
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 42(5); 2018 > Article
-
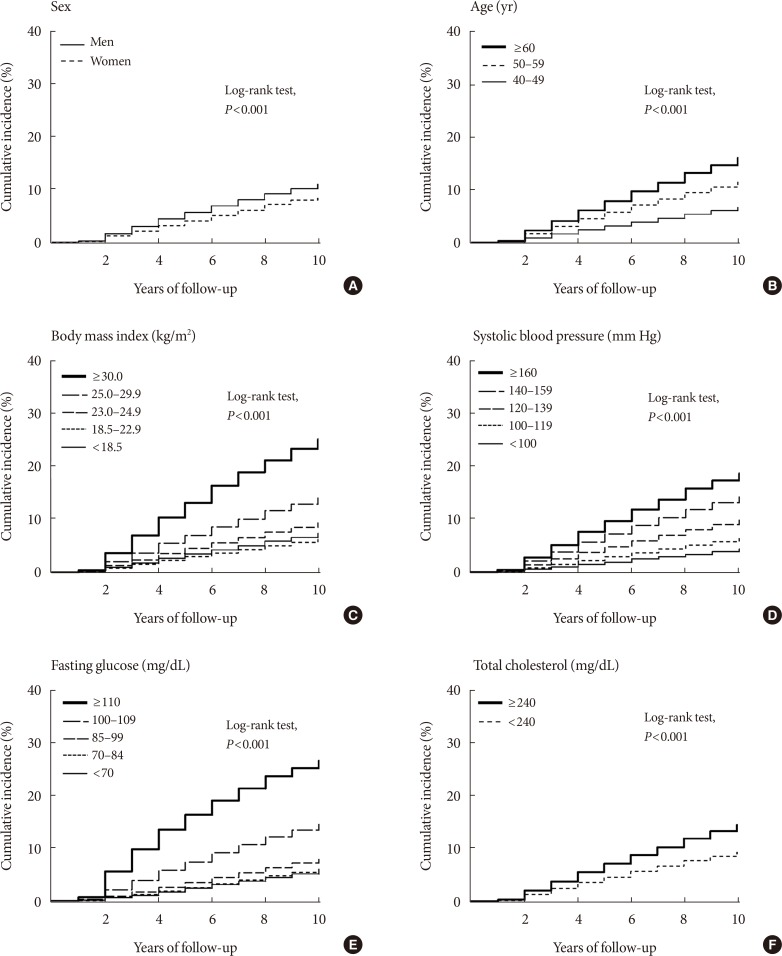
Original ArticleEpidemiology Development and Validation of the Korean Diabetes Risk Score: A 10-Year National Cohort Study
-
Kyoung Hwa Ha1,2, Yong-ho Lee3, Sun Ok Song4, Jae-woo Lee5, Dong Wook Kim6, Kyung-hee Cho7, Dae Jung Kim1,2

-
Diabetes & Metabolism Journal 2018;42(5):402-414.
DOI: https://doi.org/10.4093/dmj.2018.0014
Published online: July 6, 2018
1Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea.
2Cardiovascular and Metabolic Disease Etiology Research Center, Ajou University School of Medicine, Suwon, Korea.
3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
4Department of Endocrinology and Metabolism, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
5Department of Family Medicine, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
6Policy Research Affairs, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
7Department of Family Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- Corresponding author: Dae Jung Kim. Department of Endocrinology and Metabolism, Ajou University School of Medicine, 164 World cup-ro, Yeongtong-gu, Suwon 16499, Korea. djkim@ajou.ac.kr
Copyright © 2018 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Alanine to glycine ratio is a novel predictive biomarker for type 2 diabetes mellitus
Kwang Seob Lee, Yong‐ho Lee, Sang‐Guk Lee
Diabetes, Obesity and Metabolism.2024; 26(3): 980. CrossRef - Associations of updated cardiovascular health metrics, including sleep health, with incident diabetes and cardiovascular events in older adults with prediabetes: A nationwide population-based cohort study
Kyoung Hwa Ha, Dae Jung Kim, Seung Jin Han
Diabetes Research and Clinical Practice.2023; 203: 110820. CrossRef - Comparisons of the prediction models for undiagnosed diabetes between machine learning versus traditional statistical methods
Seong Gyu Choi, Minsuk Oh, Dong–Hyuk Park, Byeongchan Lee, Yong-ho Lee, Sun Ha Jee, Justin Y. Jeon
Scientific Reports.2023;[Epub] CrossRef - Risk prediction models for incident type 2 diabetes in Chinese people with intermediate hyperglycemia: a systematic literature review and external validation study
Shishi Xu, Ruth L. Coleman, Qin Wan, Yeqing Gu, Ge Meng, Kun Song, Zumin Shi, Qian Xie, Jaakko Tuomilehto, Rury R. Holman, Kaijun Niu, Nanwei Tong
Cardiovascular Diabetology.2022;[Epub] CrossRef - Gamma-glutamyl transferase to high-density lipoprotein cholesterol ratio: A valuable predictor of type 2 diabetes mellitus incidence
Wangcheng Xie, Bin Liu, Yansong Tang, Tingsong Yang, Zhenshun Song
Frontiers in Endocrinology.2022;[Epub] CrossRef - Low aspartate aminotransferase/alanine aminotransferase (DeRitis) ratio assists in predicting diabetes in Chinese population
Wangcheng Xie, Weidi Yu, Shanshan Chen, Zhilong Ma, Tingsong Yang, Zhenshun Song
Frontiers in Public Health.2022;[Epub] CrossRef - Prediction Models for Type 2 Diabetes Risk in the General Population: A Systematic Review of Observational Studies
Samaneh Asgari, Davood Khalili, Farhad Hosseinpanah, Farzad Hadaegh
International Journal of Endocrinology and Metabolism.2021;[Epub] CrossRef - Development of a clinical risk score for incident diabetes: A 10‐year prospective cohort study
Tae Jung Oh, Jae Hoon Moon, Sung Hee Choi, Young Min Cho, Kyong Soo Park, Nam H Cho, Hak Chul Jang
Journal of Diabetes Investigation.2021; 12(4): 610. CrossRef - Association between longitudinal blood pressure and prognosis after treatment of cerebral aneurysm: A nationwide population-based cohort study
Jinkwon Kim, Jang Hoon Kim, Hye Sun Lee, Sang Hyun Suh, Kyung-Yul Lee, Yan Li
PLOS ONE.2021; 16(5): e0252042. CrossRef - Development of a predictive risk model for all-cause mortality in patients with diabetes in Hong Kong
Sharen Lee, Jiandong Zhou, Keith Sai Kit Leung, William Ka Kei Wu, Wing Tak Wong, Tong Liu, Ian Chi Kei Wong, Kamalan Jeevaratnam, Qingpeng Zhang, Gary Tse
BMJ Open Diabetes Research & Care.2021; 9(1): e001950. CrossRef - Development and Validation of a Deep Learning Based Diabetes Prediction System Using a Nationwide Population-Based Cohort
Sang Youl Rhee, Ji Min Sung, Sunhee Kim, In-Jeong Cho, Sang-Eun Lee, Hyuk-Jae Chang
Diabetes & Metabolism Journal.2021; 45(4): 515. CrossRef - Development and validation of a new diabetes index for the risk classification of present and new-onset diabetes: multicohort study
Shinje Moon, Ji-Yong Jang, Yumin Kim, Chang-Myung Oh
Scientific Reports.2021;[Epub] CrossRef - New risk score model for identifying individuals at risk for diabetes in southwest China
Liying Li, Ziqiong Wang, Muxin Zhang, Haiyan Ruan, Linxia Zhou, Xin Wei, Ye Zhu, Jiafu Wei, Sen He
Preventive Medicine Reports.2021; 24: 101618. CrossRef - Multiple Biomarkers Improved Prediction for the Risk of Type 2 Diabetes Mellitus in Singapore Chinese Men and Women
Yeli Wang, Woon-Puay Koh, Xueling Sim, Jian-Min Yuan, An Pan
Diabetes & Metabolism Journal.2020; 44(2): 295. CrossRef - Smoking as a Target for Prevention of Diabetes
Ye Seul Yang, Tae Seo Sohn
Diabetes & Metabolism Journal.2020; 44(3): 402. CrossRef - Middle-aged men with type 2 diabetes as potential candidates for pancreatic cancer screening: a 10-year nationwide population-based cohort study
Dong-Hoe Koo, Kyung-Do Han, Hong Joo Kim, Cheol-Young Park
Acta Diabetologica.2020; 57(2): 197. CrossRef - Systematic review with meta-analysis of the epidemiological evidence relating smoking to type 2 diabetes
Peter N Lee, Katharine J Coombs
World Journal of Meta-Analysis.2020; 8(2): 119. CrossRef - Biomarker Score in Risk Prediction: Beyond Scientific Evidence and Statistical Performance
Heejung Bang
Diabetes & Metabolism Journal.2020; 44(2): 245. CrossRef - Research progress on Traditional Chinese Medicine syndromes of diabetes mellitus
Jingkang Wang, Quantao Ma, Yaqi Li, Pengfei Li, Min Wang, Tieshan Wang, Chunguo Wang, Ting Wang, Baosheng Zhao
Biomedicine & Pharmacotherapy.2020; 121: 109565. CrossRef - Cardiometabolic risk prediction algorithms for young people with psychosis: a systematic review and exploratory analysis
B. I. Perry, R. Upthegrove, O. Crawford, S. Jang, E. Lau, I. McGill, E. Carver, P. B. Jones, G. M. Khandaker
Acta Psychiatrica Scandinavica.2020; 142(3): 215. CrossRef - Impact of obesity, fasting plasma glucose level, blood pressure, and renal function on the severity of COVID-19: A matter of sexual dimorphism?
Kyungmin Huh, Rugyeom Lee, Wonjun Ji, Minsun Kang, In Cheol Hwang, Dae Ho Lee, Jaehun Jung
Diabetes Research and Clinical Practice.2020; 170: 108515. CrossRef

 KDA
KDA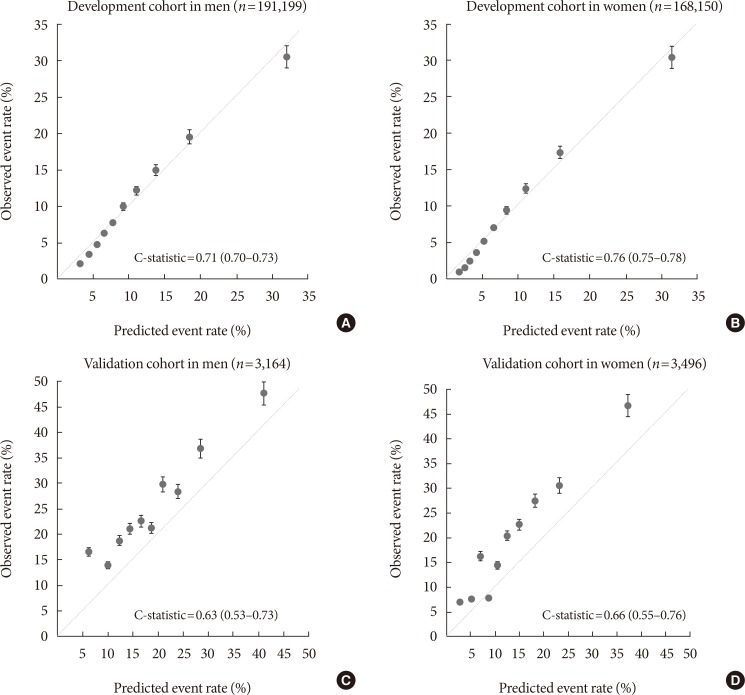
 PubReader
PubReader Cite
Cite





