
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Pathophysiology
- Deficiency of ASGR1 Alleviates Diet-Induced Systemic Insulin Resistance via Improved Hepatic Insulin Sensitivity
- Xiaorui Yu, Jiawang Tao, Yuhang Wu, Yan Chen, Penghui Li, Fan Yang, Miaoxiu Tang, Abdul Sammad, Yu Tao, Yingying Xu, Yin-Xiong Li
- Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0124 [Epub ahead of print]

- 909 View
- 63 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Insulin resistance (IR) is the key pathological basis of many metabolic disorders. Lack of asialoglycoprotein receptor 1 (ASGR1) decreased the serum lipid levels and reduced the risk of coronary artery disease. However, whether ASGR1 also participates in the regulatory network of insulin sensitivity and glucose metabolism remains unknown.
Methods
The constructed ASGR1 knockout mice and ASGR1-/- HepG2 cell lines were used to establish the animal model of metabolic syndrome and the IR cell model by high-fat diet (HFD) or drug induction, respectively. Then we evaluated the glucose metabolism and insulin signaling in vivo and in vitro.
Results
ASGR1 deficiency ameliorated systemic IR in mice fed with HFD, evidenced by improved insulin intolerance, serum insulin, and homeostasis model assessment of IR index, mainly contributed from increased insulin signaling in the liver, but not in muscle or adipose tissues. Meanwhile, the insulin signal transduction was significantly enhanced in ASGR1-/- HepG2 cells. By transcriptome analyses and comparison, those differentially expressed genes between ASGR1 null and wild type were enriched in the insulin signal pathway, particularly in phosphoinositide 3-kinase-AKT signaling. Notably, ASGR1 deficiency significantly reduced hepatic gluconeogenesis and glycogenolysis.
Conclusion
The ASGR1 deficiency was consequentially linked with improved hepatic insulin sensitivity under metabolic stress, hepatic IR was the core factor of systemic IR, and overcoming hepatic IR significantly relieved the systemic IR. It suggests that ASGR1 is a potential intervention target for improving systemic IR in metabolic disorders.
- Basic Research
- MondoA Is Required for Normal Myogenesis and Regulation of the Skeletal Muscle Glycogen Content in Mice
- Hui Ran, Yao Lu, Qi Zhang, Qiuyue Hu, Junmei Zhao, Kai Wang, Xuemei Tong, Qing Su
- Diabetes Metab J. 2021;45(3):439-451. Published online May 18, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0212
- Correction in: Diabetes Metab J 2021;45(5):797
- 6,229 View
- 191 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
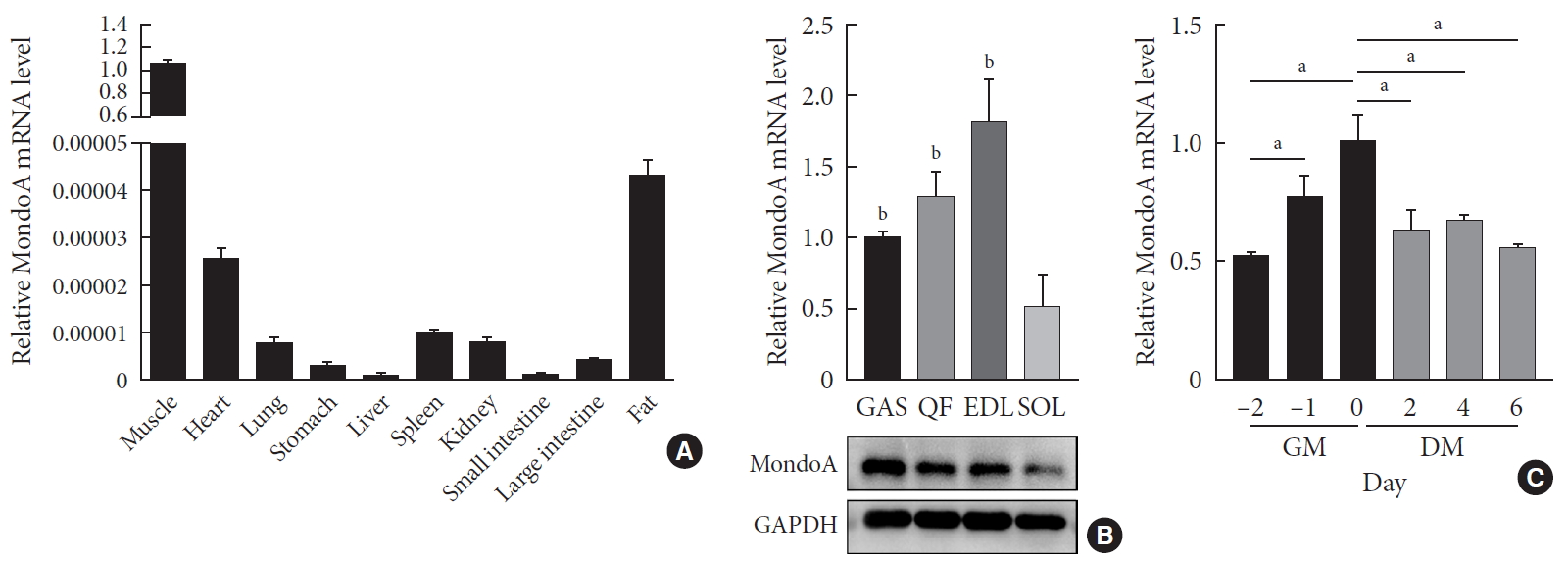
ePub Background Skeletal muscle is the largest tissue in the human body, and it plays a major role in exerting force and maintaining metabolism homeostasis. The role of muscle transcription factors in the regulation of metabolism is not fully understood. MondoA is a glucose-sensing transcription factor that is highly expressed in skeletal muscle. Previous studies suggest that MondoA can influence systemic metabolism homeostasis. However, the function of MondoA in the skeletal muscle remains unclear.
Methods We generated muscle-specific MondoA knockout (MAKO) mice and analyzed the skeletal muscle morphology and glycogen content. Along with skeletal muscle from MAKO mice, C2C12 myocytes transfected with small interfering RNA against MondoA were also used to investigate the role and potential mechanism of MondoA in the development and glycogen metabolism of skeletal muscle.
Results MAKO caused muscle fiber atrophy, reduced the proportion of type II fibers compared to type I fibers, and increased the muscle glycogen level. MondoA knockdown inhibited myoblast proliferation, migration, and differentiation by inhibiting the phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/Akt pathway. Further mechanistic experiments revealed that the increased muscle glycogen in MAKO mice was caused by thioredoxin-interacting protein (TXNIP) downregulation, which led to upregulation of glucose transporter 4 (GLUT4), potentially increasing glucose uptake.
Conclusion MondoA appears to mediate mouse myofiber development, and MondoA decreases the muscle glycogen level. The findings indicate the potential function of MondoA in skeletal muscle, linking the glucose-related transcription factor to myogenesis and skeletal myofiber glycogen metabolism.
-
Citations
Citations to this article as recorded by- The Function of MondoA and ChREBP Nutrient—Sensing Factors in Metabolic Disease
Byungyong Ahn
International Journal of Molecular Sciences.2023; 24(10): 8811. CrossRef - Normal and Neoplastic Growth Suppression by the Extended Myc Network
Edward V. Prochownik, Huabo Wang
Cells.2022; 11(4): 747. CrossRef - The Role of Mondo Family Transcription Factors in Nutrient-Sensing and Obesity
Huiyi Ke, Yu Luan, Siming Wu, Yemin Zhu, Xuemei Tong
Frontiers in Endocrinology.2021;[Epub] CrossRef
- The Function of MondoA and ChREBP Nutrient—Sensing Factors in Metabolic Disease
- A Case of Hepatic Glycogenosis in a Patient with Uncontrolled Type 1 Diabetes Mellitus.
- Seung Hwan Lee, Hyuk Sang Kwon, Jung Ah Shin, Won Chul Kim, Jeong Hoon Kim, Yoon Hee Choi, Kun Ho Yoon, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang
- Korean Diabetes J. 2006;30(1):82-86. Published online January 1, 2006
- DOI: https://doi.org/10.4093/jkda.2006.30.1.82
- 2,053 View
- 20 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - When a patient with diabetes presents with hepatomegaly and increased level of liver enzymes, glycogenosis or nonalcoholic steatohepatitis (NASH) should be considered. Glycogenosis is mainly developed in patients with type 1 diabetes, when blood glucose level is poorly controlled, when a high dosage of insulin is administered in ketoacidosis, or when glucose is given to control hypoglycemia caused by high dosage of insulin. On the other hand, the main causes of NASH, which are known to mainly affect type 2 diabetes patients, are obesity, dyslipidemia or insulin resistance. Glycogenosis differs from NASH, the former being a reversible change that improves with the control of blood glucose level and the minimum dosage requirement of insulin, and the latter being a progressive disease that may lead to fibrosis or cirrhosis of the liver. However, clinical differentiation of the two diseases is difficult and liver biopsy is helpful for making a definite diagnosis. We present a type 1 diabetes patient with poorly controlled blood glucose level, who have had a frequent history of diabetic ketoacidosis, showing hepatomegaly and a slight increase in liver enzyme level. The patient was diagnosed as diabetic glycogenosis, confirmed by liver biopsy. Strict control of the blood glucose level resulted in rapid improvement showing the reversible nature of the disease.
-
Citations
Citations to this article as recorded by- Four cases of type 1 diabetes mellitus showing sharp serum transaminase increases and hepatomegaly due to glycogenic hepatopathy
Yuichi Ikarashi, Tomomi Kogiso, Etsuko Hashimoto, Kuniko Yamamoto, Kazuhisa Kodama, Makiko Taniai, Nobuyuki Torii, Hiroko Takaike, Yasuko Uchigata, Katsutoshi Tokushige
Hepatology Research.2017;[Epub] CrossRef - Glycogenic hepatopathy in a Korean girl with poorly controlled type 1 diabetes mellitus
Hwal Rim Jeong, Young Seok Shim, Young Bae Kim, Hae Sang Lee, Jin Soon Hwang
Annals of Pediatric Endocrinology & Metabolism.2014; 19(1): 49. CrossRef - Three cases of glycogenic hepatopathy mimicking acute and relapsing hepatitis in type I diabetes mellitus
Jae Hwang Cha, Sang Ho Ra, Yu Mi Park, Yong Kwan Ji, Ji Hyun Lee, So Yeon Park, Soon Koo Baik, Sang Ok Kwon, Mee Yon Cho, Moon Young Kim
Clinical and Molecular Hepatology.2013; 19(4): 421. CrossRef - Hepatic glycogenosis in a patient with poorly controlled type 1 diabetes mellitus
Hye Young Jin, Dae-Young Kang, Jin-Ho Choi
Korean Journal of Pediatrics.2009; 52(11): 1279. CrossRef
- Four cases of type 1 diabetes mellitus showing sharp serum transaminase increases and hepatomegaly due to glycogenic hepatopathy
- The Role of Akt-1/PKBalpha on Insulin Action in 3T3-L1 Adipocyte.
- Jung Min Lee, Hyun Shik Son, Hyuk Sang Kwon, Seung Ki Kwack, Seung Hyun Ko, Sang Ah Chang, Kun Ho Yoon, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang, Prem Sharma
- Korean Diabetes J. 2002;26(4):274-285. Published online August 1, 2002
- 1,060 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
S: Akt/PKB as a serine/threonine kinase is stimulated by insulin and other growth factors. And insulin stimulates glucose uptake by promoting the translocation of glucose transporter 4 (GLUT4) to the cell membrane. But, it is not clear that Akt/PKB, a downstream target of PI 3-kinase, is involved in glucose uptake pathway. In this study, we investigated the role of Akt/PKB, especially Akt-1, on insulin action in 3T3-L1 adipocyte. METHODS: We made recombinant Ad5.Akt-1 vector by the insertion of Akt-1 gene to adenoviral vector. And then, we overexpressed Akt-1 proteins(wild type and kinase inactive type) in 3T3-L1 adipocytes by using a adenoviral transfection method. We observed the changes of glucose uptake, glycogen synthesis, activities of mitogen-activated protein kinase (MAPK, also called extracellular signal-regulated kinase), p70 ribosomal s6 protein kinase (p70s6k), and glycogen synthase kinase 3 (GSK3) according to Akt-1 activity and insulin treatment. RESULTS: First, overexpression of Akt-1 did not affect to glucose uptake, whether insulin stimulates or not. Second, overexpression of Akt-1 did not affect the phosphorylation of p44/42-MAPK, either. Third, the glycogen synthesis was increased by overexpression of Akt-1. CONCLUSION: Akt-1 activation is necessary for glycogen synthesis, but is not essential for glucose transport in 3T3-L1 adipocytes.
- The Effects of Uncoupling Protein 3 Overexpression on Glucose Metabolism in OLETF Rats in Vivo and Cultured Skeletal Muscle Cells in Vitro.
- Jeong Hee Han, Hye Seon Park, Jung Min Koh, Ha Young Kim, Ho Kyung Kang, In Kyu Lee, Joong Yeol Park, Sung Kwan Hong, Jae Dam Lee, Ki Up Lee
- Korean Diabetes J. 2001;25(6):460-468. Published online December 1, 2001
- 1,141 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
UCP3 is a mitochondrial membrane protein expressed selectively in the skeletal muscle and brown adipose tissue. Since the skeletal muscle is the main organ determining insulin sensitivity in the body, it was hypothesized that UCP3 overexpression in skeletal muscle cells would improve glucose metabolism. METHODS: An adenovirus-UCP3 was produced by a recombinant DNA method. OLETF rats were divided into 2 groups. Four rats were injected with the adenovirus- UCP3 (UCP3 group) and others were injected with the adenovirus (control group) in the skeletal muscle. The UCP3 group was provided with the same quantity of food as that consumed by the control group on the previous day. Insulin sensitivity was evaluated by the euglycemic hyperinsulinemic clamp method. In a separate experiment, glucose transport and glycogen synthesis we evaluated in C2C12 cells transfected with ether an adenovirus or the adenovirus-UCP3. RESULTS: The insulin sensitivity improved significantly and the body weight decreased in the UCP3 group. The glucose transport and glycogen synthesis were higher in the UCP3-C2C12 skeletal muscle cells at the basal state. After insulin treatment, glucose transport and glycogen synthesis were also higher in the UCP3-C2C12 cells but the increments were reduced after treatment with wortmannin, a PI3K inhibitor. CONCLUSION: Insulin sensitivity was higher in the UCP3-overexpressed OLETF rats in the in vivo study. UCP3 transfection also increased glucose transport and glycogen synthesis in the cultured skeletal muscle cells by a PI3K dependent mechanism.
- Effect of Overexpression of Gi Proteins on Insulin Actions in 3T3-L1 Adipocytes.
- Hyun Shik Son, Bong Yun Cha, Sung Dae Moon, Jung Min Lee, Ok Ki Hong, Sang Ah Chang, Yu Bae Ahn, Kun Ho Yoon, Kwang Woo Lee, Ho Young Son, Sung Koo Kang
- Korean Diabetes J. 2000;24(4):404-412. Published online January 1, 2001
- 1,037 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
It has been reported that G proteins are involved in biological actions of insulin. Especially, Gi protein is more associated with insulin actions than Gs proteins. Gi protein has at least three different subtypes of Gi 1, Gi 2 and Gi 3 protein. However, it is not certain which subtypes of Gi proteins are associated with biological actions of insulin. METHODS: To investigate which subtypes of Gi proteins are associated with insulin action, we overexpressed three different kinds of Gi protein, Gi 1, Gi 2 and Gi 3 protein, in 3T3-L1 adipocytes using DNA-polylysine-adenovirus complex transfection method. After incubating for 2 hours, 3T3-L1 adipocytes were treated with 100 nM insulin for the evaluation of biological actions of insulin. Moreover, to elucidate insulin stimulated insulin receptor autophosphorylation and IRS-1 phosphorylation, 3T3-L1 adipocytes were stimulated with 100 nM insulin for 10 minutes, homogenized and immunoprecipitated with anti-phosphotyrosine antibody. RESULTS: Transfection with Gi 2 gene resulted in increment in insulin-stimulated [3H]2-deoxyglucose (DOG) uptake without affecting basal 2-DOG uptake, but not with Gi 1 and Gi 3 gene transfection. There was unchanged glycogen synthesis rate in all three Gialphasubtypes. Insulin-induced increments of insulin receptor autophos phorylation and IRS-1 phosphorylation were found in Gi 2 protein overexpressed group, only. CONCLUSION: These results suggest that Gi 2 protein may be associated with regulation of biological actions of insulin.
- Metabolic Phenotype of Glycogen Synthase Gene Inhibition in Human Skeletal Muscle Cells.
- Jae Joon Koh, Kyong Soo Park, Jeong Mi Kim, Seong Yeon Kim, Hong Kyu Lee, Theodore P Ciaraldi, Robert R Henry
- Korean Diabetes J. 2000;24(3):331-339. Published online January 1, 2001
- 911 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Glycogen synthase (GS) is the rate-limiting enzyme controlling non-oxidative glucose disposal in skeletal muscle. Reduction in GS activity and impaired insulin responsiveness are characteristic features of skeletal muscle in type 2 diabetes that contribute to glucose intolerance. These properties also exist in human skeletal muscle cell cultures from type 2 diabetic subjects. The aim of study is to determine the effect of an isolated reduction in GS on glucose metabolism and if this change can generate a diabetes-like state. METHODS: Cultured skeletal muscle cells from non-diabetic subjects were treated with antisense oligodeoxynucleotides (ODN) to GS to interfere with expression of the gene for 6 days. GS activity, protein expression, glycogen synthesis and cellular glycogen content were measured. RESULTS: Treatment with antisense ODN reduced GS protein expression by 70% compared to control (scrambled) ODN (p<0.01). Both total GS activity and that measured at 0.1 mM G-6-P were reduced by antisense ODN treatment. Insulin responsiveness of GS was also halved. Basal GS FV0.1 was decreased in both antisense ODN and control ODN treated cells and antisense treated cells did not show increase in GS FV0.1 in response to insulin stimulation. Glucose incorporation into glycogen under basal conditions was unaltered after antisense ODN treatment, though no further stimulation in response to insulin was observed. Yet both cellular glycogen content and glycogen synthesis were lower in antisense ODN treated cells compared to control ODN treated cells. CONCLUSIONS: Reduction in GS expression in human skeletal muscle cell impair GS activity and insulin responsiveness but does not replicate the abnormalities of glycogen synthesis found in cultured diabetic skeletal muscle cells.
- Effect of Exercise Training on Insulin Sensitivity and Intracellular Glucose Metabolism in Skeletal Muscle of High Fat-fed Rats.
- Chul Hee Kim, Joong Yeol Park, Sung Kwan Hong, Kyong Soo Park, Hong Kyu Lee, Ki Up Lee
- Korean Diabetes J. 1998;22(2):231-242. Published online January 1, 2001
- 1,068 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Insulin resistance is a major characteristic of non-insulin-dependent diabetes mellitus and obesity. Many studies have indicated that increased intake of fat are associated with obesity and insulin resistance. On the other hand, chronic exercise is known to improve insulin sensitivity. However, the mechanisms by which high fat diet induces insulin resistance and exercise trainmg improves insulin sensitivity are not established. This study was undertaken to examine the mechanisms by which high fat diet and exercise training affect the insulin sensitivity in the whole body and in skeletal muscles. METHODS: Male Sprague-Dawley rats were divided into three groups: high fat sedentary group, high fat exercise group, and control(low fat sedentary) group. High fat diet consists of 66.5% fat and 12.5% carbohydrate, and control(low fat) diet consists of 12 5% fat and 66.5% carbohydrate. Exercise training was performed by swimming three hours per day. After 3 weeks, animals underwent hyperinsulinemic euglycemic clamp study to measure whole body glucose metabolic fluxes. Glycogen synthase activity and glucose-6-phosphate (G-6-P) levels were measured in skeletal muscle at the end of the clamp study. RESULTS: In the high fat diet group, whole body glycolysis and glycogen synthesis were decreased. Exercise training reversed the insulin resistance induced by high fat diet by increasing both glycolysis and glycogen synthesis. Glycogen synthase activity in skeletal muscle was reduced in high fat diet group, and it was partially reversed by exercise training. G-6-P level in skeletal muscle was increased in high fat diet group, and it was further increased by exercise training. CONCLUSION: These results suggested that the insulin resistance in high fat diet-fed rats is due to the impairment in glucose metabolism at sites distal to G-6-P, i.e. glycolysis and glycogen synthesis. In contrast, the improvement in insulin sensitivity by exercise training in high fat-fed rats is primarily due to the increased glucose metabolic flux proximal to G-6-P, i.e. glucose transport and phosphorylation.
- Effect of Troglitazone on Glycogen synthase Activity in Human skeletal Muscle Culture from Obese Non-Diabetic and Obese Non-insulin Dependent Diabetes Mellitus.
- Leslie Abrams Carter, Theodore Ciaraldi, Robert R Henry, Kyong Soo Park, Hong Kyu Lee
- Korean Diabetes J. 1997;21(3):254-261. Published online January 1, 2001
- 865 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Skeletal muscle is the principal tissue of insulin resistance in obese and non-insulin dependent diabetic(NIDDM) subjects. Troglitazone, a member of thiazolidinedione class of compounds, has been shown to improve glucose tolerance in insulin resistant state. At the cellular level, troglitazone has been shown to improve insulin action in skeletal muscle, liver and adipose tissue. However, there has been no direct assessment of mechanism of this drug in insulin resistant human skeletal muscle from obese and obese NIDDM subjects. METHODS: To determine the effect of troglitzone on skeletal muscle glycogen synthase(GS) activity in insulin resistant human skeletal muscle, muscle cultures from 7 obese non-diabetic and 8 obese NIDDM subjects were grown for 4 weeks and then fused for 4 days either with or without troglitzone(0~5ug/mL) and harvested for GS activity and protein measurement. GS activity was measured by enzymatic method and protein expression was measured by Western blot using polyclonal antibody specific for C-terminal end of GS protein, RESULTS: Troglitzone increased GS activity(GS activity at 0.1 mM G6P and fractional velocity) dose-dependently in both obese non-diabetic and type II diabetes and the increased GS activity by troglitzone was mostly basal rather than insulin-stimulated. Basal fractional velocity of GS increased 2.8+/-0.7 and 3.7 +/-1.2 fold in obese non-diabetic and type II diabetes respectively. There was no changes in GS total activity and GS protein expression in either group with troglitzone treatment.. CONCLUSION: Troglitazone has effects to improve glycogen synthase activity in skeletal tnuscle of obese and obese NIDDM subjects.
- Mechanism of Insulin Resistance : Time Dependence of the Development of Insulin Resistance in High Fat Fed Rats.
- Kyong Soo Park, Ki Up Lee, Sung Woo Park, Hong Kyu Lee, Hun Ki Min
- Korean Diabetes J. 1997;21(2):168-175. Published online January 1, 2001
- 857 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Increased FFA availability is known to induce insulin resistance by decrease in peripheral glucose utilization and increase in hepatic glucose procluction. However, there are conflicting results about the time dependence of the developrnent of insulin resistance with increased availability of FFA. METHODS: To elucidate the time dependence of the development of insulin resistance associated with increased availability of FFA, peripheral glucose utilization rate and hepatic glucose production rate were measured by euglycemic hyperinsulinemic clamp with 3-3H glucose infusion in rats fed high fat diet (1 week or 3 weeks) or control diet(ordinary chow diet). RESULTS: Basal plasrna FFA levels and steady state plasma insulin levels increased after high fat diet. After 1 week of high fat diet, suppressibility of hepatic glucose production rate by insulin was impaired(p<0.05 vs control). Insulin sensitivity index(glucose utilization rates/steady state plasma insulin concentrmtions X100) was decreased only after 3 weeks of high fat diet(p<0.05 vs control) which was accompanied by decreased glycogen synthase activity. CONCLUSION: High fat diet induces hepatic insulin resistance before peripheral insulin resistance and decreased glycogen synthase activity may contribute to the development of peripheral insulin resistance in rats fed high fat diet.

 KDA
KDA
 First
First Prev
Prev





