
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
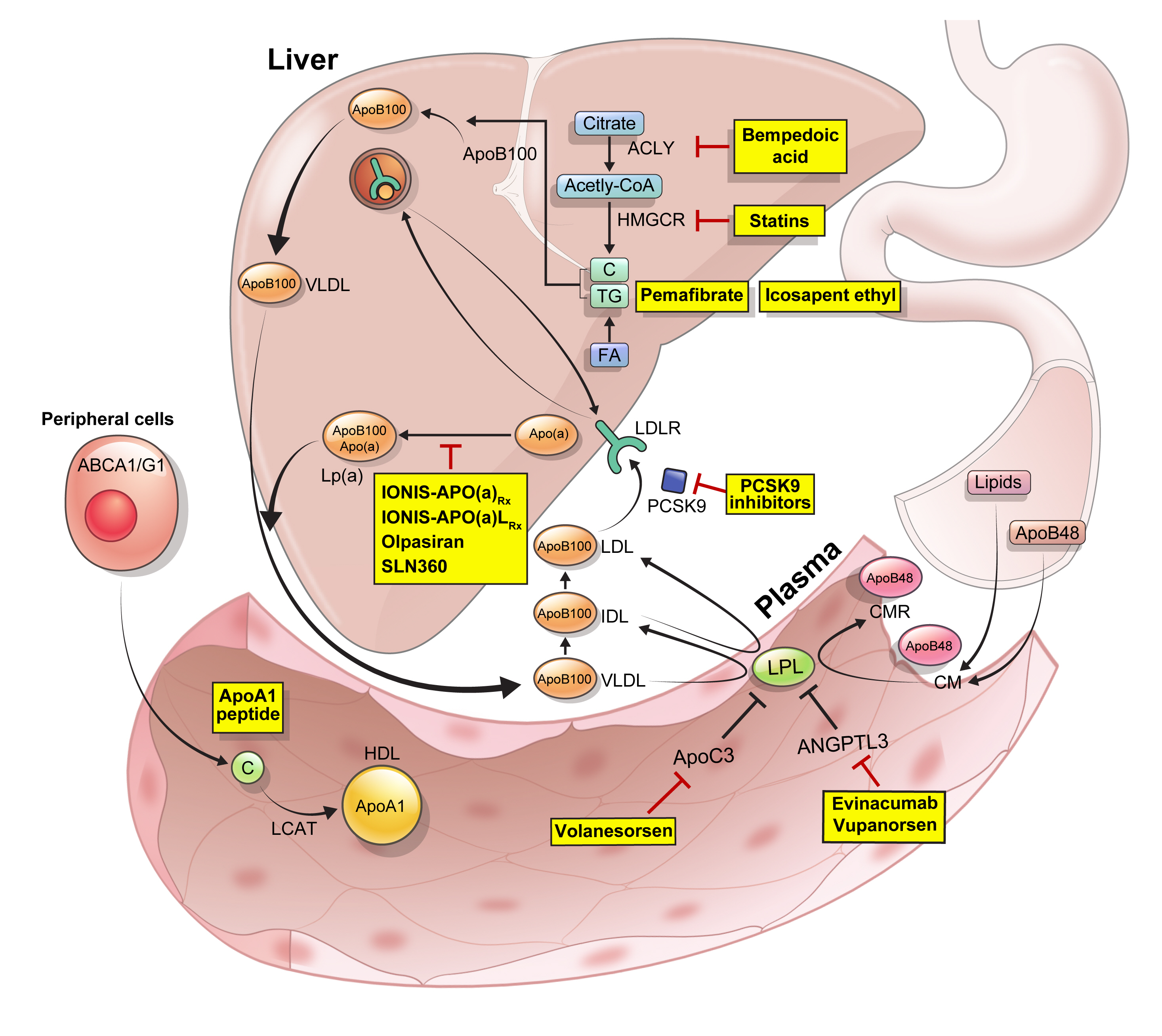
- New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
- Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
- Diabetes Metab J. 2022;46(4):517-532. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0198
- Correction in: Diabetes Metab J 2022;46(5):817
- 10,029 View
- 865 Download
- 25 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Statins are the cornerstone of the prevention and treatment of atherosclerotic cardiovascular disease (ASCVD). However, even under optimal statin therapy, a significant residual ASCVD risk remains. Therefore, there has been an unmet clinical need for novel lipid-lowering agents that can target low-density lipoprotein cholesterol (LDL-C) and other atherogenic particles. During the past decade, several drugs have been developed for the treatment of dyslipidemia. Inclisiran, a small interfering RNA that targets proprotein convertase subtilisin/kexin type 9 (PCSK9), shows comparable effects to that of PCSK9 monoclonal antibodies. Bempedoic acid, an ATP citrate lyase inhibitor, is a valuable treatment option for the patients with statin intolerance. Pemafibrate, the first selective peroxisome proliferator-activated receptor alpha modulator, showed a favorable benefit-risk balance in phase 2 trial, but the large clinical phase 3 trial (PROMINENT) was recently stopped for futility based on a late interim analysis. High dose icosapent ethyl, a modified eicosapentaenoic acid preparation, shows cardiovascular benefits. Evinacumab, an angiopoietin-like 3 (ANGPTL3) monoclonal antibody, reduces plasma LDL-C levels in patients with refractory hypercholesterolemia. Novel antisense oligonucleotides targeting apolipoprotein C3 (apoC3), ANGPTL3, and lipoprotein(a) have significantly attenuated the levels of their target molecules with beneficial effects on associated dyslipidemias. Apolipoprotein A1 (apoA1) is considered as a potential treatment to exploit the athero-protective effects of high-density lipoprotein cholesterol (HDL-C), but solid clinical evidence is necessary. In this review, we discuss the mode of action and clinical outcomes of these novel lipid-lowering agents beyond statins.
-
Citations
Citations to this article as recorded by- The role of adherence in patients with chronic diseases
Michel Burnier
European Journal of Internal Medicine.2024; 119: 1. CrossRef - Bempedoic acid: new evidence and recommendations on use
Kristina Paponja, Ivan Pećin, Željko Reiner, Maciej Banach
Current Opinion in Lipidology.2024; 35(1): 41. CrossRef - Genetic insights into repurposing statins for hyperthyroidism prevention: a drug-target Mendelian randomization study
Anqi Huang, Xinyi Wu, Jiaqi Lin, Chiju Wei, Wencan Xu
Frontiers in Endocrinology.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Neutrophil Extracellular Traps (NETs) and Atherosclerosis: Does Hypolipidemic Treatment Have an Effect?
Petros Adamidis, Despoina Pantazi, Iraklis Moschonas, Evangelos Liberopoulos, Alexandros Tselepis
Journal of Cardiovascular Development and Disease.2024; 11(3): 72. CrossRef - Modulating effects of crocin on lipids and lipoproteins: Mechanisms and potential benefits
Habib Yaribeygi, Mina Maleki, Farin Rashid-Farrokhi, Payman Raise Abdullahi, Mohammad Amin Hemmati, Tannaz Jamialahmadi, Amirhossein Sahebkar
Heliyon.2024; 10(7): e28837. CrossRef - Assessing the Benefits of Lifestyle Influences on Cardiovascu-lar Health After Acute Coronary Syndrome
Marius Rus, Claudia Elena Stanis, Paula Marian, Lilliana Oana Pobirci, Loredana Ioana Banszki, Veronica Huplea, Gheorghe Adrian Osiceanu, Bianca-Maria Pop, Gabriela Dogaru, Felicia Liana Andronie-Cioara
Balneo and PRM Research Journal.2024; 15(Vol.15, no): 660. CrossRef - Liver cancer cells as the model for developing liver-targeted RNAi therapeutics
Beibei Hou, Linhui Qin, Linfeng Huang
Biochemical and Biophysical Research Communications.2023; 644: 85. CrossRef - Insights into Causal Cardiovascular Risk Factors from Mendelian Randomization
C. M. Schooling, J. V. Zhao
Current Cardiology Reports.2023; 25(2): 67. CrossRef - Secoisolariciresinol diglucoside and anethole ameliorate lipid abnormalities, oxidative injury, hypercholesterolemia, heart, and liver conditions
Sana Noreen, Habib‐ur Rehman, Tabussam Tufail, Huma Badar Ul Ain, Chinaza Godswill Awuchi
Food Science & Nutrition.2023; 11(6): 2620. CrossRef - Colesterol remanente, riesgo vascular y prevención de la arteriosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán, Agustín Blanco, Mariano Blasco, José Luís Díaz Díaz, Ángel Díaz Rodríguez, Alipio Mangas, Vicente Pascual, Juan Pedro Botet, Pablo Pérez Martínez
Clínica e Investigación en Arteriosclerosis.2023; 35(4): 206. CrossRef - Evolving Management of Low‐Density Lipoprotein Cholesterol: A Personalized Approach to Preventing Atherosclerotic Cardiovascular Disease Across the Risk Continuum
Michael J. Wilkinson, Norman E. Lepor, Erin D. Michos
Journal of the American Heart Association.2023;[Epub] CrossRef - The cell origins of foam cell and lipid metabolism regulated by mechanical stress in atherosclerosis
Zhi Ouyang, Jian Zhong, Junyi Shen, Ye Zeng
Frontiers in Physiology.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Remnant cholesterol, vascular risk, and prevention of atherosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán
Clínica e Investigación en Arteriosclerosis (English Edition).2023; 35(4): 206. CrossRef - Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications
Marios Spanakis, Danny Alon-Ellenbogen, Petros Ioannou, Nikolaos Spernovasilis
Pharmacy.2023; 11(4): 130. CrossRef - Advances in Treatment of Dyslipidemia
Jill Dybiec, Wiktoria Baran, Bartłomiej Dąbek, Piotr Fularski, Ewelina Młynarska, Ewa Radzioch, Jacek Rysz, Beata Franczyk
International Journal of Molecular Sciences.2023; 24(17): 13288. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Preparation, characterization and in vivo pharmacokinetic study of ginsenoside Rb1-PLGA nanoparticles
Lixin Du, Huiling Lu, Yifei Xiao, Zhihua Guo, Ya Li
Scientific Reports.2023;[Epub] CrossRef - Dysregulation of Cholesterol Homeostasis in Ovarian Cancer
Zahraa Qusairy, Anne Gangloff, Shuk On Annie Leung
Current Oncology.2023; 30(9): 8386. CrossRef - Riesgo residual. Conclusiones
Ángel Cequier, José Luis Zamorano
Revista Española de Cardiología Suplementos.2023; 23: 25. CrossRef - Causal effects of circulating lipids and lipid-lowering drugs on the risk of urinary stones: a Mendelian randomization study
Zilong Tan, Jing Hong, Aochuan Sun, Mengdi Ding, Jianwu Shen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Bibliometric analysis of residual cardiovascular risk: trends and frontiers
Lin Wang, Sutong Wang, Chaoyuan Song, Yiding Yu, Yuehua Jiang, Yongcheng Wang, Xiao Li
Journal of Health, Population and Nutrition.2023;[Epub] CrossRef - Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review
Kenneth Francis Rodrigues, Wilson Thau Lym Yong, Md. Safiul Alam Bhuiyan, Shafiquzzaman Siddiquee, Muhammad Dawood Shah, Balu Alagar Venmathi Maran
Biology.2022; 11(9): 1308. CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef
- The role of adherence in patients with chronic diseases
- Obesity and Metabolic Syndrome
- Statins Increase Mitochondrial and Peroxisomal Fatty Acid Oxidation in the Liver and Prevent Non-Alcoholic Steatohepatitis in Mice
- Han-Sol Park, Jung Eun Jang, Myoung Seok Ko, Sung Hoon Woo, Bum Joong Kim, Hyun Sik Kim, Hye Sun Park, In-Sun Park, Eun Hee Koh, Ki-Up Lee
- Diabetes Metab J. 2016;40(5):376-385. Published online April 5, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.5.376
- 6,288 View
- 85 Download
- 63 Web of Science
- 126 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Non-alcoholic fatty liver disease is the most common form of chronic liver disease in industrialized countries. Recent studies have highlighted the association between peroxisomal dysfunction and hepatic steatosis. Peroxisomes are intracellular organelles that contribute to several crucial metabolic processes, such as facilitation of mitochondrial fatty acid oxidation (FAO) and removal of reactive oxygen species through catalase or plasmalogen synthesis. Statins are known to prevent hepatic steatosis and non-alcoholic steatohepatitis (NASH), but underlying mechanisms of this prevention are largely unknown.
Methods Seven-week-old C57BL/6J mice were given normal chow or a methionine- and choline-deficient diet (MCDD) with or without various statins, fluvastatin, pravastatin, simvastatin, atorvastatin, and rosuvastatin (15 mg/kg/day), for 6 weeks. Histological lesions were analyzed by grading and staging systems of NASH. We also measured mitochondrial and peroxisomal FAO in the liver.
Results Statin treatment prevented the development of MCDD-induced NASH. Both steatosis and inflammation or fibrosis grades were significantly improved by statins compared with MCDD-fed mice. Gene expression levels of peroxisomal proliferator-activated receptor α (PPARα) were decreased by MCDD and recovered by statin treatment. MCDD-induced suppression of mitochondrial and peroxisomal FAO was restored by statins. Each statin's effect on increasing FAO and improving NASH was independent on its effect of decreasing cholesterol levels.
Conclusion Statins prevented NASH and increased mitochondrial and peroxisomal FAO via induction of PPARα. The ability to increase hepatic FAO is likely the major determinant of NASH prevention by statins. Improvement of peroxisomal function by statins may contribute to the prevention of NASH.
-
Citations
Citations to this article as recorded by- Organelle stress and alterations in interorganelle crosstalk during liver fibrosis
Saloni Sinha, Nora Hassan, Robert E. Schwartz
Hepatology.2024; 79(2): 482. CrossRef - Statin therapy: a potential adjuvant to immunotherapies in hepatocellular carcinoma
Jiao Wang, Chengyu Liu, Ronghua Hu, Licheng Wu, Chuanzhou Li
Frontiers in Pharmacology.2024;[Epub] CrossRef - Recent Progress in Anti‐Tumor Nanodrugs Based on Tumor Microenvironment Redox Regulation
Lan Yao, Xiang Zhu, Yunyi Shan, Liang Zhang, Jing Yao, Hui Xiong
Small.2024;[Epub] CrossRef - Exploration of the Key Genes Involved in Non-alcoholic Fatty Liver Disease and Possible MicroRNA Therapeutic Targets
Ali Mahmoudi, Amin Jalili, Alexandra E. Butler, Seyed H. Aghaee-Bakhtiari, Tannaz Jamialahmadi, Amirhossein Sahebkar
Journal of Clinical and Experimental Hepatology.2024; 14(4): 101365. CrossRef - Inflammation drives pathogenesis of early intestinal failure-associated liver disease
Scott C. Fligor, Savas T. Tsikis, Thomas I. Hirsch, Ashish Jain, Liang Sun, Shira Rockowitz, Kathleen M. Gura, Mark Puder
Scientific Reports.2024;[Epub] CrossRef - A Systematic Review of Statins for the Treatment of Nonalcoholic Steatohepatitis: Safety, Efficacy, and Mechanism of Action
Shiqin Zhang, Xiaoling Ren, Bingzheng Zhang, Tian Lan, Bing Liu
Molecules.2024; 29(8): 1859. CrossRef - Effects of metformin and simvastatin treatment on ultrastructural features of liver macrophages in HFD mice
Darko Ciric, Tamara Kravic-Stevovic, Vladimir Bumbasirevic, Sasa Petricevic, Sofija Jovanovic, Vladimir Trajkovic, Tamara Martinovic
Ultrastructural Pathology.2023; 47(1): 1. CrossRef - Statins for the Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis
Weiwei Dai, Baohong Xu, Peng Li, Junhua Weng
American Journal of Therapeutics.2023; 30(1): e17. CrossRef - Atorvastatin Attenuates Diet-Induced Non-Alcoholic Steatohepatitis in APOE*3-Leiden Mice by Reducing Hepatic Inflammation
José A. Inia, Geurt Stokman, Elsbet J. Pieterman, Martine C. Morrison, Aswin L. Menke, Lars Verschuren, Martien P. M. Caspers, Martin Giera, J. Wouter Jukema, Anita M. van den Hoek, Hans M. G. Princen
International Journal of Molecular Sciences.2023; 24(9): 7818. CrossRef - The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease
Alexandra C. Finney, Sandeep Das, Dhananjay Kumar, M. Peyton McKinney, Bishuang Cai, Arif Yurdagul, Oren Rom
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Serum metabolomic signatures of fatty acid oxidation defects differentiate host-response subphenotypes of acute respiratory distress syndrome
Tomeka L. Suber, Stacy G. Wendell, Steven J. Mullett, Benjamin Zuchelkowski, William Bain, Georgios D. Kitsios, Bryan J. McVerry, Prabir Ray, Anuradha Ray, Rama K. Mallampalli, Yingze Zhang, Faraaz Shah, Seyed Mehdi Nouraie, Janet S. Lee
Respiratory Research.2023;[Epub] CrossRef - Statins on nonalcoholic fatty liver disease: A systematic review and meta-analysis of 14 RCTs
Haiyan Zhou, Maeda Toshiyoshi,, Wenli Zhao, Ye Zhao, Yan Zhao,
Medicine.2023; 102(26): e33981. CrossRef - Atorvastatin rescues hyperhomocysteinemia-induced cognitive deficits and neuroinflammatory gene changes
Erica M. Weekman, Sherika N. Johnson, Colin B. Rogers, Tiffany L. Sudduth, Kevin Xie, Qi Qiao, David W. Fardo, Teodoro Bottiglieri, Donna M. Wilcock
Journal of Neuroinflammation.2023;[Epub] CrossRef - Crosstalk between Lipids and Non-Alcoholic Fatty Liver Disease
Divyavani Gowda, Chandra Shekhar, Siddabasave Gowda B. Gowda, Yifan Chen, Shu-Ping Hui
Livers.2023; 3(4): 687. CrossRef - Empagliflozin: Potential Protective Effects on Hepatocytes and Liver Outcomes in Streptozotocin -Diabetic Rats.
Alia Khwaldeh, Nour Al-Sawalha, Shefa' Aljabali, Ziad Shraideh, Sokiyna Ababneh, Roba Bdeir
Biomedical and Pharmacology Journal.2023; 16(4): 2123. CrossRef - Is there a role of lipid-lowering therapies in the management of fatty liver disease?
Ismini Tzanaki, Aris P Agouridis, Michael S Kostapanos
World Journal of Hepatology.2022; 14(1): 119. CrossRef - Bifidobacterium animalis subsp. lactis A6 Enhances Fatty Acid β-Oxidation of Adipose Tissue to Ameliorate the Development of Obesity in Mice
Yanxiong Huo, Guoping Zhao, Jinwang Li, Ran Wang, Fazheng Ren, Yixuan Li, Xiaoyu Wang
Nutrients.2022; 14(3): 598. CrossRef - PharmOmics: A species- and tissue-specific drug signature database and gene-network-based drug repositioning tool
Yen-Wei Chen, Graciel Diamante, Jessica Ding, Thien Xuan Nghiem, Jessica Yang, Sung-Min Ha, Peter Cohn, Douglas Arneson, Montgomery Blencowe, Jennifer Garcia, Nima Zaghari, Paul Patel, Xia Yang
iScience.2022; 25(4): 104052. CrossRef - Pleyotropic Effects of Statins Non-Alcoholic Fat Disease of the Liver Non-Alcoholic Steatohepatitis
И.Р. Агабабян, Ш.Ш. Садыкова
Рецепт.2022; (2): 194. CrossRef - PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH)
Simona Todisco, Anna Santarsiero, Paolo Convertini, Giulio De Stefano, Michele Gilio, Vito Iacobazzi, Vittoria Infantino
Biology.2022; 11(5): 792. CrossRef - Association between organochlorine pesticides and nonalcoholic fatty liver disease in the National Health and Nutrition Examination Survey 2003–2004
Hyunji Sang, Kyu-Na Lee, Chang Hee Jung, Kyungdo Han, Eun Hee Koh
Scientific Reports.2022;[Epub] CrossRef - Integrating the contributions of mitochondrial oxidative metabolism to lipotoxicity and inflammation in NAFLD pathogenesis
Curtis C. Hughey, Patrycja Puchalska, Peter A. Crawford
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2022; 1867(11): 159209. CrossRef - Supplementation of Lycium barbarum Polysaccharide Combined with Aerobic Exercise Ameliorates High-Fat-Induced Nonalcoholic Steatohepatitis via AMPK/PPARα/PGC-1α Pathway
Dou-Dou Li, Jia-Min Ma, Ming-Jing Li, Lu-Lu Gao, Yan-Na Fan, Yan-Nan Zhang, Xiu-Juan Tao, Jian-Jun Yang
Nutrients.2022; 14(15): 3247. CrossRef - Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH)
Xiaohan Xu, Kyle L. Poulsen, Lijuan Wu, Shan Liu, Tatsunori Miyata, Qiaoling Song, Qingda Wei, Chenyang Zhao, Chunhua Lin, Jinbo Yang
Signal Transduction and Targeted Therapy.2022;[Epub] CrossRef - Regulation of Hepatic Lipid and Glucose Metabolism by INSP3R1
Rachel J. Perry
Diabetes.2022; 71(9): 1834. CrossRef - Effects of SLCO1B1 Genetic Variant on Metabolite Profile in Participants on Simvastatin Treatment
Lilian Fernandes Silva, Rowmika Ravi, Jagadish Vangipurapu, Anniina Oravilahti, Markku Laakso
Metabolites.2022; 12(12): 1159. CrossRef - Acute and chronic effects of environmental realistic concentrations of simvastatin in danio rerio: evidences of oxidative alterations and endocrine disruptive activity
D. Rebelo, A.T. Correia, B. Nunes
Environmental Toxicology and Pharmacology.2021; 81: 103522. CrossRef - Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway
In-Jin Cho, Da-Hee Oh, Jin Yoo, You-Cheol Hwang, Kyu Jeung Ahn, Ho-Yeon Chung, Soung Won Jeong, Ju-Young Moon, Sang-Ho Lee, Sung-Jig Lim, In-Kyung Jeong
Scientific Reports.2021;[Epub] CrossRef - Atorvastatin Modulates Bile Acid Homeostasis in Mice with Diet-Induced Nonalcoholic Steatohepatitis
Hana Lastuvkova, Fatemeh Alaei Faradonbeh, Jolana Schreiberova, Milos Hroch, Jaroslav Mokry, Hana Faistova, Zuzana Nova, Radomír Hyspler, Ivone Cristina Igreja Sa, Petr Nachtigal, Alzbeta Stefela, Petr Pavek, Stanislav Micuda
International Journal of Molecular Sciences.2021; 22(12): 6468. CrossRef - PPARs as Metabolic Sensors and Therapeutic Targets in Liver Diseases
Hugo Christian Monroy-Ramirez, Marina Galicia-Moreno, Ana Sandoval-Rodriguez, Alejandra Meza-Rios, Arturo Santos, Juan Armendariz-Borunda
International Journal of Molecular Sciences.2021; 22(15): 8298. CrossRef - Simvastatin affects the PPARα signaling pathway and causes oxidative stress and embryonic development interference in Mugilogobius abei
Chao Wang, Tianli Tang, Yimeng Wang, Xiangping Nie, Kaibin Li
Aquatic Toxicology.2021; 239: 105951. CrossRef - Impact of statin use on the risk and prognosis of hepatocellular carcinoma: a meta-analysis
Jianfeng Wang, Xiaogang Li
European Journal of Gastroenterology & Hepatology.2021; 33(12): 1603. CrossRef - Statins in Non-alcoholic Steatohepatitis
Jose D. Torres-Peña, Laura Martín-Piedra, Francisco Fuentes-Jiménez
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Comparison of the liver findings after simvastatin-treatment between Spontaneously Diabetic Torii-Leprfa (SDT fatty) rats and Sprague-Dawley rats
Tadakazu Takahashi, Yusuke Suzuki, Naohito Yamada, Kaoru Toyoda, Keisuke Goda, Katsunori Ryoke, Chizuru Matsuura, Akio Kobayashi, Shoichiro Sugai, Kayoko Shimoi
Fundamental Toxicological Sciences.2020; 7(1): 41. CrossRef - Current and emerging pharmacotherapeutic interventions for the treatment of liver fibrosis
Joeri Lambrecht, Leo A. van Grunsven, Frank Tacke
Expert Opinion on Pharmacotherapy.2020; 21(13): 1637. CrossRef - The Influence of Statins on the Aerobic Metabolism of Endothelial Cells
Izabela Broniarek, Karolina Dominiak, Lukasz Galganski, Wieslawa Jarmuszkiewicz
International Journal of Molecular Sciences.2020; 21(4): 1485. CrossRef - NADPH Oxidase Inhibition in Fibrotic Pathologies
Karen Bernard, Victor J. Thannickal
Antioxidants & Redox Signaling.2020; 33(6): 455. CrossRef - Pleiotropic Effects of Statins in the Light of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis
Farah Ahsan, Federico Oliveri, Harshit K Goud, Zainab Mehkari, Lubna Mohammed, Moiz Javed, Aldanah Althwanay, Ian H Rutkofsky
Cureus.2020;[Epub] CrossRef - Peroxisomal footprint in the pathogenesis of nonalcoholic steatohepatitis
S.M. Touhidul Islam, Jeseong Won, Mushfiquddin Khan, Kenneth D. Chavin, Inderjit Singh
Annals of Hepatology.2020; 19(5): 466. CrossRef - Structural Basis for Activation of Human Sirtuin 6 by Fluvastatin
Weijie You, Clemens Steegborn
ACS Medicinal Chemistry Letters.2020; 11(11): 2285. CrossRef - Specificity of transaminase activities in the prediction of drug-induced hepatotoxicity
Akio Kobayashi, Yusuke Suzuki, Shoichiro Sugai
The Journal of Toxicological Sciences.2020; 45(9): 515. CrossRef - Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease
Kate L. Bowerman, Saima Firdous Rehman, Annalicia Vaughan, Nancy Lachner, Kurtis F. Budden, Richard Y. Kim, David L. A. Wood, Shaan L. Gellatly, Shakti D. Shukla, Lisa G. Wood, Ian A. Yang, Peter A. Wark, Philip Hugenholtz, Philip M. Hansbro
Nature Communications.2020;[Epub] CrossRef - Simvastatin Reduces Hepatic Oxidative Stress and Endoplasmic Reticulum Stress in Nonalcoholic Steatohepatitis Experimental Model
Graziella Rodrigues, Andrea Janz Moreira, Silvia Bona, Elizângela Schemitt, Cláudio Augusto Marroni, Fábio Cangeri Di Naso, Alexandre Simões Dias, Thienne Rocha Pires, Jaqueline Nascimento Picada, Norma Possa Marroni
Oxidative Medicine and Cellular Longevity.2019; 2019: 1. CrossRef - Impaired Peroxisomal Fitness in Obese Mice, a Vicious Cycle Exacerbating Adipocyte Dysfunction via Oxidative Stress
Lingjuan Piao, Debra Dorotea, Songling Jiang, Eun Hee Koh, Goo Taeg Oh, Hunjoo Ha
Antioxidants & Redox Signaling.2019; 31(18): 1339. CrossRef - (5R)-5-hydroxytriptolide ameliorates liver lipid accumulation by suppressing lipid synthesis and promoting lipid oxidation in mice
Yunxia Dong, Henglei Lu, Qiang Li, Xinming Qi, Yuanchao Li, Zean Zhang, Jing Chen, Jin Ren
Life Sciences.2019; 232: 116644. CrossRef - Simvastatin alleviates bone resorption in apical periodontitis possibly by inhibition of mitophagy‐related osteoblast apoptosis
C.‐N. Yang, S.‐H. Kok, H.‐W. Wang, J. Z.‐C. Chang, E. H.‐H. Lai, C.‐T. Shun, H. Yang, M.‐H. Chen, C.‐Y. Hong, S.‐K. Lin
International Endodontic Journal.2019; 52(5): 676. CrossRef - Different Effects of Pravastatin on Preeclampsia-like Symptoms in Different Mouse Models
Jing Huai, Zi Yang, Yan-Hong Yi, Guang-Jiao Wang
Chinese Medical Journal.2018; 131(4): 461. CrossRef - Ameliorating effects of D-47, a newly developed compound, on lipid metabolism in an animal model of familial hypercholesterolemia (WHHLMI rabbits)
Shohei Tamura, Yui Koike, Hiroaki Takeda, Tomonari Koike, Yoshihiro Izumi, Ryosuke Nagasaka, Tetsuto Tsunoda, Motoo Tori, Kazuo Ogawa, Takeshi Bamba, Masashi Shiomi
European Journal of Pharmacology.2018; 822: 147. CrossRef - Fluvastatin activates sirtuin 6 to regulate sterol regulatory element-binding proteins and AMP-activated protein kinase in HepG2 cells
Ji-Hye Kim, Jun Mi Lee, Jong-Hoon Kim, Kwang Rok Kim
Biochemical and Biophysical Research Communications.2018; 503(3): 1415. CrossRef - Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study
Gyuri Kim, Suk-Yong Jang, Chung Mo Nam, Eun Seok Kang
Journal of Hepatology.2018; 68(3): 476. CrossRef - Membrane Remodeling as a Key Player of the Hepatotoxicity Induced by Co-Exposure to Benzo[a]pyrene and Ethanol of Obese Zebrafish Larvae
Muhammad Imran, Odile Sergent, Arnaud Tête, Isabelle Gallais, Martine Chevanne, Dominique Lagadic-Gossmann, Normand Podechard
Biomolecules.2018; 8(2): 26. CrossRef - Corticosterone-Induced Lipogenesis Activation and Lipophagy Inhibition in Chicken Liver Are Alleviated by Maternal Betaine Supplementation
Yun Hu, Qinwei Sun, Yan Hu, Zhen Hou, Yibo Zong, Nagmeldin A Omer, Halima Abobaker, Ruqian Zhao
The Journal of Nutrition.2018; 148(3): 316. CrossRef - Simvastatin protects against acetaminophen-induced liver injury in mice
Huan Liang, Yang Feng, Ruixia Cui, Minglong Qiu, Jingyao Zhang, Chang Liu
Biomedicine & Pharmacotherapy.2018; 98: 916. CrossRef - Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial
Laurie R Braun, Meghan N Feldpausch, Natalia Czerwonka, Julian Weiss, Karen Branch, Hang Lee, Edgar L Martinez-Salazar, Martin Torriani, Craig A Sponseller, Steven K Grinspoon, Takara L Stanley
The Journal of Clinical Endocrinology & Metabolism.2018; 103(11): 4176. CrossRef - Inactivation of SREBP-1a Phosphorylation Prevents Fatty Liver Disease in Mice: Identification of Related Signaling Pathways by Gene Expression Profiles in Liver and Proteomes of Peroxisomes
Birgit Knebel, Sonja Hartwig, Sylvia Jacob, Ulrike Kettel, Martina Schiller, Waltraud Passlack, Cornelia Koellmer, Stefan Lehr, Dirk Müller-Wieland, Jorg Kotzka
International Journal of Molecular Sciences.2018; 19(4): 980. CrossRef - MicroRNA-124 Regulates Fatty Acid and Triglyceride Homeostasis
Tyler A. Shaw, Ragunath Singaravelu, Megan H. Powdrill, Jordan Nhan, Nadine Ahmed, Dennis Özcelik, John Paul Pezacki
iScience.2018; 10: 149. CrossRef - Liver Disease in Singapore
Mark Muthiah, Chern H Chong, Seng G Lim
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 66. CrossRef - Accelerating Treatment of Skeletal Class II Malocclusion using Fixed Twin Block Appliances
Snigdha Pattanaik, Navya Puvvula, Noorjahan Mohammad
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 146. CrossRef - Effect of a Papain-based Chemomechanical Agent on Structure of Dentin and Bond Strength: Anin vitroStudy
Veena S Pai, Yashwanth Gowda, Sruthi Nair
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 161. CrossRef - Perception of Indian Dental Surgeons regarding Molar Incisor Hypomineralization
Sumita Upadhyay, Jatinder K Dhillon
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 116. CrossRef - Association between Obesity and Oral Health Status in Schoolchildren: A Survey in Five Districts of West Bengal, India
Rahul Kaul, Paras Angrish, Subrata Saha, Sonali Halder, Bhaswar Bhattacharya, Malay Mitra
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 233. CrossRef - Burden of Alcoholic Liver Disease: Bhutan Scenario
Pelden Wangchuk
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 81. CrossRef - Comparative Evaluation of Effects ofTriphala, Garlic Extracts, and Chlorhexidine Mouthwashes on SalivaryStreptococcus mutansCounts and Oral Hygiene Status
Shweta Gupta, Narendra Padiyar, Bharathi Padiyar
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 299. CrossRef - Evaluation of Dentin–Pulp Complex Response after Conservative Clinical Procedures in Primary Teeth
Thais Marchini Oliveira, Bianca Mello, Tassia C Stafuzza, Luciana Vitor, Daniela Rios, Thiago Silva, Maria Machado
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 188. CrossRef - Nonalcoholic Fatty Liver Disease: Time to Take the Bull by the Horns
Preetam Nath, Shivaram P Singh
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 47. CrossRef - F-18 Fluorodeoxyglucose Positron Emission Tomography/ Computed Tomography Findings of Isolated Gastric Tuberculosis mimicking Gastric Cancer and Lymphoma
Remzi A Akdogan, Halil Rakici, Serkan Güngör, Recep Bedir, Elif Akdogan
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 93. CrossRef - Clinical, Radiological, and Histological Assessment of Magnetic Nanoparticles as Pulpotomy Medicament in Primary Molars
Manoj K Mallela, Harivinder R Konyala, Ajay R Mareddy, N Venugopal Reddy, Keerthi P Susheela
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 283. CrossRef - Determination of ABO Blood Groups and Rh Typing from Dry Salivary Samples
Laxmi Lakade, Priyam R Velani
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 100. CrossRef - Orofacial Manifestations of Leukemic Children on Treatment: A Descriptive Study
Keerthilatha M Pai, Aparna Aggarwal
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 193. CrossRef - Mandibular Regional Odontodysplasia in an 8-year-old Boy showing Teeth Disorders, Gubernaculum Tracts, and Altered Bone Fractal Pattern
Davi de Sá Cavalcante, Cristiane SR Fonteles, Thyciana R Ribeiro, Lúcio M Kurita, Alynne Vde M Pimenta, Francisco SR Carvalho, Fábio WG Costa
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 128. CrossRef - Evaluation of Chemokines in the Gingival Crevicular Fluid of Children with Down Syndrome
Harshini Togaru, Veerakishore Kumar Reddy, Naveen K Kommineni, Prathyusha Padakandla, John P Indupalli, Swapna P Nanga
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 288. CrossRef - Hepatitis B Virus Infection among Health Care Workers in Indonesia
David H Muljono, Teguh Wijayadi, Rizalinda Sjahril
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 88. CrossRef - Comparison of the Effectiveness of Probiotic, Chlorhexidine-based Mouthwashes, and Oil Pulling Therapy on Plaque Accumulation and Gingival Inflammation in 10- to 12-year-old Schoolchildren: A Randomized Controlled Trial
Saravana K Kandaswamy, Asokan Sharath, PR Geetha Priya
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 66. CrossRef - Evaluation of Changes in Salivary pH after Intake of Different Eatables and Beverages in Children at Different Time Intervals
Ankit Pachori, Haalaswamy Kambalimath, Garima Bhambhani, Garima Malhotra
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 177. CrossRef - Customized Hybrid Bluegrass Appliance: An Innovative Technique
Ziauddin Mohammad, Apeksha Bagalkotkar, Ashank Mishra, Gopi Veerala
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 141. CrossRef - Postobstructive Cyst Formation in Pancreatic Duct affecting Surgical Approach
Alper Parlakgumus, Ali Ezer
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 99. CrossRef - Validating the Usage of Cariogram in 5- and 12-year-old School-going Children in Paonta Sahib, Himachal Pradesh, India: A 12-month Prospective Study
Manish Madan, Pallav Singhal, Anu Garg, Akash Dupper
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 110. CrossRef - Epidemiology of Chronic Hepatitis B in Turkey
Hasan Ozkan
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 73. CrossRef - A Comparative Evaluation of Time-dependent Changes on the Surface Hardness of Bulk Cure Composites: Anin vitroStudy
Anindita Sarma
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 183. CrossRef - Management of Hepatocellular Carcinoma: Bangladesh Perspective
Mohammad Noor-E-Alam
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 52. CrossRef - Trichobezoar: Ravenous for Hair
Aman Kamra
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 97. CrossRef - Comparative Evaluation of the Fracture Resistance of Two Different Fiber-reinforced Composite Restorations with Particulate Filler Composite Restorations
Vanga V Narasimha Rao, Srinivas K Chandrabhatla, Vabbala R Rajasekhar
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 277. CrossRef - A Comparative Evaluation of Efficacy ofStreptococcus mutansCounts in Saliva: Anin vivoStudy
Inder K Pandit
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 94. CrossRef - Liver Cancer in Nepal
Ananta Shrestha
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 63. CrossRef - Pre-eruptive Intracoronal Radiolucency in First Permanent Molar
Mariana C Ilha, Paulo F Kramer, Simone H Ferreira, Henrique C Ruschel
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 151. CrossRef - Comparative Evaluation of Various Temperature Changes on Stress Distribution in Class II Mesial-occlusal-distal Preparation restored with Different Restorative Materials: A Finite Element Analysis
Binita Srivastava, Neorem N Devi
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 167. CrossRef - A Survey on the Use of Antibiotics among the Dentists of Kolkata, West Bengal, India
Rahul Kaul, Paras Angrish, Subrata Saha, Ashok V Sengupta, Shantanu Mukherjee
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 122. CrossRef - Changing Etiology in Liver Cirrhosis in Sapporo, Japan
Jong-Hon Kang, Takeshi Matsui
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 77. CrossRef - Epidemiology, Genotype Distribution, Prognosis, Control, and Management of Viral Hepatitis B, C, D, and Hepatocellular Carcinoma in Mongolia
Oidov Baatarkhuu, Tsagaantsooj Gerelchimeg, Dashchirev Munkh-Orshikh, Badamnachin Batsukh, Ganbold Sarangua, Jazag Amarsanaa
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 57. CrossRef - Impact of Diabetes Mellitus Type 1 on Lebanese Families’ Quality of Life
Nahla Nassif, Balsam Noueiri
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 61. CrossRef - Pediatric Dental Appointments No-show: Rates and Reasons
Anup Panda, Rupinder Bhatia, Esha C Vora
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 171. CrossRef - A Case of Painless Excision
Rupinder Bhatia, Ipshita A Suyash
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 135. CrossRef - Manuka Honey: A Potent Cariostatic Agent–Anin vitroStudy
Sapna Konde, Javaregowda P Beena, Punyatoya Sahoo, N Sunil Raj, Narayana C Kumar
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 105. CrossRef - Reviewing of Research Finding of Hepatitis B Virus Infection in Lao People's Democratic Republic
Angkham Ounavong
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 75. CrossRef - Nonalcoholic Fatty Liver Disease: Identifying the Disease Burden in Sri Lanka
Anuradha S Dassanayake
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 69. CrossRef - Comparative Evaluation of Microhardness by Common Drinks on Esthetic Restorative Materials and Enamel: Anin vitroStudy
Manish Madan, Akash Dupper, Roli Gupta, Trilok Kainthla
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 155. CrossRef - Hepatocellular Carcinoma Surveillance: Benefit of Serum Alfa-fetoprotein in Real-world Practice
Patharapan Lersritwimanmaen, Supot Nimanong
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 83. CrossRef - Body Mass Index and Dental Caries: A Systematic Review
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 228. CrossRef - Evaluation of Surgical Options for Supernumerary Teeth in the Anterior Maxilla
Gianluca Porcaro, Luca Mirabelli, Ernesto Amosso
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 294. CrossRef - A Comparative Study to evaluate Parent's Ability to assess Dental Fear in their 6- to 10-year-old Children using Children's Fear Survey Schedule—Dental Subscale
Ritika Malhotra, Anchal Sahni
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 205. CrossRef - Estimation of Salivary Glucose, Calcium, Phosphorus, Alkaline Phosphatase, and Immunoglobulin A among Diabetic and Nondiabetic Children: A Case–Control Study
Kalyani Uppu, Suzan Sahana, Ghanashyam P Madu, Aron AK Vasa, Sowjanya Nalluri
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 71. CrossRef - Pedodontic Considerations in a Child with Attention Deficit Hyperactivity Disorder: Literature Review and a Case Report
Siddhi Sinha, Prasanna Praveen, S Prathibha Rani, Athimuthu Anantharaj
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 254. CrossRef - Salivary Cortisol and Alpha-amylase—Biomarkers of Stress in Children undergoing Extraction: Anin vivoStudy
Neha Agarwal, Shefali Chaturvedy, Saurabh Chaturvedi, Yogita Chaturvedi
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 214. CrossRef - Influence of Storage Media and Duration of Fragment in the Media on the Bond Strength of the Reattached Tooth Fragment
Prashant Jalannavar
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 83. CrossRef - Comparative Evaluation of Mucosal Vibrator with Topical Anesthetic Gel to reduce Pain during Administration of Local Anesthesia in Pediatric Patients: Anin vivoStudy
Mahima Gandhi, Garima Kalia, Khushboo Rathore
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 261. CrossRef - Management of Autistic Patients in Dental Office: A Clinical Update
Jyothi S Bommangoudar
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 219. CrossRef - Foreign Body causing Displacement of Immature Fractured Apical Root Fragment: An Unusual Case Report
Aman Moda, Rajesh Singla, Preeti M Agrawal
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 247. CrossRef - Validity and Reliability of the Hindi Version of the Modified Child Perceptions Questionnaire 11 to 14
Mohit Sharma, Prasanna Kumar, Dempsy CM Mandanna
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 271. CrossRef - Nonsyndromic Gingival Fibromatosis: A Rare Case Report
Mahima Gandhi, Akshat Vijay
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 250. CrossRef - Prevalence of Deleterious Oral Habits among 3- to 5-yearold Preschool Children in Bhubaneswar, Odisha, India
Brahmananda Dutta, Tulika Verma
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 210. CrossRef - Evaluation of Antimicrobial Activity of Two Endodontic Sealers: Zinc Oxide with Thyme Oil and Zinc Oxide Eugenol against Root Canal Microorganisms—Anin vitroStudy
Manoj Chandak, Nilima Thosar, Silpi Basak
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 79. CrossRef - Prevalence and Risk Factors for Dental Caries among Preschool Children: A Cross-sectional Study in Eastern India
Vinay K Chugh, Kushal K Sahu, Ankita Chugh
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 238. CrossRef - Squamous Papilloma on Hard Palate: Case Report and Literature Review
Penmatsa Chaitanya, Satyam Martha, Ramachandran Punithvathy, Madhusudhan Reddy
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 244. CrossRef - Comparison of Vitamin D Level of Children with Severe Early Childhood Caries and Children with No Caries
Anchal Chhonkar, Vishal Arya
International Journal of Clinical Pediatric Dentistry.2018; 11(3): 199. CrossRef - Use of “Surface Analyzer” to evaluate the Effect of Two Polishing Systems on Surface Texture of Four Newer Composites
Shefally Garg, Munish Goel, Shweta Verma, Nanika Mahajan, Bhawna Kaul, Vikas Garg
International Journal of Clinical Pediatric Dentistry.2018; 11(4): 266. CrossRef - Clinical Evaluation of Preventive Effect of Fissure Sealants on Initial Carious Lesion of Permanent Mandibular Molars Pretreated with and without Fluoride Varnish by Fluorescence Camera
Madhagudanahalli S Lakshmi, Kudlapur T Srilatha, Bhojraj Nandlal, Seema Deshmukh
International Journal of Clinical Pediatric Dentistry.2018; 11(2): 89. CrossRef - Hepatocellular Carcinoma in Malaysia and Its Changing Trend
Rosmawati Mohamed, Ruksana Raihan, Amirah Azzeri, Fatiha H Shabaruddin
Euroasian Journal of Hepato-Gastroenterology.2018; 8(1): 54. CrossRef - Advances in the Understanding and Treatment of Mitochondrial Fatty Acid Oxidation Disorders
Eric S. Goetzman
Current Genetic Medicine Reports.2017; 5(3): 132. CrossRef - Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance
Ruth C. R. Meex, Matthew J. Watt
Nature Reviews Endocrinology.2017; 13(9): 509. CrossRef - In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications
Yun Hu, Qinwei Sun, Jie Liu, Yimin Jia, Demin Cai, Abdulrahman A. Idriss, Nagmeldin A. Omer, Ruqian Zhao
Scientific Reports.2017;[Epub] CrossRef - The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease
Sheng Zhong, Yuxiang Fan, Qi Yan, Xingyu Fan, Bo Wu, Yujuan Han, Ying Zhang, Yong Chen, Huimao Zhang, Junqi Niu
Medicine.2017; 96(49): e9061. CrossRef - Atorvastatin reduces lipid accumulation in the liver by activating protein kinase A-mediated phosphorylation of perilipin 5
Xing Gao, Yang Nan, Yuanlin Zhao, Yuan Yuan, Bincheng Ren, Chao Sun, Kaiyu Cao, Ming Yu, Xuyang Feng, Jing Ye
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2017; 1862(12): 1512. CrossRef - Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis
E. Matthew Morris, Colin S. McCoin, Julie A. Allen, Michelle L. Gastecki, Lauren G. Koch, Steven L. Britton, Justin A. Fletcher, Xiarong Fu, Wen‐Xing Ding, Shawn C. Burgess, R. Scott Rector, John P. Thyfault
The Journal of Physiology.2017; 595(14): 4909. CrossRef - Use of Statins in Patients with Chronic Liver Disease and Cirrhosis: Current Views and Prospects
Jose Ignacio Vargas, Marco Arrese, Vijay H. Shah, Juan Pablo Arab
Current Gastroenterology Reports.2017;[Epub] CrossRef - Endogenous catalase delays high-fat diet-induced liver injury in mice
Lingjuan Piao, Jiyeon Choi, Guideock Kwon, Hunjoo Ha
The Korean Journal of Physiology & Pharmacology.2017; 21(3): 317. CrossRef - Clinical implications of understanding the association between oxidative stress and pediatric NAFLD
Jake P. Mann, Massimiliano Raponi, Valerio Nobili
Expert Review of Gastroenterology & Hepatology.2017; 11(4): 371. CrossRef
- Organelle stress and alterations in interorganelle crosstalk during liver fibrosis
- Diabetogenic Effect of Statins: A Double-Edged Sword?
- Ji Sung Yoon, Hyoung Woo Lee
- Diabetes Metab J. 2013;37(6):415-422. Published online December 12, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.6.415
- 3,631 View
- 43 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Statins are widely prescribed cholesterol-lowering agents, which have been demonstrated to significantly reduce cardiovascular morbidity and mortality. However, recent trials have reported that statins cause worsening of hyperglycemia and increase the risk of new-onset diabetes. The association between the diabetogenic effect of statins with intensive dose and accompanying major risk factors for diabetes has been demonstrated. However, statins do not appear to have a class effect on insulin sensitivity in non-diabetic patients. Numerous mechanisms have been suggested to explain how statins cause β-cell insulin secretory dysfunction and peripheral insulin resistance leading to incident diabetes. According to findings from an aggregate of large clinical trials, the benefits of statin treatment appear to outweigh the risk of new-onset diabetes. Therefore, it would be inappropriate to discontinue the use of statins for prevention of cardiovascular events because of its potential risk for development of incident diabetes. This review addresses the currently available evidence related to statin use and new-onset diabetes from a clinical perspective.
-
Citations
Citations to this article as recorded by- High doses of rosuvastatin induce impaired branched‐chain amino acid catabolism and lead to insulin resistance
Xue Bai, Xingzhen Long, Fang Song, Baolin Chen, Changcheng Sheng, Cailin Tang, Li Li, Jiaxing Zhang, Rui Zhang, Jiquan Zhang, Jiali Li
Experimental Physiology.2023; 108(7): 961. CrossRef - Statins and the diabetogenic effect (II)
Nicolae Bacinschi, Ina Guţu, Anastasia Caracaş, Svetlana Latuş, Stela Bacinschi-Gheorghiţă, Aurelia Bacinschi, Dumitru Ştîrba, Olesea Malancea
Farmacist.ro.2022; 5(208): 14. CrossRef - Statins and the diabetogenic effect (I)
Nicolae Bacinschi, Ina Guţu, Anastasia Caracaş, Svetlana Latuş, Stela Bacinschi-Gheorghiţă, Aurelia Bacinschi, Dumitru Ştîrba, Olesea Malancea
Farmacist.ro.2022; 4(207): 16. CrossRef - Efficacy and Safety of a Fixed-Dose Combination of Candesartan and Rosuvastatin on Blood Pressure and Cholesterol in Patients With Hypertension and Hypercholesterolemia: A Multicenter, Randomized, Double-Blind, Parallel Phase III Clinical Study
Kyoung Im Cho, Bo Hyun Kim, Yong Hyun Park, Jeong-Cheon Ahn, Sang Hyun Kim, Wook Jin Chung, Weon Kim, Il Suk Sohn, Jin Ho Shin, Yong Jin Kim, Kiyuk Chang, Cheol Woong Yu, Soe Hee Ahn, Seok Yeon Kim, Jae Kean Ryu, Jong Young Lee, Bum Kee Hong, Taek Jong Ho
Clinical Therapeutics.2019; 41(8): 1508. CrossRef - Change in ALT levels after administration of HMG‐CoA reductase inhibitors to subjects with pretreatment levels three times the upper normal limit in clinical practice
Hyunah Kim, Hyeseon Lee, Tong Min Kim, So Jung Yang, Seo Yeon Baik, Seung‐Hwan Lee, Jae‐Hyoung Cho, Hyunyong Lee, Hyeon Woo Yim, In Young Choi, Kun‐Ho Yoon, Hun‐Sung Kim
Cardiovascular Therapeutics.2018;[Epub] CrossRef - Phenotyping of Korean patients with better-than-expected efficacy of moderate-intensity statins using tensor factorization
Jingyun Choi, Yejin Kim, Hun-Sung Kim, In Young Choi, Hwanjo Yu, Katriina Aalto-Setala
PLOS ONE.2018; 13(6): e0197518. CrossRef - Management of patients with statin intolerance
Sabine Fischer, Ulrich Julius
Atherosclerosis Supplements.2017; 30: 33. CrossRef - Use of Moderate‐Intensity Statins for Low‐Density Lipoprotein Cholesterol Level above 190 mg/dL at Baseline in Koreans
Hun‐Sung Kim, Hyeseon Lee, Sue Hyun Lee, Yoo Jin Jeong, Tong Min Kim, So Jung Yang, Sun Jung Baik, Hyunah Kim, Seung‐Hwan Lee, Jae Hyoung Cho, In‐Young Choi, Kun‐Ho Yoon, Ju Han Kim
Basic & Clinical Pharmacology & Toxicology.2017; 121(4): 272. CrossRef - Uso de estatinas e o risco de Diabetes Mellitus tipo 2: Revisão Baseada na Evidência
Susana Silva, Nuno Monteiro
Revista Brasileira de Medicina de Família e Comunidade.2016; 11(38): 1. CrossRef - Association of statin use and stress-induced hyperglycemia in patients with acute ST-elevation myocardial infarction
Chen Yan, Ma Qin, Yang S Juan, Li Y Tao, Gao M dong, Zeng Zechun, Yang X Chun, Cong H Liang, Liu Yin, Meng Kang
JRSM Cardiovascular Disease.2016; 5: 204800401663944. CrossRef - Statin‐related aminotransferase elevation according to baseline aminotransferases level in real practice in Korea
H.‐S. Kim, S. H. Lee, H. Kim, S.‐H. Lee, J. H. Cho, H. Lee, H. W. Yim, S.‐H. Kim, I.‐Y. Choi, K.‐H. Yoon, J. H. Kim
Journal of Clinical Pharmacy and Therapeutics.2016; 41(3): 266. CrossRef - Statins and their increased risk of inducing diabetes
Aris P Agouridis, Michael S Kostapanos, Moses S Elisaf
Expert Opinion on Drug Safety.2015; 14(12): 1835. CrossRef - Effects ofVacciniumBerries on Serum Lipids: A Meta-Analysis of Randomized Controlled Trials
Yitong Zhu, Ya Miao, Zheying Meng, Yuan Zhong
Evidence-Based Complementary and Alternative Medicine.2015; 2015: 1. CrossRef - Type 2 Diabetes Mellitus, Metabolic Syndrome, and Mixed Dyslipidemia: How Similar, How Different, and How to Treat?
Julian Halcox, Anoop Misra
Metabolic Syndrome and Related Disorders.2015; 13(1): 1. CrossRef - How to balance cardiorenometabolic benefits and risks of statins
Soo Lim, Pyung Chun Oh, Ichiro Sakuma, Kwang Kon Koh
Atherosclerosis.2014; 235(2): 644. CrossRef - Contemporary treatment strategies for Type 2 diabetes-related macrovascular disease
Andrew MN Walker, Richard M Cubbon, Mark T Kearney
Expert Review of Endocrinology & Metabolism.2014; 9(6): 641. CrossRef
- High doses of rosuvastatin induce impaired branched‐chain amino acid catabolism and lead to insulin resistance
- Regulation of Muscle Pyruvate Dehydrogenase Complex in Insulin Resistance: Effects of Exercise and Dichloroacetate
- Dumitru Constantin-Teodosiu
- Diabetes Metab J. 2013;37(5):301-314. Published online October 17, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.5.301
- 6,225 View
- 80 Download
- 47 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Since the mitochondrial pyruvate dehydrogenase complex (PDC) controls the rate of carbohydrate oxidation, impairment of PDC activity mediated by high-fat intake has been advocated as a causative factor for the skeletal muscle insulin resistance, metabolic syndrome, and the onset of type 2 diabetes (T2D). There are also situations where muscle insulin resistance can occur independently from high-fat dietary intake such as sepsis, inflammation, or drug administration though they all may share the same underlying mechanism, i.e., via activation of forkhead box family of transcription factors, and to a lower extent via peroxisome proliferator-activated receptors. The main feature of T2D is a chronic elevation in blood glucose levels. Chronic systemic hyperglycaemia is toxic and can lead to cellular dysfunction that may become irreversible over time due to deterioration of the pericyte cell's ability to provide vascular stability and control to endothelial proliferation. Therefore, it may not be surprising that T2D's complications are mainly macrovascular and microvascular related, i.e., neuropathy, retinopathy, nephropathy, coronary artery, and peripheral vascular diseases. However, life style intervention such as exercise, which is the most potent physiological activator of muscle PDC, along with pharmacological intervention such as administration of dichloroacetate or L-carnitine can prove to be viable strategies for treating muscle insulin resistance in obesity and T2D as they can potentially restore whole body glucose disposal.
-
Citations
Citations to this article as recorded by- Oxidative stress and metabolism meet epigenetic modulation in physical exercise
José Luis García-Giménez, Irene Cánovas-Cervera, Federico V. Pallardó
Free Radical Biology and Medicine.2024; 213: 123. CrossRef - The pyruvate dehydrogenase complex: Life’s essential, vulnerable and druggable energy homeostat
Peter W. Stacpoole, Charles E. McCall
Mitochondrion.2023; 70: 59. CrossRef - Regulating mitochondrial metabolism by targeting pyruvate dehydrogenase with dichloroacetate, a metabolic messenger
Nick Schoenmann, Nicholas Tannenbaum, Ryan M. Hodgeman, Raghavan Pillai Raju
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2023; 1869(7): 166769. CrossRef - Serum metabolomics profiling by proton nuclear magnetic resonance spectrometry of the response to single oral macronutrient challenges in women with polycystic ovary syndrome (PCOS) compared with male and female controls
Héctor F. Escobar-Morreale, María Ángeles Martínez-García, María Insenser, Nicolau Cañellas, Xavier Correig, Manuel Luque-Ramírez
Biology of Sex Differences.2023;[Epub] CrossRef - Metabolomics and mitochondrial dysfunction in cardiometabolic disease
Abhishek Shastry, Kimberly Dunham-Snary
Life Sciences.2023; 333: 122137. CrossRef - Loss of metabolic flexibility as a result of overexpression of pyruvate dehydrogenase kinases in muscle, liver and the immune system: Therapeutic targets in metabolic diseases
Jae‐Han Jeon, Themis Thoudam, Eun Jung Choi, Min‐Ji Kim, Robert A Harris, In‐Kyu Lee
Journal of Diabetes Investigation.2021; 12(1): 21. CrossRef - Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy
Saleha Anwar, Anas Shamsi, Taj Mohammad, Asimul Islam, Md. Imtaiyaz Hassan
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2021; 1876(1): 188568. CrossRef - Effect of exercise training on skeletal muscle protein expression in relation to insulin sensitivity: Per‐protocol analysis of a randomized controlled trial (GO‐ACTIWE)
Lea Bruhn, Rasmus Kjøbsted, Jonas Salling Quist, Anne Sofie Gram, Mads Rosenkilde, Kristine Færch, Jørgen F.P. Wojtaszewski, Bente Stallknecht, Martin Bæk Blond
Physiological Reports.2021;[Epub] CrossRef - The Mechanism behind Influenza Virus Cytokine Storm
Yinuo Gu, Xu Zuo, Siyu Zhang, Zhuoer Ouyang, Shengyu Jiang, Fang Wang, Guoqiang Wang
Viruses.2021; 13(7): 1362. CrossRef - Suppression of Pyruvate Dehydrogenase Kinase by Dichloroacetate in Cancer and Skeletal Muscle Cells Is Isoform Specific and Partially Independent of HIF-1α
Nives Škorja Milić, Klemen Dolinar, Katarina Miš, Urška Matkovič, Maruša Bizjak, Mojca Pavlin, Matej Podbregar, Sergej Pirkmajer
International Journal of Molecular Sciences.2021; 22(16): 8610. CrossRef - The Regulatory Roles of PPARs in Skeletal Muscle Fuel Metabolism and Inflammation: Impact of PPAR Agonism on Muscle in Chronic Disease, Contraction and Sepsis
Hannah Crossland, Dumitru Constantin-Teodosiu, Paul L. Greenhaff
International Journal of Molecular Sciences.2021; 22(18): 9775. CrossRef - PPARα, δ and FOXO1 Gene Silencing Overturns Palmitate-Induced Inhibition of Pyruvate Oxidation Differentially in C2C12 Myotubes
Hung-Che Chien, Despina Constantin, Paul L. Greenhaff, Dumitru Constantin-Teodosiu
Biology.2021; 10(11): 1098. CrossRef - Molecular Mechanisms of Muscle Fatigue
Dumitru Constantin-Teodosiu, Despina Constantin
International Journal of Molecular Sciences.2021; 22(21): 11587. CrossRef - Coronavirus Disease-19 (COVID-19) and Modern Lifestyle Diseases
Pallav Sengupta, Sulagna Dutta
Biomedical and Pharmacology Journal.2021; 14(4): 2245. CrossRef - A single bout of resistance exercise improves postprandial lipid metabolism in overweight/obese men with prediabetes
Adam J. Bittel, Daniel C. Bittel, Bettina Mittendorfer, Bruce W. Patterson, Adewole L. Okunade, Jun Yoshino, Lane C. Porter, Nada A. Abumrad, Dominic N. Reeds, W. Todd Cade
Diabetologia.2020; 63(3): 611. CrossRef - An integrative approach to the regulation of mitochondrial respiration during exercise: Focus on high-intensity exercise
Jose A.L. Calbet, Saúl Martín-Rodríguez, Marcos Martin-Rincon, David Morales-Alamo
Redox Biology.2020; 35: 101478. CrossRef - PPARδ and FOXO1 Mediate Palmitate-Induced Inhibition of Muscle Pyruvate Dehydrogenase Complex and CHO Oxidation, Events Reversed by Electrical Pulse Stimulation
Hung-Che Chien, Paul L. Greenhaff, Dumitru Constantin-Teodosiu
International Journal of Molecular Sciences.2020; 21(16): 5942. CrossRef - Cancer cachexia has many symptoms but only one cause: anoxia
Tomas Koltai
F1000Research.2020; 9: 250. CrossRef - Structural basis for the inhibition of PDK2 by novel ATP- and lipoyl-binding site targeting compounds
Jihoon Kang, Haushabhau S. Pagire, Donguk Kang, Yo Han Song, In Kyu Lee, Kang Taek Lee, Chin-Ju Park, Jin Hee Ahn, Jungwook Kim
Biochemical and Biophysical Research Communications.2020; 527(3): 778. CrossRef - Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic
Livio Luzi, Maria Grazia Radaelli
Acta Diabetologica.2020; 57(6): 759. CrossRef - Changes in gene expression of lactate carriers (MCT1 and CD147) in cardiac muscle of diabetic male rats: the effect of dichloroacetate and endurance training
H. Rezaeinasab, A. Habibi, M. Nikbakht, M. Rashno, S. Shakerian
The Ukrainian Biochemical Journal.2020; 92(5): 111. CrossRef - Mitochondrial Uncoupling Coordinated With PDH Activation Safely Ameliorates Hyperglycemia via Promoting Glucose Oxidation
Haowen Jiang, Jia Jin, Yanan Duan, Zhifu Xie, Yufeng Li, Anhui Gao, Min Gu, Xinwen Zhang, Chang Peng, Chunmei Xia, Tiancheng Dong, Hui Li, Lifang Yu, Jie Tang, Fan Yang, Jingya Li, Jia Li
Diabetes.2019; 68(12): 2197. CrossRef - Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Induced by Repeated Forced Swimming in Mice
Takuya Ohba, Shinichi Domoto, Miyu Tanaka, Shinsuke Nakamura, Masamitsu Shimazawa, Hideaki Hara
Biological and Pharmaceutical Bulletin.2019; 42(7): 1140. CrossRef - The Beta Cell in Type 2 Diabetes
Ashley A. Christensen, Maureen Gannon
Current Diabetes Reports.2019;[Epub] CrossRef - Reduced expression of Twist 1 is protective against insulin resistance of adipocytes and involves mitochondrial dysfunction
Sumei Lu, Hong Wang, Rui Ren, Xiaohong Shi, Yanmei Zhang, Wanshan Ma
Scientific Reports.2018;[Epub] CrossRef - PDK4 Deficiency Suppresses Hepatic Glucagon Signaling by Decreasing cAMP Levels
Bo-Yoon Park, Jae-Han Jeon, Younghoon Go, Hye Jin Ham, Jeong-Eun Kim, Eun Kyung Yoo, Woong Hee Kwon, Nam-Ho Jeoung, Yong Hyun Jeon, Seung-Hoi Koo, Byung-Gyu Kim, Ling He, Keun-Gyu Park, Robert A. Harris, In-Kyu Lee
Diabetes.2018; 67(10): 2054. CrossRef - Differences in Muscle Metabolism Between Triathletes and Normally Active Volunteers Investigated Using Multinuclear Magnetic Resonance Spectroscopy at 7T
Radka Klepochová, Ladislav Valkovič, Thomas Hochwartner, Christoph Triska, Norbert Bachl, Harald Tschan, Siegfried Trattnig, Michael Krebs, Martin Krššák
Frontiers in Physiology.2018;[Epub] CrossRef - Defining the contribution of skeletal muscle pyruvate dehydrogenase α1 to exercise performance and insulin action
Kristoffer Svensson, Jessica R. Dent, Shahriar Tahvilian, Vitor F. Martins, Abha Sathe, Julien Ochala, Mulchand S. Patel, Simon Schenk
American Journal of Physiology-Endocrinology and Metabolism.2018; 315(5): E1034. CrossRef - Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism
María Isabel Hernández-Alvarez, Angels Díaz-Ramos, María Berdasco, Jeff Cobb, Evarist Planet, Diane Cooper, Agnieszka Pazderska, Krzystof Wanic, Declan O’Hanlon, Antonio Gomez, Laura R. de la Ballina, Manel Esteller, Manuel Palacin, Donal J. O’Gorman, Joh
Scientific Reports.2017;[Epub] CrossRef - Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer
Peter W Stacpoole
JNCI: Journal of the National Cancer Institute.2017;[Epub] CrossRef - Hyperpalatable Diet and Physical Exercise Modulate the Expression of the Glial Monocarboxylate Transporters MCT1 and 4
Luis V. Portela, Andressa W. Brochier, Clarissa B. Haas, Afonso Kopczynski de Carvalho, Jussania A. Gnoato, Eduardo R. Zimmer, Eduardo Kalinine, Luc Pellerin, Alexandre P. Muller
Molecular Neurobiology.2017; 54(8): 5807. CrossRef - Mitochondrial targeting by dichloroacetate improves outcome following hemorrhagic shock
Kumar Subramani, Sumin Lu, Marie Warren, Xiaogang Chu, Haroldo A. Toque, R. William Caldwell, Michael P. Diamond, Raghavan Raju
Scientific Reports.2017;[Epub] CrossRef - The effect of age and unilateral leg immobilization for 2 weeks on substrate utilization during moderate‐intensity exercise in human skeletal muscle
A. Vigelsø, M. Gram, R. Dybboe, A. B. Kuhlman, C. Prats, P. L. Greenhaff, D. Constantin‐Teodosiu, J. B. Birk, J. F. P. Wojtaszewski, F. Dela, J. W. Helge
The Journal of Physiology.2016; 594(8): 2339. CrossRef - Global Kinetic Analysis of Mammalian E3 Reveals pH-dependent NAD+/NADH Regulation, Physiological Kinetic Reversibility, and Catalytic Optimum
Michael A. Moxley, Daniel A. Beard, Jason N. Bazil
Journal of Biological Chemistry.2016; 291(6): 2712. CrossRef - Pyruvate dehydrogenase kinase regulates hepatitis C virus replication
Gwon-Soo Jung, Jae-Han Jeon, Yeon-Kyung Choi, Se Young Jang, Soo Young Park, Sung-Woo Kim, Jun-Kyu Byun, Mi-Kyung Kim, Sungwoo Lee, Eui-Cheol Shin, In-Kyu Lee, Yu Na Kang, Keun-Gyu Park
Scientific Reports.2016;[Epub] CrossRef - Altered heart proteome in fructose-fed Fisher 344 rats exposed to bisphenol A
S.A. Ljunggren, M. Iggland, M. Rönn, L. Lind, P.M. Lind, H. Karlsson
Toxicology.2016; 347-349: 6. CrossRef - Unacylated ghrelin restores insulin and autophagic signaling in skeletal muscle of diabetic mice
Bjorn T. Tam, Xiao M. Pei, Benjamin Y. Yung, Shea P. Yip, Lawrence W. Chan, Cesar S. Wong, Parco M. Siu
Pflügers Archiv - European Journal of Physiology.2015; 467(12): 2555. CrossRef - Sex-related differences in the effects of high-fat diets on DHEA-treated rats
Ana Lúcia Cecconello, Marcia Trapp, Ana Lúcia Hoefel, Cláudia Vieira Marques, Bruno Dutra Arbo, Gabriela Osterkamp, Luiz Carlos Rios Kucharski, Maria Flávia Marques Ribeiro
Endocrine.2015; 48(3): 985. CrossRef - Pharmacological Blockade of Cannabinoid CB1 Receptors in Diet-Induced Obesity Regulates Mitochondrial Dihydrolipoamide Dehydrogenase in Muscle
Sergio Arrabal, Miguel Angel Lucena, Miren Josune Canduela, Almudena Ramos-Uriarte, Patricia Rivera, Antonia Serrano, Francisco Javier Pavón, Juan Decara, Antonio Vargas, Elena Baixeras, Mercedes Martín-Rufián, Javier Márquez, Pedro Fernández-Llébrez, Bau
PLOS ONE.2015; 10(12): e0145244. CrossRef - A glance at … exercise and glucose uptake
Michael J. Glade, Kyl Smith
Nutrition.2015; 31(6): 893. CrossRef - Perpetual muscle PDH activation in PDH kinase knockout mice protects against high-fat feeding–induced muscle insulin resistance
Dumitru Constantin-Teodosiu, Francis B. Stephens, Paul L. Greenhaff
Proceedings of the National Academy of Sciences.2015;[Epub] CrossRef - Statin-Induced Increases in Atrophy Gene Expression Occur Independently of Changes in PGC1α Protein and Mitochondrial Content
Craig A. Goodman, Derk Pol, Evelyn Zacharewicz, Robert S. Lee-Young, Rod J. Snow, Aaron P. Russell, Glenn K. McConell, Ashok Kumar
PLOS ONE.2015; 10(5): e0128398. CrossRef -
13C metabolic flux analysis shows that resistin impairs the metabolic response to insulin in L6E9 myotubes
Shirley Guzmán, Silvia Marin, Anibal Miranda, Vitaly A Selivanov, Josep J Centelles, Romain Harmancey, Fatima Smih, Annie Turkieh, Yves Durocher, Antonio Zorzano, Philippe Rouet, Marta Cascante
BMC Systems Biology.2014;[Epub] CrossRef - Translational Research: From Biological Discovery to Public Benefit (or Not)
Michael R. Emmert-Buck
Advances in Biology.2014; 2014: 1. CrossRef - Diabetic Worker with History of Falls: A Case Study
Ann R. Lurati
Workplace Health & Safety.2014; 62(5): 175. CrossRef - Diabetic Worker With History of Falls: A Case Study
Ann R. Lurati
Workplace Health & Safety.2014; 62(5): 175. CrossRef - Mitochondrial Pyruvate Carrier 2 Hypomorphism in Mice Leads to Defects in Glucose-Stimulated Insulin Secretion
Patrick A. Vigueira, Kyle S. McCommis, George G. Schweitzer, Maria S. Remedi, Kari T. Chambers, Xiaorong Fu, William G. McDonald, Serena L. Cole, Jerry R. Colca, Rolf F. Kletzien, Shawn C. Burgess, Brian N. Finck
Cell Reports.2014; 7(6): 2042. CrossRef
- Oxidative stress and metabolism meet epigenetic modulation in physical exercise
- Effects of Lovastatin on Free Fatty Acid Oxidation in Cultured L6 Rat Skeletal Muscle Cells.
- Dong Lim Kim, Kee Ho Song, Hae Rim Kim, Suk Kyeong Kim
- Korean Diabetes J. 2007;31(3):230-235. Published online May 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.3.230
- 1,932 View
- 18 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Recent clinical studies suggest that statins improve insulin resistance and glucose metabolism in patients with metabolic syndrome and type 2 diabetes. To evaluate the possible mechanism of this action, we measured free fatty acid oxidation in cultured L6 rat skeletal muscle cell line. METHODS: Cultured L6 myotubes were treated with or without lovastatin (1, 5, 20 micrometer) for 24 hours or 48 hours and palmitate oxidation was measured. We also measured protein concentration of the cells. RESULTS: Lovastain increased palmitate oxidation in dose and time dependent manner in L6 myotubes (24 hr; 1 micrometer 119.2 +/- 11.9% of control, 5 micrometer 140.9 +/- 8.1%, 20 micrometer 150 +/- 5%, P = 0.05 vs control, respectively, 48 hr 1 micrometer 120.9 +/- 14.5%, 5 micrometer 176.6 +/- 28.2%, 20 micrometer 196.0 +/- 19.9%, P < 0.01 vs control, respectively). However, lovastatin decreased total cellular protein (24 hr: 1 micrometer 89.2 +/- 6.1% of control, 5 micrometer 79.3 +/- 7.6%, 20 micrometer 65.4 +/- 4.2%, P = 0.05 vs control, respectively, 48 hr: 1 micrometer 81.7 +/- 5.1%, 5 micrometer 58.6 +/- 11.9%, 20 micrometer 48.1 +/- 6.9%, P < 0.01 vs control, respectively). CONCLUSION: Lovastatin increased skeletal muscle free fatty acid oxidation in L6 rat skeletal muscle cells. This would be one of the mechanisms which lovastatin improves insulin resistance. -
Citations
Citations to this article as recorded by- Characterization and Mechanisms of Action of Avocado Extract Enriched in Mannoheptulose as a Candidate Calorie Restriction Mimetic
Donald K. Ingram, Paul J. Pistell, Zhong Q. Wang, Yongmei Yu, Stefan Massimino, Gary M. Davenport, Michael Hayek, George S. Roth
Journal of Agricultural and Food Chemistry.2021; 69(26): 7367. CrossRef
- Characterization and Mechanisms of Action of Avocado Extract Enriched in Mannoheptulose as a Candidate Calorie Restriction Mimetic

 KDA
KDA
 First
First Prev
Prev





