
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
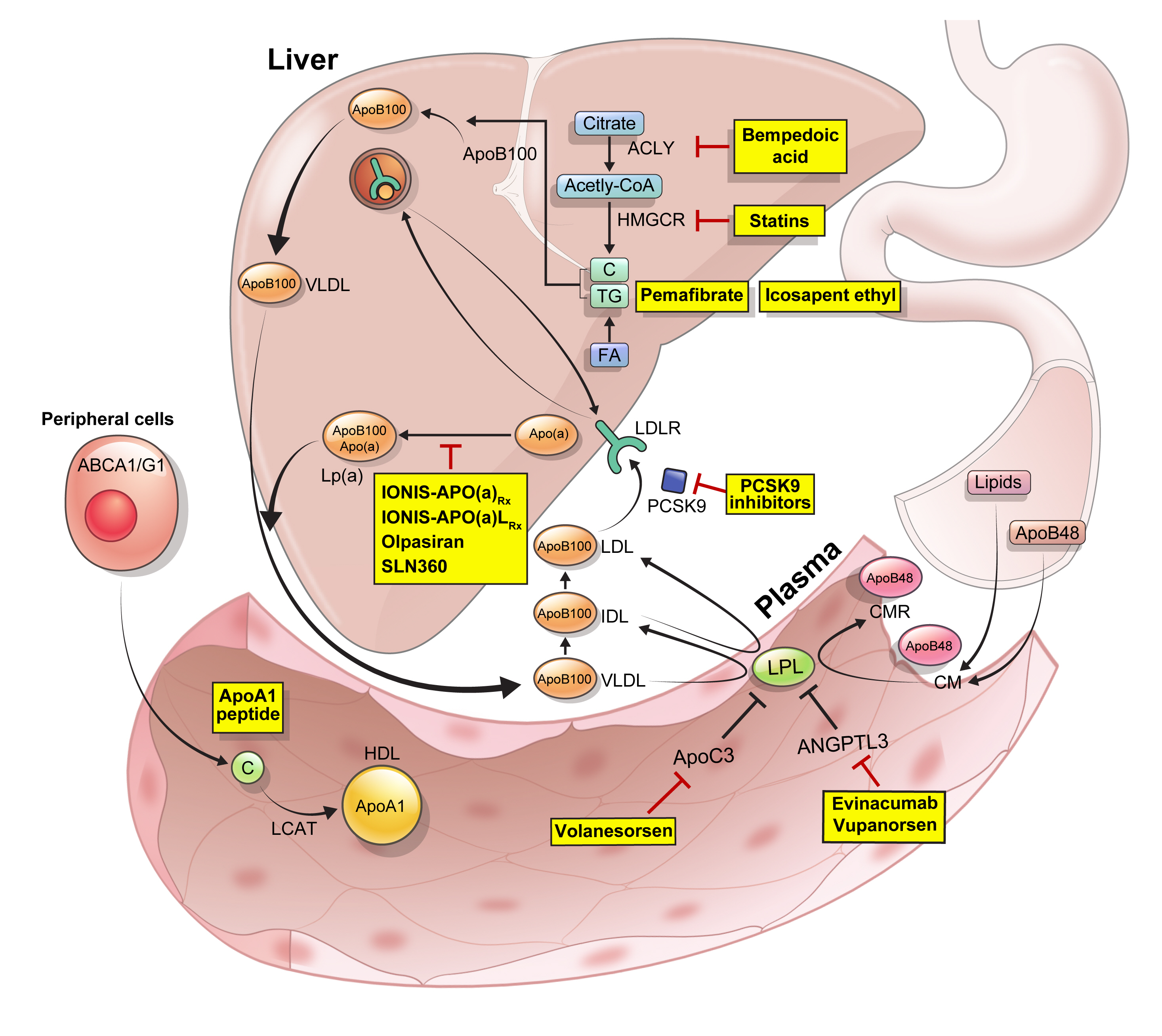
- New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
- Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
- Diabetes Metab J. 2022;46(4):517-532. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0198
- Correction in: Diabetes Metab J 2022;46(5):817
- 10,002 View
- 865 Download
- 25 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Statins are the cornerstone of the prevention and treatment of atherosclerotic cardiovascular disease (ASCVD). However, even under optimal statin therapy, a significant residual ASCVD risk remains. Therefore, there has been an unmet clinical need for novel lipid-lowering agents that can target low-density lipoprotein cholesterol (LDL-C) and other atherogenic particles. During the past decade, several drugs have been developed for the treatment of dyslipidemia. Inclisiran, a small interfering RNA that targets proprotein convertase subtilisin/kexin type 9 (PCSK9), shows comparable effects to that of PCSK9 monoclonal antibodies. Bempedoic acid, an ATP citrate lyase inhibitor, is a valuable treatment option for the patients with statin intolerance. Pemafibrate, the first selective peroxisome proliferator-activated receptor alpha modulator, showed a favorable benefit-risk balance in phase 2 trial, but the large clinical phase 3 trial (PROMINENT) was recently stopped for futility based on a late interim analysis. High dose icosapent ethyl, a modified eicosapentaenoic acid preparation, shows cardiovascular benefits. Evinacumab, an angiopoietin-like 3 (ANGPTL3) monoclonal antibody, reduces plasma LDL-C levels in patients with refractory hypercholesterolemia. Novel antisense oligonucleotides targeting apolipoprotein C3 (apoC3), ANGPTL3, and lipoprotein(a) have significantly attenuated the levels of their target molecules with beneficial effects on associated dyslipidemias. Apolipoprotein A1 (apoA1) is considered as a potential treatment to exploit the athero-protective effects of high-density lipoprotein cholesterol (HDL-C), but solid clinical evidence is necessary. In this review, we discuss the mode of action and clinical outcomes of these novel lipid-lowering agents beyond statins.
-
Citations
Citations to this article as recorded by- The role of adherence in patients with chronic diseases
Michel Burnier
European Journal of Internal Medicine.2024; 119: 1. CrossRef - Bempedoic acid: new evidence and recommendations on use
Kristina Paponja, Ivan Pećin, Željko Reiner, Maciej Banach
Current Opinion in Lipidology.2024; 35(1): 41. CrossRef - Genetic insights into repurposing statins for hyperthyroidism prevention: a drug-target Mendelian randomization study
Anqi Huang, Xinyi Wu, Jiaqi Lin, Chiju Wei, Wencan Xu
Frontiers in Endocrinology.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Neutrophil Extracellular Traps (NETs) and Atherosclerosis: Does Hypolipidemic Treatment Have an Effect?
Petros Adamidis, Despoina Pantazi, Iraklis Moschonas, Evangelos Liberopoulos, Alexandros Tselepis
Journal of Cardiovascular Development and Disease.2024; 11(3): 72. CrossRef - Modulating effects of crocin on lipids and lipoproteins: Mechanisms and potential benefits
Habib Yaribeygi, Mina Maleki, Farin Rashid-Farrokhi, Payman Raise Abdullahi, Mohammad Amin Hemmati, Tannaz Jamialahmadi, Amirhossein Sahebkar
Heliyon.2024; 10(7): e28837. CrossRef - Assessing the Benefits of Lifestyle Influences on Cardiovascu-lar Health After Acute Coronary Syndrome
Marius Rus, Claudia Elena Stanis, Paula Marian, Lilliana Oana Pobirci, Loredana Ioana Banszki, Veronica Huplea, Gheorghe Adrian Osiceanu, Bianca-Maria Pop, Gabriela Dogaru, Felicia Liana Andronie-Cioara
Balneo and PRM Research Journal.2024; 15(Vol.15, no): 660. CrossRef - Liver cancer cells as the model for developing liver-targeted RNAi therapeutics
Beibei Hou, Linhui Qin, Linfeng Huang
Biochemical and Biophysical Research Communications.2023; 644: 85. CrossRef - Insights into Causal Cardiovascular Risk Factors from Mendelian Randomization
C. M. Schooling, J. V. Zhao
Current Cardiology Reports.2023; 25(2): 67. CrossRef - Secoisolariciresinol diglucoside and anethole ameliorate lipid abnormalities, oxidative injury, hypercholesterolemia, heart, and liver conditions
Sana Noreen, Habib‐ur Rehman, Tabussam Tufail, Huma Badar Ul Ain, Chinaza Godswill Awuchi
Food Science & Nutrition.2023; 11(6): 2620. CrossRef - Colesterol remanente, riesgo vascular y prevención de la arteriosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán, Agustín Blanco, Mariano Blasco, José Luís Díaz Díaz, Ángel Díaz Rodríguez, Alipio Mangas, Vicente Pascual, Juan Pedro Botet, Pablo Pérez Martínez
Clínica e Investigación en Arteriosclerosis.2023; 35(4): 206. CrossRef - Evolving Management of Low‐Density Lipoprotein Cholesterol: A Personalized Approach to Preventing Atherosclerotic Cardiovascular Disease Across the Risk Continuum
Michael J. Wilkinson, Norman E. Lepor, Erin D. Michos
Journal of the American Heart Association.2023;[Epub] CrossRef - The cell origins of foam cell and lipid metabolism regulated by mechanical stress in atherosclerosis
Zhi Ouyang, Jian Zhong, Junyi Shen, Ye Zeng
Frontiers in Physiology.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Remnant cholesterol, vascular risk, and prevention of atherosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán
Clínica e Investigación en Arteriosclerosis (English Edition).2023; 35(4): 206. CrossRef - Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications
Marios Spanakis, Danny Alon-Ellenbogen, Petros Ioannou, Nikolaos Spernovasilis
Pharmacy.2023; 11(4): 130. CrossRef - Advances in Treatment of Dyslipidemia
Jill Dybiec, Wiktoria Baran, Bartłomiej Dąbek, Piotr Fularski, Ewelina Młynarska, Ewa Radzioch, Jacek Rysz, Beata Franczyk
International Journal of Molecular Sciences.2023; 24(17): 13288. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Preparation, characterization and in vivo pharmacokinetic study of ginsenoside Rb1-PLGA nanoparticles
Lixin Du, Huiling Lu, Yifei Xiao, Zhihua Guo, Ya Li
Scientific Reports.2023;[Epub] CrossRef - Dysregulation of Cholesterol Homeostasis in Ovarian Cancer
Zahraa Qusairy, Anne Gangloff, Shuk On Annie Leung
Current Oncology.2023; 30(9): 8386. CrossRef - Riesgo residual. Conclusiones
Ángel Cequier, José Luis Zamorano
Revista Española de Cardiología Suplementos.2023; 23: 25. CrossRef - Causal effects of circulating lipids and lipid-lowering drugs on the risk of urinary stones: a Mendelian randomization study
Zilong Tan, Jing Hong, Aochuan Sun, Mengdi Ding, Jianwu Shen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Bibliometric analysis of residual cardiovascular risk: trends and frontiers
Lin Wang, Sutong Wang, Chaoyuan Song, Yiding Yu, Yuehua Jiang, Yongcheng Wang, Xiao Li
Journal of Health, Population and Nutrition.2023;[Epub] CrossRef - Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review
Kenneth Francis Rodrigues, Wilson Thau Lym Yong, Md. Safiul Alam Bhuiyan, Shafiquzzaman Siddiquee, Muhammad Dawood Shah, Balu Alagar Venmathi Maran
Biology.2022; 11(9): 1308. CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef
- The role of adherence in patients with chronic diseases
- Basic Research
- Peroxisomal Fitness: A Potential Protective Mechanism of Fenofibrate against High Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice
- Songling Jiang, Md Jamal Uddin, Xiaoying Yu, Lingjuan Piao, Debra Dorotea, Goo Taeg Oh, Hunjoo Ha
- Diabetes Metab J. 2022;46(6):829-842. Published online June 24, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0274
- 4,948 View
- 292 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Non-alcoholic fatty liver disease (NAFLD) has been increasing in association with the epidemic of obesity and diabetes. Peroxisomes are single membrane-enclosed organelles that play a role in the metabolism of lipid and reactive oxygen species. The present study examined the role of peroxisomes in high-fat diet (HFD)-induced NAFLD using fenofibrate, a peroxisome proliferator-activated receptor α (PPARα) agonist.
Methods
Eight-week-old male C57BL/6J mice were fed either a normal diet or HFD for 12 weeks, and fenofibrate (50 mg/kg/day) was orally administered along with the initiation of HFD.
Results
HFD-induced liver injury as measured by increased alanine aminotransferase, inflammation, oxidative stress, and lipid accumulation was effectively prevented by fenofibrate. Fenofibrate significantly increased the expression of peroxisomal genes and proteins involved in peroxisomal biogenesis and function. HFD-induced attenuation of peroxisomal fatty acid oxidation was also significantly restored by fenofibrate, demonstrating the functional significance of peroxisomal fatty acid oxidation. In Ppara deficient mice, fenofibrate failed to maintain peroxisomal biogenesis and function in HFD-induced liver injury.
Conclusion
The present data highlight the importance of PPARα-mediated peroxisomal fitness in the protective effect of fenofibrate against NAFLD. -
Citations
Citations to this article as recorded by- Pharmacological potential of ginseng and ginsenosides in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
Young-Su Yi
Journal of Ginseng Research.2024; 48(2): 122. CrossRef - Fenofibrate alleviates NAFLD by enhancing the PPARα/PGC-1α signaling pathway coupling mitochondrial function
Xuemei Wang, Jieying Wang, Cao Ying, Yuan Xing, Xuan Su, Ke Men
BMC Pharmacology and Toxicology.2024;[Epub] CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD
Manuela Vitulo, Elisa Gnodi, Giulia Rosini, Raffaella Meneveri, Roberto Giovannoni, Donatella Barisani
International Journal of Molecular Sciences.2023; 24(16): 12748. CrossRef - PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
Tiantian Liu, Xinlu Chen, Ziqi Wei, Xue Han, Yujia Liu, Zhengliang Ma, Tianjiao Xia, Xiaoping Gu
Translational Neuroscience.2023;[Epub] CrossRef - Fenofibrate enhances lipid deposition via modulating PPARγ, SREBP-1c, and gut microbiota in ob/ob mice fed a high-fat diet
Ying Zhang, Xiu-Bin Jia, Yun-Chao Liu, Wen-Qian Yu, Yan-Hong Si, Shou-Dong Guo
Frontiers in Nutrition.2022;[Epub] CrossRef
- Pharmacological potential of ginseng and ginsenosides in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
- Drug/Regimen
- Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction
- Nam Hoon Kim, Sin Gon Kim
- Diabetes Metab J. 2020;44(2):213-221. Published online April 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0001
- 7,668 View
- 311 Download
- 41 Web of Science
- 42 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Fibrates, peroxisome proliferator-activated receptor-α agonists, are potent lipid-modifying drugs. Their main effects are reduction of triglycerides and increase in high-density lipoprotein levels. Several randomized controlled trials have not demonstrated their benefits on cardiovascular risk reduction, especially as an “add on” to statin therapy. However, subsequent analyses by major clinical trials, meta-analyses, and real-world evidence have proposed their potential in specific patient populations with atherogenic dyslipidemia and metabolic syndrome. Here, we have reviewed and discussed the accumulated data on fibrates to understand their current status in cardiovascular risk management.
-
Citations
Citations to this article as recorded by- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ji Yoon Kim, Jimi Choi, Sin Gon Kim
European Heart Journal - Cardiovascular Pharmacotherapy.2024; 10(2): 118. CrossRef - Role of PPARα in inflammatory response of C2C12 myotubes
Yuki Shimizu, Keiko Hamada, Tingting Guo, Chie Hasegawa, Yusuke Kuga, Katsushi Takeda, Takashi Yagi, Hiroyuki Koyama, Hiroshi Takagi, Daisuke Aotani, Hiromi Kataoka, Tomohiro Tanaka
Biochemical and Biophysical Research Communications.2024; 694: 149413. CrossRef - Obicetrapib: Reversing the Tide of CETP Inhibitor Disappointments
John J. P. Kastelein, Andrew Hsieh, Mary R. Dicklin, Marc Ditmarsch, Michael H. Davidson
Current Atherosclerosis Reports.2024; 26(2): 35. CrossRef - Metabolic Flexibility of the Heart: The Role of Fatty Acid Metabolism in Health, Heart Failure, and Cardiometabolic Diseases
Virginia Actis Dato, Stephan Lange, Yoshitake Cho
International Journal of Molecular Sciences.2024; 25(2): 1211. CrossRef - ApoB100 and Atherosclerosis: What’s New in the 21st Century?
Dimitris Kounatidis, Natalia G. Vallianou, Aikaterini Poulaki, Angelos Evangelopoulos, Fotis Panagopoulos, Theodora Stratigou, Eleni Geladari, Irene Karampela, Maria Dalamaga
Metabolites.2024; 14(2): 123. CrossRef - Follistatin-like 1 (FSTL1) levels as potential early biomarker of cardiovascular disease in a Mexican population
N. Ponce-Ruíz, J. F. Herrera-Moreno, A. E. Rojas-García, B. S. Barrón-Vivanco, C. A. González-Arias, Y. Y. Bernal-Hernández, L. Ortega-Cervantes, J. Ponce-Gallegos, J. A. Hernández-Nolasco, I. M. Medina-Díaz
Heart and Vessels.2024;[Epub] CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Coenzyme Q10 in atherosclerosis
Minjun Liao, Xueke He, Yangyang Zhou, Weiqiang Peng, Xiao-Mei Zhao, Miao Jiang
European Journal of Pharmacology.2024; 970: 176481. CrossRef - Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study
Maria Rosaria Paravati, Anna Caterina Procopio, Maja Milanović, Giuseppe Guido Maria Scarlata, Nataša Milošević, Maja Ružić, Nataša Milić, Ludovico Abenavoli
Nutrients.2024; 16(8): 1226. CrossRef - Present and Future of Dyslipidaemia Treatment—A Review
Iveta Merćep, Andro Vujević, Dominik Strikić, Ivana Radman, Ivan Pećin, Željko Reiner
Journal of Clinical Medicine.2023; 12(18): 5839. CrossRef - VLDL receptor gene therapy for reducing atherogenic lipoproteins
Ronald M. Krauss, Jonathan T. Lu, Joseph J. Higgins, Cathryn M. Clary, Ray Tabibiazar
Molecular Metabolism.2023; 69: 101685. CrossRef - The emerging role of PPAR-alpha in breast cancer
Zhiwen Qian, Lingyan Chen, Jiayu Liu, Ying Jiang, Yan Zhang
Biomedicine & Pharmacotherapy.2023; 161: 114420. CrossRef - Molecular mechanisms and therapeutic perspectives of peroxisome proliferator‐activated receptor α agonists in cardiovascular health and disease
Yujie Pu, Chak Kwong Cheng, Hongsong Zhang, Jiang‐Yun Luo, Li Wang, Brian Tomlinson, Yu Huang
Medicinal Research Reviews.2023; 43(6): 2086. CrossRef - Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism
DuoYao Cao, Zakir Khan, Xiaomo Li, Suguru Saito, Ellen A Bernstein, Aaron R Victor, Faizan Ahmed, Aoi O Hoshi, Luciana C Veiras, Tomohiro Shibata, Mingtian Che, Lei Cai, Michifumi Yamashita, Ryan E Temel, Jorge F Giani, Daniel J Luthringer, Ajit S Divakar
Cardiovascular Research.2023; 119(9): 1825. CrossRef - Rapid flow synthesis of fenofibrate via scalable flash chemistry with in-line Li recovery
Sanket A. Kawale, Dong-Chang Kang, Gwang-Noh Ahn, Amirreza Mottafegh, Ji-Ho Kang, Gi-Su Na, Dong-Pyo Kim
Chemical Engineering Journal.2023; 477: 147033. CrossRef - Effectiveness and Safety of Fenofibrate in Routine Treatment of Patients with Hypertriglyceridemia and Metabolic Syndrome
Marat V. Ezhov, Gregory P. Arutyunov
Diseases.2023; 11(4): 140. CrossRef - Development of New Genome Editing Tools for the Treatment of Hyperlipidemia
Giulio Preta
Cells.2023; 12(20): 2466. CrossRef - Exploring the hypolipidemic effects of bergenin from Saxifraga melanocentra Franch: mechanistic insights and potential for hyperlipidemia treatment
Li Zhang, Yingying Tong, Yan Fang, Jinjin Pei, Qilan Wang, Gang Li
Lipids in Health and Disease.2023;[Epub] CrossRef - Obesity and Dyslipidemia
Barbora Nussbaumerova, Hana Rosolova
Current Atherosclerosis Reports.2023; 25(12): 947. CrossRef - Bibliometric analysis of residual cardiovascular risk: trends and frontiers
Lin Wang, Sutong Wang, Chaoyuan Song, Yiding Yu, Yuehua Jiang, Yongcheng Wang, Xiao Li
Journal of Health, Population and Nutrition.2023;[Epub] CrossRef - Hypertriglyceridemia in Apoa5–/– mice results from reduced amounts of lipoprotein lipase in the capillary lumen
Ye Yang, Anne P. Beigneux, Wenxin Song, Le Phuong Nguyen, Hyesoo Jung, Yiping Tu, Thomas A. Weston, Caitlyn M. Tran, Katherine Xie, Rachel G. Yu, Anh P. Tran, Kazuya Miyashita, Katsuyuki Nakajima, Masami Murakami, Yan Q. Chen, Eugene Y. Zhen, Joonyoung R.
Journal of Clinical Investigation.2023;[Epub] CrossRef - Blood-Derived Lipid and Metabolite Biomarkers in Cardiovascular Research from Clinical Studies: A Recent Update
Dipali Kale, Amol Fatangare, Prasad Phapale, Albert Sickmann
Cells.2023; 12(24): 2796. CrossRef - Effective, disease-modifying, clinical approaches to patients with mild-to-moderate hypertriglyceridaemia
Gary F Lewis, Robert A Hegele
The Lancet Diabetes & Endocrinology.2022; 10(2): 142. CrossRef - Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study
Thomas Metzner, Deborah R. Leitner, Karin Mellitzer, Andrea Beck, Harald Sourij, Tatjana Stojakovic, Gernot Reishofer, Winfried März, Ulf Landmesser, Hubert Scharnagl, Hermann Toplak, Günther Silbernagel
Biomedicines.2022; 10(1): 193. CrossRef - Is there a role of lipid-lowering therapies in the management of fatty liver disease?
Ismini Tzanaki, Aris P Agouridis, Michael S Kostapanos
World Journal of Hepatology.2022; 14(1): 119. CrossRef - Therapeutic Strategies and Chemoprevention of Atherosclerosis: What Do We Know and Where Do We Go?
Ana Clara Aprotosoaie, Alexandru-Dan Costache, Irina-Iuliana Costache
Pharmaceutics.2022; 14(4): 722. CrossRef - The Overlooked Transformation Mechanisms of VLCFAs: Peroxisomal β-Oxidation
Qinyue Lu, Weicheng Zong, Mingyixing Zhang, Zhi Chen, Zhangping Yang
Agriculture.2022; 12(7): 947. CrossRef - Current Trends of Big Data Research Using the Korean National Health Information Database
Mee Kyoung Kim, Kyungdo Han, Seung-Hwan Lee
Diabetes & Metabolism Journal.2022; 46(4): 552. CrossRef - New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
Diabetes & Metabolism Journal.2022; 46(4): 517. CrossRef - Novel Targets for a Combination of Mechanical Unloading with Pharmacotherapy in Advanced Heart Failure
Agata Jedrzejewska, Alicja Braczko, Ada Kawecka, Marcin Hellmann, Piotr Siondalski, Ewa Slominska, Barbara Kutryb-Zajac, Magdi H. Yacoub, Ryszard T. Smolenski
International Journal of Molecular Sciences.2022; 23(17): 9886. CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef - Fenofibrate add-on to statin treatment is associated with low all-cause death and cardiovascular disease in the general population with high triglyceride levels
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Metabolism.2022; 137: 155327. CrossRef - Alterations of HDL’s to piHDL’s Proteome in Patients with Chronic Inflammatory Diseases, and HDL-Targeted Therapies
Veronika Vyletelová, Mária Nováková, Ľudmila Pašková
Pharmaceuticals.2022; 15(10): 1278. CrossRef - Cardiovascular Risk Profile and Lipid Management in the Population-Based Cohort Study LATINO: 20 Years of Real-World Data
Cristina Gavina, Daniel Seabra Carvalho, Marisa Pardal, Marta Afonso-Silva, Diana Grangeia, Ricardo Jorge Dinis-Oliveira, Francisco Araújo, Tiago Taveira-Gomes
Journal of Clinical Medicine.2022; 11(22): 6825. CrossRef - New and emerging lipid-lowering therapy
James M Backes, Daniel E Hilleman
Future Cardiology.2021; 17(8): 1407. CrossRef - Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: what do we know and what not?
Styliani Fragki, Hubert Dirven, Tony Fletcher, Bettina Grasl-Kraupp, Kristine Bjerve Gützkow, Ron Hoogenboom, Sander Kersten, Birgitte Lindeman, Jochem Louisse, Ad Peijnenburg, Aldert H. Piersma, Hans M. G. Princen, Maria Uhl, Joost Westerhout, Marco J. Z
Critical Reviews in Toxicology.2021; 51(2): 141. CrossRef -
A network pharmacology analysis on drug‐like compounds from
Ganoderma lucidum
for alleviation of atherosclerosis
Ki Kwang Oh, Md. Adnan, Dong Ha Cho
Journal of Food Biochemistry.2021;[Epub] CrossRef - Efficacy and Safety of Fenofibrate-Statin Combination Therapy in Patients With Inadequately Controlled Triglyceride Levels Despite Previous Statin Monotherapy: A Multicenter, Randomized, Double-blind, Phase IV Study
Myung Soo Park, Jong-Chan Youn, Eung Ju Kim, Ki Hoon Han, Sang Hak Lee, Sung Hea Kim, Byung Jin Kim, Sung Uk Kwon, Kyu-Hyung Ryu
Clinical Therapeutics.2021; 43(10): 1735. CrossRef - Prevalence of and Factors Associated With the Prescription of Fibrates Among Patients Receiving Lipid-Lowering Drugs in Germany
Louis Jacob, Roger-Axel Greiner, Mark Luedde, Karel Kostev
Journal of Cardiovascular Pharmacology.2021; 78(6): 885. CrossRef - Challenging Issues in the Management of Cardiovascular Risk Factors in Diabetes During the COVID-19 Pandemic: A Review of Current Literature
Leili Rahimi, Mojtaba Malek, Faramarz Ismail-Beigi, Mohammad E. Khamseh
Advances in Therapy.2020; 37(8): 3450. CrossRef - Treatment With Gemfibrozil Prevents the Progression of Chronic Kidney Disease in Obese Dahl Salt-Sensitive Rats
Corbin A. Shields, Bibek Poudel, Kasi C. McPherson, Andrea K. Brown, Ubong S. Ekperikpe, Evan Browning, Lamari Sutton, Denise C. Cornelius, Jan M. Williams
Frontiers in Physiology.2020;[Epub] CrossRef - Oxidative Stress and Inflammation in Renal and Cardiovascular Complications of Diabetes
Amelia Charlton, Jessica Garzarella, Karin A. M. Jandeleit-Dahm, Jay C. Jha
Biology.2020; 10(1): 18. CrossRef
- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
- Role of Peroxisome Proliferator-Activated Receptor α in Diabetic Nephropathy
- Sungjin Chung, Cheol Whee Park
- Diabetes Metab J. 2011;35(4):327-336. Published online August 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.4.327
- 65,535 View
- 47 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader With a developing worldwide epidemic of diabetes mellitus, the renal complications associated with diabetes have become a serious health concern. Primary therapy for treating diabetic nephropathy is a multifactorial process. Peroxisome proliferator-activated receptor alpha (PPARα) agonists have been used primarily in clinical practice for the treatment of dyslipidemia and insulin resistance. Given that PPARα expression and regulation of metabolic pathways are involved in oxidative stress, inflammation, blood pressure regulation, and the renin-angiotensin aldosterone system, PPARα likely influences the development and pathogenesis of diabetic nephropathy via indirect effects on glucose and lipid homeostasis and also by direct action on the kidneys. These findings suggest that PPARα may become an important therapeutic target for treating diabetic renal complications.
-
Citations
Citations to this article as recorded by- The Proteome of Circulating Large Extracellular Vesicles in Diabetes and Hypertension
Akram Abolbaghaei, Maddison Turner, Jean-François Thibodeau, Chet E. Holterman, Christopher R. J. Kennedy, Dylan Burger
International Journal of Molecular Sciences.2023; 24(5): 4930. CrossRef - Metformin mitigates renal dysfunction in obese insulin-resistant rats via activation of the AMPK/PPARα pathway
Laongdao Thongnak, Nattavadee Pengrattanachot, Sasivimon Promsan, Nichakorn Phengpol, Prempree Sutthasupha, Krit Jaikumkao, Anusorn Lungkaphin
Archives of Pharmacal Research.2023; 46(5): 408. CrossRef - A comprehensive insight into the molecular and cellular mechanisms of the effects of Propolis on preserving renal function: a systematic review
Paniz Anvarifard, Maryam Anbari, Alireza Ostadrahimi, Mohammadreza Ardalan, Zohreh Ghoreishi
Nutrition & Metabolism.2022;[Epub] CrossRef - APX-115, a pan-NADPH oxidase inhibitor, protects development of diabetic nephropathy in podocyte specific NOX5 transgenic mice
Eun Soo Lee, Hong Min Kim, Sun Hee Lee, Kyung Bong Ha, Yoon Soo Bae, Soo Jin Lee, Sung Hwan Moon, Eun Young Lee, Ji-Hye Lee, Choon Hee Chung
Free Radical Biology and Medicine.2020; 161: 92. CrossRef - Fenofibrate decreased microalbuminuria in the type 2 diabetes patients with hypertriglyceridemia
Xiaomeng Sun, Jia Liu, Guang Wang
Lipids in Health and Disease.2020;[Epub] CrossRef - AMPK allostery: A therapeutic target for the management/treatment of diabetic nephropathy
Kehinde Sulaimon Ayinde, Olamide Tosin Olaoba, Boyenle Ibrahim, Du Lei, Qian Lu, Xiaoxing Yin, Temitope Isaac Adelusi
Life Sciences.2020; 261: 118455. CrossRef - Greater efficacy of atorvastatin versus a non-statin lipid-lowering agent against renal injury: potential role as a histone deacetylase inhibitor
Ravi Shankar Singh, Dharmendra Kumar Chaudhary, Aradhana Mohan, Praveen Kumar, Chandra Prakash Chaturvedi, Carolyn M. Ecelbarger, Madan M. Godbole, Swasti Tiwari
Scientific Reports.2016;[Epub] CrossRef - Upregulation of SIRT1-AMPK by thymoquinone in hepatic stellate cells ameliorates liver injury
Yong Yang, Ting Bai, You-Li Yao, De-Quan Zhang, Yan-Ling Wu, Li-Hua Lian, Ji-Xing Nan
Toxicology Letters.2016; 262: 80. CrossRef - Peroxisome proliferator-activated receptor α-dependent renoprotection of murine kidney by irbesartan
Makoto Harada, Yuji Kamijo, Takero Nakajima, Koji Hashimoto, Yosuke Yamada, Hisashi Shimojo, Frank J. Gonzalez, Toshifumi Aoyama
Clinical Science.2016; 130(21): 1969. CrossRef - Anthocyanin-rich Seoritae extract ameliorates renal lipotoxicity via activation of AMP-activated protein kinase in diabetic mice
Eun Sil Koh, Ji Hee Lim, Min Young Kim, Sungjin Chung, Seok Joon Shin, Bum Soon Choi, Hye Won Kim, Seong Yeon Hwang, Sae Woong Kim, Cheol Whee Park, Yoon Sik Chang
Journal of Translational Medicine.2015;[Epub] CrossRef - Diabetic Kidney Disease: From Epidemiology to Clinical Perspectives
Cheol Whee Park
Diabetes & Metabolism Journal.2014; 38(4): 252. CrossRef - Peroxisome proliferator-activated receptor agonists in a battle against the aging kidney
Marijn M. Speeckaert, Céline Vanfraechem, Reinhart Speeckaert, Joris R. Delanghe
Ageing Research Reviews.2014; 14: 1. CrossRef - Fenofibrate and dipyridamole treatments in low-doses either alone or in combination blunted the development of nephropathy in diabetic rats
Pitchai Balakumar, Rajavel Varatharajan, Ying Hui Nyo, Raja Renushia, Devarajan Raaginey, Ann Nah Oh, Shaikh Sohrab Akhtar, Mani Rupeshkumar, Karupiah Sundram, Sokkalingam A. Dhanaraj
Pharmacological Research.2014; 90: 36. CrossRef - Differential effects of low-dose fenofibrate treatment in diabetic rats with early onset nephropathy and established nephropathy
Supriya Kadian, Nanjaian Mahadevan, Pitchai Balakumar
European Journal of Pharmacology.2013; 698(1-3): 388. CrossRef - Age-Associated Molecular Changes in the Kidney in Aged Mice
Ji Hee Lim, Eun Nim Kim, Min Young Kim, Sungjin Chung, Seok Joon Shin, Hyung Wook Kim, Chul Woo Yang, Yong-Soo Kim, Yoon Sik Chang, Cheol Whee Park, Bum Soon Choi
Oxidative Medicine and Cellular Longevity.2012; 2012: 1. CrossRef - Epidemiology of Obesity, the Metabolic Syndrome, and Chronic Kidney Disease
Rikki M. Tanner, Todd M. Brown, Paul Muntner
Current Hypertension Reports.2012; 14(2): 152. CrossRef
- The Proteome of Circulating Large Extracellular Vesicles in Diabetes and Hypertension
- Prevention of Diabetes by Fenofibrate in OLETF Rats: Hepatic Mechanism for Reducing Visceral Adiposity.
- Hye Jeong Lee, Mi Kyoung Park, Kyung Il Lee, Young Jun An, Ji Min Kim, Ja Young Park, Young Han, Sook Hee Hong, Sun Seob Choi, Young Hyun Yoo, Joon Duk Suh, Duk Kyu Kim
- Korean Diabetes J. 2007;31(1):63-74. Published online January 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.1.63
- 2,136 View
- 18 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The aim of this study is to evaluate the hepatic mechanism of fenofibrate that has the diabetes protective action in rats. METHODS: We chose OLETF rats and divided them into three groups. Fenofibrate (DF) group was fed with diet and fenofibrate (300 mg/kg/day). Paired feeding (Dd) group and free diet (DD) group were fed with diet. After 36 weeks of treatment, all the rats were sacrificed. RESULTS: The fasting blood glucose level of DF group (8.5 +/- 0.9 mmol/L) showed normal. The fasting blood glucose level of Dd group (22.4 +/- 3.0 mmol/L) and DD group (16.9 +/- 3.7 mmol/L) showed significantly increased than that of DF group (P < 0.01, respectively). The body weight, visceral adipose tissue and subcutaneous adipose tissue of DF group were significantly decreased compared to those of Dd and DD groups (P < 0.01, P < 0.05, P < 0.05). DF group showed significantly increased state-3 respiration rate, ATP synthetic activity, state-4 respiration rate and their blood beta-keton body levels than those of control groups (P < 0.01, respectively). DF group showed normal morphology of hepatocytes but DD and Dd groups showed hepatic steatosis with mitochondrial swellings. CONCLUSION: Chronic fenofibrate treatment prevents the development of diabetes in OLETF rats with inhibiting gain of body weight and abdominal adiposity. The hepatic mechanism for reducing visceral adiposity is that fenofibrate leads to increasing oxidative phosphorylation, uncoupling and ketogenesis as well as increasing beta-oxidation of fatty acids. Moreover, fenofibrate treatment prevents the development of hepatic steatosis. -
Citations
Citations to this article as recorded by- The Differences of Metabolic Syndrome Risk Factors according to Obesity and Abdominal Obesity in Elderly Korean Women
Kyung-A Shin
The Korean Journal of Clinical Laboratory Science.2016; 48(4): 304. CrossRef - Effects of Soybean and DJI Chungkukjang Powder on Blood Glucose and Serum Lipid Reduction in db/db Mice
Jae-Joon Lee, Ah-Ra Kim, Hae-Choon Chang, Hae-Ok Jung, Myung-Yul Lee
Journal of the Korean Society of Food Science and Nutrition.2012; 41(8): 1086. CrossRef - Comparative analysis of fat and muscle proteins in fenofibratefed type II diabetic OLETF rats: the fenofibrate-dependent expression of PEBP or C11orf59 protein
Jong-Ryeal Hahm, Jin-Sook Ahn, Hae-Sook Noh, Seon-Mi Baek, Ji-Hye Ha, Tae-Sik Jung, Yong-Jun An, Duk-Kyu Kim, Deok-Ryong Kim
BMB Reports .2010; 43(5): 337. CrossRef - Comparative analysis of fat and muscle proteins in fenofibratefed type II diabetic OLETF rats: the fenofibrate-dependent expression of PEBP or C11orf59 protein
Jong-Ryeal Hahm, Jin-Sook Ahn, Hae-Sook Noh, Seon-Mi Baek, Ji-Hye Ha, Tae-Sik Jung, Yong-Jun An, Duk-Kyu Kim, Deok-Ryong Kim
BMB Reports.2010; 43(5): 337. CrossRef
- The Differences of Metabolic Syndrome Risk Factors according to Obesity and Abdominal Obesity in Elderly Korean Women
- Exercise and Fenofibrate Reduces Body Adiposity Synergistically in OLETF Rats.
- Young Jun An, Hre Jeong Lee, Mi Kyoung Park, Kyung Il Lee, In Young Koh, Dong Sik Jung, Ah Young Kang, Duk Kyu Kim
- Korean Diabetes J. 2004;28(2):131-138. Published online April 1, 2004
- 942 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The PPAR alpha activator, Fenofibrate, is a pharmacological ligand, which induces beta-oxidation of long chain fatty acids in the mitochondria of hepatocytes. The beta-oxidation induced by exogenous PPAR alpha activators may be operated maximally when the sustained production of energy substrate in the liver is required by working muscles due to continued exercise. The aim of this study was to determine whether the combination therapy of exercise and Fenofibrate could synergistically reduce body adiposity in OLETF rats. METHODS: Twenty-eight male OLETF rats(13 wk old) were divided into four groups. The diet(n=7) and exercise groups(n=7) were fed with chow for 12 weeks. The Fenofibrate(n=7) and combined treatment(exercise and Fenofibrate) groups (n=7) were fed with Fenofibrate(32mg/kg/day) mixed chow for 12 weeks. The animals in the exercise and combined treatment groups were exercised by running on a treadmill for 12 weeks. At 24 weeks of age, all the rats were sacrificed, and examined by biochemical tests and had their adipose tissue weight measured. RESULTS: There were no significant changes in the retroperitoneal and subcutaneous fats between the diet and Fenofibrate groups, but there were between the diet and combined treatment groups(P<0.05). CONCLUSION: Exercise combined with Fenofibrate synergistically reduces body adiposity in OLETF rats

 KDA
KDA
 First
First Prev
Prev





