
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Review
- Metabolic Risk/Epidemiology
- Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
- Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
- Diabetes Metab J. 2024;48(2):184-195. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0168
- 2,612 View
- 364 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
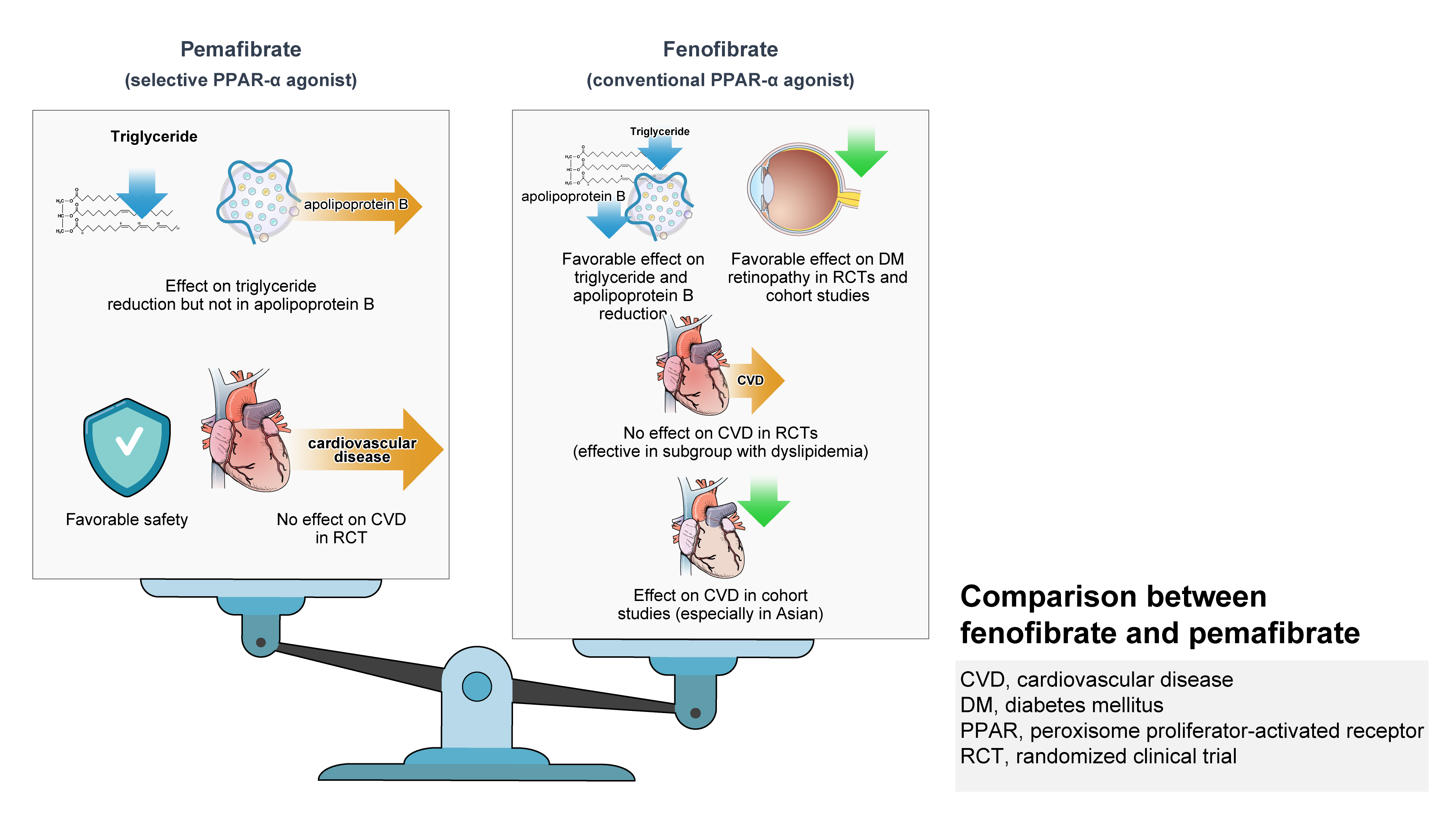
ePub - Hypertriglyceridemia and decreased high-density lipoprotein cholesterol (HDL-C) persist despite statin therapy, contributing to residual atherosclerotic cardiovascular disease (ASCVD) risk. Asian subjects are metabolically more susceptible to hypertriglyceridemia than other ethnicities. Fenofibrate regulates hypertriglyceridemia, raises HDL-C levels, and is a recommended treatment for dyslipidemia. However, data on fenofibrate use across different Asian regions are limited. This narrative review summarizes the efficacy and safety data of fenofibrate in Asian subjects with dyslipidemia and related comorbidities (diabetes, metabolic syndrome, diabetic retinopathy, and diabetic nephropathy). Long-term fenofibrate use resulted in fewer cardiovascular (CV) events and reduced the composite of heart failure hospitalizations or CV mortality in type 2 diabetes mellitus. Fenofibrate plays a significant role in improving irisin resistance and microalbuminuria, inhibiting inflammatory responses, and reducing retinopathy incidence. Fenofibrate plus statin combination significantly reduced composite CV events risk in patients with metabolic syndrome and demonstrated decreased triglyceride and increased HDL-C levels with an acceptable safety profile in those with high CV or ASCVD risk. Nevertheless, care is necessary with fenofibrate use due to possible hepatic and renal toxicities in vulnerable individuals. Long-term trials and real-world studies are needed to confirm the clinical benefits of fenofibrate in the heterogeneous Asian population with dyslipidemia.
Original Articles
- Basic Research
- Peroxisomal Fitness: A Potential Protective Mechanism of Fenofibrate against High Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice
- Songling Jiang, Md Jamal Uddin, Xiaoying Yu, Lingjuan Piao, Debra Dorotea, Goo Taeg Oh, Hunjoo Ha
- Diabetes Metab J. 2022;46(6):829-842. Published online June 24, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0274
- 5,016 View
- 293 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Non-alcoholic fatty liver disease (NAFLD) has been increasing in association with the epidemic of obesity and diabetes. Peroxisomes are single membrane-enclosed organelles that play a role in the metabolism of lipid and reactive oxygen species. The present study examined the role of peroxisomes in high-fat diet (HFD)-induced NAFLD using fenofibrate, a peroxisome proliferator-activated receptor α (PPARα) agonist.
Methods
Eight-week-old male C57BL/6J mice were fed either a normal diet or HFD for 12 weeks, and fenofibrate (50 mg/kg/day) was orally administered along with the initiation of HFD.
Results
HFD-induced liver injury as measured by increased alanine aminotransferase, inflammation, oxidative stress, and lipid accumulation was effectively prevented by fenofibrate. Fenofibrate significantly increased the expression of peroxisomal genes and proteins involved in peroxisomal biogenesis and function. HFD-induced attenuation of peroxisomal fatty acid oxidation was also significantly restored by fenofibrate, demonstrating the functional significance of peroxisomal fatty acid oxidation. In Ppara deficient mice, fenofibrate failed to maintain peroxisomal biogenesis and function in HFD-induced liver injury.
Conclusion
The present data highlight the importance of PPARα-mediated peroxisomal fitness in the protective effect of fenofibrate against NAFLD. -
Citations
Citations to this article as recorded by- Pharmacological potential of ginseng and ginsenosides in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
Young-Su Yi
Journal of Ginseng Research.2024; 48(2): 122. CrossRef - Fenofibrate alleviates NAFLD by enhancing the PPARα/PGC-1α signaling pathway coupling mitochondrial function
Xuemei Wang, Jieying Wang, Cao Ying, Yuan Xing, Xuan Su, Ke Men
BMC Pharmacology and Toxicology.2024;[Epub] CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD
Manuela Vitulo, Elisa Gnodi, Giulia Rosini, Raffaella Meneveri, Roberto Giovannoni, Donatella Barisani
International Journal of Molecular Sciences.2023; 24(16): 12748. CrossRef - PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
Tiantian Liu, Xinlu Chen, Ziqi Wei, Xue Han, Yujia Liu, Zhengliang Ma, Tianjiao Xia, Xiaoping Gu
Translational Neuroscience.2023;[Epub] CrossRef - Fenofibrate enhances lipid deposition via modulating PPARγ, SREBP-1c, and gut microbiota in ob/ob mice fed a high-fat diet
Ying Zhang, Xiu-Bin Jia, Yun-Chao Liu, Wen-Qian Yu, Yan-Hong Si, Shou-Dong Guo
Frontiers in Nutrition.2022;[Epub] CrossRef
- Pharmacological potential of ginseng and ginsenosides in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
- Exercise and Fenofibrate Reduces Body Adiposity Synergistically in OLETF Rats.
- Young Jun An, Hre Jeong Lee, Mi Kyoung Park, Kyung Il Lee, In Young Koh, Dong Sik Jung, Ah Young Kang, Duk Kyu Kim
- Korean Diabetes J. 2004;28(2):131-138. Published online April 1, 2004
- 949 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The PPAR alpha activator, Fenofibrate, is a pharmacological ligand, which induces beta-oxidation of long chain fatty acids in the mitochondria of hepatocytes. The beta-oxidation induced by exogenous PPAR alpha activators may be operated maximally when the sustained production of energy substrate in the liver is required by working muscles due to continued exercise. The aim of this study was to determine whether the combination therapy of exercise and Fenofibrate could synergistically reduce body adiposity in OLETF rats. METHODS: Twenty-eight male OLETF rats(13 wk old) were divided into four groups. The diet(n=7) and exercise groups(n=7) were fed with chow for 12 weeks. The Fenofibrate(n=7) and combined treatment(exercise and Fenofibrate) groups (n=7) were fed with Fenofibrate(32mg/kg/day) mixed chow for 12 weeks. The animals in the exercise and combined treatment groups were exercised by running on a treadmill for 12 weeks. At 24 weeks of age, all the rats were sacrificed, and examined by biochemical tests and had their adipose tissue weight measured. RESULTS: There were no significant changes in the retroperitoneal and subcutaneous fats between the diet and Fenofibrate groups, but there were between the diet and combined treatment groups(P<0.05). CONCLUSION: Exercise combined with Fenofibrate synergistically reduces body adiposity in OLETF rats
Randomized Controlled Trial
- The Effect of Micronized Fenofibrate on the Plasma Levels of Glycated LDL-C, Lp(a) and Insulin Resistance in Patients with Type 2 Diabetes Mellitus.
- Mi Kyoung Park, Duk Kyu Kim
- Korean Diabetes J. 2000;24(6):678-688. Published online January 1, 2001
- 839 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
It has been indicated that micronized fenofibrate therapy changes the atherogenic lipid profile into more favorable lipid profile in patients with type 2 diabetes and dyslipidemia. The aim of this study is to evaluate the effect of micronized fenofibrate on the plasma levels of glycated LDL-C, Lp(a), FFA and insulin resistance in patients with type 2 diabetes and dyslipidemia. METHODS: Forty-seven patients with type 2 diabetes (M/F=23/24, mean age 57 +/- 7 yrs) were studied who had relatively good glycemic index (HbA1c < 8.0%) but dyslipidemia (i.e., dyslipidemia : TG >2.25 mmol/L or HDL-C < 0.90 mmol/L or LDL-C >3.36 mmol/L). All the patients were maintained by the previous method of glucose control without change during entire period of the study. The patients were randomized to drug group (Lipidil ) or placebo group for 12 weeks and measured for fasting plasma levels of lipid, glycated LDL-C, Lp(a), insulin, C-peptide, glucose. The results were compared before and after the administration. RESULTS: Micronized fenofibrate therapy significantly reduced the plasma levels of triglyceride, total cholesterol, LDL-C, TC/HDL-C (p<0.0001), FFA (p<0.05) and ele vated the level of HDL-C (p<0.0001) after 12 weeks administration. However, no significant(-3.6%) changes were observed in the level of Lp(a) . In both groups, the plasma levels of glycated LDL-C were elevated even though the glycemic controls were good (drug group: 0.09+/-0.05 mmol/L, placebo group: 0.10+/-0.03 mmol/L), but no significant changes were noticed after administration for 12 weeks (-13.5%, +4.8%, respectively). HOMA-IR index was significantly decreased in the drug group after administration (p<0.01). The change of plasma insulin level was significantly different when compared to that of the placebo group (p<0.05). The plasma level of C-peptide and glycemic indexes (FBS and HbA1c) were not changed significant. CONCLUSION: Micronized fenofibrate therapy for 12 weeks was very effective for control of diabetic dyslipidemia. It significantly reduced FFA to improve the insulin resistance, but it didn't improve the elevated plasma level of glycated LDL-C and Lp(a).

 KDA
KDA
 First
First Prev
Prev





