- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 44(2); 2020 > Article
-
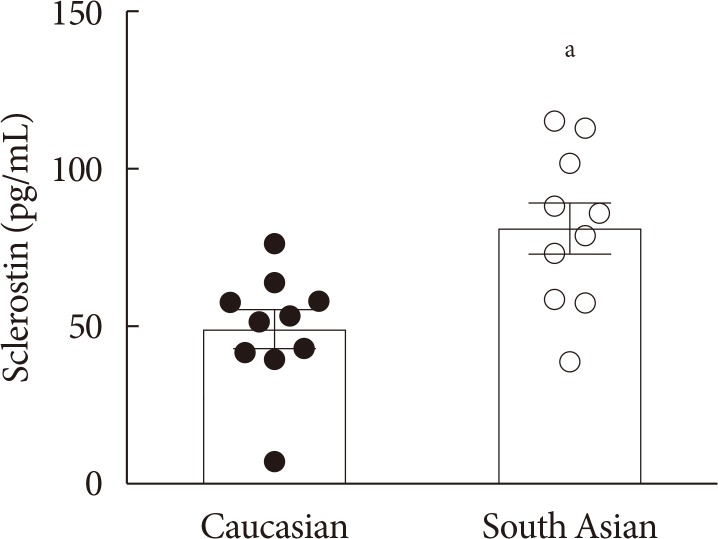
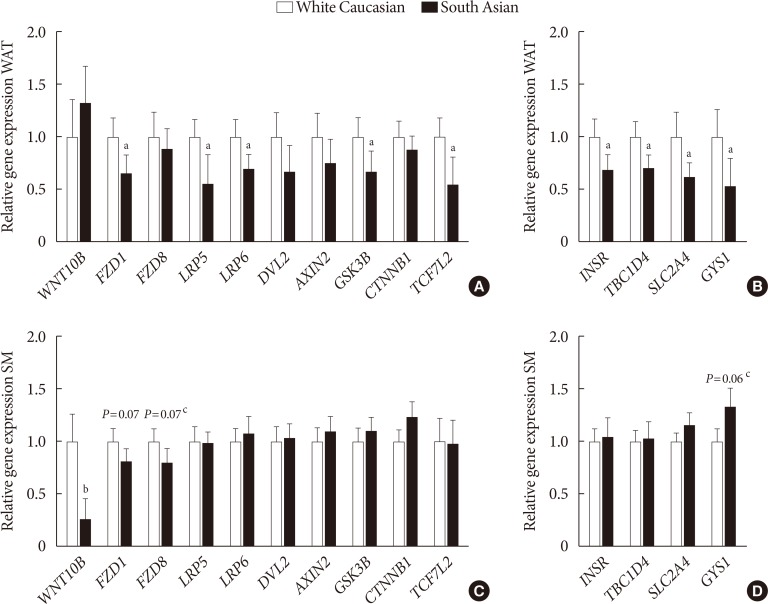
Original ArticleBasic Research Higher Plasma Sclerostin and Lower Wnt Signaling Gene Expression in White Adipose Tissue of Prediabetic South Asian Men Compared with White Caucasian Men
-
Laura G.M. Janssen1,2
 , Andrea D. van Dam1,2, Mark J.W. Hanssen3, Sander Kooijman1,2, Kimberly J. Nahon1,2, Hanneke Reinders1,2, Ingrid M. Jazet1, Wouter D. van Marken Lichtenbelt3, Patrick C.N. Rensen1,2, Natasha M. Appelman-Dijkstra1,4, Mariëtte R. Boon1,2
, Andrea D. van Dam1,2, Mark J.W. Hanssen3, Sander Kooijman1,2, Kimberly J. Nahon1,2, Hanneke Reinders1,2, Ingrid M. Jazet1, Wouter D. van Marken Lichtenbelt3, Patrick C.N. Rensen1,2, Natasha M. Appelman-Dijkstra1,4, Mariëtte R. Boon1,2 -
Diabetes & Metabolism Journal 2020;44(2):326-335.
DOI: https://doi.org/10.4093/dmj.2019.0031
Published online: October 31, 2019
1Division of Endocrinology, Department of Medicine, Leiden University Medical Center, Leiden, The Netherlands.
2Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, Leiden, The Netherlands.
3Department of Human Biology and Movement Sciences, NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht University Medical Center, Maastricht, The Netherlands.
4Center for Bone Quality, Division of Endocrinology, Leiden University Medical Center, Leiden, The Netherlands.
- Corresponding author: Laura G.M. Janssen. Division of Endocrinology, Department of Medicine, Leiden University Medical Center, P.O. Box 9600, 2300 RC Leiden, The Netherlands. l.g.m.janssen@lumc.nl
Copyright © 2020 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Insights on effects of Wnt pathway modulation on insulin signaling and glucose homeostasis for the treatment of type 2 diabetes mellitus: Wnt activation or Wnt inhibition?
Ruchi Yadav, Bhumika Patel
International Journal of Biological Macromolecules.2024; 261: 129634. CrossRef - Evaluating the correlation of sclerostin levels with obesity and type 2 diabetes in a multiethnic population living in Kuwait
Tahani Alramah, Preethi Cherian, Irina Al-Khairi, Mohamed Abu-Farha, Thangavel Alphonse Thanaraj, Ahmed N. Albatineh, Fayez Safadi, Hamad Ali, Muhammad Abdul-Ghani, Jaakko Tuomilehto, Heikki A. Koistinen, Fahd Al-Mulla, Jehad Abubaker
Frontiers in Endocrinology.2024;[Epub] CrossRef - The role of the Wnt signalling pathway in the energy metabolism of bone remodelling
Mengyuan Zhu, Zhipeng Fan
Cell Proliferation.2022;[Epub] CrossRef - Transcriptomic analysis provides a new insight: Oleuropein reverses high glucose-induced osteogenic inhibition in bone marrow mesenchymal stem cells via Wnt10b activation
An Lao, Yu Chen, Yiting Sun, Tiange Wang, Kaili Lin, Jiaqiang Liu, Jianyong Wu
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Subcutaneous adipose tissue sclerostin is reduced and Wnt signaling is enhanced following 4‐weeks of sprint interval training in young men with obesity
Nigel Kurgan, Hashim Islam, Jennifer B. L. Matusiak, Bradley J. Baranowski, Joshua Stoikos, Val A. Fajardo, Rebecca E. K. MacPherson, Brendon J. Gurd, Panagiota Klentrou
Physiological Reports.2022;[Epub] CrossRef - Circulating Levels of Sclerostin Predict Glycemic Improvement after Sleeve Gastrectomy
Federico Carbone, Elisa Nulli Migliola, Aldo Bonaventura, Alessandra Vecchié, Stefano De Vuono, Maria Anastasia Ricci, Gaetano Vaudo, Marcello Boni, Stefano Ministrini, Graziana Lupattelli, Fabrizio Montecucco
Nutrients.2021; 13(2): 623. CrossRef - Serum sclerostin concentration is associated with specific adipose, muscle and bone tissue markers in lean adolescent females with increased physical activity
Jaak Jürimäe, Vita Karvelyte, Liina Remmel, Anna-Liisa Tamm, Priit Purge, Rita Gruodyte-Raciene, Sigitas Kamandulis, Katre Maasalu, Luis Gracia-Marco, Vallo Tillmann
Journal of Pediatric Endocrinology and Metabolism.2021; 34(6): 755. CrossRef - The complex role of Wnt ligands in type 2 diabetes mellitus and related complications
Xiaobo Nie, Xiaoyun Wei, Han Ma, Lili Fan, Wei‐Dong Chen
Journal of Cellular and Molecular Medicine.2021; 25(14): 6479. CrossRef

 KDA
KDA


 PubReader
PubReader Cite
Cite