
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
- A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
- Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Young Min, Sungrae Kim
- Diabetes Metab J. 2022;46(6):855-865. Published online March 8, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0264
- 6,747 View
- 298 Download
- 6 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Thiazolidinediones (TZDs) have been associated with various safety concerns including weight gain, bladder cancer, and congestive heart failure (CHF). This study evaluated the efficacy and safety of lobeglitazone, a novel TZD in patients with type 2 diabetes mellitus (T2DM) in real practice.
Methods
In this non-interventional, multi-center, retrospective, and observational study conducted at 15 tertiary or secondary referral hospitals in Korea, a total of 2,228 patients with T2DM who received lobeglitazone 0.5 mg for more than 1 year were enrolled.
Results
Overall adverse events (AEs) occurred in 381 patients (17.10%) including edema in 1.97% (n=44). Cerebrovascular and cardiovascular diseases were identified in 0.81% (n=18) and 0.81% (n=18), respectively. One case of CHF was reported as an AE. Edema occurred in 1.97% (n=44) of patients. Hypoglycemia occurred in 2.47% (n=55) of patients. Fracture occurred in 1.17% (n=26) of all patients. Lobeglitazone significantly decreased HbA1c level, resulting in a mean treatment difference of -1.05%± 1.35% (P<0.001), and decreased total cholesterol, triglyceride, and low-density lipoprotein cholesterol. However, it increased high-density lipoprotein cholesterol, regardless of statin administration. The patients who received lobeglitazone 0.5 mg showed an apparent reduction in glycosylated hemoglobin (HbA1c) from baseline during the first 6 months of treatment. The HbA1c levels remained stable between months 6 and 42.
Conclusion
Lobeglitazone has long-term safety profile, good glycemic-lowering effect and long-term durability of glycemic control in real-world clinical settings. -
Citations
Citations to this article as recorded by- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis
Shashank R. Joshi, Saibal Das, Suja Xaviar, Shambo Samrat Samajdar, Indranil Saha, Sougata Sarkar, Shatavisa Mukherjee, Santanu Kumar Tripathi, Jyotirmoy Pal, Nandini Chatterjee
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102703. CrossRef - Will lobeglitazone rival pioglitazone? A systematic review and critical appraisal
Kalyan Kumar Gangopadhyay, Awadhesh Kumar Singh
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(4): 102747. CrossRef - Lobeglitazone
Reactions Weekly.2023; 1948(1): 262. CrossRef - Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study
Joonsang Yoo, Jimin Jeon, Minyoul Baik, Jinkwon Kim
Cardiovascular Diabetology.2023;[Epub] CrossRef - Lobeglitazone and Its Therapeutic Benefits: A Review
Balamurugan M, Sarumathy S, Robinson R
Cureus.2023;[Epub] CrossRef - Oldies but Goodies: Thiazolidinedione as an Insulin Sensitizer with Cardioprotection
Eun-Hee Cho
Diabetes & Metabolism Journal.2022; 46(6): 827. CrossRef
- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
- Drug/Regimen
- Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
- Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
- Diabetes Metab J. 2021;45(3):326-336. Published online April 19, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0272
- 9,821 View
- 413 Download
- 22 Web of Science
- 23 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
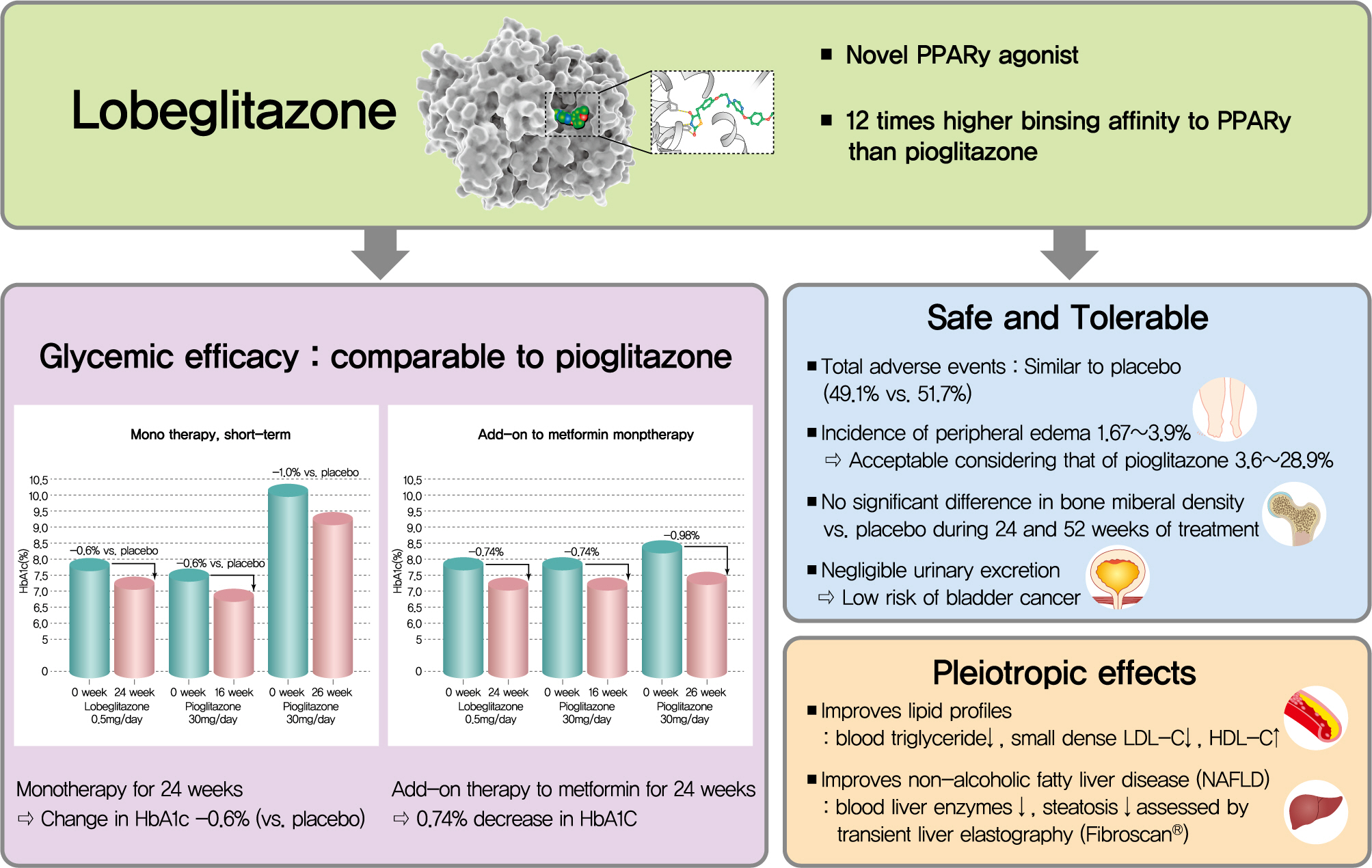
- Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and β-cell dysfunction. Among available oral antidiabetic agents, only the thiazolidinediones (TZDs) primarily target insulin resistance. TZDs improve insulin sensitivity by activating peroxisome proliferator-activated receptor γ. Rosiglitazone and pioglitazone have been used widely for T2DM treatment due to their potent glycemic efficacy and low risk of hypoglycemia. However, their use has decreased because of side effects and safety issues, such as cardiovascular concerns and bladder cancer. Lobeglitazone (Chong Kun Dang Pharmaceutical Corporation), a novel TZD, was developed to meet the demands for an effective and safe TZD. Lobeglitazone shows similar glycemic efficacy to pioglitazone, with a lower effective dose, and favorable safety results. It also showed pleiotropic effects in preclinical and clinical studies. In this article, we summarize the pharmacologic, pharmacokinetic, and clinical characteristics of lobeglitazone.
-
Citations
Citations to this article as recorded by- Etiology of Drug-Induced Edema: A Review of Dihydropyridine, Thiazolidinedione, and Other Medications Causing Edema
Evan S Sinnathamby, Bretton T Urban, Robert A Clark, Logan T Roberts, Audrey J De Witt, Danielle M Wenger, Aya Mouhaffel, Olga Willett, Shahab Ahmadzadeh, Sahar Shekoohi, Alan D Kaye, Giustino Varrassi
Cureus.2024;[Epub] CrossRef - Novel thiazolidin-4-one benzenesulfonamide hybrids as PPARγ agonists: Design, synthesis and in vivo anti-diabetic evaluation
Islam H. Ali, Rasha M. Hassan, Ahmed M. El Kerdawy, Mahmoud T. Abo-Elfadl, Heba M.I. Abdallah, Francesca Sciandra, Iman A.Y. Ghannam
European Journal of Medicinal Chemistry.2024; 269: 116279. CrossRef - The role of the methoxy group in approved drugs
Debora Chiodi, Yoshihiro Ishihara
European Journal of Medicinal Chemistry.2024; : 116364. CrossRef - Thiazolidinedione an auspicious scaffold as PPAR-γ agonist: its possible mechanism to Manoeuvre against insulin resistant diabetes mellitus
Sourav Basak, Anjali Murmu, Balaji Wamanrao Matore, Partha Pratim Roy, Jagadish Singh
European Journal of Medicinal Chemistry Reports.2024; 11: 100160. CrossRef - Efficacy and Safety of Novel Thiazolidinedione Rivoglitazone in Type-2 Diabetes a Meta-Analysis
Deep Dutta, Jyoti Kadian, Indira Maisnam, Ashok Kumar, Saptarshi Bhattacharya, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2023; 27(4): 286. CrossRef - Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis
Shashank R. Joshi, Saibal Das, Suja Xaviar, Shambo Samrat Samajdar, Indranil Saha, Sougata Sarkar, Shatavisa Mukherjee, Santanu Kumar Tripathi, Jyotirmoy Pal, Nandini Chatterjee
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102703. CrossRef - Synthesis, Characterization, and Pharmacokinetic Studies of Thiazolidine-2,4-Dione Derivatives
Bushra Ansari, Haroon Khan, Muhammad Saeed Jan, Khalaf F. Alsharif, Khalid J. Alzahrani, Umer Rashid, Abdul Saboor Pirzada, Vinod Kumar Tiwari
Journal of Chemistry.2023; 2023: 1. CrossRef - Will lobeglitazone rival pioglitazone? A systematic review and critical appraisal
Kalyan Kumar Gangopadhyay, Awadhesh Kumar Singh
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(4): 102747. CrossRef - Evaluation of pharmacokinetic interactions between lobeglitazone, empagliflozin, and metformin in healthy subjects
Heeyoung Kim, Choon Ok Kim, Hyeonsoo Park, Min Soo Park, Dasohm Kim, Taegon Hong, Yesong Shin, Byung Hak Jin
Translational and Clinical Pharmacology.2023; 31(1): 59. CrossRef - Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study
Joonsang Yoo, Jimin Jeon, Minyoul Baik, Jinkwon Kim
Cardiovascular Diabetology.2023;[Epub] CrossRef - Complementary effects of dapagliflozin and lobeglitazone on metabolism in a diet-induced obese mouse model
Yun Kyung Lee, Tae Jung Oh, Ji In Lee, Bo Yoon Choi, Hyen Chung Cho, Hak Chul Jang, Sung Hee Choi
European Journal of Pharmacology.2023; 957: 175946. CrossRef - Thiazolidinediones: Recent Development in Analytical Methodologies
Tarang Patel, Vatsal Patel
Journal of Chromatographic Science.2023;[Epub] CrossRef - Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease
Shotaro Kamata, Akihiro Honda, Isao Ishii
Biomolecules.2023; 13(8): 1264. CrossRef - Lobeglitazone inhibits LPS-induced NLRP3 inflammasome activation and inflammation in the liver
Hye-Young Seo, So-Hee Lee, Ji Yeon Park, Eugene Han, Sol Han, Jae Seok Hwang, Mi Kyung Kim, Byoung Kuk Jang, Kenji Fujiwara
PLOS ONE.2023; 18(8): e0290532. CrossRef - Insulin sensitizers in 2023: lessons learned and new avenues for investigation
Jerry R. Colca, Steven P. Tanis, Rolf F. Kletzien, Brian N. Finck
Expert Opinion on Investigational Drugs.2023; 32(9): 803. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Lobeglitazone and Its Therapeutic Benefits: A Review
Balamurugan M, Sarumathy S, Robinson R
Cureus.2023;[Epub] CrossRef - A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - Effect of the addition of thiazolidinedione to sodium-glucose cotransporter 2 inhibitor therapy on lipid levels in type 2 diabetes mellitus: a retrospective study using Korean National Health Insurance Service data
Taegyun Park, Kyungdo Han, Dongwook Shin, Jongho Park
Cardiovascular Prevention and Pharmacotherapy.2022; 4(3): 114. CrossRef - Design of Improved Antidiabetic Drugs: A Journey from Single to Multitarget Agents
Vassiliki‐Panagiota Tassopoulou, Ariadni Tzara, Angeliki P. Kourounakis
ChemMedChem.2022;[Epub] CrossRef - A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Y
Diabetes & Metabolism Journal.2022; 46(6): 855. CrossRef - Lobeglitazone Exerts Anti-Inflammatory Effect in Lipopolysaccharide-Induced Bone-Marrow Derived Macrophages
Dabin Jeong, Wan-Kyu Ko, Seong-Jun Kim, Gong-Ho Han, Daye Lee, Seung-Hun Sheen, Seil Sohn
Biomedicines.2021; 9(10): 1432. CrossRef
- Etiology of Drug-Induced Edema: A Review of Dihydropyridine, Thiazolidinedione, and Other Medications Causing Edema
- Clinical Diabetes & Therapeutics
- Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
- Soo Lim, Kyoung Min Kim, Sin Gon Kim, Doo Man Kim, Jeong-Taek Woo, Choon Hee Chung, Kyung Soo Ko, Jeong Hyun Park, Yongsoo Park, Sang Jin Kim, Hak Chul Jang, Dong Seop Choi
- Diabetes Metab J. 2017;41(5):377-385. Published online October 24, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.5.377
- 4,271 View
- 42 Download
- 19 Web of Science
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The aim of this multicenter, randomized, double-blind study was to examine the effect of lobeglitazone, a novel thiazolidinedione, on the changes in bone mineral density (BMD) in patients with type 2 diabetes mellitus.
Methods A 24-week, double-blinded phase was followed by a 28-week, open-label phase, in which the placebo group also started to receive lobeglitazone. A total of 170 patients aged 34 to 76 years were randomly assigned in a 2:1 ratio to receive lobeglitazone 0.5 mg or a matching placebo orally, once daily. BMD was assessed using dual-energy X-ray absorptiometry at week 24 and at the end of the study (week 52).
Results During the double-blinded phase, the femur neck BMD showed decreasing patterns in both groups, without statistical significance (−0.85%±0.36% and −0.78%±0.46% in the lobeglitazone and placebo groups, respectively). The treatment difference between the groups was 0.07%, which was also not statistically significant. Further, minimal, nonsignificant decreases were observed in both groups in the total hip BMD compared to values at baseline, and these differences also did not significantly differ between the groups. During the open-label phase, the BMD was further decreased, but not significantly, by −0.32% at the femur neck and by −0.60% at the total hip in the lobeglitazone group, and these changes did not significantly differ compared with the original placebo group switched to lobeglitazone.
Conclusion Our results indicate that treatment with lobeglitazone 0.5 mg over 52 weeks showed no detrimental effect on the BMD compared to the placebo.
-
Citations
Citations to this article as recorded by- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis
Shashank R. Joshi, Saibal Das, Suja Xaviar, Shambo Samrat Samajdar, Indranil Saha, Sougata Sarkar, Shatavisa Mukherjee, Santanu Kumar Tripathi, Jyotirmoy Pal, Nandini Chatterjee
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102703. CrossRef - The benefits of adipocyte metabolism in bone health and regeneration
Lisa-Marie Burkhardt, Christian H. Bucher, Julia Löffler, Charlotte Rinne, Georg N. Duda, Sven Geissler, Tim J. Schulz, Katharina Schmidt-Bleek
Frontiers in Cell and Developmental Biology.2023;[Epub] CrossRef - Will lobeglitazone rival pioglitazone? A systematic review and critical appraisal
Kalyan Kumar Gangopadhyay, Awadhesh Kumar Singh
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(4): 102747. CrossRef - Comparison of therapeutic efficacy and safety of sitagliptin, dapagliflozin, or lobeglitazone adjunct therapy in patients with type 2 diabetes mellitus inadequately controlled on sulfonylurea and metformin: Third agent study
Jun Hwa Hong, Jun Sung Moon, Kayeon Seong, Soo Lim
Diabetes Research and Clinical Practice.2023; 203: 110872. CrossRef - Bone Mineral Density Evaluation Among Type 2 Diabetic Patients in Rural Haryana, India: An Analytical Cross-Sectional Study
Nitish Khandelwal, Surbhi Rajauria, Siddhesh Pandurang Kanjalkar, Omkar Shivaji Chavanke, Sanjay Rai
Cureus.2023;[Epub] CrossRef - Lobeglitazone and Its Therapeutic Benefits: A Review
Balamurugan M, Sarumathy S, Robinson R
Cureus.2023;[Epub] CrossRef - A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Y
Diabetes & Metabolism Journal.2022; 46(6): 855. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes & Metabolism Journal.2021; 45(3): 326. CrossRef - Effect of lobeglitazone on motor function in rat model of Parkinson’s disease with diabetes co-morbidity
Kambiz Hassanzadeh, Arman Rahimmi, Mohammad Raman Moloudi, Rita Maccarone, Massimo Corbo, Esmael Izadpanah, Marco Feligioni
Brain Research Bulletin.2021; 173: 184. CrossRef - Recent Perspective on Thiazolidinedione
Won Jun Kim
The Journal of Korean Diabetes.2021; 22(2): 97. CrossRef - Use of in vitro bone models to screen for altered bone metabolism, osteopathies, and fracture healing: challenges of complex models
Sabrina Ehnert, Helen Rinderknecht, Romina H. Aspera-Werz, Victor Häussling, Andreas K. Nussler
Archives of Toxicology.2020; 94(12): 3937. CrossRef - Update on: effects of anti-diabetic drugs on bone metabolism
Guillaume Mabilleau, Béatrice Bouvard
Expert Review of Endocrinology & Metabolism.2020; 15(6): 415. CrossRef - The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: Systematic review and meta‐analysis of observational studies
Khemayanto Hidayat, Xuan Du, Meng‐Jiao Wu, Bi‐Min Shi
Obesity Reviews.2019; 20(10): 1494. CrossRef - Diabetes pharmacotherapy and effects on the musculoskeletal system
Evangelia Kalaitzoglou, John L. Fowlkes, Iuliana Popescu, Kathryn M. Thrailkill
Diabetes/Metabolism Research and Reviews.2019;[Epub] CrossRef - Morin Exerts Anti‐Arthritic Effects by Attenuating Synovial Angiogenesis via Activation of Peroxisome Proliferator Activated Receptor‐γ
Mengfan Yue, Ni Zeng, Yufeng Xia, Zhifeng Wei, Yue Dai
Molecular Nutrition & Food Research.2018;[Epub] CrossRef - The effects of diabetes therapy on bone: A clinical perspective
Karim G. Kheniser, Carmen M. Polanco Santos, Sangeeta R. Kashyap
Journal of Diabetes and its Complications.2018; 32(7): 713. CrossRef - Changes in the Bone Mineral Density of Femur Neck and Total Hip Over a 52-Week Treatment with Lobeglitazone
Da Young Lee, Ji A Seo
Diabetes & Metabolism Journal.2017; 41(5): 374. CrossRef
- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis

 KDA
KDA
 First
First Prev
Prev





