- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 46(3); 2022 > Article
-
Sulwon Lecture 2021Basic Research Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c)
-
Tae Kwan Yoon1*
 , Chan Hee Lee2*
, Chan Hee Lee2* , Obin Kwon3, Min-Seon Kim4
, Obin Kwon3, Min-Seon Kim4
-
Diabetes & Metabolism Journal 2022;46(3):402-413.
DOI: https://doi.org/10.4093/dmj.2022.0092
Published online: May 25, 2022
1Division of Endocrinology and Metabolism, Department of Internal Medicine, H+ Yangji Hospital, Seoul, Korea
2Department of of Biomedical Science & Program of Material Science for Medicine and Pharmaceutics, Hallym University, Chuncheon, Korea
3Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea
4Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
-
Corresponding author: Min-Seon Kim
 Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea E-mail: mskim@amc.seoul.kr
Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea E-mail: mskim@amc.seoul.kr - *Tae Kwan Yoon and Chan Hee Lee contributed equally to this study as first authors.
Copyright © 2022 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- MITOCHONDRIAL ADAPTATION TO EXERCISE
- POTENTIAL MECHANISMS OF EXERCISE-INDUCED MITOHORMESIS
- MITOCHONDRIA-DERIVED PEPTIDES AND THEIR BIOLOGICAL ACTIONS
- EFFECTS OF EXERCISE ON THE EXPRESSION OF MDPs
- EFFECTS OF MOTS-c TREATMENT ON EXERCISE PERFORMANCE
- MOTS-c AND EXERCISE-INDUCED THERMOGENESIS
- CONCLUSIONS
- NOTES
- REFERENCES
ABSTRACT
- Low levels of mitochondrial stress are beneficial for organismal health and survival through a process known as mitohormesis. Mitohormetic responses occur during or after exercise and may mediate some salutary effects of exercise on metabolism. Exercise-related mitohormesis involves reactive oxygen species production, mitochondrial unfolded protein response (UPRmt), and release of mitochondria-derived peptides (MDPs). MDPs are a group of small peptides encoded by mitochondrial DNA with beneficial metabolic effects. Among MDPs, mitochondrial ORF of the 12S rRNA type-c (MOTS-c) is the most associated with exercise. MOTS-c expression levels increase in skeletal muscles, systemic circulation, and the hypothalamus upon exercise. Systemic MOTS-c administration increases exercise performance by boosting skeletal muscle stress responses and by enhancing metabolic adaptation to exercise. Exogenous MOTS-c also stimulates thermogenesis in subcutaneous white adipose tissues, thereby enhancing energy expenditure and contributing to the anti-obesity effects of exercise training. This review briefly summarizes the mitohormetic mechanisms of exercise with an emphasis on MOTS-c.
- In 2016, the World Health Organization (WHO) reported that 39% of adults aged ≥18 years were overweight, and 13% were obese [1]. Additionally, this report indicated that over 340 million children and adolescents aged 5 to 19 were overweight or obese [1]. Since 1975, the prevalence of overweight and obesity in adults and children has continued to grow every year [1]. A rapid expansion in the overweight or obese population concomitantly increases the prevalence of obesity-associated disorders such as type 2 diabetes mellitus, hypertension, dyslipidemia, heart disease, and certain types of cancer [2,3]. Notably, obesity and overweight are preventable and can be alleviated by increasing physical activity [4]. Exercise reduces excessive fat mass and ultimately helps to maintain a non-obese healthy condition [4]. Exercise provides metabolic advantages by increasing oxygen consumption, insulin sensitivity, and fatty acid oxidation [5]. These beneficial effects of exercise are strongly associated with mitochondrial adaptive changes [6,7]. In this review, we will outline the latest knowledge on the role of mitochondria and the mitochondria-derived peptide (MDP) mitochondrial ORF of the 12S rRNA type-c (MOTS-c) in exercise physiology.
INTRODUCTION
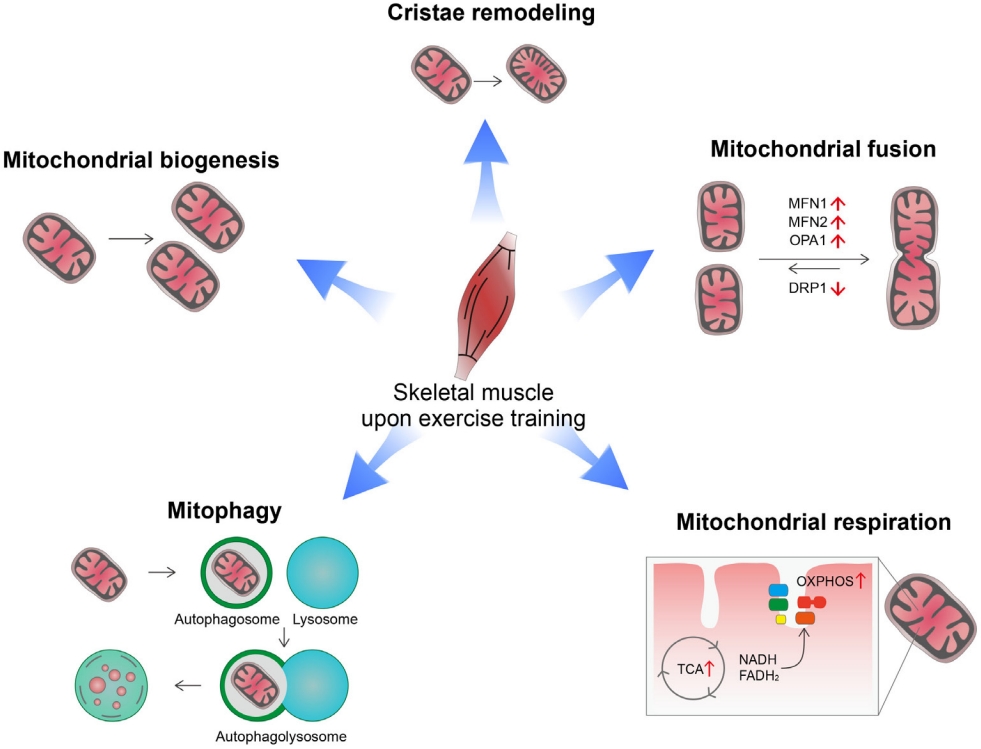
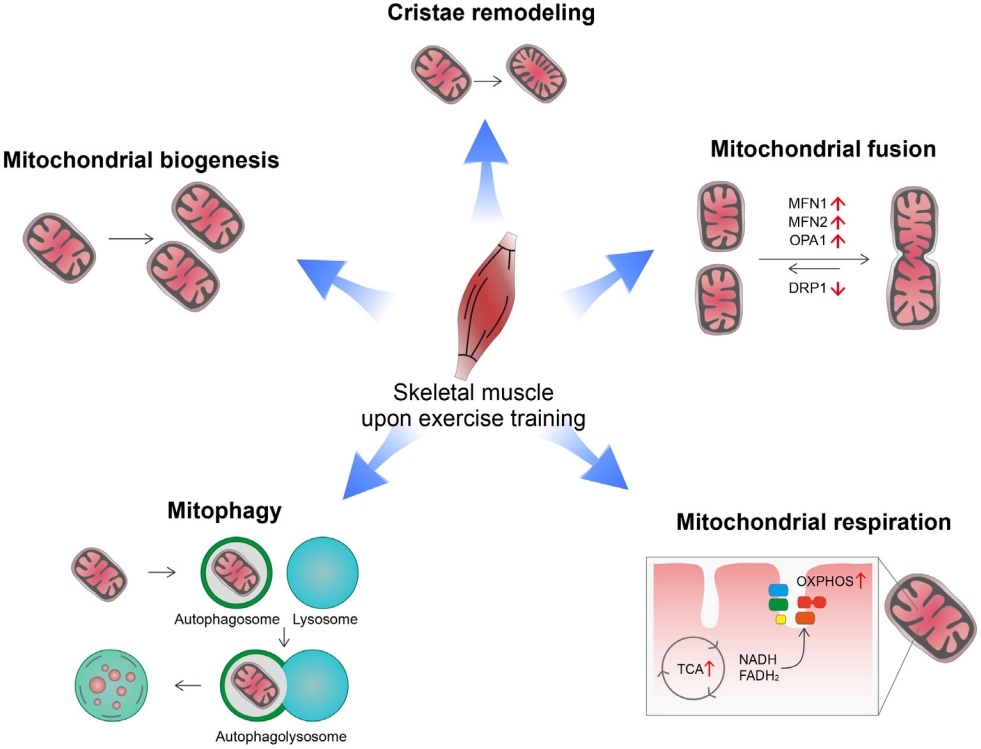
- Exercise induces a variety of changes in mitochondria depending on exercise intensity, duration, and frequency [8,9]. These include changes in mitochondrial biogenesis, mitochondrial dynamics (fusion/fission), cristae remodeling, mitophagy, mitochondrial respiration capacity, and oxidative metabolism (Fig. 1). Exercise offers metabolic advantages such as increased oxygen consumption, insulin sensitivity, and fatty acid oxidation, which are closely linked to mitochondrial functions [5,10].
- High-intensity physical training enhances mitochondrial biogenesis and improves mitochondrial functional capacity [11]. Mitochondrial biogenesis is a process that synthesizes new mitochondria and is primarily regulated by the peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1-alpha (PGC-1α) [12]. All three isoforms of PGC-1α are involved in skeletal muscle mitochondrial biogenesis [13,14]. Indeed, PGC-1α overexpression increases mitochondrial biogenesis and capillary density in skeletal muscle, which leads to increased mitochondrial catabolic metabolism and enhanced physical activity [13]. Exercise increases PGC-1α expression via the β2-adrenergic receptor and its downstream signaling pathways [15]. Additionally, intracellular Ca2+ levels increase in contracting muscles and act as a messenger for the transcriptional regulation of exercise-related genes. The calcium/calmodulin-dependent protein kinase (CaMK) largely mediates the regulatory functions of Ca2+ [16]. Physical exercise activates CaMK-II, the dominant isoform of CaMK in human skeletal muscle [17]. In turn, CaMK-II stimulates mitochondrial biogenesis and improves glucose transport by stimulating PGC-1α expression [18-20]. These findings indicate an involvement of CaMK-II in exercise-induced mitochondrial and metabolic adaptation.
- In addition to mitochondria biogenesis, endurance exercise increases the area of mitochondrial inner membranes per mitochondrial volume in human muscles [21]. Moreover, the mitochondrial cristae density correlates with whole body oxygen uptake and serves as a marker for mitochondrial respiratory capacity [21]. This mitochondrial cristae remodeling may be a part of mitochondrial adaptation for increasing mitochondrial respiration.
- Mitochondrial dynamics (i.e., fission and fusion) play critical roles in maintaining mitochondrial functions and morphology [22]. Key regulators of these processes are the cytosolic GTPase dynamin-related protein 1 (DRP1) for fission and mitofusin 1 (MFN1), MFN2, and optic atrophy 1 (OPA1) for fusion [23-25]. Exercise stimulates mitochondrial fusion by increasing the expression of mitofusins (MFN1, MFN2) in human skeletal muscles [26]. Exercise-induced changes in mitochondrial dynamics are mediated via both the PGC-1α and estrogen-related receptor α (ERRα) [26]. Moreover, inhibiting mitochondrial fusion in skeletal muscles via depletion of MFN1/MFN2 impedes exercise performance and shortens exhaustion time in mice [27]. Taken altogether, mitochondrial fusion may be critical for skeletal muscle adaptation to exercise and exercise performance.
- Autophagy is a catabolic pathway that recycles intracellular components under energy-shortage conditions and clears damaged organs and protein aggregates [28]. Exercise increases mitochondrial autophagy, i.e., mitophagy in skeletal muscle and heart tissue through AMP activated protein kinase (AMPK)- dependent mechanisms [29]. Exercise-induced mitophagy helps to maintain mitochondrial homeostasis by selective removal of damaged/dysfunctional mitochondria [29]. Therefore, mitophagy is considered an important part of mitochondrial adaptation to exercise.
MITOCHONDRIAL ADAPTATION TO EXERCISE
- Hormesis refers to salutary biological adaptations to low continuous or moderate intermittent doses of stress, which may be fatal at higher or chronic doses. Exposure to lower levels of stress protects organisms against subsequent greater stress. Mitohormesis, a compound word derived from mitochondria and hormesis, was initially demonstrated in lower organisms such as Caenorhabditis elegans [30,31]. Exposure to low levels of mitochondrial stressors extends life span and retards aging-related diseases [30,31]. Now, this phenomenon has been observed in mammals [32].
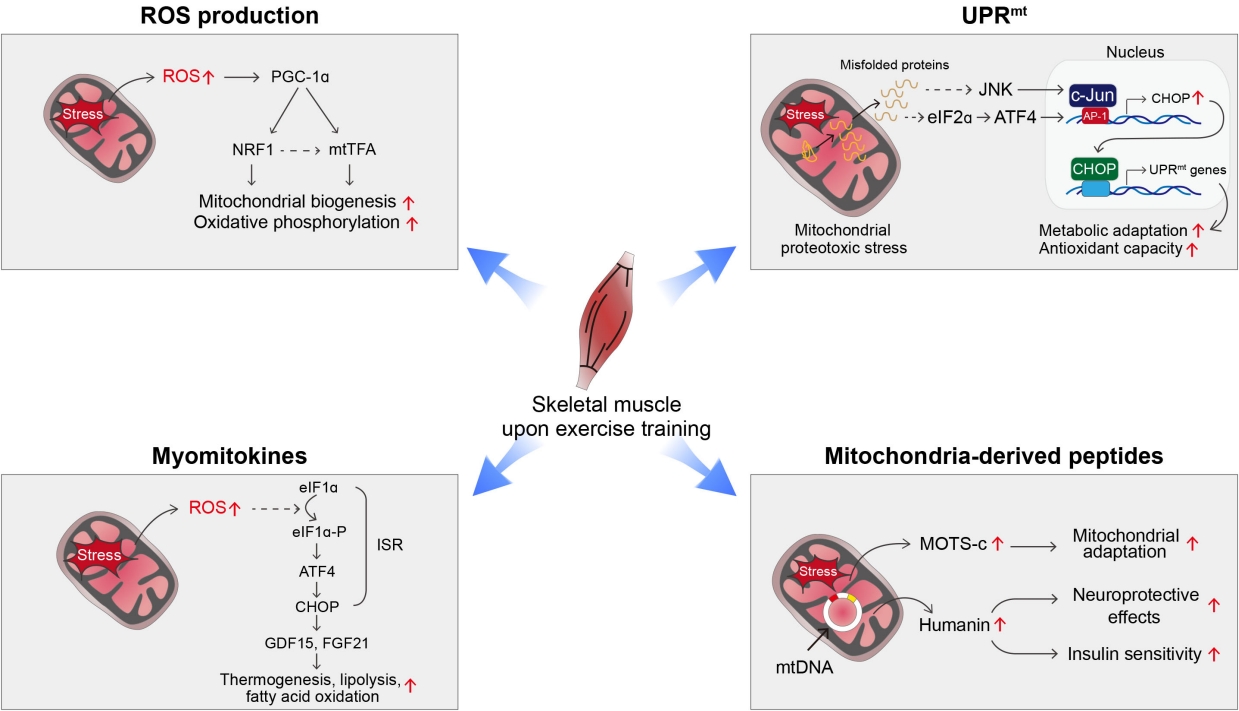
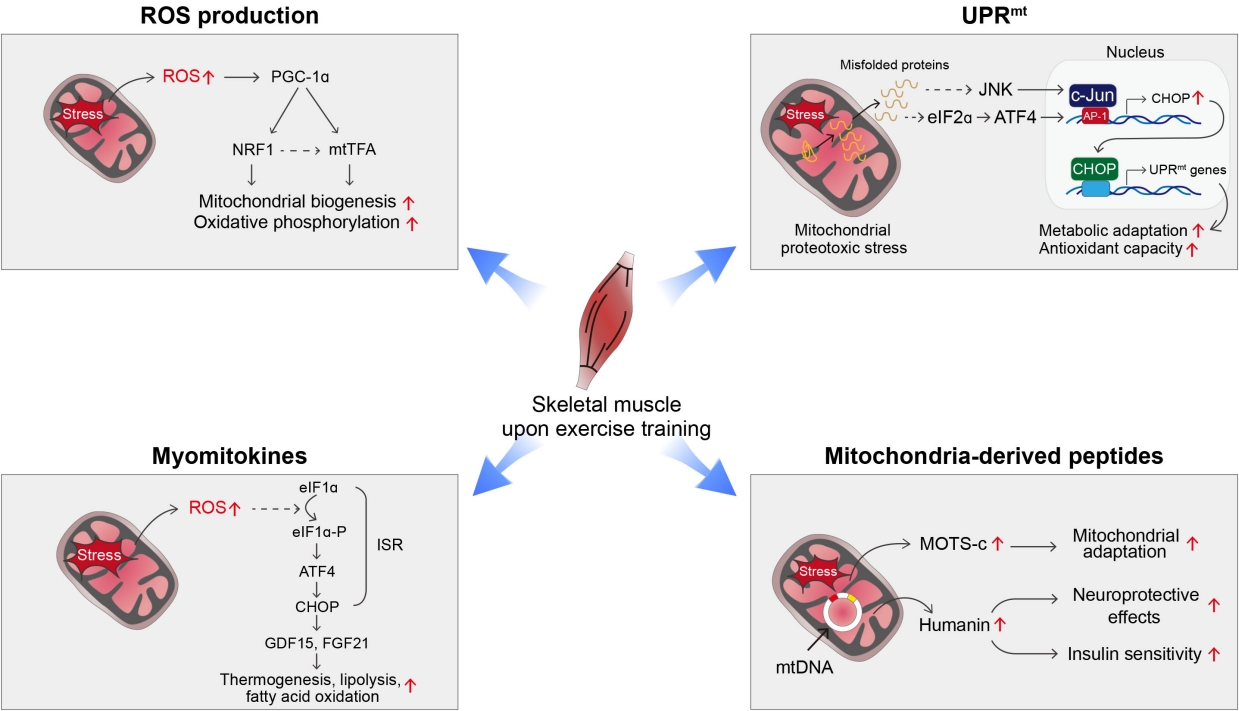
- Exercise may be a powerful way to induce mitohormesis [33]. Potential mechanisms underlying exercise-induced mitohormesis are depicted in Fig. 2. During exercise or muscle contraction, reactive oxygen species (ROS) are produced in skeletal muscle as a byproduct of mitochondrial oxidative phosphorylation (OXPHOS) [33]. Chronic ROS overproduction by excessive metabolic flux and mitochondrial dysfunction, have been implicated in the progression of human diseases such as atherosclerosis, diabetes, dementia, and cancer [34-36]. In contrast, moderate levels of ROS, such as is generated during exercise, may help maintain normal energy metabolism and health [33]. Exercise-induced ROS production increases the expression of PGC-1α, nuclear respiratory factor 1 (NRF1), and mitochondrial transcription factor A (mtTFA)/mitochondrial transcription factor A (TFAM) in skeletal muscle [33]. Moreover, supplementation with antioxidants attenuates exercise-induced PGC-1α expression, mitochondrial biogenesis, and insulin sensitivity in skeletal muscles [37]. In line with this, a chronic moderate degree of exercise training increases ROS production in the hypothalamus, a controlling center of energy balance [38]. Hypothalamic ROS production during exercise training is critical for exercise-induced thermogenesis given that central administration of antioxidants blocks exercise-induced thermogenesis [38].
- The mitochondrial unfolded protein response (UPRmt) is a type of adaptive response to mitochondrial stress that occurs during recovery from various mitochondrial insults [39-41]. Upon mitochondrial stress, unfolded or misfolded proteins accumulate in the mitochondria and are subsequently degraded by mitochondrial proteases and exported to the cytosol [39, 41]. These proteins activate retrograde signaling involving activating transcription factor associated with stress-1 (ATFS-1) in C. elegans and c-Jun N-terminal kinase (JNK) and activating transcription factor 4 (ATF4) in mammals and relay mitochondrial stress signals to the nucleus [39,41]. Consequently, the transcription of mitochondrial chaperones and proteases is stimulated to help resolve mitochondrial proteotoxic stress. Evidence suggests that induction of UPRmt improves survival and provides a health benefit [39-41]. Interestingly, UPRmt also takes place in neighboring cells or distal tissues through a process known as non-cell-autonomous UPRmt [42]. For instance, knocking down electron transport chain components in the nervous system can trigger UPRmt responses in the intestine and extend the life span of C. elegans [42]. It was recently shown that high-intensity interval training induces UPRmt in the skeletal muscle of aged mice [43]. Moreover, we observed that moderate-intensity treadmill exercise induced UPRmt in the white and brown adipose tissue (BAT) of young mice [38]. These data suggest that UPRmt may underlie the mitohormetic effect of exercise.
- Exercise-induced mitohormetic responses may be also mediated by myomitokines such as fibroblast growth factor 21 (FGF21) and growth differentiation factor 15 (GDF15) [44,45]. The expression levels of both factors in skeletal muscle are elevated by exercise-induced mitochondrial stress via the integrated stress response (ISR) involving ATF4-C/EBP-homologous protein (CHOP) pathway [44,45]. They are released into the bloodstream to stimulate adipose tissue thermogenesis, lipolysis, and fatty acid oxidation, leading to alleviation of obesity and obesity-related metabolic complications [46-48].
- Another potential endocrine mediator of exercise-induced mitohormesis is mitochondrial DNA (mtDNA)-encoded small proteins, called MDPs [49], given that their production is increased by exercise and mitochondrial stress and depends on ROS levels [38]. The roles of MDPs in exercise physiology will be described in detail in the following section.
POTENTIAL MECHANISMS OF EXERCISE-INDUCED MITOHORMESIS
- MDPs are small bioactive peptides encoded by short open-reading frames (sORF) in mtDNA. To date, eight MDPs have been identified, most of which have various cell- and organ-protective properties [38,50]. Humanin was the first MDP to be identified and is encoded by an sORF in the 16S ribosomal RNA gene called MT-RNR2 [51,52]. Humanin has been identified as a cytoprotective and anti-apoptotic factor in Alzheimer’s diseases, where it protects neurons from amyloid-β-related cytotoxicity [52,53]. Its neuroprotective effects are mediated via inhibition of JNK, activation of signal transducer and activator of transcription-3 (STAT3) and extracellular signal-regulated kinase (ERK) signaling as well as through interactions with insulin-like growth factor-binding protein 3 (IGFBP-3) [54,55]. Humanin also has beneficial roles in glucose metabolism. Indeed, humanin treatment improves glucose-stimulated insulin secretion and peripheral insulin sensitivity [56,57]. Another six small humanin‐like peptides (SHLP) are encoded in the ORFs within the same 16S rRNA gene in which humanin is located [58]. SHLP-2 and SHLP-3 have similar protective effects as humanin and improve mitochondrial metabolism by increasing oxygen consumption rate and reducing apoptosis and ROS production [58]. SHLP-2 and SHLP-3 act as insulin sensitizers [58]. Central and systemic treatment with SHLP-2 and SHLP-3 enhance the ability of insulin to inhibit hepatic glucose production and to stimulate glucose uptake [58]. Both peptides also promote adipocyte differentiation in 3T3‐L1 preadipocytes [58].
- A novel MDP named MOTS-c was recently identified [59]. Unbiased gene microarray and global metabolomics assays revealed that the folate-methionine cycle is a target of MOTS-c function [59]. By inhibiting the folate cycle at the level of 5-methyl-tetrahydrofolate (5Me-THF), MOTS-c treatment for 4 hours increases the cellular levels of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) in cultured cells [59]. Resultant AMPK activation improves glucose and fatty acid metabolism. Seven days MOTS‐c infusion significantly increases glucose clearance and the insulin‐stimulated glucose disposal rate in lean mice [59]. This effect may be mediated via through increased glucose transporter 4 (GLUT4) expression and mitochondrial fusion-induced GLUT4 translocation to the plasma membrane [59,60].
- Moreover, chronic systemic administration of MOTS‐c for 8 weeks prevents high-fat diet (HFD)‐induced obesity and insulin resistance [59]. MOTS-c treatment also improved hyperglycemia and reproductive outcomes in the mice model of gestational diabetes mellitus [61] and attenuated the development of autoimmune diabetes by suppressing T-cells-induced β-cell destruction in mice [62]. Subsequent studies have demonstrated multiple beneficial effects of MOTS-c on bone biology, inflammation, pain, vascular calcification, and myocardial remodeling where these actions seem to be largely mediated via AMPK activation [63-66].
MITOCHONDRIA-DERIVED PEPTIDES AND THEIR BIOLOGICAL ACTIONS
- Exercise-induced changes in MDP expression have been investigated in humans and mice [67-69]. In humans, acute high-intensity endurance exercise (cycling) increased plasma concentrations of humanin, SHLP-6, and MOTS-c, as well as the skeletal muscle expression of humanin and MOTS-c [67-69]. Acute exercise-induced elevations in blood and muscle MOTS-c expression returned to baseline levels several hours later [68]. Exercise-induced changes in MDPs may differ according to exercise types. Von Walden et al. [69] showed that plasma humanin levels were elevated during post-endurance exercise (cycling) but remained unaltered after resistance exercise (leg press and extension). The effects of exercise training on MDP expression are less distinct than those of acute exercise [67,70,71]. Twelve weeks of endurance exercise (Nordic walk) in older men with prediabetes increased humanin protein levels in the serum but not in skeletal muscle [70]. Sixteen weeks of combined endurance and resistance exercise increased MOTS-c levels in the blood of non-Hispanic breast cancer survivors [71]. In contrast, 2 weeks of high-intensity cycling in young men and 8 weeks of endurance exercise in women with polycystic ovary syndrome did not alter the expression levels of humanin, SHLP-2, or MOTS-c in the blood and skeletal muscles [72]. In mice, eight weeks of treadmill running in lean and obese male mice elevated the plasma and skeletal muscle expression levels of MOTS-c [73,74]. We found that in young male mice, a moderate-degree of treadmill exercise for 2 weeks increased MOTS-c expression in the hypothalamus while acute running until exhaustion had no effect [38].
- It remains unclear how exercise alters MDP expression. Upon acute exercise, humanin protein expression in skeletal muscles increases within 30 minutes without changes in its transcript levels [67]. Moreover, humanin directly binds to E3 ubiquitin-protein ligase tripartite motif 11 (TRIM11), implying the possibility of proteolysis-mediated regulation of humanin [75]. This regulatory mechanism has not been reported for MOTS-c. The expression of MDPs may be also regulated at the transcriptional level. Indeed, adiponectin increases MOTS-c expression in a sirtuin 1 (SIRT1)- and PGC-1α-dependent manner [74], suggesting the involvement of PGC-1α in the transcriptional regulation of MOTS-c.
EFFECTS OF EXERCISE ON THE EXPRESSION OF MDPs
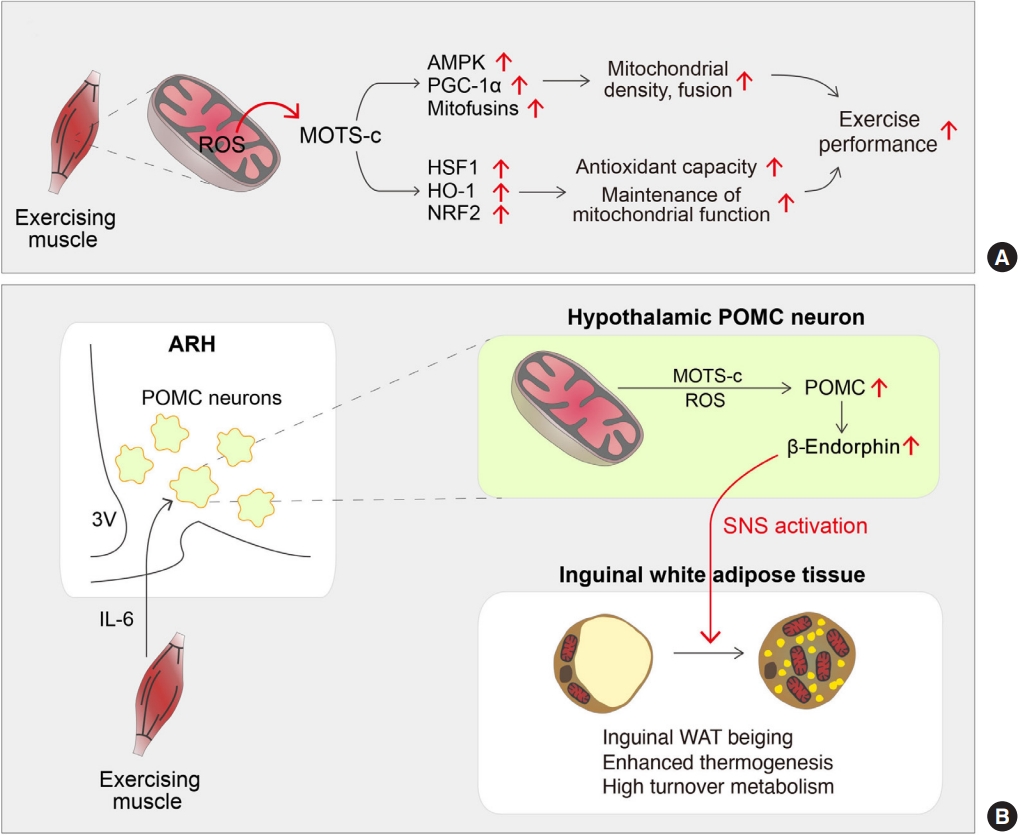
- Among MDPs, MOTS-c is the most associated with exercise. While exercise controls MOTS-c expression, MOTS-c controls exercise performance. A recent paper reported that chronic MOTS-c treatment enhanced physical activity and health span in young-, middle-, and old-age mice [68]. Mice treated with MOTS-c for 2 weeks displayed increased muscle force and stride length, along with increased lean mass and decreased fat mass [68]. However, the detailed mechanisms by which MOTS-c increases physical performance largely remain to be elucidated. Notably, it took more than 1 week to observe the effect of MOTS-c on exercise performance [68]. Thus, MOTS-c may promote adaptive responses to exercise-induced metabolic and oxidative stress in skeletal muscle (Fig. 3A). Supporting this assumption, MOTS-c treated mice exhibit increased expression of genes associated with glucose and amino acid metabolism and metabolic stress responses in skeletal muscles [68]. Particularly, the expression of heat shock factor 1 (HSF1), heme oxygenase-1 (HO-1), nuclear factor erythroid-2-related factor 2 (NFE2L2/NRF2) is significantly increased [68] and these genes are linked to exercise performance enhancement [76-78]. HSF1 is associated with maintaining protein quality and metabolism, especially under stress conditions [79]. HO-1 and NRF2 are associated with antioxidant capacity and maintenance of mitochondrial function [76-78]. Therefore, these molecules may act downstream of MOTS-c to affect physical performance. As shown previously [80], intracellular MOTS-c might translocate to the nucleus during exercise, where it may possibly modulate the transcription of those genes by interacting with transcription factors and coregulators. Additionally, MOTS-c treatment increases the expression of mitochondrial biogenesis-related molecules such as PGC-1α, NRF1, TFAM, cytochrome c oxidase subunit 4 (COX4), and translocase of outer mitochondrial membrane 20 (TOMM20) and induces mitochondrial fusion by upregulating fusion-related genes such as MFN2 and OPA1 [60]. These mitochondrial changes can also contribute to MOTS-c-enhanced physical performance. In addition, a recent paper has shown that MOTS-c treatment for 8 weeks prevented HFD-induced muscle atrophy through suppression of myostatin expression [81].
- MOTS-c is secreted from exercising muscles [68,74], and extracellular MOTS-c may act on neighboring cells or cells in remote organs like hormones [82]. It is yet unclear whether intracellular and extracellular MOTS-c are structurally different. Indeed, MOTS-c treatment stimulates fatty acid oxidation and glucose uptake in skeletal muscle cells and adipocytes through increased AMPK activity [59,83]. At the same time, MOTS-c may interact with other metabolic regulators. For example, adiponectin stimulates MOTS-c expression and vice versa [74]. These two molecules act synergistically to stimulate adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 (APPL1), SIRT1, and PGC-1α expression [74]. A recent study has demonstrated that exercise training and MOTS-c treatment have additive effects on weight loss, improvement of insulin resistance and PGC-1α upregulation [73]. Thus, MOTS-c and exercise training may have some non-overlapping effects.
EFFECTS OF MOTS-c TREATMENT ON EXERCISE PERFORMANCE
- In addition to skeletal muscle, exercise training causes beneficial adaptations to multiple organs, including adipose tissues [84,85]. The most prominent finding of exercise-induced changes in adipose tissue is ‘beiging’ or ‘browning’ in the inguinal subcutaneous white adipose tissue (iWAT) [84,85]. Adipocytes in this fat depot look like classical white adipocytes under the conditions of low thermogenic need. However, upon increased thermogenic needs induced by cold exposure, HFD feeding, and exercise, these cells adopt the features of brown adipocytes, i.e., small multilocular fat droplets, high mitochondrial density/activity, increased glucose uptake, and increased expression of thermogenic genes such as uncoupling protein-1 (UCP1), PR domain containing 16 (PRDM16), and PGC-1α. Exercise-induced changes are relatively less distinct in visceral WAT and the primary thermogenic organ BAT [85]. Therefore, exercise-induced adipose tissue thermogenesis seems to occur primarily in the iWAT. On the other hand, skeletal muscle non-shivering thermogenesis also increases during physical activity, possibly through sarcoplasmic reticulum calcium ATPase (SERCA)-dependent mechanisms [86]. It is thus curious why adipose tissue thermogenesis should be elevated despite increased skeletal muscle thermogenesis.
- The major mediator of exercise-induced thermogenesis is thought to be the sympathetic nerves innervating iWAT [87]. Other potential mediators are exercise-induced myokines, such as irisin [88], myostatin [89], meteorin-like 1 (Metrnl) [90], lactate [91], and β-aminoisobutyric acid (BAIBA) [92]. MOTS-s can also be considered a myokine involved in exercise-induced thermogenesis. Indeed, intraperitoneal injection of MOTS-c for 7 days promotes cold tolerance by stimulating non-shivering thermogenesis [93]. Mechanistically, it is thought that MOTS-c dramatically upregulates the thermogenic gene expression in BAT and induces beiging in the iWAT [93]. Interestingly, MOTS-c can stimulate thermogenic activity through central mechanisms (Fig. 3B). In our previous study [38], moderate-degree exercise training increases MOTS-c expression in hypothalamic neurons via exercise-related myokine interleukin-6. Moreover, intracerebroventricular administration of MOTS-c for 28 days induced beiging of iWAT and enhanced thermogenic gene expression [38]. These effects occurred via increased β-endorphin production in hypothalamic proopiomelanocortin (POMC) neurons and enhanced sympathetic innervation in iWAT [38]. In this process, hypothalamic neuron ROS production may be essential given that inhibition of hypothalamic ROS production during exercise training significantly inhibits the inducible thermogenic response in iWAT [38].
MOTS-c AND EXERCISE-INDUCED THERMOGENESIS
- Exercise is a type of stress to our body as it depletes energy stores, stimulates ROS production and induces mitochondrial stress responses, such as UPRmt. Mitochondrial stress responses to exercise generally yield beneficial outcomes by increasing mitochondrial anti-oxidant and protein-folding capacities [33]. These reactions are currently proven in skeletal muscles, adipose tissues, and the hypothalamus [38,43,94,95] and might also occur in other organs. Future studies are warranted to test this possibility.
- MDPs have drawn attention from many researchers as this class of peptides has numerous beneficial biological effects on the brain, cardiovascular system, glucose/energy metabolism, and bones [54,59,70,71]. Moreover, MDPs are short peptides consisting of less than 30 amino acids [50], potentially making them more therapeutically approachable. In this view, MOTS-c is a promising target as an exercise mimetic or physical performance enhancer. Indeed, MOTS-c treatment enhances physical capacity and metabolic health in both young and aged mice [68]. This effect must be confirmed in humans, especially in physically inactive elderly persons. In addition, as both MOTS-c and antidiabetic drug metformin activate AMPK [59, 96], it will be worth to test whether MOTS-c/metformin combination treatment will be more effective than monotherapy in activating AMPK and improving glucose metabolism. To be druggable, MOTS-c might be modified to degradation-resistant form for long-lasting effects. Better insight into the adaptive responses to exercise can lead us to challenging intervention for many human diseases, including obesity.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This study was supported by grants from the National Research Foundation of Korea (NRF), the Ministry of Education of Korea (2020R1A2C3004843, 2020R1A4A3078962, 2022R1C1C1004187), and the Asan Institute for Life Sciences (2019-IP0855-1).
NOTES
-
Acknowledgements
- We thank the Scientific Publications Team at Asan Medical Center.
- 1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627-42.PubMedPMC
- 2. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics 2015;33:673-89.ArticlePubMedPMCPDF
- 3. Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, et al. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J 2021;45:1-10.ArticlePubMedPMCPDF
- 4. Celik O, Yildiz BO. Obesity and physical exercise. Minerva Endocrinol (Torino) 2021;46:131-44.ArticlePubMed
- 5. Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab 2017;25:1027-36.ArticlePubMedPMC
- 6. Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta 2014;1840:1276-84.ArticlePubMed
- 7. Huertas JR, Casuso RA, Agustin PH, Cogliati S. Stay fit, stay young: mitochondria in movement: the role of exercise in the new mitochondrial paradigm. Oxid Med Cell Longev 2019;2019:7058350.ArticlePubMedPMCPDF
- 8. Hood DA, Memme JM, Oliveira AN, Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol 2019;81:19-41.ArticlePubMed
- 9. Lundby C, Jacobs RA. Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol 2016;101:17-22.ArticlePubMed
- 10. Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2011;8:92-103.ArticlePubMedPDF
- 11. Bishop DJ, Botella J, Genders AJ, Lee MJ, Saner NJ, Kuang J, et al. High-intensity exercise and mitochondrial biogenesis: current controversies and future research directions. Physiology (Bethesda) 2019;34:56-70.ArticlePubMed
- 12. Austin S, St-Pierre J. PGC1α and mitochondrial metabolism: emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 2012;125(Pt 21):4963-71.ArticlePubMedPDF
- 13. Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One 2011;6:e28290.ArticlePubMedPMC
- 14. Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 2011;1813:1269-78.ArticlePubMedPMC
- 15. Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, et al. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 2007;148:3441-8.PubMed
- 16. Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res 2007;73:631-40.ArticlePubMed
- 17. Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol 2003;553(Pt 1):303-9.ArticlePubMedPMCPDF
- 18. Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol 2006;574(Pt 3):889-903.ArticlePubMedPMC
- 19. Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 2002;296:349-52.ArticlePubMed
- 20. Joseph JS, Anand K, Malindisa ST, Oladipo AO, Fagbohun OF. Exercise, CaMKII, and type 2 diabetes. EXCLI J 2021;20:386-99.PubMedPMC
- 21. Nielsen J, Gejl KD, Hey-Mogensen M, Holmberg HC, Suetta C, Krustrup P, et al. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J Physiol 2017;595:2839-47.ArticlePubMedPMCPDF
- 22. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012;337:1062-5.ArticlePubMedPMC
- 23. Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature 2016;540:139-43.ArticlePubMedPMCPDF
- 24. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 2004;117(Pt 26):6535-46.ArticlePubMedPDF
- 25. Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol 2016;212:379-87.ArticlePubMedPMCPDF
- 26. Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 2005;567(Pt 1):349-58.PubMedPMC
- 27. Bell MB, Bush Z, McGinnis GR, Rowe GC. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J Appl Physiol (1985) 2019;126:341-53.ArticlePubMedPMC
- 28. Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol 2013;15:713-20.ArticlePubMedPMCPDF
- 29. Guan Y, Drake JC, Yan Z. Exercise-induced mitophagy in skeletal muscle and heart. Exerc Sport Sci Rev 2019;47:151-6.ArticlePubMedPMC
- 30. Yun J, Finkel T. Mitohormesis. Cell Metab 2014;19:757-66.ArticlePubMedPMC
- 31. Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 2010;45:410-8.ArticlePubMed
- 32. Cox CS, McKay SE, Holmbeck MA, Christian BE, Scortea AC, Tsay AJ, et al. Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling. Cell Metab 2018;28:776-86.ArticlePubMedPMC
- 33. Musci RV, Hamilton KL, Linden MA. Exercise-induced mitohormesis for the maintenance of skeletal muscle and healthspan extension. Sports (Basel) 2019;7:170.ArticlePubMedPMC
- 34. Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999;43:562-71.ArticlePubMed
- 35. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996;313(Pt 1):17-29.ArticlePubMedPMCPDF
- 36. Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J Alzheimers Dis 2009;17:245-57.ArticlePubMed
- 37. Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 2009;106:8665-70.ArticlePubMedPMC
- 38. Kang GM, Min SH, Lee CH, Kim JY, Lim HS, Choi MJ, et al. Mitohormesis in hypothalamic POMC neurons mediates regular exercise-induced high-turnover metabolism. Cell Metab 2021;33:334-49.ArticlePubMedPMC
- 39. Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol 2018;19:109-20.ArticlePubMedPDF
- 40. Mottis A, Herzig S, Auwerx J. Mitocellular communication: shaping health and disease. Science 2019;366:827-32.ArticlePubMed
- 41. Qureshi MA, Haynes CM, Pellegrino MW. The mitochondrial unfolded protein response: signaling from the powerhouse. J Biol Chem 2017;292:13500-6.ArticlePubMedPMC
- 42. Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 2011;144:79-91.ArticlePubMedPMC
- 43. Cordeiro AV, Peruca GF, Braga RR, Bricola RS, Lenhare L, Silva V, et al. High-intensity exercise training induces mitonuclear imbalance and activates the mitochondrial unfolded protein response in the skeletal muscle of aged mice. Geroscience 2021;43:1513-8.ArticlePubMedPMCPDF
- 44. Laurens C, Parmar A, Murphy E, Carper D, Lair B, Maes P, et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight 2020;5:e131870.ArticlePubMedPMC
- 45. Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One 2013;8:e63517.ArticlePubMedPMC
- 46. Johann K, Kleinert M, Klaus S. The role of GDF15 as a myomitokine. Cells 2021;10:2990.ArticlePubMedPMC
- 47. Kim KH, Lee MS. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J 2014;38:245-51.ArticlePubMedPMC
- 48. Kim KH, Lee MS. GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH. Biochim Biophys Acta Gen Subj 2021;1865:129834.ArticlePubMed
- 49. Woodhead J, Merry TL. Mitochondrial-derived peptides and exercise. Biochim Biophys Acta Gen Subj 2021;1865:130011.ArticlePubMed
- 50. Merry TL, Chan A, Woodhead J, Reynolds JC, Kumagai H, Kim SJ, et al. Mitochondrial-derived peptides in energy metabolism. Am J Physiol Endocrinol Metab 2020;319:E659-66.ArticlePubMedPMC
- 51. Bodzioch M, Lapicka-Bodzioch K, Zapala B, Kamysz W, Kiec-Wilk B, Dembinska-Kiec A. Evidence for potential functionality of nuclearly-encoded humanin isoforms. Genomics 2009;94:247-56.ArticlePubMed
- 52. Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci U S A 2001;98:6336-41.PubMedPMC
- 53. Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, et al. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci 2001;21:9235-45.ArticlePubMedPMC
- 54. Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A 2003;100:13042-7.ArticlePubMedPMC
- 55. Hashimoto Y, Tsuji O, Niikura T, Yamagishi Y, Ishizaka M, Kawasumi M, et al. Involvement of c-Jun N-terminal kinase in amyloid precursor protein-mediated neuronal cell death. J Neurochem 2003;84:864-77.ArticlePubMed
- 56. Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. FASEB J 2013;27:4890-8.ArticlePubMedPMCPDF
- 57. Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One 2009;4:e6334.ArticlePubMedPMC
- 58. Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 2016;8:796-809.ArticlePubMedPMC
- 59. Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 2015;21:443-54.ArticlePubMedPMC
- 60. Bhullar KS, Shang N, Kerek E, Wu K, Wu J. Mitofusion is required for MOTS-c induced GLUT4 translocation. Sci Rep 2021;11:14291.ArticlePubMedPMCPDF
- 61. Yin Y, Pan Y, He J, Zhong H, Wu Y, Ji C, et al. The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus. Pharmacol Res 2022;175:105987.ArticlePubMed
- 62. Kong BS, Min SH, Lee C, Cho YM. Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell Rep 2021;36:109447.ArticlePubMedPMC
- 63. Wei M, Gan L, Liu Z, Liu L, Chang JR, Yin DC, et al. Mitochondrial-derived peptide MOTS-c attenuates vascular calcification and secondary myocardial remodeling via adenosine monophosphate-activated protein kinase signaling pathway. Cardiorenal Med 2020;10:42-50.ArticlePubMedPDF
- 64. Yan Z, Zhu S, Wang H, Wang L, Du T, Ye Z, et al. MOTS-c in hibits osteolysis in the mouse calvaria by affecting osteocyte-osteoclast crosstalk and inhibiting inflammation. Pharmacol Res 2019;147:104381.ArticlePubMed
- 65. Ming W, Lu G, Xin S, Huanyu L, Yinghao J, Xiaoying L, et al. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem Biophys Res Commun 2016;476:412-9.ArticlePubMed
- 66. Yin X, Jing Y, Chen Q, Abbas AB, Hu J, Xu H. The intraperitoneal administration of MOTS-c produces antinociceptive and anti-inflammatory effects through the activation of AMPK pathway in the mouse formalin test. Eur J Pharmacol 2020;870:172909.ArticlePubMed
- 67. Woodhead J, D’Souza RF, Hedges CP, Wan J, Berridge MV, Cameron-Smith D, et al. High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J Appl Physiol (1985) 2020;128:1346-54.ArticlePubMedPMC
- 68. Reynolds JC, Lai RW, Woodhead J, Joly JH, Mitchell CJ, Cameron-Smith D, et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun 2021;12:470.ArticlePubMedPMCPDF
- 69. von Walden F, Fernandez-Gonzalo R, Norrbom J, Emanuelsson EB, Figueiredo VC, Gidlund EK, et al. Acute endurance exercise stimulates circulating levels of mitochondrial-derived peptides in humans. J Appl Physiol (1985) 2021;131:1035-42.ArticlePubMed
- 70. Gidlund EK, von Walden F, Venojarvi M, Riserus U, Heinonen OJ, Norrbom J, et al. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol Rep 2016;4:e13063.ArticlePubMedPMCPDF
- 71. Dieli-Conwright CM, Sami N, Norris MK, Wan J, Kumagai H, Kim SJ, et al. Effect of aerobic and resistance exercise on the mitochondrial peptide MOTS-c in Hispanic and non-Hispanic White breast cancer survivors. Sci Rep 2021;11:16916.ArticlePubMedPMCPDF
- 72. Ramanjaneya M, Jerobin J, Bettahi I, Bensila M, Aye M, Siveen KS, et al. Lipids and insulin regulate mitochondrial-derived peptide (MOTS-c) in PCOS and healthy subjects. Clin Endocrinol (Oxf) 2019;91:278-87.ArticlePubMedPDF
- 73. Yang B, Yu Q, Chang B, Guo Q, Xu S, Yi X, et al. MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochim Biophys Acta Mol Basis Dis 2021;1867:166126.ArticlePubMed
- 74. Guo Q, Chang B, Yu QL, Xu ST, Yi XJ, Cao SC. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1-SIRT1-PGC-1α. Diabetologia 2020;63:2675-88.ArticlePubMedPDF
- 75. Niikura T, Hashimoto Y, Tajima H, Ishizaka M, Yamagishi Y, Kawasumi M, et al. A tripartite motif protein TRIM11 binds and destabilizes humanin, a neuroprotective peptide against Alzheimer’s disease-relevant insults. Eur J Neurosci 2003;17:1150-8.ArticlePubMed
- 76. Alves de Souza RW, Gallo D, Lee GR, Katsuyama E, Schaufler A, Weber J, et al. Skeletal muscle heme oxygenase-1 activity regulates aerobic capacity. Cell Rep 2021;35:109018.ArticlePubMedPMC
- 77. Done AJ, Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol 2016;10:191-9.ArticlePubMedPMC
- 78. Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol 2016;594:5195-207.ArticlePubMedPMCPDF
- 79. Flockhart M, Nilsson LC, Tais S, Ekblom B, Apro W, Larsen FJ. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab 2021;33:957-70.ArticlePubMed
- 80. Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab 2018;28:516-24.ArticlePubMedPMC
- 81. Kumagai H, Coelho AR, Wan J, Mehta HH, Yen K, Huang A, et al. MOTS-c reduces myostatin and muscle atrophy signaling. Am J Physiol Endocrinol Metab 2021;320:E680-90.ArticlePubMedPMC
- 82. Lee C, Kim KH, Cohen P. MOTS-c: a novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic Biol Med 2016;100:182-7.ArticlePubMedPMC
- 83. Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med (Berl) 2019;97:473-85.ArticlePubMedPDF
- 84. Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem 2014;289:34129-40.PubMedPMC
- 85. Lehnig AC, Dewal RS, Baer LA, Kitching KM, Munoz VR, Arts PJ, et al. Exercise training induces depot-specific adaptations to white and brown adipose tissue. iScience 2019;11:425-39.ArticlePubMedPMC
- 86. Periasamy M, Herrera JL, Reis F. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab J 2017;41:327-36.ArticlePubMedPMCPDF
- 87. Vidal P, Stanford KI. Exercise-induced adaptations to adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2020;11:270.ArticlePubMedPMC
- 88. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463-8.ArticlePubMedPMCPDF
- 89. Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc 2011;43:1828-35.ArticlePubMedPMC
- 90. Alizadeh H. Myokine-mediated exercise effects: the role of myokine meteorin-like hormone (Metrnl). Growth Factors 2022;39:71-8.Article
- 91. Mendez-Gutierrez A, Aguilera CM, Osuna-Prieto FJ, Martinez-Tellez B, Rico Prados MC, Acosta FM, et al. Exercise-induced changes on exerkines that might influence brown adipose tissue metabolism in young sedentary adults. Eur J Sport Sci 2022 Apr 25 [Epub]. https://doi.org/10.1080/17461391.2022.2040597.Article
- 92. Colitti M, Boschi F, Montanari T. Dynamic of lipid droplets and gene expression in response to β-aminoisobutyric acid treatment on 3T3-L1 cells. Eur J Histochem 2018;62:2984.ArticlePubMedPMCPDF
- 93. Lu H, Tang S, Xue C, Liu Y, Wang J, Zhang W, et al. Mitochondrial-derived peptide MOTS-c increases adipose thermogenic activation to promote cold adaptation. Int J Mol Sci 2019;20:2456.ArticlePubMedPMC
- 94. Cordeiro AV, Bricola RS, Braga RR, Lenhare L, Silva V, Anaruma CP, et al. Aerobic exercise training induces the mitonuclear imbalance and UPRmt in the skeletal muscle of aged mice. J Gerontol A Biol Sci Med Sci 2020;75:2258-61.ArticlePubMedPDF
- 95. Braga RR, Crisol BM, Bricola RS, Sant’ana MR, Nakandakari S, Costa SO, et al. Exercise alters the mitochondrial proteostasis and induces the mitonuclear imbalance and UPRmt in the hypothalamus of mice. Sci Rep 2021;11:3813.ArticlePubMedPMCPDF
- 96. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167-74.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Mitochondrial-derived peptides: Antidiabetic functions and evolutionary perspectives
Satadeepa Kal, Sumana Mahata, Suborno Jati, Sushil K. Mahata
Peptides.2024; 172: 171147. CrossRef - Beneficial Effects of Low-Grade Mitochondrial Stress on Metabolic Diseases and Aging
Se Hee Min, Gil Myoung Kang, Jae Woo Park, Min-Seon Kim
Yonsei Medical Journal.2024; 65(2): 55. CrossRef - Roles of Myokines and Muscle-Derived Extracellular Vesicles in Musculoskeletal Deterioration under Disuse Conditions
Jie Zhang, Yunfang Gao, Jiangwei Yan
Metabolites.2024; 14(2): 88. CrossRef - Antifragility and antiinflammaging: Can they play a role for a healthy longevity?
Fabiola Olivieri, Francesco Prattichizzo, Fabrizia Lattanzio, Anna Rita Bonfigli, Liana Spazzafumo
Ageing Research Reviews.2023; 84: 101836. CrossRef - MOTS-c: A promising mitochondrial-derived peptide for therapeutic exploitation
Yuejun Zheng, Zilin Wei, Tianhui Wang
Frontiers in Endocrinology.2023;[Epub] CrossRef - MOTS-c: A potential anti-pulmonary fibrosis factor derived by mitochondria
Zewei Zhang, Dongmei Chen, Kaili Du, Yaping Huang, Xingzhe Li, Quwen Li, Xiaoting Lv
Mitochondrion.2023; 71: 76. CrossRef - Mitochondrial-Encoded Peptide MOTS-c, Diabetes, and Aging-Related Diseases
Byung Soo Kong, Changhan Lee, Young Min Cho
Diabetes & Metabolism Journal.2023; 47(3): 315. CrossRef - MOTS-c Serum Concentration Positively Correlates with Lower-Body Muscle Strength and Is Not Related to Maximal Oxygen Uptake—A Preliminary Study
Remigiusz Domin, Michał Pytka, Mikołaj Żołyński, Jan Niziński, Marcin Rucinski, Przemysław Guzik, Jacek Zieliński, Marek Ruchała
International Journal of Molecular Sciences.2023; 24(19): 14951. CrossRef - Unique Properties of Apicomplexan Mitochondria
Ian M. Lamb, Ijeoma C. Okoye, Michael W. Mather, Akhil B. Vaidya
Annual Review of Microbiology.2023; 77(1): 541. CrossRef

 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite