
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Type 1 Diabetes
- A New Tool to Identify Pediatric Patients with Atypical Diabetes Associated with Gene Polymorphisms
- Sophie Welsch, Antoine Harvengt, Paola Gallo, Manon Martin, Dominique Beckers, Thierry Mouraux, Nicole Seret, Marie-Christine Lebrethon, Raphaël Helaers, Pascal Brouillard, Miikka Vikkula, Philippe A. Lysy
- Received May 26, 2023 Accepted November 25, 2023 Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0166 [Epub ahead of print]
- 800 View
- 49 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
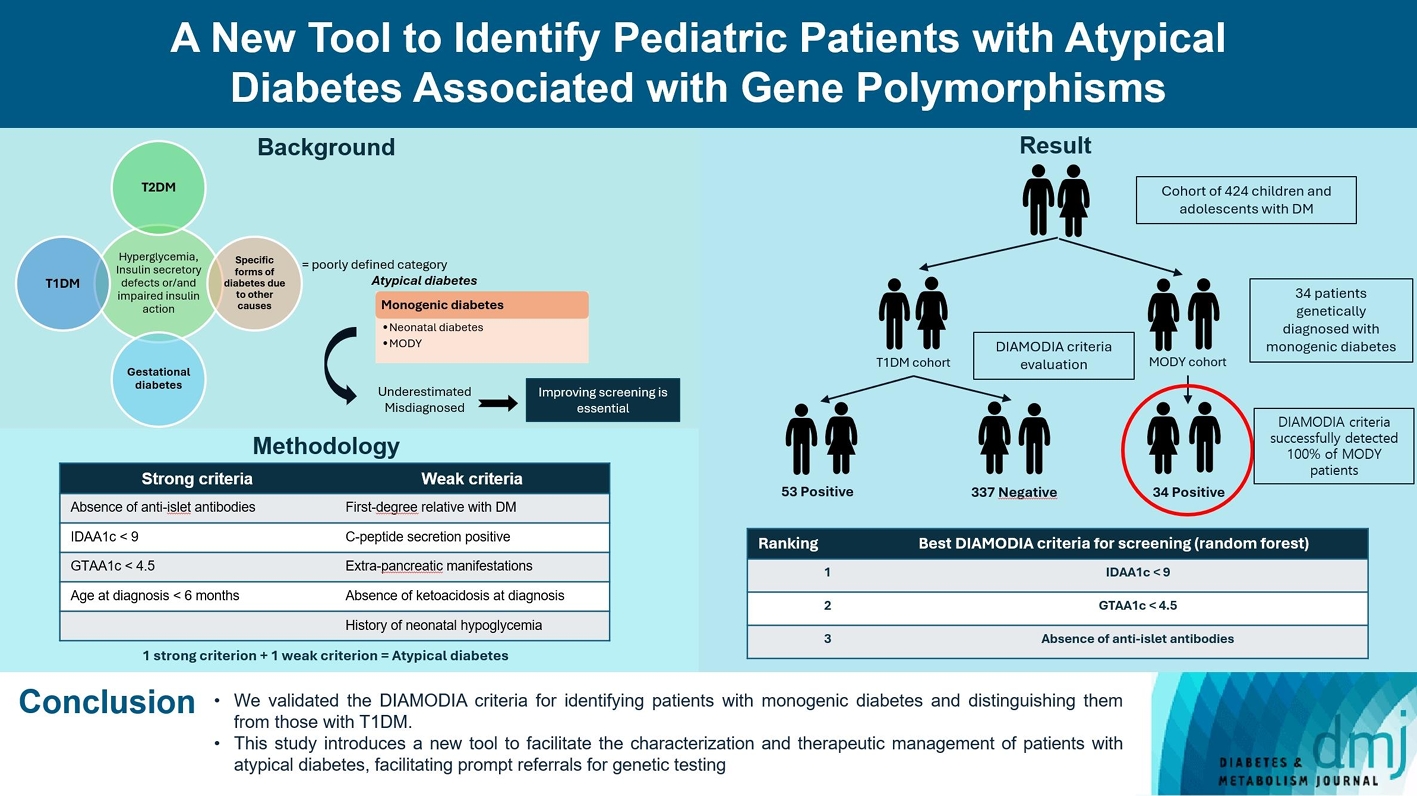
Recent diabetes subclassifications have improved the differentiation between patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus despite several overlapping features, yet without considering genetic forms of diabetes. We sought to facilitate the identification of monogenic diabetes by creating a new tool that we validated in a pediatric maturity-onset diabetes of the young (MODY) cohort.
Methods
We first created the DIAgnose MOnogenic DIAbetes (DIAMODIA) criteria based on the pre-existing, but incomplete, MODY calculator. This new score is composed of four strong and five weak criteria, with patients having to display at least one weak and one strong criterion.
Results
The effectiveness of the DIAMODIA criteria was evaluated in two patient cohorts, the first consisting of patients with confirmed MODY diabetes (n=34) and the second of patients with T1DM (n=390). These DIAMODIA criteria successfully detected 100% of MODY patients. Multiple correspondence analysis performed on the MODY and T1DM cohorts enabled us to differentiate MODY patients from T1DM. The three most relevant variables to distinguish a MODY from T1DM profile were: lower insulin-dose adjusted A1c score ≤9, glycemic target-adjusted A1c score ≤4.5, and absence of three anti-islet cell autoantibodies.
Conclusion
We validated the DIAMODIA criteria, as it effectively identified all monogenic diabetes patients (MODY cohort) and succeeded to differentiate T1DM from MODY patients. The creation of this new and effective tool is likely to facilitate the characterization and therapeutic management of patients with atypical diabetes, and promptly referring them for genetic testing which would markedly improve clinical care and counseling, as well.
- Type 1 Diabetes
- Optimal Coefficient of Variance Threshold to Minimize Hypoglycemia Risk in Individuals with Well-Controlled Type 1 Diabetes Mellitus
- Jee Hee Yoo, Seung Hee Yang, Sang-Man Jin, Jae Hyeon Kim
- Received March 14, 2023 Accepted August 12, 2023 Published online March 4, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0083 [Epub ahead of print]

- 515 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the optimal coefficient of variance (%CV) for preventing hypoglycemia based on real-time continuous glucose monitoring (rt-CGM) data in people with type 1 diabetes mellitus (T1DM) already achieving their mean glucose (MG) target.
Methods
Data from 172 subjects who underwent rt-CGM for at least 90 days and for whom 439 90-day glycemic profiles were available were analyzed. Receiver operator characteristic analysis was conducted to determine the cut-off value of %CV to achieve time below range (%TBR)<54 mg/dL <1 and =0.
Results
Overall mean glycosylated hemoglobin was 6.8% and median %TBR<54 mg/dL was 0.2%. MG was significantly higher and %CV significantly lower in profiles achieving %TBR<54 mg/dL <1 compared to %TBR<54 mg/dL ≥1 (all P<0.001). The cut-off value of %CV for achieving %TBR<54 mg/dL <1 was 37.5%, 37.3%, and 31.0%, in the whole population, MG >135 mg/dL, and ≤135 mg/dL, respectively. The cut-off value for %TBR<54 mg/dL=0% was 29.2% in MG ≤135 mg/dL. In profiles with MG ≤135 mg/dL, 94.2% of profiles with a %CV <31 achieved the target of %TBR<54 mg/dL <1, and 97.3% with a %CV <29.2 achieved the target of %TBR<54 mg/ dL=0%. When MG was >135 mg/dL, 99.4% of profiles with a %CV <37.3 achieved %TBR<54 mg/dL <1.
Conclusion
In well-controlled T1DM with MG ≤135 mg/dL, we suggest a %CV <31% to achieve the %TBR<54 mg/dL <1 target. Furthermore, we suggest a %CV <29.2% to achieve the target of %TBR<54 mg/dL =0 for people at high risk of hypoglycemia.
- Complications
- Does the Relationship of the Autonomic Symptoms Questionnaire COMPASS 31 with Cardiovascular Autonomic Tests Differ between Type 1 and Type 2 Diabetes Mellitus?
- Ilenia D’Ippolito, Marika Menduni, Cinzia D’Amato, Aikaterini Andreadi, Davide Lauro, Vincenza Spallone
- Received August 28, 2023 Accepted November 22, 2023 Published online February 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0301 [Epub ahead of print]

- 608 View
- 44 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The aim was to investigate if autonomic symptoms questionnaire Composite Autonomic Symptom Score (COMPASS) 31 has different association with cardiovascular autonomic neuropathy (CAN) and diagnostic performance between type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).
Methods
Seventy-nine participants with T1DM and 140 with T2DM completed COMPASS 31 before cardiovascular reflex tests (CARTs) for CAN, and assessment of symptoms, signs, vibration, and thermal perception thresholds for diabetic polyneuropathy (DPN) diagnosis.
Results
COMPASS 31 total weighted score (TWS) was similar in the two groups, but significantly associated with confirmed CAN only in T1DM (P=0.0056) and not T2DM group (P=0.1768) and correlated with CARTs score more strongly in T1DM (rho=0.356, P=0.0016) than in T2DM group (rho=0.084, P=0.3218) (P=0.016). Only in T1DM and not T2DM group, the area under the receiver operating characteristic curve (AUC) reached a fair diagnostic accuracy (>0.7) for confirmed CAN (0.73±0.07 vs. 0.61±0.08) and DPN (0.75±0.06 vs. 0.68±0.05), although without a significant difference. COMPASS 31 TWS (cut-off 16.44) reached acceptable diagnostic performance in T1DM, with sensitivity for confirmed CAN 81.2% and sensitivity and specificity for DPN 76.3% and 78%, compared to T2DM group (all <70%). AUC for DPN of orthostatic intolerance domain was higher in T1DM compared to T2DM group (0.73±0.05 vs. 0.58±0.04, P=0.027).
Conclusion
COMPASS 31 is more weakly related to CAN in T2DM than in T1DM, with a fair diagnostic accuracy for confirmed CAN only in T1DM. This difference supports a multifactorial origin of symptoms and should be considered when using COMPASS 31.
Review
- Pathophysiology
- Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
- Yun Kyung Cho, Chang Hee Jung
- Diabetes Metab J. 2023;47(6):757-766. Published online July 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0072

- 2,794 View
- 327 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - With the increasing use of immune-checkpoint inhibitors (ICIs), such as anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and anti-programmed cell death-1 (PD-1), for the treatment of malignancies, cases of ICI-induced type 1 diabetes mellitus (ICI-T1DM) have been reported globally. This review focuses on the features and pathogenesis of this disease. T1DM is an immune-related adverse event that occurs following the administration of anti-PD-1 or anti-programmed death ligand-1 (PDL1) alone or in combination with anti-CTLA-4. More than half of the reported cases presented as abrupt-onset diabetic ketoacidosis. The primary mechanism of ICI-T1DM is T-cell stimulation, which results from the loss of interaction between PD-1 and PD-L1 in pancreatic islet. The similarities and differences between ICI-T1DM and classical T1DM may provide insights into this disease entity. ICI-T1DM is a rare but often life-threatening medical emergency that healthcare professionals and patients need to be aware of. Early detection of and screening for this disease is imperative. At present, the only known treatment for ICI-T1DM is insulin injection. Further research into the mechanisms and risk factors associated with ICI-T1DM development may contribute to a better understanding of this disease entity and the identification of possible preventive strategies.
-
Citations
Citations to this article as recorded by- Research Advances of Immune Checkpoint Inhibitors Related Endocrine Adverse Events
晶晶 王
Advances in Clinical Medicine.2024; 14(02): 2706. CrossRef - Immune checkpoint inhibitor‑associated diabetes mellitus in patients with HCC: Report of three cases and literature review
Gaocheng Wang, Jingjing Wang, Shuilin Dong, Zhanguo Zhang, Wanguang Zhang, Jianping Zhao
Experimental and Therapeutic Medicine.2024;[Epub] CrossRef
- Research Advances of Immune Checkpoint Inhibitors Related Endocrine Adverse Events
Original Articles
- Lifestyle
- Clinical Effects of a Home Care Pilot Program for Patients with Type 1 Diabetes Mellitus: A Retrospective Cohort Study
- Sejeong Lee, KyungYi Kim, Ji Eun Kim, Yura Hyun, Minyoung Lee, Myung-Il Hahm, Sang Gyu Lee, Eun Seok Kang
- Diabetes Metab J. 2023;47(5):693-702. Published online June 22, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0170
- 1,915 View
- 138 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
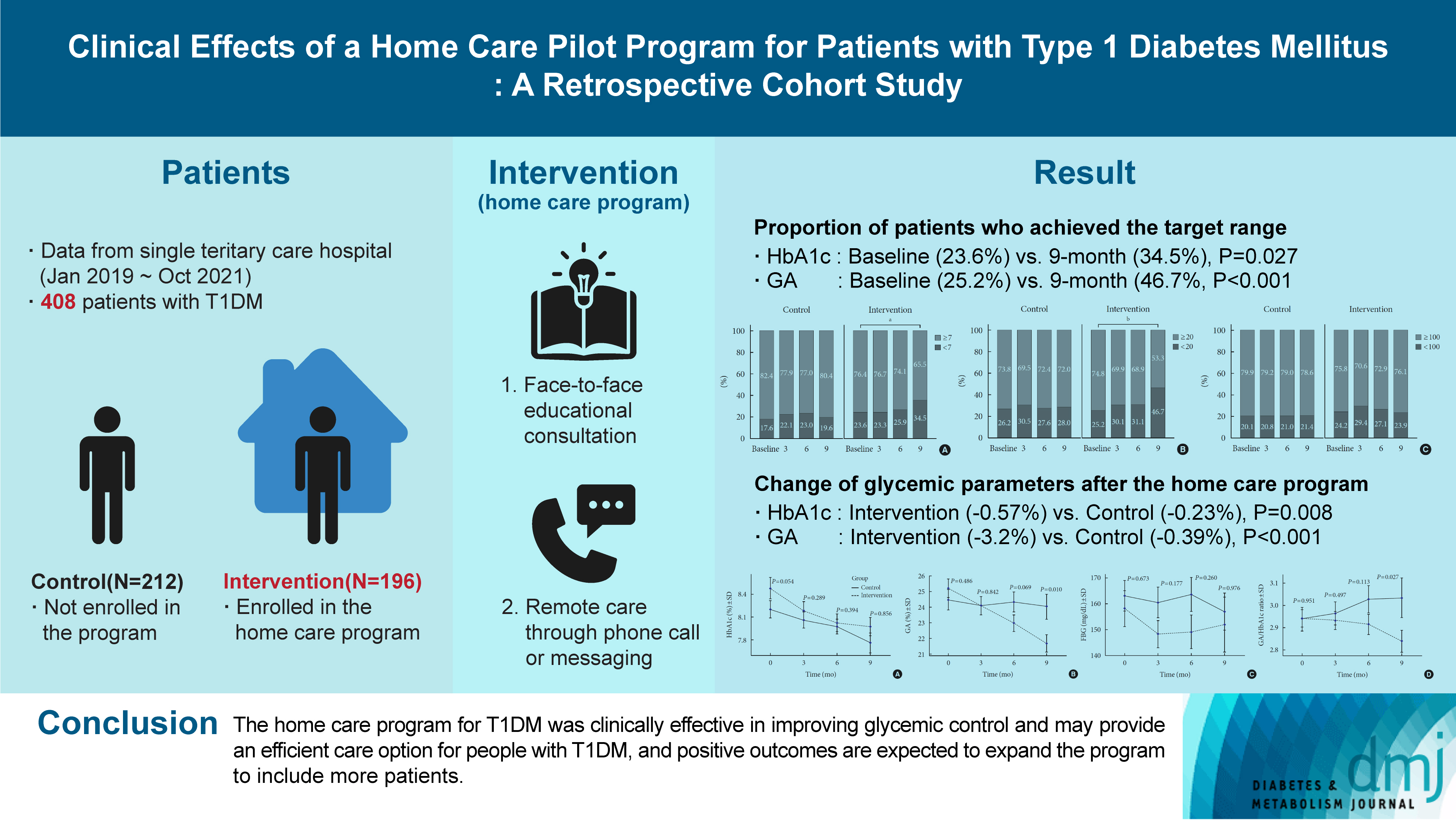
Given the importance of continuous self-care for people with type 1 diabetes mellitus (T1DM), the Ministry of Health and Welfare of Korea launched a pilot program for chronic disease management. Herein, we applied a home care pilot program to people with T1DM to investigate its effects.
Methods
This retrospective cohort study was conducted at a single tertiary hospital (January 2019 to October 2021). A multidisciplinary team comprising doctors, nurses, and clinical nutritionists provided specialized education and periodically assessed patients’ health status through phone calls or text messages. A linear mixed model adjusting for age, sex, and body mass index was used to analyze the glycemic control changes before and after implementing the program between the intervention and control groups.
Results
Among 408 people with T1DM, 196 were enrolled in the intervention group and 212 in the control group. The reduction in glycosylated hemoglobin (HbA1c) after the program was significantly greater in the intervention group than in the control group (estimated marginal mean, –0.57% vs. –0.23%, P=0.008); the same trend was confirmed for glycoalbumin (GA) (–3.2% vs. –0.39%, P<0.001). More patients achieved the target values of HbA1c (<7.0%) and GA (<20%) in the intervention group than in the control group at the 9-month follow-up (34.5% vs. 19.6% and 46.7% vs. 28.0%, respectively).
Conclusion
The home care program for T1DM was clinically effective in improving glycemic control and may provide an efficient care option for people with T1DM, and positive outcomes are expected to expand the program to include more patients. -
Citations
Citations to this article as recorded by- Glycemic outcomes and patient satisfaction and self-management improves in transition from standard to virtual multidisciplinary care
Noga Minsky, Liat Arnon Klug, Tatyana Kolobov, Elizabeth Tarshish, Yuval Shalev Many, Aviva Lipsitz, Amna Jabarin, Nicole Morozov, Dania Halperin, Moshe Shalom, Rachel Nissanholtz-Gannot, Genya Aharon-Hananel, Amir Tirosh, Orly Tamir
Diabetes Research and Clinical Practice.2024; 209: 111587. CrossRef
- Glycemic outcomes and patient satisfaction and self-management improves in transition from standard to virtual multidisciplinary care
- Guideline/Fact Sheet
- Insulin Fact Sheet in Type 1 and 2 Diabetes Mellitus and Trends of Antidiabetic Medication Use in Insulin Users with Type 2 Diabetes Mellitus: 2002 to 2019
- Jiyun Park, Gyuri Kim, Bong-Sung Kim, Kyung-Do Han, So Yoon Kwon, So Hee Park, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim
- Diabetes Metab J. 2023;47(2):211-219. Published online February 7, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0346
- 3,547 View
- 258 Download
- 1 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
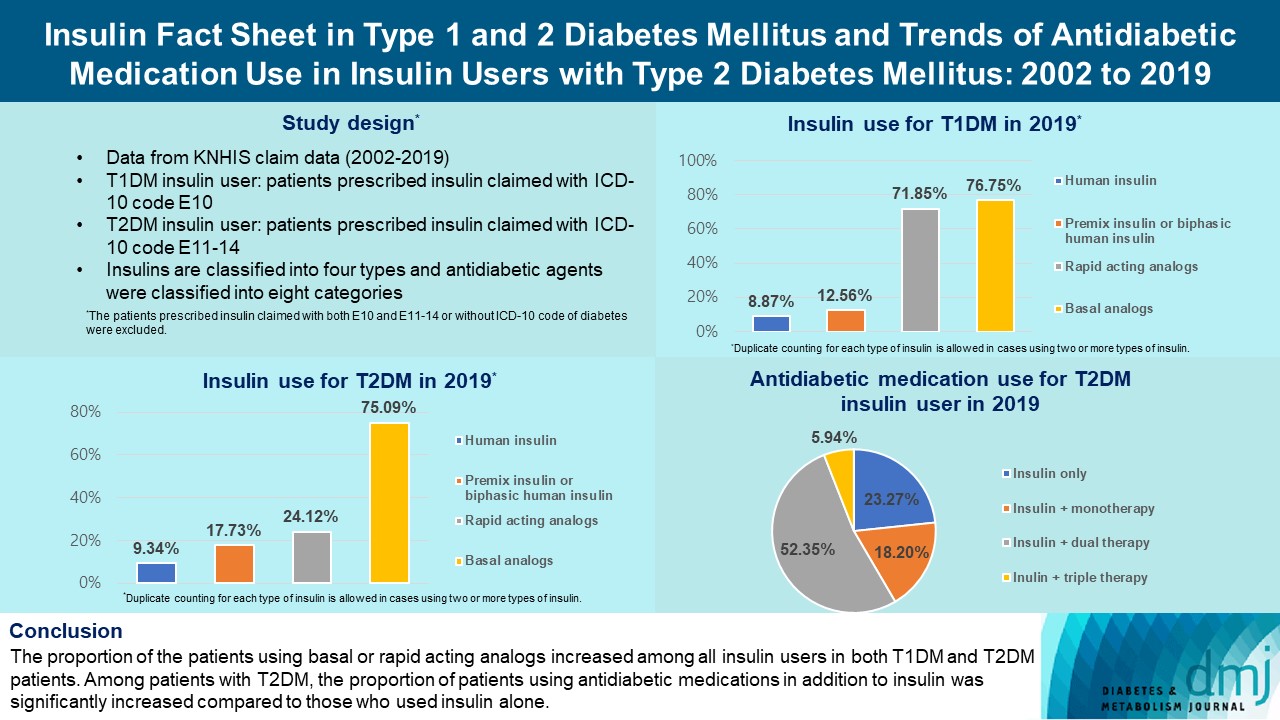
This study investigated the trends of insulin use among Korean patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). Changes in prescription of antidiabetic medications in T2DM patients taking insulin therapy were evaluated.
Methods
We analyzed data from the National Health Insurance Service database in Korea to evaluate the prevalence of insulin users and trends of insulin use in T1DM and T2DM patients from January 2002 to December 2019. We also investigated numbers and types of antidiabetic medications in insulin users with T2DM.
Results
The overall total number of insulin users increased from 2002 to 2019, reaching 348,254 for T2DM and 20,287 for T1DM in 2019 compared with 109,974 for T2DM and 34,972 for T1DM in 2002. The proportion of patients using basal analogs and short acting analogs have increased and those using human insulin, premixed insulin, or biphasic human insulin have decreased (rapid acting analogs: 71.85% and 24.12% in T1DM and T2DM, respectively, in 2019; basal analogs: 76.75% and 75.09% in T1DM and T2DM, respectively, in 2019). The use of other antidiabetic medication in addition to insulin increased for T2DM, especially in dual therapy, reaching up to 52.35% in 2019 compared with 16.72% in 2002.
Conclusion
The proportion of the patients using basal or rapid acting analogs increased among all insulin users in both T1DM and T2DM patients. Among patients with T2DM, the proportion of patients using antidiabetic medications in addition to insulin was significantly increased compared to those who used insulin alone. -
Citations
Citations to this article as recorded by- Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah B. Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Evaluation of pharmacokinetic interactions between lobeglitazone, empagliflozin, and metformin in healthy subjects
Heeyoung Kim, Choon Ok Kim, Hyeonsoo Park, Min Soo Park, Dasohm Kim, Taegon Hong, Yesong Shin, Byung Hak Jin
Translational and Clinical Pharmacology.2023; 31(1): 59. CrossRef - Smart Insulin Pen: Managing Insulin Therapy for People with Diabetes in the Digital Era
Jee Hee Yoo, Jae Hyeon Kim
The Journal of Korean Diabetes.2023; 24(4): 190. CrossRef
- Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Review
- Technology/Device
- Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
- Jee Hee Yoo, Jae Hyeon Kim
- Diabetes Metab J. 2023;47(1):27-41. Published online January 12, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0271

- 6,248 View
- 385 Download
- 11 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Continuous glucose monitoring (CGM) technology has evolved over the past decade with the integration of various devices including insulin pumps, connected insulin pens (CIPs), automated insulin delivery (AID) systems, and virtual platforms. CGM has shown consistent benefits in glycemic outcomes in type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) treated with insulin. Moreover, the combined effect of CGM and education have been shown to improve glycemic outcomes more than CGM alone. Now a CIP is the expected future technology that does not need to be worn all day like insulin pumps and helps to calculate insulin doses with a built-in bolus calculator. Although only a few clinical trials have assessed the effectiveness of CIPs, they consistently show benefits in glycemic outcomes by reducing missed doses of insulin and improving problematic adherence. AID systems and virtual platforms made it possible to achieve target glycosylated hemoglobin in diabetes while minimizing hypoglycemia, which has always been challenging in T1DM. Now fully automatic AID systems and tools for diabetes decisions based on artificial intelligence are in development. These advances in technology could reduce the burden associated with insulin treatment for diabetes.
-
Citations
Citations to this article as recorded by- Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System
Kyung-Soo Kim, Seung-Hwan Lee, Won Sang Yoo, Cheol-Young Park
Diabetes Technology & Therapeutics.2024; 26(4): 222. CrossRef - Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Recent advances in the precision control strategy of artificial pancreas
Wuyi Ming, Xudong Guo, Guojun Zhang, Yinxia Liu, Yongxin Wang, Hongmei Zhang, Haofang Liang, Yuan Yang
Medical & Biological Engineering & Computing.2024;[Epub] CrossRef - Digital Health in Diabetes and Cardiovascular Disease
Dorothy Avoke, Abdallah Elshafeey, Robert Weinstein, Chang H. Kim, Seth S. Martin
Endocrine Research.2024; : 1. CrossRef - Continuous glucose monitoring with structured education in adults with type 2 diabetes managed by multiple daily insulin injections: a multicentre randomised controlled trial
Ji Yoon Kim, Sang-Man Jin, Kang Hee Sim, Bo-Yeon Kim, Jae Hyoung Cho, Jun Sung Moon, Soo Lim, Eun Seok Kang, Cheol-Young Park, Sin Gon Kim, Jae Hyeon Kim
Diabetologia.2024;[Epub] CrossRef - Glycemic Outcomes During Early Use of the MiniMed™ 780G Advanced Hybrid Closed-Loop System with Guardian™ 4 Sensor
Toni L. Cordero, Zheng Dai, Arcelia Arrieta, Fang Niu, Melissa Vella, John Shin, Andrew S. Rhinehart, Jennifer McVean, Scott W. Lee, Robert H. Slover, Gregory P. Forlenza, Dorothy I. Shulman, Rodica Pop-Busui, James R. Thrasher, Mark S. Kipnes, Mark P. Ch
Diabetes Technology & Therapeutics.2023; 25(9): 652. CrossRef - Navigating the Seas of Glycemic Control: The Role of Continuous Glucose Monitoring in Type 1 Diabetes Mellitus
Jun Sung Moon
Diabetes & Metabolism Journal.2023; 47(3): 345. CrossRef - APSec1.0: Innovative Security Protocol Design with Formal Security Analysis for the Artificial Pancreas System
Jiyoon Kim, Jongmin Oh, Daehyeon Son, Hoseok Kwon, Philip Virgil Astillo, Ilsun You
Sensors.2023; 23(12): 5501. CrossRef - Advances and Development of Electronic Neural Interfaces
Xue Jiaxiang, Liu Zhixin
Journal of Computing and Natural Science.2023; : 147. CrossRef - Continuous Glucose Monitoring (CGM) and Metabolic Control in a Cohort of Patients with Type 1 Diabetes and Coeliac Disease
Flavia Amaro, Maria Alessandra Saltarelli, Marina Primavera, Marina Cerruto, Stefano Tumini
Endocrines.2023; 4(3): 595. CrossRef - Comparison of Glycemia Risk Index with Time in Range for Assessing Glycemic Quality
Ji Yoon Kim, Jee Hee Yoo, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2023; 25(12): 883. CrossRef - The Benefits Of Continuous Glucose Monitoring In Pregnancy
Jee Hee Yoo, Jae Hyeon Kim
Endocrinology and Metabolism.2023; 38(5): 472. CrossRef - The Growing Challenge of Diabetes Management in an Aging Society
Seung-Hwan Lee
Diabetes & Metabolism Journal.2023; 47(5): 630. CrossRef - Recent advances in artificial intelligence-assisted endocrinology and diabetes
Ioannis T. Oikonomakos, Ranjit M. Anjana, Viswanathan Mohan, Charlotte Steenblock, Stefan R. Bornstein
Exploration of Endocrine and Metabolic Disease.2023; 1(1): 16. CrossRef - An Observational Pilot Study of a Tailored Environmental Monitoring and Alert System for Improved Management of Chronic Respiratory Diseases
Mohammed Alotaibi, Fady Alnajjar, Badr A Alsayed, Tareq Alhmiedat, Ashraf M Marei, Anas Bushnag, Luqman Ali
Journal of Multidisciplinary Healthcare.2023; Volume 16: 3799. CrossRef - Smart Insulin Pen: Managing Insulin Therapy for People with Diabetes in the Digital Era
Jee Hee Yoo, Jae Hyeon Kim
The Journal of Korean Diabetes.2023; 24(4): 190. CrossRef
- Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System
Original Articles
- Type 1 Diabetes
- Performance of Fast-Acting Aspart Insulin as Compared to Aspart Insulin in Insulin Pump for Managing Type 1 Diabetes Mellitus: A Meta-Analysis
- Deep Dutta, Ritin Mohindra, Kunal Mahajan, Meha Sharma
- Diabetes Metab J. 2023;47(1):72-81. Published online June 24, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0035
- 4,894 View
- 250 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
No meta-analysis has analysed efficacy and safety of fast-acting aspart insulin (FIAsp) with insulin pump in type 1 diabetes mellitus (T1DM).
Methods
Electronic databases were searched for randomised controlled trials (RCTs) involving T1DM patients on insulin pump receiving FIAsp in intervention arm, and placebo/active comparator insulin in control arm. Primary outcome was to evaluate changes in 1- and 2-hour post-prandial glucose (1hPPG and 2hPPG). Secondary outcomes were to evaluate alterations in percentage time with blood glucose <3.9 mmol/L (hypoglycaemia), time in range (TIR) blood glucose 3.9 to 10 mmol/L, insulin requirements and adverse events.
Results
Data from four RCTs involving 640 patients was analysed. FIAsp use in insulin pump was associated with significantly greater lowering of 1hPPG (mean difference [MD], –1.35 mmol/L; 95% confidence interval [CI], –1.72 to –0.98; P<0.01; I2=63%) and 2hPPG (MD, –1.19 mmol/L; 95% CI, –1.38 to –1.00; P<0.01; I2=0%) as compared to controls. TIR was comparable among groups (MD, 1.06%; 95% CI, –3.84 to 5.96; P=0.67; I2=70%). Duration of blood glucose <3.9 mmol/L was lower in FIAsp group, approaching significance (MD, –0.91%; 95% CI, –1.84 to 0.03; P=0.06; I2=0%). Total hypoglycaemic episodes (risk ratio [RR], 1.35; 95% CI, 0.55 to 3.31; P=0.51; I2=0%), severe hypoglycaemia (RR, 2.26; 95% CI, 0.77 to 6.66; P=0.14), infusion site reactions (RR, 1.35; 95% CI, 0.63 to 2.93; P=0.77; I2=0%), and treatment-emergent adverse events (RR, 1.13; 95% CI, 0.80 to 1.60; P=0.50; I2=0%) were comparable.
Conclusion
FIAsp use in insulin pump is associated with better post-prandial glycaemic control with no increased hypoglycaemia or glycaemic variability. -
Citations
Citations to this article as recorded by- Burden and Coping Strategies of Hypoglycemia in People with Diabetes
Aris Liakos, Thomas Karagiannis, Ioannis Avgerinos, Apostolos Tsapas, Eleni Bekiari
Current Diabetes Reviews.2024;[Epub] CrossRef - Efficacy and Safety of Ultra-rapid Lispro Insulin in Managing Type-1 and Type-2 Diabetes: A Systematic Review and Meta-Analysis
Deep Dutta, Lakshmi Nagendra, Saptarshi Bhattacharya, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2023; 27(6): 467. CrossRef
- Burden and Coping Strategies of Hypoglycemia in People with Diabetes
- Type 1 Diabetes
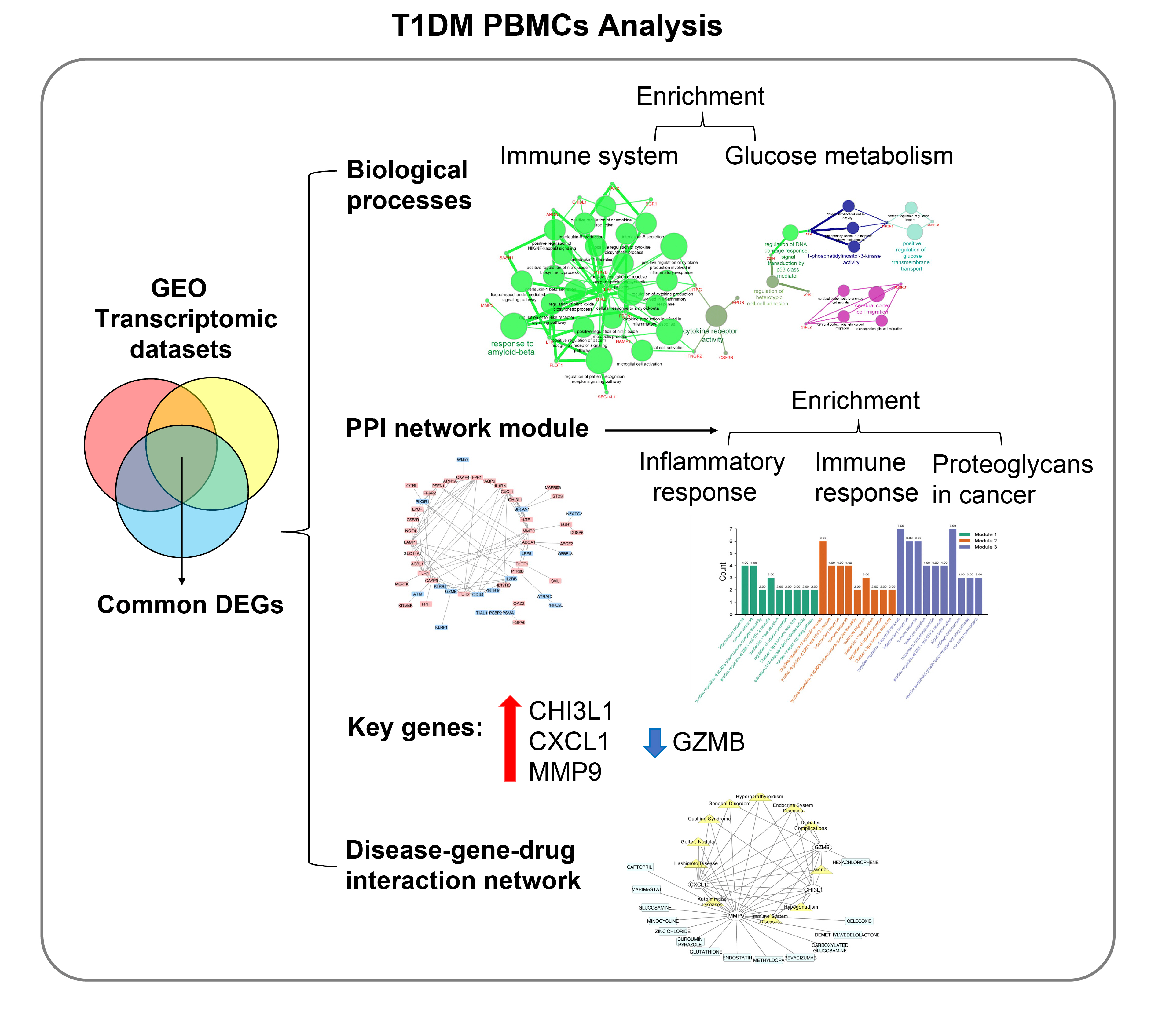
- Identification of Key Genes and Pathways in Peripheral Blood Mononuclear Cells of Type 1 Diabetes Mellitus by Integrated Bioinformatics Analysis
- Xing Li, Mingyu Liao, Jiangheng Guan, Ling Zhou, Rufei Shen, Min Long, Jiaqing Shao
- Diabetes Metab J. 2022;46(3):451-463. Published online April 1, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0018
- 6,985 View
- 292 Download
- 6 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The onset and progression of type 1 diabetes mellitus (T1DM) is closely related to autoimmunity. Effective monitoring of the immune system and developing targeted therapies are frontier fields in T1DM treatment. Currently, the most available tissue that reflects the immune system is peripheral blood mononuclear cells (PBMCs). Thus, the aim of this study was to identify key PBMC biomarkers of T1DM.
Methods
Common differentially expressed genes (DEGs) were screened from the Gene Expression Omnibus (GEO) datasets GSE9006, GSE72377, and GSE55098, and PBMC mRNA expression in T1DM patients was compared with that in healthy participants by GEO2R. Gene Ontology, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and protein-protein interaction (PPI) network analyses of DEGs were performed using the Cytoscape, DAVID, and STRING databases. The vital hub genes were validated by reverse transcription-polymerase chain reaction using clinical samples. The disease-gene-drug interaction network was built using the Comparative Toxicogenomics Database (CTD) and Drug Gene Interaction Database (DGIdb).
Results
We found that various biological functions or pathways related to the immune system and glucose metabolism changed in PBMCs from T1DM patients. In the PPI network, the DEGs of module 1 were significantly enriched in processes including inflammatory and immune responses and in pathways of proteoglycans in cancer. Moreover, we focused on four vital hub genes, namely, chitinase-3-like protein 1 (CHI3L1), C-X-C motif chemokine ligand 1 (CXCL1), matrix metallopeptidase 9 (MMP9), and granzyme B (GZMB), and confirmed them in clinical PBMC samples. Furthermore, the disease-gene-drug interaction network revealed the potential of key genes as reference markers in T1DM.
Conclusion
These results provide new insight into T1DM pathogenesis and novel biomarkers that could be widely representative reference indicators or potential therapeutic targets for clinical applications. -
Citations
Citations to this article as recorded by- Single-cell and transcriptomic analyses reveal the influence of diabetes on ovarian cancer
Zhihao Zhao, Qilin Wang, Fang Zhao, Junnan Ma, Xue Sui, Hyok Chol Choe, Peng Chen, Xue Gao, Lin Zhang
BMC Genomics.2024;[Epub] CrossRef - Bioinformatics analysis identifies TGF-β signaling pathway-associated molecular subtypes and gene signature in diabetic foot
Guanggang Du, Jie Chen, Xuezhu Zhu, Zongdong Zhu
iScience.2024; 27(3): 109094. CrossRef - Identification of Comorbidities, Genomic Associations, and Molecular Mechanisms for COVID-19 Using Bioinformatics Approaches
Shudeb Babu Sen Omit, Salma Akhter, Humayan Kabir Rana, A. R. M. Mahamudul Hasan Rana, Nitun Kumar Podder, Mahmudul Islam Rakib, Ashadun Nobi, Ali Imran
BioMed Research International.2023; 2023: 1. CrossRef - Advanced Delivery Strategies for Immunotherapy in Type I Diabetes Mellitus
Mingshu Huang, Weixing Chen, Min Wang, Yisheng Huang, Hongyu Liu, Yue Ming, Yuanxin Chen, Zhengming Tang, Bo Jia
BioDrugs.2023; 37(3): 331. CrossRef - Identification of the key genes of tuberculosis and construction of a diagnostic model via weighted gene co-expression network analysis
Baiying Li, Lifang Sun, Yaping Sun, Libo Zhen, Qi Qi, Ting Mo, Huijie Wang, Meihua Qiu, Qingshan Cai
Journal of Infection and Chemotherapy.2023; 29(11): 1046. CrossRef - Probing biological network in concurrent carcinomas and Type-2 diabetes for potential biomarker screening: An advanced computational paradigm
Abdullah Al Marzan, Shatila Shahi, Md Sakil Arman, Md Zafrul Hasan, Ajit Ghosh
Advances in Biomarker Sciences and Technology.2023; 5: 89. CrossRef - Transcriptional analysis of human peripheral blood mononuclear cells stimulated by Mycobacterium tuberculosis antigen
Jing Wei, Fangzheng Guo, Yamin Song, Kun Xu, Feiyang Lin, Kangsheng Li, Baiqing Li, Zhongqing Qian, Xiaojing Wang, Hongtao Wang, Tao Xu
Frontiers in Cellular and Infection Microbiology.2023;[Epub] CrossRef - Combining bioinformatics and machine learning algorithms to identify and analyze shared biomarkers and pathways in COVID-19 convalescence and diabetes mellitus
Jinru Shen, Yaolou Wang, Xijin Deng, Si Ri Gu Leng Sana
Frontiers in Endocrinology.2023;[Epub] CrossRef - Transcriptome analysis of peripheral blood mononuclear cells in patients with type 1 diabetes mellitus
Zhaoxiang Wang, Li Zhang, Fengyan Tang, Zhongming Yang, Mengzhu Wang, Jue Jia, Dong Wang, Ling Yang, Shao Zhong, Guoyue Yuan
Endocrine.2022; 78(2): 270. CrossRef
- Single-cell and transcriptomic analyses reveal the influence of diabetes on ovarian cancer
Short Communications
- Others
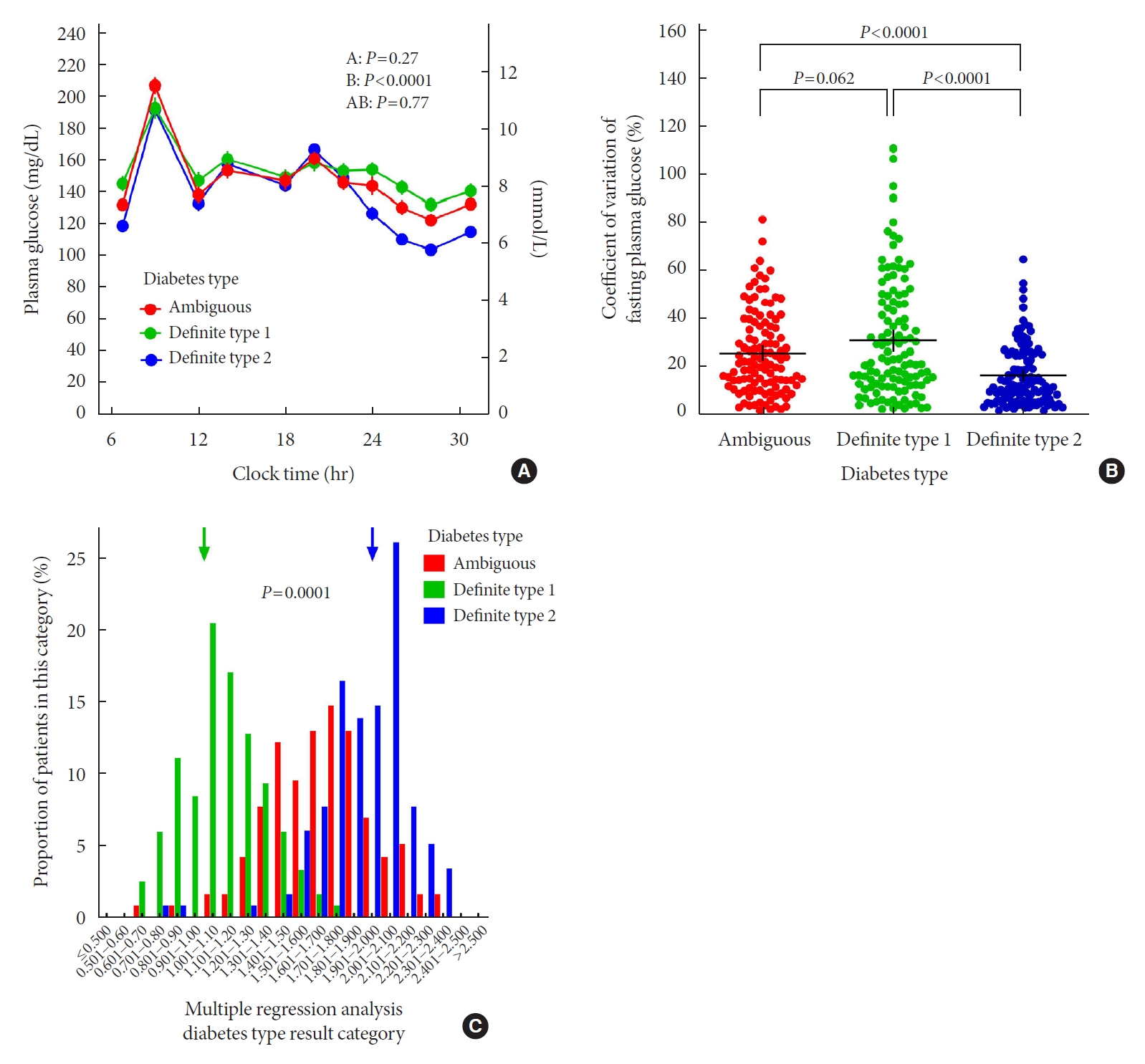
- Comparison of Insulin-Treated Patients with Ambiguous Diabetes Type with Definite Type 1 and Type 2 Diabetes Mellitus Subjects: A Clinical Perspective
- Insa Laspe, Juris J. Meier, Michael A. Nauck
- Diabetes Metab J. 2023;47(1):140-146. Published online March 22, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0322
- 65,535 View
- 183 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - In clinical practice, the distinction between type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) can be challenging, leaving patients with “ambiguous” diabetes type. Insulin-treated patients (n=115) previously diagnosed with T2DM had to be re-classified based on clinical phenotype and laboratory results, and were operationally defined as having an ambiguous diabetes type. They were compared against patients with definite T1DM and T2DM regarding 12 clinical and laboratory features typically different between diabetes types. Characteristics of patients with ambiguous diabetes type, representing approximately 6% of all patients with T1DM or T2DM seen at our specialized clinic, fell in between those of patients with definite T1DM and T2DM, both regarding individual features and with respect to a novel classification based on multi-variable regression analysis (P<0.0001). In conclusion, a substantial proportion of diabetes patients in a tertiary care centre presented with an “ambiguous” diabetes type. Their clinical characteristics fall in between those of definite T1DM or T2DM patients.
- Pathophysiology
- Glutamic Acid Decarboxylase and Tyrosine Phosphatase-Related Islet Antigen-2 Positivity among Children and Adolescents with Diabetes in Korea
- Ka Young Kim, Min Seung Kim, Yun Jeong Lee, Young Ah Lee, Seong Yong Lee, Choong Ho Shin, Jae Hyun Kim
- Diabetes Metab J. 2022;46(6):948-952. Published online March 8, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0332
- 4,518 View
- 183 Download
- 3 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Autoantibodies against glutamic acid decarboxylase (GADA), tyrosine phosphatase-related islet antigen 2 (IA2A), insulin (INSA), and islet cells (ICA) are critical for determining the type of diabetes and management strategy in new-onset diabetes mellitus (NODM), but there have been few reports of all diabetes-associated autoantibody (DAA) in Korea. We retrospectively analyzed 193 patients with NODM aged 0 to 18 years who were followed at two tertiary centers in Korea (2017 to 2021). Patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) were 93 (48.2%) and 100 (51.8%), respectively. In T1DM patients, the DAA positivity rate was 94.6%; prevalence of GADA, IA2A, INSA, and ICA was 71.0%, 71.0%, 31.2%, and 10.8%, respectively; and IA2A added 10.7% point autoantibody positivity (83.9% for GADA+INSA+ICA and 94.6% for GADA+INSA+ICA+IA2A). Among the patients with T2DM, 12 (12.0%) were positive for DAA, and all were positive for INSA. These findings suggest that DAA at diagnosis, especially GADA and IA2A, is useful for classifying diabetes in Korean children and adolescents.
-
Citations
Citations to this article as recorded by- Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
Yun Kyung Cho, Chang Hee Jung
Diabetes & Metabolism Journal.2023; 47(6): 757. CrossRef - Diagnostic and Therapeutic Strategies of Type 2 Diabetes Mellitus in Youth
Hwa Young Kim, Jae Hyun Kim
The Ewha Medical Journal.2022;[Epub] CrossRef
- Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
Brief Report
- Technology/Device
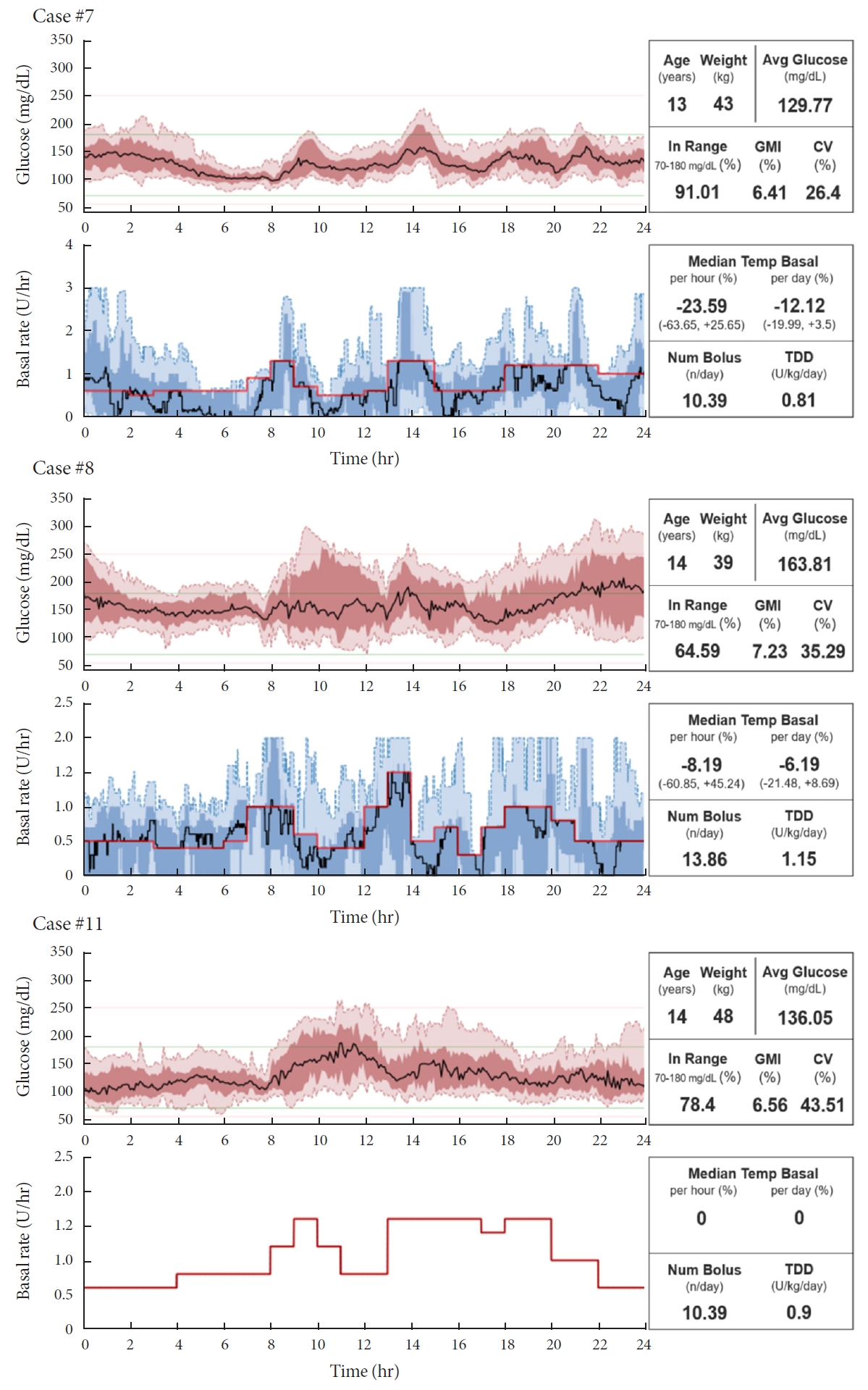
- Do-It-Yourself Open Artificial Pancreas System in Children and Adolescents with Type 1 Diabetes Mellitus: Real-World Data
- Min Sun Choi, Seunghyun Lee, Jiwon Kim, Gyuri Kim, Sung Min Park, Jae Hyeon Kim
- Diabetes Metab J. 2022;46(1):154-159. Published online November 23, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0011
- 5,300 View
- 192 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Few studies have been conducted among Asian children and adolescents with type 1 diabetes mellitus (T1DM) using do-it-yourself artificial pancreas system (DIY-APS). We evaluated real-world data of pediatric T1DM patients using DIY-APS. Data were obtained for 10 patients using a DIY-APS with algorithms. We collected sensor glucose and insulin delivery data from each participant for a period of 4 weeks. Average glycosylated hemoglobin was 6.2%±0.3%. The mean percentage of time that glucose level remained in the target range of 70 to 180 mg/dL was 82.4%±7.8%. Other parameters including time above range, time below range and mean glucose were also within the recommended level, similar to previous commercial and DIY-APS studies. However, despite meeting the target range, unadjusted gaps were still observed between the median basal setting and temporary basal insulin, which should be handled by healthcare providers.
-
Citations
Citations to this article as recorded by- Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes & Metabolism Journal.2023; 47(1): 27. CrossRef - Open-source automated insulin delivery systems (OS-AIDs) in a pediatric population with type 1 diabetes in a real-life setting: the AWeSoMe study group experience
Judith Nir, Marianna Rachmiel, Abigail Fraser, Yael Lebenthal, Avivit Brener, Orit Pinhas-Hamiel, Alon Haim, Eve Stern, Noa Levek, Tal Ben-Ari, Zohar Landau
Endocrine.2023; 81(2): 262. CrossRef - Efficacy and safety of Android artificial pancreas system use at home among adults with type 1 diabetes mellitus in China: protocol of a 26-week, free-living, randomised, open-label, two-arm, two-phase, crossover trial
Mengyun Lei, Beisi Lin, Ping Ling, Zhigu Liu, Daizhi Yang, Hongrong Deng, Xubin Yang, Jing Lv, Wen Xu, Jinhua Yan
BMJ Open.2023; 13(8): e073263. CrossRef - Barriers to Uptake of Open-Source Automated Insulin Delivery Systems: Analysis of Socioeconomic Factors and Perceived Challenges of Caregivers of Children and Adolescents With Type 1 Diabetes From the OPEN Survey
Antonia Huhndt, Yanbing Chen, Shane O’Donnell, Drew Cooper, Hanne Ballhausen, Katarzyna A. Gajewska, Timothée Froment, Mandy Wäldchen, Dana M. Lewis, Klemens Raile, Timothy C. Skinner, Katarina Braune
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - Toward Personalized Hemoglobin A1c Estimation for Type 2 Diabetes
Namho Kim, Da Young Lee, Wonju Seo, Nan Hee Kim, Sung-Min Park
IEEE Sensors Journal.2022; 22(23): 23023. CrossRef
- Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Review
- Technology/Device
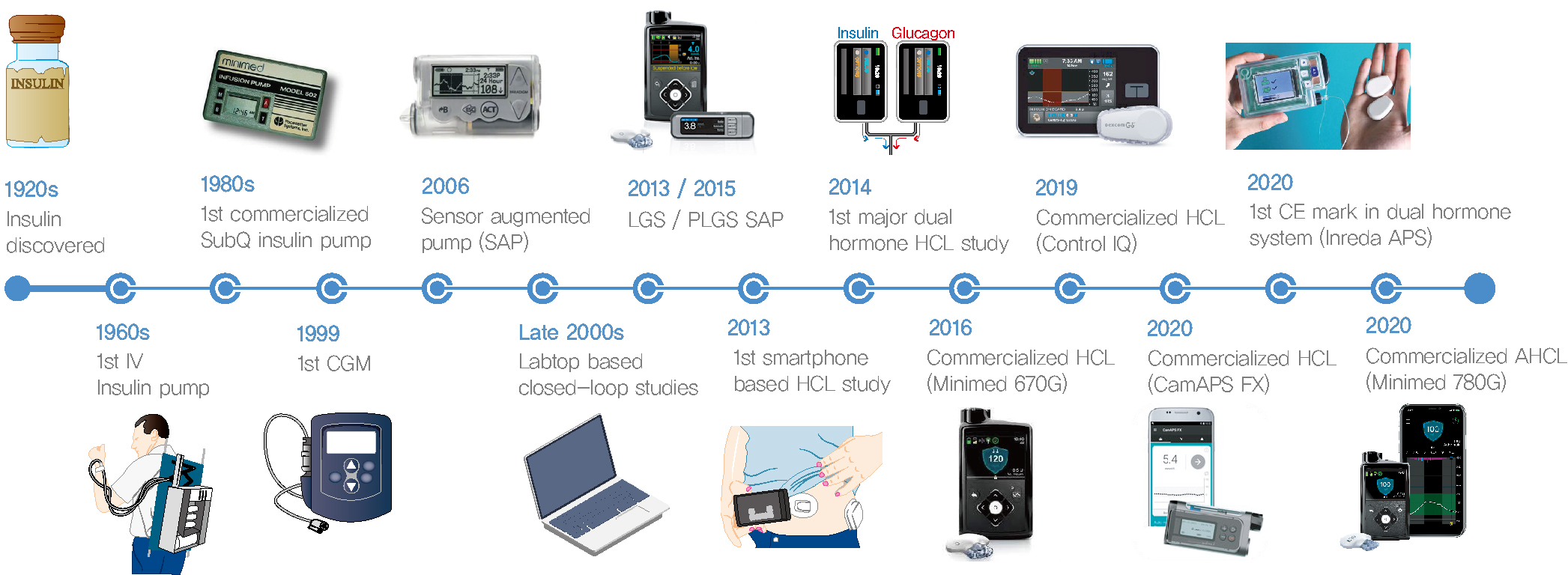
- Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
- Sun Joon Moon, Inha Jung, Cheol-Young Park
- Diabetes Metab J. 2021;45(6):813-839. Published online November 22, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0177
- 14,446 View
- 797 Download
- 29 Web of Science
- 28 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Since Banting and Best isolated insulin in the 1920s, dramatic progress has been made in the treatment of type 1 diabetes mellitus (T1DM). However, dose titration and timely injection to maintain optimal glycemic control are often challenging for T1DM patients and their families because they require frequent blood glucose checks. In recent years, technological advances in insulin pumps and continuous glucose monitoring systems have created paradigm shifts in T1DM care that are being extended to develop artificial pancreas systems (APSs). Numerous studies that demonstrate the superiority of glycemic control offered by APSs over those offered by conventional treatment are still being published, and rapid commercialization and use in actual practice have already begun. Given this rapid development, keeping up with the latest knowledge in an organized way is confusing for both patients and medical staff. Herein, we explore the history, clinical evidence, and current state of APSs, focusing on various development groups and the commercialization status. We also discuss APS development in groups outside the usual T1DM patients and the administration of adjunct agents, such as amylin analogues, in APSs.
-
Citations
Citations to this article as recorded by- Integration of a Safety Module to Prevent Rebound Hypoglycemia in Closed-Loop Artificial Pancreas Systems
María F. Villa-Tamayo, Patricio Colmegna, Marc D. Breton
Journal of Diabetes Science and Technology.2024; 18(2): 318. CrossRef - The effects of acute hyperglycaemia on sports and exercise performance in type 1 diabetes: A systematic review and meta-analysis
Bonar McGuire, Hashim Dadah, Dominic Oliver
Journal of Science and Medicine in Sport.2024; 27(2): 78. CrossRef - A new approach to stabilize diabetes systems with time-varying delays and disturbance rejection
S. Syafiie, Fahd Alharbi, Abdullah Ali Alshehri, Bassam Hasanain
Journal of the Franklin Institute.2024; 361(1): 543. CrossRef - Effects of Low-Dose Glucagon on Subcutaneous Insulin Absorption in Pigs
Ingrid Anna Teigen, Marte Kierulf Åm, Misbah Riaz, Sverre Christian Christiansen, Sven Magnus Carlsen
Current Therapeutic Research.2024; 100: 100736. CrossRef - Robust Online Correlation Method for Identification of a Nonparametric Model of Type 1 Diabetes
Martin Dodek, Eva Miklovičová
IEEE Access.2024; 12: 35899. CrossRef - 100 Years of insulin: A chemical engineering perspective
B. Wayne Bequette
Korean Journal of Chemical Engineering.2023; 40(1): 1. CrossRef - Efficacy of intermittent short‐term use of a real‐time continuous glucose monitoring system in non‐insulin–treated patients with type 2 diabetes: A randomized controlled trial
Sun Joon Moon, Kyung‐Soo Kim, Woo Je Lee, Mi Yeon Lee, Robert Vigersky, Cheol‐Young Park
Diabetes, Obesity and Metabolism.2023; 25(1): 110. CrossRef - Identifiable prediction animal model for the bi-hormonal intraperitoneal artificial pancreas
Karim Davari Benam, Hasti Khoshamadi, Marte Kierulf Åm, Øyvind Stavdahl, Sebastien Gros, Anders Lyngvi Fougner
Journal of Process Control.2023; 121: 13. CrossRef - Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes & Metabolism Journal.2023; 47(1): 27. CrossRef - CGM accuracy: Contrasting CE marking with the governmental controls of the USA (FDA) and Australia (TGA): A narrative review
John S Pemberton, Emma G Wilmot, Katharine Barnard‐Kelly, Lalantha Leelarathna, Nick Oliver, Tabitha Randell, Craig E Taplin, Pratik Choudhary, Peter Adolfsson
Diabetes, Obesity and Metabolism.2023; 25(4): 916. CrossRef - Evaluation of awareness and attitude of paediatric nursing students, nurses, and adolescents regarding type one diabetes advanced devices and virtual nursing
Howaida Moawad Ahmed Ali
Kontakt.2023; 25(2): 100. CrossRef - Predicting the output error of the suboptimal state estimator to improve the performance of the MPC-based artificial pancreas
Martin Dodek, Eva Miklovičová
Control Theory and Technology.2023; 21(4): 541. CrossRef - A Markov Model of Gap Occurrence in Continuous Glucose Monitoring Data for Realistic in Silico Clinical Trials
Martina Vettoretti, Martina Drecogna, Simone Del Favero, Andrea Facchinetti, Giovanni Sparacino
Computer Methods and Programs in Biomedicine.2023; 240: 107700. CrossRef - Drug delivery breakthrough technologies – A perspective on clinical and societal impact
Beate Bittner, Manuel Sánchez-Félix, Dennis Lee, Athanas Koynov, Joshua Horvath, Felix Schumacher, Simon Matoori
Journal of Controlled Release.2023; 360: 335. CrossRef - Importance of continuous glucose monitoring in the treatment of diabetes mellitus
Sun Joon Moon, Won-Young Lee
Journal of the Korean Medical Association.2023; 66(7): 432. CrossRef - Constrained Versus Unconstrained Model Predictive Control for Artificial Pancreas
Chiara Toffanin, Lalo Magni
IEEE Transactions on Control Systems Technology.2023; 31(5): 2288. CrossRef - Intelligent Insulin vs. Artificial Intelligence for Type 1 Diabetes: Will the Real Winner Please Stand Up?
Valentina Maria Cambuli, Marco Giorgio Baroni
International Journal of Molecular Sciences.2023; 24(17): 13139. CrossRef - Artificial Intelligence in Efficient Diabetes Care
Gopal Bhagwan Khodve, Sugato Banerjee
Current Diabetes Reviews.2023;[Epub] CrossRef - The artificial pancreas: two alternative approaches to achieve a fully closed-loop system with optimal glucose control
M. K. Åm, I. A. Teigen, M. Riaz, A. L. Fougner, S. C. Christiansen, S. M. Carlsen
Journal of Endocrinological Investigation.2023; 47(3): 513. CrossRef - Multivariable Automated Insulin Delivery System for Handling Planned and Spontaneous Physical Activities
Mohammad Reza Askari, Mohammad Ahmadasas, Andrew Shahidehpour, Mudassir Rashid, Laurie Quinn, Minsun Park, Ali Cinar
Journal of Diabetes Science and Technology.2023; 17(6): 1456. CrossRef - Advanced Technology (Continuous Glucose Monitoring and Advanced Hybrid Closed-Loop Systems) in Diabetes from the Perspective of Gender Differences
Maria Grazia Nuzzo, Marciano Schettino
Diabetology.2023; 4(4): 519. CrossRef - Artificial Pancreas under a Zone Model Predictive Control based on Gaussian Process models: toward the personalization of the closed loop
Marco Polver, Beatrice Sonzogni, Mirko Mazzoleni, Fabio Previdi, Antonio Ferramosca
IFAC-PapersOnLine.2023; 56(2): 9642. CrossRef - Personalized Constrained MPC for glucose regulation
Chiara Toffanin, Lalo Magni
IFAC-PapersOnLine.2023; 56(2): 9648. CrossRef - Automated Insulin Delivery Systems in Children and Adolescents With Type 1 Diabetes: A Systematic Review and Meta-analysis of Outpatient Randomized Controlled Trials
Baoqi Zeng, Le Gao, Qingqing Yang, Hao Jia, Feng Sun
Diabetes Care.2023; 46(12): 2300. CrossRef - Novel Glycemic Index Based on Continuous Glucose Monitoring to Predict Poor Clinical Outcomes in Critically Ill Patients: A Pilot Study
Eun Yeong Ha, Seung Min Chung, Il Rae Park, Yin Young Lee, Eun Young Choi, Jun Sung Moon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Dual‐hormone artificial pancreas for glucose control in type 1 diabetes: A meta‐analysis
Baoqi Zeng, Hao Jia, Le Gao, Qingqing Yang, Kai Yu, Feng Sun
Diabetes, Obesity and Metabolism.2022; 24(10): 1967. CrossRef - Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Systematic Review
Alezandra Torres-Castaño, Amado Rivero-Santana, Lilisbeth Perestelo-Pérez, Andrea Duarte-Díaz, Analia Abt-Sacks, Vanesa Ramos-García, Yolanda Álvarez-Pérez, Ana M. Wäagner, Mercedes Rigla, Pedro Serrano-Aguilar
Applied Sciences.2022; 12(20): 10262. CrossRef - History of insulin treatment of pediatric patients with diabetes in Korea
Jae Hyun Kim, Choong Ho Shin, Sei Won Yang
Annals of Pediatric Endocrinology & Metabolism.2021; 26(4): 237. CrossRef
- Integration of a Safety Module to Prevent Rebound Hypoglycemia in Closed-Loop Artificial Pancreas Systems
Original Article
- Type 1 Diabetes
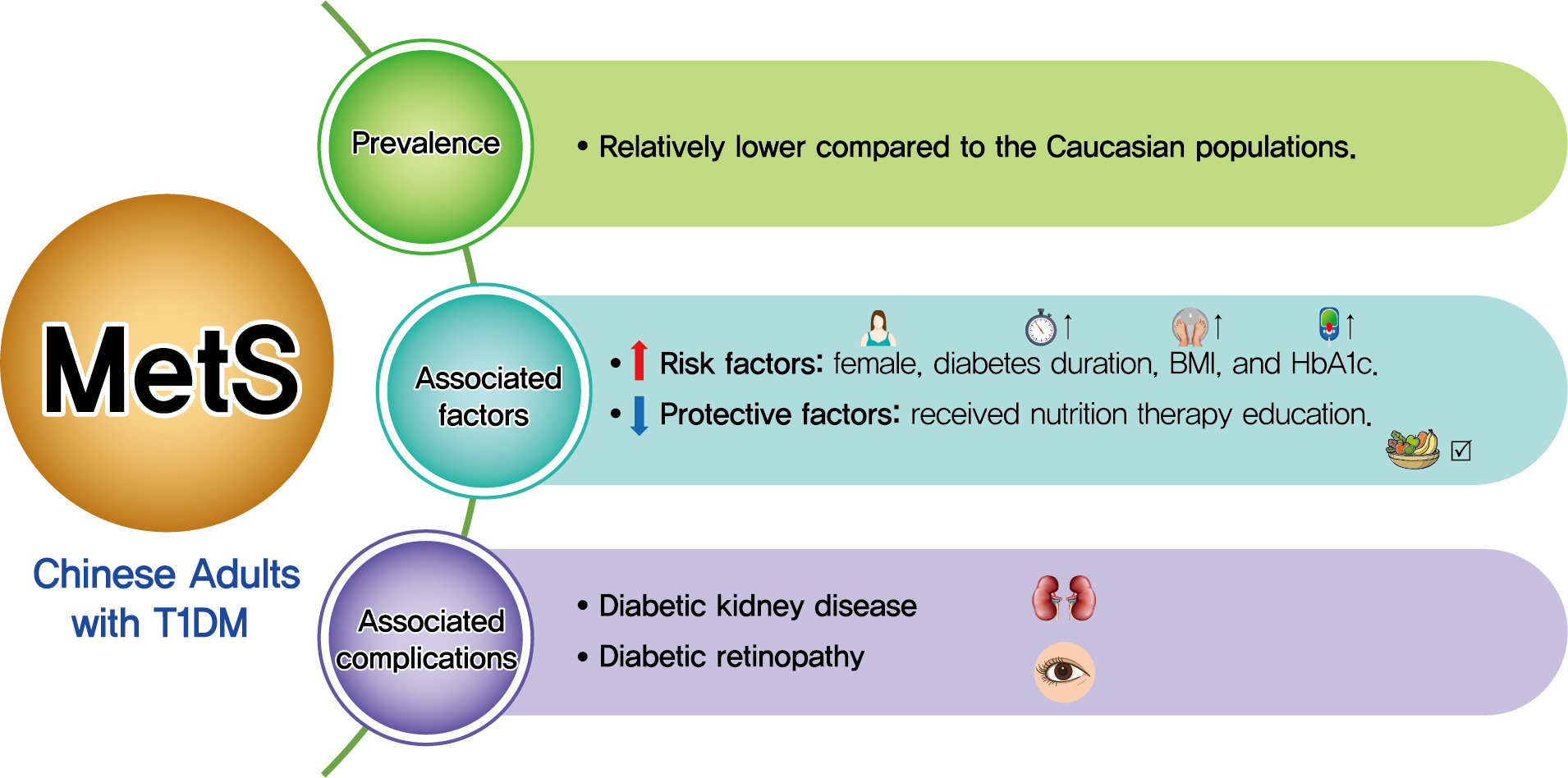
- Association between Metabolic Syndrome and Microvascular Complications in Chinese Adults with Type 1 Diabetes Mellitus
- Qianwen Huang, Daizhi Yang, Hongrong Deng, Hua Liang, Xueying Zheng, Jinhua Yan, Wen Xu, Xiangwen Liu, Bin Yao, Sihui Luo, Jianping Weng
- Diabetes Metab J. 2022;46(1):93-103. Published online August 31, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0240
- 5,665 View
- 203 Download
- 6 Web of Science
- 8 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
Both type 1 diabetes mellitus (T1DM) and metabolic syndrome (MetS) are associated with an elevated risk of morbidity and mortality yet with increasing heterogeneity. This study primarily aimed to evaluate the prevalence of MetS among adult patients with T1DM in China and investigate its associated risk factors, and relationship with microvascular complications.
Methods
We included adult patients who had been enrolled in the Guangdong T1DM Translational Medicine Study conducted from June 2010 to June 2015. MetS was defined according to the updated National Cholesterol Education Program criterion. Logistic regression models were used to estimate the odds ratio (OR) for the association between MetS and the risk of diabetic kidney disease (DKD) and diabetic retinopathy (DR).
Results
Among the 569 eligible patients enrolled, the prevalence of MetS was 15.1%. While female gender, longer diabetes duration, higher body mass index, and glycosylated hemoglobin A1c (HbA1c) were risk factors associated with MetS (OR, 2.86, 1.04, 1.14, and 1.23, respectively), received nutrition therapy education was a protective factor (OR, 0.46). After adjustment for gender, age, diabetes duration, HbA1c, socioeconomic and lifestyle variables, MetS status was associated with an increased risk of DKD and DR (OR, 2.14 and 3.72, respectively; both P<0.05).
Conclusion
Although the prevalence of MetS in adult patients with T1DM in China was relatively low, patients with MetS were more likely to have DKD and DR. A comprehensive management including lifestyle modification might reduce their risk of microvascular complications in adults with T1DM. -
Citations
Citations to this article as recorded by- Prevalence of Metabolic Syndrome and Its Risk Factors Influence on Microvascular Complications in Patients With Type 1 and Type 2 Diabetes Mellitus
Asad Riaz, Shoaib Asghar, Salman Shahid, Haider Tanvir, Muhammad Hamza Ejaz, Mamuna Akram
Cureus.2024;[Epub] CrossRef - Simplified integration of optimal self-management behaviors is associated with improved HbA1c in patients with type 1 diabetes
C. Deng, Y. Xie, F. Liu, X. Tang, L. Fan, X. Yang, Y. Chen, Z. Zhou, X. Li
Journal of Endocrinological Investigation.2024;[Epub] CrossRef - Dynamic Changes in Metabolic Status Are Associated With Risk of Ocular Motor Cranial Nerve Palsies
Daye Diana Choi, Kyung-Ah Park, Kyungdo Han, Sei Yeul Oh
Journal of Neuro-Ophthalmology.2023;[Epub] CrossRef - Development and validation of an age-sex-ethnicity-specific metabolic syndrome score in the Chinese adults
Shujuan Yang, Bin Yu, Wanqi Yu, Shaoqing Dai, Chuanteng Feng, Ying Shao, Xing Zhao, Xiaoqing Li, Tianjing He, Peng Jia
Nature Communications.2023;[Epub] CrossRef - Association of Endotoxemia with Low-Grade Inflammation, Metabolic Syndrome and Distinct Response to Lipopolysaccharide in Type 1 Diabetes
Aleksejs Fedulovs, Leonora Pahirko, Kaspars Jekabsons, Liga Kunrade, Jānis Valeinis, Una Riekstina, Valdis Pīrāgs, Jelizaveta Sokolovska
Biomedicines.2023; 11(12): 3269. CrossRef - Association between Metabolic Syndrome and Microvascular Complications in Chinese Adults with Type 1 Diabetes Mellitus (Diabetes Metab J 2022;46:93-103)
Qianwen Huang, Sihui Luo
Diabetes & Metabolism Journal.2022; 46(3): 515. CrossRef - Association between Metabolic Syndrome and Microvascular Complications in Chinese Adults with Type 1 Diabetes Mellitus (Diabetes Metab J 2022;46:93-103)
Gyuri Kim
Diabetes & Metabolism Journal.2022; 46(3): 512. CrossRef - Metabolic syndrome associated with higher glycemic variability in type 1 diabetes: A multicenter cross-sectional study in china
Keyu Guo, Liyin Zhang, Jianan Ye, Xiaohong Niu, Hongwei Jiang, Shenglian Gan, Jian Zhou, Lin Yang, Zhiguang Zhou
Frontiers in Endocrinology.2022;[Epub] CrossRef
- Prevalence of Metabolic Syndrome and Its Risk Factors Influence on Microvascular Complications in Patients With Type 1 and Type 2 Diabetes Mellitus
Short Communication
- Type 1 Diabetes
- Real-World Analysis of Therapeutic Outcome in Type 1 Diabetes Mellitus at a Tertiary Care Center
- Antonia Kietaibl, Michaela Riedl, Latife Bozkurt
- Diabetes Metab J. 2022;46(1):149-153. Published online July 6, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0267
- 4,357 View
- 144 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin replacement in type 1 diabetes mellitus (T1DM) needs intensified treatment, which can either be performed by multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). This retrospective analysis of a real-world scenario aimed to evaluate whether glycaemic and cardiovascular risk factors could be controlled with CSII outclass MDI as suggested by recent evidence. Data from patients with either insulin pump (n=68) or injection (n=224) therapy at an Austrian tertiary care centre were analysed between January 2016 and December 2017. There were no significant differences with regard to the latest glycosylated hemoglobin, cardiovascular risk factor control or diabetes-associated late complications. Hypoglycaemia was less frequent (P<0.001), sensor-augmented therapy was more common (P=0.003) and mean body mass index (BMI) was higher (P=0.002) with CSII treatment. This retrospective analysis of real-world data in T1DM did not demonstrate the superiority of insulin pump treatment with regard to glycaemic control or cardiovascular risk factor control.

 KDA
KDA
 First
First Prev
Prev





