
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Metabolic Risk/Epidemiology
- Gestational Diabetes Mellitus and Its Implications across the Life Span
- Brandy Wicklow, Ravi Retnakaran
- Diabetes Metab J. 2023;47(3):333-344. Published online February 8, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0348
- 5,378 View
- 420 Download
- 6 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Gestational diabetes mellitus (GDM) has historically been perceived as a medical complication of pregnancy that also serves as a harbinger of maternal risk of developing type 2 diabetes mellitus (T2DM) in the future. In recent decades, a growing body of evidence has detailed additional lifelong implications that extend beyond T2DM, including an elevated risk of ultimately developing cardiovascular disease. Furthermore, the risk factors that mediate this lifetime cardiovascular risk are evident not only after delivery but are present even before the pregnancy in which GDM is first diagnosed. The concept thus emerging from these data is that the diagnosis of GDM enables the identification of women who are already on an enhanced track of cardiometabolic risk that starts early in life. Studies of the offspring of pregnancies complicated by diabetes now suggest that the earliest underpinnings of this cardiometabolic risk profile may be determined in utero and may first manifest clinically in childhood. Accordingly, from this perspective, GDM is now seen as a chronic metabolic disorder that holds implications across the life span of both mother and child.
-
Citations
Citations to this article as recorded by- ATP5me alleviates high glucose-induced myocardial cell injury
Qingsha Hou, Fang Yan, Xiuling Li, Huanling Liu, Xiang Yang, Xudong Dong
International Immunopharmacology.2024; 129: 111626. CrossRef - Prevalence and Predictors of Gestational Diabetes Mellitus and Overt Diabetes in Pregnancy: A Secondary Analysis of Nationwide Data from India
Saurav Basu, Vansh Maheshwari, Rutul Gokalani, Chandrakant Lahariya
Preventive Medicine: Research & Reviews.2024; 1(1): 52. CrossRef - Serum betaine and dimethylglycine in mid-pregnancy and the risk of gestational diabetes mellitus: a case-control study
Ziqing Zhou, Yao Yao, Yanan Sun, Xin Wang, Shang Huang, Jianli Hou, Lijun Wang, Fengxiang Wei
Endocrine.2024;[Epub] CrossRef - Quality assessment of videos on social media platforms related to gestational diabetes mellitus in China: A cross-section study
Qin-Yu Cai, Jing Tang, Si-Zhe Meng, Yi Sun, Xia Lan, Tai-Hang Liu
Heliyon.2024; 10(7): e29020. CrossRef - Inflammation and decreased cardiovagal modulation are linked to stress and depression at 36th week of pregnancy in gestational diabetes mellitus
Manoharan Renugasundari, Gopal Krushna Pal, Latha Chaturvedula, Nivedita Nanda, K. T. Harichandrakumar, Thiyagarajan Durgadevi
Scientific Reports.2023;[Epub] CrossRef - Women with gestational diabetes mellitus, controlled for plasma glucose level, exhibit maternal and fetal dyslipidaemia that may warrant treatment
Barbara J. Meyer, Colin Cortie, Marloes Dekker-Nitert, Helen L. Barrett, Dilys J. Freeman
Diabetes Research and Clinical Practice.2023; 204: 110929. CrossRef - Pregnancy diet to prevent gestational diabetes: study design and dietary assessments
Sylvia H. Ley
The American Journal of Clinical Nutrition.2023; 118(5): 847. CrossRef
- ATP5me alleviates high glucose-induced myocardial cell injury
- Metabolic Risk/Epidemiology
- Prevalence of Type 2 Diabetes Mellitus among Korean Children, Adolescents, and Adults Younger than 30 Years: Changes from 2002 to 2016
- Yong Hee Hong, In-Hyuk Chung, Kyungdo Han, Sochung Chung, on Behalf of the Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity
- Diabetes Metab J. 2022;46(2):297-306. Published online October 26, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0038
- 9,404 View
- 346 Download
- 9 Web of Science
- 12 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
Despite the importance of and social concern regarding prevention of diabetes at younger ages, limited data are available. This study sought to analyze changes in the prevalence of type 2 diabetes mellitus (T2DM) in Koreans younger than 30 years according to sex, age, and level of income.
Methods
The dataset analyzed in this study was derived from health insurance claims recorded in the National Health Insurance Service (NHIS) database. Participants’ level of income was categorized as low (quintile 1, <20% of insurance premium) or others (quintile 2–5).
Results
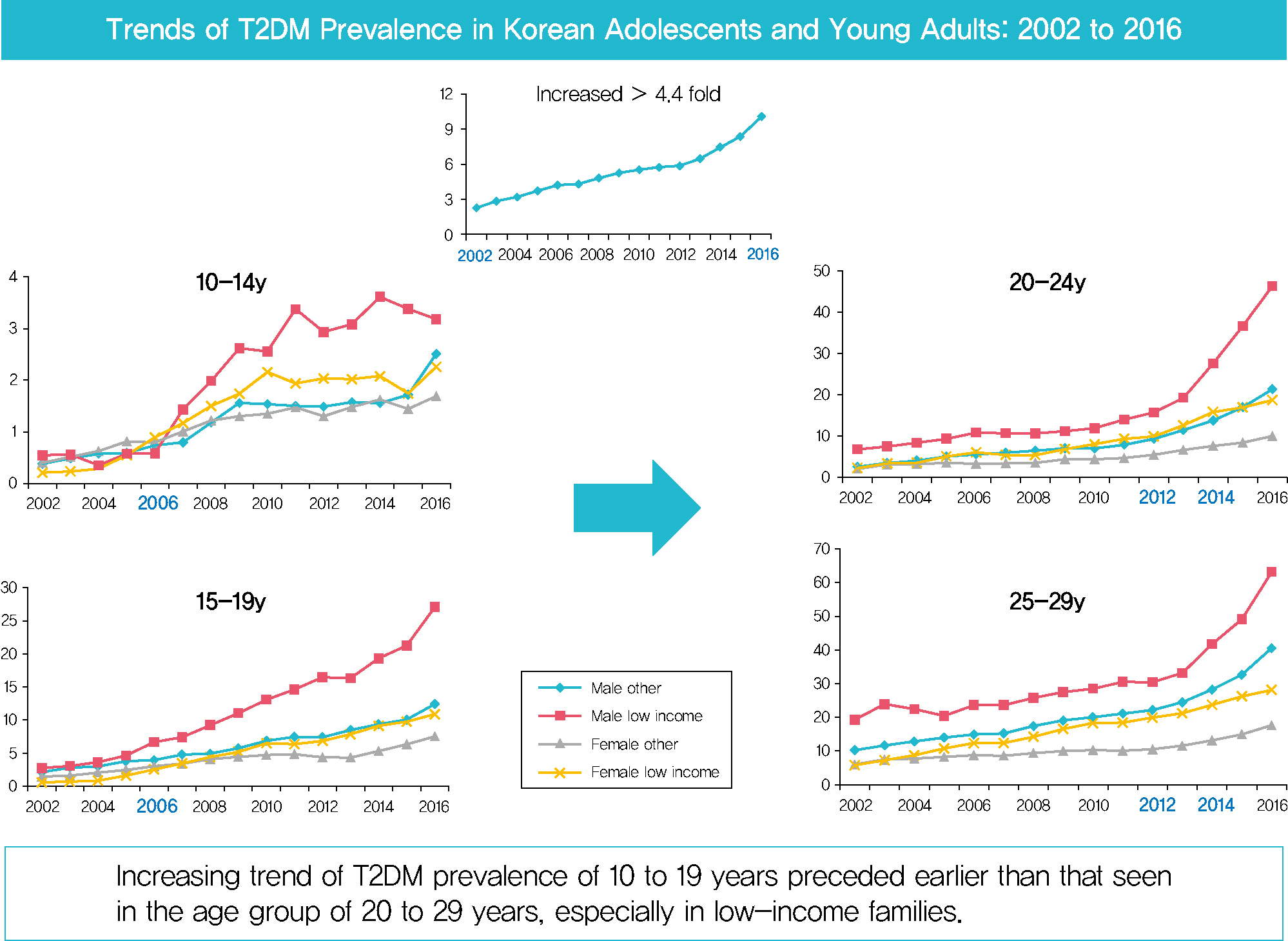
In males and females, the prevalence of T2DM per 10,000 people steadily increased from 2.57 in 2002 to 11.41 in 2016, and from 1.96 in 2002 to 8.63 in 2016. The prevalence of T2DM in girls was higher in the age group of 5 to 14 years. Even though the prevalence was higher among those older than 20 years, the increase had started earlier, in the early 2000s, in younger age group. Adolescents aged 10 to 19 years in low-income families showed a remarkable increase in prevalence of T2DM, especially in boys.
Conclusion
The prevalence of T2DM in young Koreans increased more than 4.4-fold from 2002 to 2016, and the increase started in the early 2000s in younger age groups and in low-income families. This is the first study to examine the trend in prevalence of T2DM in children, adolescents, and young adults in Korea. Future studies and collaborations with social support systems to prevent T2DM at an early age group should be performed. -
Citations
Citations to this article as recorded by- SCORE and SCORE2 in East Asian Population
JungMin Choi, Soseul Sung, Sue K. Park, Seyong Park, Hyoyeong Kim, Myeong-Chan Cho, Bryan Williams, Hae-Young Lee
JACC: Asia.2024; 4(4): 265. CrossRef - Chronic disease management program applied to type 2 diabetes patients and prevention of diabetic complications: a retrospective cohort study using nationwide data
Min Kyung Hyun, Jang Won Lee, Seung-Hyun Ko
BMC Public Health.2023;[Epub] CrossRef - Clinical and pathological characteristics of DKD patients with early-onset type 2 diabetes
Liang Wu, Yi-Yang Zhao, Meng-Rui Li, Dong-Yuan Chang, Ming-Hui Zhao, Min Chen
Journal of Diabetes and its Complications.2023; 37(8): 108520. CrossRef - Type 2 Diabetes and Its Association With Psychiatric Disorders in Young Adults in South Korea
Min-Kyung Lee, Su-Young Lee, Seo-Young Sohn, Jiyeon Ahn, Kyungdo Han, Jae-Hyuk Lee
JAMA Network Open.2023; 6(6): e2319132. CrossRef - Glycemic control and complications of type 2 diabetes mellitus in children and adolescents during the COVID-19 outbreak
Kyeong Eun Oh, Yu Jin Kim, Ye Rim Oh, Eungu Kang, Hyo-Kyoung Nam, Young-Jun Rhie, Kee-Hyoung Lee
Annals of Pediatric Endocrinology & Metabolism.2023; 28(4): 275. CrossRef - Position Statement on the Appropriateness and Significance of Adding the Glycated Hemoglobin Test to the National Health Examination
Ji Hye Kim, Dae Jung Kim, Jaehyun Kim, Sangjoon Park, Kyunghoon Lee, Jun Goo Kang, Eu Jeong Ku, Su Kyoung Kwon, Won Jun Kim, Young Sang Lyu, Jang Won Son, Young Sil Eom, Kyung Ae Lee, Jeongrim Lee, Jung Min Lee, Jung Hwa Lee, Jung Hwa Jung, Hochan Cho, Da
The Journal of Korean Diabetes.2023; 24(4): 178. CrossRef - Trends and Risk Factors of Metabolic Syndrome among Korean Adolescents, 2007 to 2018 (Diabetes Metab J 2021;45:880-9)
Dae Jung Kim
Diabetes & Metabolism Journal.2022; 46(2): 349. CrossRef - Prevalence trends of type 1 and type 2 diabetes in children and adolescents in North Rhine-Westphalia, the most populous federal state in Germany, 2002-2020
C. Baechle, A. Stahl-Pehe, N. Prinz, T. Meissner, C. Kamrath, R.W. Holl, J. Rosenbauer
Diabetes Research and Clinical Practice.2022; 190: 109995. CrossRef - Diagnostic and Therapeutic Strategies of Type 2 Diabetes Mellitus in Youth
Hwa Young Kim, Jae Hyun Kim
The Ewha Medical Journal.2022;[Epub] CrossRef - Factors Affecting High-Risk for Diabetes among Korean Adolescents: An Analysis Using the Eighth Korea National Health and Nutrition Examination Survey (2020)
Kyung-Sook Bang, Sang-Youn Jang, Ji-Hye Choe
Children.2022; 9(8): 1249. CrossRef - Characteristics of Glycemic Control and Long-Term Complications in Patients with Young-Onset Type 2 Diabetes
Han-sang Baek, Ji-Yeon Park, Jin Yu, Joonyub Lee, Yeoree Yang, Jeonghoon Ha, Seung Hwan Lee, Jae Hyoung Cho, Dong-Jun Lim, Hun-Sung Kim
Endocrinology and Metabolism.2022; 37(4): 641. CrossRef - 젊은 2형 당뇨병 환자의 관리
재현 배
Public Health Weekly Report.2022; 15(35): 2474. CrossRef
- SCORE and SCORE2 in East Asian Population
- Metabolic Risk/Epidemiology
- Maternal Hyperglycemia during Pregnancy Increases Adiposity of Offspring
- Hye Rim Chung, Joon Ho Moon, Jung Sub Lim, Young Ah Lee, Choong Ho Shin, Joon-Seok Hong, Soo Heon Kwak, Sung Hee Choi, Hak Chul Jang
- Diabetes Metab J. 2021;45(5):730-738. Published online February 22, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0154
- 5,754 View
- 180 Download
- 6 Web of Science
- 6 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
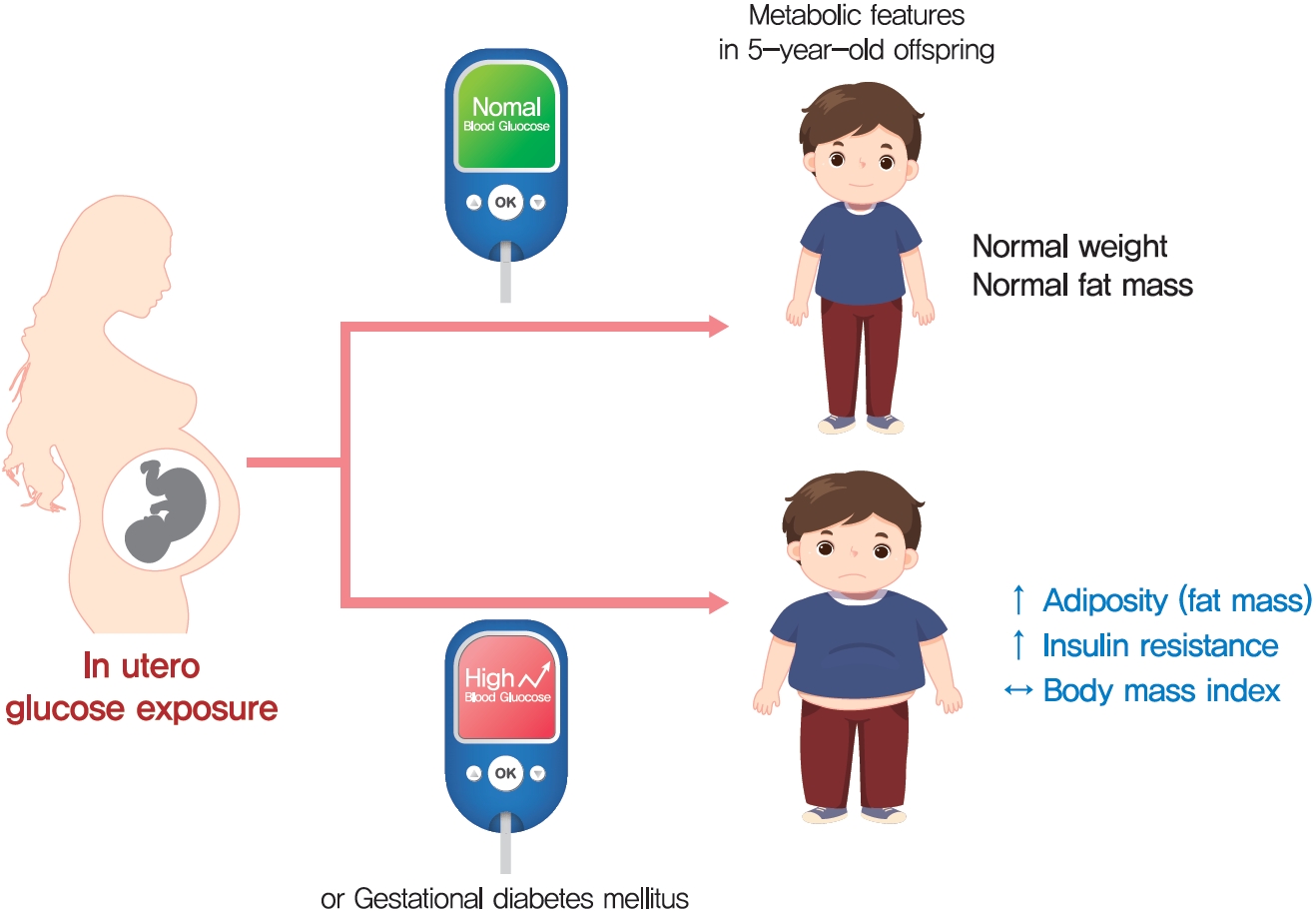
The effect of intrauterine hyperglycemia on fat mass and regional fat proportion of the offspring of mothers with gestational diabetes mellitus (OGDM) remains to be determined.
Methods
The body composition of OGDM (n=25) and offspring of normoglycemic mothers (n=49) was compared using dualenergy X-ray absorptiometry at age 5 years. The relationship between maternal glucose concentration during a 100 g oral glucose tolerance test (OGTT) and regional fat mass or proportion was analyzed after adjusting for maternal prepregnancy body mass index (BMI).
Results
BMI was comparable between OGDM and control (median, 16.0 kg/m2 vs. 16.1 kg/m2 ). Total, truncal, and leg fat mass were higher in OGDM compared with control (3,769 g vs. 2,245 g, P=0.004; 1,289 g vs. 870 g, P=0.017; 1,638 g vs. 961 g, P=0.002, respectively), whereas total lean mass was lower in OGDM (15,688 g vs. 16,941 g, P=0.001). Among OGDM, total and truncal fat mass were correlated with fasting and 3-hour glucose concentrations of maternal 100 g OGTT during pregnancy (total fat mass, r=0.49, P=0.018 [fasting], r=0.473, P=0.023 [3-hour]; truncal fat mass, r=0.571, P=0.004 [fasting], r=0.558, P=0.006 [3-hour]), but there was no correlation between OGDM leg fat mass and maternal OGTT during pregnancy. Regional fat indices were not correlated with concurrent maternal 75 g OGTT values.
Conclusion
Intrauterine hyperglycemia is associated with increased fat mass, especially truncal fat, in OGDM aged 5 years. -
Citations
Citations to this article as recorded by- Advances in free fatty acid profiles in gestational diabetes mellitus
Haoyi Du, Danyang Li, Laura Monjowa Molive, Na Wu
Journal of Translational Medicine.2024;[Epub] CrossRef - High-fat diet during pregnancy lowers fetal weight and has a long-lasting adverse effect on brown adipose tissue in the offspring
Mihoko Yamaguchi, Jun Mori, Nozomi Nishida, Satoshi Miyagaki, Yasuhiro Kawabe, Takeshi Ota, Hidechika Morimoto, Yusuke Tsuma, Shota Fukuhara, Takehiro Ogata, Takuro Okamaura, Naoko Nakanishi, Masahide Hamaguchi, Hisakazu Nakajima, Michiaki Fukui, Tomoko I
Journal of Developmental Origins of Health and Disease.2023; 14(2): 261. CrossRef - Prediction of gestational diabetes mellitus in Asian women using machine learning algorithms
Byung Soo Kang, Seon Ui Lee, Subeen Hong, Sae Kyung Choi, Jae Eun Shin, Jeong Ha Wie, Yun Sung Jo, Yeon Hee Kim, Kicheol Kil, Yoo Hyun Chung, Kyunghoon Jung, Hanul Hong, In Yang Park, Hyun Sun Ko
Scientific Reports.2023;[Epub] CrossRef - Effects of early standardized management on the growth trajectory of offspring with gestational diabetes mellitus at 0–5 years old: a preliminary longitudinal study
Bingbing Guo, Jingjing Pei, Yin Xu, Yajie Wang, Xinye Jiang
Scientific Reports.2023;[Epub] CrossRef - Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications
Joon Ho Moon, Hak Chul Jang
Diabetes & Metabolism Journal.2022; 46(1): 3. CrossRef - Increased Pro-Inflammatory T Cells, Senescent T Cells, and Immune-Check Point Molecules in the Placentas of Patients With Gestational Diabetes Mellitus
Yea Eun Kang, Hyon-Seung Yi, Min-Kyung Yeo, Jung Tae Kim, Danbit Park, Yewon Jung, Ok Soon Kim, Seong Eun Lee, Ji Min Kim, Kyong Hye Joung, Ju Hee Lee, Bon Jeong Ku, Mina Lee, Hyun Jin Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef
- Advances in free fatty acid profiles in gestational diabetes mellitus
- Epidemiology
- Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents
- Buyun Liu, Hans-Joachim Lehmler, Yangbo Sun, Guifeng Xu, Qi Sun, Linda G. Snetselaar, Robert B. Wallace, Wei Bao
- Diabetes Metab J. 2019;43(1):59-75. Published online February 19, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0045
- 5,979 View
- 155 Download
- 92 Web of Science
- 92 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Bisphenol F (BPF) and bisphenol S (BPS) are increasingly used as substitutes for bisphenol A (BPA), an environmental obesogen. However, health effects of BPF and BPS remain unclear. In this study, we evaluated the associations of BPA, BPF, and BPS with obesity in children and adolescents.
Methods We used data from the U.S. National Health and Nutrition Examination Survey 2013 to 2014, a nationally representative study. We included 745 participants aged 6 to 17 years old. General obesity was defined based on the 2000 Centers for Disease Control and Prevention body mass index-for-age growth charts for the United States. Abdominal obesity was defined as waist-to-height ratio ≥0.5.
Results After adjustment for demographic, socioeconomic and lifestyle factors, and urinary creatinine levels, the odds ratio of general obesity comparing the highest with lowest quartile of urinary bisphenol levels was 1.74 (95% confidence interval [CI], 0.92 to 3.31) for BPA, 1.54 (95% CI, 1.02 to 2.32) for BPF, and 1.36 (95% CI, 0.53 to 3.51) for BPS. Moreover, the associations were stronger in boys than in girls for BPA and BPF. Similar results were observed for abdominal obesity.
Conclusion This study for the first time showed that exposure to BPF, a commonly used substitute for BPA, was positively associated with higher risk of obesity in children and adolescents. The association of BPA and BPF with general and abdominal obesity was primarily observed in boys, suggesting a possible sex difference. Further investigations on the underlying mechanisms are needed.
-
Citations
Citations to this article as recorded by- Obesogenic effects of six classes of emerging contaminants
Siying Wu, Chaoyu Tong, Jing Liu
Journal of Environmental Sciences.2025; 151: 252. CrossRef - Bisphenol S, bisphenol F, bisphenol a exposure and body composition in US adults
Buyun Liu, Yuxiang Yan, Juan Xie, Jian Sun, Hans-Joachim Lehmler, Leonardo Trasande, Robert B. Wallace, Wei Bao
Chemosphere.2024; 346: 140537. CrossRef - Sex and Gender Differences on the Impact of Metabolism-Disrupting Chemicals on Obesity: A Systematic Review
Massimo D’Archivio, Lucia Coppola, Roberta Masella, Alessia Tammaro, Cinzia La Rocca
Nutrients.2024; 16(2): 181. CrossRef - The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies
Maria Dalamaga, Dimitrios Kounatidis, Dimitrios Tsilingiris, Natalia G. Vallianou, Irene Karampela, Sotiria Psallida, Athanasios G. Papavassiliou
International Journal of Molecular Sciences.2024; 25(1): 675. CrossRef - Sex-specific associations of bisphenol A and its substitutes with body fat distribution among US adults: NHANES 2011–2016
Shili Zhang, Lingyan Dai, Ziyu Wan, Zhiwei Huang, Mengchen Zou, Haixia Guan
Environmental Science and Pollution Research.2024; 31(5): 7948. CrossRef - EDC mixtures during pregnancy and body fat at 7 years of age in a Swedish cohort, the SELMA study
Katherine Svensson, Chris Gennings, Christian Lindh, Hannu Kiviranta, Panu Rantakokko, Sverre Wikström, Carl-Gustaf Bornehag
Environmental Research.2024; 248: 118293. CrossRef - Chemical Composition of Leachates from Hydraulic Fracturing Proppants from Surficial Releases in Southeastern New Mexico
Matthew S. Varonka, Terry G. Gregston, Michael Villalobos, Jacqueline P. Green, William H. Orem
Environmental Science & Technology Letters.2024; 11(3): 243. CrossRef - Associations of bisphenol A exposure with metabolic syndrome and its components: A systematic review and meta‐analysis
Tianli Xiao, Zehua Huang, Chanjuan Zheng, Binh Quach, Yulian Zhu, Feifei Li, Wei Liang, Julien Baker, Christoph Reichetzeder, Berthold Hocher, Yide Yang
Obesity Reviews.2024;[Epub] CrossRef - Relationship of bisphenol A substitutes bisphenol F and bisphenol S with adiponectin/leptin ratio among children from the environment and development of children cohort
Hye Jin Lee, Yun Jeong Lee, Youn-Hee Lim, Hwa Young Kim, Bung-Nyun Kim, Johanna Inhyang Kim, Yong Min Cho, Yun-Chul Hong, Choong Ho Shin, Young Ah Lee
Environment International.2024; 185: 108564. CrossRef - SWATH-MS reveals that Bisphenol A and its analogs regulate pathways leading to disruption in Insulin signaling and fatty acid metabolism
Shabda Kulsange, Monika Sharma, Babasaheb Sonawane, Meera R. Jaiswal, Mahesh Kulkarni, B. Santhakumari
Food and Chemical Toxicology.2024; : 114667. CrossRef - Exposure to Bisphenol A, S, and F and its Association with Obesity and Diabetes Mellitus in General Adults of Korea: Korean National Environmental Health Survey (KoNEHS) 2015–2017
Min Kyong Moon, Min Joo Kim, Inae Lee, Sunmi Kim, Sohyeon Choi, Jeongim Park, Yoon Hee Cho, Sooyeon Hong, Jiyoung Yoo, Hyunwoong Park, Gi Jeong Cheon, Young Joo Park, Kyungho Choi
Exposure and Health.2023; 15(1): 53. CrossRef - Evaluation of toxicological effects of bisphenol S with an in vitro human bone marrow mesenchymal stem cell: Implications for bone health
Mei Li, Tenglong Li, Juan Yin, Chunfeng Xie, Jianyun Zhu
Toxicology.2023; 484: 153408. CrossRef - In silico profiling of endocrine-disrupting potential of bisphenol analogues and their halogenated transformation products
Karolina Nowak, Žiga Jakopin
Food and Chemical Toxicology.2023; 173: 113623. CrossRef - Transient developmental exposure to low doses of bisphenol F negatively affects neurogliogenesis and olfactory behaviour in adult mice
Pieter Vancamp, Lucile Butruille, Anni Herranen, Anita Boelen, Jean-Baptiste Fini, Barbara A. Demeneix, Sylvie Remaud
Environment International.2023; 172: 107770. CrossRef - Development of human dermal PBPK models for the bisphenols BPA, BPS, BPF, and BPAF with parallel-layered skin compartment: Basing on dermal administration studies in humans
Man Hu, Zhichun Zhang, Yining Zhang, Ming Zhan, Weidong Qu, Gengsheng He, Ying Zhou
Science of The Total Environment.2023; 868: 161639. CrossRef - Postnatal exposure to Bisphenol S induces liver injury in mice: Possible implication of PPARγ receptor
Bessem Mornagui, Raja Rezg, Fadoua Neffati, Mohamed Fadhel Najjar, Ahmed Rejeb
Toxicology and Industrial Health.2023; 39(5): 237. CrossRef - JAK3/STAT5b/PPARγ Pathway Mediates the Association between Di(2-ethylhexyl) Phthalate Exposure and Lipid Metabolic Disorder in Chinese Adolescent Students
Qi Xu, Shuang Ding, Wen Qi, Xueting Zhang, Meng Zhang, Jiqiang Xing, Aipeng Ju, Liting Zhou, Lin Ye
Chemical Research in Toxicology.2023; 36(5): 725. CrossRef - Bisphenol A substitutes and childhood obesity at 7 years: a cross-sectional study in Shandong, China
Minyan Chen, Cheng Lv, Shanyu Zhang, Lap Ah Tse, Xinyu Hong, Xi Liu, Yu Ding, Ping Xiao, Ying Tian, Yu Gao
Environmental Science and Pollution Research.2023; 30(29): 73174. CrossRef - Association between Bisphenol A exposure and body composition parameters in children
Yong Guo, Cui Liu, Yu-Hong Deng, Jing Ning, Li Yu, Jie-Ling Wu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Urinary neonicotinoid insecticides and adiposity measures among 7-year-old children in northern China: A cross-sectional study
Zhenping Lu, Yi Hu, Lap Ah Tse, Jinxia Yu, Zhuanning Xia, Xiaoning Lei, Yan Zhang, Rong Shi, Ying Tian, Yu Gao
International Journal of Hygiene and Environmental Health.2023; 251: 114188. CrossRef - Mechanism of Bisphenol F Affecting Motor System and Motor Activity in Zebrafish
Yeonhwa Kim, Seong Soon Kim, Byeong Heon Park, Kyu-Seok Hwang, Myung Ae Bae, Sung-Hee Cho, Suhyun Kim, Hae-Chul Park
Toxics.2023; 11(6): 477. CrossRef - The effects of trans fat diet intake on metabolic parameters and pancreatic tissue in offspring of prenatal bisphenol A exposed rats
Hala Abulehia, Noor Shafina Mohd Nor, Siti Hamimah Sheikh Abdul Kadir, Mardiana Abdul Aziz, Sarah Zulkifli
Scientific Reports.2023;[Epub] CrossRef - Genetic background in the rat affects endocrine and metabolic outcomes of bisphenol F exposure
Valerie A Wagner, Katie L Holl, Karen C Clark, John J Reho, Melinda R Dwinell, Hans-Joachim Lehmler, Hershel Raff, Justin L Grobe, Anne E Kwitek
Toxicological Sciences.2023; 194(1): 84. CrossRef - Association of parabens and bisphenols with lung function in children aged 5–12 years from Shanghai, China
Yi Hu, Hao Chen, Yuan Tian, Dan Wu, Angela Vinturache, Guodong Ding, Guangjun Yu
International Journal of Hygiene and Environmental Health.2023; 252: 114210. CrossRef - Bisphenol A substitutes and obesity: a review of the epidemiology and pathophysiology
Shane V. Varghese, Julianne M. Hall
Frontiers in Endocrinology.2023;[Epub] CrossRef - Levels of Bisphenol A and its analogs in nails, saliva, and urine of children: a case control study
Yolanda Gálvez-Ontiveros, Inmaculada Moscoso-Ruiz, Vega Almazán Fernández de Bobadilla, Celia Monteagudo, Rafael Giménez-Martínez, Lourdes Rodrigo, Alberto Zafra-Gómez, Ana Rivas
Frontiers in Nutrition.2023;[Epub] CrossRef - Exposure to Bisphenol A and Its Analogs among Thai School-Age Children
Nattakarn Numsriskulrat, Thanawan Teeranathada, Chansuda Bongsebandhu-Phubhakdi, Suphab Aroonparkmongkol, Kyungho Choi, Vichit Supornsilchai
Toxics.2023; 11(9): 761. CrossRef - Bisphenol analogues inhibit human and rat 17β-hydroxysteroid dehydrogenase 1: 3D-quantitative structure-activity relationship (3D-QSAR) and in silico docking analysis
Sailing Chen, Shaowei Wang, Jingyi Zheng, Han Lu, Huiqian Chen, Yunbing Tang, Nan Wang, Yang Zhu, Yiyan Wang, Ping Duan, Ren-shan Ge
Food and Chemical Toxicology.2023; 181: 114052. CrossRef - Associations of prenatal exposure to bisphenols with BMI growth trajectories in offspring within the first two years: evidence from a birth cohort study in China
Chao Xiong, Kai Chen, Lu-Li Xu, Yi-Ming Zhang, Hua Liu, Meng-Lan Guo, Zhi-Guo Xia, Yu-Ji Wang, Xiao-Feng Mu, Xiao-Xuan Fan, Jing-Quan Chen, Yu-Ru Liu, Yuan-Yuan Li, Wei Xia, You-Jie Wang, Ai-Fen Zhou
World Journal of Pediatrics.2023;[Epub] CrossRef - Ecotoxicological Evaluation of Bisphenol A and Alternatives: A Comprehensive In Silico Modelling Approach
Liadys Mora Lagares, Marjan Vračko
Journal of Xenobiotics.2023; 13(4): 719. CrossRef - Bisphenol A (BPA) and Cardiovascular or Cardiometabolic Diseases
Jeong-Hun Kang, Daisuke Asai, Riki Toita
Journal of Xenobiotics.2023; 13(4): 775. CrossRef - Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on the available evidence in relation to the potential obesogenic activity of certain chemical compounds that may be present in foods
Ana María Rivas Velasco, Irene Bretón Lesmes, Araceli Díaz Perales, Ángel Gil Izquierdo, María José González Muñoz, Victoria Moreno Arribas, María del Puy Portillo Baquedano, Silvia Pichardo Sánchez
Food Risk Assess Europe.2023;[Epub] CrossRef - Regulatory and academic studies to derive reference values for human health: The case of bisphenol S
Claire Beausoleil, Brigitte Le Magueresse-Battistoni, Catherine Viguié, Sylvie Babajko, Marie-Chantal Canivenc-Lavier, Nicolas Chevalier, Claude Emond, René Habert, Nicole Picard-Hagen, Sakina Mhaouty-Kodja
Environmental Research.2022; 204: 112233. CrossRef - Urinary bisphenol concentrations and its association with metabolic disorders in the US and Korean populations
Ji Yoon Choi, Jiyun Lee, Da-An Huh, Kyong Whan Moon
Environmental Pollution.2022; 295: 118679. CrossRef - Associations of mid-childhood bisphenol A and bisphenol S exposure with mid-childhood and adolescent obesity
Priya Gajjar, Yun Liu, Nan Li, Jessie P. Buckley, Aimin Chen, Bruce P. Lanphear, Heidi J. Kalkwarf, Kim M. Cecil, Kimberly Yolton, Joseph M. Braun
Environmental Epidemiology.2022; 6(1): e187. CrossRef - Profile of Environmental Chemicals in the Korean Population—Results of the Korean National Environmental Health Survey (KoNEHS) Cycle 3, 2015–2017
Sun Kyoung Jung, Wookhee Choi, Sung Yeon Kim, Sooyeon Hong, Hye Li Jeon, Youngkyung Joo, Chulwoo Lee, Kyungho Choi, Sungkyoon Kim, Kee-Jae Lee, Jiyoung Yoo
International Journal of Environmental Research and Public Health.2022; 19(2): 626. CrossRef - The bisphenol F and bisphenol S and cardiovascular disease: results from NHANES 2013–2016
Ruihua Wang, Qiaoyuan Fei, Shan Liu, Xueqiong Weng, Huanzhu Liang, Yingying Wu, Lin Wen, Guang Hao, Guangwen Cao, Chunxia Jing
Environmental Sciences Europe.2022;[Epub] CrossRef - Bisphenols A and its analogues induce genotoxic damage in marine and freshwater amphipods
Serena Cosentino, Federica Aureli, Valentina Iannilli
Environmental Advances.2022; 7: 100183. CrossRef - Impact of environmental pollution on the obesogenic environment
Adriana Martínez-Esquivel, Daniela Joyce Trujillo-Silva, V Gabriela Cilia-López
Nutrition Reviews.2022; 80(7): 1787. CrossRef - Effects of BPZ and BPC on Oxidative Stress of Zebrafish under Different pH Conditions
Ying Han, Yumeng Fei, Mingxin Wang, Yingang Xue, Yuxuan Liu
Molecules.2022; 27(5): 1568. CrossRef - Race-specific associations of urinary phenols and parabens with adipokines in midlife women: The Study of Women's Health Across the Nation (SWAN)
Seulbi Lee, Carrie Karvonen-Gutierrez, Bhramar Mukherjee, William H. Herman, Sung Kyun Park
Environmental Pollution.2022; 303: 119164. CrossRef - Are BPA Substitutes as Obesogenic as BPA?
Fabiana Oliviero, Alice Marmugi, Catherine Viguié, Véronique Gayrard, Nicole Picard-Hagen, Laila Mselli-Lakhal
International Journal of Molecular Sciences.2022; 23(8): 4238. CrossRef - Aptamer-Based Biosensors for the Analytical Determination of Bisphenol A in Foodstuffs
Marica Erminia Schiano, Avazbek Abduvakhidov, Michela Varra, Stefania Albrizio
Applied Sciences.2022; 12(8): 3752. CrossRef - Bisphenol A exposure induces multiple effects in DOPC membrane models
Mateus D. Maximino, Cibely S. Martin, Priscila Aléssio
Journal of Molecular Liquids.2022; 359: 119253. CrossRef - Bisphenol S induces Agrp expression through GPER1 activation and alters transcription factor expression in immortalized hypothalamic neurons: A mechanism distinct from BPA-induced upregulation
Katherine J. Xu, Neruja Loganathan, Denise D. Belsham
Molecular and Cellular Endocrinology.2022; 552: 111630. CrossRef - Bisphenol S Alters the Steroidome in the Preovulatory Follicle, Oviduct Fluid and Plasma in Ewes With Contrasted Metabolic Status
Ophélie Téteau, Philippe Liere, Antoine Pianos, Alice Desmarchais, Olivier Lasserre, Pascal Papillier, Claire Vignault, Marie-Emilie Lebachelier de la Riviere, Virginie Maillard, Aurélien Binet, Svetlana Uzbekova, Marie Saint-Dizier, Sebastien Elis
Frontiers in Endocrinology.2022;[Epub] CrossRef - Relationship between bisphenol A, bisphenol S, and bisphenol F and serum uric acid concentrations among school-aged children
Yun Jeong Lee, Youn-Hee Lim, Choong Ho Shin, Bung-Nyun Kim, Johanna Inhyang Kim, Yun-Chul Hong, Yong Min Cho, Young Ah Lee, Pasquale Avino
PLOS ONE.2022; 17(6): e0268503. CrossRef - Associations of bisphenol exposure with thyroid hormones in pregnant women: a prospective birth cohort study in China
Huishen Huang, Jun Liang, Peng Tang, Chuanxiang Yu, Haoran Fan, Qian Liao, Jinghua Long, Dongxiang Pan, Xiaoyun Zeng, Shun Liu, Dongping Huang, Xiaoqiang Qiu
Environmental Science and Pollution Research.2022; 29(58): 87170. CrossRef - Association between urinary concentrations of bisphenol A substitutes and diabetes in adults
Rafael Moreno-Gómez-Toledano, Esperanza Vélez-Vélez, María I Arenas, Marta Saura, Ricardo J Bosch
World Journal of Diabetes.2022; 13(7): 521. CrossRef - Uncovering the functions of plasma proteins in ulcerative colitis and identifying biomarkers for BPA-induced severe ulcerative colitis: A plasma proteome analysis
Chen Huang, Yuqin Wang, Xiao Lin, Ting Fung Chan, Keng Po Lai, Rong Li
Ecotoxicology and Environmental Safety.2022; 242: 113897. CrossRef - Relationship between emergent BPA-substitutes and renal and cardiovascular diseases in adult population
Rafael Moreno-Gómez-Toledano
Environmental Pollution.2022; 313: 120106. CrossRef - Climate change and the water quality threats posed by the emerging contaminants per- and polyfluoroalkyl substances (PFAS) and microplastics
Malcolm J. Gander
Water International.2022; : 1. CrossRef - Endocrine disruptor chemicals as obesogen and diabetogen: Clinical and mechanistic evidence
Niyazi Emre Kurşunoğlu, Banu Pinar Sarer Yurekli
World Journal of Clinical Cases.2022; 10(31): 11226. CrossRef - Exposure to Bisphenol A Substitutes, Bisphenol S and Bisphenol F, and Its Association with Developing Obesity and Diabetes Mellitus: A Narrative Review
Hend F. Alharbi, Raya Algonaiman, Rana Alduwayghiri, Thamer Aljutaily, Reham M. Algheshairy, Abdulkarim S. Almutairi, Razan M. Alharbi, Leena A. Alfurayh, Amjad A. Alshahwan, Amjad F. Alsadun, Hassan Barakat
International Journal of Environmental Research and Public Health.2022; 19(23): 15918. CrossRef - Influence of BPA exposure, measured in saliva, on childhood weight
Leticia Heras-González, Diana Espino, Maria Jose Jimenez-Casquet, Alejandro Lopez-Moro, Fatima Olea-Serrano, Miguel Mariscal-Arcas
Frontiers in Endocrinology.2022;[Epub] CrossRef - Bisphenol S enhances gap junction intercellular communication in ovarian theca cells
Jeremy Gingrich, Yong Pu, Brad L. Upham, Madeline Hulse, Sarah Pearl, Denny Martin, Anita Avery, Almudena Veiga-Lopez
Chemosphere.2021; 263: 128304. CrossRef - Exposure to bisphenols and asthma morbidity among low-income urban children with asthma
Lesliam Quirós-Alcalá, Nadia N. Hansel, Meredith McCormack, Antonia M. Calafat, Xiaoyun Ye, Roger D. Peng, Elizabeth C. Matsui
Journal of Allergy and Clinical Immunology.2021; 147(2): 577. CrossRef - Evaluation of the effects of low nanomolar bisphenol A-like compounds’ levels on early human embryonic development and lipid metabolism with human embryonic stem cell in vitro differentiation models
Xiaoxing Liang, Renjun Yang, Nuoya Yin, Francesco Faiola
Journal of Hazardous Materials.2021; 407: 124387. CrossRef - Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers
Jessica L. Levasseur, Stephanie C. Hammel, Kate Hoffman, Allison L. Phillips, Sharon Zhang, Xiaoyun Ye, Antonia M. Calafat, Thomas F. Webster, Heather M. Stapleton
Environment International.2021; 147: 106317. CrossRef - Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period
Mark P. Green, Alexandra J. Harvey, Bethany J. Finger, Gerard A. Tarulli
Environmental Research.2021; 194: 110694. CrossRef - Environmental Factors Involved in Maternal Morbidity and Mortality
Abee L. Boyles, Brandiese E. Beverly, Suzanne E. Fenton, Chandra L. Jackson, Anne Marie Z. Jukic, Vicki L. Sutherland, Donna D. Baird, Gwen W. Collman, Darlene Dixon, Kelly K. Ferguson, Janet E. Hall, Elizabeth M. Martin, Thaddeus T. Schug, Alexandra J. W
Journal of Women's Health.2021; 30(2): 245. CrossRef - Bisphenol-S and Bisphenol-F alter mouse pancreatic β-cell ion channel expression and activity and insulin release through an estrogen receptor ERβ mediated pathway
Laura Marroqui, Juan Martinez-Pinna, Manuel Castellano-Muñoz, Reinaldo S. dos Santos, Regla M. Medina-Gali, Sergi Soriano, Ivan Quesada, Jan-Ake Gustafsson, José A. Encinar, Angel Nadal
Chemosphere.2021; 265: 129051. CrossRef - Urinary bisphenol A concentrations and the risk of obesity in Korean adults
Shinje Moon, Moon Young Seo, Kyungho Choi, Yoon-seok Chang, Shin-Hye Kim, Mi Jung Park
Scientific Reports.2021;[Epub] CrossRef - Transcriptomic pathway and benchmark dose analysis of Bisphenol A, Bisphenol S, Bisphenol F, and 3,3',5,5'-Tetrabromobisphenol A in H9 human embryonic stem cells
Vian Peshdary, Cheryl A. Hobbs, Timothy Maynor, Kim Shepard, Remi Gagné, Andrew Williams, Byron Kuo, Nikolai Chepelev, Leslie Recio, Carole Yauk, Ella Atlas
Toxicology in Vitro.2021; 72: 105097. CrossRef - Prenatal exposure to bisphenols and cognitive function in children at 7 years of age in the Swedish SELMA study
Carl-Gustaf Bornehag, Elin Engdahl, Maria Unenge Hallerbäck, Sverre Wikström, Christian Lindh, Joëlle Rüegg, Eva Tanner, Chris Gennings
Environment International.2021; 150: 106433. CrossRef - Urinary bisphenol A levels in prepubertal children with exogenous obesity according to presence of metabolic syndrome
Esra Aktağ, Kadriye Yurdakök, Siddika Songül Yalçın, Nurgün Kandemir
Journal of Pediatric Endocrinology and Metabolism.2021; 34(4): 495. CrossRef - Metabolic pathways, alterations in miRNAs expression and effects of genetic polymorphisms of bisphenol a analogues: A systematic review
Viviana Ramírez, Yolanda Gálvez-Ontiveros, Patricia Porras-Quesada, Luis Javier Martinez-Gonzalez, Ana Rivas, María Jesús Álvarez-Cubero
Environmental Research.2021; 197: 111062. CrossRef - Dietary quality and bisphenols: trends in bisphenol A, F, and S exposure in relation to the Healthy Eating Index using representative data from the NHANES 2007–2016
Irene van Woerden, Devon C Payne-Sturges, Corrie M Whisner, Meg Bruening
The American Journal of Clinical Nutrition.2021; 114(2): 669. CrossRef - Bisphenol A and its effects on the systemic organs of children
Sarah Zulkifli, Amirah Abdul Rahman, Siti Hamimah Sheikh Abdul Kadir, Noor Shafina Mohd Nor
European Journal of Pediatrics.2021; 180(10): 3111. CrossRef - Bisphenols' occurrence in bivalves as sentinel of environmental contamination
Elena Baralla, Valeria Pasciu, Maria Vittoria Varoni, Maria Nieddu, Roberto Demuro, Maria Piera Demontis
Science of The Total Environment.2021; 785: 147263. CrossRef - Bisphenol F Exposure in Adolescent Heterogeneous Stock Rats Affects Growth and Adiposity
Valerie A Wagner, Karen C Clark, Leslie Carrillo-Sáenz, Katie A Holl, Miriam Velez-Bermudez, Derek Simonsen, Justin L Grobe, Kai Wang, Andrew Thurman, Leah C Solberg Woods, Hans-Joachim Lehmler, Anne E Kwitek
Toxicological Sciences.2021; 181(2): 246. CrossRef - Factors Associated with Exposure to Dietary Bisphenols in Adolescents
Virginia Robles-Aguilera, Yolanda Gálvez-Ontiveros, Lourdes Rodrigo, Inmaculada Salcedo-Bellido, Margarita Aguilera, Alberto Zafra-Gómez, Celia Monteagudo, Ana Rivas
Nutrients.2021; 13(5): 1553. CrossRef - Impact of short-term change of adiposity on risk of high blood pressure in children: Results from a follow-up study in China
Yi-de Yang, Ming Xie, Yuan Zeng, Shuqian Yuan, Haokai Tang, Yanhui Dong, Zhiyong Zou, Bin Dong, Zhenghe Wang, Xiangli Ye, Xiuqin Hong, Qiu Xiao, Jun Ma, Raffaella Buzzetti
PLOS ONE.2021; 16(9): e0257144. CrossRef - Life-Time Environmental Chemical Exposure and Obesity: Review of Epidemiological Studies Using Human Biomonitoring Methods
Nayan Chandra Mohanto, Yuki Ito, Sayaka Kato, Michihiro Kamijima
Frontiers in Endocrinology.2021;[Epub] CrossRef - Italian Children Exposure to Bisphenol A: Biomonitoring Data from the LIFE PERSUADED Project
Sabrina Tait, Fabrizia Carli, Luca Busani, Demetrio Ciociaro, Veronica Della Latta, Annalisa Deodati, Enrica Fabbrizi, Anna Paola Pala, Francesca Maranghi, Roberta Tassinari, Giacomo Toffol, Stefano Cianfarani, Amalia Gastaldelli, Cinzia La Rocca
International Journal of Environmental Research and Public Health.2021; 18(22): 11846. CrossRef - Bisphenol A disrupts apolipoprotein E expression through estrogen-related receptor gamma and DNA methlylation in the liver of male rare minnow Gobiocypris rarus
Yingying Zhang, Zhu Zhu, Qiao Liu, Meng Zhang, Hui Yang, Wenzhi Wei
Ecotoxicology and Environmental Safety.2021; 228: 113041. CrossRef - Metabolic Syndrome and Endocrine Disrupting Chemicals: An Overview of Exposure and Health Effects
Elsi Haverinen, Mariana F. Fernandez, Vicente Mustieles, Hanna Tolonen
International Journal of Environmental Research and Public Health.2021; 18(24): 13047. CrossRef - Obesogens in Children—An Uncharted Territory
Mirjam Močnik, Nataša Marčun Varda
Metabolites.2021; 11(12): 882. CrossRef - Synthetic Chemicals and Cardiometabolic Health Across the Life Course Among Vulnerable Populations: a Review of the Literature from 2018 to 2019
Symielle A. Gaston, Linda S. Birnbaum, Chandra L. Jackson
Current Environmental Health Reports.2020; 7(1): 30. CrossRef - Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line
M.Á. Martínez, J. Blanco, J. Rovira, V. Kumar, J.L. Domingo, M. Schuhmacher
Food and Chemical Toxicology.2020; 140: 111298. CrossRef - Urinary bisphenol A and its analogues and haemato-biochemical alterations of pregnant women in Korea
Sora Kang, Bo Hye Shin, Jeoung A Kwon, Chan Wha Lee, Eun Kyo Park, Eun Young Park, Byungmi Kim
Environmental Research.2020; 182: 109104. CrossRef - Historical exposure to non-persistent environmental pollutants and risk of type 2 diabetes in a Spanish sub-cohort from the European Prospective Investigation into Cancer and Nutrition study
E. Salamanca-Fernández, L.M. Iribarne-Durán, M. Rodríguez-Barranco, F. Vela-Soria, N. Olea, M.J. Sánchez-Pérez, J.P. Arrebola
Environmental Research.2020; 185: 109383. CrossRef - Association Between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults
Wei Bao, Buyun Liu, Shuang Rong, Susie Y. Dai, Leonardo Trasande, Hans-Joachim Lehmler
JAMA Network Open.2020; 3(8): e2011620. CrossRef - Using three statistical methods to analyze the association between exposure to 9 compounds and obesity in children and adolescents: NHANES 2005-2010
Bangsheng Wu, Yi Jiang, Xiaoqing Jin, Li He
Environmental Health.2020;[Epub] CrossRef - How was the Diabetes Metabolism Journal added to MEDLINE?
Hye Jin Yoo
Science Editing.2020; 7(2): 201. CrossRef - Adipogenic effects of prenatal exposure to bisphenol S (BPS) in adult F1 male mice
Young-Ah Ahn, Hwayoung Baek, Miso Choi, Junbo Park, Soo Jin Son, Hyun Ju Seo, Jaeyun Jung, Je Kyung Seong, Jaehyouk Lee, Sungkyoon Kim
Science of The Total Environment.2020; 728: 138759. CrossRef - Bisphenol A Analogues in Food and Their Hormonal and Obesogenic Effects: A Review
Andújar, Gálvez-Ontiveros, Zafra-Gómez, Rodrigo, Álvarez-Cubero, Aguilera, Monteagudo, Rivas
Nutrients.2019; 11(9): 2136. CrossRef - Toxicological considerations of nano-sized plastics
PA Stapleton
AIMS Environmental Science.2019; 6(5): 367. CrossRef - Bisphenol A and adiposity measures in peripubertal boys from the INMA-Granada cohort
Vicente Mustieles, Maribel Casas, Patricia Ferrando-Marco, Olga Ocón-Hernández, Iris Reina-Pérez, Andrea Rodríguez-Carrillo, Fernando Vela-Soria, Rocío Pérez-Lobato, Eva María Navarrete-Muñoz, Carmen Freire, Nicolás Olea, Mariana F. Fernández
Environmental Research.2019; 173: 443. CrossRef - Trends and disparities in urinary BPA concentrations among U.S. emerging adults
Irene van Woerden, Meg Bruening, Jessica Montresor-López, Devon C. Payne-Sturges
Environmental Research.2019; 176: 108515. CrossRef - Concern about the Safety of Bisphenol A Substitutes
Min Kyong Moon
Diabetes & Metabolism Journal.2019; 43(1): 46. CrossRef - Urinary Bisphenols and Obesity Prevalence Among U.S. Children and Adolescents
Melanie H Jacobson, Miriam Woodward, Wei Bao, Buyun Liu, Leonardo Trasande
Journal of the Endocrine Society.2019; 3(9): 1715. CrossRef
- Obesogenic effects of six classes of emerging contaminants
- Effects of Exercise Alone on Insulin Sensitivity and Glucose Tolerance in Obese Youth
- SoJung Lee, YoonMyung Kim
- Diabetes Metab J. 2013;37(4):225-232. Published online August 14, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.4.225
- 3,927 View
- 38 Download
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader As with the dramatic increases in childhood obesity over the past decades, the incidence of type 2 diabetes has increased among children and adolescents in the United States. Insulin resistance is a common feature of childhood obesity and increases the risk of type 2 diabetes, metabolic syndrome, and atherogenic lipoprotein profile in obese youth. Although cross-sectional studies report beneficial effects of physical activity or cardiorespiratory fitness on insulin sensitivity, the role of regular exercise alone (e.g., no calorie restriction) as a strategy to reduce the risk of type 2 diabetes is unclear in obese children and adolescents. In this mini review, we examined the independent effects of various exercise on glucose tolerance and insulin sensitivity in obese youth.
-
Citations
Citations to this article as recorded by- Obesity blunts insulin sensitivity improvements and attenuates strength gains following resistance training in nondiabetic men
Ramzi A. Al-Horani, Khaled M. Alsays, Osama Abo Alrob
European Journal of Applied Physiology.2024; 124(5): 1425. CrossRef - Lifestyle and Pharmacological Interventions and Treatment Indications for the Management of Obesity in Children and Adolescents
Despina Herouvi, George Paltoglou, Alexandra Soldatou, Christina Kalpia, Spyridon Karanasios, Kyriaki Karavanaki
Children.2023; 10(7): 1230. CrossRef - Predictors of postprandial glycaemia, insulinaemia and insulin resistance in adolescents
Ryan A. Williams, Karah J. Dring, Simon B. Cooper, John G. Morris, Caroline Sunderland, Mary E. Nevill
British Journal of Nutrition.2021; 125(10): 1101. CrossRef - New insights into the pharmacological treatment of pediatric patients with type 2 diabetes
Tatsuhiko Urakami
Clinical Pediatric Endocrinology.2018; 27(1): 1. CrossRef - Effect of 7 days of exercise on exogenous carbohydrate oxidation and insulin resistance in children with obesity
Lisa Chu, Katherine M. Morrison, Michael C. Riddell, Sandeep Raha, Brian W. Timmons
Applied Physiology, Nutrition, and Metabolism.2018; 43(7): 677. CrossRef - 12-Week aerobic exercise and nutritional program minimized the presence of the 64Arg allele on insulin resistance
Gerusa E. Milano-Gai, Lupe Furtado-Alle, Jorge Mota, Leilane Lazarotto, Gisele E. Milano, Ricardo R. de Souza Lehtonen, Ana C.K. Titski, Íncare C. Jesus, Luciane V. Tureck, Rosana B. Radominski, Manuel João Coelho-e-Silva, Neiva Leite
Journal of Pediatric Endocrinology and Metabolism.2018; 31(9): 1033. CrossRef - ADRB2 Gln27Glu polymorphism influenced changes in leptin but not body composition or metabolic and other inflammatory parameters after twelve weeks of combined training in overweight adolescents
Neiva Leite, Larissa Rosa da Silva, Íncare Correa de Jesus, Wendell Arthur Lopes, Claudia Regina Cavaglieri, Cássio Leandro Consentino, Rosana Bento Radominski, Ricardo Lehtonen Rodrigues de Souza, Luciane Viater Tureck, Lupe Furtado-Alle
Motriz: Revista de Educação Física.2018;[Epub] CrossRef - Patterns of Physical Activity Adherence by Adolescents With Diabetes or Obesity Enrolled in a Personalized Community-Based Intervention
Sara F. Michaliszyn, Melinda Higgins, Melissa Spezia Faulkner
The Diabetes Educator.2018; 44(6): 519. CrossRef - Diet Quality and Mortality Risk in Metabolically Obese Normal-Weight Adults
Yong-Moon Mark Park, Teresa T. Fung, Susan E. Steck, Jiajia Zhang, Linda J. Hazlett, Kyungdo Han, Seung-Hwan Lee, Anwar T. Merchant
Mayo Clinic Proceedings.2016; 91(10): 1372. CrossRef - Mediterranean diet and mortality risk in metabolically healthy obese and metabolically unhealthy obese phenotypes
Y-M Park, S E Steck, T T Fung, J Zhang, L J Hazlett, K Han, A T Merchant
International Journal of Obesity.2016; 40(10): 1541. CrossRef - Efeitos metabólicos do exercício físico na obesidade infantil: uma visão atual
Santiago Tavares Paes, João Carlos Bouzas Marins, Ana Eliza Andreazzi
Revista Paulista de Pediatria.2015; 33(1): 122. CrossRef - Metabolic effects of exercise on childhood obesity: a current view
Santiago Tavares Paes, João Carlos Bouzas Marins, Ana Eliza Andreazzi
Revista Paulista de Pediatria (English Edition).2015; 33(1): 122. CrossRef - Impact of regular physical activity on blood glucose control and cardiovascular risk factors in adolescents with type 2 diabetes mellitus - a multicenter study of 578 patients from 225 centres
A Herbst, T Kapellen, E Schober, C Graf, T Meissner, RW Holl
Pediatric Diabetes.2015; 16(3): 204. CrossRef - Impact of exercise training without caloric restriction on inflammation, insulin resistance and visceral fat mass in obese adolescents
M. Mendelson, A.‐S. Michallet, D. Monneret, C. Perrin, F. Estève, P. R. Lombard, P. Faure, P. Lévy, A. Favre‐Juvin, J.‐L. Pépin, B. Wuyam, P. Flore
Pediatric Obesity.2015; 10(4): 311. CrossRef - Early-life sleep deprivation persistently depresses melatonin production and bio-energetics of the pineal gland: potential implications for the development of metabolic deficiency
Li-You Chen, Cheng Tiong, Chung-Hung Tsai, Wen-Chieh Liao, Shun-Fa Yang, Su-Chung Youn, Fu-Der Mai, Hung-Ming Chang
Brain Structure and Function.2015; 220(2): 663. CrossRef - Whey Protein Improves Exercise Performance and Biochemical Profiles in Trained Mice
WEN-CHYUAN CHEN, WEN-CHING HUANG, CHIEN-CHAO CHIU, YU-KAI CHANG, CHI-CHANG HUANG
Medicine & Science in Sports & Exercise.2014; 46(8): 1517. CrossRef - Type 2 diabetes in the child and adolescent
Phil Zeitler, Junfen Fu, Nikhil Tandon, Kristen Nadeau, Tatsuhiko Urakami, Timothy Barrett, David Maahs
Pediatric Diabetes.2014; 15(S20): 26. CrossRef - ENDOCRINOLOGY AND ADOLESCENCE: Aerobic exercise reduces insulin resistance markers in obese youth: a meta-analysis of randomized controlled trials
Antonio García-Hermoso, Jose M Saavedra, Yolanda Escalante, Mairena Sánchez-López, Vicente Martínez-Vizcaíno
European Journal of Endocrinology.2014; 171(4): R163. CrossRef
- Obesity blunts insulin sensitivity improvements and attenuates strength gains following resistance training in nondiabetic men
- Humoral Immunological Marks in Patients with Child-onset and Adult-onset Type 1 Diabetes.
- Hyun Dae Yoon, Jae Hong Kim, Jung Hyun Oh, Jin Chul Park, Sang Yub Nam, Ji Soon Yoon, Kyu Chang Won, In Ho Cho, Choong Ki Lee, Joong Yeol Park, Sung Kwan Hong, Ki Up Lee, Hyoung Woo Lee
- Korean Diabetes J. 2000;24(4):444-456. Published online January 1, 2001
- 1,236 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Type 1 diabetes mellitus is an autoimmune disease in which serum antibodies against islet antigens have been recognized. These antibodies include cytoplasmic islet cell antibodies (ICA), and glutamic acid decarboxylase (GAD)65 antibodies and IA2 antibodies. It has been reported that the prevalence of these autoantibodies is different among Caucacian and Asian and Korean type 1 diabetes patients. And the natural course of type 1 diabetes can differ according to the age of onset. But, in contrast to the classic juvenile onset type 1 diabetes, the adult onset type 1 diabetes is poorly characterized about clinical and autoimmune differences at presentation. Thus, this study was perfomed to evaluate clinical and autoimmune characteristics at presentation in subjects with either child onset or adult onset type 1 diabetes and to establish an autoimmune pathogenesis in Korean type 1 diabetes. METHOD: We examined the clinical characteristics of child onset type 1 diabetes (n=32) and adult onset type 1 diabetes (n=40) retrospectively. At the same time, ICA from these patients was measured by standard indirect immunofluorescence, GADA and IA2A from these patients were measured by radioimmunoassay. RESULTS: The mean duration of disease was longer in the adult onset and their serum fasting C-peptide concentration at diagnosis were higer. The prevalence of ICA, GADA, IA2A in sera from 32 patients with child onset type 1 diabetes was 50%, 38% and 31% respectively. And, the prevalence of ICA, GADA and IA2A in sera from 40 patients with adult onset type 1 diabetes was 30%, 25% and 18% respectively.The prevalence of ICA, GADA and IA2A in sera from 39 patients with typical type 1 diabetes was 46%, 30% and 16% respectively. And, the prevalence of ICA, GADA and IA2A in sera from 33 patients with atypical type 1 diabetes was 30%, 30% and 25% respectively. The concordance rate of ICA and GADA in child onset and adult onset diabetes was 81% (26/32), 80% (32/40) respectively. In a subset of these patients with recent onset type 1 diabetes (duration of diabetes < or = 1 year), the prevalence of ICA, GADA and IA2A was 75% (3/4), 75% (3/4), 100% (1/1) respectively, in the child onset type 1 diabetes. CONCLUSION: These observations show that autoantibodies in Korean patients with child onset type 1 diabetes is similar compaired with other Asian groups but is lower than Caucasian patients with type 1 diabetes and the prevalence of humoral immunologic makers in child onset type 1 diabetes was higher than that of adult onset diabetes. These results suggest that autoimmune response is a significant cause of Korean type 1 diabetes but other factors except autoimmunity may play an important role in the pathogenesis of Korean type 1 diabetes.

 KDA
KDA
 First
First Prev
Prev





