
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
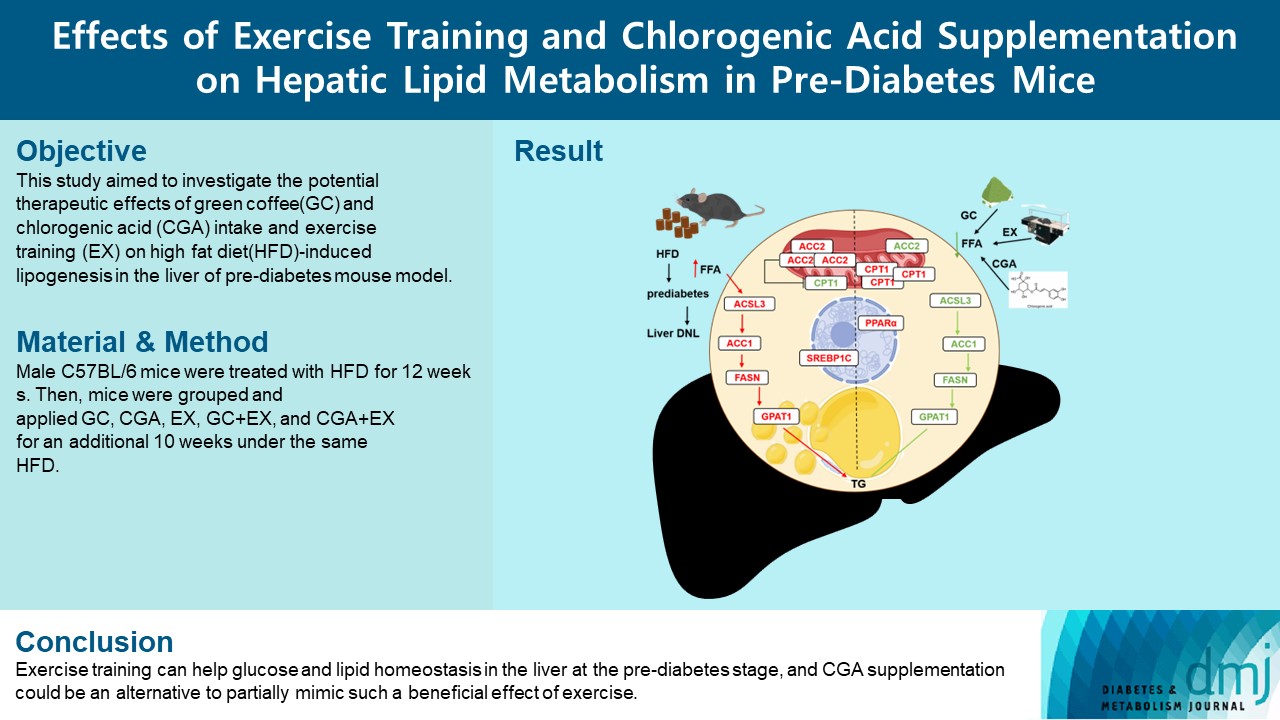
- Effects Of Exercise Training And Chlorogenic Acid Supplementation On Hepatic Lipid Metabolism In Prediabetes Mice
- Samaneh Shirkhani, Sayyed Mohammad Marandi, Mohammad Hossein Nasr-Esfahani, Seung Kyum Kim
- Diabetes Metab J. 2023;47(6):771-783. Published online September 8, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0265
- 2,288 View
- 169 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Since prediabetes is a risk factor for metabolic syndromes, it is important to promote a healthy lifestyle to prevent prediabetes. This study aimed to determine the effects of green coffee (GC), chlorogenic acid (CGA) intake, and exercise training (EX) on hepatic lipid metabolism in prediabetes male C57BL/6 mice.
Methods
Forty-nine mice were randomly divided into two groups feeding with a normal diet (n=7) or a high-fat diet (HFD, n=42) for 12 weeks. Then, HFD mice were further divided into six groups (n=7/group): control (pre-D), GC, CGA, EX, GC+EX, and CGA+EX. After additional 10 weeks under the same diet, plasma, and liver samples were obtained.
Results
HFD-induced prediabetes conditions with increases in body weight, glucose, insulin, insulin resistance, and lipid profiles were alleviated in all treatment groups. Acsl3, a candidate gene identified through an in silico approach, was lowered in the pre-D group, while treatments partly restored it. HFD induced adverse alterations of de novo lipogenesis- and β oxidation-associated molecules in the liver. However, GC and CGA supplementation and EX reversed or ameliorated these changes. In most cases, GC or CGA supplementation combined with EX has no synergistic effect and the GC group had similar results to the CGA group.
Conclusion
These findings suggest that regular exercise is an effective non-therapeutic approach for prediabetes, and CGA supplementation could be an alternative to partially mimic the beneficial effects of exercise on prediabetes. -
Citations
Citations to this article as recorded by- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients
Perioperative Precision Medicine.2023;[Epub] CrossRef
- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients
- Pathophysiology
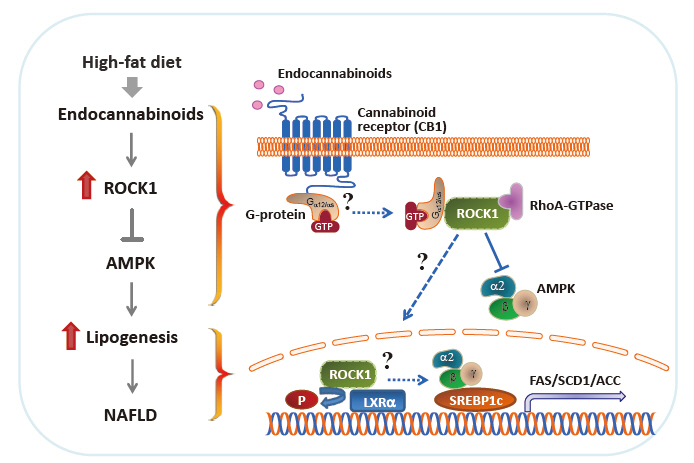
- Rho-Kinase as a Therapeutic Target for Nonalcoholic Fatty Liver Diseases
- Inês Sousa-Lima, Hyun Jeong Kim, John Jones, Young-Bum Kim
- Diabetes Metab J. 2021;45(5):655-674. Published online September 30, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0197
- 5,794 View
- 171 Download
- 7 Web of Science
- 7 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- Nonalcoholic fatty liver disease (NAFLD) is a major public health problem and the most common form of chronic liver disease, affecting 25% of the global population. Although NAFLD is closely linked with obesity, insulin resistance, and type 2 diabetes mellitus, knowledge on its pathogenesis remains incomplete. Emerging data have underscored the importance of Rho-kinase (Rho-associated coiled-coil-containing kinase [ROCK]) action in the maintenance of normal hepatic lipid homeostasis. In particular, pharmacological blockade of ROCK in hepatocytes or hepatic stellate cells prevents the progression of liver diseases such as NAFLD and fibrosis. Moreover, mice lacking hepatic ROCK1 are protected against obesity-induced fatty liver diseases by suppressing hepatic de novo lipogenesis. Here we review the roles of ROCK as an indispensable regulator of obesity-induced fatty liver disease and highlight the key cellular pathway governing hepatic lipid accumulation, with focus on de novo lipogenesis and its impact on therapeutic potential. Consequently, a comprehensive understanding of the metabolic milieu linking to liver dysfunction triggered by ROCK activation may help identify new targets for treating fatty liver diseases such as NAFLD.
-
Citations
Citations to this article as recorded by- THE ROLE OF N6-METHYLADENOSINE METHYLTRANSFERASE RBM15 IN NONALCOHOLIC FATTY LIVER DISEASE
Shiqing Li, Shengyi Lian, Wei Cheng, Tao Zhang, Xiaobing Gong
Shock.2024; 61(2): 311. CrossRef - Exploring the potential of drug repurposing for liver diseases: A comprehensive study
Fares E.M. Ali, Mustafa Ahmed Abdel-Reheim, Emad H.M. Hassanein, Mostafa K. Abd El-Aziz, Hanan S. Althagafy, Khalid S.A. Badran
Life Sciences.2024; : 122642. CrossRef - Targeting of G-protein coupled receptor 40 alleviates airway hyperresponsiveness through RhoA/ROCK1 signaling pathway in obese asthmatic mice
Xixi Lin, Like Wang, Xiaojie Lu, Yuanyuan Zhang, Rongying Zheng, Ruijie Chen, Weixi Zhang
Respiratory Research.2023;[Epub] CrossRef - Selectivity matters: selective ROCK2 inhibitor ameliorates established liver fibrosis via targeting inflammation, fibrosis, and metabolism
Alexandra Zanin-Zhorov, Wei Chen, Julien Moretti, Melanie S. Nyuydzefe, Iris Zhorov, Rashmi Munshi, Malavika Ghosh, Cindy Serdjebi, Kelli MacDonald, Bruce R. Blazar, Melissa Palmer, Samuel D. Waksal
Communications Biology.2023;[Epub] CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Paeoniflorin alleviates liver injury in hypercholesterolemic rats through the ROCK/AMPK pathway
Tong Liu, Ning Zhang, Lingya Kong, Sijie Chu, Ting Zhang, Guangdi Yan, Donglai Ma, Jun Dai, Zhihong Ma
Frontiers in Pharmacology.2022;[Epub] CrossRef - Fasudil Increased the Sensitivity to Gefitinib in NSCLC by Decreasing Intracellular Lipid Accumulation
Tingting Liao, Jingjing Deng, Wenjuan Chen, Juanjuan Xu, Guanghai Yang, Mei Zhou, Zhilei Lv, Sufei Wang, Siwei Song, Xueyun Tan, Zhengrong Yin, Yumei Li, Yang Jin
Cancers.2022; 14(19): 4709. CrossRef
- THE ROLE OF N6-METHYLADENOSINE METHYLTRANSFERASE RBM15 IN NONALCOHOLIC FATTY LIVER DISEASE
- Obesity and Metabolic Syndrome
- Inhibition of Serotonin Synthesis Induces Negative Hepatic Lipid Balance
- Jun Namkung, Ko Eun Shong, Hyeongseok Kim, Chang-Myung Oh, Sangkyu Park, Hail Kim
- Diabetes Metab J. 2018;42(3):233-243. Published online April 25, 2018
- DOI: https://doi.org/10.4093/dmj.2017.0084
- 4,690 View
- 84 Download
- 24 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Hepatic steatosis is caused by metabolic stress associated with a positive lipid balance, such as insulin resistance and obesity. Previously we have shown the anti-obesity effects of inhibiting serotonin synthesis, which eventually improved insulin sensitivity and hepatic steatosis. However, it is not clear whether serotonin has direct effect on hepatic lipid accumulation. Here, we showed the possibility of direct action of serotonin on hepatic steatosis.
Methods Mice were treated with para-chlorophenylalanine (PCPA) or LP-533401 to inhibit serotonin synthesis and fed with high fat diet (HFD) or high carbohydrate diet (HCD) to induce hepatic steatosis. Hepatic triglyceride content and gene expression profiles were analyzed.
Results Pharmacological and genetic inhibition of serotonin synthesis reduced HFD-induced hepatic lipid accumulation. Furthermore, short-term PCPA treatment prevented HCD-induced hepatic steatosis without affecting glucose tolerance and browning of subcutaneous adipose tissue. Gene expression analysis revealed that the expressions of genes involved in
de novo lipogenesis and triacylglycerol synthesis were downregulated by short-term PCPA treatment as well as long-term PCPA treatment.Conclusion Short-term inhibition of serotonin synthesis prevented hepatic lipid accumulation without affecting systemic insulin sensitivity and energy expenditure, suggesting the direct steatogenic effect of serotonin in liver.
-
Citations
Citations to this article as recorded by- A new serotonin 2A receptor antagonist with potential benefits in Non-Alcoholic Fatty Liver Disease
Lucia Sessa, Simona Concilio, Jesús Fominaya, Daniela Eletto, Stefano Piotto, Xavier Busquets
Life Sciences.2023; 314: 121315. CrossRef - Serotonin reuptake transporter deficiency promotes liver steatosis and impairs intestinal barrier function in obese mice fed a Western‐style diet
Louisa Filipe Rosa, Eva Haasis, Annkathrin Knauss, Daria Guseva, Stephan C. Bischoff
Neurogastroenterology & Motility.2023;[Epub] CrossRef - Probiotics counteract hepatic steatosis caused by ketogenic diet and upregulate AMPK signaling in a model of infantile epilepsy
Chunlong Mu, Naghmeh Nikpoor, Thomas A. Tompkins, Jong M. Rho, Morris H. Scantlebury, Jane Shearer
eBioMedicine.2022; 76: 103838. CrossRef - Research Progresses for 5-Hydroxytryptamine in Lipid Metabolism
钰婷 吴
Bioprocess.2022; 12(01): 1. CrossRef - Platelet-Activating Factor Promotes the Development of Non-Alcoholic Fatty Liver Disease
Hang Yin, Anhua Shi, Junzi Wu
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 2003. CrossRef - Involvement of the liver-gut peripheral neural axis in nonalcoholic fatty liver disease pathologies via hepatic HTR2A
Takashi Owaki, Kenya Kamimura, Masayoshi Ko, Itsuo Nagayama, Takuro Nagoya, Osamu Shibata, Chiyumi Oda, Shinichi Morita, Atsushi Kimura, Takeki Sato, Toru Setsu, Akira Sakamaki, Hiroteru Kamimura, Takeshi Yokoo, Shuji Terai
Disease Models & Mechanisms.2022;[Epub] CrossRef - Pancreatic Sirtuin 3 Deficiency Promotes Hepatic Steatosis by Enhancing 5-Hydroxytryptamine Synthesis in Mice With Diet-Induced Obesity
Xing Ming, Arthur C.K. Chung, Dandan Mao, Huanyi Cao, Baoqi Fan, Willy K.K. Wong, Chin Chung Ho, Heung Man Lee, Kristina Schoonjans, Johan Auwerx, Guy A. Rutter, Juliana C.N. Chan, Xiao Yu Tian, Alice P.S. Kong
Diabetes.2021; 70(1): 119. CrossRef - Peripheral Selective Oxadiazolylphenyl Alanine Derivatives as Tryptophan Hydroxylase 1 Inhibitors for Obesity and Fatty Liver Disease
Eun Jung Bae, Won Gun Choi, Haushabhau S. Pagire, Suvarna H. Pagire, Saravanan Parameswaran, Jun-Ho Choi, Jihyeon Yoon, Won-il Choi, Ji Hun Lee, Jin Sook Song, Myung Ae Bae, Mijin Kim, Jae-Han Jeon, In-Kyu Lee, Hail Kim, Jin Hee Ahn
Journal of Medicinal Chemistry.2021; 64(2): 1037. CrossRef - Modulation of serotonin in the gut-liver neural axis ameliorates the fatty and fibrotic changes in non-alcoholic fatty liver
Masayoshi Ko, Kenya Kamimura, Takashi Owaki, Takuro Nagoya, Norihiro Sakai, Itsuo Nagayama, Yusuke Niwa, Osamu Shibata, Chiyumi Oda, Shinichi Morita, Atsushi Kimura, Ryosuke Inoue, Toru Setsu, Akira Sakamaki, Takeshi Yokoo, Shuji Terai
Disease Models & Mechanisms.2021;[Epub] CrossRef - Metabolic Disturbances in Rat Sublines with Constitutionally Altered Serotonin Homeostasis
Maja Kesić, Petra Baković, Ranko Stojković, Jasminka Štefulj, Lipa Čičin-Šain
International Journal of Molecular Sciences.2021; 22(10): 5400. CrossRef - Role of 5-HT degradation in acute liver injury induced by carbon tetrachloride
Yu-Xin Zhang, Chen Li, Xiu-Rui Liang, Jia-Qi Jin, Yi Zhang, Fan Xu, Jing Guan, Ying-Ying Ma, Xiao-Nan Ma, Run-Kun Liu, Ji-Hua Fu
European Journal of Pharmacology.2021; 908: 174355. CrossRef - Fluoxetine-induced hepatic lipid accumulation is linked to elevated serotonin production
Ahmed Ayyash, Alison C. Holloway
Canadian Journal of Physiology and Pharmacology.2021; 99(9): 983. CrossRef - Brain-gut-liver interactions across the spectrum of insulin resistance in metabolic fatty liver disease
Eleni Rebelos, Patricia Iozzo, Maria Angela Guzzardi, Maurizia Rossana Brunetto, Ferruccio Bonino
World Journal of Gastroenterology.2021; 27(30): 4999. CrossRef - Inhibiting serotonin signaling through HTR2B in visceral adipose tissue improves obesity-related insulin resistance
Won Gun Choi, Wonsuk Choi, Tae Jung Oh, Hye-Na Cha, Inseon Hwang, Yun Kyung Lee, Seung Yeon Lee, Hyemi Shin, Ajin Lim, Dongryeol Ryu, Jae Myoung Suh, So-Young Park, Sung Hee Choi, Hail Kim
Journal of Clinical Investigation.2021;[Epub] CrossRef - Green tea and selenium-enriched green tea ameliorates non-alcoholic fatty liver disease through peripheral 5-hydroxytryptamine signals in high-fat diet-fed mice
Lin Zhang, Jia-Ying Xu, Ya-Fang Du, Zhang-Min Wang, Jian-Xiang Li, N. Ou-Yang, Yan Wang, Xue-Bin Yin, Li-Qiang Qin
International Food Research Journal.2021; 28(5): 996. CrossRef - Inhibition of serotonin synthesis: A novel therapeutic paradigm
Michael Bader
Pharmacology & Therapeutics.2020; 205: 107423. CrossRef - One-Carbon Metabolism in Fatty Liver Disease and Fibrosis: One-Carbon to Rule Them All
da Silva Robin P, Eudy Brandon J, Deminice Rafael
The Journal of Nutrition.2020; 150(5): 994. CrossRef - Design, Synthesis, and Biological Evaluation of New Peripheral 5HT2A Antagonists for Nonalcoholic Fatty Liver Disease
Minhee Kim, Inseon Hwang, Haushabhau S. Pagire, Suvarna H. Pagire, Wonsuk Choi, Won Gun Choi, Jihyeon Yoon, Won Mi Lee, Jin Sook Song, Eun Kyung Yoo, Seung Mi Lee, Mi-jin Kim, Myung Ae Bae, Dooseop Kim, Heejong Lee, Eun-Young Lee, Jae-Han Jeon, In-Kyu Lee
Journal of Medicinal Chemistry.2020; 63(8): 4171. CrossRef - Hepatic fibrosis is associated with total proteinuria in Korean patients with type 2 diabetes
Eugene Han, Yongin Cho, Kyung-won Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-wan Lee
Medicine.2020; 99(33): e21038. CrossRef - The Role of the Gut Microbiota in Lipid and Lipoprotein Metabolism
Yijing Yu, Fitore Raka, Khosrow Adeli
Journal of Clinical Medicine.2019; 8(12): 2227. CrossRef - Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule
Julian M Yabut, Justin D Crane, Alexander E Green, Damien J Keating, Waliul I Khan, Gregory R Steinberg
Endocrine Reviews.2019; 40(4): 1092. CrossRef - Serotonin signals through a gut-liver axis to regulate hepatic steatosis
Wonsuk Choi, Jun Namkung, Inseon Hwang, Hyeongseok Kim, Ajin Lim, Hye Jung Park, Hye Won Lee, Kwang-Hyub Han, Seongyeol Park, Ji-Seon Jeong, Geul Bang, Young Hwan Kim, Vijay K. Yadav, Gerard Karsenty, Young Seok Ju, Chan Choi, Jae Myoung Suh, Jun Yong Par
Nature Communications.2018;[Epub] CrossRef
- A new serotonin 2A receptor antagonist with potential benefits in Non-Alcoholic Fatty Liver Disease

 KDA
KDA
 First
First Prev
Prev





