choi, Hyun-Kyung Moon
The Korean Journal of Nutrition.2010; 43(5): 463. CrossRef - Vitamin D and Diabetes Mellitus
Jung Hyun Noh
Korean Diabetes Journal.2009; 33(4): 276. CrossRef
- Comparison of Biological Markers and Lifestyle Factors on the Presence of Diabetes Mellitus in Middle-aged adults
- Relationship Between Serum Bilirubin Levels and Coronary Atherosclerosis in Patients with Type 2 Diabetes.
- Jun Sung Moon, Woo Jin Chang, Chan Hee Lee, Ji Eun Lee, Kyung Ah Chun, Ji Sung Yoon, Ihn Ho Cho, Hyoung Woo Lee, Kyu Chang Won
- Korean Diabetes J. 2008;32(4):338-345. Published online August 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.4.338
- 2,523 View
- 18 Download
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Lipid oxidation and formation of oxygen radicals have been identified to be the important factors of atherogenesis. Because bilirubin, a potent physiological antioxidant inhibits lipid oxidation, it is suggested that low serum concentrations of bilirubin is associated with atherosclerosis. The aim of this study was to evaluate the relationship between bilirubin levels and coronary atherosclerosis. METHODS: The coronary calcium score (CCS) of 172 subjects (male 63, mean age 60.5 +/- 1.0) with type 2 diabetes were evaluated in Yeungnam University Hospital between January 2005 and February 2007. The subjects were divided into two groups with CCS 10 as the cut off. RESULTS: Higher CCS was significantly associated with lower bilirubin (P < 0.05), but after adjusted with age, no longer correlation were seen (P = 0.121). To determine the relationship between subclinical coronary atherosclerosis and bilirubin, the subjects with previous history of cardiovascular disease were excluded. In 138 subjects (male 54, mean age 58.4 +/- 1.1), higher CCS was significantly associated with lower levels of bilirubin. After adjusted with age, duration of diabetes, and history of hypertension, CCS was also inversely related with bilirubin (P < 0.05). CONCLUSION: These results suggest that lower levels of bilirubin might be considered as a risk factor of coronary artery disease, especially in type 2 diabetics without cardiovascular disease. -
Citations
Citations to this article as recorded by- Effects of Ginseng By-Products Supplementation on Performance,
Blood Biochemical Profiles, Organ Development, and Stress Parameter in
Broiler under Heat Stress Condition
Jun-Ho Lee, Ji-Won Yoon, Bong-Ki Kim, Hee-Bok Park, Kyu-Sang Lim, Ji-Hyuk Kim
Korean Journal of Poultry Science.2022; 49(4): 255. CrossRef - Correlation of Serum Bilirubin Levels in Type 2 Diabetes Mellitus Patients with and without Diabetic Retinopathy
Johncy John, Gajaraj Tulsidas Naik, Suria C. Rashmi, Sheetal Vaijanath Zille, Swetha Sampangi Iyer, Meghana Neeralagi, Asma M.K
Journal of Evolution of Medical and Dental Sciences.2021; 10(45): 4013. CrossRef - Association of SNPs in the UGT1A gene cluster with total bilirubin and mortality in the Diabetes Heart Study
Amanda J. Cox, Maggie C.-Y. Ng, Jianzhao Xu, Carl D. Langefeld, Kenneth L. Koch, Paul A. Dawson, J. Jeffrey Carr, Barry I. Freedman, Fang-Chi Hsu, Donald W. Bowden
Atherosclerosis.2013; 229(1): 155. CrossRef - The Association between Low Serum Bilirubin and Carotid Atherosclerosis in Subjects with Type 2 Diabetes
Byoung Hyun Park, Hye Jung Nho, Chung Gu Cho
Endocrinology and Metabolism.2012; 27(2): 126. CrossRef - Association of Serum Total Bilirubin with Serum High Sensitivity C-reactive Protein in Middle-aged Men
Kiwoong Yu, Cheolhwan Kim, Eunju Sung, Hocheol Shin, Hyewon Lee
Korean Journal of Family Medicine.2011; 32(6): 327. CrossRef - The Relationship among Homocysteine, Bilirubin, and Diabetic Retinopathy
Ho Chan Cho
Diabetes & Metabolism Journal.2011; 35(6): 595. CrossRef - Relationship Between Serum Bilirubin Levels and Coronary Atherosclerosis in Patients with Type 2 Diabetes (Korean Diabetes Journal 32(4):338-345, 2008)
Soo Lim
Korean Diabetes Journal.2008; 32(5): 462. CrossRef
- Effects of Ginseng By-Products Supplementation on Performance,
Blood Biochemical Profiles, Organ Development, and Stress Parameter in
Broiler under Heat Stress Condition
- Proliferation and Differentiation of Pancreatic beta Cells in L-type Calcium Channel alpha(1D) Subunit (Ca(v)1.3) Heterozygous Knock Out Mice After Partial Pancreatectomy.
- Yoon Hee Choi, Il Hee Yun, Sun Hee Suh, Dong Jun Lim, Jae Hyuung Cho, Hyuk Sang Kwon, Bong Yun Cha, Ho Young Son, Chung Gyu Park, Kun Ho Yoon
- Korean Diabetes J. 2007;31(3):208-219. Published online May 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.3.208
- 2,014 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
S: L-type voltage-dependent calcium channel (LTCC) plays a crucial role in insulin secretion from pancreatic beta cells through Ca2+ influx. In the recent report, LTCC Ca(v)1.3 subtype homozygous knock out mice showed impairment of postnatal pancreatic beta cell development as well as insulin secretion. METHODS: We performed 90% partial pancreatectomy in heterozygous Ca(v)1.3 knock out mice to investigate the effect of partial deficiency of Ca(v)1.3 gene on beta cell regeneration in the adult. Glucose homeostasis, metabolic profiles including serum insulin and lipid levels and morphologic changes of pancreatic islets were studied. RESULTS: 90% Partial pancreatectomy induced glucose intolerance only in the heterozygous knock out mice at 8 weeks after surgery. Distribution of islet size was significantly different between two groups after partial pancreatectomy; median value of islet size of heterozygote was larger than that of wild type (642.8 micrometer2 vs 1459.8 micrometer2, P < 0.01). The frequency of single beta cell unit, considered as a unit of beta cell neogenesis, was much lower in heterozygote than that of wild type (41% vs 23.3%, P < 0.05). CONCLUSION: These data suggest that Ca(v)1.3 gene deficiency is specifically associated with impairment of beta cell regeneration, especially neogensis and eventual glucose intolerance in the 90% partial pancreatectomized mice.
- Value of Coronary Calcium Score in Type 2 Diabetics.
- Ji Eun Lee, Mi Jung Eun, Kyung Ah Chun, Jae Hong Kim, Ji Sung Yoon, Ihn Ho Cho, Kyu Chang Won, Hyoung Woo Lee
- Korean Diabetes J. 2006;30(4):303-311. Published online July 1, 2006
- DOI: https://doi.org/10.4093/jkda.2006.30.4.303
- 2,335 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Cardiovascular disease including coronary heart disease (CHD) is the most common cause of morbidity and mortality in patients with diabetes. But traditional risk factor assessment is limited to predict CHD in asymptomatic high-risk individuals. In this study, relationship between coronary calcium score (CCS) and CHD was evaluated to determine value of coronary artery calcification detected by multi-slice spiral computed tomography to predict CHD in high risk asymptomatic patients with type 2 diabetes. METHODS: 127 patients were enrolled who admitted in Yeungnam University Hospital between December 2004 and May 2005. Standard cardiovascular risk factors and the CCS measured by multi-slice spiral computed tomography were assessed. RESULTS: Enrolled subjects were consisted of 56 subjects with diabetes and 71 subjects without diabetes. The mean CCS was significantly greater in patients with diabetes than without diabetics (P < 0.01). In both groups, patients with higher CCS had higher prevalence of CHD (P < 0.05). In all subjects, LDL cholesterol levels and CCS were significantly associated in multi-variate analysis (P < 0.05). In patients without diabetes, age was only associated with presence of CHD (P < 0.05). CCS was only associated with CHD in patients with diabetes, even after adjusting for the effects of age, LDL cholesterol and CRP (P < 0.05). CONCLUSION: Therefore, multi-slice spiral computed tomography can non-invasively and accurately detect coronary calcification. By detection of coronary artery calcification, it may be possible to predict coronary heart disease early in high-risk asymptomatic patients with type 2 diabetes.
- Association of High Intracellular Calcium Levels with Insulin Resistance in Women with Polycystic Ovary Syndrome.
- Jee Young Oh, Hye Jin Lee, Young Sun Hong, Hye Won Chung, Yeon Ah Sung
- Korean Diabetes J. 2004;28(2):101-110. Published online April 1, 2004
- 1,203 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Insulin resistance is an intrinsic defect of polycystic ovary syndrome (PCOS), and elevated levels of cytosolic free calcium in insulin target cells may cause insulin resistance. To our knowledge, the relationship between intracellular calcium and insulin resistance in PCOS has not been investigated. The purpose of this study was to determine whether the levels of intracelluar calcium are changed and if they have any association with insulin resistance in women with PCOS. METHODS: The intracellular calcium levels in the platelets and the insulin sensitivity were measured by fluorescent spectrophotometry and the euglycemic hyperinsulinemic clamp technique, respectively, in 16 women with PCOS and 6 normal cycling women. A 2h, 75 g oral glucose tolerance test was performed to determine the glucose tolerance. RESULTS: The insulin sensitivity measured by the glucose disposal rate(the M-value), was significantly lower in women with PCOS(4.6+/-1.5mg/kg/min vs. 7.0+/-1.3mg/kg/min, p<0.01), but the intracellular calcium levels were significantly higher in women with PCOS compared to the controls(122.7+/-36.7 vs 59.1+/-29.3mmol/L, p<0.01). When the women with PCOS were divided into the overweight or obese(n=9, BMI ?23kg/m2) and lean(n=7, BMI<23kg/m2) groups, both groups had significantly lower M values compared to the control subjects(3.9+/-1.3, 5.5+/-1.2 vs. 7.0+/-1.3mumg/kg/min, p<0.001), and these levels between the overweight/obese and lean PCOS groups showed a significant difference(p<0.001). The overweight/ obese and lean women with PCOS had significantly higher levels of intracellular calcium compared to the control subjects(131.3+/-39.6, 111.7+/-31.8 vs. 59.1+/-29.3nmol/L, p<0.01), but these levels did not differ significantly between the overweight/obese and lean women with PCOS. The intracellular calcium levels showed a significant positive correlation with age, and a negative correlation with the M value(r=-0.55, p<0.05). The BMI-adjusted partial correlation showed marginal significance between elevated levels of intracellular calcium and insulin sensitivity (r=-0.47, p=0.07). CONCLUSION: Women with PCOS showed both insulin resistance and increased levels of intracellular calcium compared to the control subjects. Increased levels of intracellular calcium were associated with insulin resistance in women with PCOS.
- Effect of Glucose Concentrations on the Cell Proliferation and Expression of L-type Calcium Channel mRNA in Cultured Rat Aortic Vascular Smooth Muscle Cells.
- Young Jung Cho, Hyung Joon Yoo, Hong Woo Nam, Ji Young Suh, In Kyung Jeong, Sung Hee Ihm, Hyeon Kyu Kim, Cheol Young Park, Jae Myung Yoo, Doo Man Kim, Moon Gi Choi, Sung Woo Park
- Korean Diabetes J. 2003;27(3):253-259. Published online June 1, 2003
- 1,177 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Vascular smooth muscle cell (VSMC) proliferation is one of the major pathogenic mechanisms for atherosclerosis. It is known that L-type calcium channels play a role in VSMC proliferation in diabetic rats. However, there have been no studies that show an association between the L-type calcium channels and the VSMC proliferation due to various glucose concentrations in the culture media. Therefore, the association between the voltage-dependent L-type calcium channels of the VSMCs, and the growth of vascular smooth muscle cells, was examined. METHODS: Rat aortic VSMCs were isolated from the aorta of Sprague-Dawley and OLETF rats, using an enzymic method. The VSMCs were cultured in various concentrations of glucose (5.5, 11.0, 16.6, 25, 30 and 40 mM). The VSMCs (1x10(4) cells in 24-well plates) were incubated in the presence of Bay K 8644 (10(-6)M), both with and without verapamil (10(-6)M), for 48 hours. The proliferation was then assessed by the MTT (methylthiazole tetrazolium) assay, and the expression of L-type calcium channel mRNA by RT-PCR. RESULTS: The vascular smooth muscle cell proliferation was significantly increased, in a dose-dependent manner, with glucose concentrations below 25 mM in both in a dose-dependent manner, with glucose concentrations below 25 mM in both kinds of rat. However, the increase in the VSMC proliferation of the OLETF rat was significantly higher than in the Sprague-Dawley rat. After the Bay K 8644 treatment, with the same glucose concentration, the VSMC proliferation and the expression of L-type calcium channel mRNA were significantly increased in both kinds of rat. After treatment with verapamil, the increased VSMC proliferation and expression of L-type calcium channel mRNA, due to the Bay K 8644, were suppressed to control levels in both kinds of rat. CONCLUSION: The results suggest that below certain concentrations of glucose, 25 mM, the L-type calcium channels may play a role in the VSMC proliferation of OLETF and Sprague-Dawley rats. The growth of the VSMCs in OLETF rats, due to various glucose concentrations (< 25 mM), was significantly higher than in the Sprague-Dawley rats.

 KDA
KDA
 First
First Prev
Prev





