
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 37(4); 2013 > Article
-
Original ArticleGenetics Association of Estrogen Receptor α Genes
Pvu II andXba I Polymorphisms with Type 2 Diabetes Mellitus in the Inpatient Population of a Hospital in Southern Iran - Farzaneh Mohammadi1, Mohammad Pourahmadi2, Mohadeseh Mosalanejad1, Houshang Jamali1, Mohamed Amin Ghobadifar3, Saeideh Erfanian4
-
Diabetes & Metabolism Journal 2013;37(4):270-277.
DOI: https://doi.org/10.4093/dmj.2013.37.4.270
Published online: August 14, 2013
1Department of Biology, Jahrom Branch, Islamic Azad University, Jahrom, Iran.
2Department of Anatomy, Jahrom University of Medical Sciences, Jahrom, Iran.
3Student Research Committee, Jahrom University of Medical Sciences, Jahrom, Iran.
4Research Laboratory, Jahrom University of Medical Sciences, Jahrom, Iran.
- Corresponding author: Saeideh Erfanian. Research Laboratory, Jahrom University of Medical Sciences, Motahari Ave, Jahrom, Iran. erfanian_85@yahoo.com
Copyright © 2013 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Estrogen plays a fundamental role in the pathogenesis of type 2 diabetes mellitus (T2DM). Very few studies have shown the association between estrogen receptor α (ERα), PvuII and XbaI gene polymorphisms with T2DM in both men and women. We evaluated the hypothesis that PvuII and XbaI polymorphisms of ERα gene may be associated with T2DM in adult.
-
Methods
- From spring of 2010 to the fall of 2011, a case-control study was performed at clinical centers of Jahrom University of Medical Sciences. We included 174 patients with T2DM including men and women and 174 age, sex, and body mass index frequency-matched health controls. We analyzed the PvuII and XbaI polymorphisms of ERα by using the polymerase chain reaction-based restriction fragment length polymorphism method.
-
Results
- No significant differences between demographic characteristics of control and patients groups were observed. Allele frequencies of both PvuII and XbaI polymorphisms were significantly different between patients and control subjects (P=0.014 vs. P=0.002, respectively). When the group was separated into women and men, logistic regression analysis of genotype distribution of PvuII (pp vs. Pp+PP) in both sexes revealed that there was no significant association of PvuII genotype with men (odds ratio [OR], 1.67; confidence interval [CI], 0.86 to 3.28; P=0.89) and women (OR, 0.96; CI, 0.53 to 1.74; P=0.12).
-
Conclusion
- PvuII and XbaI polymorphisms in ERα are related with T2DM in the inpatient population.
- Type 2 diabetes mellitus (T2DM) is a public health problem in the world with a high prevalence which is the most noticeable disease in developing countries [1]. T2DM is characterized formally by insulin resistance or insulin deficiency in patients with high blood glucose [2]. T2DM is a multifactorial disease such that both environmental and genetic factors lead to its pathogenesis [3]. Hence, it is essential to recognize the population with genetic inclination and protect them from exposure to environmental risks.
- Sex hormones may act as an important role in patients with diabetes mellitus. Different studies showed that estrogen can inhibit the deduction of insulin dependent diabetes, modulates insulin secretion, regulates calcium signals through plasma membrane receptors, and regulates K-ATP channel activity [4,5]. Estrogen shows its physiological effect via the estrogen receptors (ERs) which might prevent osteoporosis, menopause syndrome, diabetes, arteriosclerosis, etc. [6-8]. There are two main forms of ER including ERα and ERβ. ERα gene is located on chromosome 6q25.1 whereas ERβ gene is located on chromosome 14q22-24 [9]. ERα gene encompasses 140 kb of DNA including eight exons, encodes a protein of 595 amino acids and its molecular weight is about 66 kDa. The first intron of the gene, like a promoter, usually involves a larger number of regulatory sequences than other introns do. Several single nucleotide polymorphism (SNPs) or sequence variations have been identified in the ERα gene and its association with increased or decreased frequencies of various diseases has been shown in several cases. Estrogen-related receptor has been proposed to modulate estrogen signaling, and the proposal has recently been reviewed from a metabolic perspective in this journal [10]. Thus, the gene encoding ERα gene is a potential candidate gene for susceptibility to T2DM. The PvuII and XbaI restriction fragment length polymorphisms (RFLPs) are common markers for genetic analysis of the ERα that are located in intron 1 of the ERα [7,9].
- An association has been found among the PvuII and XbaI with different pathological conditions, including prostate and breast cancer, cardiovascular disorders, severe preeclampsia, and osteoporosis [11-15]. PvuII and XbaI may affect the alteration of protein expression that results from splicing of ER mRNA. Results are still conflicting and the transcriptional regulation of ERα is as yet unclear. Nevertheless, only a small number of regulatory regions have been characterized as well [16]. Possible practical mechanisms referred to PvuII and XbaI polymorphisms include a change of ERα gene expression by influencing on alternative splicing of ERα gene and altering binding of transcription factors. In the last few years, ERα polymorphisms attracted huge interest and the PvuII and XbaI polymorphisms are the most widely investigated issues. Nevertheless, it is still unknown how the polymorphisms of ERα gene may act as genetic markers of diabetes. Since the evidence regarding this issue is scarce, we performed the present study to find the association between PvuII and XbaI polymorphisms and T2DM in the inpatient population of a hospital in southern Iran.
INTRODUCTION
- Study design
- The target subjects of present based descriptive cross-sectional survey consisted of women and men aged 35 to 65 years residing in Jahrom, Iran between the spring of 2010 and the fall of 2011. Of them, 174 adults with T2DM were compared with 174 nondiabetic ones as controls. Control subjects were frequency matched to patients by sex, age, and body mass index (BMI). All of case subjects were selected from patients who admitted to clinical centers in Jahrom, and control subjects were randomly selected from the healthy patients of our parent study [17]. We included the healthy subjects in the analyses of present study who were all normal fasting glucose and normal glucose tolerance. Randomization was done by a computer-based random digit generator. None of the patients and control subjects received hormone replacement therapy, had history of a sex hormone dependent disease, and had significant renal dysfunction and liver damage. All of the participants consented to donate biological specimens for present study. Patients were diagnosed as T2DM according to the World Health Organization criteria 1999 which is adopted by the American Diabetes Association 1997 standard. They were treated by drugs based on National Institute for Health and Clinical Excellence guideline 87. The study protocol was approved by the Ethics Committee of Jahrom University of Medical Sciences and all of the participants gave their written informed consent.
- Biochemical analysis
- Body height and weight were measured in participants, then the measurements were used to calculate BMI. After 12-hour fasting, blood samples were selected from the patients. For diagnosis of T2DM, a standard oral glucose tolerance test was performed (fasting blood glucose [FBG] ≥7.0 mmol/L). Individuals whose FBG was lower than 5.6 mmol/L (less than 7.8 mmol/L 2 hours after the test) were put in the controls. Others with 7.0 mmol/L>FBG≥5.6 mmol/L (7.9 to 11 mmol/L 2 hours after the test) were excluded. Serum levels of high density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglyceride (TG) were determined by using standard methods of commercial kits (Pars Azmon, Tehran, Iran). Low density lipoprotein cholesterol (LDL-C) was calculated based on to the Friedewald formula. The lipid and glucose data of patients were not analyzed in present study, because most of patients were in treatment by drugs.
- Genotyping
- All of the participants were genotyped for the PvuII and XbaI polymorphisms which are also known as T/C, rs2234693, and A/G, rs9340799, respectively. Polymerase chain reaction (PCR)-based RFLP assays were used to analyze the presence of PvuII and XbaI polymorphisms within ERα gene. A 1.37 kb DNA fragment which contains the two polymorphic sites was amplified by using forward, 5'-CTG CCA CCC TAT CTG TAT CTT TTC CTA TTC TCC-3', and reverse, 5'-TCT TTC TCT GCC ACC CTG GCG TCG ATT ATC TGA-3' primers. PCR amplification was carried out in a 20 µL reaction mixture with each primer as the following steps: an initial denaturation step at 95℃ for 5 minutes; followed by 40 cycles of 95℃ for 1 minute, 62℃ for 1 minute, and 72℃ for 1 minute; and finally elongation at 72℃ for 10 minutes. The PCR products were completely digested with the restriction endonucleases PvuII and XbaI separately for 16 hours at 37℃. Digested products electrophoresed in a 2% agarose gel which was stained with ethidium bromide. Genotype of heterozugous Pp and Xx exhibited fragments 1,300,850, and 450 bp lengths, while the XbaI is approximately 50 bp away from PvuII polymorphism site. Capital P or X and lower-case p or x represent the absence of restriction site and the presence of the restriction site, respectively.
- Statistical analysis
- Results are reported as mean±standard deviation (SD) or median for quantitative variables, and percentages for categorical variables. A 2-sided of P values of 0.05 or less was considered statistically significant. All the statistical analyses were performed using SPSS version 14.0 for Windows (SPSS Inc., Chicago, IL, USA). The groups were compared using the Student t-test and the chi-square test (or Fisher exact test if required) for continuous and categorical variables, respectively. Allele frequencies were calculated for each genotype by allele counting. With the observed number of events/nonevents or cases/noncases and the assumption of 2-sided values of P values of 0.05 or less, we calculated the hazard ratio/odds ratio (OR) which could be detected in present studies at 90% power. Logistic regression analysis was done to indentify the association of risk factors including age and sex with genotypes differences in the studied groups.
METHODS
- One hundred and seventy-four patients with T2DM and 174 controls without diabetes were genotyped for the PvuII and XbaI gene polymorphisms in ERα. All of participants were aged 35 to 65 years. Although most of women were between the ages of 40 and 65, but all of them were in premenopause. No significant differences between demographic characteristics of control and patients groups were observed. Seventy-nine men (45.40%) had T2DM and 82 men (47.12%) were in control group. The average age of the participants in the present study was 55.82±10.6. The clinical data and baseline characteristics of participants are shown in Table 1.
- PvuII and XbaI polymorphisms were detected in the study. The frequencies of PP, Pp, pp, XX, Xx, and xx genotypes and alleles in control subjects and the patients were investigated. Both frequencies of p and x alleles were higher than P and X ones. Allele frequencies of both PvuII and XbaI polymorphisms were significantly different between patients and control subjects (P=0.014 vs. P=0.002, respectively), so Table 2 showed that PvuII and XbaI variants were associated to the T2DM. Logistic regression analysis of genotype distribution of PvuII (pp vs. Pp+PP) in both sexes revealed that there was no significantly association in men (P=0.89) and women (P=0.12). When the sex factor was controlled by logistic regression, analysis of genotype distribution of PvuII (pp vs. Pp+PP) found no significant differences in 35 to 44 years (OR, 0.95; 95% confidence interval [CI], 0.48 to 1.85; P=0.88), 45 to 54 years (OR, 1.1; 95% CI, 0.52 to 2.32; P=0.81), and 55 to 65 years age group (OR, 2.32; 95% CI, 0.88 to 6.07; P=0.08), but OR of gene polymorphism ascend with aging (Table 3).
- Logistic regressions showed that there was no significant association between men and women in genotype distribution of XbaI (P=0.32 vs. P=0.39). Moreover, after adjusting the sex factor by logistic regression analysis, data showed no significant differences in 35 to 44 years (OR, 1.17; 95% CI, 0.60 to 2.25; P=0.63), 45 to 54 years (OR, 1.33; 95% CI, 0.63 to 2.81; P=0.45), and 55 to 65 years age group (OR, 1.45; 95% CI, 0.55 to 3.80; P=0.44) in genotype distribution of XbaI (Table 4).
- Analysis of the association between gene polymorphisms and clinical characteristics in healthy group showed that PvuII and XbaI genotypes were significantly related to high level of FBS (PvuII: OR, 2.7, 95% CI, 1.05 to 6.95, P=0.03; XbaI: OR, 2.51, 95% CI, 1.03 to 6.15, P=0.04) in women and (PvuII: OR, 2.99, 95% CI, 1.31 to 6.80, P=0.008; XbaI: OR, 2.66, 95% CI, 1.20 to 5.90, P=0.01) in 55 to 65 years age group with PP, Pp, XX, and Xx genotypes than in those with pp and xx genotypes. After adjusting age or sex by logistic regression analysis in control group, data showed that PvuII genotype (PP vs. Pp) was significantly related to high level of TC and TG (TC: OR, 3.11, 95% CI, 1.37 to 7.07, P=0.005; TG: OR, 3.07, 95% CI, 1.34 to 7.05, P=0.007) in women and (TC: OR, 2.91, 95% CI, 1.35 to 6.25, P=0.005; TG: OR, 3.32, 95% CI, 1.51 to 7.31, P=0.002) in 55 to 65 years age group (Table 5). Moreover, logistic regression analysis showed that XbaI genotype (XX vs. Xx) was significantly related to high level of TC and TG (TC: OR, 3.07, 95% CI, 1.44 to 6.54, P=0.003; TG: OR, 2.61, 95% CI, 1.25 to 5.45, P=0.009) in women (Table 6).
RESULTS
- T2DM, which is caused by both acquired and genetic abnormalities, is a multifactorial disease that affects insulin secretion and insulin sensitivity. Hallmarks of T2DM are insulin resistance in liver, skeletal muscle and fat, combined with insulin insufficiency owing to a slump in β-cell function [18]. Recognition of the susceptibility genes for T2DM may come to initial prevention of disease. Genetic changes have an additive and partial effect on T2DM. Moreover, environmental factors play a fundamental role in facilitating or reprieving the expression of the disease.
- The distribution of PvuII and XbaI polymorphisms in our study showed an increased ratio of Pp to pp and that of Xx to xx genotypes and a reduced frequency of PP and XX genotypes of patients in comparison to that of Asian and Caucasian populations of European ancestry, even though African population showed a lower frequency of pp and xx genotypes [11,18-21]. As a matter of fact, linkage disequilibrium differential degree among different racial populations may explain previous contradiction among ERα gene polymorphism studies [22]. These frequencies of genotypes may be due to incomplete disequilibrium and may result from multiple mutations and recombination that have occurred at or between polymorphic sites.
- Sex steroids apparently have a considerable role in insulin resistance risk. Role of estradiol in adjusting energy metabolism has been reported by recent studies, which revealed new evidences about the important role of ERs [23]. ERs seem to play a role in the occurrence or prevention of T2DM. ERα is a member of the nuclear hormone receptor superfamily and regulates the transcription of target genes in response to estrogen [24]. In the present study, there was an association between PvuII and XbaI polymorphisms in ERα and T2DM. There are few studies on the relationship of PvuII and XbaI polymorphisms with diabetes. Our study reports a significant association between both ERα gene polymorphisms (PvuII and XbaI) and T2DM in both men and women subjects of the inpatient population. A gender specific association has been reported from Hungrian and Chinease populations [5,23]. A study of 100 Iranian with T2DM and control group showed no significant association between PvuII and XbaI polymorphisms with diabetes in women [8]. In a study of Swedish population, no significant association was found between PvuII and XbaI polymorphisms and T2DM [25]. Our finding did not correlate with these studies, while a study in China supports our study in which Huang et al. [20] reported that PvuII gene polymorphism in ERα is associated with T2DM. However, in contrast to our data, they did not find any association between XbaI polymorphisms and T2DM. The exact reasons behind these discrepancies are not entirely clear; recruitment procedures and differences in environmental background and/or genetics may have played a role.
- PvuII and XbaI genes polymorphisms are possible markers for several diseases. These genotypic variations have been implicated in development of multitudinous diseases, among which are osteoporosis, cancers, cardiovascular diseases, neurodegenerative diseases, and lupus erythematosus [11,12,26]. Unfortunately, how these genetic polymorphisms influence receptor activity is still unknown. It has been suggested that a binding site produced by C allele of PvuII is for B-myb transcription factor. Estrogen induces B-myb's expression which can accrue transcription the construct 10-fold of a downstream reporter [27]. It is concluded that C allele might produce ERα isoforms or amplify the ERα transcription. A to G transition of XbaI may also have an effect on expression and alteration of the ERα.
- Huang et al. [20] confirmed that PvuII and XbaI polymorphisms were more frequent in female with T2DM, but our data showed no significant differences of PvuII and XbaI polymorphisms distribution between female and male. The reason for this discrepancy can be ascribed to several factors such as sample size, the study design, gene-environment interactions, and population heterogeneity. After logistic regression analysis, OR showed that PvuII and XbaI polymorphisms in ERα increased with the aging; although it could be because small sample size in our study rendered this association statistically insignificant. FBS is an important factor to judge the T2DM, so data from healthy group suggest the possibility that both PvuII and XbaI polymorphisms in ERα is significantly related to the development of female with T2DM, and improved with increasing age. It suggests that PvuII and XbaI polymorphisms in ERα might be a risk factor for T2DM in women. Nevertheless, because the data is from the control group, the results can be associated with the metabolic syndrome, not with T2DM, thus it is a logic jump to assert that the polymorphisms are related to T2DM. On the contrary, these finding shows that difference in the locations of the two SNPs does not influence in the roles. However, further studies are needed to confirm these finding.
- An association between obesity, hypertriglyceridemia, and T2DM has been detected since the early 1960s [28,29]. It is clear that estrogen affects the lipoprotein metabolism in many potential beneficial ways. Estrogen increases the rates of all lipoprotein fractions formation so it leads to an increase in HDL-C, a decrease in LDL-C, and an increase in the mRNA for LDL receptor [6]. Our data revealed that PvuII polymorphisms are related to high serum lipids of TC and TG, that is high serum lipid can increase the risk of T2DM in healthy group. Several other studies including a study of Italians indicating that PvuII polymorphisms are strongly associated with familial hypercholesterolemia, confirmed our finding [30-33].
- The present study had some advantages and limitations. The first advantage of this study is that our studied population was collection of a homogeneous sample well characterized controls and cases that increases the sensitivity of detecting the associations. The second advantage of this study is that we do not dichotomize continuous variables data which gives an additional impact on exactness. We acknowledge that the number of sample size might be small in the population and this doesn't allow drawing any definitive conclusions. Therefore, a larger population is required to establish a definitive role for these variations in the inpatient population.
- In conclusion, findings of present study suggest the possibility that PvuII and XbaI polymorphisms in ERα are related with T2DM in inpatient men and women population. It would be invaluable to conduct studies in other heterogeneous populations in order to check the replication validity of present findings. It is suggested that future studies focus on the role of ERα in the progression and development of different diseases which may help to identify therapeutic or diagnostic markers.
DISCUSSION
-
Acknowledgements
- The authors acknowledge the authorities of Jahrom University of Medical Sciences for financial support.
ACKNOWLEDGMENTS
- 1. Morita T, Tabata S, Mineshita M, Mizoue T, Moore MA, Kono S. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces health study. Asian Pac J Cancer Prev 2005;6:485-489. PubMed
- 2. Khan RM, Ch TS, Ahmad M, Pash MM. Assessment of awareness about diabetes mellitus among adult populace of lahore: a preventive approach required to combat the disease in Pakistan. Pak J Med Health Sci 2009;3:121-124.
- 3. Rodriguez-Gutierrez R, Gonzalez-Saldivar G, Gonzalez-Gonzalez JG. Diagnosis of diabetes. N Engl J Med 2013;368:192-193. Article
- 4. Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, Wild RA, Wang W, Lee ET. The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in american Indian postmenopausal women: the strong heart study. Diabetes Care 2002;25:500-504. PubMed
- 5. Speer G, Cseh K, Winkler G, Vargha P, Braun E, Takacs I, Lakatos P. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. Eur J Endocrinol 2001;144:385-389. ArticlePubMed
- 6. Di Croce L, Bruscalupi G, Trentalance A. Independent behavior of rat liver LDL receptor and HMGCoA reductase under estrogen treatment. Biochem Biophys Res Commun 1996;224:345-350. ArticlePubMed
- 7. Yaich L, Dupont WD, Cavener DR, Parl FF. Analysis of the PvuII restriction fragment-length polymorphism and exon structure of the estrogen receptor gene in breast cancer and peripheral blood. Cancer Res 1992;52:77-83. PubMed
- 8. Golkhu S, Ghaedi M, Taghvaie NM, Boroumand MA, Davoodi G, Aminzadegan A, Poorgoli L, Fathollahi MS. Genetic polymorphisms of estrogen receptors in Iranian women with diabetes and coronary artery disease. Iran J Med Sci 2009;34:208-212.
- 9. Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997;138:863-870. ArticlePubMed
- 10. Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab J 2012;36:328-335. ArticlePubMedPMC
- 11. Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 1999;8:843-854. PubMed
- 12. Shearman AM, Cooper JA, Kotwinski PJ, Miller GJ, Humphries SE, Ardlie KG, Jordan B, Irenze K, Lunetta KL, Schuit SC, Uitterlinden AG, Pols HA, Demissie S, Cupples LA, Mendelsohn ME, Levy D, Housman DE. Estrogen receptor alpha gene variation is associated with risk of myocardial infarction in more than seven thousand men from five cohorts. Circ Res 2006;98:590-592. PubMed
- 13. Tanaka Y, Sasaki M, Kaneuchi M, Shiina H, Igawa M, Dahiya R. Polymorphisms of estrogen receptor alpha in prostate cancer. Mol Carcinog 2003;37:202-208. ArticlePubMed
- 14. Tempfer CB, Schneeberger C, Huber JC. Applications of polymorphisms and pharmacogenomics in obstetrics and gynecology. Pharmacogenomics 2004;5:57-65. ArticlePubMed
- 15. Molvarec A, Nagy B, Kovacs M, Walentin S, Imreh E, Rigo J Jr, Szalay J, Fust G, Prohaszka Z, Karadi I. Lipid, haemostatic and inflammatory variables in relation to the estrogen receptor alpha (ESR1) PvuII and XbaI gene polymorphisms. Clin Chim Acta 2007;380:157-164. PubMed
- 16. Penolazzi L, Lambertini E, Aguiari G, del Senno L, Piva R. Cis element 'decoy' against the upstream promoter of the human estrogen receptor gene. Biochim Biophys Acta 2000;1492:560-567. ArticlePubMed
- 17. Zarei S, Bigizadeh S, Pourahmadi M, Ghobadifar MA. Chronic pain and its determinants: a population-based study in Southern Iran. Korean J Pain 2012;25:245-253. ArticlePubMedPMC
- 18. Ganasyam SR, Rao TB, Murthy YS, Jyothy A, Sujatha M. Association of estrogen receptor-alpha gene & metallothionein-1 gene polymorphisms in type 2 diabetic women of andhra pradesh. Indian J Clin Biochem 2012;27:69-73. PubMedPMC
- 19. Matsushita H, Kurabayashi T, Tomita M, Tanaka K. Effects of vitamin D and estrogen receptor gene polymorphisms on the changes in lumbar bone mineral density with multiple pregnancies in Japanese women. Hum Reprod 2004;19:59-64. ArticlePubMed
- 20. Huang Q, Wang TH, Lu WS, Mu PW, Yang YF, Liang WW, Li CX, Lin GP. Estrogen receptor alpha gene polymorphism associated with type 2 diabetes mellitus and the serum lipid concentration in Chinese women in Guangzhou. Chin Med J (Engl) 2006;119:1794-1801. ArticlePubMed
- 21. Araujo KL, de Rezende LCS, Letícia Soncini, Daltoe RD, Madeira KP, Paes MF, Herkenhoff FL, Range LB, Silva IV. Prevalence of estrogen receptor alpha PvuII (c454-397T>C) and XbaI (c454A>G) polymorphisms in a population of Brazilian women. Braz Arch Biol Technol 2011;54:1151-1158.Article
- 22. Newton KM, LaCroix AZ, Heckbert SR, Abraham L, McCulloch D, Barlow W. Estrogen therapy and risk of cardiovascular events among women with type 2 diabetes. Diabetes Care 2003;26:2810-2816. ArticlePubMedPDF
- 23. Xiang K, Wang Y, Zheng T, Jia W, Li J, Chen L, Shen K, Wu S, Lin X, Zhang G, Wang C, Wang S, Lu H, Fang Q, Shi Y, Zhang R, Xu J, Weng Q. Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the Chinese: significant linkage to chromosome 6q21-q23 and chromosome 1q21-q24. Diabetes 2004;53:228-234. PubMed
- 24. Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol 1995;13:217-226. PubMed
- 25. Dahlman I, Vaxillaire M, Nilsson M, Lecoeur C, Gu HF, Cavalcanti-Proenca C, Efendic S, Ostenson CG, Brismar K, Charpentier G, Gustafsson JA, Froguel P, Dahlman-Wright K, Steffensen KR. Estrogen receptor alpha gene variants associate with type 2 diabetes and fasting plasma glucose. Pharmacogenet Genomics 2008;18:967-975. ArticlePubMed
- 26. Brandi ML, Becherini L, Gennari L, Racchi M, Bianchetti A, Nacmias B, Sorbi S, Mecocci P, Senin U, Govoni S. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer's disease. Biochem Biophys Res Commun 1999;265:335-338. PubMed
- 27. Jeng MH, Shupnik MA, Bender TP, Westin EH, Bandyopadhyay D, Kumar R, Masamura S, Santen RJ. Estrogen receptor expression and function in long-term estrogen-deprived human breast cancer cells. Endocrinology 1998;139:4164-4174. ArticlePubMed
- 28. Albrink MJ, Meigs JW. The relationship between serum triglycerides and skinfold thickness in obese subjects. Ann N Y Acad Sci 1965;131:673-683. ArticlePubMed
- 29. Pollare T, Lithell H, Berne C. Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism 1990;39:167-174. ArticlePubMed
- 30. Sand P, Luckhaus C, Schlurmann K, Gotz M, Deckert J. Untangling the human estrogen receptor gene structure. J Neural Transm 2002;109:567-583. ArticlePubMedPDF
- 31. Deng HW, Li J, Li JL, Johnson M, Gong G, Recker RR. Association of VDR and estrogen receptor genotypes with bone mass in postmenopausal Caucasian women: different conclusions with different analyses and the implications. Osteoporos Int 1999;9:499-507. ArticlePubMedPDF
- 32. Daga A, Fabbi M, Mattioni T, Bertolini S, Corte G. Pvu II polymorphism of low density lipoprotein receptor gene and familial hypercholesterolemia. Study of Italians. Arteriosclerosis 1988;8:845-850. ArticlePubMed
- 33. Kikuchi T, Hashimoto N, Kawasaki T, Uchiyama M. Association of serum low-density lipoprotein metabolism with oestrogen receptor gene polymorphisms in healthy children. Acta Paediatr 2000;89:42-45. ArticlePubMed
REFERENCES

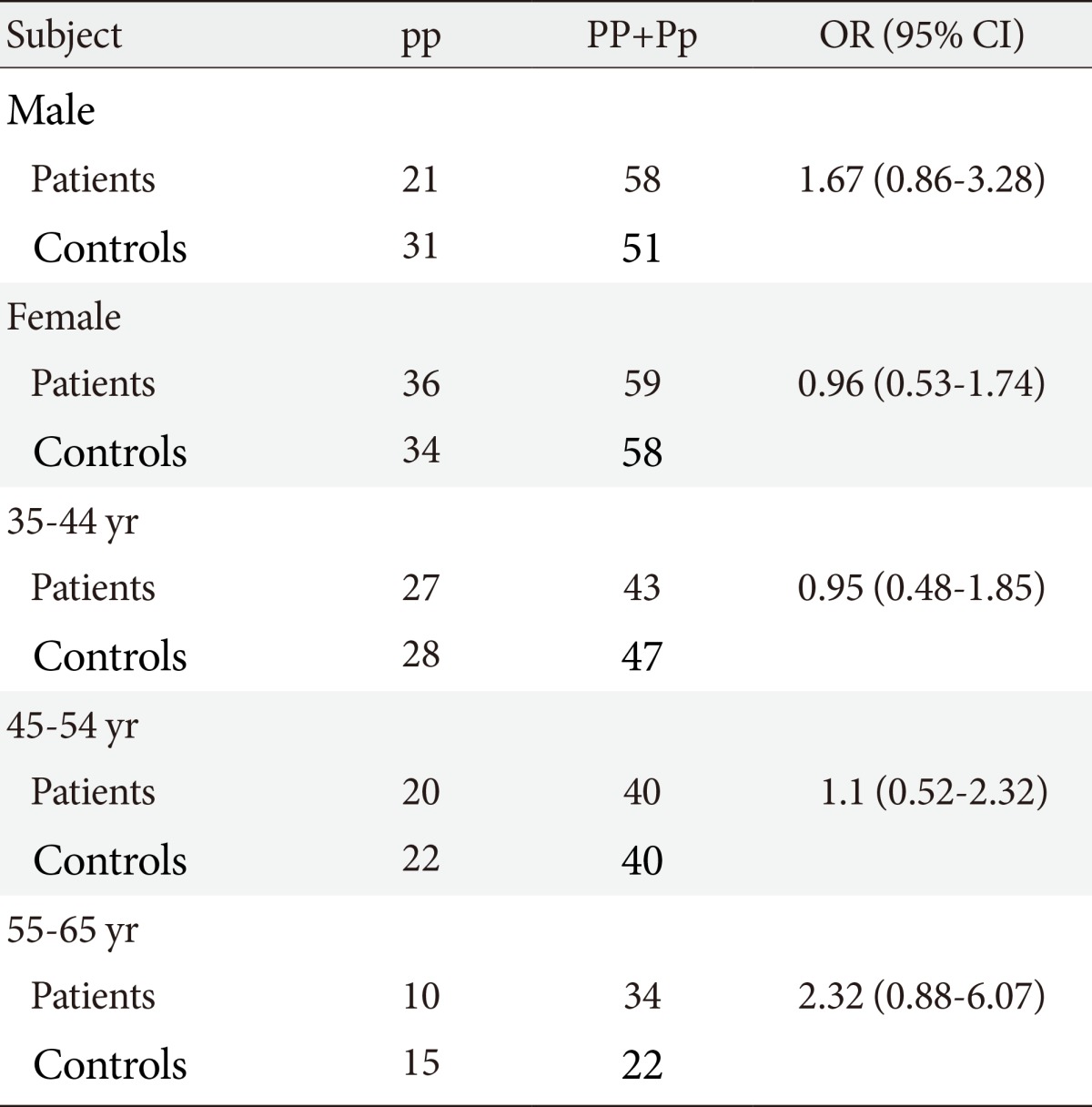

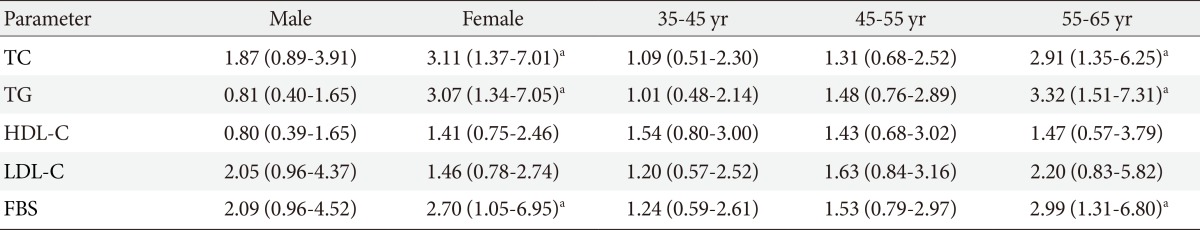
Values are presented as odds ratio (95% confidence interval).
TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; FBG, fasting blood glucose.
aP<.05, three age groups had been controlled by sex, and female and male groups had been controlled by age.
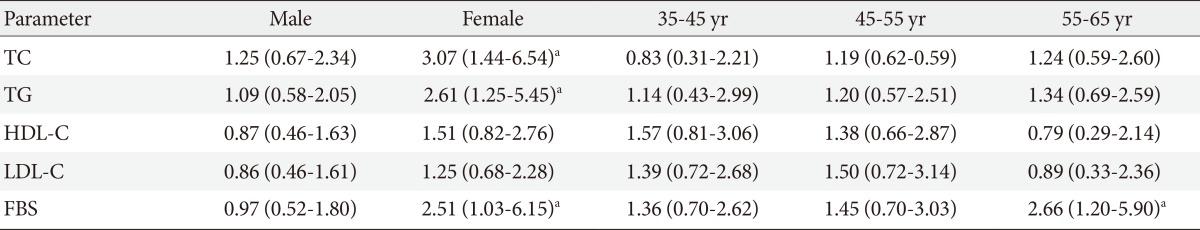
Values are presented as odds ratio (95% confidence interval).
TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; FBG, fasting blood glucose.
aP<0.05, three age groups had been controlled by sex, and female and male groups had been controlled by age.
Figure & Data
References
Citations

- Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population
Iman Akhlaghipour, Amir Reza Bina, Mohammad Reza Mogharrabi, Ali Fanoodi, Amir Reza Ebrahimian, Soroush Khojasteh Kaffash, Atefeh Babazadeh Baghan, Mohammad Erfan Khorashadizadeh, Negin Taghehchian, Meysam Moghbeli
Human Genomics.2022;[Epub] CrossRef - Estrogen receptor 1 gene polymorphisms (PvuII and XbaI) are associated with type 2 diabetes in Palestinian women
Suheir Ereqat, Stéphane Cauchi, Khaled Eweidat, Muawiyah Elqadi, Abedelmajeed Nasereddin
PeerJ.2019; 7: e7164. CrossRef - Association between Genetic Variants and Diabetes Mellitus in Iranian Populations: A Systematic Review of Observational Studies
Mehrnoosh Khodaeian, Samaneh Enayati, Ozra Tabatabaei-Malazy, Mahsa M. Amoli
Journal of Diabetes Research.2015; 2015: 1. CrossRef - Genetic polymorphism of estrogen receptor alpha gene in Egyptian women with type II diabetes mellitus
Tarek M.K. Motawi, Mahmoud A. El-Rehany, Sherine M. Rizk, Maggie M. Ramzy, Doaa M. el-Roby
Meta Gene.2015; 6: 36. CrossRef

 KDA
KDA
 PubReader
PubReader Cite
Cite



