
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug Regimen
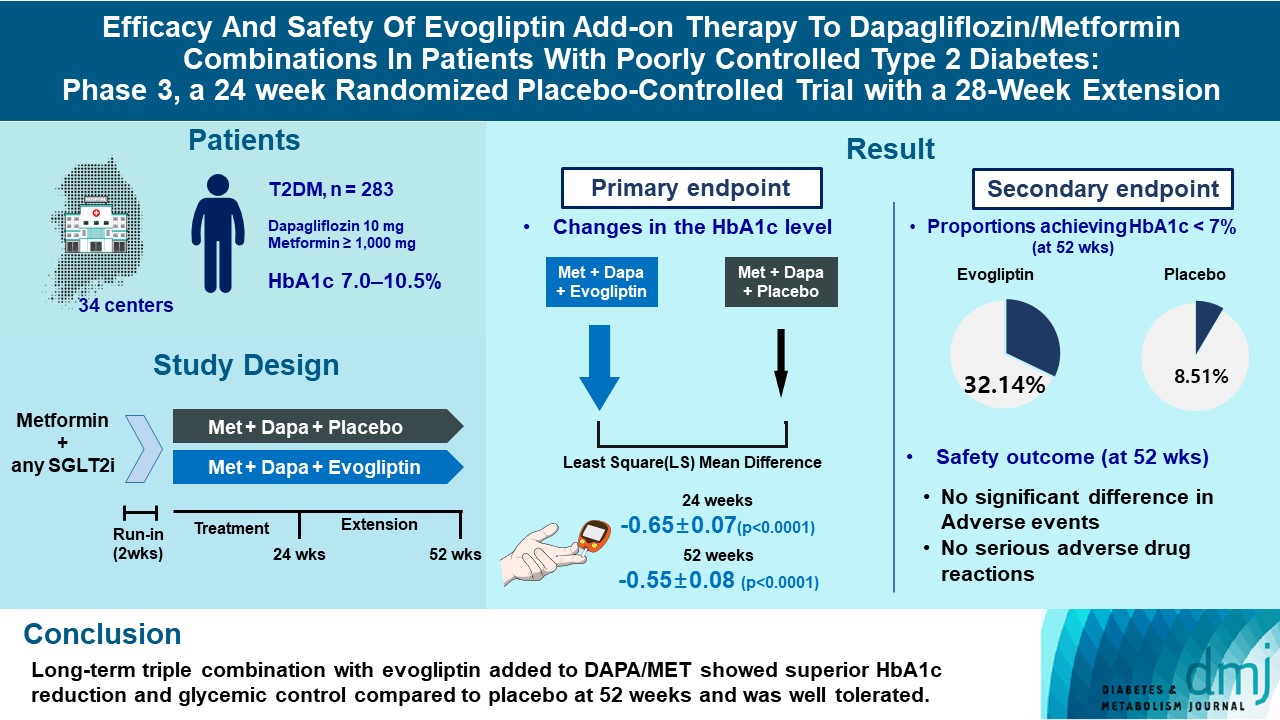
- Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extension
- Jun Sung Moon, Il Rae Park, Hae Jin Kim, Choon Hee Chung, Kyu Chang Won, Kyung Ah Han, Cheol-Young Park, Jong Chul Won, Dong Jun Kim, Gwan Pyo Koh, Eun Sook Kim, Jae Myung Yu, Eun-Gyoung Hong, Chang Beom Lee, Kun-Ho Yoon
- Diabetes Metab J. 2023;47(6):808-817. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0387
- 2,576 View
- 281 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigates the long-term efficacy and safety of evogliptin add-on therapy in patients with inadequately controlled type 2 diabetes mellitus (T2DM) previously received dapagliflozin and metformin (DAPA/MET) combination.
Methods
In this multicenter randomized placebo-controlled phase 3 trial, patients with glycosylated hemoglobin (HbA1c) levels 7.0% to 10.5% (n=283) previously used DAPA 10 mg plus MET (≥1,000 mg) were randomly assigned to the evogliptin 5 mg once daily or placebo group (1:1). The primary endpoint was the difference in the HbA1c level from baseline at week 24, and exploratory endpoints included the efficacy and safety of evogliptin over 52 weeks (trial registration: ClinicalTrials.gov NCT04170998).
Results
Evogliptin add-on to DAPA/MET therapy was superior in HbA1c reduction compared to placebo at weeks 24 and 52 (least square [LS] mean difference, –0.65% and –0.55%; 95% confidence interval [CI], –0.79 to –0.51 and –0.71 to –0.39; P<0.0001). The proportion of patients achieving HbA1c <7% was higher in the triple combination group at week 52 (32.14% vs. 8.51% in placebo; odds ratio, 5.62; P<0.0001). Evogliptin significantly reduced the fasting glucose levels and mean daily glucose levels with improvement in homeostatic model assessment of β-cell function (LS mean difference, 9.04; 95% CI, 1.86 to 16.21; P=0.0138). Adverse events were similar between the groups, and no serious adverse drug reactions were reported in the evogliptin group.
Conclusion
Long-term triple combination with evogliptin added to DAPA/MET showed superior HbA1c reduction and glycemic control compared to placebo at 52 weeks and was well tolerated.
- Basic Research
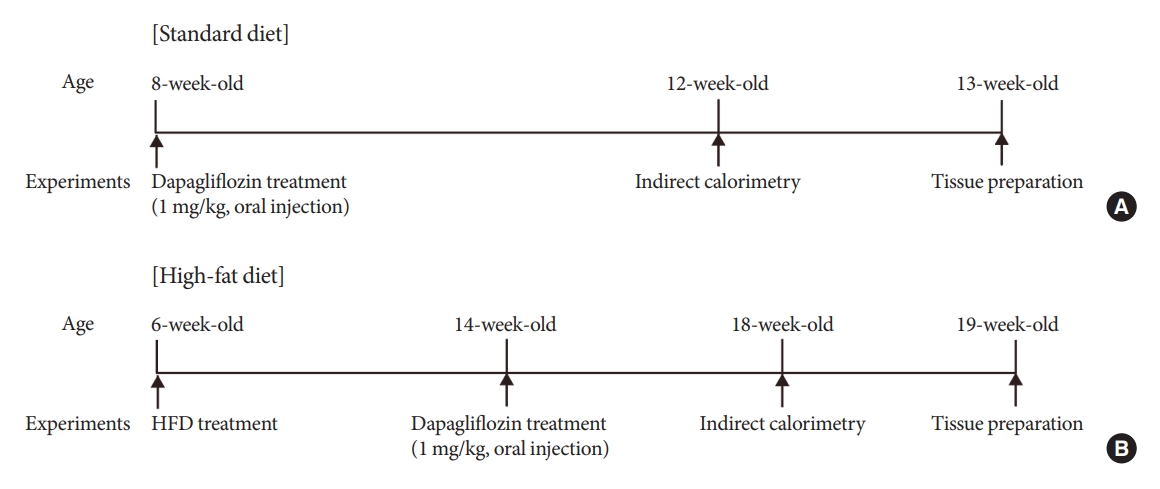
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2023;47(6):784-795. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0261
- 1,408 View
- 149 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are currently used to treat patients with diabetes. Previous studies have demonstrated that treatment with SGLT-2 inhibitors is accompanied by altered metabolic phenotypes. However, it has not been investigated whether the hypothalamic circuit participates in the development of the compensatory metabolic phenotypes triggered by the treatment with SGLT-2 inhibitors.
Methods
Mice were fed a standard diet or high-fat diet and treated with dapagliflozin, an SGLT-2 inhibitor. Food intake and energy expenditure were observed using indirect calorimetry system. The activity of hypothalamic neurons in response to dapagliflozin treatment was evaluated by immunohistochemistry with c-Fos antibody. Quantitative real-time polymerase chain reaction was performed to determine gene expression patterns in the hypothalamus of dapagliflozin-treated mice.
Results
Dapagliflozin-treated mice displayed enhanced food intake and reduced energy expenditure. Altered neuronal activities were observed in multiple hypothalamic nuclei in association with appetite regulation. Additionally, we found elevated immunosignals of agouti-related peptide neurons in the paraventricular nucleus of the hypothalamus.
Conclusion
This study suggests the functional involvement of the hypothalamus in the development of the compensatory metabolic phenotypes induced by SGLT-2 inhibitor treatment. -
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
Diabetes & Metabolism Journal.2024; 48(1): 159. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Drug Regimen
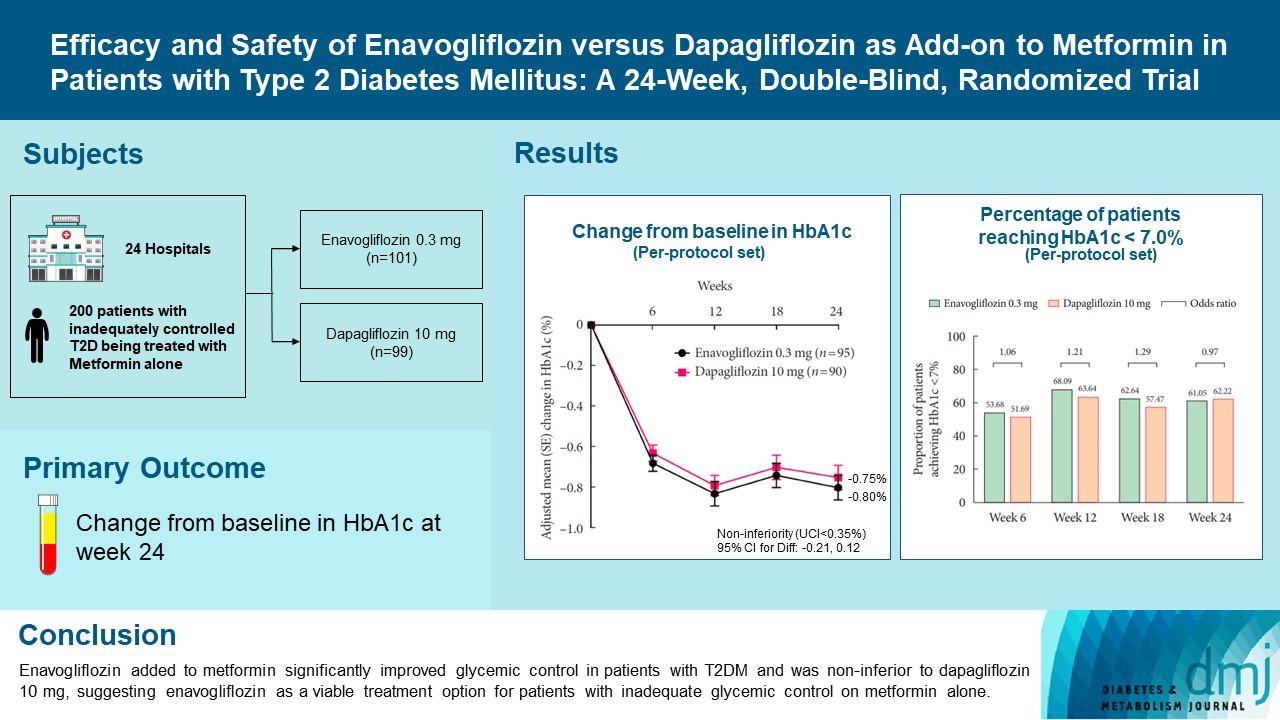
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Kyung Ah Han, Yong Hyun Kim, Doo Man Kim, Byung Wan Lee, Suk Chon, Tae Seo Sohn, In Kyung Jeong, Eun-Gyoung Hong, Jang Won Son, Jae Jin Nah, Hwa Rang Song, Seong In Cho, Seung-Ah Cho, Kun Ho Yoon
- Diabetes Metab J. 2023;47(6):796-807. Published online February 9, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0315
- 40,043 View
- 572 Download
- 4 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Enavogliflozin is a novel sodium-glucose cotransporter-2 inhibitor currently under clinical development. This study evaluated the efficacy and safety of enavogliflozin as an add-on to metformin in Korean patients with type 2 diabetes mellitus (T2DM) against dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, 200 patients were randomized to receive enavogliflozin 0.3 mg/day (n=101) or dapagliflozin 10 mg/day (n=99) in addition to ongoing metformin therapy for 24 weeks. The primary objective of the study was to prove the non-inferiority of enavogliflozin to dapagliflozin in glycosylated hemoglobin (HbA1c) change at week 24 (non-inferiority margin of 0.35%) (Clinical trial registration number: NCT04634500).
Results
Adjusted mean change of HbA1c at week 24 was –0.80% with enavogliflozin and –0.75% with dapagliflozin (difference, –0.04%; 95% confidence interval, –0.21% to 0.12%). Percentages of patients achieving HbA1c <7.0% were 61% and 62%, respectively. Adjusted mean change of fasting plasma glucose at week 24 was –32.53 and –29.14 mg/dL. An increase in urine glucose-creatinine ratio (60.48 vs. 44.94, P<0.0001) and decrease in homeostasis model assessment of insulin resistance (–1.85 vs. –1.31, P=0.0041) were significantly greater with enavogliflozin than dapagliflozin at week 24. Beneficial effects of enavogliflozin on body weight (–3.77 kg vs. –3.58 kg) and blood pressure (systolic/diastolic, –5.93/–5.41 mm Hg vs. –6.57/–4.26 mm Hg) were comparable with those of dapagliflozin, and both drugs were safe and well-tolerated.
Conclusion
Enavogliflozin added to metformin significantly improved glycemic control in patients with T2DM and was non-inferior to dapagliflozin 10 mg, suggesting enavogliflozin as a viable treatment option for patients with inadequate glycemic control on metformin alone. -
Citations
Citations to this article as recorded by- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Young Sang Lyu, Sangmo Hong, Si Eun Lee, Bo Young Cho, Cheol-Young Park
Cardiovascular Diabetology.2024;[Epub] CrossRef - A 52‐week efficacy and safety study of enavogliflozin versus dapagliflozin as an add‐on to metformin in patients with type 2 diabetes mellitus: ENHANCE‐M extension study
Tae Seo Sohn, Kyung‐Ah Han, Yonghyun Kim, Byung‐Wan Lee, Suk Chon, In‐Kyung Jeong, Eun‐Gyoung Hong, Jang Won Son, JaeJin Na, Jae Min Cho, Seong In Cho, Wan Huh, Kun‐Ho Yoon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - The effect of renal function on the pharmacokinetics and pharmacodynamics of enavogliflozin, a potent and selective sodium‐glucose cotransporter‐2 inhibitor, in type 2 diabetes
Sae Im Jeong, Mu Seong Ban, Jun‐Gi Hwang, Min‐Kyu Park, Soo Lim, Sejoong Kim, Soon Kil Kwon, Yoonjin Kim, Jae Min Cho, Jae Jin Na, Wan Huh, Jae‐Yong Chung
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: A systematic review and meta-analysis
Deep Dutta, B.G. Harish, Beatrice Anne, Lakshmi Nagendra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102816. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - Prospects of using sodium-glucose co-transporter-2 (SGLT-2) inhibitors in patients with metabolic-associated fatty liver disease (MAFLD)
Iryna Kostitska, Nadia Protas, Liliia Petrovska
Diabetes Obesity Metabolic Syndrome.2023; (5): 8. CrossRef - Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
Sang Youl Rhee
Diabetes & Metabolism Journal.2023; 47(6): 769. CrossRef
- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
- Drug/Regimen
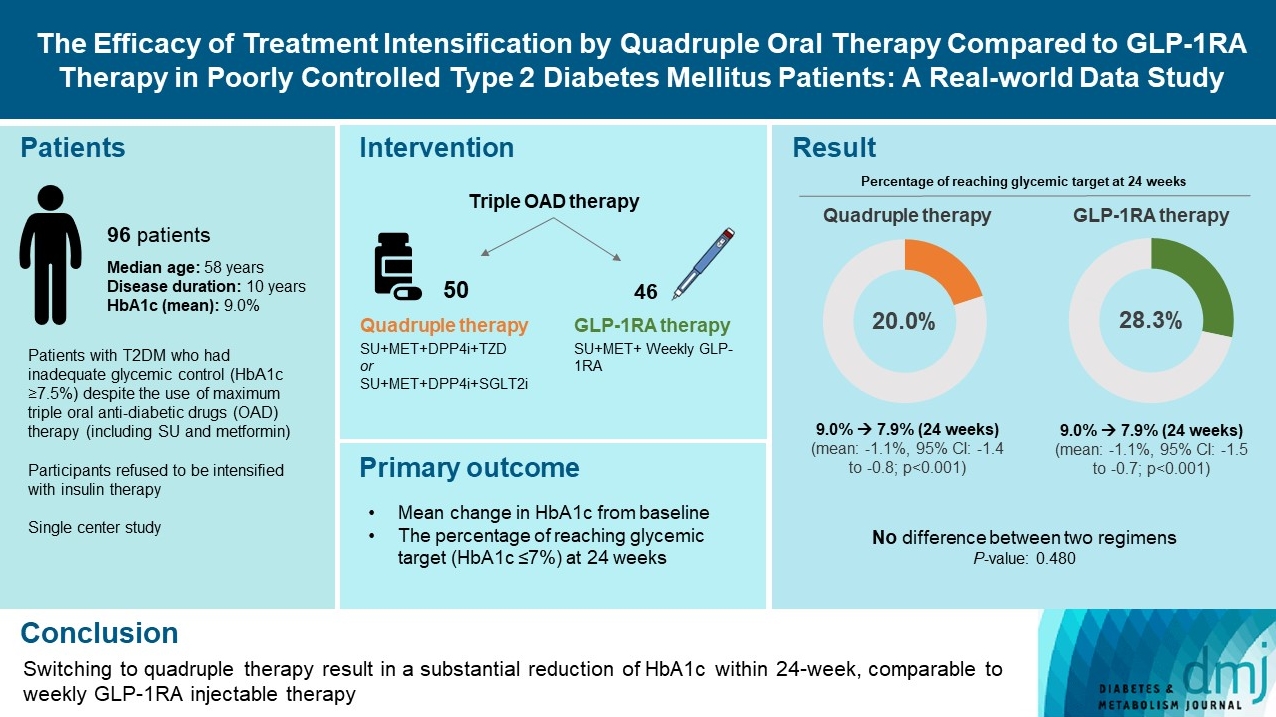
- The Efficacy of Treatment Intensification by Quadruple Oral Therapy Compared to GLP-1RA Therapy in Poorly Controlled Type 2 Diabetes Mellitus Patients: A Real-World Data Study
- Minyoung Kim, Hosu Kim, Kyong Young Kim, Soo Kyoung Kim, Junghwa Jung, Jong Ryeal Hahm, Jaehoon Jung, Jong Ha Baek
- Diabetes Metab J. 2023;47(1):135-139. Published online April 29, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0373
- 7,520 View
- 296 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - We compared the glycemic efficacy of treatment intensification between quadruple oral antidiabetic drug therapy and once-weekly glucagon-like peptide-1 receptor agonist (GLP-1RA)-based triple therapy in patients with poorly controlled type 2 diabetes mellitus refractory to triple oral therapy. For 24 weeks, changes in glycosylated hemoglobin (HbA1c) from baseline were compared between the two treatment groups. Of all 96 patients, 50 patients were treated with quadruple therapy, and 46 were treated with GLP-1RA therapy. Reductions in HbA1c for 24 weeks were comparable (in both, 1.1% reduction from baseline; P=0.59). Meanwhile, lower C-peptide level was associated with a lower glucose-lowering response of GLP-1RA therapy (R=0.3, P=0.04) but not with quadruple therapy (R=–0.13, P=0.40). HbA1c reduction by GLP-1RA therapy was inferior to that by quadruple therapy in the low C-peptide subgroup (mean, –0.1% vs. –1.3%; P=0.04). Treatment intensification by switching to quadruple oral therapy showed similar glucose-lowering efficacy to weekly GLP-1RA-based triple therapy. Meanwhile, the therapeutic response was affected by C-peptide levels in the GLP-1RA therapy group but not in the quadruple therapy group.
- Drug/Regimen
- Comparison of Serum Ketone Levels and Cardiometabolic Efficacy of Dapagliflozin versus Sitagliptin among Insulin-Treated Chinese Patients with Type 2 Diabetes Mellitus
- Chi-Ho Lee, Mei-Zhen Wu, David Tak-Wai Lui, Darren Shing-Hei Chan, Carol Ho-Yi Fong, Sammy Wing-Ming Shiu, Ying Wong, Alan Chun-Hong Lee, Joanne King-Yan Lam, Yu-Cho Woo, Karen Siu-Ling Lam, Kelvin Kai-Hang Yiu, Kathryn Choon-Beng Tan
- Diabetes Metab J. 2022;46(6):843-854. Published online April 28, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0319
- 5,002 View
- 257 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Insulin-treated patients with long duration of type 2 diabetes mellitus (T2DM) are at increased risk of ketoacidosis related to sodium-glucose co-transporter 2 inhibitor (SGLT2i). The extent of circulating ketone elevation in these patients remains unknown. We conducted this study to compare the serum ketone response between dapagliflozin, an SGLT2i, and sitagliptin, a dipeptidyl peptidase-4 inhibitor, among insulin-treated T2DM patients.
Methods
This was a randomized, open-label, active comparator-controlled study involving 60 insulin-treated T2DM patients. Participants were randomized 1:1 for 24-week of dapagliflozin 10 mg daily or sitagliptin 100 mg daily. Serum β-hydroxybutyrate (BHB) levels were measured at baseline, 12 and 24 weeks after intervention. Comprehensive cardiometabolic assessments were performed with measurements of high-density lipoprotein cholesterol (HDL-C) cholesterol efflux capacity (CEC), vibration-controlled transient elastography and echocardiography.
Results
Among these 60 insulin-treated participants (mean age 58.8 years, diabetes duration 18.2 years, glycosylated hemoglobin 8.87%), as compared with sitagliptin, serum BHB levels increased significantly after 24 weeks of dapagliflozin (P=0.045), with a median of 27% increase from baseline. Change in serum BHB levels correlated significantly with change in free fatty acid levels. Despite similar glucose lowering, dapagliflozin led to significant improvements in body weight (P=0.006), waist circumference (P=0.028), HDL-C (P=0.041), CEC (P=0.045), controlled attenuation parameter (P=0.007), and liver stiffness (P=0.022). Average E/e’, an echocardiographic index of left ventricular diastolic dysfunction, was also significantly lower at 24 weeks in participants treated with dapagliflozin (P=0.037).
Conclusion
Among insulin-treated T2DM patients with long diabetes duration, compared to sitagliptin, dapagliflozin modestly increased ketone levels and was associated with cardiometabolic benefits. -
Citations
Citations to this article as recorded by- Serum thrombospondin‐2 level changes with liver stiffness improvement in patients with type 2 diabetes
Jimmy Ho Cheung Mak, David Tak‐Wai Lui, Carol Ho‐Yi Fong, Chloe Yu‐Yan Cheung, Ying Wong, Alan Chun‐Hong Lee, Ruby Lai‐Chong Hoo, Aimin Xu, Kathryn Choon‐Beng Tan, Karen Siu‐Ling Lam, Chi‐Ho Lee
Clinical Endocrinology.2024; 100(3): 230. CrossRef - SGLT-2 inhibitors as novel treatments of multiple organ fibrosis
Junpei Hu, Jianhui Teng, Shan Hui, Lihui Liang
Heliyon.2024; 10(8): e29486. CrossRef - Effect of sodium-glucose cotransporter protein-2 inhibitors on left ventricular hypertrophy in patients with type 2 diabetes: A systematic review and meta-analysis
Yao Wang, Yujie Zhong, Zhehao Zhang, Shuhao Yang, Qianying Zhang, Bingyang Chu, Xulin Hu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Effects of SGLT2 inhibitors on hepatic fibrosis and steatosis: A systematic review and meta-analysis
Peipei Zhou, Ying Tan, Zhenning Hao, Weilong Xu, Xiqiao Zhou, Jiangyi Yu
Frontiers in Endocrinology.2023;[Epub] CrossRef - The impact of sodium-glucose Cotransporter-2 inhibitors on lipid profile: A meta-analysis of 28 randomized controlled trials
Gang Fan, Dian long Guo, Hong Zuo
European Journal of Pharmacology.2023; 959: 176087. CrossRef
- Serum thrombospondin‐2 level changes with liver stiffness improvement in patients with type 2 diabetes
- Drug/Regimen
- Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study
- Seung-Hwan Lee, Kyung-Wan Min, Byung-Wan Lee, In-Kyung Jeong, Soon-Jib Yoo, Hyuk-Sang Kwon, Yoon-Hee Choi, Kun-Ho Yoon
- Diabetes Metab J. 2021;45(3):339-348. Published online May 28, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0203

- 8,287 View
- 332 Download
- 12 Web of Science
- 15 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
Background Glycemic variability is associated with the development of diabetic complications and hypoglycemia. However, the effect of sodium-glucose transporter 2 (SGLT2) inhibitors on glycemic variability is controversial. We aimed to examine the effect of dapagliflozin as an add-on therapy to insulin on the glycemic variability assessed using continuous glucose monitoring (CGM) in subjects with type 2 diabetes mellitus.
Methods In this multicenter, placebo-controlled, double-blind, randomized study, 84 subjects received 10 mg of dapagliflozin (
n =41) or the placebo (n =43) for 12 weeks. CGM was performed before and after treatment to compare the changes in glycemic variability measures (standard deviation [SD], mean amplitude of glycemic excursions [MAGEs]).Results At week 12, significant reductions in glycosylated hemoglobin (−0.74%±0.66% vs. 0.01%±0.65%,
P <0.001), glycated albumin (−3.94%±2.55% vs. −0.67%±2.48%,P <0.001), and CGM-derived mean glucose (−41.6±39.2 mg/dL vs. 1.1±46.2 mg/dL,P <0.001) levels were observed in the dapagliflozin group compared with the placebo group. SD and MAGE were significantly decreased in the dapagliflozin group, but not in the placebo group. However, the difference in ΔSD and ΔMAGE failed to reach statistical significance between two groups. No significant differences in the incidence of safety endpoints were observed between the two groups.Conclusion Dapagliflozin effectively decreased glucose levels, but not glucose variability, after 12 weeks of treatment in participants with type 2 diabetes mellitus receiving insulin treatment. The role of SGLT2 inhibitors in glycemic variability warrants further investigations.
-
Citations
Citations to this article as recorded by- Selective sodium-glucose cotransporter-2 inhibitors in the improvement of hemoglobin and hematocrit in patients with type 2 diabetes mellitus: a network meta-analysis
Yuanyuan Luo, Ruojing Bai, Wei Zhang, Guijun Qin
Frontiers in Endocrinology.2024;[Epub] CrossRef - Continuous Glucose Monitoring Profiles and Health Outcomes After Dapagliflozin Plus Saxagliptin vs Insulin Glargine
Donald C Simonson, Marcia A Testa, Ella Ekholm, Maxwell Su, Tina Vilsbøll, Serge A Jabbour, Marcus Lind
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Impact of empagliflozin on insulin needs in patients with heart failure and diabetes: An EMPEROR‐Pooled analysis
Khawaja M. Talha, Jennifer Green, Gerasimos Filippatos, Stuart Pocock, Faiez Zannad, Martina Brueckmann, Elke Schueler, Anne Pernille Ofstad, João Pedro Ferreira, Stefan D. Anker, Javed Butler, Julio Rosenstock, Milton Packer
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Risk of Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Treated with Dapagliflozin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Zhigui Zheng, Dongyuan He, Jianguo Chen, Xiaohui Xie, Yunan Lu, Binbin Wu, Xinxin Jiang
Clinical Drug Investigation.2023; 43(4): 209. CrossRef - Effect of SGLT2 Inhibitors and Metformin on Inflammatory and Prognostic
Biomarkers in Type 2 Diabetes Patients
Yang Cao, Ning Liang, Ting Liu, Jingai Fang, Xiaodong Zhang
Endocrine, Metabolic & Immune Disorders - Drug Targets.2023; 23(4): 530. CrossRef - What is Glycaemic Variability and which Pharmacological Treatment Options are Effective? A Narrative Review
Juan Miguel Huertas Cañas, Maria Alejandra Gomez Gutierrez, Andres Bedoya Ossa
European Endocrinology.2023; 19(2): 4. CrossRef - La variabilité glycémique : un facteur de risque singulier à conjuguer au pluriel
Louis Monnier, Claude Colette, Fabrice Bonnet, David Owens
Médecine des Maladies Métaboliques.2022; 16(1): 15. CrossRef - Association between Variability of Metabolic Risk Factors and Cardiometabolic Outcomes
Min Jeong Park, Kyung Mook Choi
Diabetes & Metabolism Journal.2022; 46(1): 49. CrossRef - Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on serum urate levels in patients with and without diabetes: a systematic review and meta-regression of 43 randomized controlled trials
Alicia Swee Yan Yip, Shariel Leong, Yao Hao Teo, Yao Neng Teo, Nicholas L. X. Syn, Ray Meng See, Caitlin Fern Wee, Elliot Yeung Chong, Chi-Hang Lee, Mark Y. Chan, Tiong-Cheng Yeo, Raymond C. C. Wong, Ping Chai, Ching-Hui Sia
Therapeutic Advances in Chronic Disease.2022; 13: 204062232210835. CrossRef - Hypoglycemic agents and glycemic variability in individuals with type 2 diabetes: A systematic review and network meta-analysis
SuA Oh, Sujata Purja, Hocheol Shin, Minji Kim, Eunyoung Kim
Diabetes and Vascular Disease Research.2022; 19(3): 147916412211068. CrossRef - The Clinical Effect of Dapagliflozin in Patients with Angiographically Confirmed Coronary Artery Disease and Concomitant Type 2 Diabetes Mellitus
Yana Yu. Dzhun, Yevhen Yu. Marushko, Yanina A. Saienko, Nadiya M. Rudenko, Borys M. Mankovsky
Ukrainian Journal of Cardiovascular Surgery.2022; 30(3): 35. CrossRef - Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives
Alessandro Bellis, Ciro Mauro, Emanuele Barbato, Antonio Ceriello, Antonio Cittadini, Carmine Morisco
International Journal of Molecular Sciences.2021; 22(2): 775. CrossRef - Glycemic Variability Impacted by SGLT2 Inhibitors and GLP 1 Agonists in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis
Heeyoung Lee, Se-eun Park, Eun-Young Kim
Journal of Clinical Medicine.2021; 10(18): 4078. CrossRef - Effect of Dapagliflozin on Glycemic Variability in Patients with Type 2 Diabetes under Insulin Glargine Combined with Other Oral Hypoglycemic Drugs
Menghui Luo, Xiaocen Kong, Huiying Wang, Xiaofang Zhai, Tingting Cai, Bo Ding, Yun Hu, Ting Jing, Xiaofei Su, Huiqin Li, Jianhua Ma, Yoshifumi Saisho
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Time in Range from Continuous Glucose Monitoring: A Novel Metric for Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes & Metabolism Journal.2020; 44(6): 828. CrossRef
- Selective sodium-glucose cotransporter-2 inhibitors in the improvement of hemoglobin and hematocrit in patients with type 2 diabetes mellitus: a network meta-analysis
- A Novel Therapeutic Agent for Type 2 Diabetes Mellitus: SGLT2 Inhibitor
- Chang Hee Jung, Jung Eun Jang, Joong-Yeol Park
- Diabetes Metab J. 2014;38(4):261-273. Published online August 20, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.4.261
- 7,293 View
- 127 Download
- 62 Web of Science
- 58 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Type 2 diabetes mellitus (T2DM) is a complex endocrine and metabolic disorder, and a major public health problem that is rapidly increasing in prevalence. Although a wide range of pharmacotherapies for glycemic control is now available, management of T2DM remains complex and challenging. The kidneys contribute immensely to glucose homeostasis by reabsorbing glucose from the glomerular filtrate. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a new class of antidiabetic agents that inhibit glucose absorption from the kidney independent of insulin, offer a unique opportunity to improve the outcomes of patients with T2DM. In this review, we provide an overview of two globally-approved SGLT2 inhibitors, dapagliflozin and canagliflozin, and discuss their effects and safety. This information will help clinicians to decide whether these drugs will benefit their patients.
-
Citations
Citations to this article as recorded by- Clinical outcomes with the use of sodium-glucose cotransporter-2 inhibitors in patients with atrial fibrillation and type 2 diabetes mellitus: a multi-centre, real-world cohort study
Jaehyuk Jang, Soyoon Park, Soohyun Kim, Sung-Hwan Kim, Yong-Seog Oh, Young Kyoung Sa, Youmi Hwang, Sung-Won Jang, Sang-Hyun Ihm, Young Choi
European Journal of Preventive Cardiology.2024; 31(3): 320. CrossRef - Quantitative analysis of low-content impurity crystal forms in canagliflozin tablets by NIR solid-state analysis technique
Mingdi Liu, Jichao Liu, Qiuhong Wang, Ping Song, Haichao Li, Zan Sun, Chenglong Shi, Weibing Dong
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2024; 311: 124000. CrossRef - A Randomized Crossover Trial of Mixed Meal Tolerance Test Response in People with Type 1 Diabetes on Insulin Pump Therapy and YG1699 or Dapagliflozin
Pablo Lapuerta, Silvia Urbina, Jiaojuan He, Alyssa Wittle, Chenghai Li, Tong Li, Helen Wang, Marcus Hompesch
Clinical Pharmacology & Therapeutics.2024;[Epub] CrossRef - Canagliflozin independently reduced plasma volume from conventional diuretics in patients with type 2 diabetes and chronic heart failure: a subanalysis of the CANDLE trial
Kazuki Shiina, Hirofumi Tomiyama, Atsushi Tanaka, Takumi Imai, Itaru Hisauchi, Isao Taguchi, Akira Sezai, Shigeru Toyoda, Kaoru Dohi, Haruo Kamiya, Keisuke Kida, Toshihisa Anzai, Taishiro Chikamori, Koichi Node, Masayoshi Ajioka, Junya Ako, Rie Amano, Mit
Hypertension Research.2023; 46(2): 495. CrossRef - Ecofriendly appraisal of stability‐indicating high‐performance chromatographic assay of canagliflozin and metformin with their toxic impurities; in silico toxicity prediction
Raghda A. Emam, Aml A. Emam
Journal of Separation Science.2023;[Epub] CrossRef - Effect of sodium‐glucose cotransporter 2 inhibitors on the rate of decline in kidney function: A systematic review and meta‐analysis
Yanbei Duo, Junxiang Gao, Tao Yuan, Weigang Zhao
Journal of Diabetes.2023; 15(1): 58. CrossRef - Quantitative analysis of low content polymorphic impurities in canagliflozin tablets by PXRD, NIR, ATR-FITR and Raman solid-state analysis techniques combined with stoichiometry
Mingdi Liu, Jichao Liu, Qiuhong Wang, Ping Song, Haichao Li, Songgu Wu, Junbo Gong
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2023; 293: 122458. CrossRef - SGLT2 Inhibitors vs. GLP-1 Agonists to Treat the Heart, the Kidneys and the Brain
Bartosz Rolek, Mateusz Haber, Magdalena Gajewska, Sylwester Rogula, Arkadiusz Pietrasik, Aleksandra Gąsecka
Journal of Cardiovascular Development and Disease.2023; 10(8): 322. CrossRef - Cardiorenal Protections of SGLT2 Inhibitors in the Treatment of Type 2
Diabetes
Somayeh Nazari, Hossein Mirkhani
Current Diabetes Reviews.2023;[Epub] CrossRef - Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extensio
Jun Sung Moon, Il Rae Park, Hae Jin Kim, Choon Hee Chung, Kyu Chang Won, Kyung Ah Han, Cheol-Young Park, Jong Chul Won, Dong Jun Kim, Gwan Pyo Koh, Eun Sook Kim, Jae Myung Yu, Eun-Gyoung Hong, Chang Beom Lee, Kun-Ho Yoon
Diabetes & Metabolism Journal.2023; 47(6): 808. CrossRef - Novel perspective on cardiovascular adverse event profiles due to treatment with sodium-glucose transport protein 2 (SGLT2) inhibitors in the Japanese Adverse Drug Event Report (JADER) database
Koji Suematsu
Personalized Medicine Universe.2023; 12: 23. CrossRef - The fate of flavonoids after oral administration: a comprehensive overview of its bioavailability
Abid Naeem, Yang Ming, Hu Pengyi, Kang Yong Jie, Liu Yali, Zhang Haiyan, Xiao Shuai, Li Wenjing, Wu Ling, Zhang Ming Xia, Liu Shan Shan, Zheng Qin
Critical Reviews in Food Science and Nutrition.2022; 62(22): 6169. CrossRef - Add-on therapy with dapagliflozin in routine outpatient care of type 2 diabetes patients from Turkey: a retrospective cohort study on HbA1c, body weight, and blood pressure outcomes
Derun Taner Ertugrul, Erdal Kan, Cigdem Bahadir Tura, Haci Bayram Tugtekin, Hayati Ayakta, Mehmet Celebioglu, Ceren Yılmaz, Onur Utebay, Ilhan Yetkin, Eren Gurkan, Kerem Sezer, Ramazan Gen, Suleyman Ozcaylak, Yildiz Okuturlar, Mehmet Coskun, Nilgun Govec
International Journal of Diabetes in Developing Countries.2022; 42(1): 147. CrossRef - Sodium–glucose cotransporter 2 inhibitors do not increase the risk of fractures in real‐world clinical practice in Korea: A national observational cohort study
Kyoung Hwa Ha, Dae Jung Kim, Yong Jun Choi
Journal of Diabetes Investigation.2022; 13(6): 986. CrossRef - Luseogliflozin preserves the pancreatic beta-cell mass and function in db/db mice by improving mitochondrial function
Yuki Yamauchi, Akinobu Nakamura, Takashi Yokota, Kiyohiko Takahashi, Shinichiro Kawata, Kazuhisa Tsuchida, Kazuno Omori, Hiroshi Nomoto, Hiraku Kameda, Kyu Yong Cho, Toshihisa Anzai, Shinya Tanaka, Yasuo Terauchi, Hideaki Miyoshi, Tatsuya Atsumi
Scientific Reports.2022;[Epub] CrossRef - Blood glucose levels and bodyweight change after dapagliflozin administration
Hyunah Kim, Seung‐Hwan Lee, Hyunyong Lee, Hyeon Woo Yim, Jae‐Hyoung Cho, Kun‐Ho Yoon, Hun‐Sung Kim
Journal of Diabetes Investigation.2021; 12(9): 1594. CrossRef - Dapagliflozin Improves Cardiac Hemodynamics and Mitigates Arrhythmogenesis in Mitral Regurgitation‐Induced Myocardial Dysfunction
Yu‐Wen Lin, Chin‐Yu Chen, Jhih‐Yuan Shih, Bor‐Chih Cheng, Ching‐Ping Chang, Mao‐Tsun Lin, Chung‐Han Ho, Zhih‐Cherng Chen, Sudeshna Fisch, Wei‐Ting Chang
Journal of the American Heart Association.2021;[Epub] CrossRef - Cardiovascular Safety of Sodium Glucose Cotransporter 2 Inhibitors as Add-on to Metformin Monotherapy in Patients with Type 2 Diabetes Mellitus
Ja Young Jeon, Kyoung Hwa Ha, Dae Jung Kim
Diabetes & Metabolism Journal.2021; 45(4): 505. CrossRef - Ipragliflozin, an SGLT2 Inhibitor, Ameliorates High-Fat Diet-Induced Metabolic Changes by Upregulating Energy Expenditure through Activation of the AMPK/ SIRT1 Pathway
Ji-Yeon Lee, Minyoung Lee, Ji Young Lee, Jaehyun Bae, Eugene Shin, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Diabetes & Metabolism Journal.2021; 45(6): 921. CrossRef - SGLT2i: beyond the glucose-lowering effect
Lihua Ni, Cheng Yuan, Guopeng Chen, Changjiang Zhang, Xiaoyan Wu
Cardiovascular Diabetology.2020;[Epub] CrossRef - Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial
Eugene Han, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Journal of Clinical Medicine.2020; 9(1): 259. CrossRef - Stability Indicating, pH and pKa Dependent HPLC–DAD Method for the Simultaneous Determination of Weakly Ionizable Empagliflozin, Dapagliflozin and Canagliflozin in Pharmaceutical Formulations
Shahzad Sharif, Rashida Bashir, Ahmad Adnan, Sabiha Mansoor, Izaz Ahmad, Ayoub Rashid Ch, Muhammad Saqlain Tahir
Chromatographia.2020; 83(12): 1453. CrossRef - Cardiorenal protection with SGLT2: Lessons from the cardiovascular outcome trials
José Silva‐Cardoso, Omar Sheikh, Mouhamed Nashawi, Son Pham, Kelly M. Gallegos, Laith R. Dinkha, Robert J. Chilton
Journal of Diabetes.2020; 12(4): 279. CrossRef - Effect of the Sodium-Glucose Cotransporter 2 Inhibitor, Dapagliflozin, on Genitourinary Infection in an Animal Model of Type 2 Diabetes
Jin Bong Choi, Je Mo Yoo, Ye-Jee Lee, Jae Woong Kim, Seung-Ju Lee, Hee Youn Kim, Dong Sup Lee, Seung-Hyun Ko, Hyun-Sop Choe
International Neurourology Journal.2020; 24(1): 21. CrossRef - Sodium‐glucose cotransporter 2 inhibitors regulate ketone body metabolism via inter‐organ crosstalk
Jin Hee Kim, Minyoung Lee, Soo Hyun Kim, So Ra Kim, Byung‐Wan Lee, Eun Seok Kang, Bong‐Soo Cha, Jin Won Cho, Yong‐ho Lee
Diabetes, Obesity and Metabolism.2019; 21(4): 801. CrossRef - Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Jae Hyun Bae, Eun-Gee Park, Sunhee Kim, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Scientific Reports.2019;[Epub] CrossRef - SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart
Chenguang Li, Jie Zhang, Mei Xue, Xiaoyu Li, Fei Han, Xiangyang Liu, Linxin Xu, Yunhong Lu, Ying Cheng, Ting Li, Xiaochen Yu, Bei Sun, Liming Chen
Cardiovascular Diabetology.2019;[Epub] CrossRef - An update of SGLT1 and SGLT2 inhibitors in early phase diabetes-type 2 clinical trials
Ernest Adeghate, Sahar Mohsin, Faisal Adi, Fares Ahmed, Ali Yahya, Huba Kalász, Kornelia Tekes, Ernest A. Adeghate
Expert Opinion on Investigational Drugs.2019; 28(9): 811. CrossRef - Stability‐indicating chromatographic and chemometric methods for environmentally benign determination of canagliflozin and its major degradation product; A comparative study and greenness assessment
Aml A. Emam, Nada S. Abdelwahab
Biomedical Chromatography.2019;[Epub] CrossRef - A Lower Baseline Urinary Glucose Excretion Predicts a Better Response to the Sodium Glucose Cotransporter 2 Inhibitor
You-Cheol Hwang, Jae Hyeon Kim, Byung-Wan Lee, Woo Je Lee
Diabetes & Metabolism Journal.2019; 43(6): 898. CrossRef - Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors
Ji-Yeon Lee, Yongin Cho, Minyoung Lee, You Jin Kim, Yong-ho Lee, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
Diabetes & Metabolism Journal.2019; 43(2): 158. CrossRef - Comparative Efficacy and Safety Among Sodium-glucose Cotransporter-2 Inhibitors in Type 2 Diabetes – Results from a Retrospective Single-centre Study
Manash P Baruah, Sanjay Kalra
European Endocrinology.2019; 15(2): 113. CrossRef - Canagliflozin stability study and ecofriendly chromatographic determination of its degradation product: A comparative study
Aml A. Emam
Journal of Separation Science.2018; 41(4): 822. CrossRef - Efficacy and tolerability of novel triple combination therapy in drug-naïve patients with type 2 diabetes from the TRIPLE-AXEL trial: protocol for an open-label randomised controlled trial
Nam Hoon Kim, Soo Lim, Soo Heon Kwak, Min Kyong Moon, Jun Sung Moon, Yong-ho Lee, Ho Chan Cho, Juneyoung Lee, Sin Gon Kim
BMJ Open.2018; 8(9): e022448. CrossRef - Short-term outcomes of patients with Type 2 diabetes mellitus treated with canagliflozin compared with sitagliptin in a real-world setting
YL Shao, KH Yee, SK, Koh, YF Wong, LY Yeoh, S Low, CF Sum
Singapore Medical Journal.2018; 59(5): 251. CrossRef - Clinical implications of current cardiovascular outcome trials with sodium glucose cotransporter-2 (SGLT2) inhibitors
Soo Lim, Robert H. Eckel, Kwang Kon Koh
Atherosclerosis.2018; 272: 33. CrossRef - Effect of Empagliflozin, a Selective Sodium-Glucose Cotransporter 2 Inhibitor, on Kidney and Peripheral Nerves in Streptozotocin-Induced Diabetic Rats
Kyung Ae Lee, Heung Yong Jin, Na Young Lee, Yu Ji Kim, Tae Sun Park
Diabetes & Metabolism Journal.2018; 42(4): 338. CrossRef - The effect of Ramadan fasting and continuing sodium-glucose co-transporter-2 (SGLT2) inhibitor use on ketonemia, blood pressure and renal function in Muslim patients with type 2 diabetes
Yanli Shao, Gwyneth Joy Lim, Chin Lian Chua, Yip Fong Wong, Ester Chai Kheng Yeoh, Serena Kiat Mun Low, Chee Fang Sum
Diabetes Research and Clinical Practice.2018; 142: 85. CrossRef - Effect of Dapagliflozin on Alanine Aminotransferase Improvement in Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease
Dug-Hyun Choi, Chan-Hee Jung, Ji-Oh Mok, Chul-Hee Kim, Sung-Koo Kang, Bo-Yeon Kim
Endocrinology and Metabolism.2018; 33(3): 387. CrossRef - Comparison between sodium–glucose cotransporter 2 inhibitors and pioglitazone as additions to insulin therapy in type 2 diabetes patients: A systematic review with an indirect comparison meta‐analysis
Yun Kyung Cho, Ye‐Jee Kim, Yu Mi Kang, Seung Eun Lee, Joong‐Yeol Park, Woo Je Lee, Chang Hee Jung
Journal of Diabetes Investigation.2018; 9(4): 882. CrossRef - Characteristics of Dapagliflozin Responders: A Longitudinal, Prospective, Nationwide Dapagliflozin Surveillance Study in Korea
Eugene Han, Ari Kim, Sung Jae Lee, Je-Yon Kim, Jae Hyeon Kim, Woo Je Lee, Byung-Wan Lee
Diabetes Therapy.2018; 9(4): 1689. CrossRef - Combining SGLT2 Inhibition With a Thiazolidinedione Additively Attenuate the Very Early Phase of Diabetic Nephropathy Progression in Type 2 Diabetes Mellitus
Eugene Han, Eugene Shin, Gyuri Kim, Ji-Yeon Lee, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Frontiers in Endocrinology.2018;[Epub] CrossRef - Clinical parameters affecting dapagliflozin response in patients with type 2 diabetes
J.-Y. Lee, G. Kim, S.R. Kim, Y.-H. Lee, B.-W. Lee, B.-S. Cha, E.S. Kang
Diabetes & Metabolism.2017; 43(2): 191. CrossRef - Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds
Daniella B.R. Insuela, Vinicius F. Carvalho
European Journal of Pharmacology.2017; 812: 64. CrossRef - Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J‐STEP/INS): Results of a 16‐week randomized, double‐blind, placebo‐controlled multicentre trial
Yasuo Terauchi, Masahiro Tamura, Masayuki Senda, Ryoji Gunji, Kohei Kaku
Diabetes, Obesity and Metabolism.2017; 19(10): 1397. CrossRef - A comparison of effects of DPP-4 inhibitor and SGLT2 inhibitor on lipid profile in patients with type 2 diabetes
Seon-Ah Cha, Yong-Moon Park, Jae-Seung Yun, Tae-Seok Lim, Ki-Ho Song, Ki-Dong Yoo, Yu-Bae Ahn, Seung-Hyun Ko
Lipids in Health and Disease.2017;[Epub] CrossRef - Morning Spot Urine Glucose-to-Creatinine Ratios Predict Overnight Urinary Glucose Excretion in Patients With Type 2 Diabetes
So Ra Kim, Yong-ho Lee, Sang-Guk Lee, Sun Hee Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee, Jeong-Ho Kim, Byung-Wan Lee
Annals of Laboratory Medicine.2017; 37(1): 9. CrossRef - The Relationship between Increases in Morning Spot Urinary Glucose Excretion and Decreases in HbA1C in Patients with Type 2 Diabetes After Taking an SGLT2 Inhibitor: A Retrospective, Longitudinal Study
So Ra Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Diabetes Therapy.2017; 8(3): 601. CrossRef - Clinical and Genetic Features of Patients With Type 2 Diabetes and Renal Glycosuria
Siqian Gong, Jiandong Guo, Xueyao Han, Meng Li, Lingli Zhou, Xiaoling Cai, Yu Zhu, Yingying Luo, Simin Zhang, Xianghai Zhou, Yumin Ma, Linong Ji
The Journal of Clinical Endocrinology & Metabolism.2017; 102(5): 1548. CrossRef - Effect of Sodium-Glucose Co-Transporter 2 Inhibitor, Dapagliflozin, on Renal Renin-Angiotensin System in an Animal Model of Type 2 Diabetes
Seok Joon Shin, Sungjin Chung, Soo Jung Kim, Eun-Mi Lee, Young-Hye Yoo, Ji-Won Kim, Yu-Bae Ahn, Eun-Sook Kim, Sung-Dae Moon, Myung-Jun Kim, Seung-Hyun Ko, Kwang-Hyun Baek
PLOS ONE.2016; 11(11): e0165703. CrossRef - Sodium–glucose cotransporter 2 inhibitors: an evidence-based practice approach to their use in the natural history of type 2 diabetes
Stanley S. Schwartz, Intekhab Ahmed
Current Medical Research and Opinion.2016; 32(5): 907. CrossRef - Insulin resistance, role of metformin and other non-insulin therapies in pediatric type 1 diabetes
Fida Bacha, Sara Klinepeter Bartz
Pediatric Diabetes.2016; 17(8): 545. CrossRef - Renal threshold for glucose reabsorption predicts diabetes improvement by sodium‐glucose cotransporter 2 inhibitor therapy
Aya Osaki, Shuichi Okada, Tsugumichi Saito, Eijiro Yamada, Kumeo Ono, Yawara Niijima, Hiroto Hoshi, Masanobu Yamada
Journal of Diabetes Investigation.2016; 7(5): 751. CrossRef - Sodium-glucose co-transporter inhibition in the treatment of diabetes: Sweetening the pot
Carlos A Alvarez, Ian J Neeland, Darren K McGuire
Diabetes and Vascular Disease Research.2015; 12(2): 74. CrossRef - A simple and sensitive high performance liquid chromatography assay with a fluorescence detector for determination of canagliflozin in human plasma
Muzaffar Iqbal, Nasr Y. Khalil, Amer M. Alanazi, Khalid A. Al-Rashood
Analytical Methods.2015; 7(7): 3028. CrossRef - Efficacy and safety of sodium–glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years
Xiang-Yang Liu, Ning Zhang, Rui Chen, Jia-Guo Zhao, Pei Yu
Journal of Diabetes and its Complications.2015; 29(8): 1295. CrossRef - Severe hypercalcemia and hypernatremia in a patient treated with canagliflozin
Arshpreet Kaur, Stephen J Winters
Endocrinology, Diabetes & Metabolism Case Reports.2015;[Epub] CrossRef - Pharmacological treatment and therapeutic perspectives of metabolic syndrome
Soo Lim, Robert H. Eckel
Reviews in Endocrine and Metabolic Disorders.2014; 15(4): 329. CrossRef
- Clinical outcomes with the use of sodium-glucose cotransporter-2 inhibitors in patients with atrial fibrillation and type 2 diabetes mellitus: a multi-centre, real-world cohort study

 KDA
KDA
 First
First Prev
Prev





