
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
- Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
- Diabetes Metab J. 2020;44(2):222-233. Published online April 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0053
- 7,527 View
- 169 Download
- 17 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Impaired β-cell function is the key pathophysiology of type 2 diabetes mellitus, and chronic exposure of nutrient excess could lead to this tragedy. For preserving β-cell function, it is essential to understand the cause and mechanisms about the progression of β-cells failure. Glucotoxicity, lipotoxicity, and glucolipotoxicity have been suggested to be a major cause of β-cell dysfunction for decades, but not yet fully understood. Fatty acid translocase cluster determinant 36 (CD36), which is part of the free fatty acid (FFA) transporter system, has been identified in several tissues such as muscle, liver, and insulin-producing cells. Several studies have reported that induction of CD36 increases uptake of FFA in several cells, suggesting the functional interplay between glucose and FFA in terms of insulin secretion and oxidative metabolism. However, we do not currently know the regulating mechanism and physiological role of CD36 on glucolipotoxicity in pancreatic β-cells. Also, the downstream and upstream targets of CD36 related signaling have not been defined. In the present review, we will focus on the expression and function of CD36 related signaling in the pancreatic β-cells in response to hyperglycemia and hyperlipidemia (ceramide) along with the clinical studies on the association between CD36 and metabolic disorders.
-
Citations
Citations to this article as recorded by- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
Alpana Mukhuty, Samanwita Mandal, Chandrani Fouzder, Snehasis Das, Dipanjan Chattopadhyay, Tanmay Majumdar, Rakesh Kundu
Molecular and Cellular Endocrinology.2024; 581: 112112. CrossRef - CD36 gene variant rs1761667(G/A) as a biomarker in obese type 2 diabetes mellitus cases
Ashwin Kumar Shukla, Amreen Shamsad, Atar Singh Kushwah, Shalini Singh, Kauser Usman, Monisha Banerjee
Egyptian Journal of Medical Human Genetics.2024;[Epub] CrossRef - CD36 regulates macrophage and endothelial cell activation and multinucleate giant cell formation in anti neutrophil cytoplasm antibody vasculitis
Xiang Zhang, Catherine King, Alexander Dowell, Paul Moss, Lorraine Harper, Dimitrios Chanouzas, Xiong-zhong Ruan, Alan David Salama
Clinical Immunology.2024; 260: 109914. CrossRef - The association of soluble cluster of differentiation 36 with metabolic diseases: A potential biomarker and therapeutic target
Yun Li, Yaxi Chen, Xiong Z. Ruan
Pediatric Discovery.2023;[Epub] CrossRef - The role of candidate transport proteins in β‐cell long‐chain fatty acid uptake: Where are we now?
Christina Clavelo‐Farrow, Patricia Thomas
Diabetic Medicine.2023;[Epub] CrossRef - SARS-CoV-2 in the pancreas and the impaired islet function in COVID-19 patients
Ningfei Ji, Mingshun Zhang, Liang Ren, Yunyun Wang, Bicheng Hu, Jie Xiang, Yingyun Gong, Chaojie Wu, Guoqiang Qu, Wenqiu Ding, Zhiqiang Yin, Shan Li, Zhengxia Wang, Lianzheng Zhou, Xueqin Chen, Yuan Ma, Jinhai Tang, Yun Liu, Liang Liu, Mao Huang
Emerging Microbes & Infections.2022; 11(1): 1115. CrossRef - Is imaging-based muscle quantity associated with risk of diabetes? A meta-analysis of cohort studies
Shanhu Qiu, Xue Cai, Yang Yuan, Bo Xie, Zilin Sun, Tongzhi Wu
Diabetes Research and Clinical Practice.2022; 189: 109939. CrossRef - Lipotoxicity in a Vicious Cycle of Pancreatic Beta Cell Exhaustion
Vladimir Grubelnik, Jan Zmazek, Matej Završnik, Marko Marhl
Biomedicines.2022; 10(7): 1627. CrossRef - Association of cluster determinant 36, scavenger receptor class B type 1, and major facilitator superfamily domain containing the 2a genetic polymorphism with serum lipid profile in aging population with type 2 diabetes mellitus
Xixiang Wang, Xiaojun Ma, Jingjing Xu, Yujie Guo, Shaobo Zhou, Huiyan Yu, Linhong Yuan
Frontiers in Nutrition.2022;[Epub] CrossRef - CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages
Noorzaileen Eileena Zaidi, Nur Aima Hafiza Shazali, Thean-Chor Leow, Mohd Azuraidi Osman, Kamariah Ibrahim, Wan-Hee Cheng, Kok-Song Lai, Nik Mohd Afizan Nik Abd Rahman
Cells.2022; 11(22): 3556. CrossRef - The Past and Present Lives of the Intraocular Transmembrane Protein CD36
Rucui Yang, Qingping Liu, Mingzhi Zhang
Cells.2022; 12(1): 171. CrossRef - Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus
Sachin Kumar, Tapan Behl, Monika Sachdeva, Aayush Sehgal, Shilpa Kumari, Arun Kumar, Gagandeep Kaur, Harlokesh Narayan Yadav, Simona Bungau
Life Sciences.2021; 264: 118661. CrossRef - Contribution of rs3211938 polymorphism at CD36 to glucose levels, oxidized low-density lipoproteins, insulin resistance, and body mass index in Mexican mestizos with type-2 diabetes from western Mexico
Beatriz Teresita Martín-Márquez, Flavio Sandoval-Garcia, Mónica Vazquez-Del Mercado, Erika-Aurora Martínez-García, Fernanda-Isadora Corona-Meraz, Ana-Lilia Fletes-Rayas, Soraya-Amalí Zavaleta-Muñiz
Nutrición Hospitalaria.2021;[Epub] CrossRef - Investigating the association of CD36 gene polymorphisms (rs1761667 and rs1527483) with T2DM and dyslipidemia: Statistical analysis, machine learning based prediction, and meta-analysis
Ma’mon M. Hatmal, Walhan Alshaer, Ismail S. Mahmoud, Mohammad A. I. Al-Hatamleh, Hamzeh J. Al-Ameer, Omar Abuyaman, Malek Zihlif, Rohimah Mohamud, Mais Darras, Mohammad Al Shhab, Rand Abu-Raideh, Hilweh Ismail, Ali Al-Hamadi, Ali Abdelhay, Kanhaiya Singh
PLOS ONE.2021; 16(10): e0257857. CrossRef - Misregulation of Wnt Signaling Pathways at the Plasma Membrane in Brain and Metabolic Diseases
Mustafa Karabicici, Yagmur Azbazdar, Evin Iscan, Gunes Ozhan
Membranes.2021; 11(11): 844. CrossRef
- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
- Basic Research
-
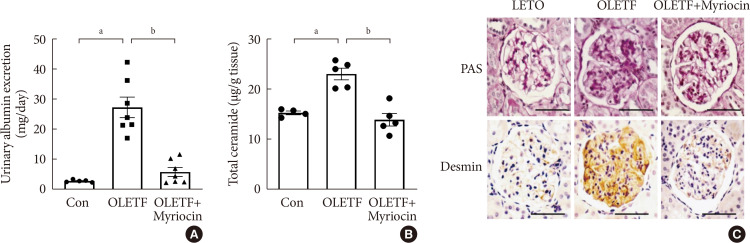
- Inhibition of Ceramide Accumulation in Podocytes by Myriocin Prevents Diabetic Nephropathy
- Chang-Yun Woo, Ji Yeon Baek, Ah-Ram Kim, Chung Hwan Hong, Ji Eun Yoon, Hyoun Sik Kim, Hyun Ju Yoo, Tae-Sik Park, Ranjan Kc, Ki-Up Lee, Eun Hee Koh
- Diabetes Metab J. 2020;44(4):581-591. Published online November 4, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0063
- 6,168 View
- 165 Download
- 26 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Ceramides are associated with metabolic complications including diabetic nephropathy in patients with diabetes. Recent studies have reported that podocytes play a pivotal role in the progression of diabetic nephropathy. Also, mitochondrial dysfunction is known to be an early event in podocyte injury. Thus, we tested the hypothesis that ceramide accumulation in podocytes induces mitochondrial damage through reactive oxygen species (ROS) production in patients with diabetic nephropathy.
Methods We used Otsuka Long Evans Tokushima Fatty (OLETF) rats and high-fat diet (HFD)-fed mice. We fed the animals either a control- or a myriocin-containing diet to evaluate the effects of the ceramide. Also, we assessed the effects of ceramide on intracellular ROS generation and on podocyte autophagy in cultured podocytes.
Results OLETF rats and HFD-fed mice showed albuminuria, histologic features of diabetic nephropathy, and podocyte injury, whereas myriocin treatment effectively treated these abnormalities. Cultured podocytes exposed to agents predicted to be risk factors (high glucose, high free fatty acid, and angiotensin II in combination [GFA]) showed an increase in ceramide accumulation and ROS generation in podocyte mitochondria. Pretreatment with myriocin reversed GFA-induced mitochondrial ROS generation and prevented cell death. Myriocin-pretreated cells were protected from GFA-induced disruption of mitochondrial integrity.
Conclusion We showed that mitochondrial ceramide accumulation may result in podocyte damage through ROS production. Therefore, this signaling pathway could become a pharmacological target to abate the development of diabetic kidney disease.
-
Citations
Citations to this article as recorded by- Interplay of lipid metabolism and inflammation in podocyte injury
Zilv Luo, Zhaowei Chen, Jijia Hu, Guohua Ding
Metabolism.2024; 150: 155718. CrossRef - Associations of plasma sphingolipids with measures of insulin sensitivity, β-cell function, and incident diabetes in Japanese Americans
Ji Cheol Bae, Pandora L. Wander, Rozenn N. Lemaitre, Amanda M. Fretts, Colleen M. Sitlani, Hai H. Bui, Melissa K. Thomas, Donna Leonetti, Wilfred Y. Fujimoto, Edward J. Boyko, Kristina M. Utzschneider
Nutrition, Metabolism and Cardiovascular Diseases.2024; 34(3): 633. CrossRef - A review of the mechanisms of abnormal ceramide metabolism in type 2 diabetes mellitus, Alzheimer’s disease, and their co-morbidities
Yun Pan, Jieying Li, Panjie Lin, Lihua Wan, Yiqian Qu, Lingyong Cao, Lei Wang
Frontiers in Pharmacology.2024;[Epub] CrossRef - Ceramides and mitochondrial homeostasis
Song Ding, Guorui Li, Tinglv Fu, Tianyu Zhang, Xiao Lu, Ning Li, Qing Geng
Cellular Signalling.2024; 117: 111099. CrossRef - Reduced sphingolipid biosynthesis modulates proteostasis networks to enhance longevity
Nathaniel L. Hepowit, Eric Blalock, Sangderk Lee, Kimberly M. Bretland, Jason A. MacGurn, Robert C. Dickson
Aging.2023; 15(2): 472. CrossRef - Protective effect of natural products in the metabolic-associated kidney diseases via regulating mitochondrial dysfunction
Peng Liu, Yao Chen, Jing Xiao, Wenhui Zhu, Xiaoming Yan, Ming Chen
Frontiers in Pharmacology.2023;[Epub] CrossRef - BCAA insufficiency leads to premature ovarian insufficiency via ceramide‐induced elevation of ROS
Xiao Guo, Yuemeng Zhu, Lu Guo, Yiwen Qi, Xiaocheng Liu, Jinhui Wang, Jiangtao Zhang, Linlin Cui, Yueyang Shi, Qichu Wang, Cenxi Liu, Guangxing Lu, Yilian Liu, Tao Li, Shangyu Hong, Yingying Qin, Xuelian Xiong, Hao Wu, Lin Huang, He Huang, Chao Gu, Bin Li,
EMBO Molecular Medicine.2023;[Epub] CrossRef - Chinese herbal medicine and its active compounds in attenuating renal injury via regulating autophagy in diabetic kidney disease
Peng Liu, Wenhui Zhu, Yang Wang, Guijie Ma, Hailing Zhao, Ping Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Integrated gas chromatography‐mass spectrometry and ultra‐high‐performance liquid chromatography‐mass spectrometry renal metabolomics and lipidomics deciphered the metabolic regulation mechanism of Gushudan on kidney‐yang‐deficiency‐syndrome rats
Qing Lu, Jing Zhang, Ling Xin, Yanwei Lou, Feng Qin, Longshan Zhao, Zhili Xiong
Journal of Separation Science.2023;[Epub] CrossRef - Advances in the pharmacological study of Chinese herbal medicine to alleviate diabetic nephropathy by improving mitochondrial oxidative stress
Ming Chen, Yao Chen, Wenhui Zhu, Xiaoming Yan, Jing Xiao, Peiqing Zhang, Peng Liu, Ping Li
Biomedicine & Pharmacotherapy.2023; 165: 115088. CrossRef - Rodent models to study type 1 and type 2 diabetes induced human diabetic nephropathy
Amit Talukdar, Mandira Basumatary
Molecular Biology Reports.2023; 50(9): 7759. CrossRef - Art2 mediates selective endocytosis of methionine transporters during adaptation to sphingolipid depletion
Nathaniel L. Hepowit, Bradley Moon, Adam C. Ebert, Robert C. Dickson, Jason A. MacGurn
Journal of Cell Science.2023;[Epub] CrossRef - Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease
Alla Mitrofanova, Sandra Merscher, Alessia Fornoni
Nature Reviews Nephrology.2023; 19(10): 629. CrossRef - Research progress of autophagy in pathogenesis of diabetes nephropathy
Shengnan Zeng, Ying Li
Diabetic Nephropathy.2023; 3(3): 51. CrossRef - Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease
Judy Baek, Chenchen He, Farsad Afshinnia, George Michailidis, Subramaniam Pennathur
Nature Reviews Nephrology.2022; 18(1): 38. CrossRef - Podocyte Bioenergetics in the Development of Diabetic Nephropathy: The Role of Mitochondria
Irena Audzeyenka, Agnieszka Bierżyńska, Abigail C Lay
Endocrinology.2022;[Epub] CrossRef - Acylcarnitines: Can They Be Biomarkers of Diabetic Nephropathy?
Xiaodie Mu, Min Yang, Peiyao Ling, Aihua Wu, Hua Zhou, Jingting Jiang
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 247. CrossRef - Research Progress on Natural Products’ Therapeutic Effects on Atrial Fibrillation by Regulating Ion Channels
Jinshan He, Sicong Li, Yumeng Ding, Yujia Tong, Xuebin Li, Simona Saponara
Cardiovascular Therapeutics.2022; 2022: 1. CrossRef - Mechanisms of podocyte injury and implications for diabetic nephropathy
Federica Barutta, Stefania Bellini, Gabriella Gruden
Clinical Science.2022; 136(7): 493. CrossRef - A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury
Norishi Ueda
International Journal of Molecular Sciences.2022; 23(7): 4010. CrossRef - Implications of Sphingolipid Metabolites in Kidney Diseases
Shamroop kumar Mallela, Sandra Merscher, Alessia Fornoni
International Journal of Molecular Sciences.2022; 23(8): 4244. CrossRef - Role of ceramides in the pathogenesis of diabetes mellitus and its complications
Nawajes Mandal, Richard Grambergs, Koushik Mondal, Sandip K. Basu, Faiza Tahia, Sam Dagogo-Jack
Journal of Diabetes and its Complications.2021; 35(2): 107734. CrossRef - Rotten to the Cortex: Ceramide-Mediated Lipotoxicity in Diabetic Kidney Disease
Rebekah J. Nicholson, Marcus G. Pezzolesi, Scott A. Summers
Frontiers in Endocrinology.2021;[Epub] CrossRef - Enhancing lifespan of budding yeast by pharmacological lowering of amino acid pools
Nathaniel L. Hepowit, Jessica K. A. Macedo, Lyndsay E. A. Young, Ke Liu, Ramon C. Sun, Jason A. MacGurn, Robert C. Dickson
Aging.2021; 13(6): 7846. CrossRef - New insights into renal lipid dysmetabolism in diabetic kidney disease
Alla Mitrofanova, George Burke, Sandra Merscher, Alessia Fornoni
World Journal of Diabetes.2021; 12(5): 524. CrossRef - Excessively Enlarged Mitochondria in the Kidneys of Diabetic Nephropathy
Kiyoung Kim, Eun-Young Lee
Antioxidants.2021; 10(5): 741. CrossRef - Mechanistic insights into the role of serum-glucocorticoid kinase 1 in diabetic nephropathy: A systematic review
Saba Noor, Taj Mohammad, Gulam M. Ashraf, Joviana Farhat, Anwar L. Bilgrami, Mathew Suji Eapen, Sukhwinder Singh Sohal, Dharmendra Kumar Yadav, Md Imtaiyaz Hassan
International Journal of Biological Macromolecules.2021; 193: 562. CrossRef - The Updates of Podocyte Lipid Metabolism in Proteinuric Kidney Disease
Yu Sun, Sijia Cui, Yunfeng Hou, Fan Yi
Kidney Diseases.2021; 7(6): 438. CrossRef - Saturated fatty acids induce insulin resistance in podocytes through inhibition of IRS1 via activation of both IKKβ and mTORC1
Benoit Denhez, Marina Rousseau, Crysta Spino, David-Alexandre Dancosst, Marie-Ève Dumas, Andréanne Guay, Farah Lizotte, Pedro Geraldes
Scientific Reports.2020;[Epub] CrossRef
- Interplay of lipid metabolism and inflammation in podocyte injury
- Effects of Endurance Exercise and High-Fat Diet on Insulin Resistance and Ceramide Contents of Skeletal Muscle in Sprague-Dawley Rats
- Hyun Lyung Jung, Ho Youl Kang
- Korean Diabetes J. 2010;34(4):244-252. Published online August 31, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.4.244
- 3,276 View
- 28 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background We evaluated the effects of endurance exercise and a high-fat diet on insulin resistance and ceramide contents of skeletal muscle in Sprague-Dawley rats.
Methods We randomly divided 32 rats into four groups: control (CON,
n = 8), high fat diet (HF,n = 8), exercise (Ex, 24 m/min for 2 hours, 5 days/wk,n = 8), HF/Ex (n = 8). After 4-week treatments, plasma lipid profiles, glucose and insulin concentrations were measured. The triglycerides (TG), ceramide, and glucose transporter 4 (GLUT-4) contents were measured in the skeletal muscle. The rate of glucose transport was determined under submaximal insulin concentration during the muscle incubation.Results Free fatty acid levels were significantly higher in CON and HF than Ex (
P = 0.032). Plasma glucose levels in HF were significantly higher than the two Ex groups (P = 0.002), and insulin levels were significantly higher in HF than in other three groups (P = 0.021). Muscular TG concentrations were significantly higher in HF than CON and Ex and also in HF/Ex than Ex, respectively (P = 0.005). Hepatic TG concentrations were significantly higher in HF than other three groups but Ex was significantly lower than HF/Ex (P = 0.000). Muscular ceramide content in HF was significantly greater than that in either Ex or HF/Ex (P = 0.031). GLUT-4 levels in CON and HF were significantly lower than those in Ex and HF/Ex (P = 0.009,P = 0.003). The glucose transport rate in submaximal insulin concentration was lower in CON than in either Ex or HF/Ex (P = 0.043), but not different from HF.Conclusion This study suggests that high fat diet for 4 weeks selectively impairs insulin resistance, but not glucose transport rate, GLUT-4 and ceramide content in skeletal muscle per se. However, endurance exercise markedly affects the content of ceramide and insulin resistance in muscle.
-
Citations
Citations to this article as recorded by- Early warning for inactive ovaries based on liver function index, serum MDA, IL-6, FGF21 and ANGPTL8 in dairy cows
Zhijie Wang, Yuxi Song, Feng Zhang, Chang Zhao, Shixin Fu, Cheng Xia, Yunlong Bai
Italian Journal of Animal Science.2022; 21(1): 113. CrossRef - Metabolic Syndrome is Associated with Ceramide Accumulation in Visceral Adipose Tissue of Women with Morbid Obesity
Barbara Choromańska, Piotr Myśliwiec, Hady Razak Hady, Jacek Dadan, Hanna Myśliwiec, Adrian Chabowski, Agnieszka Mikłosz
Obesity.2019; 27(3): 444. CrossRef - Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse
Alexey M. Petrov, Maria N. Shalagina, Vladimir A. Protopopov, Valeriy G. Sergeev, Sergey V. Ovechkin, Natalia G. Ovchinina, Alexey V. Sekunov, Andrey L. Zefirov, Guzalia F. Zakirjanova, Irina G. Bryndina
International Journal of Molecular Sciences.2019; 20(19): 4860. CrossRef - Lipids in psychiatric disorders and preventive medicine
Miriam Schneider, Beth Levant, Martin Reichel, Erich Gulbins, Johannes Kornhuber, Christian P. Müller
Neuroscience & Biobehavioral Reviews.2017; 76: 336. CrossRef - A mitochondrial-targeted ubiquinone modulates muscle lipid profile and improves mitochondrial respiration in obesogenic diet-fed rats
Charles Coudray, Gilles Fouret, Karen Lambert, Carla Ferreri, Jennifer Rieusset, Agnieszka Blachnio-Zabielska, Jérôme Lecomte, Raymond Ebabe Elle, Eric Badia, Michael P. Murphy, Christine Feillet-Coudray
British Journal of Nutrition.2016; 115(7): 1155. CrossRef - The Metabolic Implications of Glucocorticoids in a High-Fat Diet Setting and the Counter-Effects of Exercise
Emily Dunford, Michael Riddell
Metabolites.2016; 6(4): 44. CrossRef - Effects of Exercise Intensity on PGC-1α, PPAR-γ, and Insulin Resistance in Skeletal Muscle of High Fat Diet-fed Sprague-Dawley Rats
Hyun-Lyung Jung, Ho-Youl Kang
Journal of the Korean Society of Food Science and Nutrition.2014; 43(7): 963. CrossRef - The roles of aerobic exercise training and suppression IL-6 gene expression by RNA interference in the development of insulin resistance
Hui Tang, Min-hao Xie, Yu Lei, Liang Zhou, Yu-ping Xu, Jian-guang Cai
Cytokine.2013; 61(2): 394. CrossRef - Long-Term Consequences of Developmental Alcohol Exposure on Brain Structure and Function: Therapeutic Benefits of Physical Activity
Anna Klintsova, Gillian Hamilton, Karen Boschen
Brain Sciences.2012; 3(4): 1. CrossRef
- Early warning for inactive ovaries based on liver function index, serum MDA, IL-6, FGF21 and ANGPTL8 in dairy cows

 KDA
KDA
 First
First Prev
Prev





