
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Previous issues
- Page Path
- HOME > Browse > Previous issues
Reviews
- Lifestyle
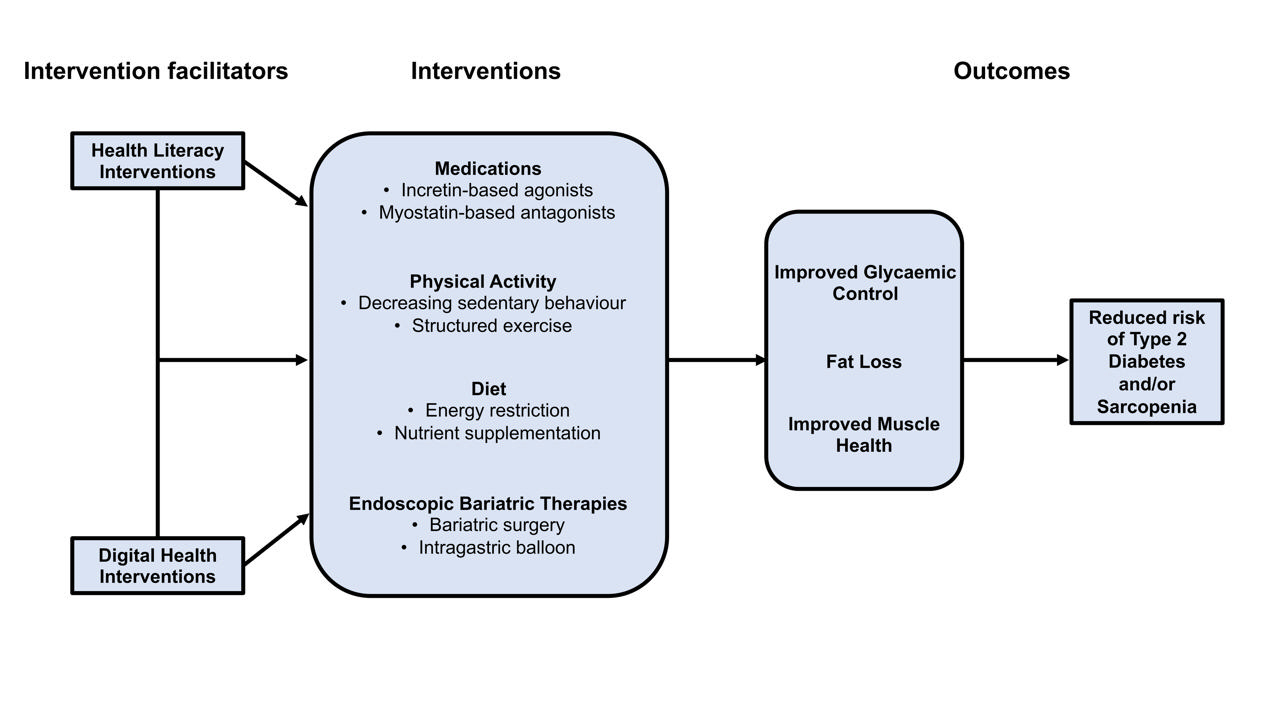
- Type 2 Diabetes Mellitus and Sarcopenia as Comorbid Chronic Diseases in Older Adults: Established and Emerging Treatments and Therapies
- Jakub Mesinovic, Jackson J. Fyfe, Jason Talevski, Michael J. Wheeler, Gloria K.W. Leung, Elena S. George, Melkamu T. Hunegnaw, Costas Glavas, Paul Jansons, Robin M. Daly, David Scott
- Diabetes Metab J. 2023;47(6):719-742. Published online September 14, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0112
- 4,547 View
- 438 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Type 2 diabetes mellitus (T2DM) and sarcopenia (low skeletal muscle mass and function) share a bidirectional relationship. The prevalence of these diseases increases with age and they share common risk factors. Skeletal muscle fat infiltration, commonly referred to as myosteatosis, may be a major contributor to both T2DM and sarcopenia in older adults via independent effects on insulin resistance and muscle health. Many strategies to manage T2DM result in energy restriction and subsequent weight loss, and this can lead to significant declines in muscle mass in the absence of resistance exercise, which is also a first-line treatment for sarcopenia. In this review, we highlight recent evidence on established treatments and emerging therapies targeting weight loss and muscle mass and function improvements in older adults with, or at risk of, T2DM and/or sarcopenia. This includes dietary, physical activity and exercise interventions, new generation incretin-based agonists and myostatin-based antagonists, and endoscopic bariatric therapies. We also highlight how digital health technologies and health literacy interventions can increase uptake of, and adherence to, established and emerging treatments and therapies in older adults with T2DM and/or sarcopenia.
-
Citations
Citations to this article as recorded by- Fucoidan ameliorates diabetic skeletal muscle atrophy through PI3K/Akt pathway
Caixia Li, Yaping Liu, Mingzhi Yang, Haoyue Huang, Lulu Tang, Yufan Miao, Wenjie Li, Xing Li
Journal of Functional Foods.2024; 114: 106076. CrossRef
- Fucoidan ameliorates diabetic skeletal muscle atrophy through PI3K/Akt pathway
- Complications
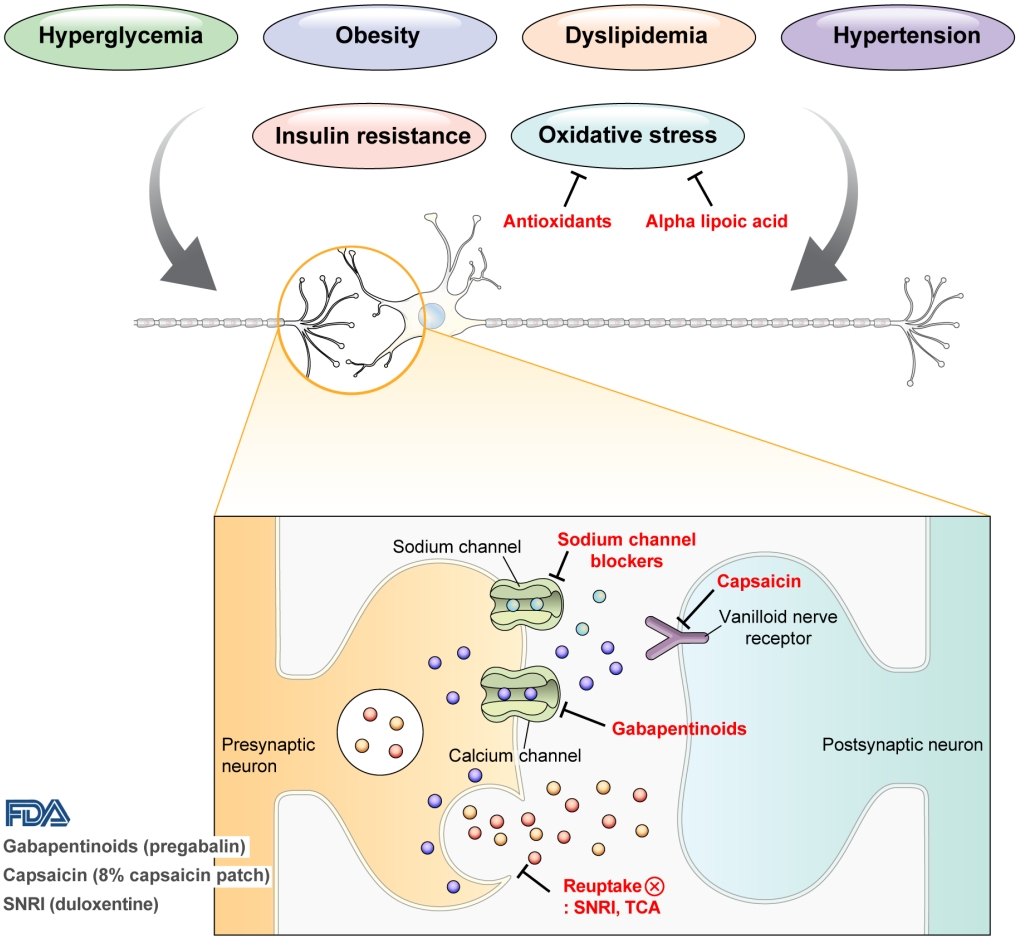
- Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy
- Han Na Jang, Tae Jung Oh
- Diabetes Metab J. 2023;47(6):743-756. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0018
- 3,905 View
- 546 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes. The lifetime prevalence of DPN is thought to be >50%, and 15%–25% of patients with diabetes experience neuropathic pain, referred to as “painful DPN.” Appropriate treatment of painful DPN is important because this pain contributes to a poor quality of life by causing sleep disturbance, anxiety, and depression. The basic principle for the management of painful DPN is to control hyperglycemia and other modifiable risk factors, but these may be insufficient for preventing or improving DPN. Because there is no promising diseasemodifying medication for DPN, the pain itself needs to be managed when treating painful DPN. Drugs for neuropathic pain, such as gabapentinoids, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, alpha-lipoic acid, sodium channel blockers, and topical capsaicin, are used for the management of painful DPN. The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, tapentadol, and the 8% capsaicin patch as drugs for the treatment of painful DPN. Recently, spinal cord stimulation using electrical stimulation is approved by the FDA for the treatment for painful DPN. This review describes the currently available pharmacological and nonpharmacological treatments for painful DPN.
-
Citations
Citations to this article as recorded by- J-2156, a small molecule somatostatin type 4 receptor agonist, alleviated hindpaw hypersensitivity in the streptozotocin-induced rat model of painful diabetic neuropathy but with a 2-fold decrease in potency at an advanced stage in the model, mimicking mo
A. Kuo, M. Z. Imam, R. Li, L. Lin, A. Raboczyj, A. E. Bohmer, J. R. Nicholson, L. Corradini, M. T. Smith
Frontiers in Pharmacology.2024;[Epub] CrossRef - The Chronic Wound–Related Pain Model
Kevin Woo
Clinics in Geriatric Medicine.2024;[Epub] CrossRef
- J-2156, a small molecule somatostatin type 4 receptor agonist, alleviated hindpaw hypersensitivity in the streptozotocin-induced rat model of painful diabetic neuropathy but with a 2-fold decrease in potency at an advanced stage in the model, mimicking mo
- Pathophysiology
- Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
- Yun Kyung Cho, Chang Hee Jung
- Diabetes Metab J. 2023;47(6):757-766. Published online July 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0072

- 2,774 View
- 326 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - With the increasing use of immune-checkpoint inhibitors (ICIs), such as anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and anti-programmed cell death-1 (PD-1), for the treatment of malignancies, cases of ICI-induced type 1 diabetes mellitus (ICI-T1DM) have been reported globally. This review focuses on the features and pathogenesis of this disease. T1DM is an immune-related adverse event that occurs following the administration of anti-PD-1 or anti-programmed death ligand-1 (PDL1) alone or in combination with anti-CTLA-4. More than half of the reported cases presented as abrupt-onset diabetic ketoacidosis. The primary mechanism of ICI-T1DM is T-cell stimulation, which results from the loss of interaction between PD-1 and PD-L1 in pancreatic islet. The similarities and differences between ICI-T1DM and classical T1DM may provide insights into this disease entity. ICI-T1DM is a rare but often life-threatening medical emergency that healthcare professionals and patients need to be aware of. Early detection of and screening for this disease is imperative. At present, the only known treatment for ICI-T1DM is insulin injection. Further research into the mechanisms and risk factors associated with ICI-T1DM development may contribute to a better understanding of this disease entity and the identification of possible preventive strategies.
-
Citations
Citations to this article as recorded by- Research Advances of Immune Checkpoint Inhibitors Related Endocrine Adverse Events
晶晶 王
Advances in Clinical Medicine.2024; 14(02): 2706. CrossRef - Immune checkpoint inhibitor‑associated diabetes mellitus in patients with HCC: Report of three cases and literature review
Gaocheng Wang, Jingjing Wang, Shuilin Dong, Zhanguo Zhang, Wanguang Zhang, Jianping Zhao
Experimental and Therapeutic Medicine.2024;[Epub] CrossRef
- Research Advances of Immune Checkpoint Inhibitors Related Endocrine Adverse Events
Special Editorial
- Ensuring Heathy Lives with the Korea Disease Control and Prevention Agency: Partnership with the Korean Diabetes Association
- Youngmee Jee
- Diabetes Metab J. 2023;47(6):767-768. Published online November 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0406
- 811 View
- 97 Download
Editorial
- Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
- Sang Youl Rhee
- Diabetes Metab J. 2023;47(6):769-770. Published online November 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0351
- 890 View
- 107 Download
Original Articles
- Basic Research
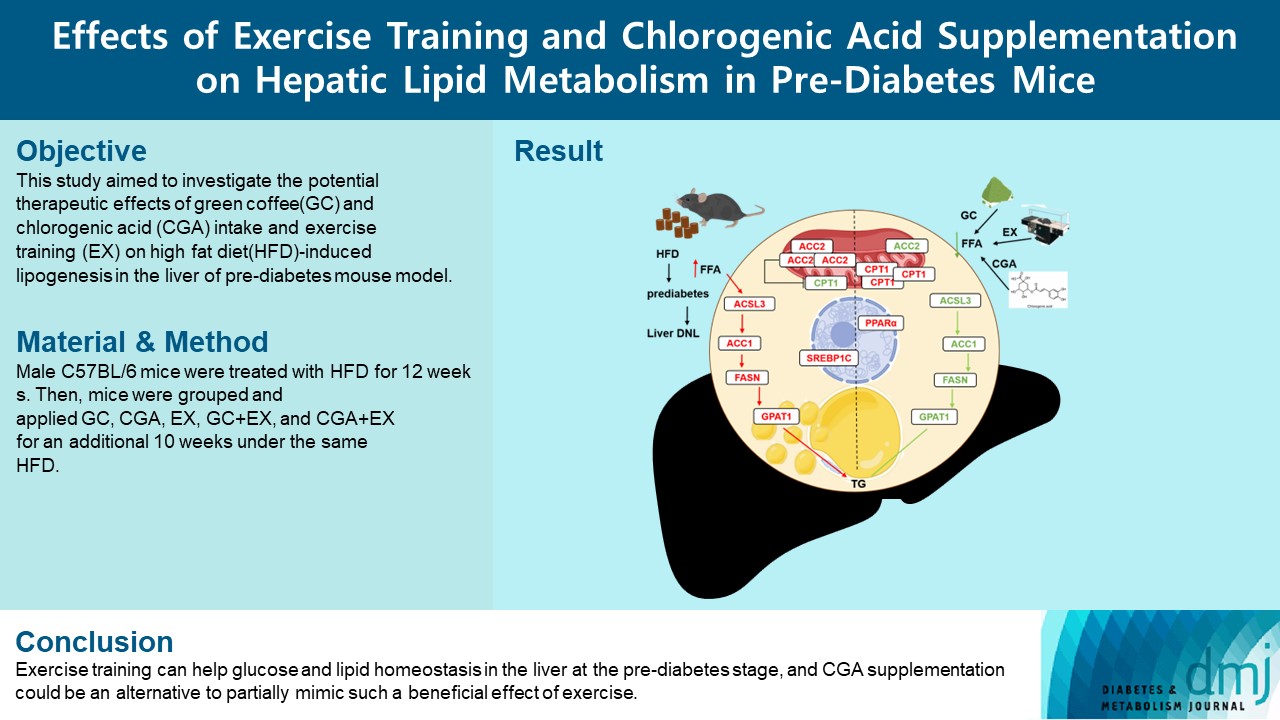
- Effects Of Exercise Training And Chlorogenic Acid Supplementation On Hepatic Lipid Metabolism In Prediabetes Mice
- Samaneh Shirkhani, Sayyed Mohammad Marandi, Mohammad Hossein Nasr-Esfahani, Seung Kyum Kim
- Diabetes Metab J. 2023;47(6):771-783. Published online September 8, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0265
- 2,262 View
- 167 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Since prediabetes is a risk factor for metabolic syndromes, it is important to promote a healthy lifestyle to prevent prediabetes. This study aimed to determine the effects of green coffee (GC), chlorogenic acid (CGA) intake, and exercise training (EX) on hepatic lipid metabolism in prediabetes male C57BL/6 mice.
Methods
Forty-nine mice were randomly divided into two groups feeding with a normal diet (n=7) or a high-fat diet (HFD, n=42) for 12 weeks. Then, HFD mice were further divided into six groups (n=7/group): control (pre-D), GC, CGA, EX, GC+EX, and CGA+EX. After additional 10 weeks under the same diet, plasma, and liver samples were obtained.
Results
HFD-induced prediabetes conditions with increases in body weight, glucose, insulin, insulin resistance, and lipid profiles were alleviated in all treatment groups. Acsl3, a candidate gene identified through an in silico approach, was lowered in the pre-D group, while treatments partly restored it. HFD induced adverse alterations of de novo lipogenesis- and β oxidation-associated molecules in the liver. However, GC and CGA supplementation and EX reversed or ameliorated these changes. In most cases, GC or CGA supplementation combined with EX has no synergistic effect and the GC group had similar results to the CGA group.
Conclusion
These findings suggest that regular exercise is an effective non-therapeutic approach for prediabetes, and CGA supplementation could be an alternative to partially mimic the beneficial effects of exercise on prediabetes. -
Citations
Citations to this article as recorded by- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients
Perioperative Precision Medicine.2023;[Epub] CrossRef
- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients
- Basic Research
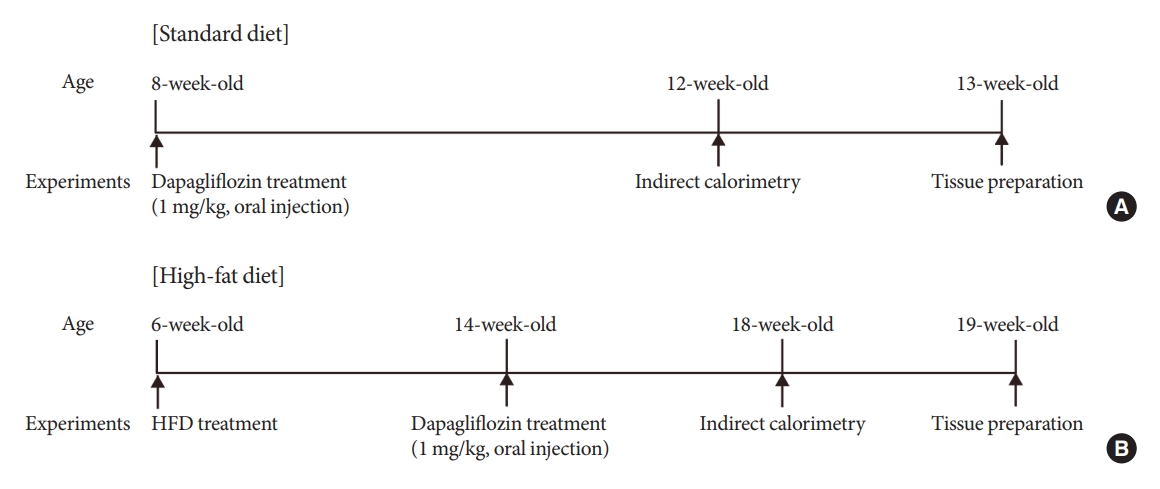
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2023;47(6):784-795. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0261
- 1,403 View
- 149 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are currently used to treat patients with diabetes. Previous studies have demonstrated that treatment with SGLT-2 inhibitors is accompanied by altered metabolic phenotypes. However, it has not been investigated whether the hypothalamic circuit participates in the development of the compensatory metabolic phenotypes triggered by the treatment with SGLT-2 inhibitors.
Methods
Mice were fed a standard diet or high-fat diet and treated with dapagliflozin, an SGLT-2 inhibitor. Food intake and energy expenditure were observed using indirect calorimetry system. The activity of hypothalamic neurons in response to dapagliflozin treatment was evaluated by immunohistochemistry with c-Fos antibody. Quantitative real-time polymerase chain reaction was performed to determine gene expression patterns in the hypothalamus of dapagliflozin-treated mice.
Results
Dapagliflozin-treated mice displayed enhanced food intake and reduced energy expenditure. Altered neuronal activities were observed in multiple hypothalamic nuclei in association with appetite regulation. Additionally, we found elevated immunosignals of agouti-related peptide neurons in the paraventricular nucleus of the hypothalamus.
Conclusion
This study suggests the functional involvement of the hypothalamus in the development of the compensatory metabolic phenotypes induced by SGLT-2 inhibitor treatment. -
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
Diabetes & Metabolism Journal.2024; 48(1): 159. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Drug Regimen
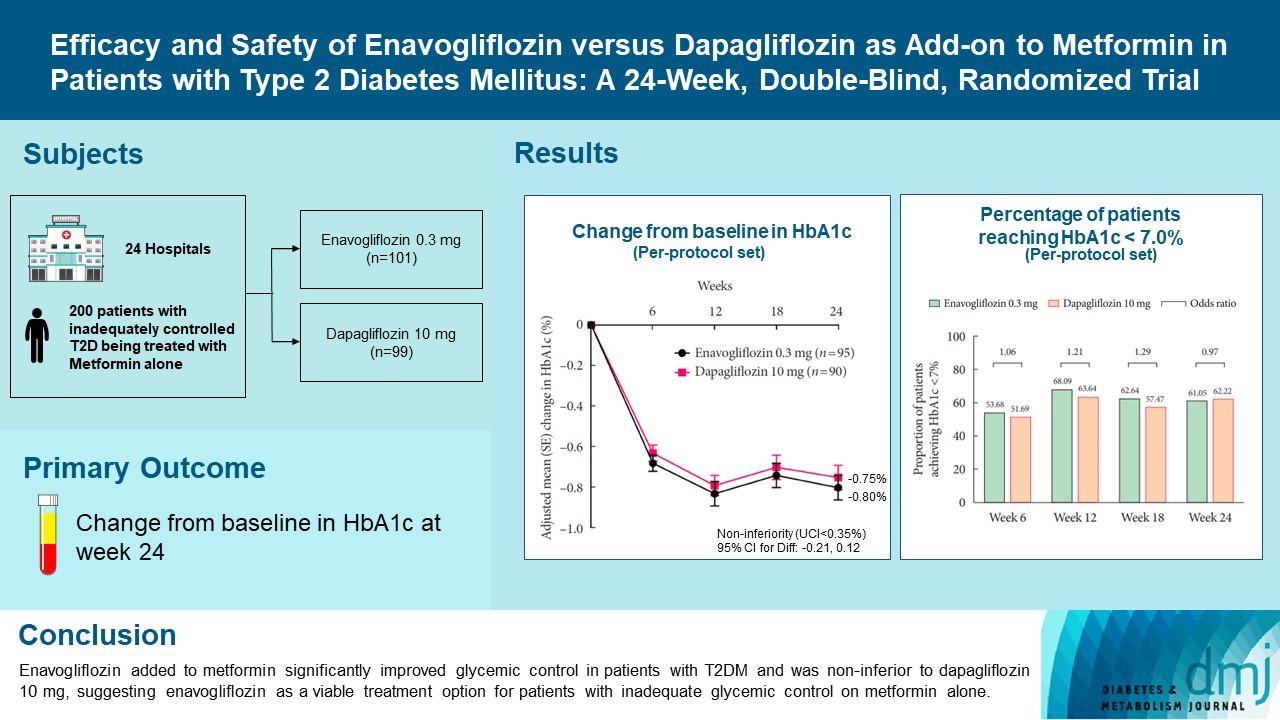
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Kyung Ah Han, Yong Hyun Kim, Doo Man Kim, Byung Wan Lee, Suk Chon, Tae Seo Sohn, In Kyung Jeong, Eun-Gyoung Hong, Jang Won Son, Jae Jin Nah, Hwa Rang Song, Seong In Cho, Seung-Ah Cho, Kun Ho Yoon
- Diabetes Metab J. 2023;47(6):796-807. Published online February 9, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0315
- 40,037 View
- 572 Download
- 4 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Enavogliflozin is a novel sodium-glucose cotransporter-2 inhibitor currently under clinical development. This study evaluated the efficacy and safety of enavogliflozin as an add-on to metformin in Korean patients with type 2 diabetes mellitus (T2DM) against dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, 200 patients were randomized to receive enavogliflozin 0.3 mg/day (n=101) or dapagliflozin 10 mg/day (n=99) in addition to ongoing metformin therapy for 24 weeks. The primary objective of the study was to prove the non-inferiority of enavogliflozin to dapagliflozin in glycosylated hemoglobin (HbA1c) change at week 24 (non-inferiority margin of 0.35%) (Clinical trial registration number: NCT04634500).
Results
Adjusted mean change of HbA1c at week 24 was –0.80% with enavogliflozin and –0.75% with dapagliflozin (difference, –0.04%; 95% confidence interval, –0.21% to 0.12%). Percentages of patients achieving HbA1c <7.0% were 61% and 62%, respectively. Adjusted mean change of fasting plasma glucose at week 24 was –32.53 and –29.14 mg/dL. An increase in urine glucose-creatinine ratio (60.48 vs. 44.94, P<0.0001) and decrease in homeostasis model assessment of insulin resistance (–1.85 vs. –1.31, P=0.0041) were significantly greater with enavogliflozin than dapagliflozin at week 24. Beneficial effects of enavogliflozin on body weight (–3.77 kg vs. –3.58 kg) and blood pressure (systolic/diastolic, –5.93/–5.41 mm Hg vs. –6.57/–4.26 mm Hg) were comparable with those of dapagliflozin, and both drugs were safe and well-tolerated.
Conclusion
Enavogliflozin added to metformin significantly improved glycemic control in patients with T2DM and was non-inferior to dapagliflozin 10 mg, suggesting enavogliflozin as a viable treatment option for patients with inadequate glycemic control on metformin alone. -
Citations
Citations to this article as recorded by- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Young Sang Lyu, Sangmo Hong, Si Eun Lee, Bo Young Cho, Cheol-Young Park
Cardiovascular Diabetology.2024;[Epub] CrossRef - A 52‐week efficacy and safety study of enavogliflozin versus dapagliflozin as an add‐on to metformin in patients with type 2 diabetes mellitus: ENHANCE‐M extension study
Tae Seo Sohn, Kyung‐Ah Han, Yonghyun Kim, Byung‐Wan Lee, Suk Chon, In‐Kyung Jeong, Eun‐Gyoung Hong, Jang Won Son, JaeJin Na, Jae Min Cho, Seong In Cho, Wan Huh, Kun‐Ho Yoon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - The effect of renal function on the pharmacokinetics and pharmacodynamics of enavogliflozin, a potent and selective sodium‐glucose cotransporter‐2 inhibitor, in type 2 diabetes
Sae Im Jeong, Mu Seong Ban, Jun‐Gi Hwang, Min‐Kyu Park, Soo Lim, Sejoong Kim, Soon Kil Kwon, Yoonjin Kim, Jae Min Cho, Jae Jin Na, Wan Huh, Jae‐Yong Chung
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: A systematic review and meta-analysis
Deep Dutta, B.G. Harish, Beatrice Anne, Lakshmi Nagendra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102816. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - Prospects of using sodium-glucose co-transporter-2 (SGLT-2) inhibitors in patients with metabolic-associated fatty liver disease (MAFLD)
Iryna Kostitska, Nadia Protas, Liliia Petrovska
Diabetes Obesity Metabolic Syndrome.2023; (5): 8. CrossRef - Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
Sang Youl Rhee
Diabetes & Metabolism Journal.2023; 47(6): 769. CrossRef
- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
- Drug Regimen
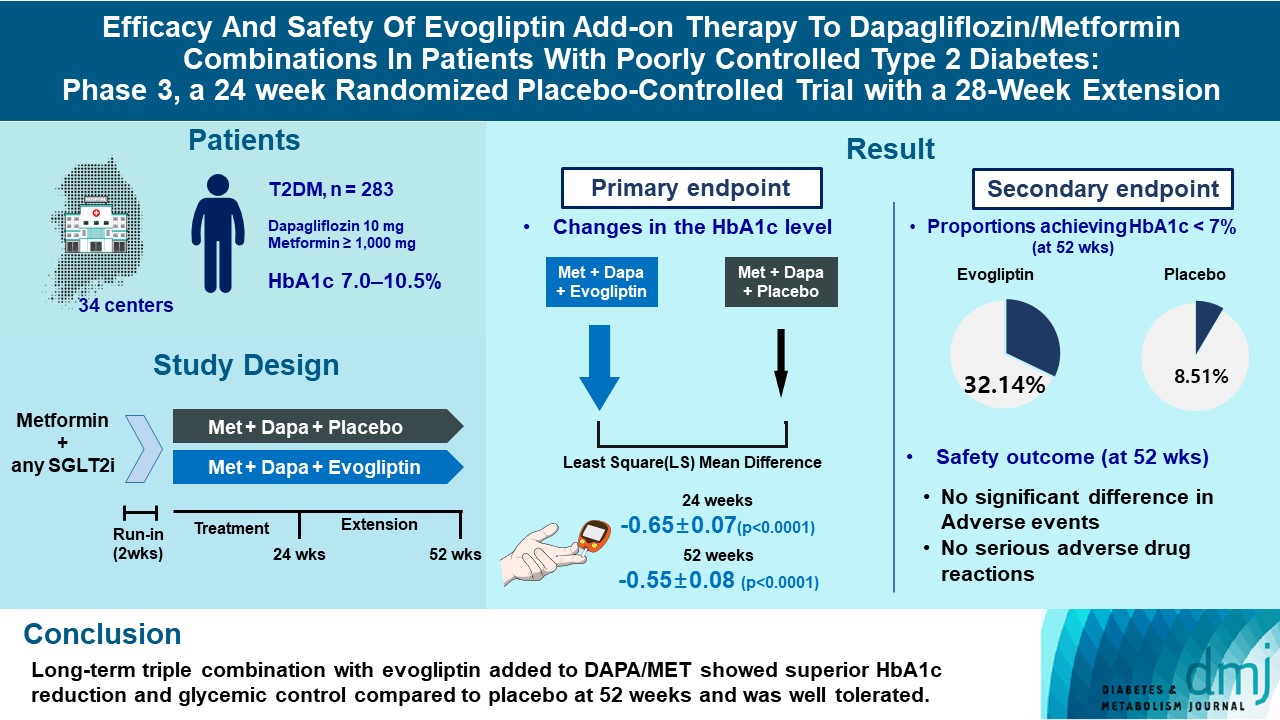
- Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extension
- Jun Sung Moon, Il Rae Park, Hae Jin Kim, Choon Hee Chung, Kyu Chang Won, Kyung Ah Han, Cheol-Young Park, Jong Chul Won, Dong Jun Kim, Gwan Pyo Koh, Eun Sook Kim, Jae Myung Yu, Eun-Gyoung Hong, Chang Beom Lee, Kun-Ho Yoon
- Diabetes Metab J. 2023;47(6):808-817. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0387
- 2,569 View
- 281 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigates the long-term efficacy and safety of evogliptin add-on therapy in patients with inadequately controlled type 2 diabetes mellitus (T2DM) previously received dapagliflozin and metformin (DAPA/MET) combination.
Methods
In this multicenter randomized placebo-controlled phase 3 trial, patients with glycosylated hemoglobin (HbA1c) levels 7.0% to 10.5% (n=283) previously used DAPA 10 mg plus MET (≥1,000 mg) were randomly assigned to the evogliptin 5 mg once daily or placebo group (1:1). The primary endpoint was the difference in the HbA1c level from baseline at week 24, and exploratory endpoints included the efficacy and safety of evogliptin over 52 weeks (trial registration: ClinicalTrials.gov NCT04170998).
Results
Evogliptin add-on to DAPA/MET therapy was superior in HbA1c reduction compared to placebo at weeks 24 and 52 (least square [LS] mean difference, –0.65% and –0.55%; 95% confidence interval [CI], –0.79 to –0.51 and –0.71 to –0.39; P<0.0001). The proportion of patients achieving HbA1c <7% was higher in the triple combination group at week 52 (32.14% vs. 8.51% in placebo; odds ratio, 5.62; P<0.0001). Evogliptin significantly reduced the fasting glucose levels and mean daily glucose levels with improvement in homeostatic model assessment of β-cell function (LS mean difference, 9.04; 95% CI, 1.86 to 16.21; P=0.0138). Adverse events were similar between the groups, and no serious adverse drug reactions were reported in the evogliptin group.
Conclusion
Long-term triple combination with evogliptin added to DAPA/MET showed superior HbA1c reduction and glycemic control compared to placebo at 52 weeks and was well tolerated.
- Drug Regimen
- The Efficacy and Safety of Moderate-Intensity Rosuvastatin with Ezetimibe versus High-Intensity Rosuvastatin in High Atherosclerotic Cardiovascular Disease Risk Patients with Type 2 Diabetes Mellitus: A Randomized, Multicenter, Open, Parallel, Phase 4 Study
- Jun Sung Moon, Il Rae Park, Sang Soo Kim, Hye Soon Kim, Nam Hoon Kim, Sin Gon Kim, Seung Hyun Ko, Ji Hyun Lee, Inkyu Lee, Bo Kyeong Lee, Kyu Chang Won
- Diabetes Metab J. 2023;47(6):818-825. Published online November 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0171

- 2,364 View
- 242 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate the efficacy and safety of moderate-intensity rosuvastatin/ezetimibe combination compared to highintensity rosuvastatin in high atherosclerotic cardiovascular disease (ASCVD) risk patients with type 2 diabetes mellitus (T2DM).
Methods
This study was a randomized, multicenter, open, parallel phase 4 study, and enrolled T2DM subjects with an estimated 10-year ASCVD risk ≥7.5%. The primary endpoint was the low-density lipoprotein cholesterol (LDL-C) change rate after 24-week rosuvastatin 10 mg/ezetimibe 10 mg treatment was non-inferior to that of rosuvastatin 20 mg. The achievement proportion of 10-year ASCVD risk <7.5% or comprehensive lipid target (LDL-C <70 mg/dL, non-high-density lipoprotein cholesterol <100 mg/dL, and apolipoprotein B <80 mg/dL) without discontinuation, and several metabolic parameters were explored as secondary endpoints.
Results
A hundred and six participants were assigned to each group. Both groups showed significant reduction in % change of LDL-C from baseline at week 24 (–63.90±6.89 vs. –55.44±6.85, combination vs. monotherapy, p=0.0378; respectively), but the combination treatment was superior to high-intensity monotherapy in LDL-C change (%) from baseline (least square [LS] mean difference, –8.47; 95% confidence interval, –16.44 to –0.49; p=0.0378). The combination treatment showed a higher proportion of achieved comprehensive lipid targets rather than monotherapy (85.36% vs. 62.22% in monotherapy, p=0.015). The ezetimibe combination significantly improved homeostasis model assessment of β-cell function even without A1c changes (LS mean difference, 17.13; p=0.0185).
Conclusion
In high ASCVD risk patients with T2DM, the combination of moderate-intensity rosuvastatin and ezetimibe was not only non-inferior but also superior to improving dyslipidemia with additional benefits compared to high-intensity rosuvastatin monotherapy.
- Technology/Device
- Clinical and Lifestyle Determinants of Continuous Glucose Monitoring Metrics in Insulin-Treated Patients with Type 2 Diabetes Mellitus
- Da Young Lee, Namho Kim, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Jihee Kim, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Sung-Min Park, Nan Hee Kim
- Diabetes Metab J. 2023;47(6):826-836. Published online August 24, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0273

- 1,787 View
- 191 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There was limited evidence to evaluate the association between lifestyle habits and continuous glucose monitoring (CGM) metrics. Thus, we aimed to depict the behavioral and metabolic determinants of CGM metrics in insulin-treated patients with type 2 diabetes mellitus (T2DM).
Methods
This is a prospective observational study. We analyzed data from 122 insulin-treated patients with T2DM. Participants wore Dexcom G6 and Fitbit, and diet information was identified for 10 days. Multivariate-adjusted logistic regression analysis was performed for the simultaneous achievement of CGM-based targets, defined by the percentage of time in terms of hyper, hypoglycemia and glycemic variability (GV). Intake of macronutrients and fiber, step counts, sleep, postprandial C-peptide-to-glucose ratio (PCGR), information about glucose lowering medications and metabolic factors were added to the analyses. Additionally, we evaluated the impact of the distribution of energy and macronutrient during a day, and snack consumption on CGM metrics.
Results
Logistic regression analysis revealed that female, participants with high PCGR, low glycosylated hemoglobin (HbA1c) and daytime step count had a higher probability of achieving all targets based on CGM (odds ratios [95% confidence intervals] which were 0.24 [0.09 to 0.65], 1.34 [1.03 to 1.25], 0.95 [0.9 to 0.99], and 1.15 [1.03 to 1.29], respectively). And participants who ate snacks showed a shorter period of hyperglycemia and less GV compared to those without.
Conclusion
We confirmed that residual insulin secretion, daytime step count, HbA1c, and women were the most relevant determinants of adequate glycemic control in insulin-treated patients with T2DM. In addition, individuals with snack consumption were exposed to lower times of hyperglycemia and GV. -
Citations
Citations to this article as recorded by- Explanatory variables of objectively measured 24-h movement behaviors in people with prediabetes and type 2 diabetes: A systematic review
Lotte Bogaert, Iris Willems, Patrick Calders, Eveline Dirinck, Manon Kinaupenne, Marga Decraene, Bruno Lapauw, Boyd Strumane, Margot Van Daele, Vera Verbestel, Marieke De Craemer
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2024; 18(4): 102995. CrossRef
- Explanatory variables of objectively measured 24-h movement behaviors in people with prediabetes and type 2 diabetes: A systematic review
- Cardiovascular Risk/Epidemiology
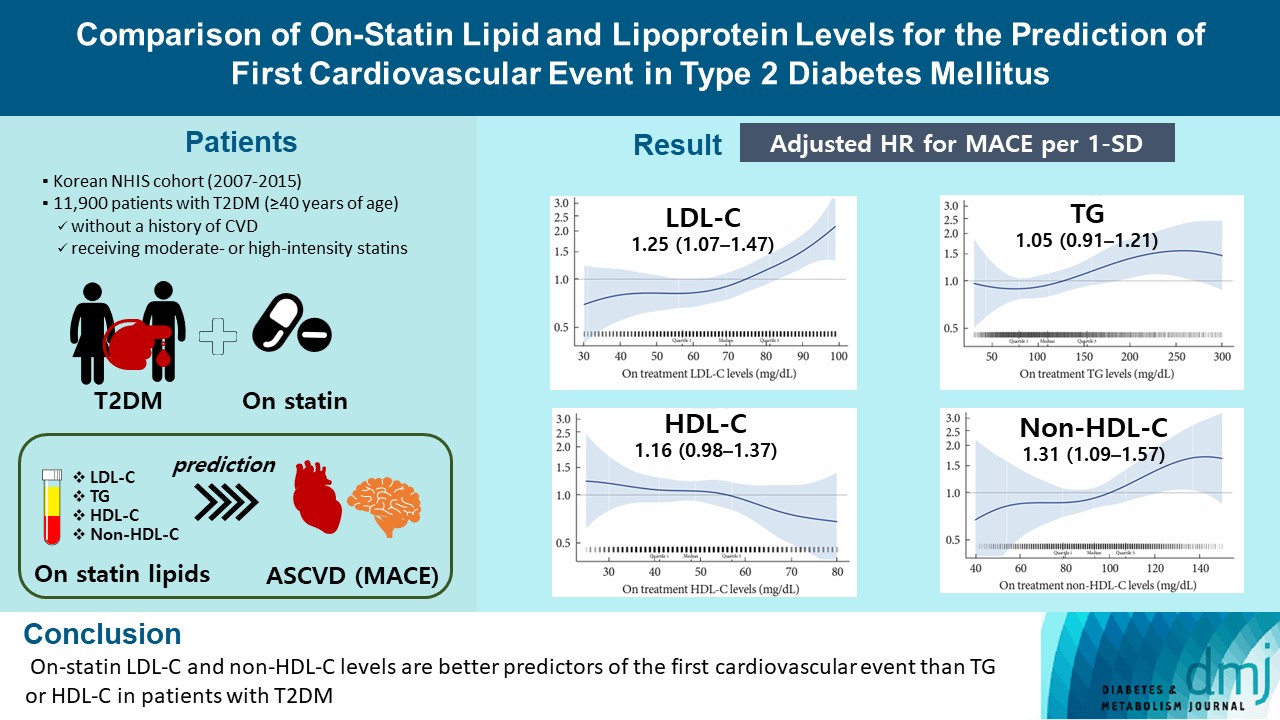
- Comparison of on-Statin Lipid and Lipoprotein Levels for the Prediction of First Cardiovascular Event in Type 2 Diabetes Mellitus
- Ji Yoon Kim, Jimi Choi, Sin Gon Kim, Nam Hoon Kim
- Diabetes Metab J. 2023;47(6):837-845. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0217
- 1,443 View
- 173 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
A substantial cardiovascular disease risk remains even after optimal statin therapy. Comparative predictiveness of major lipid and lipoprotein parameters for cardiovascular events in patients with type 2 diabetes mellitus (T2DM) who are treated with statins is not well documented.
Methods
From the Korean Nationwide Cohort, 11,900 patients with T2DM (≥40 years of age) without a history of cardiovascular disease and receiving moderate- or high-intensity statins were included. The primary outcome was the first occurrence of major adverse cardiovascular events (MACE) including ischemic heart disease, ischemic stroke, and cardiovascular death. The risk of MACE was estimated according to on-statin levels of low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), highdensity lipoprotein cholesterol (HDL-C), and non-HDL-C.
Results
MACE occurred in 712 patients during a median follow-up period of 37.9 months (interquartile range, 21.7 to 54.9). Among patients achieving LDL-C levels less than 100 mg/dL, the hazard ratios for MACE per 1-standard deviation change in ontreatment values were 1.25 (95% confidence interval [CI], 1.07 to 1.47) for LDL-C, 1.31 (95% CI, 1.09 to 1.57) for non-HDL-C, 1.05 (95% CI, 0.91 to 1.21) for TG, and 1.16 (95% CI, 0.98 to 1.37) for HDL-C, after adjusting for potential confounders and lipid parameters mutually. The predictive ability of on-statin LDL-C and non-HDL-C for MACE was prominent in patients at high cardiovascular risk or those with LDL-C ≥70 mg/dL.
Conclusion
On-statin LDL-C and non-HDL-C levels are better predictors of the first cardiovascular event than TG or HDL-C in patients with T2DM.
- Metabolic Risk/Epidemiology
- Differential Impact of Obesity on the Risk of Diabetes Development in Two Age Groups: Analysis from the National Health Screening Program
- Tae Kyung Yoo, Kyung-Do Han, Yang-Hyun Kim, Ga Eun Nam, Sang Hyun Park, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2023;47(6):846-858. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0242

- 1,183 View
- 141 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The effect of obesity on the development of type 2 diabetes mellitus (DM) in different age groups remains unclear. We assessed the impact of obesity on the development of DM for two age groups (40-year-old, middle age; 66-year-old, older adults) in the Korean population.
Methods
We analyzed Korean National Health Insurance Service data of 4,145,321 Korean adults with 40- and 66-year-old age without DM, between 2009 and 2014. Participants were followed up until 2017 or until the diagnosis of DM. We assessed the risk of DM based on the body mass index and waist circumference of the participants. Multiple confounding factors were adjusted.
Results
The median follow-up duration was 5.6 years. The association of general and abdominal obesity with the risk of DM development was stronger in the 40-year-old group (general obesity: hazard ratio [HR], 3.566, 95% confidence interval [CI], 3.512 to 3.622; abdominal obesity: HR, 3.231; 95% CI, 3.184 to 3.278) than in the 66-year-old group (general obesity: HR, 1.739; 95% CI, 1.719 to 1.759; abdominal obesity: HR, 1.799; 95% CI, 1.778 to 1.820). In the 66-year-old group, abdominal obesity had a stronger association with the development of DM as compared to general obesity. In the 40-year-old group, general obesity had a stronger association with the risk of DM development than abdominal obesity.
Conclusion
The influence of general and abdominal obesity on the development of DM differed according to age. In older adults, abdominal obesity had a stronger association with DM development than general obesity.
- Metabolic Risk/Epidemiology
- Prediabetes Progression and Regression on Objectively- Measured Physical Function: A Prospective Cohort Study
- Shanhu Qiu, Yiming Zhu, Bo Xie, Wenji Chen, Duolao Wang, Xue Cai, Zilin Sun, Tongzhi Wu
- Diabetes Metab J. 2023;47(6):859-868. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0377

- 1,288 View
- 122 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Prediabetes leads to declines in physical function in older adults, but the impact of prediabetes progression or regression on physical function is unknown. This study assessed this longitudinal association, with physical function objectivelymeasured by grip strength, walking speed, and standing balance, based on the Health and Retirement Study enrolling United States adults aged >50 years.
Methods
Participants with prediabetes were followed-up for 4-year to ascertain prediabetes status alteration (maintained, regressed, or progressed), and another 4-year to assess their impacts on physical function. Weak grip strength was defined as <26 kg for men and <16 kg for women, slow walking speed was as <0.8 m/sec, and poor standing balance was as an uncompleted fulltandem standing testing. Logistic and linear regression analyses were performed.
Results
Of the included 1,511 participants with prediabetes, 700 maintained as prediabetes, 306 progressed to diabetes, and 505 regressed to normoglycemia over 4 years. Grip strength and walking speed were declined from baseline during the 4-year followup, regardless of prediabetes status alteration. Compared with prediabetes maintenance, prediabetes progression increased the odds of developing weak grip strength by 89% (95% confidence interval [CI], 0.04 to 2.44) and exhibited larger declines in grip strength by 0.85 kg (95% CI, –1.65 to –0.04). However, prediabetes progression was not related to impairments in walking speed or standing balance. Prediabetes regression also did not affect any measures of physical function.
Conclusion
Prediabetes progression accelerates grip strength decline in aging population, while prediabetes regression may not prevent physical function decline due to aging.
- Complications
- The Risk of Shoulder Adhesive Capsulitis in Individuals with Prediabetes and Type 2 Diabetes Mellitus: A Longitudinal Nationwide Population-Based Study
- Jong-Ho Kim, Bong-Seoung Kim, Kyung-do Han, Hyuk-Sang Kwon
- Diabetes Metab J. 2023;47(6):869-878. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0275

- 1,524 View
- 145 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study aimed to investigate the association between type 2 diabetes mellitus (T2DM) and shoulder adhesive capsulitis (AC) using a large-scale, nationwide, population-based cohort in the Republic of Korea.
Methods
A total of 3,471,745 subjects aged over 20 years who underwent a National Health Insurance Service medical checkup between 2009 and 2010 were included in this study, and followed from the date of their medical checkup to the end of 2018. Subjects were classified into the following four groups based on the presence of dysglycemia and history of diabetes medication: normal, prediabetes, newly diagnosed T2DM (new-T2DM), and T2DM (claim history for antidiabetic medication). The endpoint was new-onset AC during follow-up. The incidence rates (IRs) in 1,000 person-years and hazard ratios (HRs) of AC for each group were analyzed using Cox proportional hazard regression models.
Results
The IRs of AC were 9.453 (normal), 11.912 (prediabetes), 14.933 (new-T2DM), and 24.3761 (T2DM). The adjusted HRs of AC in the prediabetes, new-T2DM, and T2DM groups were 1.084 (95% confidence interval [CI], 1.075 to 1.094), 1.312 (95% CI, 1.287 to 1.337), and 1.473 (95% CI, 1.452 to 1.494) compared to the normal group, respectively. This secular trend of the HRs of AC according to T2DM status was statistically significant (P<0.0001).
Conclusion
This large-scale, longitudinal, nationwide, population-based cohort study of 3,471,745 subjects confirmed that the risk of AC increases in prediabetic subjects and is associated with T2DM status.

 KDA
KDA


 First
First Prev
Prev





