Non-Insulin Antidiabetes Treatment in Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Article information
Abstract
In order to evaluate the efficacy and side effects of the non-insulin antidiabetes medications as an adjunct treatment in type 1 diabetes mellitus (T1DM), we conducted systematic searches in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials for randomized controlled trials published between the date of inception and March 2020 to produce a systematic review and meta-analysis. Overall, 57 studies were included. Compared with placebo, antidiabetes agents in adjunct to insulin treatment resulted in significant reduction in glycosylated hemoglobin (weighted mean difference [WMD], –0.30%; 95% confidence interval [CI], –0.34 to –0.25%; P<0.01) and body weight (WMD, –2.15 kg; 95% CI, –2.77 to –1.53 kg; P<0.01), and required a significantly lower dosage of insulin (WMD, –5.17 unit/day; 95% CI, –6.77 to –3.57 unit/day; P<0.01). Compared with placebo, antidiabetes agents in adjunct to insulin treatment increased the risk of hypoglycemia (relative risk [RR], 1.04; 95% CI, 1.01 to 1.08; P=0.02) and gastrointestinal side effects (RR, 1.99; 95% CI, 1.61 to 2.46; P<0.01) in patients with T1DM. Compared with placebo, the use of non-insulin antidiabetes agents in addition to insulin could lead to glycemic improvement, weight control and lower insulin dosage, while they might be associated with increased risks of hypoglycemia and gastrointestinal side effects in patients with T1DM.
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is characterized by destruction of the pancreatic β-cells [1], resulting in severely impaired or absent insulin secretion combined with abnormal α-cell function, with excess glucagon in the fasting and postprandial state [2-4]. Tight glycemic control for T1DM patients recommended for its long-term effects on reducing the risk of microvascular complications [5] and macrovascular diseases [6,7]. However, intensive insulin therapy has its own limitations, which makes it difficult for patients with T1DM to achieve good glycemic control. Hypoglycemia is the main concern of intensive insulin therapy, with rates of severe hypoglycemia ranging between 115 and 320 events per 100 patient-years in patients with T1DM [8,9]. Intensive insulin therapy also contributes to weight gain and the obesity-related cardiometabolic consequences: hypertension, dyslipidemia, atherosclerosis, and subsequent cardiovascular events [10-14]. The fear of hypoglycemia and weight gain may promote suboptimal dosing of insulin, which may lead to diabetic ketoacidosis, a fatal complication [15,16], in patients with T1DM.
Therefore, researchers kept on finding potential adjunct medications to insulin treatment for better treatment strategies. In recent years, the off-label use of antidiabetes drugs treating type 2 diabetes mellitus (T2DM) was given to patients with T1DM, since it was expected that the addition of non-insulin therapies might help patients with T1DM to achieve better glycemic control with possible reduction in hypoglycemia, body weight, insulin dose, and glucose variability. Several clinical trials and meta-analysis were done to show the efficacy and safety of these drugs such as glucagon-like peptide-1 receptor agonist (GLP-1RA), dipeptidyl peptide 4 inhibitor (DPP-4i), sodium glucose cotransporter 2 inhibitor (SGLT-2i), metformin, thiazolidinedione (TZD), and alpha glucosidase inhibitors (AGI), as add-on therapy with insulin. However, comprehensive evaluations of these drugs in terms of glycemic control, body weight control, as well as the risk of adverse effects and comparisons among different kinds of drugs were not well studied yet. Therefore, the aim of this meta-analysis was to evaluate the efficacy and side effects of these off-label used medications as an adjunct treatment to insulin in patients with T1DM.
METHODS
Study design
A systematic review with meta-analysis was conducted by using a prespecified study protocol which was registered in International Prospective Register of Systematic Reviews (PROSPERO) and traced with CRD42018095253. Analyses were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for conducting and reporting meta-analyses of randomized controlled trials (RCTs).
Search strategy
RCTs publicly available by March 2020, evaluating the effects of additional non-insulin antidiabetes drug in T1DM patients were included. The MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched using a highly sensitive search. Database specific subject headings (such as MeSH terms) and free texts terms were used to search for potentially eligible studies. The free texts including the following terms: metformin, acarbose, volglibose, miglitol, rosiglitazone, pioglitazone, exenatide, liraglutide, lixisenatide, dulaglutide, albiglutide, taspoglutide, semaglutide, sitagliptin, saxagliptin, vildagliptin, linagliptin, alogliptin, trelagliptin, teneligliptin, omarigliptin, dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, luseogliflozin, ertugliflozin, remogliflozin, sotagliflozin, pramlintide. ClinicalTrials.gov was also searched to identify additional eligible clinical trials and confirmed relevant data for all eligible published trials.
Study selection and data extraction
Double blind and placebo controlled RCTs evaluating the adjunction treatment of a non-insulin antidiabetes medications in T1DM were included. Studies evaluating treatment with single armed trials, active controlled trials, observational studies, retrospective studies, trials in T2DM and trials with study length less than 4 weeks were all excluded.
Two review authors (L.N. and W.Y.) independently and in duplicate screened titles/abstracts and full texts for eligible trials, assessed risk of bias and extracted the following data from each publication using a standardized form: publication data (title, first author, year, and source of publication), study design, baseline characteristics of the study population (sample size, gender, age, duration of T1DM, body mass index, and glycosylated hemoglobin [HbA1c]), description of the study drugs and the dosages and treatment duration. Primary outcomes were measured as change from baseline to study endpoint for HbA1c, risk of hypoglycemia. Secondary outcomes were measured as fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) changes from baseline, body weight change from baseline, the risk of severe hypoglycemia, risk of nocturnal hypoglycemia, and risk of other side effects. Disagreements or discrepancies were resolved by discussion among the two review authors and a third investigator (X.C.). If a published trial did not report the relevant outcomes, the registry report data from ClinicalTrials.gov would be abstracted and used.
Assessment of risk of bias
Two reviewers independently assessed risk of bias for each trial using the Cochrane Collaboration tool. Each RCT was rated as having a low, high, or unclear risk of bias for the following aspects: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias.
Statistical analysis
The meta-analysis was performed by computing the weighted mean difference (WMD) and 95% confidence intervals (CIs) to evaluate the changes of HbA1c, FPG, PPG from baseline for the treatment of each antidiabetes drug group. The risk of hypoglycemia, the risk of severe hypoglycemia, risk of ketoacidosis and the risks of other side effects were expressed in relative risk (RR) or odds ratio (OR) and 95% CIs. A P<0.05 was considered statistically significant for all analyses. The betweenstudy heterogeneity was distributed as the chi-square statistics. I2 statistics was used to quantify the percentage of total variation across studies that was attributable to heterogeneity rather than to chance. If the I2 was >50% indicating significant heterogeneity, a random-effects model was used; otherwise, a fixed-effects model was used. Publication bias was assessed via a visual inspection of the funnel plot. Sensitivity analysis was performed by including and excluding the low-level studies or the small sample sizes (less than 20 participants). Subgroup analysis was made according to the class of antidiabetes agents or according to the type of RCTs. All statistical analyses were performed with the STATA statistical software package version 13.1 (Stata Corp, College Station, TX, USA) and the Review Manager statistical software package version 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark).
RESULTS
Characteristics and methodological quality of included studies
Overall, 57 studies were included in the meta-analysis in T1DM with antidiabetes agents in adjunct to insulin treatment (Fig. 1). Among these studies, 14 studies compared metformin with placebo in adjunct to insulin treatment, seven studies compared AGI with placebo, five studies compared TZD with placebo, six studies compared GLP-1RA with placebo, six studies compared DPP-4i with placebo, 13 studies compared SGLT-2i, and six studies compared pramlintide with placebo in adjunct to insulin treatment (Supplementary Table 1).

Flow diagram of the included studies. MET, metformin; AGI, alpha glucosidase inhibitor; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; DPP-4i, dipeptidyl peptide 4 inhibitor; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Overall, the risk of bias was high or unclear for random sequence generation in 32 trials (55.2%); concealment of treatment allocation in 29 trials (50.0%); masking of participants, masking of investigators, or both in 0 trials (0.0%); masking of outcome assessment in 0 trials (0.0%); completeness of outcome reporting in five trials (8.6%); and selective reporting of outcomes in one trial (1.7%) (Supplementary Table 2). The visual inspection of the funnel plots indicated an even distribution of the variables.
Glycemic control and weight control
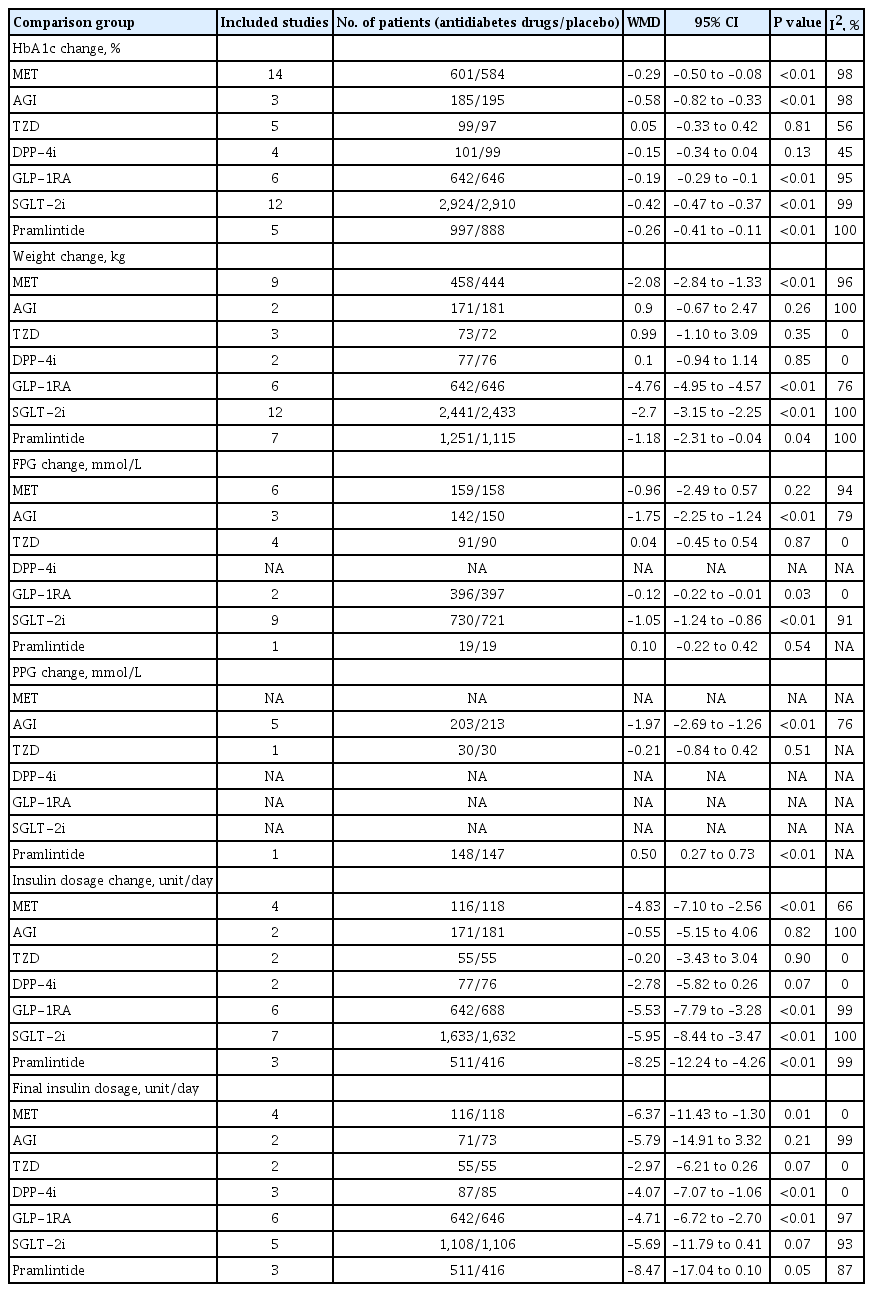
Overall, compared with placebo, antidiabetes agents in adjunct to insulin treatment resulted in significant reduction in HbA1c (WMD, –0.32%; 95% CI, –0.36% to –0.28%; P<0.01) and body weight (WMD, –2.17 kg; 95% CI, –2.78 to –1.57 kg; P<0.01). Compared with placebo, metformin exhibited significant reduction in HbA1c (WMD, –0.29%; 95% CI, –0.50% to –0.08%; P<0.01) and body weight (WMD, –2.08 kg; 95% CI, –2.84 to –1.33 kg; P<0.01), AGI led to significant reduction in HbA1c (WMD, –0.58%; 95% CI, –0.82% to –0.33%; P<0.01) but not in body weight (WMD, 0.9 kg; 95% CI, –0.67 to 2.47 kg; P=0.26), TZD resulted in non-significant increases in HbA1c (WMD, 0.05%; 95% CI, –0.33% to 0.42%; P=0.81) and body weight (WMD, 0.99 kg; 95% CI, –1.10 to 3.09 kg; P=0.35), DPP-4i led to non-significant reduction in HbA1c (WMD, –0.15%; 95% CI, –0.34% to 0.04%; P=0.13), and body weight (WMD, 0.1 kg; 95% CI, –0.94 to 1.14 kg; P=0.85), GLP-1RA contributed to significant reduction in HbA1c (WMD, –0.19%; 95% CI, –0.29% to –0.1%; P<0.01) as well as in body weight (WMD, –4.76 kg; 95% CI, –4.95 to 4.57 kg; P<0.01), SGLT-2i conferred significant reduction in HbA1c (WMD, –0.42%; 95% CI, –0.47% to –0.37%; P<0.01) and body weight (WMD, –2.70 kg; 95% CI, –3.15 to –2.25 kg; P<0.01), pramlintide resulted in significant reduction in HbA1c (WMD, –0.26%; 95% CI, –0.41% to –0.11%; P<0.01), but not in body weight (WMD, –1.18 kg; 95% CI, –2.31 to –0.04 kg; P=0.04) (Table 1, Fig. 2, and Supplementary Fig. 1).
Comparisons between antidiabetes drugs and placebo as an adjunct treatment to insulin in T1DM patients in terms of HbA1c changes, weight changes, blood pressure changes, and dosage of insulin changes

Comparison of glycosylated hemoglobin (HbA1c) change from baseline between antidiabetes agent and placebo in patients with type 1 diabetes mellitus. SD, standard deviation; IV, inverse variance; CI, confidence interval; MET, metformin; AGI, alpha glucosidase inhibitor; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; DPP-4i, dipeptidyl peptide 4 inhibitor; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Dosage of insulin
Compared with placebo, in adjunct to insulin treatment, antidiabetes agent treatment was associated with a more decreased dosage of insulin change from baseline (WMD, –5.17 unit/day; 95% CI, –6.77 to –3.57 unit/day; P<0.01). As for individual medications, treatment with metformin, GLP-1RA, SGLT-2i, or pramlintide, required a more decreased dosage of insulin change from baseline respectively (WMD –4.83,–5.53, –5.95,–8.25 unit/day, respectively), while treatment with AGI, TZD, or DPP-4i did not show significant decrease in the dosage of insulin change from baseline (Table 1 and Supplementary Fig. 2).
Corrected by placebo effect, indirect comparisons between each two kinds of active antidiabetes agents in terms of HbA1c changes from baseline indicated significant difference between TZD versus SGLT-2i, which in favor of SGLT-2i treatment (mean difference [MD], 0.39; P=0.003), significant difference between GLP-1RA and SGLT-2i, which in favor of SGLT-2i treatment (MD, 0.22; P=0.012), and significant difference between DPP-4i and SGLT-2i, which also in favor of SGLT-2i treatment (MD, 0.28; P=0.01) (Supplementary Table 3).
Risk of hypoglycemia and other adverse effects
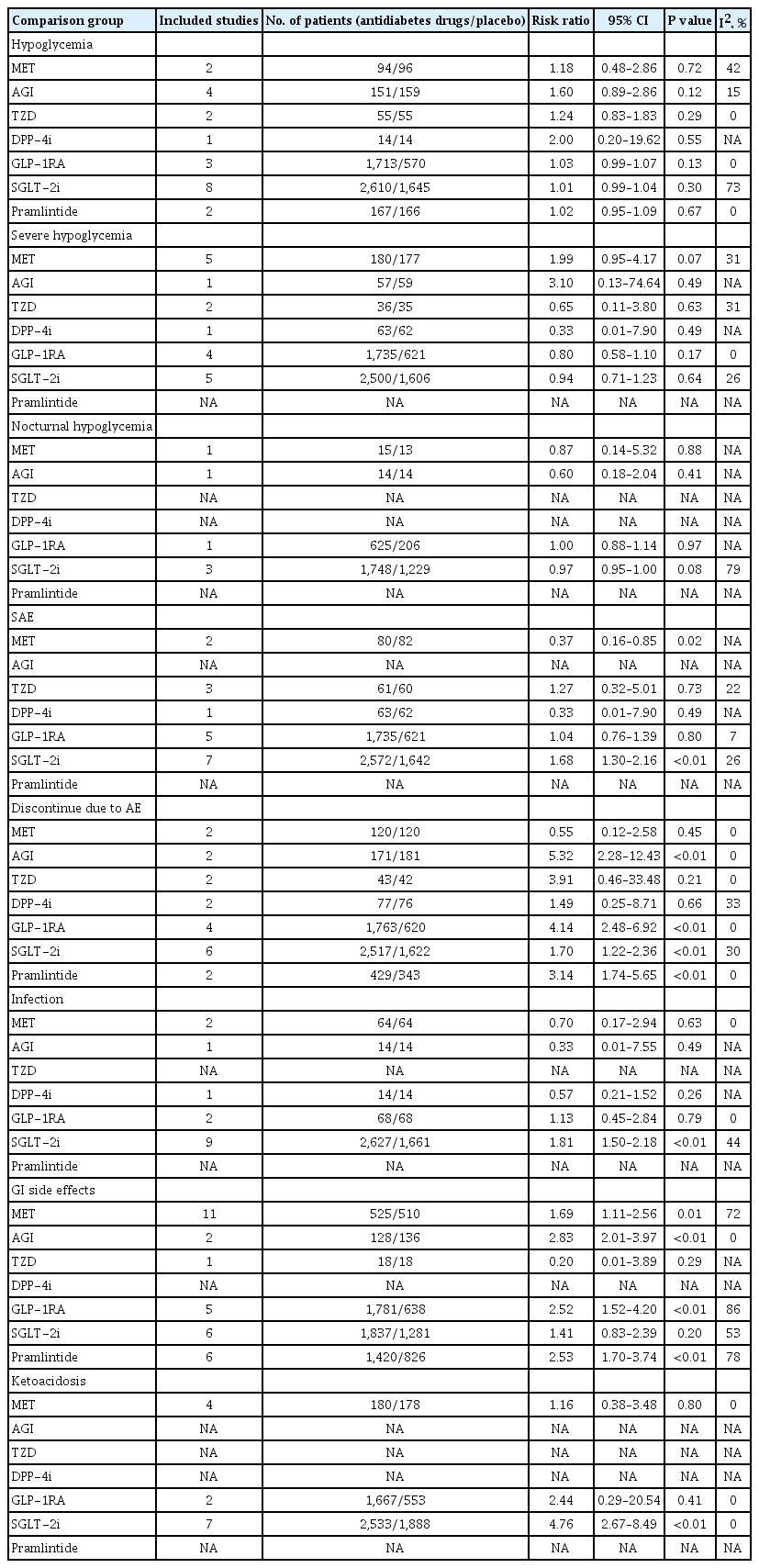
Overall, in patients with T1DM, compared with placebo, in adjunct to insulin treatment, antidiabetes agent increased the risk of hypoglycemia (RR, 1.04; 95% CI, 1.01 to 1.08; P=0.02). Separately, treatment with metformin, AGI, TZD, GLP-1RA, DPP-4i, SGLT-2i, or pramlintide, did not increase the risk of hypoglycemia (Fig. 3). In terms of ketoacidosis, compared with placebo, the risk of ketoacidosis (RR, 3.44; 95% CI, 2.01 to 5.89; P<0.01) was increased in patients with active agent in adjunct to insulin treatment. Separately, treatment with metformin or GLP-1RA, did not increase the risk of ketoacidosis, while treatment with SGLT-2i increased the risk of ketoacidosis (RR, 4.76; 95% CI, 2.67 to 8.49; P<0.01) (Supplementary Fig. 3). There was no reported case of ketoacidosis with other antidiabetes treatments. As for gastrointestinal (GI) side effects, overall, compared with placebo, antidiabetes agent in adjunct to insulin treatment increased the risk of GI side effects (RR, 1.99; 95% CI, 1.61 to 2.46; P<0.01) (Supplementary Fig. 4). Separately, treatment with metformin, AGI, GLP-1RA, or pramlintide, did increase the risk of GI side effects (Table 2).
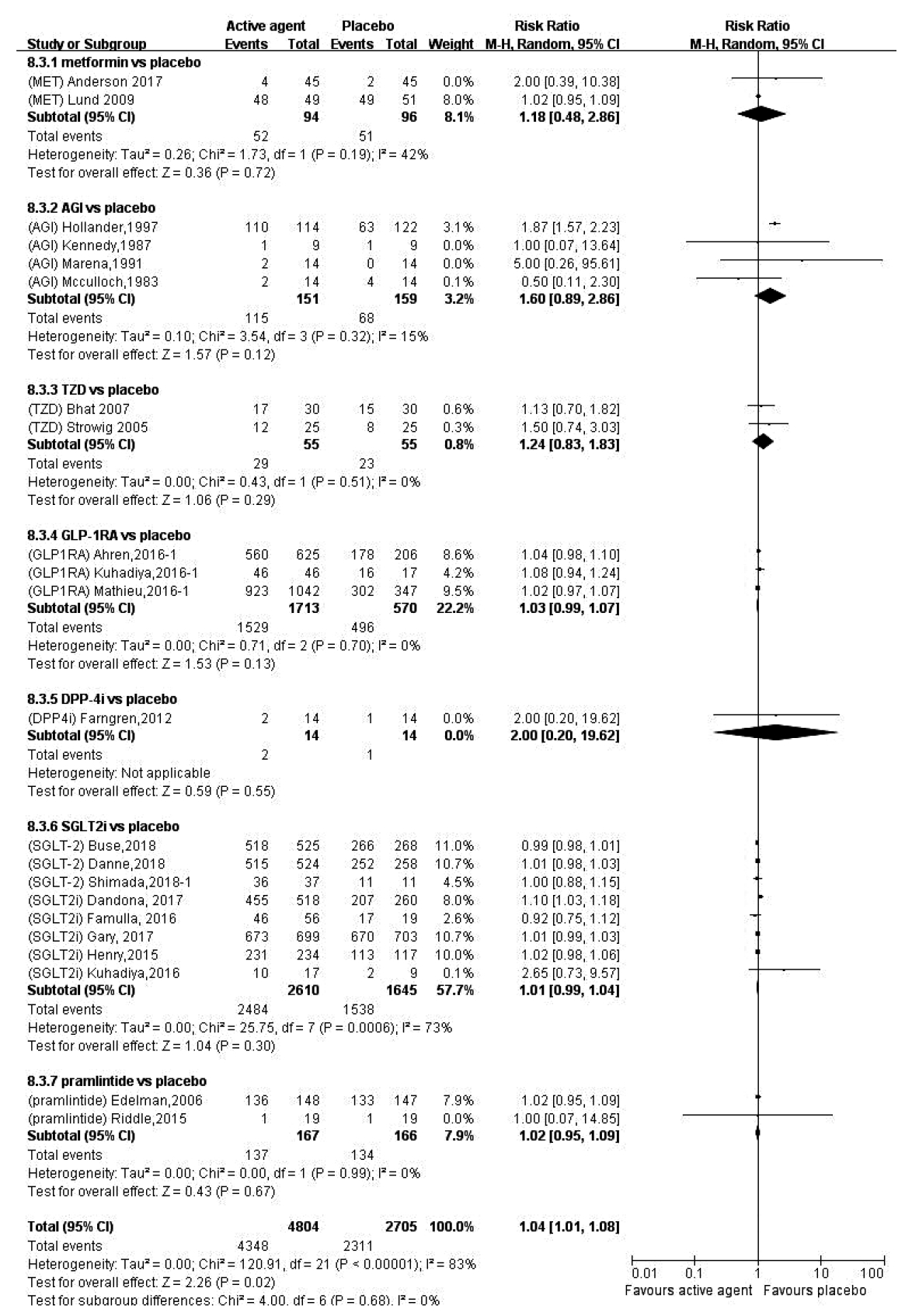
Comparison of the risk of hypoglycemia between antidiabetes agent and placebo in patients with type 1 diabetes mellitus. W-H, Mantel-Haenszel; CI, confidence interval; MET, metformin; AGI, alpha glucosidase inhibitor; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; DPP-4i, dipeptidyl peptide 4 inhibitor; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Comparisons between antidiabetes drugs and placebo as an adjunct treatment to insulin in T1DM patients in terms of adverse effects
In total, the efficacy and safety features of each antidiabetes medication when used as an adjunct treatment to insulin in patients with T1DM were summarized in Table 3. As is shown, compared with placebo, treatment with metformin decreased HbA1c, body weight, insulin dosage and the risk of serious adverse event (SAE), but increased the risk of GI side effect. Compared with placebo, GLP-1RAs decreased HbA1c, body weight, FPG, and insulin dosage, but increased the risks of discontinue due to adverse event (AE) and GI side effect. Compared with placebo, SGLT2 inhibitors decreased HbA1c, body weight, FPG, and insulin dosage, but increased the risks of SAE, discontinue due to AE, infection, and ketoacidosis. Compared with placebo, pramlintide decreased HbA1c, body weight, and insulin dosage, but increased the risks of discontinue due to AE and GI side effect.
Subgroup analysis stratified by age
Subgroup analysis of the management of T1DM between children or adolescents and adults indicated that in patients ≥18 years old, compared with placebo group, HbA1c was decreased significantly (WMD, –0.29%; 95% CI, –0.33% to –0.25%) in the antidiabetes treatment group, while in patients <18 years old, HbA1c changes were not significant between antidiabetes treatment and control groups. However, the between group variance test did not show any significant difference of WMD between patients ≥18 years old and patients <18 years old (P=0.80). In terms of body weight changes, insulin dosage changes, the risk of hypoglycemia, the risk of GI side effect and the risk of ketoacidosis, the variance tests did not show any significant difference of WMD between patients ≥18 years old and patients with <18 years old either (Table 4).
DISCUSSION
It was well known that the factors as reduction in HbA1c, improved weight control, decreasing in insulin doses, reduction in the risk of hypoglycemia and ketoacidosis were associated with improvements in common challenges with T1DM management [17,18]. This meta-analysis evaluated all these factors in T1DM with insulin treatment in combination with biguanides, AGIs, TZDs, GLP-1RA, DPP-4i, SGLT-2i, or pramlintide, which indicated that compared with placebo, most of these antidiabetes agents led to significant decrease in HbA1c, dosage of insulin and body weight with increased risks of hypoglycemia and GI side effects.
In terms of improving glycemic control, it was expected that these adjunct treatments with antidiabetes drugs approved for the treatment of T2DM could also produce benefits in patients with T1DM, since tight glycemic control was demonstrated to have long-term positive effects on the development and progression of macro- and microvascular complications [6,7]. Results from our meta-analysis indicated that compared with placebo, antidiabetes agents as metformin, GLP-1RA, SGLT-2i, AGI, and pramlintide as adjunct therapy with insulin exhibited significant reductions in HbA1c in patients with T1DM, but not DPP-4i or TZD. It was suggested that the adjunct therapies with metformin, AGI, GLP-1RA, SGLT-2i, and pramlintide, by improving of insulin resistance, suppressing glucagon, delaying gastric emptying, and decreasing glucose absorption in both the gut and kidney, which are all potential targets in improving glucose control, might offer potential benefit in treating individuals with T1DM [19]. Several previously reported meta-analysis indicated that adding antidiabetes drugs could reduce HbA1c significantly but others did not show any improvement in HbA1c level [19-24].
In terms of hypoglycemia, which was found to be associated with intensive insulin therapy and was the most significant impediment to improve glycemic control in individuals with T1DM [5,25,26], this meta-analysis showed that compared with placebo, the risk of hypoglycaemia was slightly increased in T1DM patients with the adjunct treatment of overall active agents but not with separate treatment of metformin, AGI, DPP-4i, GLP-1RA, TZD, SGLT-2i, or pramlintide although the glycemic control was improved. Ideally, the treatment for T1DM should improve HbA1c without increasing risk of hypoglycemia. What we found from this meta-analysis might gave us the promising results that by using these adjunct treatments separately with insulin treatment, patients with T1DM might get benefit from better glycemic control without increasing the risk of hypoglycemia.
Another concern was about the weight control in overweight individuals with T1DM. It was reported that the prevalence of overweight and obesity in T1DM individuals was increasing in parallel with the global population trends in weight gain [27-30], which might be related to over-insulinization, recurrent hypoglycemia and defensive snacking [31]. Therefore, to achieve body weight control target in T1DM patients, combination therapy using drugs with actions complementary to insulin could be appropriate [32]. According to our meta-analysis, it was suggested that compared with placebo, the adjunct treatment of metformin, GLP-1RA, SGLT-2i, and pramlintide could exhibit less weight gain in combination with insulin. Possible mechanisms for treatment of biguanides, GLP-1RA, SGLT-2i, and pramlintide realizing weight control in combination therapy with insulin might be similar as they acted for weight control in patients with T2DM.
Furthermore, adjunct therapies are expected to be helpful in the regard of decreasing the insulin dosage. In contrast, higher insulin doses and the use of intensive insulin therapy in patients with T1DM were reported to be associated with increases in obesity and insulin resistance during the course of therapy [33], which was a complicating factor when intensive insulin management was attempted [34]. Results from this meta-analysis suggested that with the use of adjunct therapies of metformin, GLP-1RA, SGLT-2i, and pramlintide, compared to placebo, the insulin dose decreased a lot, but not for the treatment of AGI, DPP-4i, or TZD. This finding was in accordance with previous reports [19-24].
It was also expected that adjunct therapies might improve the risk of ketoacidosis in patients with T1DM, which is a life-threatening AE. According to this meta-analysis, compared with placebo, the risk of ketoacidosis was significantly higher in patients with the adjunct therapies of SGLT-2i, but was not associated with the adjunct therapies of biguanides, or GLP-1RA. Possible mechanisms for this increased risk of ketoacidosis might be associated with the euglycemic ketoacidosis that caused by using SGLT-2i.
In terms of other adverse effects, it was suggested by our meta-analysis that the risk of GI side effects was significantly higher with the adjunct therapies of biguanides, AGI, GLP-1RA, and pramlintide, all of which were similar as reported in patients with T2DM using these kinds of antidiabetes drugs.
Since there are differences in the management of T1DM between children and adults, we conducted the subgroup analysis between children or adolescents and adults but did not find any significant difference between these two groups regarding the efficacy and safety of antidiabetes drugs. Moreover, we found that antidiabetes drugs did not affect HbA1c change, weight change, insulin dosage change, hypoglycemia, or ketoacidosis in pediatric T1DM patients, which might inspire us that the use of non-insulin antidiabetes drugs in pediatrics should be carefully evaluated, especially for patients with T1DM.
As a meta-analysis, this study has some limitations. Firstly, different types of studies with variable baseline characteristics of patients with T1DM were included in this meta-analysis, which might be associated with the heterogeneity between studies. We have performed sensitivity analyses according to different classes of active agents to control the bias. Secondly, the number of patients or the number of studies in some classes of active agent included in this meta-analysis was small, which might also be associated with the heterogeneity between studies. Another limitation was that we could not evaluate whether the cost-effectiveness or the quality of life was also improved directly by these data collected from RCTs, however, by improving glycemic control, weight control and reduction in insulin dosage, it was expected that the cost-effectiveness or the quality of life of patients with T1DM might be improved accordingly. Moreover, we were unable to report the improvement of insulin sensitivity or the changes in β-cell function in this meta-analysis because of the limited data.
But still, we have provided a comprehensive review of literature for the efficacy and safety of non-insulin antidiabetic medication in patients with T1DM, suggesting that metformin, GLP-1RAs, and SGLT2 inhibitors also resulted in reduction of glucose level, body weight, insulin dosage without increasing the risks of hypoglycemia and adverse effects, which might be promising treatment candidates for patients with T1DM. However, as the limited data for the efficacy of cardiovascular outcome and microvascular outcome, or the limited data for the cost-effectiveness evaluation, or the limited data for the evaluation of risk to benefit ratio, the use of these drugs in adjunct to insulin in T1DM patients has not been approved yet. Further research is needed to overcome the above limitations and outcome trials are needed to be carry out as those carried out in patients with T2DM to demonstrated the cardiovascular benefit and microvascular benefits.
CONCLUSIONS
Comparisons between the intriguing use of antidiabetes medications and placebo for T1DM from our meta-analysis showed that the use of antidiabetes medications in adjunction to insulin treatment could confer glycemic improvement, weight control and reduction in insulin dosage while might be associated with increased risks of hypoglycemia and GI side effects in patients with T1DM.
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0171.
Trials in type 1 diabetes mellitus comparing anti-diabetes agent versus placebo in adjunct to insulin treatment included in this meta-analysis
Risk of bias of included randomized controlled trial studies
Indirect comparisons between anti-diabetes drugs in T1DM patients in terms of HbA1c changes corrected by placebo
Comparison of weight change from baseline between anti-diabetes agent and placebo in patients with type 1 diabetes mellitus. SD, standard deviation; IV, inverse variance; CI, confidence interval; MET, metformin; AGI, alpha glucosidase inhibitor; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; DPP-4i, dipeptidyl peptide 4 inhibitor; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Comparison of dosage of insulin change from baseline between anti-diabetes agent and placebo in patients with type 1 diabetes mellitus. SD, standard deviation; IV, inverse variance; CI, confidence interval; MET, metformin; AGI, alpha glucosidase inhibitor; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; DPP-4i, dipeptidyl peptide 4 inhibitor; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Comparison of the risk of ketoacidosis between anti-diabetes agent and placebo in patients with type 1 diabetes mellitus. W-H, Mantel-Haenszel; CI, confidence interval; MET, metformin; GLP-1RA, glucagon-like peptide-1 receptor agonist; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Comparison of the risk of GI side effects between anti-diabetes agent and placebo in patients with type 1 diabetes mellitus. W-H, Mantel-Haenszel; CI, confidence interval; MET, metformin; AGI, alpha glucosidase inhibitor; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Notes
CONFLICTS OF INTEREST
Linong Ji has received fees for lecture presentations from AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi-Aventis, and Takeda. Linong Ji has received consulting fees from companies including AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi-Aventis, and Takeda. Linong Ji has received grants/research support from AstraZeneca, Bristol-Myers Squibb, Merck, Novartis, and Sanofi-Aventis. There are no other disclosures for other authors.
FUNDING
This work was supported by National Key Research and Development Program of China (No. 2016YFC1304901), National Natural Science Foundation of China (No. 81970698 and No. 81970708) and Beijing Natural Science Foundation (No. 7202216). The funding agencies had no roles in the study design, data collection or analysis, decision to publish or preparation of the manuscript.
Acknowledgements
We thank the doctors, nurses and technicians for their practical work during the study at Department of Endocrinology and Metabolism in Peking University People’s Hospital.