
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Articles
- Drug/Regimen
- Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial
- Ji-Yeon Park, Joonyub Lee, Yoon-Hee Choi, Kyung Wan Min, Kyung Ah Han, Kyu Jeung Ahn, Soo Lim, Young-Hyun Kim, Chul Woo Ahn, Kyung Mook Choi, Kun-Ho Yoon, the Practical Evidence of Antidiabetic Combination Therapy in Korea (PEAK) study investigators
- Received August 7, 2023 Accepted November 30, 2023 Published online April 23, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0259 [Epub ahead of print]

- 191 View
- 8 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Guidelines for switching to triple combination therapy directly after monotherapy failure are limited. This study investigated the efficacy, long-term sustainability, and safety of either mono or dual add-on therapy using alogliptin and pioglitazone for patients with type 2 diabetes mellitus (T2DM) who did not achieve their target glycemic range with metformin monotherapy.
Methods
The Practical Evidence of Antidiabetic Combination Therapy in Korea (PEAK) was a multicenter, placebo-controlled, double-blind, randomized trial. A total of 214 participants were randomized to receive alogliptin+pioglitazone (Alo+Pio group, n=70), alogliptin (Alo group, n=75), or pioglitazone (Pio group, n=69). The primary outcome was the difference in glycosylated hemoglobin (HbA1c) levels between the three groups at baseline to 24 weeks. For durability, the achievement of HbA1c levels <7% and <6.5% was compared in each group. The number of adverse events was investigated for safety.
Results
After 24 weeks of treatment, the change of HbA1c in the Alo+Pio, Alo, and Pio groups were –1.38%±0.08%, –1.03%±0.08%, and –0.84%±0.08%, respectively. The Alo+Pio group had significantly lower HbA1c levels than the other groups (P=0.0063, P<0.0001) and had a higher proportion of patients with target HbA1c achievement. In addition, insulin sensitivity and β-cell function, lipid profiles, and other metabolic indicators were also improved. There were no significant safety issues in patients treated with triple combination therapy.
Conclusion
Early combination triple therapy showed better efficacy and durability than the single add-on (dual) therapy. Therefore, combination therapy with metformin, alogliptin, and pioglitazone is a valuable early treatment option for T2DM poorly controlled with metformin monotherapy.
- Type 1 Diabetes
- A New Tool to Identify Pediatric Patients with Atypical Diabetes Associated with Gene Polymorphisms
- Sophie Welsch, Antoine Harvengt, Paola Gallo, Manon Martin, Dominique Beckers, Thierry Mouraux, Nicole Seret, Marie-Christine Lebrethon, Raphaël Helaers, Pascal Brouillard, Miikka Vikkula, Philippe A. Lysy
- Received May 26, 2023 Accepted November 25, 2023 Published online March 22, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0166 [Epub ahead of print]
- 779 View
- 48 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
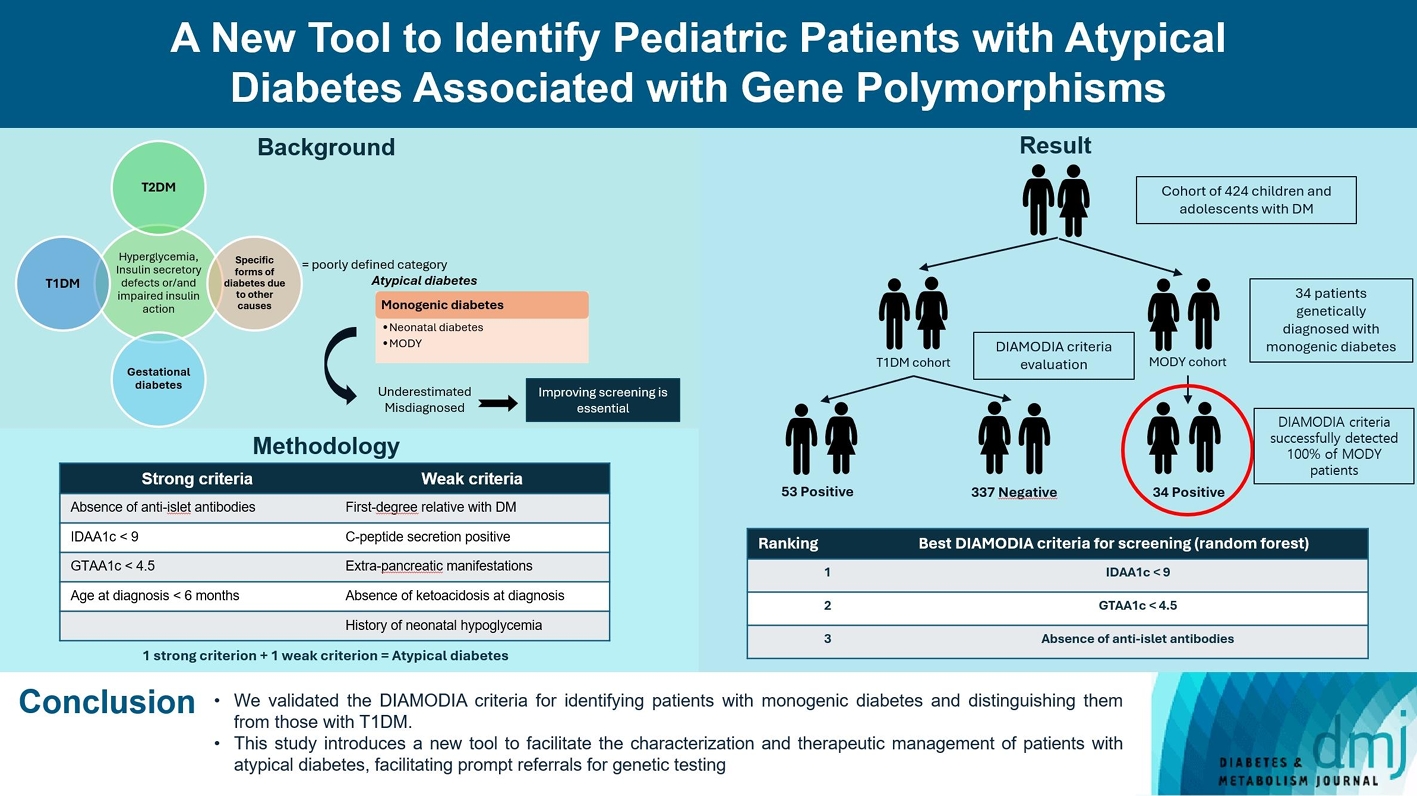
Recent diabetes subclassifications have improved the differentiation between patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus despite several overlapping features, yet without considering genetic forms of diabetes. We sought to facilitate the identification of monogenic diabetes by creating a new tool that we validated in a pediatric maturity-onset diabetes of the young (MODY) cohort.
Methods
We first created the DIAgnose MOnogenic DIAbetes (DIAMODIA) criteria based on the pre-existing, but incomplete, MODY calculator. This new score is composed of four strong and five weak criteria, with patients having to display at least one weak and one strong criterion.
Results
The effectiveness of the DIAMODIA criteria was evaluated in two patient cohorts, the first consisting of patients with confirmed MODY diabetes (n=34) and the second of patients with T1DM (n=390). These DIAMODIA criteria successfully detected 100% of MODY patients. Multiple correspondence analysis performed on the MODY and T1DM cohorts enabled us to differentiate MODY patients from T1DM. The three most relevant variables to distinguish a MODY from T1DM profile were: lower insulin-dose adjusted A1c score ≤9, glycemic target-adjusted A1c score ≤4.5, and absence of three anti-islet cell autoantibodies.
Conclusion
We validated the DIAMODIA criteria, as it effectively identified all monogenic diabetes patients (MODY cohort) and succeeded to differentiate T1DM from MODY patients. The creation of this new and effective tool is likely to facilitate the characterization and therapeutic management of patients with atypical diabetes, and promptly referring them for genetic testing which would markedly improve clinical care and counseling, as well.
- Complications
- Switching from Conventional Fibrates to Pemafibrate Has Beneficial Effects on the Renal Function of Diabetic Subjects with Chronic Kidney Disease
- Rimi Izumihara, Hiroshi Nomoto, Kenichi Kito, Yuki Yamauchi, Kazuno Omori, Yui Shibayama, Shingo Yanagiya, Aika Miya, Hiraku Kameda, Kyu Yong Cho, So Nagai, Ichiro Sakuma, Akinobu Nakamura, Tatsuya Atsumi, on Behalf of the PARM-TD Study Group
- Received October 15, 2023 Accepted November 22, 2023 Published online February 29, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0370 [Epub ahead of print]
- 703 View
- 129 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Fibrates have renal toxicity limiting their use in subjects with chronic kidney disease (CKD). However, pemafibrate has fewer toxic effects on renal function. In the present analysis, we evaluated the effects of pemafibrate on the renal function of diabetic subjects with or without CKD in a real-world clinical setting.
Methods
We performed a sub-analysis of data collected during a multi-center, prospective, observational study of the effects of pemafibrate on lipid metabolism in subjects with type 2 diabetes mellitus complicated by hypertriglyceridemia (the PARM-T2D study). The participants were allocated to add pemafibrate to their existing regimen (ADD-ON), switch from their existing fibrate to pemafibrate (SWITCH), or continue conventional therapy (CTRL). The changes in estimated glomerular filtration rate (eGFR) over 52 weeks were compared among these groups as well as among subgroups created according to CKD status.
Results
Data for 520 participants (ADD-ON, n=166; SWITCH, n=96; CTRL, n=258) were analyzed. Of them, 56.7% had CKD. The eGFR increased only in the SWITCH group, and this trend was also present in the CKD subgroup (P<0.001). On the other hand, eGFR was not affected by switching in participants with severe renal dysfunction (G3b or G4) and/or macroalbuminuria. Multivariate analysis showed that being older and a switch from fenofibrate were associated with elevation in eGFR (both P<0.05).
Conclusion
A switch to pemafibrate may be associated with an elevation in eGFR, but to a lesser extent in patients with poor renal function.
- Drug/Regimen
- Efficacy and Safety of IDegAsp in a Real-World Korean Population with Type 2 Diabetes Mellitus
- Shinae Kang, Yu-Bae Ahn, Tae Keun Oh, Won-Young Lee, Sung Wan Chun, Boram Bae, Amine Dahaoui, Jin Sook Jeong, Sungeun Jung, Hak Chul Jang
- Received August 24, 2023 Accepted November 22, 2023 Published online February 27, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0297 [Epub ahead of print]
- 644 View
- 42 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
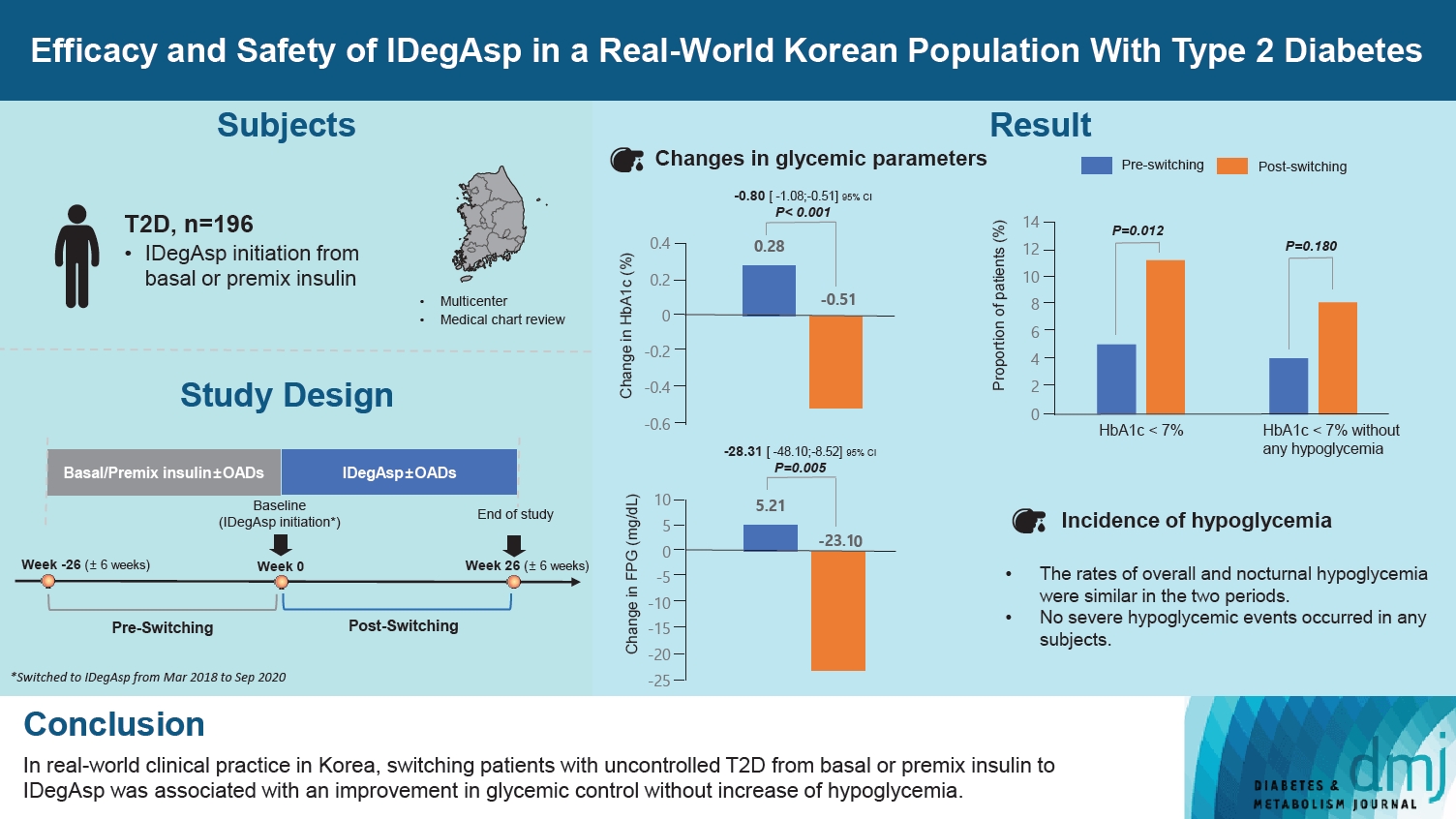
This study investigated the real-world efficacy and safety of insulin degludec/insulin aspart (IDegAsp) in Korean adults with type 2 diabetes mellitus (T2DM), whose insulin treatment was switched to IDegAsp.
Methods
This was a multicenter, retrospective, observational study comprising two 26-week treatment periods, before and after switching to IDegAsp, respectively. Korean adults with uncontrolled T2DM treated with basal or premix insulin (±oral antidiabetic drugs) were enrolled. The primary objective was to compare the degree of glycosylated hemoglobin (HbA1c) change in each 26-week observation period. The analyses included changes in HbA1c, fasting plasma glucose (FPG), body weight, proportion of participants achieving HbA1c <7.0%, hypoglycemic events, and total daily insulin dose (ClinicalTrials.gov, number NCT04656106).
Results
In total, 196 adults (mean age, 65.95 years; mean T2DM duration, 18.99 years) were analyzed. The change in both HbA1c and FPG were significantly different between the pre-switching and the post-switching period (0.28% vs. –0.51%, P<0.001; 5.21 mg/dL vs. –23.10 mg/dL, P=0.005), respectively. After switching, the rate of achieving HbA1c <7.0% was significantly improved (5.10% at baseline vs. 11.22% with IDegAsp, P=0.012). No significant differences (before vs. after switching) were observed in body weight change, and total daily insulin dose. The rates of overall and severe hypoglycemia were similar in the two periods.
Conclusion
In real-world clinical practice in Korea, the change of insulin regimen to IDegAsp was associated with an improvement in glycemic control without increase of hypoglycemia, supporting the use of IDegAsp for patients with T2DM uncontrolled with basal or premix insulin.
- Complications
- Does the Relationship of the Autonomic Symptoms Questionnaire COMPASS 31 with Cardiovascular Autonomic Tests Differ between Type 1 and Type 2 Diabetes Mellitus?
- Ilenia D’Ippolito, Marika Menduni, Cinzia D’Amato, Aikaterini Andreadi, Davide Lauro, Vincenza Spallone
- Received August 28, 2023 Accepted November 22, 2023 Published online February 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0301 [Epub ahead of print]

- 601 View
- 43 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The aim was to investigate if autonomic symptoms questionnaire Composite Autonomic Symptom Score (COMPASS) 31 has different association with cardiovascular autonomic neuropathy (CAN) and diagnostic performance between type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).
Methods
Seventy-nine participants with T1DM and 140 with T2DM completed COMPASS 31 before cardiovascular reflex tests (CARTs) for CAN, and assessment of symptoms, signs, vibration, and thermal perception thresholds for diabetic polyneuropathy (DPN) diagnosis.
Results
COMPASS 31 total weighted score (TWS) was similar in the two groups, but significantly associated with confirmed CAN only in T1DM (P=0.0056) and not T2DM group (P=0.1768) and correlated with CARTs score more strongly in T1DM (rho=0.356, P=0.0016) than in T2DM group (rho=0.084, P=0.3218) (P=0.016). Only in T1DM and not T2DM group, the area under the receiver operating characteristic curve (AUC) reached a fair diagnostic accuracy (>0.7) for confirmed CAN (0.73±0.07 vs. 0.61±0.08) and DPN (0.75±0.06 vs. 0.68±0.05), although without a significant difference. COMPASS 31 TWS (cut-off 16.44) reached acceptable diagnostic performance in T1DM, with sensitivity for confirmed CAN 81.2% and sensitivity and specificity for DPN 76.3% and 78%, compared to T2DM group (all <70%). AUC for DPN of orthostatic intolerance domain was higher in T1DM compared to T2DM group (0.73±0.05 vs. 0.58±0.04, P=0.027).
Conclusion
COMPASS 31 is more weakly related to CAN in T2DM than in T1DM, with a fair diagnostic accuracy for confirmed CAN only in T1DM. This difference supports a multifactorial origin of symptoms and should be considered when using COMPASS 31.
Sulwon Lecture 2023
- Metabolic Risk/Epidemiology
- Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
- Inha Jung, Dae-Jeong Koo, Won-Young Lee
- Received October 4, 2023 Accepted December 26, 2024 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0350 [Epub ahead of print]
- 965 View
- 59 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
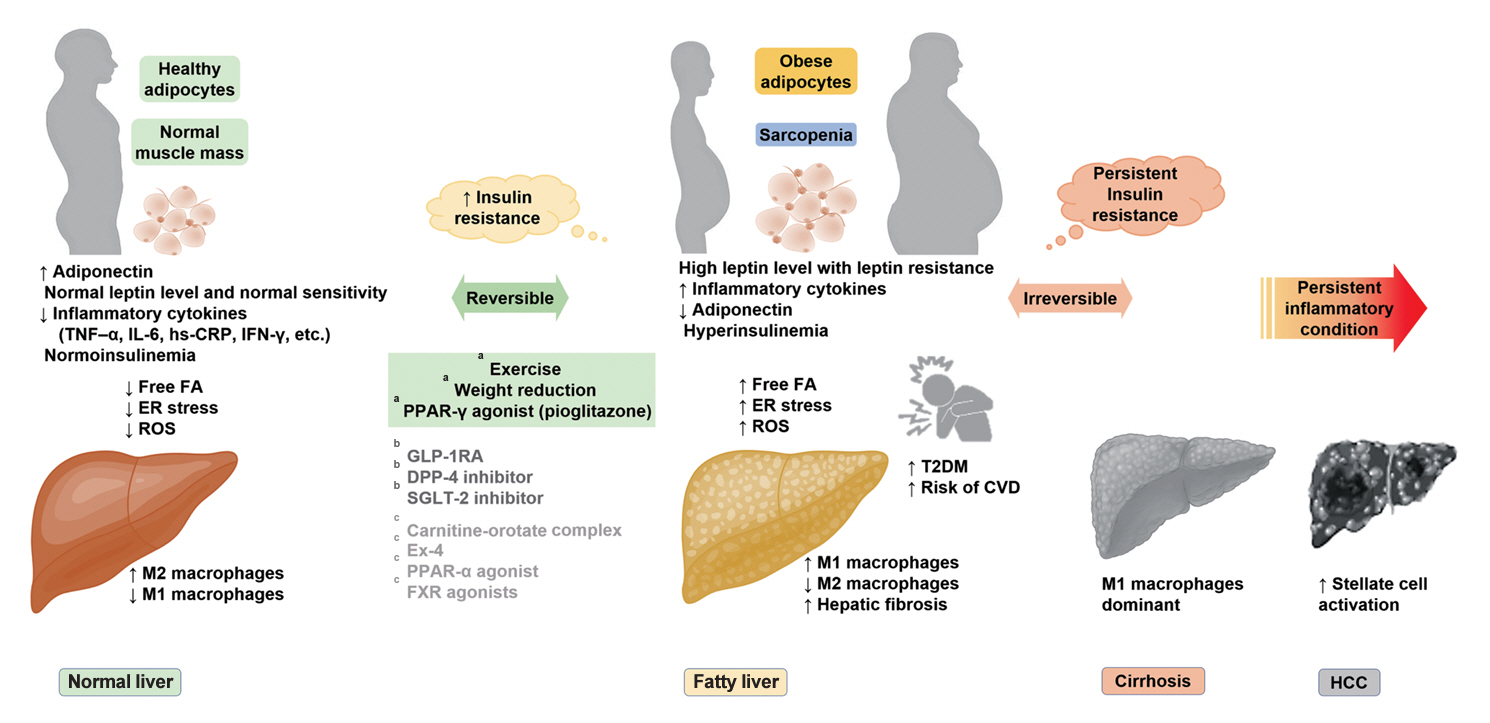
ePub - It has been generally accepted that insulin resistance (IR) and reduced insulin secretory capacity are the basic pathogenesis of type 2 diabetes mellitus (T2DM). In addition to genetic factors, the persistence of systemic inflammation caused by obesity and the associated threat of lipotoxicity increase the risk of T2DM. In particular, the main cause of IR is obesity and subjects with T2DM have a higher body mass index (BMI) than normal subjects according to recent studies. The prevalence of T2DM with IR has increased with increasing BMI during the past three decades. According to recent studies, homeostatic model assessment of IR was increased compared to that of the 1990s. Rising prevalence of obesity in Korea have contributed to the development of IR, non-alcoholic fatty liver disease and T2DM and cutting this vicious cycle is important. My colleagues and I have investigated this pathogenic mechanism on this theme through clinical and experimental studies over 20 years and herein, I would like to summarize some of our studies with deep gratitude for receiving the prestigious 2023 Sulwon Award.
Original Articles
- Drug/Regimen
- Pioglitazone as Add-on THERAPY in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Ji Hye Heo, Kyung Ah Han, Jun Hwa Hong, Hyun-Ae Seo, Eun-Gyoung Hong, Jae Myung Yu, Hye Seung Jung, Bong-Soo Cha
- Received September 1, 2023 Accepted October 25, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0314 [Epub ahead of print]
- 1,199 View
- 114 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
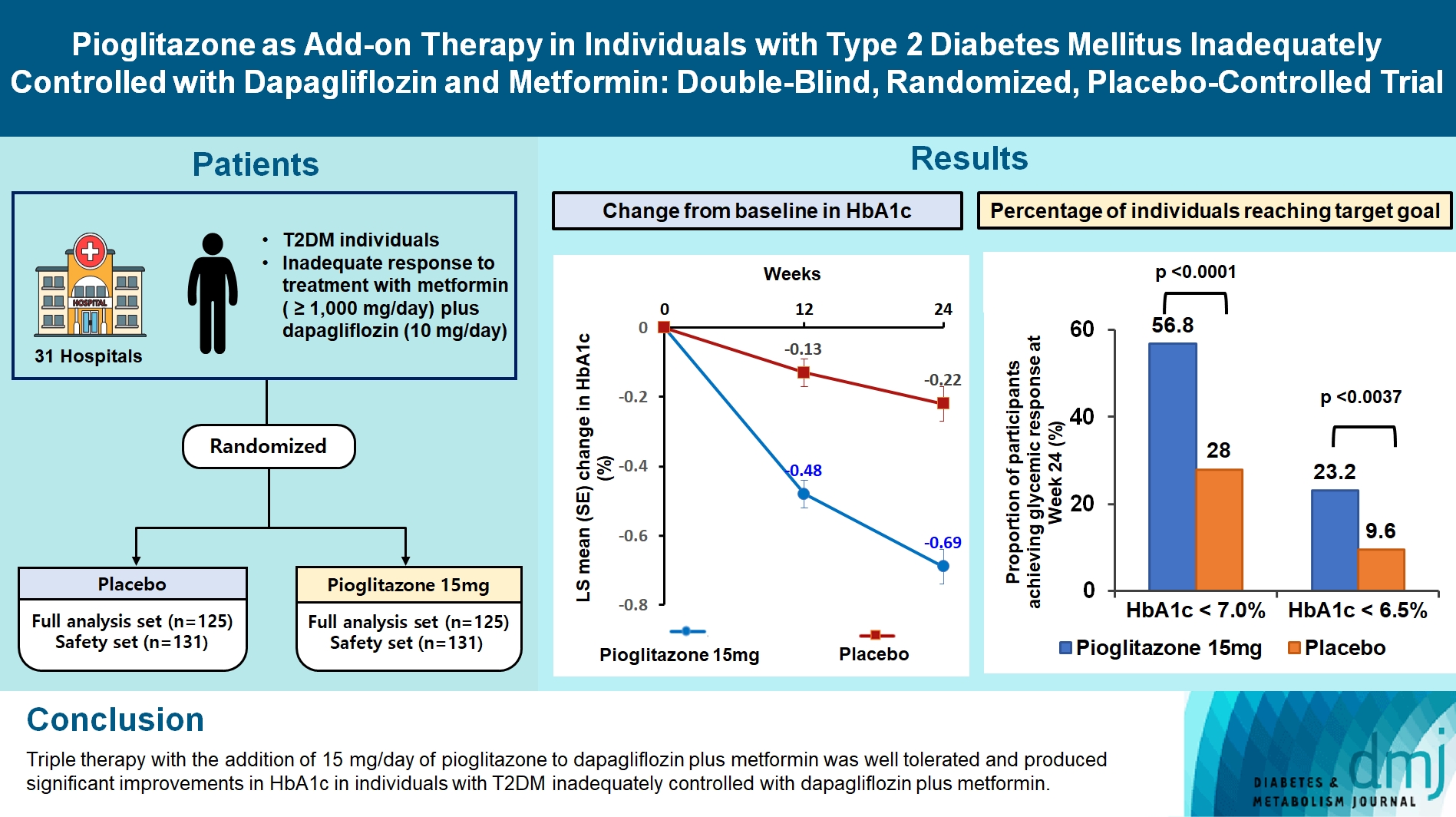
This study assessed the efficacy and safety of triple therapy with pioglitazone 15 mg add-on versus placebo in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, patients with T2DM with an inadequate response to treatment with metformin (≥1,000 mg/day) plus dapagliflozin (10 mg/day) were randomized to receive additional pioglitazone 15 mg/day (n=125) or placebo (n=125) for 24 weeks. The primary endpoint was the change in glycosylated hemoglobin (HbA1c) levels from baseline to week 24 (ClinicalTrials.gov identifier: NCT05101135).
Results
At week 24, the adjusted mean change from baseline in HbA1c level compared with placebo was significantly greater with pioglitazone treatment (–0.47%; 95% confidence interval, –0.61 to –0.33; P<0.0001). A greater proportion of patients achieved HbA1c <7% or <6.5% at week 24 with pioglitazone compared to placebo as add-on to 10 mg dapagliflozin and metformin (56.8% vs. 28% for HbA1c <7%, and 23.2% vs. 9.6% for HbA1c <6.5%; P<0.0001 for all). The addition of pioglitazone also significantly improved triglyceride, highdensity lipoprotein cholesterol levels, and homeostatic model assessment of insulin resistance levels, while placebo did not. The incidence of treatment-emergent adverse events was similar between the groups, and the incidence of fluid retention-related side effects by pioglitazone was low (1.5%).
Conclusion
Triple therapy with the addition of 15 mg/day of pioglitazone to dapagliflozin plus metformin was well tolerated and produced significant improvements in HbA1c in patients with T2DM inadequately controlled with dapagliflozin plus metformin.
- Lifestyle
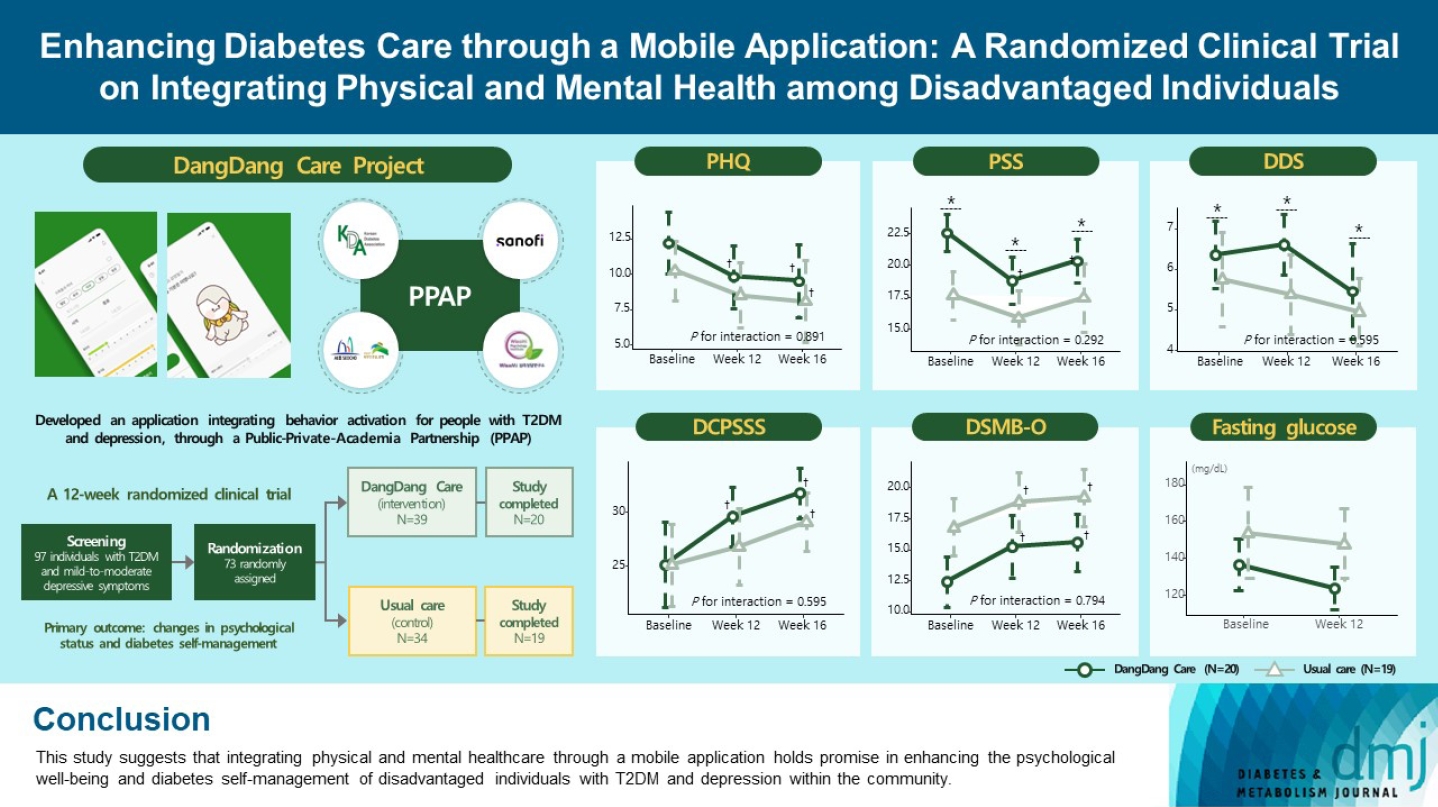
- Enhancing Diabetes Care through a Mobile Application: A Randomized Clinical Trial on Integrating Physical and Mental Health among Disadvantaged Individuals
- Jae Hyun Bae, Eun Hee Park, Hae Kyung Lee, Kun Ho Yoon, Kyu Chang Won, Hyun Mi Kim, Sin Gon Kim
- Received August 24, 2023 Accepted October 16, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0298 [Epub ahead of print]
- 689 View
- 95 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study examines integrating physical and mental healthcare for disadvantaged persons with type 2 diabetes mellitus and mild-to-moderate depression in the community, using a mobile application within a public-private-academic partnership.
Methods
The Korean Diabetes Association has developed a mobile application combining behavioral activation for psychological well-being and diabetes self-management, with conventional medical therapy. Participants were randomly assigned to receive the application with usual care or only usual care. Primary outcomes measured changes in psychological status and diabetes selfmanagement through questionnaires at week 12 from the baseline. Secondary outcomes assessed glycemic and lipid control, with psychological assessments at week 16.
Results
Thirty-nine of 73 participants completed the study (20 and 19 in the intervention and control groups, respectively) and were included in the analysis. At week 12, the intervention group showed significant reductions in depression severity and perceived stress compared to the control group. Additionally, they reported increased perceived social support and demonstrated improved diabetes self-care behavior. These positive effects persisted through week 16, with the added benefit of reduced anxiety. While fasting glucose levels in the intervention group tended to improve, no other significant differences were observed in laboratory assessments between the groups.
Conclusion
This study provides compelling evidence for the potential efficacy of a mobile application that integrates physical and mental health components to address depressive symptoms and enhance diabetes self-management in disadvantaged individuals with type 2 diabetes mellitus and depression. Further research involving larger and more diverse populations is warranted to validate these findings and solidify their implications.
- Drug/Regimen
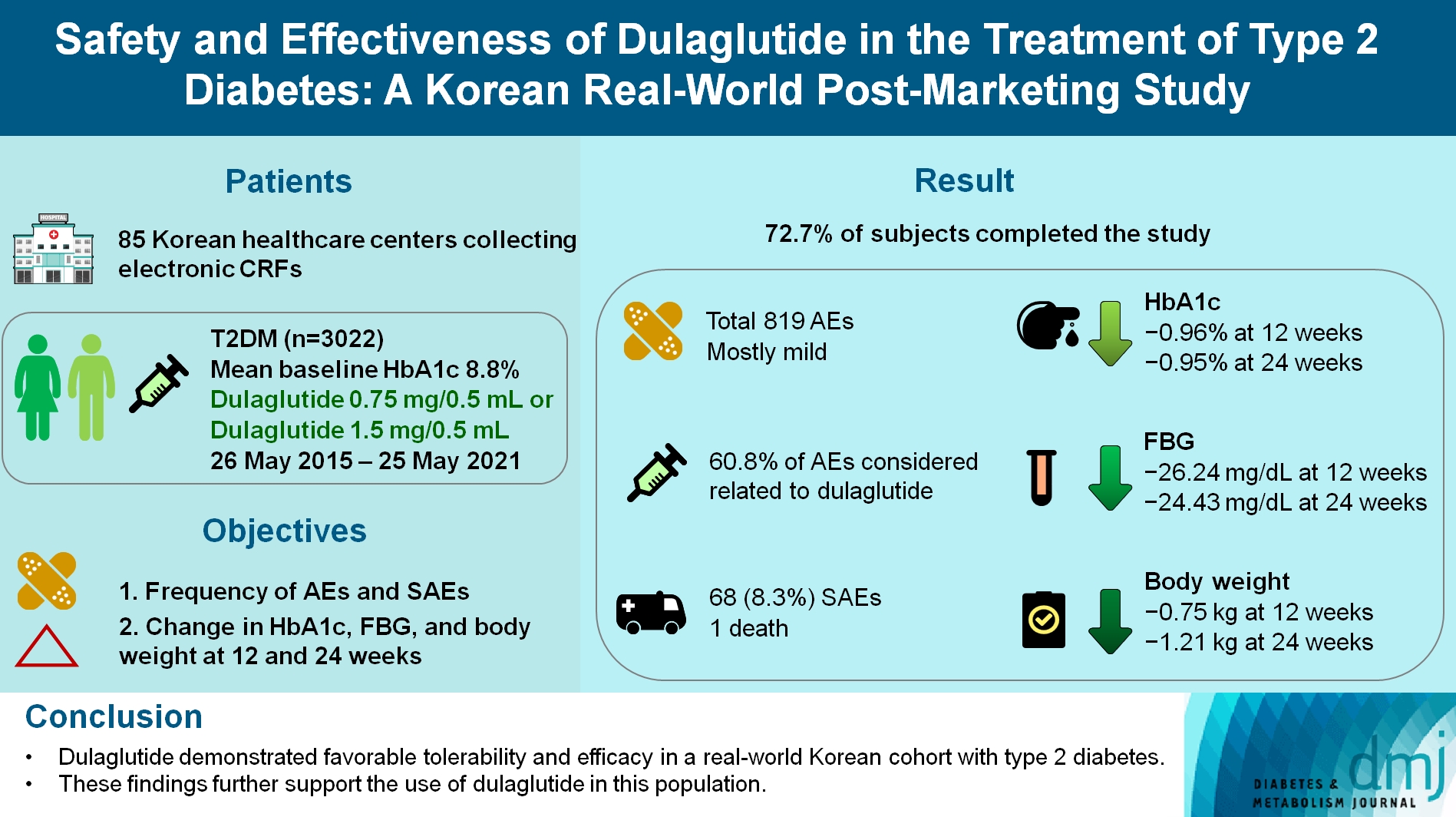
- Safety and Effectiveness of Dulaglutide in the Treatment of Type 2 Diabetes Mellitus: A Korean Real-World Post-Marketing Study
- Jeonghee Han, Woo Je Lee, Kyu Yeon Hur, Jae Hyoung Cho, Byung Wan Lee, Cheol-Young Park
- Received February 3, 2023 Accepted July 10, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0030 [Epub ahead of print]
- 593 View
- 53 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader - Background
To investigate the real-world safety and effectiveness of dulaglutide in Korean adults with type 2 diabetes mellitus (T2DM).
Methods
This was a real-world, prospective, non-interventional post-marketing safety study conducted from May 26, 2015 to May 25, 2021 at 85 Korean healthcare centers using electronic case data. Data on patients using dulaglutide 0.75 mg/0.5 mL or the dulaglutide 1.5 mg/0.5 mL single-use pens were collected and pooled. The primary objective was to report the frequency and proportion of adverse and serious adverse events that occurred. The secondary objective was to monitor the effectiveness of dulaglutide at 12 and 24 weeks by evaluating changes in glycosylated hemoglobin (HbA1c ), fasting plasma glucose, and body weight.
Results
Data were collected from 3,067 subjects, and 3,022 subjects who received ≥1 dose (of any strength) of dulaglutide were included in the safety analysis set (53% female, mean age 56 years; diabetes duration 11.2 years, mean HbA1c 8.8%). The number of adverse events reported was 819; of these, 68 (8.3%) were serious adverse events. One death was reported. Adverse events were mostly mild in severity; 60.81% of adverse events were considered related to dulaglutide. This study was completed by 72.73% (2,198/3,022) of subjects. At 12/24 weeks there were significant (P<0.0001) reductions from baseline in least-squares mean HbA1c (0.96%/0.95%), fasting blood glucose (26.24/24.43 mg/dL), and body weight (0.75/1.21 kg).
Conclusion
Dulaglutide was generally well tolerated and effective in real-world Korean individuals with T2DM. The results from this study contribute to the body of evidence for dulaglutide use in this population.
Review
- Metabolic Risk/Epidemiology
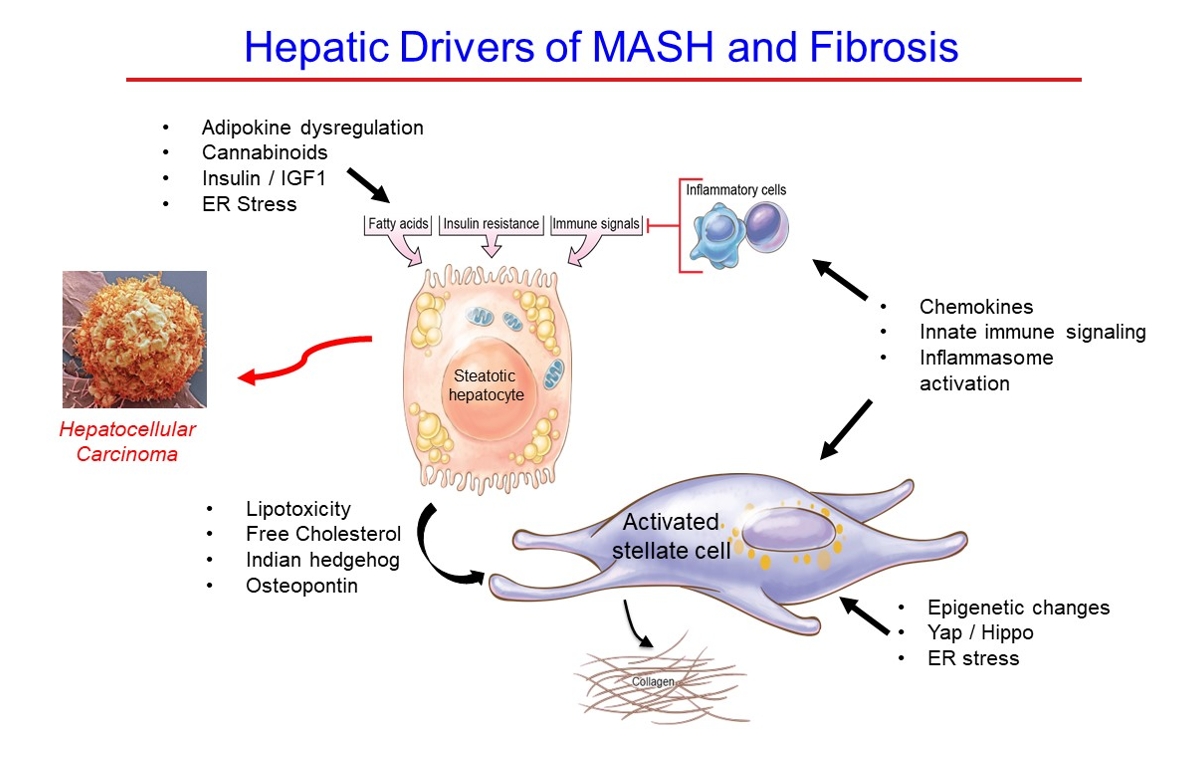
- Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome
- Scott L. Friedman
- Diabetes Metab J. 2024;48(2):161-169. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0240
- 2,246 View
- 273 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Metabolic dysfunction-associated steatotic (fatty) liver disease (MASLD), previously termed non-alcoholic fatty liver disease, is a worldwide epidemic that can lead to hepatic inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). The disease is typically a component of the metabolic syndrome that accompanies obesity, and is often overlooked because the liver manifestations are clinically silent until late-stage disease is present (i.e., cirrhosis). Moreover, Asian populations, including Koreans, have a higher fraction of patients who are lean, yet their illness has the same prognosis or worse than those who are obese. Nonetheless, ongoing injury can lead to hepatic inflammation and ballooning of hepatocytes as classic features. Over time, fibrosis develops following activation of hepatic stellate cells, the liver’s main fibrogenic cell type. The disease is usually more advanced in patients with type 2 diabetes mellitus, indicating that all diabetic patients should be screened for liver disease. Although there has been substantial progress in clarifying pathways of injury and fibrosis, there no approved therapies yet, but current research seeks to uncover the pathways driving hepatic inflammation and fibrosis, in hopes of identifying new therapeutic targets. Emerging molecular methods, especially single cell sequencing technologies, are revolutionizing our ability to clarify mechanisms underlying MASLD-associated fibrosis and HCC.
Original Articles
- Cardiovascular Risk/Epidemiology
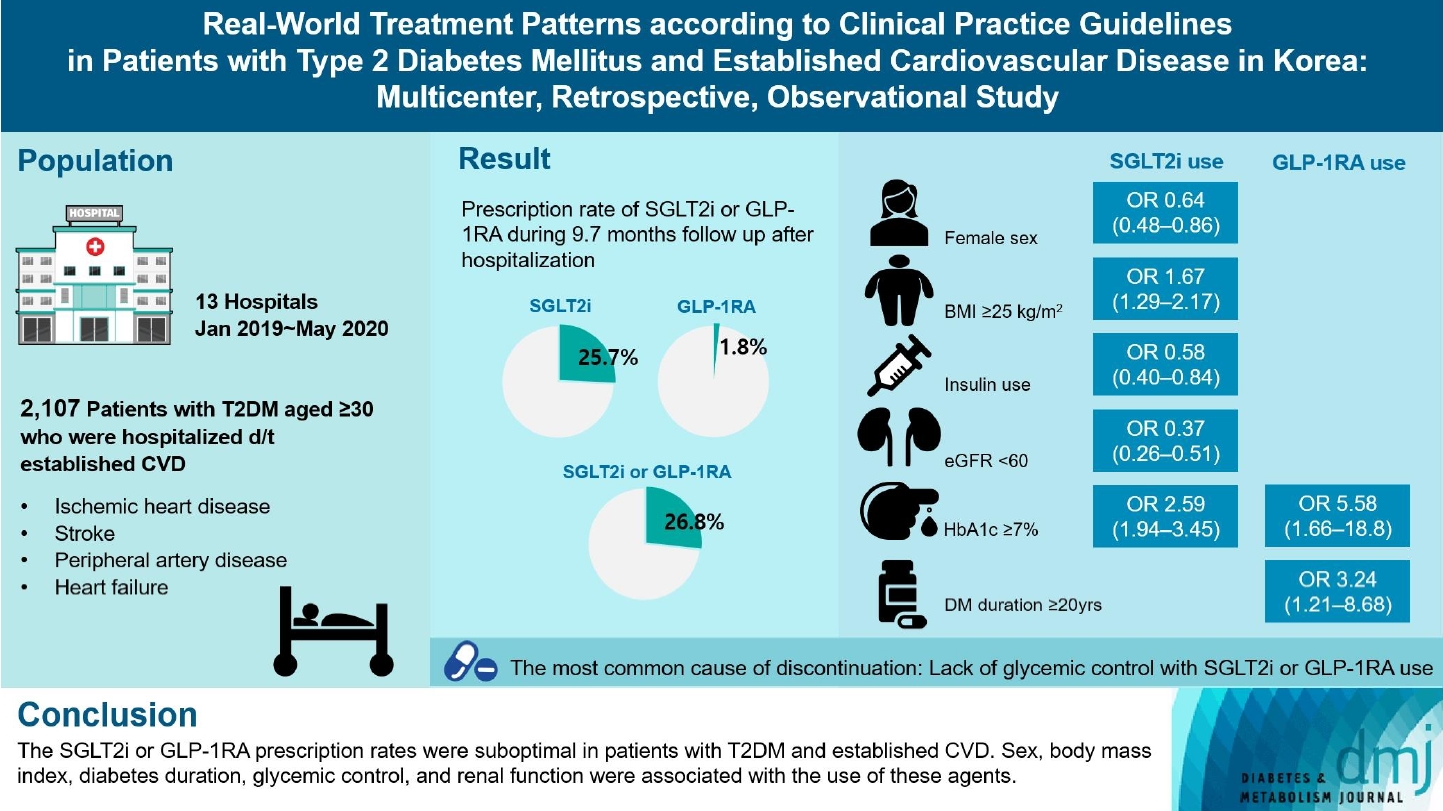
- Real-World Treatment Patterns according to Clinical Practice Guidelines in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease in Korea: Multicenter, Retrospective, Observational Study
- Ye Seul Yang, Nam Hoon Kim, Jong Ha Baek, Seung-Hyun Ko, Jang Won Son, Seung-Hwan Lee, Sang Youl Rhee, Soo-Kyung Kim, Tae Seo Sohn, Ji Eun Jun, In-Kyung Jeong, Chong Hwa Kim, Keeho Song, Eun-Jung Rhee, Junghyun Noh, Kyu Yeon Hur, Committee of Clinical Practice Guidelines, Korean Diabetes Association
- Diabetes Metab J. 2024;48(2):279-289. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0225
- 1,102 View
- 148 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Recent diabetes management guidelines recommend that sodium-glucose cotransporter 2 inhibitors (SGLT2is) or glucagon-like peptide 1 receptor agonists (GLP-1RAs) with proven cardiovascular benefits should be prioritized for combination therapy in patients with type 2 diabetes mellitus (T2DM) and established cardiovascular disease (CVD). This study was aimed at evaluating SGLT2i or GLP-1RA usage rates and various related factors in patients with T2DM and established CVD.
Methods
We enrolled adults with T2DM aged ≥30 years who were hospitalized due to established CVD from January 2019 to May 2020 at 13 secondary and tertiary hospitals in Korea in this retrospective observational study.
Results
Overall, 2,050 patients were eligible for analysis among 2,107 enrolled patients. The mean patient age, diabetes duration, and glycosylated hemoglobin level were 70.0 years, 12.0 years, and 7.5%, respectively. During the mean follow-up duration of 9.7 months, 25.7% of the patients were prescribed SGLT2is after CVD events. However, only 1.8% were prescribed GLP-1RAs. Compared with SGLT2i non-users, SGLT2i users were more frequently male and obese. Furthermore, they had a shorter diabetes duration but showed worse glycemic control and better renal function at the time of the event. GLP-1RA users had a longer duration of diabetes and worse glycemic control at the time of the event than GLP-1RA non-users.
Conclusion
The SGLT2i or GLP-1RA prescription rates were suboptimal in patients with T2DM and established CVD. Sex, body mass index, diabetes duration, glycemic control, and renal function were associated with the use of these agents. -
Citations
Citations to this article as recorded by- Enhancing Patient Outcomes: Prioritizing SGLT2is and GLP-1RAs in Diabetes with CVD
Gwanpyo Koh
Diabetes & Metabolism Journal.2024; 48(2): 208. CrossRef
- Enhancing Patient Outcomes: Prioritizing SGLT2is and GLP-1RAs in Diabetes with CVD
- Drug/Regimen
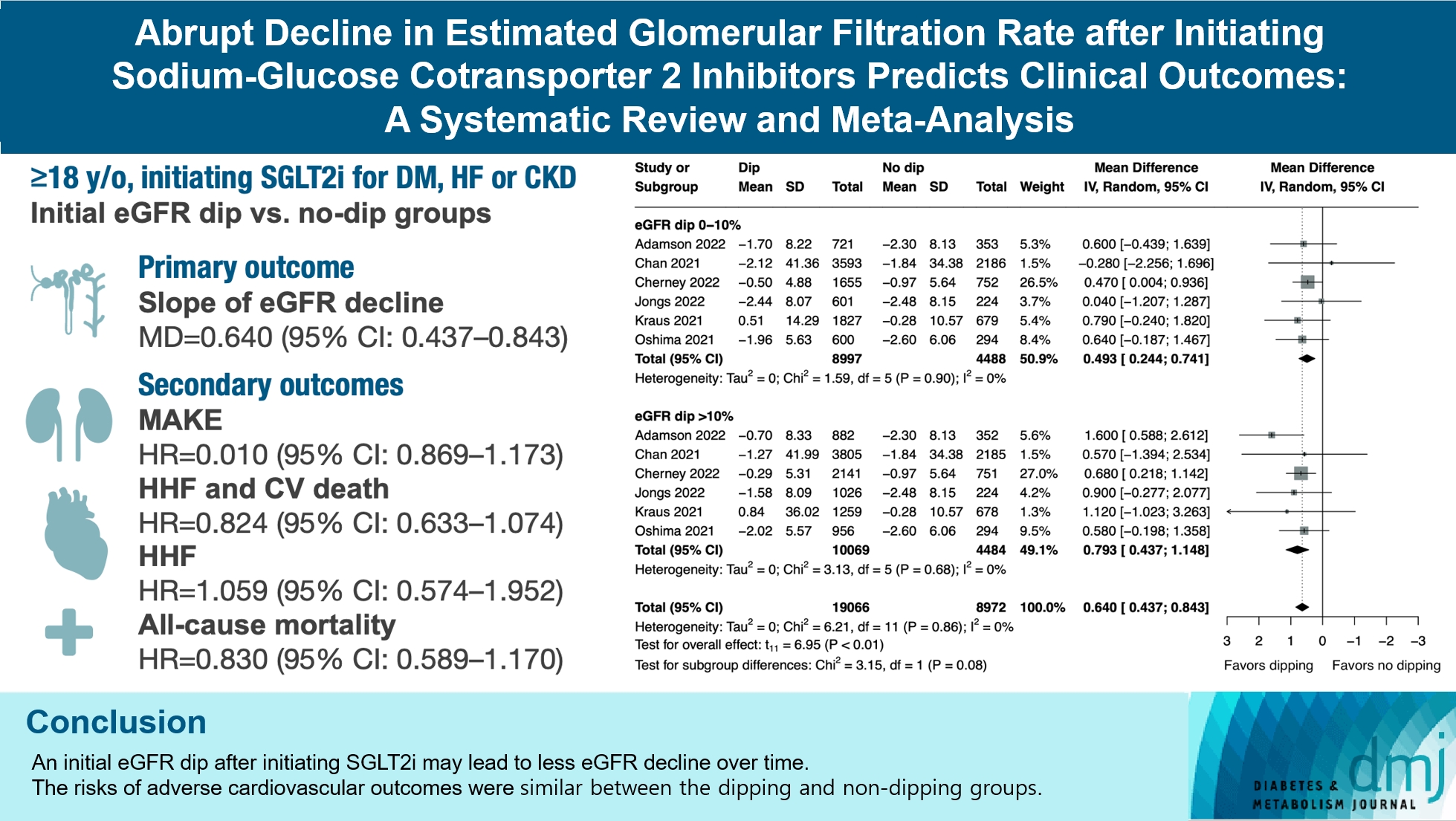
- Abrupt Decline in Estimated Glomerular Filtration Rate after Initiating Sodium-Glucose Cotransporter 2 Inhibitors Predicts Clinical Outcomes: A Systematic Review and Meta-Analysis
- Min-Hsiang Chuang, Yu-Shuo Tang, Jui-Yi Chen, Heng-Chih Pan, Hung-Wei Liao, Wen-Kai Chu, Chung-Yi Cheng, Vin-Cent Wu, Michael Heung
- Diabetes Metab J. 2024;48(2):242-252. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0201
- 1,510 View
- 202 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The initiation of sodium-glucose cotransporter-2 inhibitors (SGLT2i) typically leads to a reversible initial dip in estimated glomerular filtration rate (eGFR). The implications of this phenomenon on clinical outcomes are not well-defined.
Methods
We searched MEDLINE, Embase, and Cochrane Library from inception to March 23, 2023 to identify randomized controlled trials and cohort studies comparing kidney and cardiovascular outcomes in patients with and without initial eGFR dip after initiating SGLT2i. Pooled estimates were calculated using random-effect meta-analysis.
Results
We included seven studies in our analysis, which revealed that an initial eGFR dip following the initiation of SGLT2i was associated with less annual eGFR decline (mean difference, 0.64; 95% confidence interval [CI], 0.437 to 0.843) regardless of baseline eGFR. The risk of major adverse kidney events was similar between the non-dipping and dipping groups but reduced in patients with a ≤10% eGFR dip (hazard ratio [HR], 0.915; 95% CI, 0.865 to 0.967). No significant differences were observed in the composite of hospitalized heart failure and cardiovascular death (HR, 0.824; 95% CI, 0.633 to 1.074), hospitalized heart failure (HR, 1.059; 95% CI, 0.574 to 1.952), or all-cause mortality (HR, 0.83; 95% CI, 0.589 to 1.170). The risk of serious adverse events (AEs), discontinuation of SGLT2i due to AEs, kidney-related AEs, and volume depletion were similar between the two groups. Patients with >10% eGFR dip had increased risk of hyperkalemia compared to the non-dipping group.
Conclusion
Initial eGFR dip after initiating SGLT2i might be associated with less annual eGFR decline. There were no significant disparities in the risks of adverse cardiovascular outcomes between the dipping and non-dipping groups.
- Others
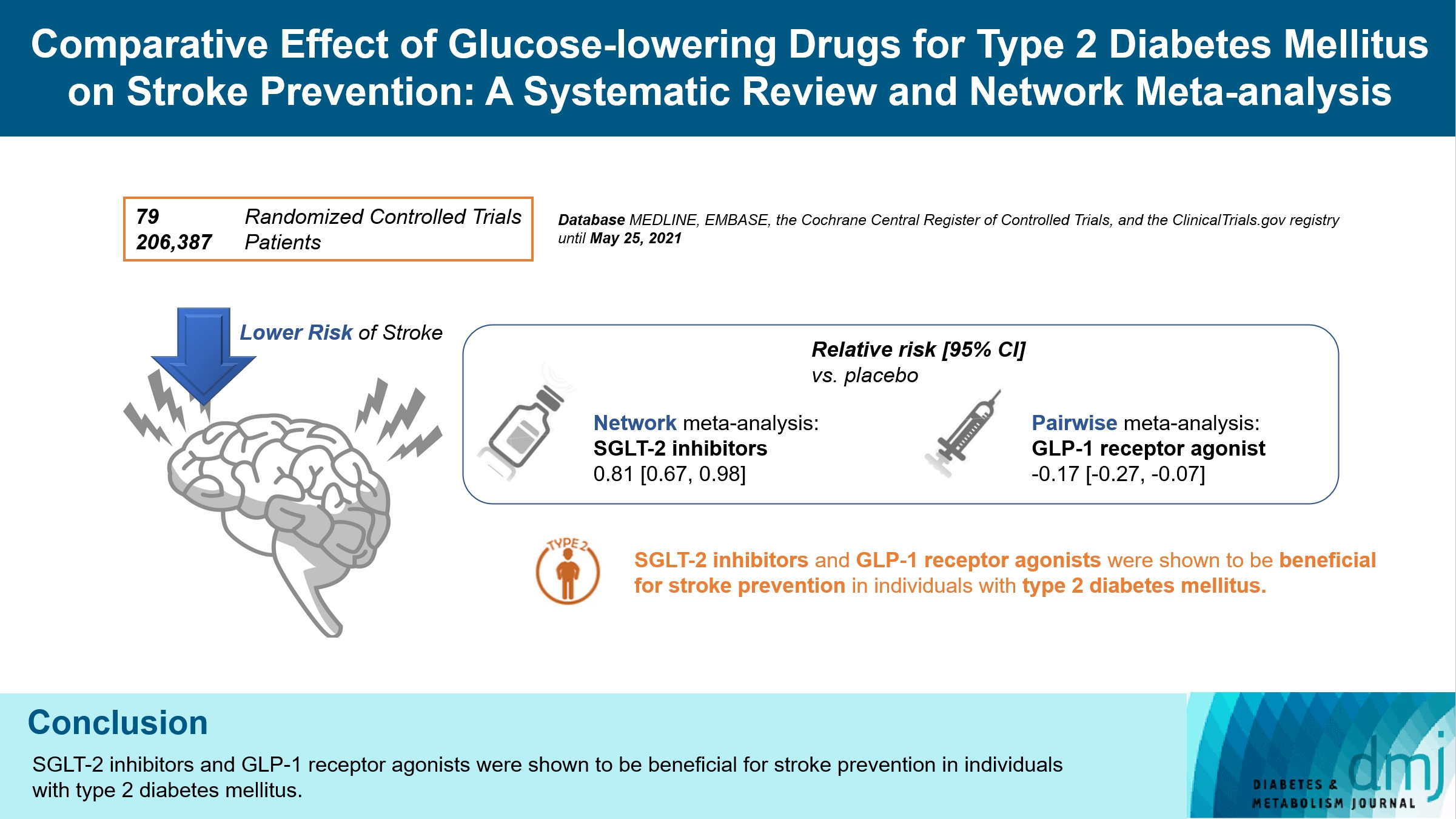
- Comparative Effect of Glucose-Lowering Drugs for Type 2 Diabetes Mellitus on Stroke Prevention: A Systematic Review and Network Meta-Analysis
- Ji Soo Kim, Gyeongsil Lee, Kyung-Il Park, Seung-Won Oh
- Diabetes Metab J. 2024;48(2):312-320. Published online January 26, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0421
- 1,213 View
- 202 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There is still a lack of research on which diabetic drugs are more effective in preventing stroke. Our network metaanalysis aimed to compare cerebrovascular benefits among glucose-lowering treatments.
Methods
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the ClinicalTrials.gov registry for clinical trials from inception through May 25, 2021. We included both prespecified cerebrovascular outcomes and cerebrovascular events reported as severe adverse events. Subgroup analyses were conducted by stroke subtype, publication type, age of patients, baseline glycosylated hemoglobin (HbA1c), duration of type 2 diabetes mellitus, and cardiovascular risks.
Results
Of 2,861 reports and 1,779 trials screened, 79 randomized controlled trials comprising 206,387 patients fulfilled the inclusion criteria. In the pairwise meta-analysis, the use of glucagon-like peptide-1 (GLP-1) agonist was associated with a lower risk of total stroke compared with placebo (relative risk [RR], –0.17; 95% confidence interval [CI], –0.27 to –0.07). In the network meta- analysis, only the use of sodium-glucose cotransporter-2 (SGLT-2) inhibitor was associated with a reduction of total stroke, compared with placebo (RR, 0.81; 95% CI, 0.67 to 0.98). In the subgroup analyses, the use of SGLT-2 inhibitor and GLP-1 agonist was associated with a lower risk of stroke in those with high HbA1c (≥8.0) and low-risk of cardiovascular disease, respectively.
Conclusion
SGLT-2 inhibitors and GLP-1 agonists were shown to be beneficial for stroke prevention in patients with type 2 diabetes mellitus. -
Citations
Citations to this article as recorded by- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes
Jae-Han Jeon
Diabetes & Metabolism Journal.2024; 48(2): 213. CrossRef - Reply to comment on: Association of glucose-lowering drugs with incident stroke and transient ischaemic attacks in primary care patients with type 2 diabetes: disease analyser database
Wolfgang Rathmann, Karel Kostev
Acta Diabetologica.2024;[Epub] CrossRef
- SGLT2 Inhibitors and GLP-1 Agonists: A Beacon of Hope for Stroke Prevention in Diabetes
Review
- Pathophysiology
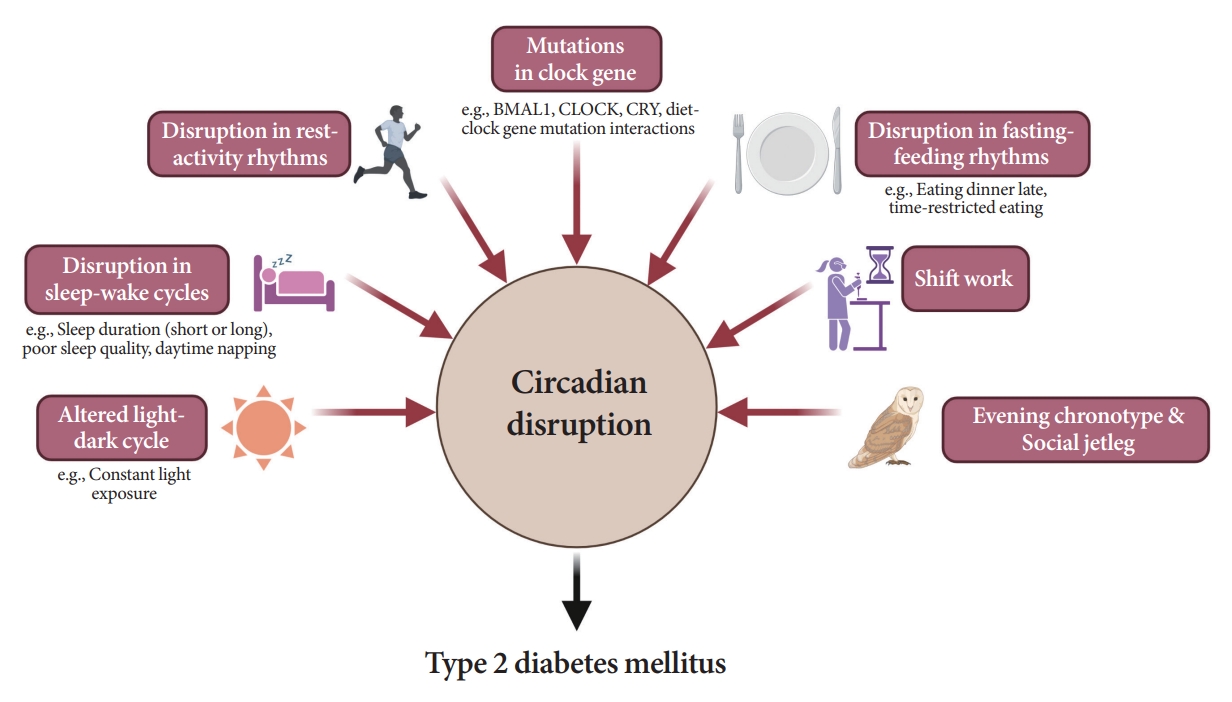
- Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
- Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
- Diabetes Metab J. 2024;48(1):37-52. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0193
- 2,159 View
- 219 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Novel strategies are required to reduce the risk of developing diabetes and/or clinical outcomes and complications of diabetes. In this regard, the role of the circadian system may be a potential candidate for the prevention of diabetes. We reviewed evidence from animal, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of diabetes. The circadian clock governs genetic, metabolic, hormonal, and behavioral signals in anticipation of cyclic 24-hour events through interactions between a “central clock” in the suprachiasmatic nucleus and “peripheral clocks” in the whole body. Currently, circadian rhythmicity in humans can be subjectively or objectively assessed by measuring melatonin and glucocorticoid levels, core body temperature, peripheral blood, oral mucosa, hair follicles, rest-activity cycles, sleep diaries, and circadian chronotypes. In this review, we summarized various circadian misalignments, such as altered light-dark, sleep-wake, rest-activity, fasting-feeding, shift work, evening chronotype, and social jetlag, as well as mutations in clock genes that could contribute to the development of diabetes and poor glycemic status in patients with diabetes. Targeting critical components of the circadian system could deliver potential candidates for the treatment and prevention of type 2 diabetes mellitus in the future.
Original Article
- Cardiovascular Risk/Epidemiology
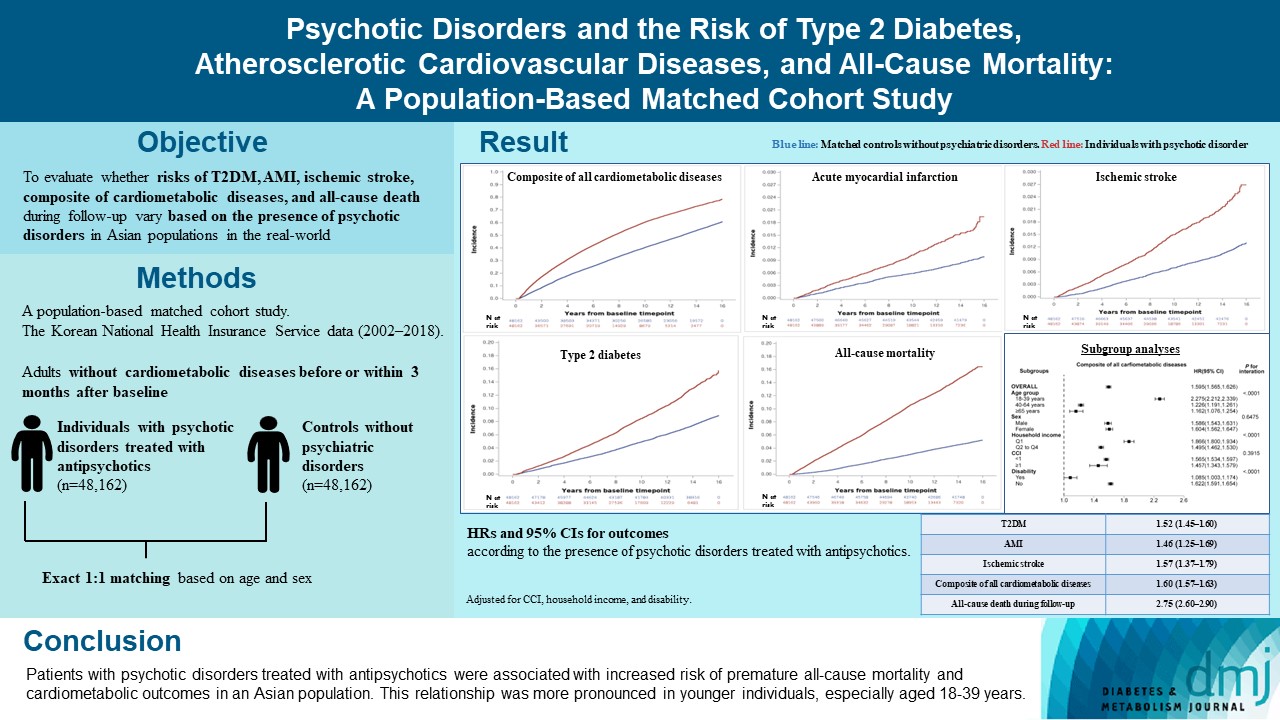
- Psychotic Disorders and the Risk of Type 2 Diabetes Mellitus, Atherosclerotic Cardiovascular Diseases, and All-Cause Mortality: A Population-Based Matched Cohort Study
- You-Bin Lee, Hyewon Kim, Jungkuk Lee, Dongwoo Kang, Gyuri Kim, Sang-Man Jin, Jae Hyeon Kim, Hong Jin Jeon, Kyu Yeon Hur
- Diabetes Metab J. 2024;48(1):122-133. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0431
- 1,090 View
- 144 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The effects of psychotic disorders on cardiometabolic diseases and premature death need to be determined in Asian populations.
Methods
In this population-based matched cohort study, the Korean National Health Insurance Service database (2002 to 2018) was used. The risk of type 2 diabetes mellitus (T2DM), acute myocardial infarction (AMI), ischemic stroke, composite of all cardiometabolic diseases, and all-cause death during follow-up was compared between individuals with psychotic disorders treated with antipsychotics (n=48,162) and 1:1 matched controls without psychiatric disorders among adults without cardiometabolic diseases before or within 3 months after baseline.
Results
In this cohort, 53,683 composite cases of all cardiometabolic diseases (during median 7.38 years), 899 AMI, and 1,216 ischemic stroke cases (during median 14.14 years), 7,686 T2DM cases (during median 13.26 years), and 7,092 deaths (during median 14.23 years) occurred. The risk of all outcomes was higher in subjects with psychotic disorders than matched controls (adjusted hazard ratios [95% confidence intervals]: 1.522 [1.446 to 1.602] for T2DM; 1.455 [1.251 to 1.693] for AMI; 1.568 [1.373 to 1.790] for ischemic stroke; 1.595 [1.565 to 1.626] for composite of all cardiometabolic diseases; and 2.747 [2.599 to 2.904] for all-cause mortality) during follow-up. Similar patterns of associations were maintained in subgroup analyses but more prominent in younger individuals (P for interaction <0.0001) when categorized as those aged 18–39, 40–64, or ≥65 years.
Conclusion
Patients with psychotic disorders treated with antipsychotics were associated with increased risk of premature allcause mortality and cardiometabolic outcomes in an Asian population. This relationship was more pronounced in younger individuals, especially aged 18 to 39 years.

 KDA
KDA
 First
First Prev
Prev





