- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 44(4); 2020 > Article
-
ReviewBasic Research Role of CRTC2 in Metabolic Homeostasis: Key Regulator of Whole-Body Energy Metabolism?
-
Hye-Sook Han
 , Yongmin Kwon, Seung-Hoi Koo
, Yongmin Kwon, Seung-Hoi Koo
-
Diabetes & Metabolism Journal 2020;44(4):498-508.
DOI: https://doi.org/10.4093/dmj.2019.0200
Published online: March 5, 2020
Division of Life Sciences, College of Life Sciences & Biotechnology, Korea University, Seoul, Korea.
- Corresponding author: Seung-Hoi Koo. Division of Life Sciences, College of Life Sciences & Biotechnology, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Korea. koohoi@korea.ac.kr
• Received: October 30, 2019 • Accepted: November 18, 2019
Copyright © 2020 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- IDENTIFICATION OF CRTC PROTEINS
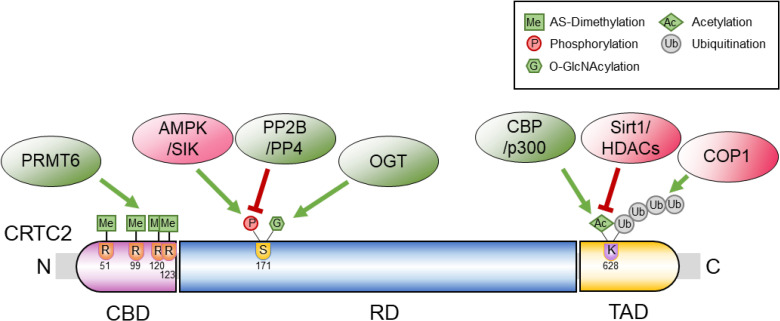
- THE STRUCTURE OF CRTC2, AND CRITICAL RESIDUES FOR ITS REGULATION
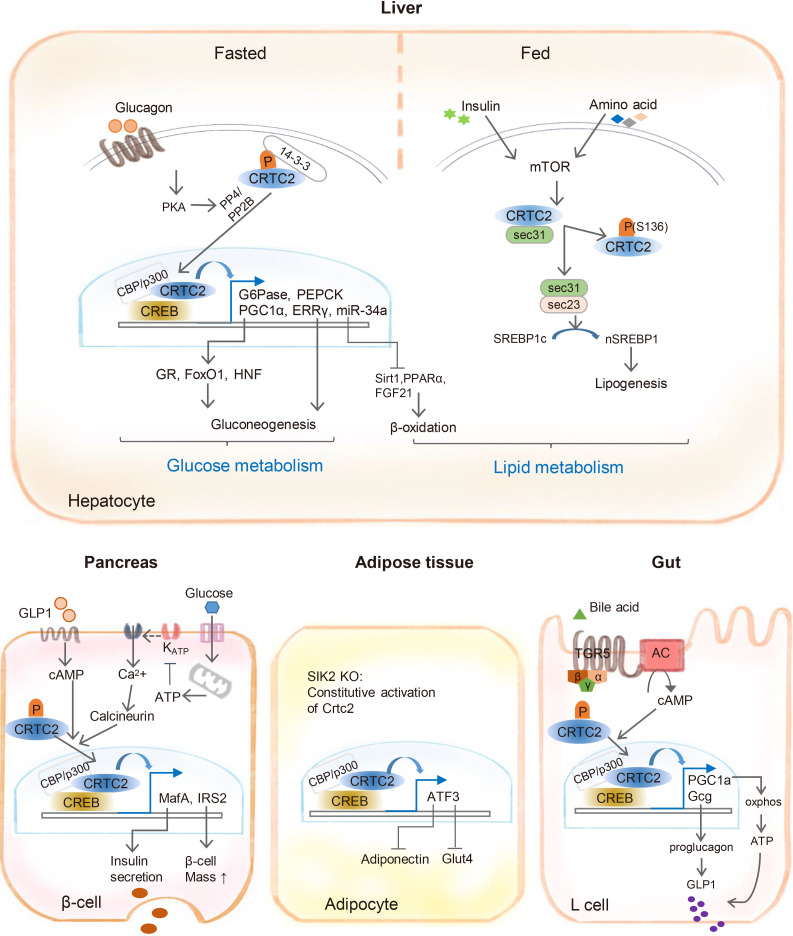
- THE ROLE OF CRTC2 IN THE CONTROL OF METABOLIC PATHWAYS
- CRTC2 AS A TRANSCRIPTIONAL COACTIVATOR FOR OTHER bZIP TRANSCRIPTION FACTORS
- FUNCTIONS OF OTHER CRTC PROTEINS: HOMOLOGUES AND PARALOGUES OF CRTC2
- CONCLUSIONS
- ACKNOWLEDGMENTS
- NOTES
- REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Integration of genomic and transcriptomic data of inbred mouse models for polygenic obesity and leanness revealed “obese” and “lean” candidate alleles in polyadenylation signals
Martin Šimon, Špela Mikec, Nicholas M. Morton, Santosh S. Atanur, Simon Horvat, Tanja Kunej
Gene Reports.2024; 35: 101903. CrossRef - Mylabris phalerata induces the apoptosis and cell cycle delay in HCC, and potentiates the effect of sorafenib based on the molecular and network pharmacology approach
Young Woo Kim, Seon Been Bak, Su Youn Baek, Il Kon Kim, Won-Yung Lee, Un-Jung Yun, Kwang-Il Park
Molecular & Cellular Toxicology.2023; 19(4): 731. CrossRef - Emerging Role of SMILE in Liver Metabolism
Nanthini Sadasivam, Kamalakannan Radhakrishnan, Hueng-Sik Choi, Don-Kyu Kim
International Journal of Molecular Sciences.2023; 24(3): 2907. CrossRef - PIMT regulates hepatic gluconeogenesis in mice
Bandish Kapadia, Soma Behera, Sireesh T. Kumar, Tapan Shah, Rebecca Kristina Edwin, Phanithi Prakash Babu, Partha Chakrabarti, Kishore V.L. Parsa, Parimal Misra
iScience.2023; 26(3): 106120. CrossRef - Biological functions of CRTC2 and its role in metabolism-related diseases
Hong-Yu Zheng, Yan-Xia Wang, Kun Zhou, Hai-Lin Xie, Zhong Ren, Hui-Ting Liu, Yang-Shao Ou, Zhi-Xiang Zhou, Zhi-Sheng Jiang
Journal of Cell Communication and Signaling.2023; 17(3): 495. CrossRef - An insulin-regulated arrestin domain protein controls hepatic glucagon action
Sezin Dagdeviren, Megan F. Hoang, Mohsen Sarikhani, Vanessa Meier, Jake C. Benoit, Marinna C. Okawa, Veronika Y. Melnik, Elisabeth M. Ricci-Blair, Natalie Foot, Randall H. Friedline, Xiaodi Hu, Lauren A. Tauer, Arvind Srinivasan, Maxim B. Prigozhin, Sudha
Journal of Biological Chemistry.2023; 299(8): 105045. CrossRef - The Pleiotropic Face of CREB Family Transcription Factors
Md. Arifur Rahman Chowdhury, Jungeun An, Sangyun Jeong
Molecules and Cells.2023; 46(7): 399. CrossRef - It is a branched road to adipose tissue aging
N. Touitou, B. Lerrer, H. Y. Cohen
Nature Aging.2023; 3(8): 911. CrossRef - Impaired BCAA catabolism in adipose tissues promotes age-associated metabolic derangement
Hye-Sook Han, Eunyong Ahn, Eun Seo Park, Tom Huh, Seri Choi, Yongmin Kwon, Byeong Hun Choi, Jueun Lee, Yoon Ha Choi, Yujin L. Jeong, Gwang Bin Lee, Minji Kim, Je Kyung Seong, Hyun Mu Shin, Hang-Rae Kim, Myeong Hee Moon, Jong Kyoung Kim, Geum-Sook Hwang, S
Nature Aging.2023; 3(8): 982. CrossRef - Exploring the diagnostic value, prognostic value, and biological functions of NPC gene family members in hepatocellular carcinoma based on a multi-omics analysis
Keheng Chen, Xin Zhang, Huixin Peng, Fengdie Huang, Guangyu Sun, Qijiang Xu, Lusheng Liao, Zhiyong Xing, Yanping Zhong, Zhichao Fang, Meihua Liao, Shihua Luo, Wencheng Chen, Mingyou Dong
Functional & Integrative Genomics.2023;[Epub] CrossRef - MicroRNA regulation of AMPK in nonalcoholic fatty liver disease
Hao Sun, Jongsook Kim Kemper
Experimental & Molecular Medicine.2023; 55(9): 1974. CrossRef - Serine active site containing protein 1 depletion alters lipid metabolism and protects against high fat diet-induced obesity in mice
Miaomiao Du, Xueyun Li, Fangyi Xiao, Yinxu Fu, Yu Shi, Sihan Guo, Lifang Chen, Lu Shen, Lan Wang, Huang Cheng, Hao Li, Anran Xie, Yaping Zhou, Kaiqiang Yang, Hezhi Fang, Jianxin Lyu, Qiongya Zhao
Metabolism.2022; 134: 155244. CrossRef - cAMP Signaling in Cancer: A PKA-CREB and EPAC-Centric Approach
Muhammad Bilal Ahmed, Abdullah A. A. Alghamdi, Salman Ul Islam, Joon-Seok Lee, Young-Sup Lee
Cells.2022; 11(13): 2020. CrossRef - Hepatic Sam68 Regulates Systemic Glucose Homeostasis and Insulin Sensitivity
Aijun Qiao, Wenxia Ma, Ying Jiang, Chaoshan Han, Baolong Yan, Junlan Zhou, Gangjian Qin
International Journal of Molecular Sciences.2022; 23(19): 11469. CrossRef - The Role of Small Heterodimer Partner-Interacting Leucine Zipper
(SMILE) as a Transcriptional Corepressor in Hepatic Glucose and Lipid
Metabolism
Woo-Ram Park, Byungyoon Choi, Nanthini Sadasivam, Don-Kyu Kim
Trends in Agriculture & Life Sciences.2022; 60: 7. CrossRef - AMPK Localization: A Key to Differential Energy Regulation
Qonita Afinanisa, Min Kyung Cho, Hyun-A Seong
International Journal of Molecular Sciences.2021; 22(20): 10921. CrossRef

 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite