
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
- Diabetes Metab J. 2024;48(2):231-241. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0366

- 1,826 View
- 171 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Administration of pancreatic endoplasmic reticulum kinase inhibitor (PERKi) improved insulin secretion and hyperglycemia in obese diabetic mice. In this study, autophagic balance was studied whether to mediate it.
Methods
Human islets were isolated from living patients without diabetes. PERKi GSK2606414 effects were evaluated in the islets under glucolipotoxicity by palmitate. Islet insulin contents and secretion were measured. Autophagic flux was assessed by microtubule associated protein 1 light chain 3 (LC3) conversion, a red fluorescent protein (RFP)-green fluorescent protein (GFP)- LC3 tandem assay, and P62 levels. For mechanical analyses, autophagy was suppressed using 3-methyladenine in mouse islets. Small interfering RNA for an autophagy-related gene autophagy related 7 (Atg7) was transfected to interfere autophagy.
Results
PERKi administration to mice decreased diabetes-induced P62 levels in the islets. Glucolipotoxicity significantly increased PERK phosphorylation by 70% and decreased insulin contents by 50% in human islets, and addition of PERKi (40 to 80 nM) recovered both. PERKi also enhanced glucose-stimulated insulin secretion (6-fold). PERKi up-regulated LC3 conversion suppressed by glucolipotoxicity, and down-regulated P62 contents without changes in P62 transcription, indicating enhanced autophagic flux. Increased autophagosome-lysosome fusion by PERKi was visualized in mouse islets, where PERKi enhanced ATG7 bound to LC3. Suppression of Atg7 eliminated PERKi-induced insulin contents and secretion.
Conclusion
This study provided functional changes of human islets with regard to autophagy under glucolipotoxicity, and suggested modulation of autophagy as an anti-diabetic mechanism of PERKi.
- Pathophysiology
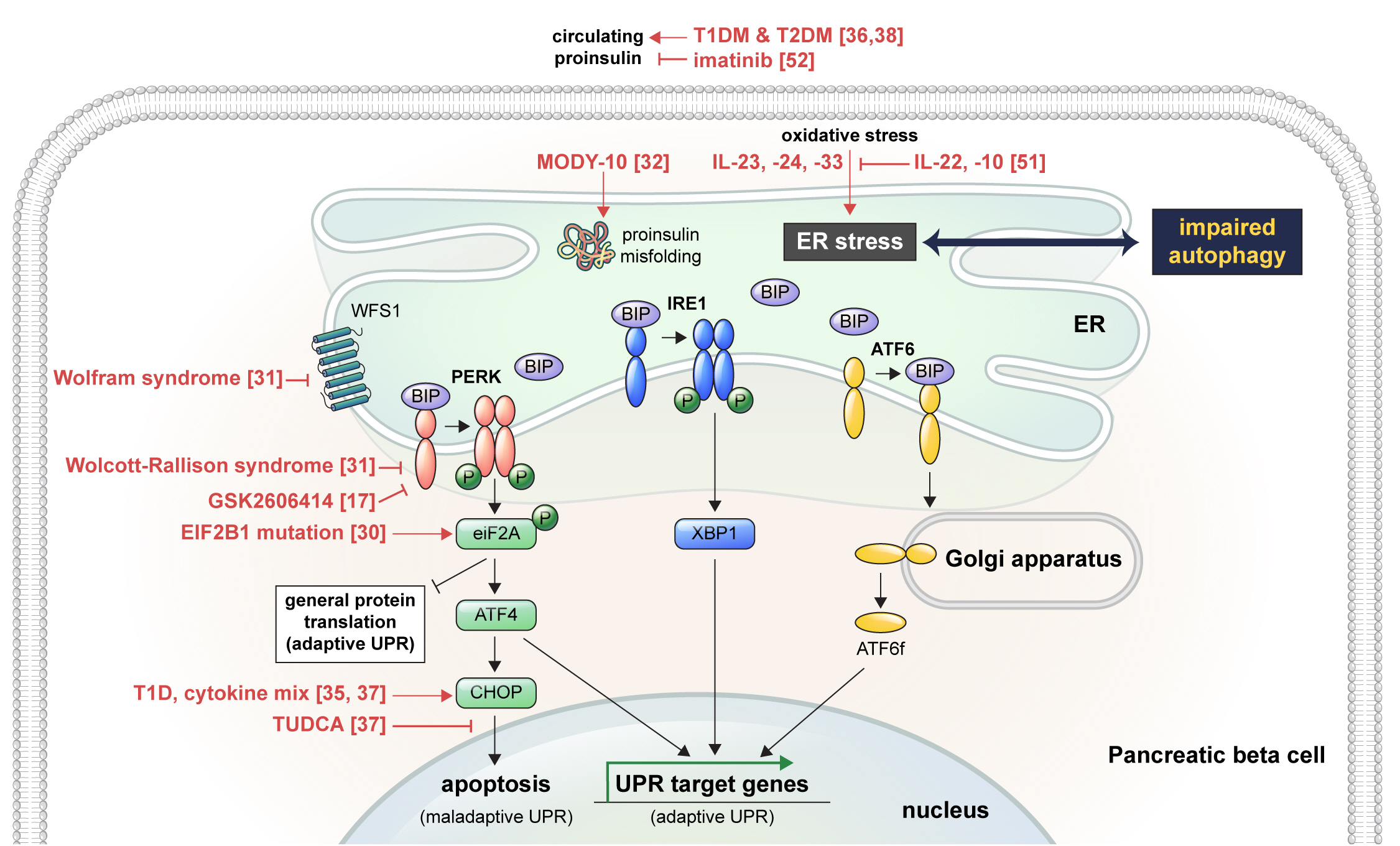
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Seoil Moon, Hye Seung Jung
- Diabetes Metab J. 2022;46(4):533-542. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0070
- 4,579 View
- 250 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Pancreatic beta cell homeostasis is crucial for the synthesis and secretion of insulin; disruption of homeostasis causes diabetes, and is a treatment target. Adaptation to endoplasmic reticulum (ER) stress through the unfolded protein response (UPR) and adequate regulation of autophagy, which are closely linked, play essential roles in this homeostasis. In diabetes, the UPR and autophagy are dysregulated, which leads to beta cell failure and death. Various studies have explored methods to preserve pancreatic beta cell function and mass by relieving ER stress and regulating autophagic activity. To promote clinical translation of these research results to potential therapeutics for diabetes, we summarize the current knowledge on ER stress and autophagy in human insulin-secreting cells.
-
Citations
Citations to this article as recorded by- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
Diabetes & Metabolism Journal.2024; 48(2): 231. CrossRef - Endoplasmic reticulum stress: A possible connection between intestinal inflammation and neurodegenerative disorders
Giorgio Vivacqua, Romina Mancinelli, Stefano Leone, Rosa Vaccaro, Ludovica Garro, Simone Carotti, Ludovica Ceci, Paolo Onori, Luigi Pannarale, Antonio Franchitto, Eugenio Gaudio, Arianna Casini
Neurogastroenterology & Motility.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Pancreatic islet remodeling in cotadutide-treated obese mice
Renata Spezani, Thatiany Souza Marinho, Luiz E. Macedo Cardoso, Marcia Barbosa Aguila, Carlos Alberto Mandarim-de-Lacerda
Life Sciences.2023; 327: 121858. CrossRef - Modulation of Unfolded Protein Response Restores Survival and Function of β-Cells Exposed to the Endocrine Disruptor Bisphenol A
Laura Maria Daian, Gabriela Tanko, Andrei Mircea Vacaru, Luiza Ghila, Simona Chera, Ana-Maria Vacaru
International Journal of Molecular Sciences.2023; 24(3): 2023. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Identification and analysis of type 2 diabetes-mellitus-associated autophagy-related genes
Kun Cui, Zhizheng Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between autophagy and insulin resistance: evidence from different tissues
Asie Sadeghi, Maryam Niknam, Mohammad Amin Momeni-Moghaddam, Maryam Shabani, Hamid Aria, Alireza Bastin, Maryam Teimouri, Reza Meshkani, Hamed Akbari
European Journal of Medical Research.2023;[Epub] CrossRef - Beta cell lipotoxicity in the development of type 2 diabetes: the need for species-specific understanding
Patricia Thomas, Meurig T. Gallagher, Gabriela Da Silva Xavier
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Association of Estrogen Receptor α Genes
Pvu II andXba I Polymorphisms with Type 2 Diabetes Mellitus in the Inpatient Population of a Hospital in Southern Iran - Farzaneh Mohammadi, Mohammad Pourahmadi, Mohadeseh Mosalanejad, Houshang Jamali, Mohamed Amin Ghobadifar, Saeideh Erfanian
- Diabetes Metab J. 2013;37(4):270-277. Published online August 14, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.4.270
- 3,429 View
- 36 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Estrogen plays a fundamental role in the pathogenesis of type 2 diabetes mellitus (T2DM). Very few studies have shown the association between estrogen receptor α (ERα),
Pvu II andXba I gene polymorphisms with T2DM in both men and women. We evaluated the hypothesis thatPvu II andXba I polymorphisms of ERα gene may be associated with T2DM in adult.Methods From spring of 2010 to the fall of 2011, a case-control study was performed at clinical centers of Jahrom University of Medical Sciences. We included 174 patients with T2DM including men and women and 174 age, sex, and body mass index frequency-matched health controls. We analyzed the
Pvu II andXba I polymorphisms of ERα by using the polymerase chain reaction-based restriction fragment length polymorphism method.Results No significant differences between demographic characteristics of control and patients groups were observed. Allele frequencies of both
Pvu II andXba I polymorphisms were significantly different between patients and control subjects (P =0.014 vs.P =0.002, respectively). When the group was separated into women and men, logistic regression analysis of genotype distribution ofPvu II (pp vs. Pp+PP) in both sexes revealed that there was no significant association ofPvu II genotype with men (odds ratio [OR], 1.67; confidence interval [CI], 0.86 to 3.28;P =0.89) and women (OR, 0.96; CI, 0.53 to 1.74;P =0.12).Conclusion Pvu II andXba I polymorphisms in ERα are related with T2DM in the inpatient population.-
Citations
Citations to this article as recorded by- Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population
Iman Akhlaghipour, Amir Reza Bina, Mohammad Reza Mogharrabi, Ali Fanoodi, Amir Reza Ebrahimian, Soroush Khojasteh Kaffash, Atefeh Babazadeh Baghan, Mohammad Erfan Khorashadizadeh, Negin Taghehchian, Meysam Moghbeli
Human Genomics.2022;[Epub] CrossRef - Estrogen receptor 1 gene polymorphisms (PvuII and XbaI) are associated with type 2 diabetes in Palestinian women
Suheir Ereqat, Stéphane Cauchi, Khaled Eweidat, Muawiyah Elqadi, Abedelmajeed Nasereddin
PeerJ.2019; 7: e7164. CrossRef - Association between Genetic Variants and Diabetes Mellitus in Iranian Populations: A Systematic Review of Observational Studies
Mehrnoosh Khodaeian, Samaneh Enayati, Ozra Tabatabaei-Malazy, Mahsa M. Amoli
Journal of Diabetes Research.2015; 2015: 1. CrossRef - Genetic polymorphism of estrogen receptor alpha gene in Egyptian women with type II diabetes mellitus
Tarek M.K. Motawi, Mahmoud A. El-Rehany, Sherine M. Rizk, Maggie M. Ramzy, Doaa M. el-Roby
Meta Gene.2015; 6: 36. CrossRef
- Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population

 KDA
KDA
 First
First Prev
Prev





