Glycemic Effects of Rebaudioside A and Erythritol in People with Glucose Intolerance
Article information
Abstract
Background
Rebaudioside A and erythritol are nonnutritive sweeteners. There have been several studies of their glycemic effects, but the outcomes remain controversial. The purpose of this study was to evaluate the glycemic effects of rebaudioside A and erythritol as a sweetener in people with glucose intolerance.
Methods
This trial evaluated the glycemic effect after 2 weeks of consumption of rebaudioside A and erythritol as sweeteners in a pre-diabetic population. The patients were evaluated for fructosamine, fasting plasma glucose, C-peptide, insulin, and 2-hour plasma glucose before and after consumption of sweetener. The primary outcome was a change in fructosamine levels from the baseline to the end of treatment. Secondary outcomes were the changes in levels of fasting plasma glucose and 2-hour plasma glucose.
Results
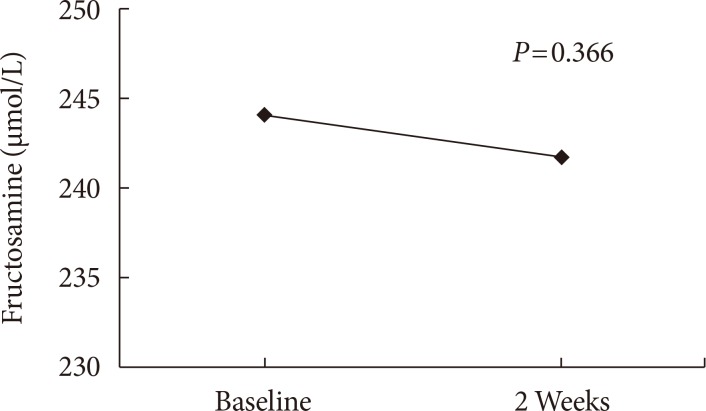
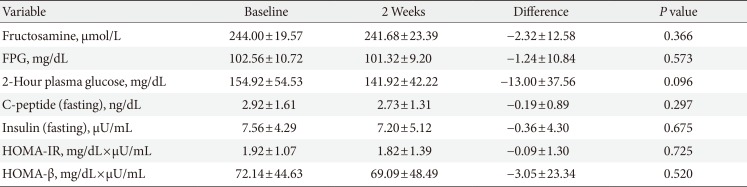
From the baseline to the end of experiment, the changes in fructosamine levels after consumption of rebaudioside A and erythritol, did not differ significantly (244.00±19.57 vs. 241.68±23.39 µmol/L, P=0.366). The change in levels from the baseline to end of the study for rebaudioside A and erythritol were fasting plasma glucose (102.56±10.72 vs. 101.32±9.20 mg/dL), 2-hour plasma glucose (154.92±54.53 vs. 141.92±42.22 mg/dL), insulin (7.56±4.29 vs. 7.20±5.12 IU/mL), and C-peptide (2.92±1.61 vs. 2.73±1.31 ng/mL), respectively, and also did not differ significantly (P>0.05 for all).
Conclusion
Our study suggests that consumption of rebaudioside A and erythritol does not alter the glucose homeostasis in people with glucose intolerance.
INTRODUCTION
Sugar-free and reduced-calorie foods and beverages are garnering more interest due to the increasing rates of obesity in several countries [1]. It was reported that globally, the demand for alternatives to sugar is increasing at a rate of 8% per year. The market is showing more and more interest in natural sweeteners compared to synthetic ones [2]. Currently, several synthetic non-nutritive sweeteners have been approved for use as food ingredients by the U.S. Food and Drug Administration. Non-nutritive sweeteners are sugar substitutes that have zero calories and do not raise blood glucose levels. They may be the preferred choice for reducing carbohydrate and calorie intake in people with diabetes.
Steviol glycosides are natural constituents of the plant Stevia rebaudiana Bertoni [3]. Stevioside, rebaudioside A, rebaudioside C, and dulcoside A are the major components of the S. rebaudiana leaf [4]. Rebaudioside A is well known for sweetness(250 to 300 times sweeter than sucrose), and is popular as a new, non-caloric sweetener in several countries [5].
Erythritol (four-carbon sugar alcohol) is produced from corn or wheat starch by enzymatic hydrolysis that yields glucose, which is fermented by safe and suitable food-grade osmophilic yeast (either Moniliella pollinis or Trichosporonoides megachliensis), and has also been used as a low-calorie sweetener in the food industry. The sweetness of erythritol is about 70% to 80% that of sucrose, and its calories are only 10% of the calories in sucrose [6].
Several studies have reported that stevioside and stevia extracts may have a hypoglycemic effect, particularly among individuals with type 2 diabetes [78910]. However, more recent studies have not provided any clear evidence to support the previous results [1112]. Previous studies have also shown that consumption of erythritol did not affect glucose homeostasis [1314], but so far, the glycemic effects of rebaudioside A and erythritol remains controversial. To date, there are no data about the glycemic effects of rebaudioside A and erythritol in Korea. Therefore, the purpose of this study was to evaluate the glycemic effects of rebaudioside A and erythritol in people with glucose intolerance.
METHODS
Subjects
This was a single, open trial conducted at two research sites in Korea. The inclusion criteria were patients 18 to 74 years old who were glucose intolerant (fasting plasma glucose of 100 to 125 mg/dL). The exclusion criteria were individuals with overt diabetes, treatment of oral hypoglycemic agents within the previous 3 months, previous history of gastrointestinal resection operation (except appendectomy), severe diabetic complications, severe liver failure, renal failure, risk of hypoglycemia, poorly controlled hypertension, severe cardiovascular disease, severe trauma or infection, patients in a perioperative period, history of drug allergy, excessive alcohol consumers, pregnant women or women that have the possibility of becoming pregnant, secondary hypertension or malignant hypertension (New York Heart Association class III or IV), bilateral renal artery stenosis, single kidney, post-kidney transplantation, angina, myocardial infarction, cardiac surgery, or stroke in the past 6 months, and a recent history of a revascularization within the previous 3 months. This study protocol was approved by the Institutional Review Board (IRB) of Pusan National University Hospital (IRB No. 1105-004-001). Signed written informed consent was obtained from all subjects.
Methods
A total of 25 subjects were enrolled for the study. Once enrolled, they stopped having any similar sweetening agents for at least 1 week before start of study (washout period). A single pack of sweetener contained 16 mg of rebaudioside A and 986 mg of erythritol. All subjects consumed 2 packs of sweetener dissolved in water, twice a day (after breakfast and dinner) for 2 weeks. Body weight and food consumption were monitored at the baseline and at the end of the study. Subjects were instructed to maintain a stable diet during the course of the study. Diet records completed at the baseline and at the end of the study were used to confirm dietary stability. Subjects reported any adverse events that occurred during the study.
Fructosamine, fasting level of blood glucose, insulin, C-peptide, and 2-hour plasma glucose levels were measured at the baseline and at the end of the study period. The subjects fasted at least 8 hours prior to measurement of levels for fasting plasma glucose, insulin, and C-peptide. Compliance was assessed by pack count and subject interview. Percent compliance was calculated as 100 times the number of doses consumed divided by the expected number of doses.
The primary outcome in the short study protocol was the change in fructosamine for blood glucose monitoring from the baseline to the end of treatment. Fasting plasma glucose, 2-hour plasma glucose, C-peptide, and insulin at the baseline and at the end of treatment were obtained for secondary outcomes.
Serum fructosamine was measured using a nitroblue tetrazolium calorimetric method (Roche Diagnostics, Mannheim, Germany). Fasting blood glucose and 2-hour plasma glucose levels were measured using the hexokinase method (Roche Diagnostics). Serum insulin and C-peptide levels were measured by the electrochemiluminescence immunoassay method (Roche Diagnostics).
Insulin resistance and pancreatic β-cell function were calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) and the homeostasis model assessment of β-cell function (HOMA-β), by the following formulas, respectively.
HOMA-IR=[fasting insulin (µU/mL)×fasting plasma glucose (mmol/L)]/22.5
HOMA-β=fasting insulin (µU/mL)/[fasting plasma glucose (mmol/L)–3.5]
Statistical analysis
We performed a paired t-test to compare the difference from the baseline. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). All tests of statistical significance were completed at the 5% level and were two-tailed tests.
RESULTS
Patient characteristics
Data was collected from 25 individuals. There were seven men and 18 women included in the study group. All subjects, except one person, had previous illness. At baseline, the mean height was 159.17±8.15 cm, and the mean body weight was 66.57±10.35 kg. Mean systolic and diastolic blood pressure were 126.84±10.57 and 77.92±9.80 mm Hg. Mean heart rate was 71.24±10.82 beats/min. Mean fructosamine level was 244.00±19.57 µmol/L. The mean fasting plasma glucose level and mean 2-hour plasma glucose level were 102.56±10.72 and 154.92±54.53 mg/dL, respectively. Mean product compliance was 92.53%±7.86%. Table 1 shows the baseline characteristics of all subjects.
Primary outcome
The change in fructosamine levels from the baseline to 2 weeks are shown in Table 2, Fig. 1 (244.00±19.57 and 241.68±23.39 µmol/L, respectively). The fructosamine level did not differ significantly between the baseline and 2 weeks during the period of study (P=0.366).

Change of fructosamine, fasting plasma glucose, 2-hour plasma glucose, fasting C-peptide, and fasting insulin level at baseline and 2 weeks
Secondary outcome
Fasting plasma glucose, insulin, C-peptide, and 2-hour plasma glucose from the baseline to 2 weeks are shown in Table 2. There were no significant differences between the baseline and the end of the study for fasting plasma glucose (102.56±10.72 and 101.32±9.20 mg/dL, P=0.573) (Fig. 2A), insulin (7.56±4.29 and 7.20±5.12 µU/mL, P=0.675), and C-peptide (2.92±1.61 and 2.73±1.31 ng/dL, P=0.297), respectively. Although the 2-hour plasma glucose levels were reduced by 13.0 mg per deciliter after consuming sweetener for 2 weeks, it was not statistically significant (154.92±54.53 and 141.92±42.22 mg/dL, P=0.096) (Fig. 2B).
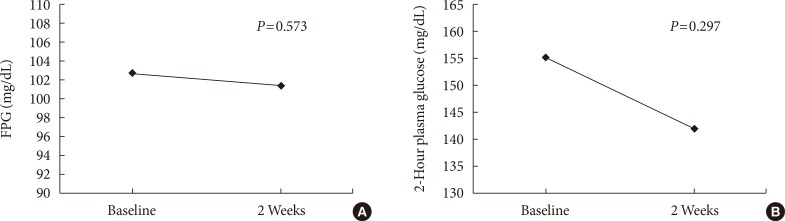
Mean change from the baseline to 2 weeks in (A) fasting plasma glucose (FPG), (B) 2-hour plasma glucose.
There were no significant differences between the baseline and 2 weeks for HOMA-IR (1.92±1.07 and 1.82±1.39 mg/dL×µU/mL, P=0.725) or HOMA-β (72.14±44.63 and 69.09 ±48.49 mg/dL×µU/mL, P=0.520) (Fig. 3). Assessments of changes in blood pressure, body weight, and fasting total cholesterol showed no differences.
Adverse events
There were two mild adverse events during the study, both involving abdominal discomfort. Both adverse events recovered spontaneously. One subject completed the study, but the other subject stopped taking the sweetener.
DISCUSSION
In this study, we found that consumption of rebaudioside A and erythritol for 2 weeks did not affect glucose homeostasis in individuals with glucose intolerance. There were no significant differences in the change of the fructosamine from the baseline to the end of the study. This result showed that taking rebaudioside A and erythritol for 2 weeks did not affect the blood glucose level. We found a tendency toward a decline in the level of fasting plasma glucose, C-peptide, insulin, and 2-hour plasma glucose during the study, but these figures were not statistically significant. We calculated HOMA-IR and HOMA-β to evaluate insulin resistance and β-cell function. HOMA-IR and HOMA-β did not differ significantly from the baseline to the end of the study. These results suggest that rebaudioside A and erythritol did not affect the β-cell function and insulin resistance.
There was some evidence that taking rebaudioside A did not affect glucose control in non-diabetics and people with type 1 and type 2 diabetes. In a 3-month study of subjects with type 2 diabetes, it was reported that fasting blood glucose and glycosylated hemoglobin (HbA1c) were not significantly lowered by intake of 1,500 mg/day of stevioside, when compared with placebo [12]. In another study, 250 mg of steviol glycoside was administered to a group of type 1 and type 2 diabetics and non-diabetics three times daily for 3 months in a double-blind, placebo-controlled trial. After 3 months, there were no significant changes in glucose level and HbA1c [15].
Several studies reported that stevioside and stevia extracts may have a hypoglycemic effect in patients with type 2 diabetes [7910]. There were some hypotheses about hypoglycemic effects of rebaudioside A. First, rebaudioside A may stimulate insulin secretion. Stevioside was shown to stimulate glucose-dependent insulin secretion from islets in an in vitro mouse model [7]. A previous study demonstrated that rebaudioside A directly stimulates insulin secretion from pancreatic β-cells [16]. Therefore, rebaudioside A has insulinotropic effects, and may play a potential role as treatment in type 2 diabetes. Second, rebaudioside A may improve insulin sensitivity. Stevioside was shown to have direct effects on insulin sensitivity in 3T3-L1 adipocytes via an increase in glucose uptake and enhanced expression of proteins involved in the insulin-signaling pathway [17]. Lastly, rebaudioside A may enhance insulin utilization. It was shown that stevioside could enhance insulin utilization in insulin-deficient rats due to decreasing phosphoenolpyruvate carboxykinase (PEPCK) gene expression in rat liver, by slowing down gluconeogenesis [10]. The glycemic effect of rebaudioside A is still not clear, but it seems that consumption of rebaudioside A did not worsen glycemic control in individuals with and without diabetes.
Several studies were performed to investigate the potential effect that the ingestion of erythritol might have on carbohydrate metabolism in non-diabetics and diabetics. In non-diabetics, erythritol was dissolved in water and ingested as a single dose of 0.3 g/kg body weight. Analysis of blood performed at 0.5, 1, 2, 3, 8, and 24 hours after erythritol administration showed no changes in insulin or glucose concentrations [13]. In diabetic individuals, erythritol, in a single oral dose of 20 g dissolved in 100 mL of water, was ingested by five fasting patients, followed by the ingestion of normal meals approximately 3 and 12 hours later. Serum insulin and glucose were measured prior to and for up to 24 hours after erythritol administration. Analysis of the serum values showed that neither insulin nor glucose concentrations were changed in response to erythritol administration [14]. These studies in non-diabetics and diabetics demonstrate that erythritol does not adversely affect glycemic homeostasis.
Fructosamine, a measure of glycated serum proteins, is used as a glycemic control indicator representing average glycemia over the previous 2 to 3 weeks [181920]. Because the half-life of serum albumin is about 14 days, fructosamine reflects short-term (about 2 weeks) control of blood glucose level [21]. Previous studies have shown that fructosamine levels strongly correlate with other measures of glycemic control, including HbA1c [18], fasting glucose [222324], and oral glucose tolerance test [2526]. Measuring fructosamine has been used as an alternative to measuring HbA1c for assessment of glycemic control. Therefore, we checked the change in fructosamine levels for short-term glycemic control monitoring.
Calculating HOMA-IR and HOMA-β for evaluating insulin resistance and β-cell function were distinctive in previous studies. Homeostasis model assessment is a method for assessing β-cell function and insulin resistance from basal glucose and insulin concentrations [27]. Homeostasis model assessment has been widely validated and applied for quantifying insulin resistance and secretion [28].
Rebaudioside A and erythritol were well tolerated and generally had no effect on laboratory measurements. Assessments of change in renal function test, liver function test, and fasting total cholesterol showed no difference. A small, significant decrease was noted for the basophil count from the baseline to the end of treatment. However, the total white cell count showed no difference from the baseline to end of treatment.
This was the first study about the glycemic effects of short-term consumption of rebaudioside A and erythritol in individuals with glucose intolerance in Korea. There were some limitations in this trial. First, the sample size was small. Second, the design of this trial was single, open trial. Third, duration of consumption was short-term periods of only 2 weeks. Fourth, we did not restrict physical activity, which could largely influence the blood glucose levels. Finally, a standard test meal was not supplemented during the study. Although subjects were instructed to maintain a stable diet and record their diet during the study, the free diet could affect the glucose homeostasis more than a standard test meal. Further studies are required to assess the long-term glycemic effect of sweeteners in diet.
In conclusion, we suggest that consumption of rebaudioside A and erythritol for 2 weeks does not alter glucose homeostasis in people having glucose intolerance, and may be promising alternatives as sweeteners in these individuals.
Notes
CONFLICTS OF INTEREST: This trial was supported by grants from the Han Wha Pharmaceuticals Co. Ltd., Seoul, Republic of Korea.