- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 43(4); 2019 > Article
-
Original ArticleClinical Diabetes & Therapeutics Effectiveness and Safety of Adding Basal Insulin Glargine in Patients with Type 2 Diabetes Mellitus Exhibiting Inadequate Response to Metformin and DPP-4 Inhibitors with or without Sulfonylurea
-
Yu Mi Kang1
 , Chang Hee Jung1
, Chang Hee Jung1 , Seung-Hwan Lee2, Sang-Wook Kim3, Kee-Ho Song4, Sin Gon Kim5, Jae Hyeon Kim6, Young Min Cho7, Tae Sun Park8, Bon Jeong Ku9, Gwanpyo Koh10, Dol Mi Kim11, Byung-Wan Lee12
, Seung-Hwan Lee2, Sang-Wook Kim3, Kee-Ho Song4, Sin Gon Kim5, Jae Hyeon Kim6, Young Min Cho7, Tae Sun Park8, Bon Jeong Ku9, Gwanpyo Koh10, Dol Mi Kim11, Byung-Wan Lee12 , Joong-Yeol Park1
, Joong-Yeol Park1
-
Diabetes & Metabolism Journal 2019;43(4):432-446.
DOI: https://doi.org/10.4093/dmj.2018.0092
Published online: June 19, 2019
1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
3Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea.
4Department of Internal Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
5Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
6Division of Endocrinology and Metabolism, Department of Medicine, Thyroid Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
7Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
8Division of Endocrinology and Metabolism, Department of Internal Medicine, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea.
9Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea.
10Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea.
11Medical Department of Diabetes and Cardiovascular, Sanofi-Korea, Seoul, Korea.
12Division of Endocrinology and Metabolism, Department of Internal Medicine, Graduate School, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding author: Joong-Yeol Park. Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea. jypark@amc.seoul.kr
- Corresponding author: Byung-Wan Lee. Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. bwanlee@yuhs.ac.kr
- *Yu Mi Kang and Chang Hee Jung contributed equally to this study as first authors.
Copyright © 2019 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- We aimed to investigate the effectiveness and safety of adding basal insulin to initiating dipeptidyl peptidase-4 (DPP-4) inhibitor and metformin and/or sulfonylurea (SU) in achieving the target glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2DM).
-
Methods
- This was a single-arm, multicenter, 24-week, open-label, phase 4 study in patients with inadequately controlled (HbA1c ≥7.5%) T2DM despite the use of DPP-4 inhibitor and metformin. A total of 108 patients received insulin glargine while continuing oral antidiabetic drugs (OADs). The primary efficacy endpoint was the percentage of subjects achieving HbA1c ≤7.0%. Other glycemic profiles were also evaluated, and the safety endpoints were adverse events (AEs) and hypoglycemia.
-
Results
- The median HbA1c at baseline (8.9%; range, 7.5% to 11.1%) decreased to 7.6% (5.5% to 11.7%) at 24 weeks. Overall, 31.7% subjects (n=33) achieved the target HbA1c level of ≤7.0%. The mean differences in body weight and fasting plasma glucose were 1.2±3.4 kg and 56.0±49.8 mg/dL, respectively. Hypoglycemia was reported in 36 subjects (33.3%, 112 episodes), all of which were fully recovered. There was no serious AE attributed to insulin glargine. Body weight change was significantly different between SU users and nonusers (1.5±2.5 kg vs. −0.9±6.0 kg, P=0.011).
-
Conclusion
- The combination add-on therapy of insulin glargine, on metformin and DPP-4 inhibitors with or without SU was safe and efficient in reducing HbA1c levels and thus, is a preferable option in managing T2DM patients exhibiting dysglycemia despite the use of OADs.
- Patients with longstanding type 2 diabetes mellitus (T2DM) exhibit progressive β-cell dysfunction [1], and the impaired insulin secretion challenges the maintenance of the glycemic profile within the recommended glycosylated hemoglobin (HbA1c) goal of ≤7%, as suggested by the American Diabetes Association; hence, adjustment of therapy is often advised in these patients [2].
- Theoretically, therapies overcoming the decline in pancreatic β-cell function without causing much weight gain and hypoglycemic episodes are desirable [3]. In these regards, dipeptidyl peptidase 4 (DPP-4) inhibitors have emerged as a promising class of antidiabetic agents for the treatment of insulinopenic T2DM patients, because they exert protective roles on pancreatic β-cells while inhibiting glucagon secretion by α-cells [4], and also because they target multiple extrapancreatic organs via incretin-related mechanisms [5]. In reality, however, use of DPP-4 inhibitors achieves the HbA1c goal in only approximately 40% of patients [3], thereby leaving insulin the mainstay of treatment in T2DM patients with insulinopenia.
- Although the effectiveness of insulin therapy has been well established by various clinical studies, its use is associated with risks of hypoglycemia and weight gain [6]. DPP-4 inhibitors and insulin exert their antidiabetic effects via different mechanisms, and their complementary effects therefore make the combination of these two agents a logical treatment option [7]. This combination might enable tighter glycemic control with a relatively low risk of hypoglycemia, because the basal insulin would theoretically supplement endogenous insulin production and alleviate the load on β-cells, facilitating recovery of the endogenous insulin response when it is required [8]. In fact, several clinical trials have demonstrated the value of adding DPP-4 inhibitors to the treatment regimen of T2DM patients already receiving insulin treatment [9101112]. In these trials, an additional reduction in HbA1c levels of 0.5% to 0.6% was achieved without an apparent weight gain or an increase in the incidence of hypoglycemia, implying that this combination is both efficacious and safe [9101112]. However, all of these trials were designed to determine the effect of adding DPP-4 inhibitors to the treatment regimen of patients using insulin. Research into the effects of adding insulin in insulin-free subjects who are already taking DPP-4 inhibitors or other oral antidiabetic drugs (OADs) has been limited [8].
- Insulin glargine is an analogue of human insulin that has been modified to provide a consistent level of plasma insulin over a long period, thereby resulting in a relatively lower rate of weight gain and incidence of hypoglycemia [13]. However, to date, the effect of adding insulin glargine to DPP-4 inhibitors and other OADs has not been evaluated. Thus, in this study, we aimed to investigate the effectiveness and safety of adding insulin glargine to DPP-4 inhibitors and metformin and/or sulfonylureas (SUs) in achieving the target HbA1c in T2DM patients.
INTRODUCTION
- Patients and trial design
- This study was a phase 4, multicenter, open-label, single-arm study. Study participants were recruited from 11 university hospitals throughout Korea (ClinicalTrials.gov number, NCT-02027753). Participants who gave written informed consent were screened using inclusion and exclusion criteria. Men and women aged ≥20 years with inadequately controlled T2DM (i.e., HbA1c ≥7.5%) despite a combination therapy of a DPP-4 inhibitor and metformin±SU for at least 3 months were eligible for this study.
- Major exclusion criteria included the following: diabetes other than T2DM; history of continuous basal insulin administration within the preceding 1 year; history of diabetic ketoacidosis within 1 year; history of admission for myocardial infarction, stroke, or heart failure within the preceding 3 months; history of drug or alcohol abuse within the preceding 6 months; body weight (BW) change ≥5 kg within the preceding 3 months; history of hypoglycemic unawareness; medication history of drugs that can influence of glucose metabolism. The complete exclusion criteria are listed in the Supplementary methods.
- Every author has received the approval of an Institutional Review Board (IRB No. 2014-0087, KC13MFMV0619, 2013-09-006, KUH1010517, 2013-08-0033, SMC2013-09-001, 2013-2116, CUH-2013-09-008, CNUH2013-09-008, 2013-10-005, 2013-0699-001) of each institution. All procedures followed were in accordance with the ethical standards of the responsible committee and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients.
- Treatment
- After a 2-week screening period, eligible patients were enrolled for treatment with insulin glargine and a stable dose of OADs for 24 weeks. In general, the doses and dosing frequency of OADs were kept at the patient's pre-study levels. However, a reduction in dose or discontinuation of the SU was allowed when hypoglycemia was predicted, at the discretion of the investigators. Insulin glargine was injected subcutaneously once daily between 8:00 PM and 10:00 PM, preferably at a fixed time. The recommended first dose was either 0.2 U/kg or 10 U. All subjects received appropriate education on the administration of insulin on at least two occasions prior to the first administration.
- The insulin dose was titrated at 3-day intervals using a treat-to-target approach, based on the subject's fasting self-monitored blood glucose (SMBG) measurements. This dose was calculated according to the median of fasting SMBG results from the 3 previous consecutive days, with a target fasting SMBG level of 70 to 130 mg/dL. If fasting SMBG was in the range 130 to 160 mg/dL, the insulin dose was increased by 2 U, and for each 30 mg/dL above that range, a further 2 U (up to a maximum of 6 U) was added. If the fasting SMBG was in the range 50 to 70 mg/dL, a dose reduction of 4 U was recommended. In cases of symptomatic hypoglycemia or a fasting SMBG level of ≤50 mg/dL, the dose was adjusted at the discretion of the investigators. Subjects were advised to contact the investigators when their fasting SMBG levels were ≤50 or >250 mg/dL. They were also required to record the dose and timing of every injection, and investigators double-checked these records by inspecting the used and currently loaded insulin cartridges. No specific counseling on dietary or lifestyle modification was provided for the study subjects. All laboratory measurements were collected and analyzed centrally.
- Effectiveness and safety assessments
- HbA1c and fasting plasma glucose (FPG) were measured at weeks 12 and 24 of the study. The primary efficacy endpoint was the percentage of patients who met the HbA1c ≤7% at week 24. Secondary efficacy endpoints included the proportion of subjects reaching the HbA1c target of ≤7% at week 12, the change in HbA1c, and the rate of change of HbA1c at weeks 12 and 24, compared with baseline. The proportions of subjects with HbA1c ≤6.5% at weeks 12 and 24, FPG, 2-hour postprandial plasma glucose (2h-PPG) at weeks 12 and 24, total daily insulin dose at week 24, mean fasting SMBG on 3 consecutive days during weeks 12 and 24, 7-point SMBG levels during weeks 4, 12, and 24, and BW changes were also assessed during the study period.
- The safety outcomes included adverse events (AEs) and hypoglycemic episodes. AEs were evaluated and coded according to MedDRA version 18.0 (McLean, VA, USA). Serious AE (SAE) was defined as one causing death or threatening life, or if one of the following conditions was met: (1) hospitalization or extension of hospital stay; (2) persistent or significant disability or incapacity; (3) congenital malformation or anomaly; or (4) any other significant medical event.
- Asymptomatic hypoglycemia was defined as a measured blood glucose level of ≤50 mg/dL without typical signs of hypoglycemia. Symptomatic hypoglycemia was defined as the presence of the typical clinical manifestations of hypoglycemia, with or without a measured blood glucose level of ≤70 mg/dL. Severe symptomatic hypoglycemia was defined by the presence of hypoglycemic symptoms that were severe enough to require help for recovery and a measured blood glucose level of ≤36 mg/dL, or by the presence of clinical symptoms that were rapidly alleviated following administration of an oral form of carbohydrate, intravenous glucose, or glucagon. Serious symptomatic hypoglycemia was classified as SAE and was defined if at least one of the following criteria was met: (1) loss of consciousness such that parenteral treatment by others was required; (2) seizure resulting from symptomatic hypoglycemia; or (3) visit to an emergency department or hospitalization for the treatment of hypoglycemia.
- Statistical analysis
- To test the primary efficacy endpoint (i.e., percentage of patients who met the HbA1c ≤7% at 24 weeks), the required sample size for the trial was 108 patients to allow 90% power to detect the difference in the response rate at 24 weeks between a null hypothesis of 22% versus an alternative of 37%, using a two-sided alpha level of 0.05 with a withdrawal rate assumed to be approximately 15%. The proportion (%) and its 95% confidence interval (CI) of subjects who met the target HbA1c of ≤7% at the completion of the 24 week treatment period and the binomial test were used to test the significant difference of target rate of 22%. A between-group difference was compared using a chi-square test or Fisher's exact test.
- The main analysis for effectiveness was performed on the full analysis set, in which all patients exposed to at least one dose of treatment and the HbA1c levels at 24 week were measured. The safety set consisted of all subjects who received at least one dose of treatment. Stratified analysis was conducted on combined treatment with SU.
- Continuous variables are summarized in observed values, mean±standard deviation. Categorical variables are summarized in observed values and proportion, and denominator was determined according to analysis group. All safety analysis was conducted in summary unless mentioned specifically, and all tests were performed at two-sided, 5% significance level. In all analyses on demographic information and basic information, primary and secondary parameters, and safety parameters, a subgroup analysis was conducted according to combined treatment with SU, and comparative analysis using analysis of covariance (ANCOVA) was made to see if there was difference between groups. Patients who were taking SU at screening but discontinued administering SU until one day prior to the initiation of the study drug (insulin glargine) were categorized as SU nonusers. Conversely, patients who have administered SU at screening period but discontinued SU after the day of study drug administration remained in the SU combination group (SU users). Changes in 7-point SMBG measurements at weeks 12 and 24 from baseline were evaluated by repeated measures analysis of variance (ANOVA). ANCOVA was used to analyze the between-group difference with baseline SMBG levels as covariates.
METHODS
- Patients and trial design
- Demographic information and baseline characteristics of subjects enrolled in this clinical trial are summarized in Table 1 and the patient flow is illustrated in Fig. 1. Of 104 subjects, 87 (83.7%) subjects were taking SU, and 17 (16.3%) subjects did not take SU during the study period. Three (17.7% of SU nonusers) subjects in whom SU was prescribed previously and then discontinued prior to study initiation were categorized as SU nonusers. Overall, there were more female (n=61, 58.65%) than male subjects (n=43, 41.35%), and this trend was similarly observed among both SU users and nonusers (Table 1). The mean age of the study subjects was 58.0±10.0 years, and the mean duration of diabetes was 11.1±6.8 years (Table 1). The mean BMI of total subjects was 26.4±3.4 kg/m2, but the SU nonusers had higher BMI at baseline, and a shorter history of diabetes, compared with the SU users (Table 1).
- Effectiveness
- Among the 104 subjects who were exposed to at least one dose of treatment, 33 subjects (31.7%) reached the HbA1c target of ≤7.0% after 24 weeks (Table 2). The percentages of subjects who reached the HbA1c goal of ≤7% were significantly higher than 22%, disproving the null hypothesis of this study (P=0.028). In addition, the results were not significantly different between SU users (31.0%) and SU nonusers (35.3%; P=0.730) (Table 2).
- At week 12, 27.9% of the subjects had reached HbA1c ≤7.0% (95% CI, 19.5 to 37.5), and 27.6% SU users and 29.4% SU nonusers reached the target HbA1c without a statistically significant difference (P=1.000) (Table 2). Mean HbA1c significantly decreased by −1.3%±0.9% at week 12 (P<0.001) and by −1.2%±0.9% at week 24 from baseline (P<0.001) (Table 2). There was no significant difference in the mean HbA1c changes between SU users and nonusers over the study period (Table 2).
- At weeks 12 and week 24, the FPG levels significantly decreased by 59.9±49.5 and 56.0±49.8 mg/dL, respectively, from baseline, with no significant difference between the FPG levels of SU users and nonusers (Table 2). Likewise, 2h-PPG was also significantly decreased by 68.9±81.5 mg/dL at week 12 and 68.6±77.1 mg/dL at week 24, and the use of SU did not significantly affect the results (Table 2).
- The mean initial insulin dose after screening was 10.2±0.8 U, and the mean daily insulin dose at week 24 was 18.6±12.6 U (Supplementary Table 1). The mean daily insulin dose at week 24 was higher in non-SU users (i.e., 18.0±12.4 U in SU users vs. 21.8±13.5 U in SU nonusers), but this difference did not reach statistical significance (P=0.223) (Supplementary Table 1). The mean fasting SMBG measured on 3 consecutive days during weeks 12 and 24 significantly decreased from baseline by 50.6±43.6 and 51.1±41.7 mg/dL, respectively (P<0.001) (Supplementary Table 1), but the administration of SU as part of the regimen did not affect this parameter.
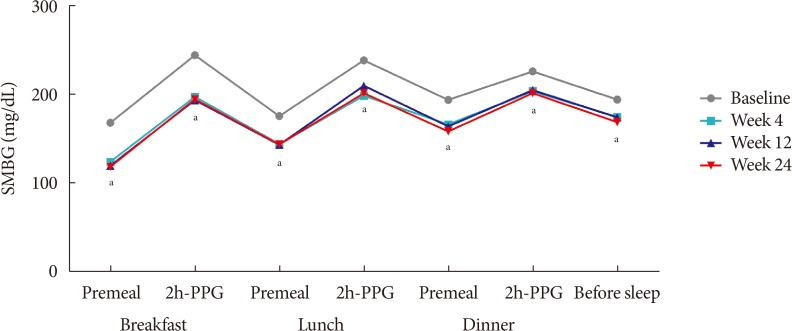
- The changes observed in the 7-point SMBG profile during the study period, which were measured immediately before breakfast, lunch, and dinner, 2 hours after each meal, and at bedtime, are illustrated in Fig. 2. The 7-point SMBG significantly decreased by 4 weeks after treatment, and these levels remained similar until the 24 week of treatment (Fig. 2).
- The mean BW of the study subjects was 69.0±12.7 kg at baseline and 70.2±13.1 kg at 24 weeks, making the mean BW change 1.2±3.4 kg (P<0.001) (Table 2). Subgroup analysis revealed that, over the 24 weeks of treatment, BW increased by 1.5±2.5 kg among SU users whereas it decreased by 0.9±6.0 kg in SU nonusers and that these differences were statistically significant (P=0.011) (Table 2).
- Safety endpoints
- Of the 108 subjects in the safety analysis set, 112 hypoglycemic episodes in 36 subjects (33.33%) were reported (Supplementary Table 2). Among these episodes, 84 were of symptomatic daytime hypoglycemia, 22 were of symptomatic nocturnal hypoglycemia, and six were of asymptomatic hypoglycemia (Supplementary Table 2). Severe symptomatic hypoglycemia occurred in three episodes, consisting of one episode of severe daytime hypoglycemia, and two episodes of severe nocturnal hypoglycemia (Supplementary Table 2). All 112 hypoglycemic episodes were fully recovered, and in 92 cases (82.1%), oral glucose administration was required for recovery. Among SU users, 97 hypoglycemic episodes were recorded in 30 subjects (33.3%), whereas 15 hypoglycemic episodes were reported in six subjects (33.3%) among SU nonusers. The incidence of hypoglycemic episodes did not differ between SU users and nonusers (P>0.05 for all categories of hypoglycemic episodes) (Supplementary Table 2). Any episode of serious symptomatic hypoglycemia was not reported throughout the study.
- All AEs were mild or moderate; no severe AEs were recorded throughout the study period. Of 86 AEs reported, 71 (82.6%) were mild, 15 (17.4%) were of moderate severity (Supplementary Table 3), in which four AEs (4.7%) were evaluated as definitely, probably or possibly related to insulin glargine (Supplementary Table 4). Three cases (3.5%) resulted in permanent discontinuation of administration; administration of insulin glargine was temporarily discontinued in one case (1.5%) and insulin dose was reduced in one case (1.2%). Eight SAEs were reported, none of which were related to insulin glargine use. No statistically significant difference was found in AEs and adverse drug reactions between SU users and SU nonusers (Supplementary Table 3).
RESULTS
- Many patients with T2DM exhibit progressive β-cell dysfunction, meaning that it becomes increasingly difficult to achieve or maintain adequate glycemic control using only the currently available OADs [14]. Following the failure of OADs alone to maintain euglycemia, additive insulin administration can help improve glycemic control and prevent chronic diabetic complications, especially if initiated early in the course of the disease [15]. Moreover, insulin administration can overcome glucotoxicity and thereby preserve β-cell mass and function [16]. However, the risks of hypoglycemia and weight gain are major safety concerns that challenge clinicians when initiating insulin therapy [6]; therefore, it is important to develop treatment regimens that minimize these side effects, while maximizing the benefit of insulins.
- A regimen consisting of basal insulin and OAD is simple and safe, and also incorporates the option of subsequent stepwise intensification, once the patients accept the insulin injection [17]. In addition, the combination of basal insulin and a DPP-4 inhibitor is a logical therapeutic option, given their complementary effects [8]. However, to date, research into the effects of adding insulin to therapeutic regimens involving DPP-4 inhibitors±other OADs has been limited [8].
- This study was designed to evaluate the effectiveness and safety of introducing insulin glargine to T2DM patients who had been failed to control dysglycemia with DPP-4 inhibitors+metformin±SU. After 24 weeks of administering insulin glargine in addition to their pre-study OADs, 31.73% subjects had achieved their HbA1c goal of ≤7% (Table 2). Moreover, secondary outcome analysis revealed a significant reduction in FPG over the study period. Other secondary measures including 2h-PPG, fasting SMBG for 3 consecutive days, and 7-point SMBG levels, were consistent with the primary endpoint, further highlighting the effectiveness of adding insulin glargine to existing OAD-based treatment regimen. A subgroup analysis revealed that these findings stayed unchanged, regardless of the simultaneous administration of SU (Table 2 and Supplementary Table 1). These results suggest that the addition of insulin glargine is an effective add-on to such a regimen, alleviating hyperglycemia that was not controlled with DPP-4 inhibitor and metformin±SU. In addition, cases of atherosclerosis and inflammation of the great toe were classified as definitely related AEs in this study (Supplementary Table 4). However, from a clinical perspective, these events might be more attributable to the underlying T2DM itself than the study drug administration alone. Therefore, the addition of insulin glargine to DPP-4 inhibitor+metformin is considered relatively safe and tolerable, regardless of the concomitant use of SU (Supplementary Tables 2 and 3).
- Our study results are in line with previous studies, in which the safety and effectiveness of combining insulin with OADs including DPP-4 inhibitors were demonstrated. In a study of 296 T2DM patients with inadequate glycemic control (mean HbA1c 8.4%) requiring high doses of basal insulin, addition of 50 mg of vildagliptin twice daily resulted in a modest reduction of HbA1c (−0.5%±0.1%) compared with placebo (−0.3%±0.1%), with fewer hypoglycemic events [10]. Another study of 390 longstanding T2DM patients on insulin therapy with the mean HbA1c level of 9.3%, showed that the addition of either 12.5 or 25 mg/day of alogliptin significantly reduced HbA1c by −0.63% and −0.71%, respectively, compared with the placebo group [11]. During this study, the insulin doses used were unchanged and the mean FPG was little affected by the treatment (i.e., 1.8 mg/dL increase with 12.5 mg and −10.8 mg/dL with 25 mg alogliptin), suggesting that the reduction in HbA1c is primarily due to the control of postprandial hyperglycemia by alogliptin [11]. Although there have been no studies that have evaluated the effectiveness and safety of administering basal insulin alongside pre-study DPP-4 inhibitor/metformin, one study simultaneously initiated basal insulin and DPP-4 inhibitor [18]. In this study of 222 insulin-free, T2DM patients whose hyperglycemia was inadequately controlled (mean HbA1c, 8.5%) by metformin±a second OAD, subjects were randomized to either an insulin determir+sitagliptin+ metformin arm or a sitagliptin+metformin±SU arm. After 26 weeks of treatment, patients who had initiated basal insulin with sitagliptin and metformin showed significantly higher reductions in HbA1c, FPG, and SMBG compared with those who had received sitagliptin+metformin±SU [18]. In this study, no severe hypoglycemia was reported and AEs were less common in the group that was on insulin therapy [18]. Taken together with our findings, these results strongly support the effectiveness and safety of the insulin+DPP-4 inhibitor regimen for the treatment of patients with longstanding T2DM, as well as the hypothesis that the benefits of this combination might be derived from their complementary effects.
- In theory, the benefit of combining basal insulin and DPP-4 inhibitors largely depends on the control of fasting glucose control by basal insulin and the correction of PPG excursions by DPP-4 inhibitors [8]. However, in the present study, 2h-PPG levels were significantly reduced by 68.9±81.5 and 68.6±77.1 mg/dL after 12 and 24 weeks of treatment with insulin glargine, respectively, despite the continuation of pre-study OADs (Table 2). This suggests that administration of basal insulin and a DPP-4 inhibitor together can have a synergistic effect to lower postprandial hyperglycemia.
- In the present study, the mean BW change was 1.15±3.38 (Table 2), which is comparable to that from other studies which had evaluated the effect of insulin glargine-based combination therapies [171819]. In addition, because administration of insulin with SU has always raised a concern of severe hypoglycemia and additive weight gain [20], we ran subgroup analyses according to the administration of SU. As a result, addition of insulin glargine in subjects taking SU did not significantly increase the incidence of hypoglycemic episodes (Supplementary Table 2). When insulin glargine was added, subjects taking SU showed a mean weight gain of 1.53±2.51 kg (Table 2), whereas those without SU in their regimen showed a statistically non-significant weight loss of −0.86±5.95 kg (Table 2) over the study period. Therefore, this difference (i.e., adjusted mean between-group difference, 2.35 kg; P=0.011) (Table 2) ascertains the weight neutral effect of adding insulin glargine in SU-free OAD combination, despite a slightly additive weight gain when combined with SU. Thus, our results provide further evidence to support the early addition of basal insulin to T2DM treatment regimens without significant fear of hypoglycemia and weight gain.
- There are several strengths of the current study. First, it was the first study to demonstrate the effectiveness and safety of adding insulin glargine to pre-study DPP-4 inhibitor/metformin regimens, thereby supporting the safety and effectiveness of adding basal insulin to treatment regimens based on DPP-4 inhibitors and metformin±SU. Specifically, the major benefit of adding basal insulin to the combination of metformin and DPP-4 inhibitors were lack of severe hypoglycemia, regardless of the SU combination and its neutral or favorable effect on the BW when SU is not combined. Second, because the pre-study OAD administration was not altered at the commencement of the study and was mostly maintained throughout the study period, the results of this study should reflect the benefit of adding insulin glargine to existing treatment regimens alone.
- However, this study has several limitations. First, this was a single-arm clinical trial; hence, we could not compare the effect of adding insulin glargine to a placebo group, because the use of the latter would have been a major ethical violation. Therefore, a few potential bias due to the nature of this study should be considered when interpreting our study results. For example, the poor glycemic control at baseline of the subjects could possibly predispose towards a beneficial effect of the addition of insulin glargine over time. Because subjects' dietary patterns or physical activity were not assessed throughout the study, we cannot rule out the possibility that the favorable study result is at least partly attributable to the lifestyle changes due to high motivation and treatment adherence associated with the initiation of new insulin regimen. Second, because it was carried out in a single country, our results may not necessarily apply to people with other races and different backgrounds. However, given that a large multi-national clinical trial has already proven the effectiveness and safety of insulin glargine [21], the principal findings of the present study are likely to be applicable to populations from other backgrounds. Lastly, the subgroup analyses between SU users and nonusers should be carefully interpreted as the number of SU nonusers was much smaller. In addition, because the SU nonusers exhibited higher BMI and shorter duration of diabetes compared to the SU users, the potential difference in endogenous insulin secretory capacity between the two groups should also be considered.
- In conclusion, this study was the first to demonstrate that the addition of insulin glargine to treatment regimens based on DPP-4 inhibitors and metformin for T2DM with inadequate glycemic control can lead to a significant improvement. The combination of insulin glargine alongside DPP-4 inhibitors and metformin was relatively safe and well-tolerable, resulting in minimal weight gain, and these beneficial effects remained significant regardless of the concomitant use of SU. This combination is also simple to apply, making it a practical approach for patients, while also permitting stepwise intensification. Nevertheless, the effects of combining insulin with a treatment regimen based on DPP-4 inhibitors, should be further explored and confirmed in future larger scale clinical studies.
DISCUSSION
-
Acknowledgements
- None
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: Sponsorship for this study and article processing charges was funded by Sanofi-Aventis Korea. Dol Mi Kim is an employee at Sanofi-Aventis Korea. No potential conflict of interest relevant to this article was reported except for her.
-
AUTHOR CONTRIBUTIONS:
NOTES
SUPPLEMENTARY MATERIALS
- 1. Stumvoll M, Goldstein BJ, van Haeften TW. Pathogenesis of type 2 diabetes. Endocr Res 2007;32:19-37. ArticlePubMed
- 2. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-149. ArticlePubMedPDF
- 3. Esposito K, Cozzolino D, Bellastella G, Maiorino MI, Chiodini P, Ceriello A, Giugliano D. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab 2011;13:594-603. ArticlePubMed
- 4. Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004;89:2078-2084. ArticlePubMed
- 5. Gallwitz B. Extra-pancreatic effects of incretin-based therapies. Endocrine 2014;47:360-371. ArticlePubMedPDF
- 6. Makimattila S, Nikkila K, Yki-Jarvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999;42:406-412. ArticlePubMedPDF
- 7. Charbonnel B, Schweizer A, Dejager S. Combination therapy with DPP-4 inhibitors and insulin in patients with type 2 diabetes mellitus: what is the evidence? Hosp Pract (1995) 2013;41:93-107. ArticlePubMed
- 8. Vora J. Combining incretin-based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care 2013;36(Suppl 2):S226-S232. PubMedPMC
- 9. Barnett AH, Charbonnel B, Li J, Donovan M, Fleming D, Iqbal N. Saxagliptin add-on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52-week safety and efficacy. Clin Drug Investig 2013;33:707-717.ArticlePubMedPDF
- 10. Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007;50:1148-1155. ArticlePubMedPDF
- 11. Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab 2009;11:1145-1152. ArticlePubMed
- 12. Vilsboll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD, Amatruda JM, Engel SS, Katz L. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:167-177. ArticlePubMed
- 13. Barnett AH. Insulin glargine in the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag 2006;2:59-67. ArticlePubMedPMC
- 14. Meneghini LF. Early insulin treatment in type 2 diabetes: what are the pros? Diabetes Care 2009;32(Suppl 2):S266-S269. PubMedPMC
- 15. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-1589. ArticlePubMed
- 16. Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 1985;28:119-121. ArticlePubMedPDF
- 17. Meneghini L, Kesavadev J, Demissie M, Nazeri A, Hollander P. Once-daily initiation of basal insulin as add-on to metformin: a 26-week, randomized, treat-to-target trial comparing insulin detemir with insulin glargine in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:729-736. ArticlePubMed
- 18. Hollander P, Raslova K, Skjoth TV, Rastam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the TRANSITION randomized controlled trial. Diabetes Obes Metab 2011;13:268-275. ArticlePubMed
- 19. Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631-636. PubMed
- 20. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association. European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203. ArticlePubMedPMCPDF
- 21. Khunti K, Srinivasan BT, Shutler S, Davies MJ. Effect of insulin glargine on glycaemic control and weight in obese and non-obese people with type 2 diabetes: data from the AT.LANTUS trial. Diabetes Obes Metab 2010;12:683-688. ArticlePubMed
REFERENCES
Mean 7-point self-monitored blood glucose (SMBG) profiles during the study period. 2h-PPG, 2-hour postprandial plasma glucose. aP<0.05.
Demographic and baseline characteristics
Primary and secondary efficacy endpoints
Figure & Data
References
Citations

- Glycaemic control with add‐on thiazolidinedione or a sodium‐glucose co‐transporter‐2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: A 24‐week, randomized controlled trial
Jaehyun Bae, Ji Hye Huh, Minyoung Lee, Yong‐Ho Lee, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2021; 23(2): 609. CrossRef - Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease
Kyung-Soo Kim, Byung-Wan Lee
Clinical and Molecular Hepatology.2020; 26(4): 430. CrossRef

 KDA
KDA
 PubReader
PubReader Cite
Cite