
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Diabetes Promotes Myocardial Fibrosis via AMPK/EZH2/PPAR-γ Signaling Pathway
- Shan-Shan Li, Lu Pan, Zhen-Ye Zhang, Meng-Dan Zhou, Xu-Fei Chen, Ling-Ling Qian, Min Dai, Juan Lu, Zhi-Ming Yu, Shipeng Dang, Ru-Xing Wang
- Received February 3, 2023 Accepted November 13, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0031 [Epub ahead of print]

- 887 View
- 54 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Diabetes-induced cardiac fibrosis is one of the main mechanisms of diabetic cardiomyopathy. As a common histone methyltransferase, enhancer of zeste homolog 2 (EZH2) has been implicated in fibrosis progression in multiple organs. However, the mechanism of EZH2 in diabetic myocardial fibrosis has not been clarified.
Methods
In the current study, rat and mouse diabetic model were established, the left ventricular function of rat and mouse were evaluated by echocardiography and the fibrosis of rat ventricle was evaluated by Masson staining. Primary rat ventricular fibroblasts were cultured and stimulated with high glucose (HG) in vitro. The expression of histone H3 lysine 27 (H3K27) trimethylation, EZH2, and myocardial fibrosis proteins were assayed.
Results
In STZ-induced diabetic ventricular tissues and HG-induced primary ventricular fibroblasts in vitro, H3K27 trimethylation was increased and the phosphorylation of EZH2 was reduced. Inhibition of EZH2 with GSK126 suppressed the activation, differentiation, and migration of cardiac fibroblasts as well as the overexpression of the fibrotic proteins induced by HG. Mechanical study demonstrated that HG reduced phosphorylation of EZH2 on Thr311 by inactivating AMP-activated protein kinase (AMPK), which transcriptionally inhibited peroxisome proliferator-activated receptor γ (PPAR-γ) expression to promote the fibroblasts activation and differentiation.
Conclusion
Our data revealed an AMPK/EZH2/PPAR-γ signal pathway is involved in HG-induced cardiac fibrosis.
- Pathophysiology
- Rho-Kinase as a Therapeutic Target for Nonalcoholic Fatty Liver Diseases
- Inês Sousa-Lima, Hyun Jeong Kim, John Jones, Young-Bum Kim
- Diabetes Metab J. 2021;45(5):655-674. Published online September 30, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0197
- 5,793 View
- 171 Download
- 7 Web of Science
- 7 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
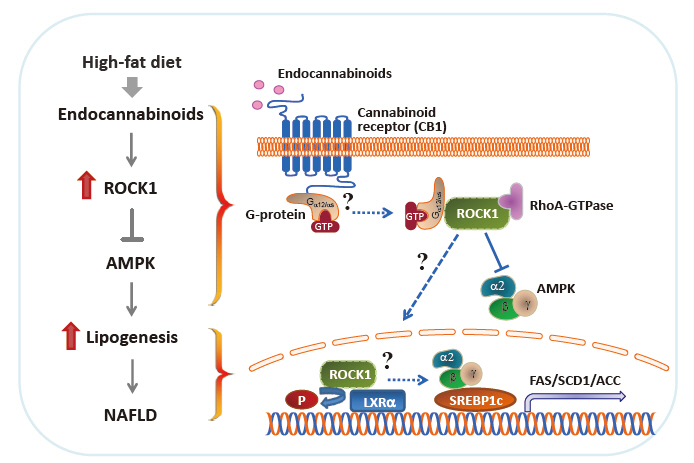
- Nonalcoholic fatty liver disease (NAFLD) is a major public health problem and the most common form of chronic liver disease, affecting 25% of the global population. Although NAFLD is closely linked with obesity, insulin resistance, and type 2 diabetes mellitus, knowledge on its pathogenesis remains incomplete. Emerging data have underscored the importance of Rho-kinase (Rho-associated coiled-coil-containing kinase [ROCK]) action in the maintenance of normal hepatic lipid homeostasis. In particular, pharmacological blockade of ROCK in hepatocytes or hepatic stellate cells prevents the progression of liver diseases such as NAFLD and fibrosis. Moreover, mice lacking hepatic ROCK1 are protected against obesity-induced fatty liver diseases by suppressing hepatic de novo lipogenesis. Here we review the roles of ROCK as an indispensable regulator of obesity-induced fatty liver disease and highlight the key cellular pathway governing hepatic lipid accumulation, with focus on de novo lipogenesis and its impact on therapeutic potential. Consequently, a comprehensive understanding of the metabolic milieu linking to liver dysfunction triggered by ROCK activation may help identify new targets for treating fatty liver diseases such as NAFLD.
-
Citations
Citations to this article as recorded by- THE ROLE OF N6-METHYLADENOSINE METHYLTRANSFERASE RBM15 IN NONALCOHOLIC FATTY LIVER DISEASE
Shiqing Li, Shengyi Lian, Wei Cheng, Tao Zhang, Xiaobing Gong
Shock.2024; 61(2): 311. CrossRef - Exploring the potential of drug repurposing for liver diseases: A comprehensive study
Fares E.M. Ali, Mustafa Ahmed Abdel-Reheim, Emad H.M. Hassanein, Mostafa K. Abd El-Aziz, Hanan S. Althagafy, Khalid S.A. Badran
Life Sciences.2024; : 122642. CrossRef - Targeting of G-protein coupled receptor 40 alleviates airway hyperresponsiveness through RhoA/ROCK1 signaling pathway in obese asthmatic mice
Xixi Lin, Like Wang, Xiaojie Lu, Yuanyuan Zhang, Rongying Zheng, Ruijie Chen, Weixi Zhang
Respiratory Research.2023;[Epub] CrossRef - Selectivity matters: selective ROCK2 inhibitor ameliorates established liver fibrosis via targeting inflammation, fibrosis, and metabolism
Alexandra Zanin-Zhorov, Wei Chen, Julien Moretti, Melanie S. Nyuydzefe, Iris Zhorov, Rashmi Munshi, Malavika Ghosh, Cindy Serdjebi, Kelli MacDonald, Bruce R. Blazar, Melissa Palmer, Samuel D. Waksal
Communications Biology.2023;[Epub] CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Paeoniflorin alleviates liver injury in hypercholesterolemic rats through the ROCK/AMPK pathway
Tong Liu, Ning Zhang, Lingya Kong, Sijie Chu, Ting Zhang, Guangdi Yan, Donglai Ma, Jun Dai, Zhihong Ma
Frontiers in Pharmacology.2022;[Epub] CrossRef - Fasudil Increased the Sensitivity to Gefitinib in NSCLC by Decreasing Intracellular Lipid Accumulation
Tingting Liao, Jingjing Deng, Wenjuan Chen, Juanjuan Xu, Guanghai Yang, Mei Zhou, Zhilei Lv, Sufei Wang, Siwei Song, Xueyun Tan, Zhengrong Yin, Yumei Li, Yang Jin
Cancers.2022; 14(19): 4709. CrossRef
- THE ROLE OF N6-METHYLADENOSINE METHYLTRANSFERASE RBM15 IN NONALCOHOLIC FATTY LIVER DISEASE
- Complications
- Therapeutic Effects of Fibroblast Growth Factor-21 on Diabetic Nephropathy and the Possible Mechanism in Type 1 Diabetes Mellitus Mice
- Wenya Weng, Tingwen Ge, Yi Wang, Lulu He, Tinghao Liu, Wanning Wang, Zongyu Zheng, Lechu Yu, Chi Zhang, Xuemian Lu
- Diabetes Metab J. 2020;44(4):566-580. Published online May 15, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0089
- 5,919 View
- 102 Download
- 12 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
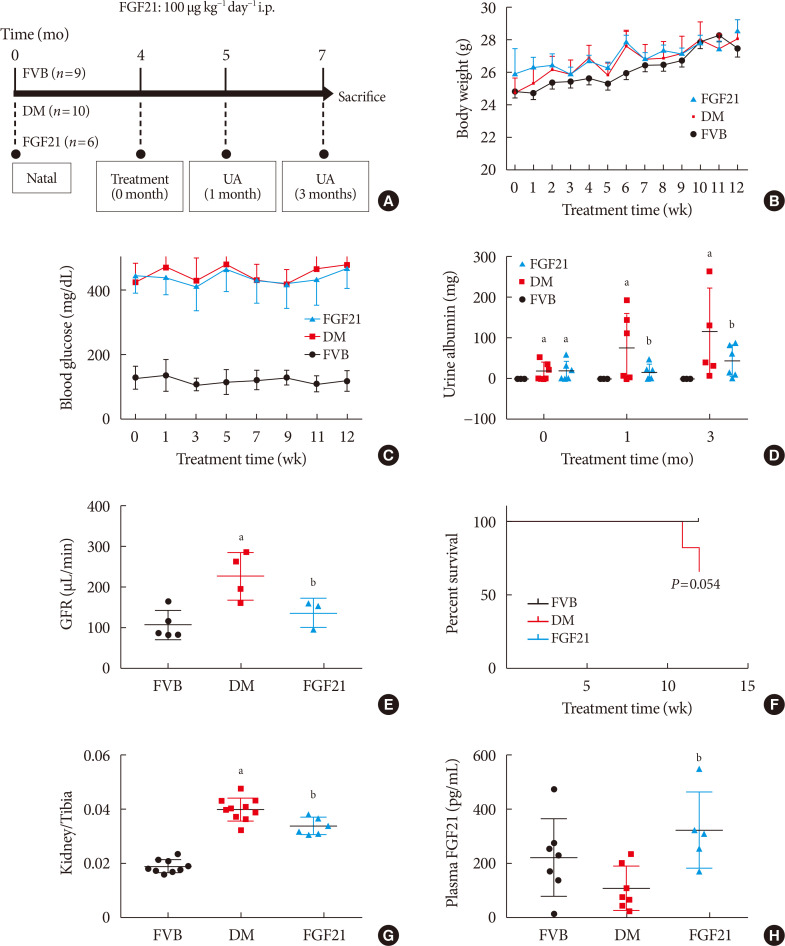
ePub Background Fibroblast growth factor 21 (FGF21) has been only reported to prevent type 1 diabetic nephropathy (DN) in the streptozotocin-induced type 1 diabetes mellitus (T1DM) mouse model. However, the FVB (Cg)-Tg (Cryaa-Tag, Ins2-CALM1) 26OVE/PneJ (OVE26) transgenic mouse is a widely recommended mouse model to recapture the most important features of T1DM nephropathy that often occurs in diabetic patients. In addition, most previous studies focused on exploring the preventive effect of FGF21 on the development of DN. However, in clinic, development of therapeutic strategy has much more realistic value compared with preventive strategy since the onset time of DN is difficult to be accurately predicted. Therefore, in the present study OVE26 mice were used to investigate the potential therapeutic effects of FGF21 on DN.
Methods Four-month-old female OVE26 mice were intraperitoneally treated with recombinant FGF21 at a dose of 100 µg/kg/day for 3 months. The diabetic and non-diabetic control mice were treated with phosphate-buffered saline at the same volume. Renal functions, pathological changes, inflammation, apoptosis, oxidative stress and fibrosis were examined in mice of all groups.
Results The results showed that severe renal dysfunction, morphological changes, inflammation, apoptosis, and fibrosis were observed in OVE26 mice. However, all the renal abnormalities above in OVE26 mice were significantly attenuated by 3-month FGF21 treatment associated with improvement of renal adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) activity and sirtuin 1 (SIRT1) expression.
Conclusion Therefore, this study demonstrated that FGF21 might exert therapeutic effects on DN through AMPK-SIRT1 pathway.
-
Citations
Citations to this article as recorded by- Fibroblast growth factor 21 alleviates unilateral ureteral obstruction-induced renal fibrosis by inhibiting Wnt/β-catenin signaling pathway
Wenhui Zhong, Yuheng Jiang, Huizhen Wang, Xiang Luo, Tao Zeng, Huimi Huang, Ling Xiao, Nan Jia, Aiqing Li
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research.2024; 1871(2): 119620. CrossRef - Urinary Excretion of Biomolecules Related to Cell Cycle, Proliferation, and Autophagy in Subjects with Type 2 Diabetes and Chronic Kidney Disease
Anton I. Korbut, Vyacheslav V. Romanov, Vadim V. Klimontov
Biomedicines.2024; 12(3): 487. CrossRef - New developments in the biology of fibroblast growth factors
David M. Ornitz, Nobuyuki Itoh
WIREs Mechanisms of Disease.2022;[Epub] CrossRef - SIRT1–SIRT7 in Diabetic Kidney Disease: Biological Functions and Molecular Mechanisms
Wenxiu Qi, Cheng Hu, Daqing Zhao, Xiangyan Li
Frontiers in Endocrinology.2022;[Epub] CrossRef - Research Progress of Fibroblast Growth Factor 21 in Fibrotic Diseases
Min-Qi Jia, Cha-Xiang Guan, Jia-Hao Tao, Yong Zhou, Liang-Jun Yan
Oxidative Medicine and Cellular Longevity.2022; 2022: 1. CrossRef - Metabolic-associated fatty liver disease increases the risk of end-stage renal disease in patients with biopsy-confirmed diabetic nephropathy: a propensity-matched cohort study
Yutong Zou, Lijun Zhao, Junlin Zhang, Yiting Wang, Yucheng Wu, Honghong Ren, Tingli Wang, Yuancheng Zhao, Huan Xu, Lin Li, Nanwei Tong, Fang Liu
Acta Diabetologica.2022; 60(2): 225. CrossRef - FGF21 and Chronic Kidney Disease
João Victor Salgado, Miguel Angelo Goes, Natalino Salgado Filho
Metabolism.2021; 118: 154738. CrossRef - The Multiple Roles of Fibroblast Growth Factor in Diabetic Nephropathy
Junyu Deng, Ye Liu, Yiqiu Liu, Wei Li, Xuqiang Nie
Journal of Inflammation Research.2021; Volume 14: 5273. CrossRef - Therapeutic effect and mechanism of combined use of FGF21 and insulin on diabetic nephropathy
Fanrui Meng, Yukai Cao, Mir Hassan Khoso, Kai Kang, Guiping Ren, Wei Xiao, Deshan Li
Archives of Biochemistry and Biophysics.2021; 713: 109063. CrossRef - FGF19 and FGF21 for the Treatment of NASH—Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human
Emma Henriksson, Birgitte Andersen
Frontiers in Endocrinology.2020;[Epub] CrossRef - FGF21: An Emerging Therapeutic Target for Non-Alcoholic Steatohepatitis and Related Metabolic Diseases
Erik J. Tillman, Tim Rolph
Frontiers in Endocrinology.2020;[Epub] CrossRef
- Fibroblast growth factor 21 alleviates unilateral ureteral obstruction-induced renal fibrosis by inhibiting Wnt/β-catenin signaling pathway
- Pathophysiology
- Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Hong Il Kim, Ji Seon Lee, Byung Kook Kwak, Won Min Hwang, Min Joo Kim, Young-Bum Kim, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2019;43(6):854-866. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0179
- 6,660 View
- 115 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
Methods NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
Results We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
Conclusion This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
-
Citations
Citations to this article as recorded by- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
Si-min Zhou, Xin-ming Yao, Yi Cheng, Yu-jie Xing, Yue Sun, Qiang Hua, Shu-jun Wan, Xiang-jian Meng
Heliyon.2024; 10(2): e24432. CrossRef - Reduced Expression Level of Protein PhosphatasePPM1EServes to Maintain Insulin Secretion in Type 2 Diabetes
Sevda Gheibi, Luis Rodrigo Cataldo, Alexander Hamilton, Mi Huang, Sebastian Kalamajski, Malin Fex, Hindrik Mulder
Diabetes.2023; 72(4): 455. CrossRef - Metformin restores prohormone processing enzymes and normalizes aberrations in secretion of proinsulin and insulin in palmitate‐exposed human islets
Quan Wen, Azazul Islam Chowdhury, Banu Aydin, Mudhir Shekha, Rasmus Stenlid, Anders Forslund, Peter Bergsten
Diabetes, Obesity and Metabolism.2023; 25(12): 3757. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Metformin disrupts insulin secretion, causes proapoptotic and oxidative effects in rat pancreatic beta‐cells in vitro
Maíra M.R. Valle, Eloisa Aparecida Vilas‐Boas, Camila F. Lucena, Simone A. Teixeira, Marcelo N. Muscara, Angelo R. Carpinelli
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Protection by metformin against severe Covid-19: An in-depth mechanistic analysis
Nicolas Wiernsperger, Abdallah Al-Salameh, Bertrand Cariou, Jean-Daniel Lalau
Diabetes & Metabolism.2022; 48(4): 101359. CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Overexpression of miR-297b-5p Promotes Metformin-Mediated Protection Against Stearic Acid-Induced Senescence by Targeting Igf1r
Qingrui Zhao, Shenghan Su, Yuqing Lin, Xuebei Li, Lingfeng Dan, Yunjin Zhang, Chunxiao Yang, Xiaohan Li, Yimeng Dong, Chenchen Geng, Changhao Sun, Xia Chu, Huimin Lu
SSRN Electronic Journal .2022;[Epub] CrossRef - Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Domenico Conza, Paola Mirra, Gaetano Calì, Luigi Insabato, Francesca Fiory, Francesco Beguinot, Luca Ulianich
Cells.2021; 10(5): 1067. CrossRef - NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction
Suma Elumalai, Udayakumar Karunakaran, Jun-Sung Moon, Kyu-Chang Won
Cells.2021; 10(7): 1573. CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Different Effects of Metformin and A769662 on Sodium Iodate-Induced Cytotoxicity in Retinal Pigment Epithelial Cells: Distinct Actions on Mitochondrial Fission and Respiration
Chi-Ming Chan, Ponarulselvam Sekar, Duen-Yi Huang, Shu-Hao Hsu, Wan-Wan Lin
Antioxidants.2020; 9(11): 1057. CrossRef - Metformin Reduces Lipotoxicity-Induced Meta-Inflammation in β-Cells through the Activation of GPR40-PLC-IP3 Pathway
Ximei Shen, Beibei Fan, Xin Hu, Liufen Luo, Yuanli Yan, Liyong Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef
- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
- Complications
- Autophagy: A Novel Therapeutic Target for Diabetic Nephropathy
- Shinji Kume, Daisuke Koya
- Diabetes Metab J. 2015;39(6):451-460. Published online December 11, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.6.451
- 4,793 View
- 54 Download
- 79 Web of Science
- 73 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetic nephropathy is a leading cause of end stage renal disease and its occurance is increasing worldwide. The most effective treatment strategy for the condition is intensive treatment to strictly control glycemia and blood pressure using renin-angiotensin system inhibitors. However, a fraction of patients still go on to reach end stage renal disease even under such intensive care. New therapeutic targets for diabetic nephropathy are, therefore, urgently needed. Autophagy is a major catabolic pathway by which mammalian cells degrade macromolecules and organelles to maintain intracellular homeostasis. The accumulation of damaged proteins and organelles is associated with the pathogenesis of diabetic nephropathy. Autophagy in the kidney is activated under some stress conditions, such as oxidative stress and hypoxia in proximal tubular cells, and occurs even under normal conditions in podocytes. These and other accumulating findings have led to a hypothesis that autophagy is involved in the pathogenesis of diabetic nephropathy. Here, we review recent findings underpinning this hypothesis and discuss the advantages of targeting autophagy for the treatment of diabetic nephropathy.
-
Citations
Citations to this article as recorded by- Aging and Diabetic Kidney Disease: Emerging Pathogenetic
Mechanisms and Clinical Implications
Yi Chen, Yashpal S. Kanwar, Xueqin Chen, Ming Zhan
Current Medicinal Chemistry.2024; 31(6): 697. CrossRef - Metformin inhibits high glucose‐induced apoptosis of renal podocyte through regulating miR‐34a/SIRT1 axis
Xudong Zhuang, Zhuye Sun, Huasheng Du, Tianhui Zhou, Jing Zou, Wei Fu
Immunity, Inflammation and Disease.2024;[Epub] CrossRef - Placenta-derived mesenchymal stem cells protect against diabetic kidney disease by upregulating autophagy-mediated SIRT1/FOXO1 pathway
Honghong Liu, Jiao Wang, Guanru Yue, Jixiong Xu
Renal Failure.2024;[Epub] CrossRef - Epigenetic Regulation of Autophagy in Bone Metabolism
Yazhou Zhang, Qianqian Wang, Hongjia Xue, Yujin Guo, Shanshan Wei, Fengfeng Li, Linqiang Gong, Weiliang Pan, Pei Jiang
Function.2024;[Epub] CrossRef - The interaction between lncRNAs and transcription factors regulating autophagy in human cancers: A comprehensive and therapeutical survey
Saade Abdalkareem Jasim, Yasir Qasim Almajidi, Reyadh R. Al‐Rashidi, Ahmed Hjazi, Irfan Ahmad, Ahmed Hussien Radie Alawadi, Enas R. Alwaily, Hashem O. Alsaab, Ali Haslany, Mohamood Hameed
Cell Biochemistry and Function.2024;[Epub] CrossRef - The beneficial effects of astragaloside IV on ameliorating diabetic kidney disease
Yiwei Gao, Xin Su, Taiqi Xue, Ning Zhang
Biomedicine & Pharmacotherapy.2023; 163: 114598. CrossRef - #2601 THE MOLECULAR EFFECT OF SGLT2I ON THE AUTOPHAGY PATHWAY IN TYPE II DIABETES MELLITUS AND ITS VASCULAR COMPLICATIONS
Offir Ertracht, Raneen Saad, Hagar Tadmor, Farid Nakhoul, Nakhoul Nakhoul
Nephrology Dialysis Transplantation.2023;[Epub] CrossRef - Role of oxidative stress in diabetes-induced complications and their management with antioxidants
Hasandeep Singh, Rajanpreet Singh, Arshdeep Singh, Harshbir Singh, Gurpreet Singh, Sarabjit Kaur, Balbir Singh
Archives of Physiology and Biochemistry.2023; : 1. CrossRef - Stress can affect mitochondrial energy metabolism and AMPK/SIRT1 signaling pathway in rats
An-ran Zhao, Jie Li, Si-qi Wang, Li-hua Bian, Wen-jing Li, Jian-you Guo
Brain Research Bulletin.2023; 203: 110770. CrossRef - Emerging links between FOXOs and diabetic complications
Urvi M. Parmar, Manjiri P. Jalgaonkar, Aayush J. Kansara, Manisha J. Oza
European Journal of Pharmacology.2023; 960: 176089. CrossRef - Pathophysiology of diabetic kidney disease and autophagy: A review
Jiawei Yu, Yan Liu, Hongjie Li, Peirong Zhang
Medicine.2023; 102(30): e33965. CrossRef - Microcystin-LR-induced autophagy via miR-282–5p/PIK3R1 pathway in Eriocheir sinensis hepatopancreas
Yuning Zhang, Jiancao Gao, Liping Cao, Jinliang Du, Gangchun Xu, Pao Xu
Ecotoxicology and Environmental Safety.2023; 267: 115661. CrossRef - Global research trends and hot spots on autophagy and kidney diseases: a bibliometric analysis from 2000 to 2022
Sinan Ai, Yake Li, Huijuan Zheng, Zhen Wang, Weijing Liu, JiaYin Tao, Yaotan Li, Yaoxian Wang
Frontiers in Pharmacology.2023;[Epub] CrossRef - Administration of mesenchymal stem cells in diabetic kidney disease: mechanisms, signaling pathways, and preclinical evidence
Yuexin Zhu, Manyu Luo, Xue Bai, Yan Lou, Ping Nie, Shan Jiang, Jicui Li, Bing Li, Ping Luo
Molecular and Cellular Biochemistry.2022; 477(8): 2073. CrossRef - Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS
Ting Hu, Ju Lu, Changyan Wu, Tianxiao Duan, Peng Luo
Molecules.2022; 27(9): 2806. CrossRef - Asiatic acid from Cyclocarya paliurus regulates the autophagy–lysosome system via directly inhibiting TGF-β type I receptor and ameliorates diabetic nephropathy fibrosis
Xuan-xuan Zhang, Yao Liu, Su-su Xu, Ru Yang, Cui-hua Jiang, Li-ping Zhu, Yin-ying Xu, Ke Pan, Jian Zhang, Zhi-qi Yin
Food & Function.2022; 13(10): 5536. CrossRef - Therapeutic Potential of Resveratrol in Diabetic Nephropathy According
to Molecular Signaling
Marziyeh Salami, Raziyeh Salami, Alireza Mafi, Mohammad-Hossein Aarabi, Omid Vakili, Zatollah Asemi
Current Molecular Pharmacology.2022; 15(5): 716. CrossRef - Impact of SGLT2 inhibitors on the kidney in people with type 2 diabetes and severely increased albuminuria
Nasir Shah, Vlado Perkovic, Sradha Kotwal
Expert Review of Clinical Pharmacology.2022; 15(7): 827. CrossRef - Autophagy-nutrient sensing pathways in diabetic complications
Urvi M. Parmar, Manjiri P. Jalgaonkar, Yogesh A. Kulkarni, Manisha J. Oza
Pharmacological Research.2022; 184: 106408. CrossRef - The Molecular Effects of SGLT2i Empagliflozin on the Autophagy Pathway in Diabetes Mellitus Type 2 and Its Complications
Ranin Saad, Hagar Tadmor, Offir Ertracht, Nakhoul Nakhoul, Farid Nakhoul, Farber Evgeny, Shaul Atar, Bernd Stratmann
Journal of Diabetes Research.2022; 2022: 1. CrossRef - What’s New in the Molecular Mechanisms of Diabetic Kidney Disease: Recent Advances
Kimio Watanabe, Emiko Sato, Eikan Mishima, Mariko Miyazaki, Tetsuhiro Tanaka
International Journal of Molecular Sciences.2022; 24(1): 570. CrossRef - Autophagy blockade mechanistically links proton pump inhibitors to worsened diabetic nephropathy and aborts the renoprotection of metformin/enalapril
Dalia Kamal Mostafa, Mohamed Mostafa Khedr, Mervat Kamel Barakat, Amany Abdelbary Abdellatif, Amal Mohamed Elsharkawy
Life Sciences.2021; 265: 118818. CrossRef - Epigenetic modulation of autophagy genes linked to diabetic nephropathy by administration of isorhamnetin in Type 2 diabetes mellitus rats
Marwa Matboli, Doaa Ibrahim, Amany H Hasanin, Mohamed K Hassan, Eman K Habib, Miram M Bekhet, Ahmed M Afifi, Sanaa Eissa
Epigenomics.2021; 13(3): 187. CrossRef - Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-κB signaling pathway to improve diabetic nephropathy
Yuxiang Liu, Wenyuan Liu, Ziyuan Zhang, Yaling Hu, Xiaodong Zhang, Yanyan Sun, Qingqing Lei, Dalin Sun, Ting Liu, Yanjun Fan, Hui Li, Wujie Ding, Jingai Fang
Renal Failure.2021; 43(1): 128. CrossRef - Geniposide Improves Diabetic Nephropathy by Enhancing ULK1-Mediated Autophagy and Reducing Oxidative Stress through AMPK Activation
Theodomir Dusabimana, Eun Jung Park, Jihyun Je, Kyuho Jeong, Seung Pil Yun, Hye Jung Kim, Hwajin Kim, Sang Won Park
International Journal of Molecular Sciences.2021; 22(4): 1651. CrossRef - Update on diagnosis, pathophysiology, and management of diabetic kidney disease
Mai Sugahara, Wai Lun Will Pak, Tetsuhiro Tanaka, Sydney C. W. Tang, Masaomi Nangaku
Nephrology.2021; 26(6): 491. CrossRef - Life-Long Hyperbilirubinemia Exposure and Bilirubin Priming Prevent In Vitro Metabolic Damage
Annalisa Bianco, Serena Pinci, Claudio Tiribelli, Cristina Bellarosa
Frontiers in Pharmacology.2021;[Epub] CrossRef - NADH/NAD+ Redox Imbalance and Diabetic Kidney Disease
Liang-Jun Yan
Biomolecules.2021; 11(5): 730. CrossRef - Circular RNAs act as regulators of autophagy in cancer
Zhixia Zhou, Yinfeng Zhang, Jinning Gao, Xiaodan Hao, Chan Shan, Jing Li, Cuiyun Liu, Yin Wang, Peifeng Li
Molecular Therapy - Oncolytics.2021; 21: 242. CrossRef - Sarsasapogenin restores podocyte autophagy in diabetic nephropathy by targeting GSK3β signaling pathway
Xi-zhi Li, Hong Jiang, Liu Xu, Yi-qi Liu, Jia-wei Tang, Jia-sen Shi, Xiu-juan Yu, Xue Wang, Lei Du, Qian Lu, Cheng-lin Li, Yao-wu Liu, Xiao-xing Yin
Biochemical Pharmacology.2021; 192: 114675. CrossRef - Dietary Restriction for Kidney Protection: Decline in Nephroprotective Mechanisms During Aging
Nadezda V. Andrianova, Marina I. Buyan, Anastasia K. Bolikhova, Dmitry B. Zorov, Egor Y. Plotnikov
Frontiers in Physiology.2021;[Epub] CrossRef - Induction of PDCD4 by albumin in proximal tubule epithelial cells potentiates proteinuria-induced dysfunctional autophagy by negatively targeting Atg5
Ezra Kombo Osoro, Xiaojuan Du, Dong Liang, Xi Lan, Riaz Farooq, Fumeng Huang, Wenhua Zhu, Jiajun Ren, Muhammad Sadiq, Lifang Tian, Xudong Yang, Dongmin Li, Shemin Lu
Biochemistry and Cell Biology.2021; 99(5): 617. CrossRef - Overexpressing STAMP2 attenuates diabetic renal injuries via upregulating autophagy in diabetic rats
Fang-qiang Song, Ming Song, Wei-xuan Ma, Zhan Gao, Yun Ti, Xu Zhang, Bo-ang Hu, Ming Zhong, Wei Zhang, Ying Yu
Biochemical and Biophysical Research Communications.2021; 579: 47. CrossRef - Mitochondrial Regulation of Diabetic Kidney Disease
Daniel L. Galvan, Koki Mise, Farhad R. Danesh
Frontiers in Medicine.2021;[Epub] CrossRef - Diabetic kidney disease update: Pathogenesis and treatment overview for clinicians
Elmukhtar Habas, Abdel-Naser Elzouki
Journal of Diabetes and Endocrine Practice.2021; 04(03): 107. CrossRef - VDR/Atg3 Axis Regulates Slit Diaphragm to Tight Junction Transition via p62-Mediated Autophagy Pathway in Diabetic Nephropathy
Bin Wang, Jing-yi Qian, Tao-tao Tang, Li-lu Lin, Nan Yu, Hong-lei Guo, Wei-jie Ni, Ling-Li Lv, Yi Wen, Zuo-Lin Li, Min Wu, Jing-Yuan Cao, Bi-Cheng Liu
Diabetes.2021; 70(11): 2639. CrossRef - SIRT1: Mechanism and Protective Effect in Diabetic Nephropathy
Jing Ji, Pengyu Tao, Qian Wang, Lingxing Li, Yuzhen Xu
Endocrine, Metabolic & Immune Disorders - Drug Targets.2021; 21(5): 835. CrossRef - SIRT1 Alleviates Aldosterone-Induced Podocyte Injury by Suppressing Mitochondrial Dysfunction and NLRP3 Inflammasome Activation
Mingzhu Jiang, Min Zhao, Mi Bai, Juan Lei, Yanggang Yuan, Songming Huang, Yue Zhang, Guixia Ding, Zhanjun Jia, Aihua Zhang
Kidney Diseases.2021; 7(4): 293. CrossRef Salvianolic Acid B Improves Chronic Mild Stress-Induced Depressive Behaviors in Rats: Involvement of AMPK/SIRT1 Signaling Pathway
Dehua Liao, Yun Chen, Yujin Guo, Changshui Wang, Ni Liu, Qian Gong, Yingzhou Fu, Yilan Fu, Lizhi Cao, Dunwu Yao, Pei Jiang
Journal of Inflammation Research.2020; Volume 13: 195. CrossRef- Long non-coding RNAs and pyroptosis
Dong He, Jun Zheng, Jia Hu, Juan Chen, Xing Wei
Clinica Chimica Acta.2020; 504: 201. CrossRef - Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy
Fang Wang, Ran Li, Linlin Zhao, Shuang Ma, Guijun Qin
European Journal of Pharmacology.2020; 885: 173387. CrossRef - Beneficial Effect of Chloroquine and Amodiaquine on Type 1 Diabetic Tubulopathy by Attenuating Mitochondrial Nox4 and Endoplasmic Reticulum Stress
Jun Mo Kang, Hyun-Seob Lee, Junghyun Kim, Dong Ho Yang, Hye Yun Jeong, Yu Ho Lee, Dong-Jin Kim, Seon Hwa Park, MinJi Sung, Jaehee Kim, Hyun-Ju An, Sang Ho Lee, So-Young Lee
Journal of Korean Medical Science.2020;[Epub] CrossRef - Glucagon-like peptide-1 alleviates diabetic kidney disease through activation of autophagy by regulating AMP-activated protein kinase-mammalian target of rapamycin pathway
Shuangli Yang, Chuman Lin, Xiaoyun Zhuo, Jiyu Wang, Shitao Rao, Wen Xu, Yanzhen Cheng, Li Yang
American Journal of Physiology-Endocrinology and Metabolism.2020; 319(6): E1019. CrossRef - Inhibition of soluble epoxide hydrolase attenuates renal tubular mitochondrial dysfunction and ER stress by restoring autophagic flux in diabetic nephropathy
Xu-shun Jiang, Xing-yang Xiang, Xue-mei Chen, Jun-ling He, Ting Liu, Hua Gan, Xiao-gang Du
Cell Death & Disease.2020;[Epub] CrossRef - Orientin Protects Podocytes from High Glucose Induced Apoptosis through Mitophagy
Zi‐Li Kong, Kui Che, Jian‐Xia Hu, Ying Chen, Yun‐Yang Wang, Xiang Wang, Wen‐Shan Lü, Yan‐Gang Wang, Jing‐Wei Chi
Chemistry & Biodiversity.2020;[Epub] CrossRef - Sex-Specific Metabolic Changes in Peripheral Organs of Diabetic Mice
Xi Zhang, Hangying Xu, Jie Ning, Hui Ji, Junjie Yan, Yafei Zheng, Qingqing Xu, Chen Li, Liangcai Zhao, Hong Zheng, Hongchang Gao
Journal of Proteome Research.2020; 19(8): 3011. CrossRef - Liver X receptor activation induces podocyte injury via inhibiting autophagic activity
Ziyi Zhang, Shengjie Tang, Weiwei Gui, Xihua Lin, Fenping Zheng, Fang Wu, Hong Li
Journal of Physiology and Biochemistry.2020; 76(2): 317. CrossRef - Autophagy plays a protective role duringPseudomonas aeruginosa-induced apoptosis via ROS–MAPK pathway
Lu Han, Qinmei Ma, Jialin Yu, Zhaoqian Gong, Chenjie Ma, Yanan Xu, Guangcun Deng, Xiaoling Wu
Innate Immunity.2020; 26(7): 580. CrossRef - Autophagy in diabetic nephropathy: a review
Elias A. T. Koch, Rola Nakhoul, Farid Nakhoul, Nakhoul Nakhoul
International Urology and Nephrology.2020; 52(9): 1705. CrossRef - P2Y2R contributes to the development of diabetic nephropathy by inhibiting autophagy response
Theodomir Dusabimana, So Ra Kim, Eun Jung Park, Jihyun Je, Kyuho Jeong, Seung Pil Yun, Hye Jung Kim, Hwajin Kim, Sang Won Park
Molecular Metabolism.2020; 42: 101089. CrossRef - Cardioprotective Effects of Taurisolo® in Cardiomyoblast H9c2 Cells under High-Glucose and Trimethylamine N-Oxide Treatment via De Novo Sphingolipid Synthesis
Stefania Lama, Vincenzo Monda, Maria Rosaria Rizzo, Marco Dacrema, Maria Maisto, Giuseppe Annunziata, Gian Carlo Tenore, Ettore Novellino, Paola Stiuso, Laura Sartiani
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef - Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy
Yu Ho Lee, Sang Hoon Kim, Jun Mo Kang, Jin Hyung Heo, Dong-Jin Kim, Seon Hwa Park, MinJi Sung, Jaehee Kim, Jisu Oh, Dong Ho Yang, Sang Ho Lee, So-Young Lee
American Journal of Physiology-Renal Physiology.2019; 317(4): F767. CrossRef - Critical role of mitochondrial dysfunction and impaired mitophagy in diabetic nephropathy
Sugandh Saxena, Alpana Mathur, Poonam Kakkar
Journal of Cellular Physiology.2019; 234(11): 19223. CrossRef - High Dose Vitamin E Attenuates Diabetic Nephropathy via Alleviation of Autophagic Stress
Yuxue Zhao, Wenting Zhang, Qi Jia, Zhendong Feng, Jing Guo, Xueting Han, Yuning Liu, Hongcai Shang, Yaoxian Wang, Wei Jing Liu
Frontiers in Physiology.2019;[Epub] CrossRef - Caffeic Acid Modulates miR-636 Expression in Diabetic Nephropathy Rats
Ahmed M. Salem, Aya S. Ragheb, Marwa G. A. Hegazy, Marwa Matboli, Sanaa Eissa
Indian Journal of Clinical Biochemistry.2019; 34(3): 296. CrossRef - Mechanistic Understanding of the Engineered Nanomaterial-Induced Toxicity on Kidney
Haiyang Zhao, Luxin Li, Huilu Zhan, Yanhui Chu, Bingbing Sun
Journal of Nanomaterials.2019; 2019: 1. CrossRef - Diabetic nephropathy: An update on pathogenesis and drug development
Vikram Rao A/L B Vasanth Rao, Sean Hong Tan, Mayuren Candasamy, Subrat Kumar Bhattamisra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 754. CrossRef - Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway
Qian Yu, Minda Zhang, Lifen Qian, Dan Wen, Guanzhong Wu
Life Sciences.2019; 225: 1. CrossRef - Catalpol Ameliorates Podocyte Injury by Stabilizing Cytoskeleton and Enhancing Autophagy in Diabetic Nephropathy
Yan Chen, Qingpu Liu, Zengfu Shan, Wangyang Mi, Yingying Zhao, Meng Li, Baiyan Wang, Xiaoke Zheng, Weisheng Feng
Frontiers in Pharmacology.2019;[Epub] CrossRef - Role of sirtuin-1 in diabetic nephropathy
Wanning Wang, Weixia Sun, Yanli Cheng, Zhonggao Xu, Lu Cai
Journal of Molecular Medicine.2019; 97(3): 291. CrossRef - Energy restriction in renal protection
Si-Yang Wang, Guang-Yan Cai, Xiang-Mei Chen
British Journal of Nutrition.2018; 120(10): 1149. CrossRef - The dysregulated autophagy signaling is partially responsible for defective podocyte development in wt1a mutant zebrafish
Xuemei Zhang, Qiaohong Lin, Fan Ren, Jin Zhang, Farman Ullah Dawar, Jie Mei
Aquaculture and Fisheries.2018; 3(3): 99. CrossRef - Supplementation of Abelmoschus manihot Ameliorates Diabetic Nephropathy and Hepatic Steatosis by Activating Autophagy in Mice
Hwajin Kim, Theodomir Dusabimana, So Kim, Jihyun Je, Kyuho Jeong, Min Kang, Kye Cho, Hye Kim, Sang Park
Nutrients.2018; 10(11): 1703. CrossRef - Acute Kidney Injury and Progression of Diabetic Kidney Disease
Samuel Mon-Wei Yu, Joseph V. Bonventre
Advances in Chronic Kidney Disease.2018; 25(2): 166. CrossRef - Cardioprotective effects of dietary rapamycin on adult female C57BLKS/J‐Leprdb mice
Peter C. Reifsnyder, Sergey Ryzhov, Kevin Flurkey, Rea P. Anunciado‐Koza, Ian Mills, David E. Harrison, Robert A. Koza
Annals of the New York Academy of Sciences.2018; 1418(1): 106. CrossRef - Viability of primary cultured podocytes is associated with extracellular high glucose-dependent autophagy downregulation
Irena Audzeyenka, Dorota Rogacka, Agnieszka Piwkowska, Stefan Angielski, Maciej Jankowski
Molecular and Cellular Biochemistry.2017; 430(1-2): 11. CrossRef - Autophagy Protects against Palmitic Acid-Induced Apoptosis in Podocytes in vitro
Xu-shun Jiang, Xue-mei Chen, Jiang-min Wan, Hai-bo Gui, Xiong-zhong Ruan, Xiao-gang Du
Scientific Reports.2017;[Epub] CrossRef - Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes
Yu Liu, Jia Zhang, Yangjia Wang, Xiangjun Zeng
Cell Death & Disease.2017; 8(8): e3006. CrossRef - Autophagy and its link to type II diabetes mellitus
Jai-Sing Yang, Chi-Cheng Lu, Sheng-Chu Kuo, Yuan-Man Hsu, Shih-Chang Tsai, Shih-Yin Chen, Yng-Tay Chen, Ying-Ju Lin, Yu-Chuen Huang, Chao-Jung Chen, Wei-De Lin, Wen-Lin Liao, Wei-Yong Lin, Yu-Huei Liu, Jinn-Chyuan Sheu, Fuu-Jen Tsai
BioMedicine.2017; 7(2): 8. CrossRef - Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy
Shan-Shan Huang, Da-Fa Ding, Sheng Chen, Cheng-Long Dong, Xiao-Long Ye, Yang-Gang Yuan, Ya-Min Feng, Na You, Jia-Rong Xu, Heng Miao, Qiang You, Xiang Lu, Yi-Bing Lu
Scientific Reports.2017;[Epub] CrossRef - Long non-coding RNAs involved in autophagy regulation
Lixian Yang, Hanying Wang, Qi Shen, Lifeng Feng, Hongchuan Jin
Cell Death & Disease.2017; 8(10): e3073. CrossRef - Treatment of diabetic kidney disease: current and future targets
Mi-Kyung Kim
The Korean Journal of Internal Medicine.2017; 32(4): 622. CrossRef - MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in db/db mice
Yanru Zhao, Zhongwei Yin, Huaping Li, Jiahui Fan, Shenglan Yang, Chen Chen, Dao Wen Wang
Aging Cell.2017; 16(2): 387. CrossRef
- Aging and Diabetic Kidney Disease: Emerging Pathogenetic
Mechanisms and Clinical Implications
- Rosiglitazone Activates AMPK and Improves Non-Alcoholic Fatty Liver Disease in OLETF Rats.
- Eun Hee Cho, Ki Up Lee
- Korean Diabetes J. 2008;32(2):141-148. Published online April 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.2.141
- 1,797 View
- 29 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Insulin resistance is very common in patients with nonalcoholic fatty liver disease (NAFLD). Glitazones improve insulin sensitivity by acting as a selective agonist of the peroxisome proliferators -activated receptor gamma (PPAR gamma), and were shown to activate AMP-activated protein kinase (AMPK) in skeletal muscle and the liver. Glitazones were also shown to reduce hepatic lipogenesis. The aim of this study was to investigate whether the protective mechanism of rosiglitazone on NAFLD is associated with AMPK activation. METHODS: Twelve OLETF rats were divided into 2 groups (control, treatment, n = 6 each). LETO rats served as controls. At 35 weeks of age, treatment group received rosiglitazone 4 mg/kg daily for 3 days. Fasting plasma glucose, insulin, free fatty acid, lactate and triglycerides were measured. Liver tissues from each group were processed for histological and hepatic triglyceride content analysis and western blotting. RESULTS: Fasting plasma glucose, insulin and triglycerides levels were significantly lower in treatment group than in control group. Histologic examination disclosed decreased hepatic steatosis in treatment group. Hepatic triglyceride content was also decreased in treatment group. Sterol regulatory binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) expression were increased and AMPK phosphorylation was reduced in OLETF rats compared with LETO rats, and these changes were reversed by rosiglitazone treatment. CONCLUSION: Rosiglitazone reduced hepatic steatosis in OLETF rats, and activated AMPK in the liver. These results suggest the role of AMPK activation in the protective action of rosiglitazone on NAFLD. -
Citations
Citations to this article as recorded by- Small Rice Bowl-Based Meal Plan for Energy and Marcronutrient Intake in Korean Men with Type 2 Diabetes: A Pilot Study
Hee Jung Ahn, Kyung Ah Han, Jin Young Jang, Jae Hyuk Lee, Kang Seo Park, Kyung Wan Min
Diabetes & Metabolism Journal.2011; 35(3): 273. CrossRef
- Small Rice Bowl-Based Meal Plan for Energy and Marcronutrient Intake in Korean Men with Type 2 Diabetes: A Pilot Study
- AICAR Reversed the Glucolipotoxicity Induced beta-cell Dysfunction through Suppression of PPAR-gamma-coactivator-1 (PGC-1) Overexpression.
- Hyuk Sang Kwon, Ji Won Kim, Heon Seok Park, Seung Hyun Ko, Bong Yun Cha, Ho Young Son, Kun Ho Yoon
- Korean Diabetes J. 2007;31(4):310-318. Published online July 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.4.310
- 2,020 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Glucolipotoxicity plays an important role in the progression of type 2 diabetes mellitus via inducing insulin secretory dysfunction. Expression of insulin gene in pancreatic beta cell might be regulated by AMP-activated protein kinase (AMPK), which is recognized as a key molecule of energy metabolism. We studied the effects of AMPK on glucolipotoxicity-induced beta-cell dysfunction by suppression of PPAR-gamma-coactivator-1 (PGC-1) in vitro and in vivo. Method: Glucolipotoxicity was induced by 33.3 mM glucose and 0.6 mM (palmitate and oleate) for 3 days in isolated rat islets. Messenger RNA (mRNA) expressions of beta-cell specific gene like insulin, BETA2/NeuroD and PGC-1 induced by glucolipotoxic condition and their changes with 5-aminoimidazole-4-carboxy-amide-1-D-ribofuranoside (AICAR) treatment were investigated using RT-PCR. We also examined glucose stimulated insulin secretion in same conditions. Furthermore, SD rats were submitted to a 90% partial pancreatectomy (Px) and randomized into two groups; Ad-GFP-infected Px rats (n = 3) and Ad-siPGC- 1-infected Px rats (n = 3). Then, the Px rats were infected with Ad-GFP or Ad-siPGC-1 (1 x 10(9) pfu) via celiac artery. After 12 days of viral infection, we measured body weight and performed the intraperitoneal glucose tolerance test (IP-GTT). RESULTS: Glucolipotoxicity resulted in blunting of glucose-stimulated insulin secretion, which was recovered by the AICAR treatment in vitro. Suppression in their expressions of insulin and BETA2/NeuroD gene by glucolipotoxic condition were improved with AICAR treatment. However, PGC-1alpha expression was gradually increased by glucolipotoxicity, and suppressed by AICAR treatment. Overexpression of PGC-1 using an adenoviral vector in freshly isolated rat islets suppressed insulin gene expression. We also confirmed the function of PGC-1 using an Ad-siPGC-1 in vivo. Direct infection of Ad-siPGC-1 in 90% pancreatectomized rats significantly improved glucose tolerance and increased body weight. CONCLUSION: AMPK could protect against glucolipotoxicity induced beta-cell dysfunction and the suppression of PGC-1 gene expression might involved in the insulin regulatory mechanism by AMPK.
- Hypothalamic AMPK Activity in Diabetic Rats.
- Churl Namkoong, Min Seon Kim, Woo Je Lee, Pil Geum Jang, Seong Min Han, Eun Hee Koh, Joong Yeol Park, Ki Up Lee
- Korean Diabetes J. 2004;28(6):468-477. Published online December 1, 2004
- 929 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
AMP-activated protein kinase (AMPK) acts as a cellular energy sensor that is activated during states of low energy charge and it regulates the various metabolic pathways to reestablish the normal cellular energy balance. It has recently been demonstrated that AMPK activity is altered by the state of energy metabolism in the hypothalamic neurons and this mediates the feeding response. METHODS: Diabetes was induced by an intra-peritoneal injection of streptozotocin (STZ) in Sprague-Dawley rats. The diabetic rats were maintained for 3 weeks with or without insulin treatment. 3 weeks later, we collected hypothalamus and we then assayed the phosphorylation of AMPK and the activity of acetyl CoA carboxylase (ACC) and isoform-specfic AMPK. To determine the effect of hypothalamic AMPK inhibition on diabetic hyperphagia, we administered an AMPK inhibitor, compound C, into the third ventricle in the STZ-induced diabetic rats. RESULTS: Phosphorylation of AMPK, which is a marker of AMPK activation, increased in the hypothalamus of the STZ-induced diabetic rats (DR). Moreover, 2-AMPK activity, but not 1-AMPK activity, increased by 2-fold in hypothalamus of the DRs. Phosphorylation of hypothalamic acetyl CoA carboxylase (ACC), a key downstream enzyme of AMPK, also increased in the DRs and this caused a reduction in ACC activity. Insulin treatment completely reversed the diabetesinduced changes in the hypothalamic AMPK and ACC, suggesting that insulin deficiency was associated with the changes in hypothalamic AMPK and ACC. Inhibition of AMPK by an intracerebroventricular administration of AMPK inhibitor, compound C, attenuated the development of diabetic hyperphagia and reduced the blood glucose levels in DRs. CONCLUSION: We have demonstrated that hypothalamic AMPK activity increased in the DRs, and inhibition of hypothalamic AMPK activity attenuated the development of diabetic hyperphagia. These data indicate that the enhanced hypothalamic AMPK activity may contribute to the development of diabetic hyperphagia

 KDA
KDA
 First
First Prev
Prev





