
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
- Diabetes Metab J. 2024;48(2):231-241. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0366

- 1,670 View
- 153 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Administration of pancreatic endoplasmic reticulum kinase inhibitor (PERKi) improved insulin secretion and hyperglycemia in obese diabetic mice. In this study, autophagic balance was studied whether to mediate it.
Methods
Human islets were isolated from living patients without diabetes. PERKi GSK2606414 effects were evaluated in the islets under glucolipotoxicity by palmitate. Islet insulin contents and secretion were measured. Autophagic flux was assessed by microtubule associated protein 1 light chain 3 (LC3) conversion, a red fluorescent protein (RFP)-green fluorescent protein (GFP)- LC3 tandem assay, and P62 levels. For mechanical analyses, autophagy was suppressed using 3-methyladenine in mouse islets. Small interfering RNA for an autophagy-related gene autophagy related 7 (Atg7) was transfected to interfere autophagy.
Results
PERKi administration to mice decreased diabetes-induced P62 levels in the islets. Glucolipotoxicity significantly increased PERK phosphorylation by 70% and decreased insulin contents by 50% in human islets, and addition of PERKi (40 to 80 nM) recovered both. PERKi also enhanced glucose-stimulated insulin secretion (6-fold). PERKi up-regulated LC3 conversion suppressed by glucolipotoxicity, and down-regulated P62 contents without changes in P62 transcription, indicating enhanced autophagic flux. Increased autophagosome-lysosome fusion by PERKi was visualized in mouse islets, where PERKi enhanced ATG7 bound to LC3. Suppression of Atg7 eliminated PERKi-induced insulin contents and secretion.
Conclusion
This study provided functional changes of human islets with regard to autophagy under glucolipotoxicity, and suggested modulation of autophagy as an anti-diabetic mechanism of PERKi.
- Basic Research
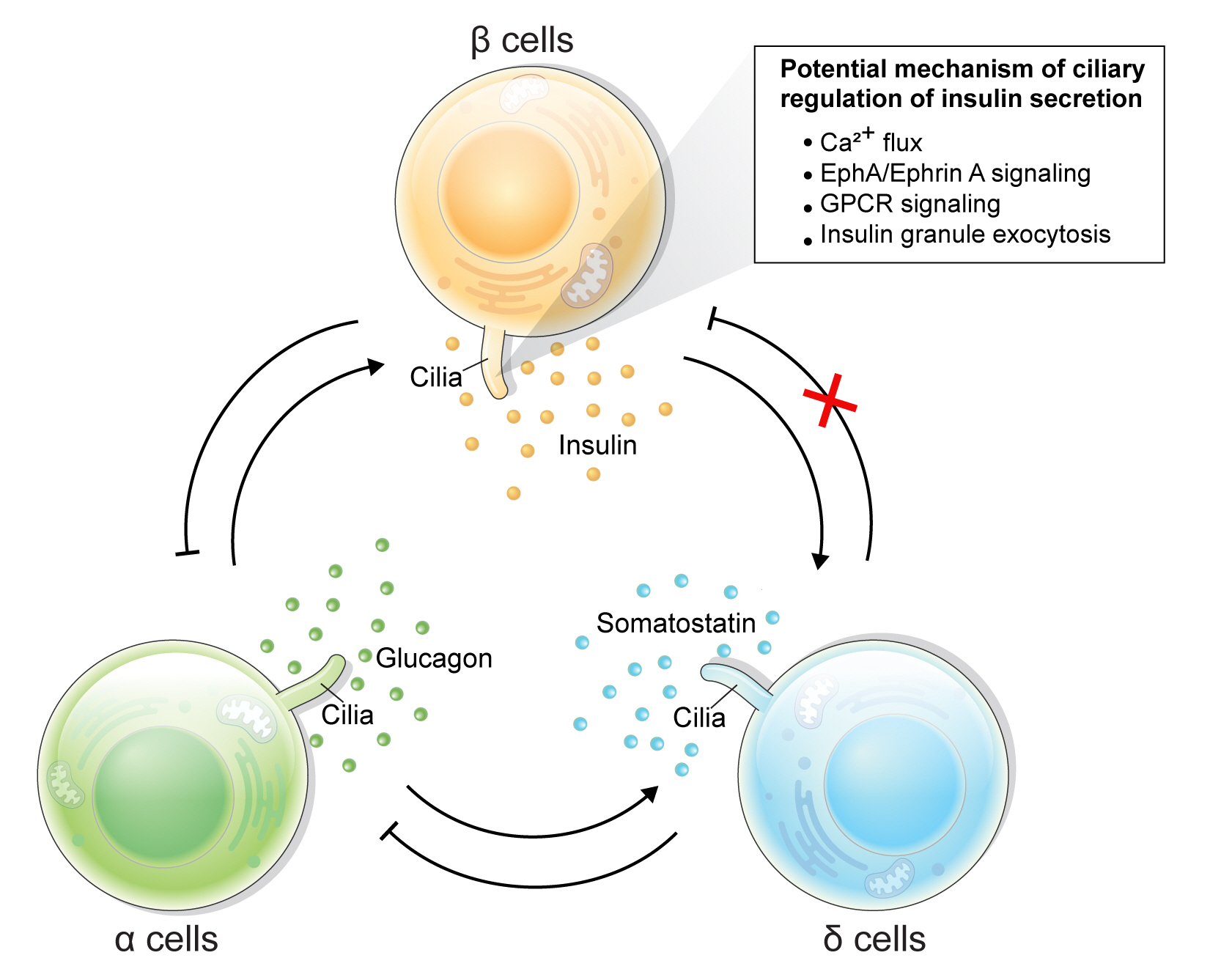
- Rediscovering Primary Cilia in Pancreatic Islets
- Eun Young Lee, Jing W. Hughes
- Diabetes Metab J. 2023;47(4):454-469. Published online April 28, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0442
- 2,592 View
- 240 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Primary cilia are microtubule-based sensory and signaling organelles on the surfaces of most eukaryotic cells. Despite their early description by microscopy studies, islet cilia had not been examined in the functional context until recent decades. In pancreatic islets as in other tissues, primary cilia facilitate crucial developmental and signaling pathways in response to extracellular stimuli. Many human developmental and genetic disorders are associated with ciliary dysfunction, some manifesting as obesity and diabetes. Understanding the basis for metabolic diseases in human ciliopathies has been aided by close examination of cilia action in pancreatic islets at cellular and molecular levels. In this article, we review the evidence for ciliary expression on islet cells, known roles of cilia in pancreas development and islet hormone secretion, and summarize metabolic manifestations of human ciliopathy syndromes. We discuss emerging data on primary cilia regulation of islet cell signaling and the structural basis of cilia-mediated cell crosstalk, and offer our interpretation on the role of cilia in glucose homeostasis and human diseases.
-
Citations
Citations to this article as recorded by- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
Samantha E. Adamson, Zipeng A. Li, Jing W. Hughes
Islets.2023;[Epub] CrossRef
- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
- Basic Research
- Differentiation of Microencapsulated Neonatal Porcine Pancreatic Cell Clusters in Vitro Improves Transplant Efficacy in Type 1 Diabetes Mellitus Mice
- Gyeong-Jin Cheon, Heon-Seok Park, Eun-Young Lee, Min Jung Kim, Young-Hye You, Marie Rhee, Ji-Won Kim, Kun-Ho Yoon
- Diabetes Metab J. 2022;46(5):677-688. Published online February 7, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0202
- 4,520 View
- 252 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Neonatal porcine pancreatic cell clusters (NPCCs) have been proposed as an alternative source of β cells for islet transplantation because of their low cost and growth potential after transplantation. However, the delayed glucose lowering effect due to the immaturity of NPCCs and immunologic rejection remain as a barrier to NPCC’s clinical application. Here, we demonstrate accelerated differentiation and immune-tolerant NPCCs by in vitro chemical treatment and microencapsulation.
Methods
NPCCs isolated from 3-day-old piglets were cultured in F-10 media and then microencapsulated with alginate on day 5. Differentiation of NPCCs is facilitated by media supplemented with activin receptor-like kinase 5 inhibitor II, triiodothyronine and exendin-4 for 2 weeks. Marginal number of microencapsulated NPCCs to cure diabetes with and without differentiation were transplanted into diabetic mice and observed for 8 weeks.
Results
The proportion of insulin-positive cells and insulin mRNA levels of NPCCs were significantly increased in vitro in the differentiated group compared with the undifferentiated group. Blood glucose levels decreased eventually after transplantation of microencapsulated NPCCs in diabetic mice and normalized after 7 weeks in the differentiated group. In addition, the differentiated group showed nearly normal glucose tolerance at 8 weeks after transplantation. In contrast, neither blood glucose levels nor glucose tolerance were improved in the undifferentiated group. Retrieved graft in the differentiated group showed greater insulin response to high glucose compared with the undifferentiated group.
Conclusion
in vitro differentiation of microencapsulated immature NPCCs increased the proportion of insulin-positive cells and improved transplant efficacy in diabetic mice without immune rejection. -
Citations
Citations to this article as recorded by- Dual-targeted nano-encapsulation of neonatal porcine islet-like cell clusters with triiodothyronine-loaded bifunctional polymersomes
Sang Hoon Lee, Minse Kim, Eun-Jin Lee, Sun Mi Ahn, Yu-Rim Ahn, Jaewon Choi, Jung-Taek Kang, Hyun-Ouk Kim
Discover Nano.2024;[Epub] CrossRef - Long‐term efficacy of encapsulated xenogeneic islet transplantation: Impact of encapsulation techniques and donor genetic traits
Heon‐Seok Park, Eun Young Lee, Young‐Hye You, Marie Rhee, Jong‐Min Kim, Seong‐Soo Hwang, Poong‐Yeon Lee
Journal of Diabetes Investigation.2024;[Epub] CrossRef
- Dual-targeted nano-encapsulation of neonatal porcine islet-like cell clusters with triiodothyronine-loaded bifunctional polymersomes
- Basic Research
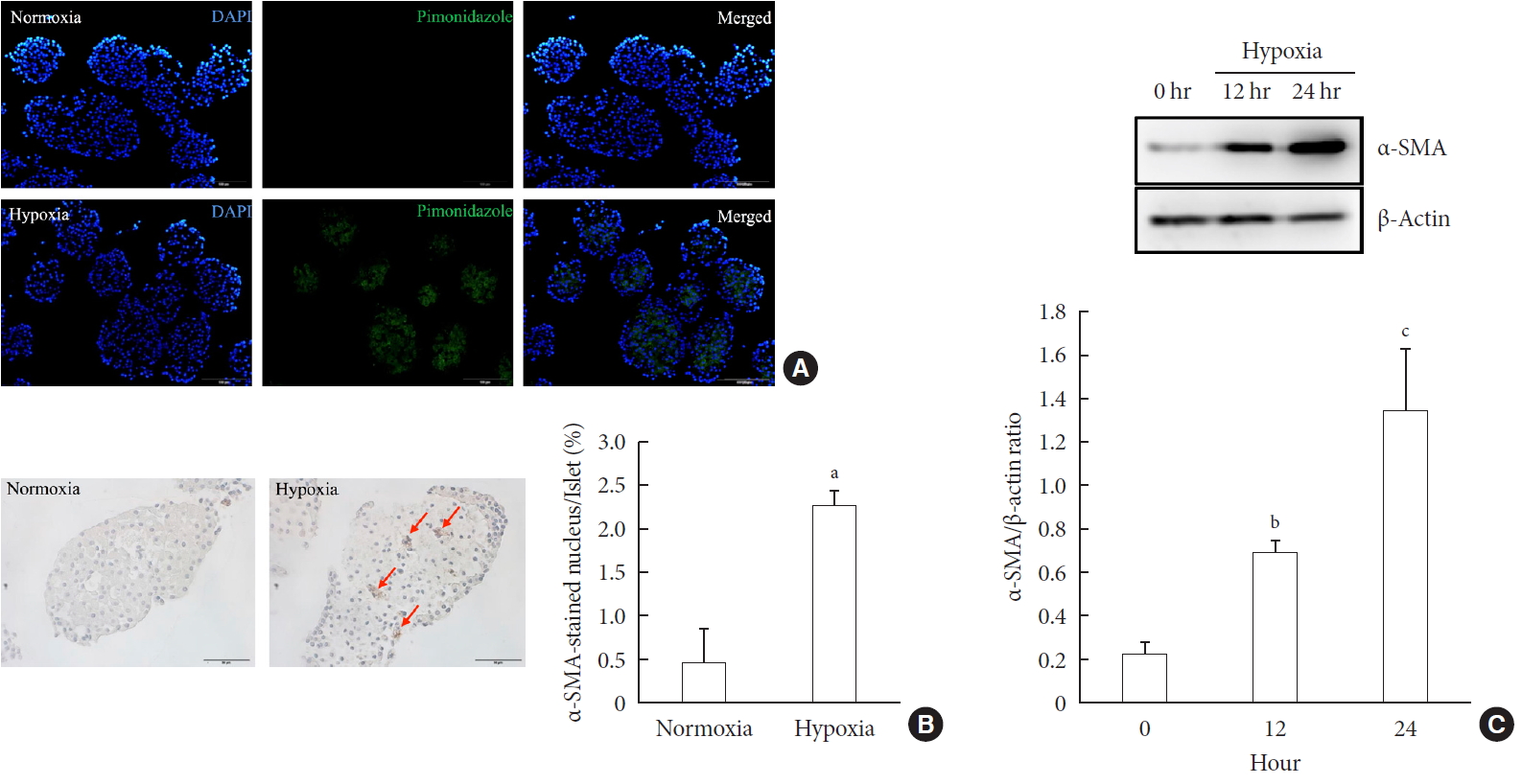
- Hypoxia Increases β-Cell Death by Activating Pancreatic Stellate Cells within the Islet
- Jong Jin Kim, Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
- Diabetes Metab J. 2020;44(6):919-927. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0181
- 5,932 View
- 146 Download
- 15 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Hypoxia can occur in pancreatic islets in type 2 diabetes mellitus. Pancreatic stellate cells (PSCs) are activated during hypoxia. Here we aimed to investigate whether PSCs within the islet are also activated in hypoxia, causing β-cell injury.
Methods Islet and primary PSCs were isolated from Sprague Dawley rats, and cultured in normoxia (21% O2) or hypoxia (1% O2). The expression of α-smooth muscle actin (α-SMA), as measured by immunostaining and Western blotting, was used as a marker of PSC activation. Conditioned media (hypoxia-CM) were obtained from PSCs cultured in hypoxia.
Results Islets and PSCs cultured in hypoxia exhibited higher expressions of α-SMA than did those cultured in normoxia. Hypoxia increased the production of reactive oxygen species. The addition of N-acetyl-L-cysteine, an antioxidant, attenuated the hypoxia-induced PSC activation in islets and PSCs. Islets cultured in hypoxia-CM showed a decrease in cell viability and an increase in apoptosis.
Conclusion PSCs within the islet are activated in hypoxia through oxidative stress and promote islet cell death, suggesting that hypoxia-induced PSC activation may contribute to β-cell loss in type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- Effects of hypoxia in the diabetic corneal stroma microenvironment
Purnima Sharma, Jian-Xing Ma, Dimitrios Karamichos
Experimental Eye Research.2024; 240: 109790. CrossRef - Visualizing hypoxic modulation of beta cell secretions via a sensor augmented oxygen gradient
Kai Duan, Mengyang Zhou, Yong Wang, Jose Oberholzer, Joe F. Lo
Microsystems & Nanoengineering.2023;[Epub] CrossRef - Pancreatic stellate cells promote pancreatic β-cell death through exosomal microRNA transfer in hypoxia
Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
Molecular and Cellular Endocrinology.2023; 572: 111947. CrossRef - Pancreatic stellate cells in pancreatic cancer: as potential targets for future therapy
Zhengfeng Wang, Ru He, Shi Dong, Wence Zhou
Frontiers in Oncology.2023;[Epub] CrossRef - Recent advances in the development of bioartificial pancreas using 3D bioprinting for the treatment of type 1 diabetes: a review
Anushikha Ghosh, Arka Sanyal, Abhik Mallick
Exploration of Medicine.2023; : 886. CrossRef - Pancreas and islet morphology in cystic fibrosis: clues to the etiology of cystic fibrosis-related diabetes
Sarah S. Malik, Diksha Padmanabhan, Rebecca L. Hull-Meichle
Frontiers in Endocrinology.2023;[Epub] CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - HIF-1 and NRF2; Key Molecules for Malignant Phenotypes of Pancreatic Cancer
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Cancers.2022; 14(2): 411. CrossRef - Pancreatic Stellate Cells and Metabolic Alteration: Physiology and Pathophysiology
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Frontiers in Physiology.2022;[Epub] CrossRef - Exosomal miR-140–3p and miR-143–3p from TGF-β1-treated pancreatic stellate cells target BCL2 mRNA to increase β-cell apoptosis
Xiangyun Zhu, Dechen Liu, Guoqing Li, Mengmeng Zhi, Ji Sun, Liang Qi, Jingbo Li, Stephen J. Pandol, Ling Li
Molecular and Cellular Endocrinology.2022; 551: 111653. CrossRef - Mitochondria oxidative stress mediated nicotine-promoted activation of pancreatic stellate cells by regulating mitochondrial dynamics
Yue Yuan, Zhiren Li, Miaomiao Li, Tong Jin, Xiaoyun Zhang, Xinjuan Liu, Jianyu Hao
Toxicology in Vitro.2022; 84: 105436. CrossRef - Antioxidant Mitoquinone Alleviates Chronic Pancreatitis via Anti-Fibrotic and Antioxidant Effects
Miaomiao Li, Yue Yuan, Xue Han, Xinjuan Liu, Weizhen Zhang, Jianyu Hao
Journal of Inflammation Research.2022; Volume 15: 4409. CrossRef - Diabetic Ferroptosis and Pancreatic Cancer: Foe or Friend?
Le Li, Xing-jia Yu, Lei Gao, Long Cheng, Bei Sun, Gang Wang
Antioxidants & Redox Signaling.2022; 37(16-18): 1206. CrossRef - Melatonin Induces Apoptosis and Modulates Cyclin Expression and MAPK Phosphorylation in Pancreatic Stellate Cells Subjected to Hypoxia
Matias Estaras, Manuel R. Gonzalez-Portillo, Miguel Fernandez-Bermejo, Jose M. Mateos, Daniel Vara, Gerardo Blanco-Fernandez, Diego Lopez-Guerra, Vicente Roncero, Gines M. Salido, Antonio González
International Journal of Molecular Sciences.2021; 22(11): 5555. CrossRef - Integrated pancreatic microcirculatory profiles of streptozotocin‐induced and insulin‐administrated type 1 diabetes mellitus
Yuan Li, Bingwei Li, Bing Wang, Mingming Liu, Xiaoyan Zhang, Ailing Li, Jian Zhang, Honggang Zhang, Ruijuan Xiu
Microcirculation.2021;[Epub] CrossRef - Pancreatic stellate cells - rising stars in pancreatic pathologies
P Hrabák, M Kalousová, T Krechler, T Zima
Physiological Research.2021; (S4): S597. CrossRef
- Effects of hypoxia in the diabetic corneal stroma microenvironment
- Basic Research
- A Novel Pancreatic Imaging Window for Stabilized Longitudinal
In Vivo Observation of Pancreatic Islets in Murine Model - Inwon Park, Sujung Hong, Yoonha Hwang, Pilhan Kim
- Diabetes Metab J. 2020;44(1):193-198. Published online May 29, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0268
- 4,677 View
- 137 Download
- 13 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Longitudinal imaging of murine pancreas is technically challenging due to the mechanical softness of the tissue influenced by peristalsis. Here, we report a novel pancreatic imaging window for long-term stabilized cellular-level observation of the islets in the pancreas
in vivo . By spatially separating the pancreas from the bowel movement and physiologic respiration with a metal plate integrated in the imaging window, we successfully tracked the pancreatic islets up to three weeks and visualized the dumbbell-shape transformation from the single islet. This window can be a useful tool for long-term cellular-level visualization of the microstructure in the pancreas.-
Citations
Citations to this article as recorded by-
Apollo-NADP
+
reveals in vivo adaptation of NADPH/NADP
+
metabolism in electrically activated pancreatic β cells
Cindy V. Bui, Curtis W. Boswell, Brian Ciruna, Jonathan V. Rocheleau
Science Advances.2023;[Epub] CrossRef - Intravital imaging of the functions of immune cells in the tumor microenvironment during immunotherapy
Xuwen Peng, Yuke Wang, Jie Zhang, Zhihong Zhang, Shuhong Qi
Frontiers in Immunology.2023;[Epub] CrossRef - Minimizing Motion Artifacts in Intravital Microscopy Using the Sedative Effect of Dexmedetomidine
Youngkyu Kim, Minju Cho, Bjorn Paulson, Sung-Hoon Kim, Jun Ki Kim
Microscopy and Microanalysis.2022; 28(5): 1679. CrossRef - SWIP—a stabilized window for intravital imaging of the murine pancreas
Wei Du, Christian Adkisson, Xianjun Ye, Camille L. Duran, Benson Chellakkan Selvanesan, Claudia Gravekamp, Maja H. Oktay, John C. McAuliffe, John S. Condeelis, Nicole C. Panarelli, Robert J. Norgard, Yogev Sela, Ben Z. Stanger, David Entenberg
Open Biology.2022;[Epub] CrossRef - Intravital longitudinal cellular visualization of oral mucosa in a murine model based on rotatory side-view confocal endomicroscopy
Sujung Hong, Jingu Lee, Jieun Moon, Eunji Kong, Jehwi Jeon, Yeon soo Kim, Hyung-Ryong Kim, Pilhan Kim
Biomedical Optics Express.2022; 13(8): 4160. CrossRef - Improved in vivo imaging method for individual islets across the mouse pancreas reveals a heterogeneous insulin secretion response to glucose
Henriette Frikke-Schmidt, Peter Arvan, Randy J. Seeley, Corentin Cras-Méneur
Scientific Reports.2021;[Epub] CrossRef - The frontier of live tissue imaging across space and time
Qiang Huang, Aliesha Garrett, Shree Bose, Stephanie Blocker, Anne C. Rios, Hans Clevers, Xiling Shen
Cell Stem Cell.2021; 28(4): 603. CrossRef - Intravital Laser-scanning Two-photon and Confocal Microscopy for Biomedical Research
Jieun Moon, Pilhan Kim
Medical Lasers.2021; 10(1): 1. CrossRef - The Eye as a Transplantation Site to Monitor Pancreatic Islet Cell Plasticity
Erwin Ilegems, Per-Olof Berggren
Frontiers in Endocrinology.2021;[Epub] CrossRef - Longitudinal Intravital Imaging of Tumor-Infiltrating Lymphocyte Motility in Breast Cancer Models
Inwon Park, Sujung Hong, Joon Seok, Stephani Edwina Lucia, Eunjoo Song, Mingyo Kim, Eunji Kong, Howon Seo, Yoonha Hwang, Soyeon Ahn, Seonghye Kim, Dong-Hyun Jang, Jae Hyuk Lee, Su-Hyung Park, Pilhan Kim, You Hwan Jo
Journal of Breast Cancer.2021; 24(5): 463. CrossRef - Intravital longitudinal imaging of hepatic lipid droplet accumulation in a murine model for nonalcoholic fatty liver disease
Jieun Moon, Eunji Kong, Jingu Lee, Jinjoo Jung, Eunha Kim, Seung Bum Park, Pilhan Kim
Biomedical Optics Express.2020; 11(9): 5132. CrossRef
-
Apollo-NADP
+
reveals in vivo adaptation of NADPH/NADP
+
metabolism in electrically activated pancreatic β cells
- Pathophysiology
-
- Essential Role of Protein Arginine Methyltransferase 1 in Pancreas Development by Regulating Protein Stability of Neurogenin 3
- Kanghoon Lee, Hyunki Kim, Joonyub Lee, Chang-Myung Oh, Heein Song, Hyeongseok Kim, Seung-Hoi Koo, Junguee Lee, Ajin Lim, Hail Kim
- Diabetes Metab J. 2019;43(5):649-658. Published online April 8, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0232
- 5,192 View
- 70 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Protein arginine methyltransferase 1 (PRMT1) is a major enzyme responsible for the formation of methylarginine in mammalian cells. Recent studies have revealed that PRMT1 plays important roles in the development of various tissues. However, its role in pancreas development has not yet been elucidated.
Methods Pancreatic progenitor cell-specific
Prmt1 knock-out (Prmt1 PKO) mice were generated and characterized for their metabolic and histological phenotypes and their levels ofNeurog3 gene expression and neurogenin 3 (NGN3) protein expression. Protein degradation assays were performed in mPAC cells.Results Prmt1 PKO mice showed growth retardation and a severely diabetic phenotype. The pancreatic size and β-cell mass were significantly reduced inPrmt1 PKO mice. Proliferation of progenitor cells during the secondary transition was decreased and endocrine cell differentiation was impaired. These defects in pancreas development could be attributed to the sustained expression of NGN3 in progenitor cells. Protein degradation assays in mPAC cells revealed that PRMT1 was required for the rapid degradation of NGN3.Conclusion PRMT1 critically contributes to pancreas development by destabilizing the NGN3 protein.
-
Citations
Citations to this article as recorded by- Arginine 65 methylation of Neurogenin 3 by PRMT1 is required for pancreatic endocrine development of hESCs
Gahyang Cho, Kwangbeom Hyun, Jieun Choi, Eunji Shin, Bumsoo Kim, Hail Kim, Jaehoon Kim, Yong-Mahn Han
Experimental & Molecular Medicine.2023; 55(7): 1506. CrossRef - Protein arginine methyltransferase 1 in the generation of immune megakaryocytes: A perspective review
Xinyang Zhao, Zechen Chong, Yabing Chen, X. Long Zheng, Qian-Fei Wang, Yueying Li
Journal of Biological Chemistry.2022; 298(11): 102517. CrossRef - Arginine 65 Methylation of Neurogenin 3 by PRMT1 Is Required for Pancreatic Endocrine Development of hESCs
Gahyang Cho, Kwangbeom Hyun, Jieun Choi, Eun Ji Shin, Bumsoo Kim, Hail Kim, Jaehoon Kim, Yong-Mahn Han
SSRN Electronic Journal .2022;[Epub] CrossRef - Protein Arginine Methyltransferase 1 Is Essential for the Meiosis of Male Germ Cells
Sahar Waseem, Sudeep Kumar, Kanghoon Lee, Byoung-Ha Yoon, Mirang Kim, Hail Kim, Keesook Lee
International Journal of Molecular Sciences.2021; 22(15): 7951. CrossRef - Proteome-Wide Alterations of Asymmetric Arginine Dimethylation Associated With Pancreatic Ductal Adenocarcinoma Pathogenesis
Meijin Wei, Chaochao Tan, Zhouqin Tang, Yingying Lian, Ying Huang, Yi Chen, Congwei Chen, Wen Zhou, Tao Cai, Jiliang Hu
Frontiers in Cell and Developmental Biology.2020;[Epub] CrossRef
- Arginine 65 methylation of Neurogenin 3 by PRMT1 is required for pancreatic endocrine development of hESCs
- Islet Studies and Transplantation
- Alginate-Catechol Cross-Linking Interferes with Insulin Secretion Capacity in Isolated Murine Islet Cells
- Yu-Sik Kim, Seung-Woo Cho, Bomin Ko, Jisoo Shin, Chul Woo Ahn
- Diabetes Metab J. 2018;42(2):164-168. Published online March 28, 2018
- DOI: https://doi.org/10.4093/dmj.2018.42.2.164
- 3,838 View
- 57 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Over the past three decades, human pancreatic islet isolation and transplantation techniques have developed as a routine clinical procedure for selected patients with type 1 diabetes mellitus. However, due to the donor shortage and required chronic systemic immunosuppression, the widespread application of islet transplantation is limited. To overcome these limitations, providing a physical barrier to transplanted islet cells with encapsulating biomaterial has emerged as a promising approach to enhance engraftment and promote islet survival post-transplantation. Alginate has been considered to be a reliable biomaterial, as it enhances islet survival and does not hamper hormone secretion. Alginate-catechol (Al-CA) hydrogel was reported to provide high mechanical strength and chemical stability without deformation over a wide range of pH values. In this study, we, demonstrated, for the first time in the literature, that encapsulation of murine pancreatic islet cells with Al-CA hydrogel does not induce cytotoxicity
ex vivo for an extended period; however, it does markedly abate glucose-stimulated insulin secretion. Catechol should not be considered as a constituent for alginate gelation for encapsulating islet cells in the application of islet transplantation.-
Citations
Citations to this article as recorded by- Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives
Chandan Maity, Nikita Das
Topics in Current Chemistry.2022;[Epub] CrossRef - Alginate: Enhancement Strategies for Advanced Applications
Alejandro Hurtado, Alaa A. A. Aljabali, Vijay Mishra, Murtaza M. Tambuwala, Ángel Serrano-Aroca
International Journal of Molecular Sciences.2022; 23(9): 4486. CrossRef - Alginate Functionalization for the Microencapsulation of Insulin Producing Cells
N. A. Len’shina, A. N. Konev, A. A. Baten’kin, P. S. Bardina, E. I. Cherkasova, A. V. Kashina, E. V. Zagainova, V. E. Zagainov, S. A. Chesnokov
Polymer Science, Series B.2021; 63(6): 640. CrossRef - Strategies to Functionalize the Anionic Biopolymer Na-Alginate without Restricting Its Polyelectrolyte Properties
Luca Szabó, Sandrine Gerber-Lemaire, Christine Wandrey
Polymers.2020; 12(4): 919. CrossRef - Catechol-Functionalized Alginate Nanoparticles as Mucoadhesive Carriers for Intravesical Chemotherapy
Nitjawan Sahatsapan, Tanasait Ngawhirunpat, Theerasak Rojanarata, Praneet Opanasopit, Prasopchai Patrojanasophon
AAPS PharmSciTech.2020;[Epub] CrossRef
- Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives
- Cell Replacement and Regeneration Therapy for Diabetes
- Hee-Sook Jun
- Korean Diabetes J. 2010;34(2):77-83. Published online April 30, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.2.77
- 2,268 View
- 26 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Reduction of beta cell function and a beta cell mass is observed in both type 1 and type 2 diabetes. Therefore, restoration of this deficiency might be a therapeutic option for treatment of diabetes. Islet transplantation has benefits, such as reduced incidence of hypoglycemia and achievement of insulin independence. However, the major drawback is an insufficient supply of islet donors. Transplantation of cells differentiated
in vitro orin vivo regeneration of insulin-producing cells are possible approaches for beta cell/islet regenerative therapy. Embryonic and adult stem cells, pancreatic ductal progenitor cells, acinar cells, and other endocrine cells have been shown to differentiate into pancreatic beta cells. Formation of fully functional beta cells and the safety of these cells are critical issues for successful clinical application.-
Citations
Citations to this article as recorded by- Direct Reprogramming of Mice Skin Fibroblasts into Insulin-Producing CellsIn Vitro
Israa S. Salman, Ahmed Majeed Al-Shammari, Mukhtar Khamis Haba
Cellular Reprogramming.2022; 24(5): 271. CrossRef - Effects of β-like cell autotransplantation through hepatic arterial intervention on diabetic dogs
Yongxu Mu, Zhiming Hao, Junfeng He, Ruiqiang Yan, Haiyan Liu, Lei Zhang, Heming Liu, Xiaoyan Hu, Qiming Li
Artificial Cells, Nanomedicine, and Biotechnology.2016; 44(5): 1333. CrossRef - Meeting the Need for Regenerative Therapies I: Target-Based Incidence and Its Relationship to U.S. Spending, Productivity, and Innovation
Nancy Parenteau, Janet Hardin-Young, William Shannon, Patrick Cantini, Alan Russell
Tissue Engineering Part B: Reviews.2012; 18(2): 139. CrossRef - Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation
Su-Jung Kim, Yong-Soo Choi, Eun-Sun Ko, Sang-Min Lim, Chang-Woo Lee, Dong-Il Kim
Journal of Bioscience and Bioengineering.2012; 113(6): 771. CrossRef
- Direct Reprogramming of Mice Skin Fibroblasts into Insulin-Producing CellsIn Vitro
- Effects of Anti-Vascular Endothelial Growth Factor (VEGF) on Pancreatic Islets in Mouse Model of Type 2 Diabetes Mellitus.
- Ji Won Kim, Dong Sik Ham, Heon Seok Park, Yu Bai Ahn, Ki Ho Song, Kun Ho Yoon, Ki Dong Yoo, Myung Jun Kim, In Kyung Jeong, Seung Hyun Ko
- Korean Diabetes J. 2009;33(3):185-197. Published online June 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.3.185
- 2,289 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Vascular endothelial growth factor (VEGF) is associated with the development of diabetic complications. However, it is unknown whether systemic VEGF treatment has any effects on the pancreatic islets in an animal model of type 2 diabetes mellitus. METHODS: Anti-VEGF peptide (synthetic ATWLPPR, VEGF receptor type 2 antagonist) was injected into db/db mice for 12 weeks. We analyzed pancreatic islet morphology and quantified beta-cell mass. Endothelial cell proliferation and the severity of islet fibrosis were also measured. VEGF expression in isolated islets was determined using Western blot analysis. RESULTS: When anti-VEGF was administered, db/db mice exhibited more severe hyperglycemia and associated delayed weight gain than non-treated db/db mice. Pancreas weight and pancreatic beta-cell mass were also significantly decreased in the anti-VEGF-treated group. VEGF and VEGF receptor proteins (types 1 and 2) were expressed in the pancreatic islets, and their expression was significantly increased in the db/db group compared with the db/dm group. However, the elevated VEGF expression was significantly reduced by anti-VEGF treatment compared with the db/db group. The anti-VEGF-treated group had more prominent islet fibrosis and islet destruction than db/db mice. Intra-islet endothelial cell proliferation was also remarkably reduced by the anti-VEGF peptide. CONCLUSION: Inhibition of VEGF action by the VEGF receptor 2 antagonist not only suppressed the proliferation of intra-islet endothelial cells but also accelerated pancreatic islet destruction and aggravated hyperglycemia in a type 2 diabetes mouse model. Therefore, the potential effects of anti-VEGF treatment on pancreatic beta cell damage should be considered.

 KDA
KDA
 First
First Prev
Prev





