
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Previous issues
- Page Path
- HOME > Browse > Previous issues
- Drug/Regimen
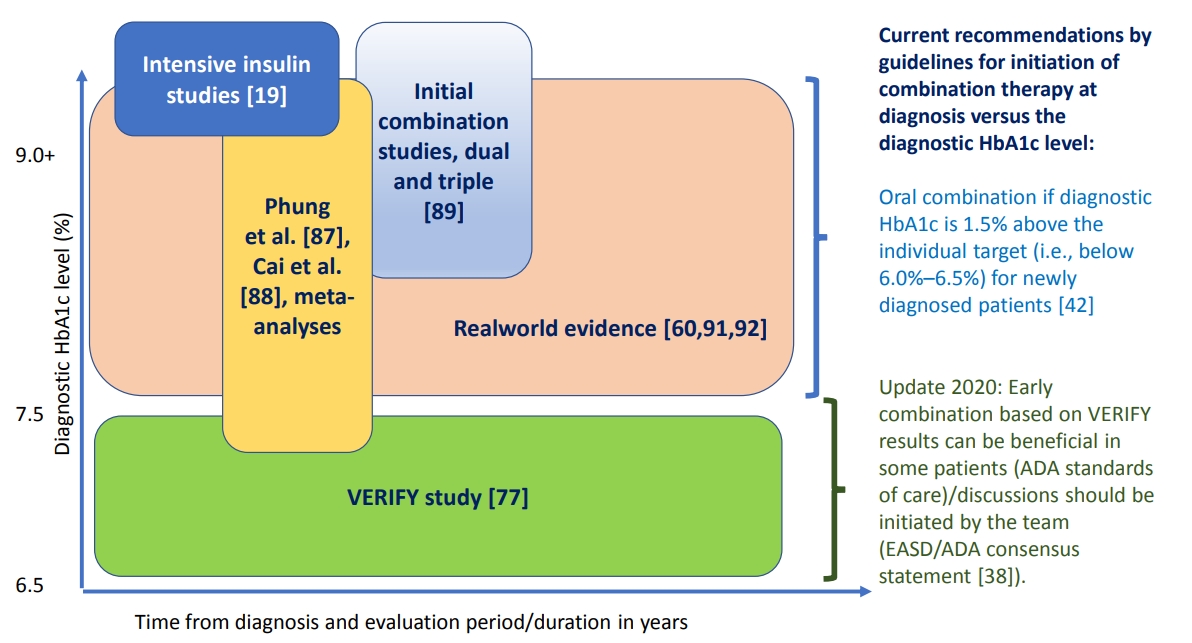
- Evaluating the Evidence behind the Novel Strategy of Early Combination from Vision to Implementation
- Päivi Maria Paldánius
- Diabetes Metab J. 2020;44(6):785-801. Published online September 15, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0179
- 7,109 View
- 287 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Type 2 diabetes mellitus (T2DM) is a complex and progressive chronic disease characterised by elevating hyperglycaemia and associated need to gradually intensify therapy in order to achieve and maintain glycaemic control. Treating hyperglycaemia with sequential therapy is proposed to allow holistic assessment of the efficacy and risk-to-benefit ratio of each added component. However, there is an array of evidence supporting the scientific rationale for using synergistic, earlier, modern drug combinations to achieve glycaemic goals, delay the deterioration of glycaemic control, and, therefore, potentially preserve or slow down the declining β-cell function. Additionally, implementation of early combination(s) may lead to opportunities to combat clinical inertia and other hurdles to optimised disease management outcomes. This review aims to discuss the latest empirical evidence for long-term clinical benefits of this novel strategy of early combination in people with newly diagnosed T2DM versus the current widely-implemented treatment paradigm, which focuses on control of hyperglycaemia using lifestyle interventions followed by sequentially intensified (mostly metformin-based) monotherapy. The recent reported Vildagliptin Efficacy in combination with metfoRmin For earlY treatment of T2DM (VERIFY) study results have provided significant new evidence confirming long-term glycaemic durability and tolerability of a specific early combination in the management of newly diagnosed, treatment-naïve patients worldwide. These results have also contributed to changes in clinical treatment guidelines and standards of care while clinical implementation and individualised treatment decisions based on VERIFY results might face barriers beyond the existing scientific evidence.
- Drug/Regimen
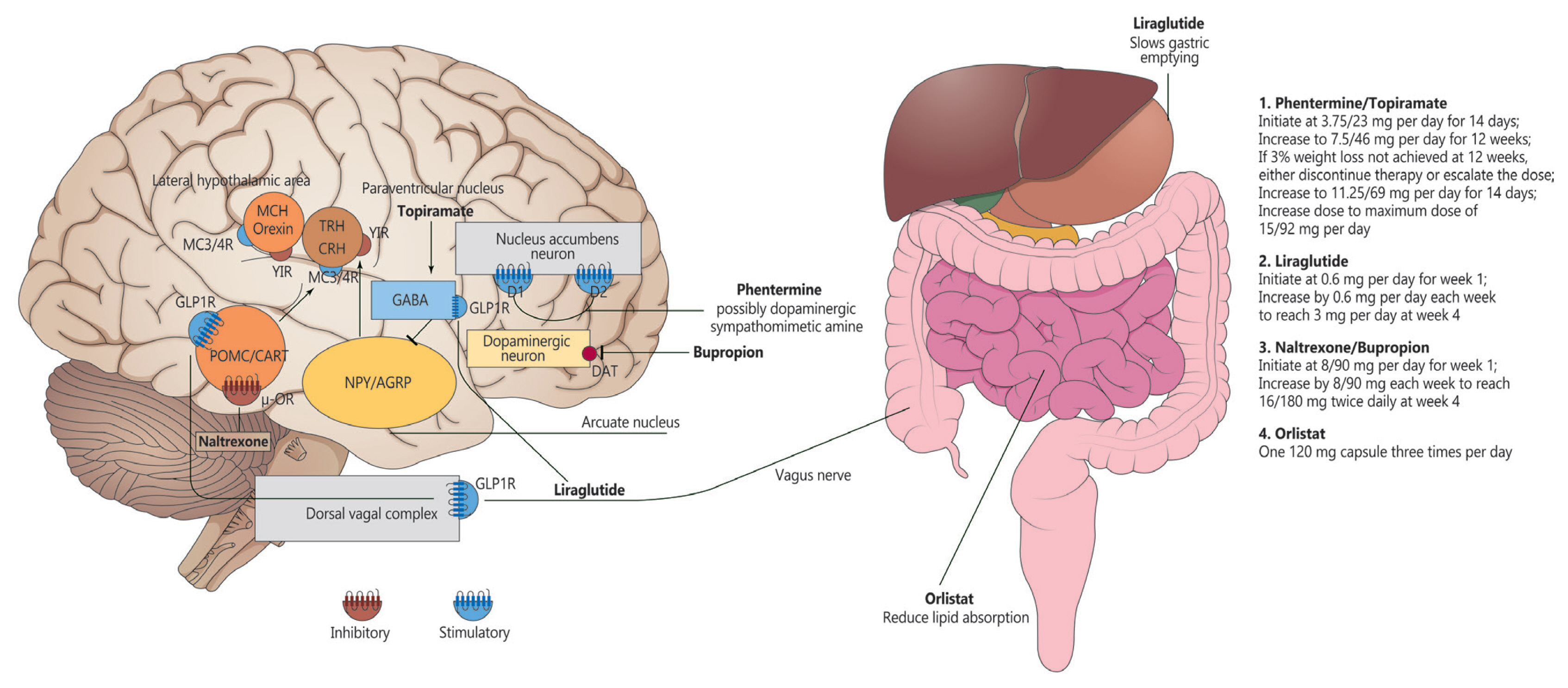
- Comprehensive Review of Current and Upcoming Anti-Obesity Drugs
- Jang Won Son, Sungrae Kim
- Diabetes Metab J. 2020;44(6):802-818. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0258
- 15,251 View
- 974 Download
- 54 Web of Science
- 61 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Obesity is among the leading causes of morbidity and mortality worldwide and its prevalence continues to increase globally. Because obesity is a chronic, complex, and heterogeneous disease influenced by genetic, developmental, biological, and environmental factors, it is necessary to approach obesity with an integrated and comprehensive treatment strategy. As it is difficult to achieve and sustain successful long-term weight loss in most patients with obesity through lifestyle modifications (e.g., diet, exercise, and behavioral therapy), pharmacological approaches to the treatment of obesity should be considered as an adjunct therapy. Currently, four drugs (orlistat, naltrexone extended-release [ER]/bupropion ER, phentermine/topiramate controlled-release, and liraglutide) can be used long-term (>12 weeks) to promote weight loss by suppressing appetite or decreasing fat absorption. Pharmacotherapy for obesity should be conducted according to a proper assessment of the clinical evidence and customized to individual patients considering the characteristics of each drug and comorbidities associated with obesity. In this review, we discuss the mechanisms of action, efficacy, and safety of these available long-term anti-obesity drugs and introduce other potential agents under investigation. Furthermore, we discuss the need for research on personalized obesity medicine.
-
Citations
Citations to this article as recorded by- Shedding light on weight loss: A narrative review of medications for treating obesity
Haritha Darapaneni, Samridhi Lakhanpal, Hiren Chhayani, Kinna Parikh, Meet Patel, Vasu Gupta, Fnu Anamika, Ripudaman Munjal, Rohit Jain
Romanian Journal of Internal Medicine.2024; 62(1): 3. CrossRef - Projected health and economic effects of the increase in childhood obesity during the COVID-19 pandemic in England: The potential cost of inaction
Iván Ochoa-Moreno, Ravita Taheem, Kathryn Woods-Townsend, Debbie Chase, Keith M. Godfrey, Neena Modi, Mark Hanson, Rebecca F. Baggaley
PLOS ONE.2024; 19(1): e0296013. CrossRef - Metabolic-associated fatty liver disease: a selective review of pathogenesis, diagnostic approaches, and therapeutic strategies
Mohammad Habibullah, Khaleed Jemmieh, Amr Ouda, Mohammad Zulqurnain Haider, Mohammed Imad Malki, Abdel-Naser Elzouki
Frontiers in Medicine.2024;[Epub] CrossRef - A review of an investigational drug retatrutide, a novel triple agonist agent for the treatment of obesity
Manmeet Kaur, Saurav Misra
European Journal of Clinical Pharmacology.2024; 80(5): 669. CrossRef - Gut microbiota and therapy for obesity and type 2 diabetes
Luyao Zhang, Pai Wang, Juan Huang, Yanpeng Xing, F. Susan Wong, Jian Suo, Li Wen
Frontiers in Endocrinology.2024;[Epub] CrossRef - Adipocyte-targeted delivery of rosiglitazone with localized photothermal therapy for the treatment of diet-induced obesity in mice
Yunxiao Zhang, Maoqi Luo, Yaxin Jia, Tingting Gao, Li Deng, Tao Gong, Zhirong Zhang, Xi Cao, Yao Fu
Acta Biomaterialia.2024;[Epub] CrossRef - Efficacy of endoscopic resuturing versus pharmacotherapy to treat weight recidivism after endoscopic sleeve gastroplasty
Kaveh Hajifathalian, Okeefe Simmons, Mohamed Abu-Hammour, Kamal Hassan, Reem Z. Sharaiha
Gastrointestinal Endoscopy.2023; 98(6): 944. CrossRef - Gardenia fruit and Eucommia leaves combination improves hyperlipidemia and hyperglycemia via pancreatic lipase and AMPK-PPARα and Keap-1-Nrf2-HO-1 regulation
Xiaotong Su, Shun Hao, Wenna Li, Xu Li, Zhentao Mo, Yiqi Li, Lu Xiao, Wenjun Wang, Feng Wang
Journal of Functional Foods.2023; 100: 105394. CrossRef - Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis
Yan Li, Wenjuan Yang, Yuanyuan Zheng, Weiqi Dai, Jie Ji, Liwei Wu, Ziqi Cheng, Jie Zhang, Jingjing Li, Xuanfu Xu, Jianye Wu, Mingwei Yang, Jiao Feng, Chuanyong Guo
Journal of Experimental & Clinical Cancer Research.2023;[Epub] CrossRef - Diet-induced gut dysbiosis and inflammation: Key drivers of obesity-driven NASH
Gideon G. Kang, Natalie L. Trevaskis, Andrew J. Murphy, Mark A. Febbraio
iScience.2023; 26(1): 105905. CrossRef - The gut microbiota in obesity and weight management: microbes as friends or foe?
Matthias Van Hul, Patrice D. Cani
Nature Reviews Endocrinology.2023; 19(5): 258. CrossRef - Phase I studies of the safety, tolerability, pharmacokinetics and pharmacodynamics of the dual glucagon receptor/glucagon‐like peptide‐1 receptor agonistBI456906
Arvid Jungnik, Jorge Arrubla Martinez, Leona Plum‐Mörschel, Christoph Kapitza, Daniela Lamers, Claus Thamer, Corinna Schölch, Michael Desch, Anita M. Hennige
Diabetes, Obesity and Metabolism.2023; 25(4): 1011. CrossRef - From Metabolic Syndrome to Type 2 Diabetes in Youth
Dario Iafusco, Roberto Franceschi, Alice Maguolo, Salvatore Guercio Nuzio, Antonino Crinò, Maurizio Delvecchio, Lorenzo Iughetti, Claudio Maffeis, Valeria Calcaterra, Melania Manco
Children.2023; 10(3): 516. CrossRef - Excess body weight: Novel insights into its roles in obesity comorbidities
Xiang Zhang, Suki Ha, Harry Cheuk-Hay Lau, Jun Yu
Seminars in Cancer Biology.2023; 92: 16. CrossRef - Striatal dopamine D2-like receptors availability in obesity and its modulation by bariatric surgery: a systematic review and meta-analysis
Gabriela Ribeiro, Ana Maia, Gonçalo Cotovio, Francisco P. M. Oliveira, Durval C. Costa, Albino J. Oliveira-Maia
Scientific Reports.2023;[Epub] CrossRef - Cost-effectiveness analysis of five anti-obesity medications from a US payer's perspective
Ainhoa Gómez Lumbreras, Malinda S. Tan, Lorenzo Villa-Zapata, Sabrina Ilham, Jacob C. Earl, Daniel C. Malone
Nutrition, Metabolism and Cardiovascular Diseases.2023; 33(6): 1268. CrossRef - Analysis of Serious Weight Gain in Patients Using Alectinib for ALK-Positive Lung Cancer
Simon P. de Leeuw, Melinda A. Pruis, Barend J. Sikkema, Mostafa Mohseni, G. D. Marijn Veerman, Marthe S. Paats, Daphne W. Dumoulin, Egbert F. Smit, Annemie M.W. J. Schols, Ron H.J. Mathijssen, Elisabeth F.C. van Rossum, Anne-Marie C. Dingemans
Journal of Thoracic Oncology.2023; 18(8): 1017. CrossRef - Role of flavonoids in controlling obesity: molecular targets and mechanisms
Anns Mahboob, Samson Mathews Samuel, Arif Mohamed, Mohmmad Younus Wani, Sofiane Ghorbel, Nabil Miled, Dietrich Büsselberg, Ali Chaari
Frontiers in Nutrition.2023;[Epub] CrossRef - The N-degron pathway mediates lipophagy: The chemical modulation of lipophagy in obesity and NAFLD
Eui Jung Jung, Ki Woon Sung, Tae Hyun Bae, Hee-Yeon Kim, Ha Rim Choi, Sung Hyun Kim, Chan Hoon Jung, Su Ran Mun, Yeon Sung Son, Shin Kim, Young Ho Suh, Anna Kashina, Joo-Won Park, Yong Tae Kwon
Metabolism.2023; 146: 155644. CrossRef - Bupropion Mediated Effects on Depression, Attention Deficit Hyperactivity Disorder, and Smoking Cessation
Austin Clark, Brendan Tate, Bretton Urban, Ryan Schroeder, Sonja Gennuso, Shahab Ahmadzadeh, David McGregor, Brook Girma, Sahar Shekoohi, Alan D. Kaye
Health Psychology Research.2023;[Epub] CrossRef - Recent advancements in pharmacological strategies to modulate energy balance for combating obesity
Benudhara Pati, Satyabrata Sendh, Bijayashree Sahu, Sunil Pani, Nivedita Jena, Naresh Chandra Bal
RSC Medicinal Chemistry.2023; 14(8): 1429. CrossRef - Management of abdominal wall hernias in patients with severe obesity
Omar M. Ghanem, Sean Orenstein, S. Julie-Ann Lloyd, Amin Andalib, Alice Race, Holly Ann Burt, Farah Husain, Matthew Goldblatt, Matthew Kroh
Surgical Endoscopy.2023; 37(9): 6619. CrossRef - Obesity-related hypertension and chronic kidney disease: from evaluation to management
Mi-Hyang Jung, Sang-Hyun Ihm
Kidney Research and Clinical Practice.2023; 42(4): 431. CrossRef - Antiepileptics pharmacotherapy or antidiabetics may hold potential in treatment of epileptic patients with diabetes mellitus: A narrative review
Marwan AL-NİMER, Saeed AL-ZUHAİRY
Hacettepe University Journal of the Faculty of Pharmacy.2023;[Epub] CrossRef - Medicamentos antidiabéticos utilizados com a finalidade de perda de peso
Sâmia Moreira de ANDRADE, Maria Victória Macedo de ANDRADE, Pedro Henrique Almeida de FARIA, Luís Marcelo Vieira ROSA, Plínio Robson Cavalcante COSTA, Luiz Gustavo Freitas PIRES
Diversitas Journal.2023;[Epub] CrossRef - Treatment of hypothalamic obesity in people with hypothalamic injury: new drugs are on the horizon
Christian L. Roth, Anna Zenno
Frontiers in Endocrinology.2023;[Epub] CrossRef - Obesity: What’s Old, What’s New and How We Manage It: An Integrated Approach in Pharmacy Practice
Ângelo Jesus
FarmaJournal.2023; 8(2): 45. CrossRef - Integrating Pharmacotherapy and Psychotherapy for Weight Loss
Marla Sanzone, Morgan Sammons
Journal of Health Service Psychology.2023; 49(4): 177. CrossRef - Effect of Lingguizhugan decoction in activating fat mobilization in obesity
Yubin YANG, Yunlong WANG, Zhengbang SUN, Ting DU, Ying YANG, Jiaojiao CHEN, Meiling WANG, Hairong LI, Jian QIN
Journal of Holistic Integrative Pharmacy.2022; 3(1): 68. CrossRef - Computational approaches to predicting treatment response to obesity using neuroimaging
Leonard Kozarzewski, Lukas Maurer, Anja Mähler, Joachim Spranger, Martin Weygandt
Reviews in Endocrine and Metabolic Disorders.2022; 23(4): 773. CrossRef - Delivery of miRNAs to the adipose organ for metabolic health
Karin Kornmueller, Ez-Zoubir Amri, Marcel Scheideler, Ruth Prassl
Advanced Drug Delivery Reviews.2022; 181: 114110. CrossRef - Purple sweet potato leaf extracts suppress adipogenic differentiation of human bone marrow–derived mesenchymal stem cells
Masakazu Ishii, Nao Ikeda, Haruka Miyata, Manami Takahashi, Masahiro Nishimura
Journal of Food Biochemistry.2022;[Epub] CrossRef - The Anti-Adiposity Mechanisms of Ampelopsin and Vine Tea Extract in High Fat Diet and Alcohol-Induced Fatty Liver Mouse Models
Jianbo Wu, Kenchi Miyasaka, Wakana Yamada, Shogo Takeda, Norihito Shimizu, Hiroshi Shimoda
Molecules.2022; 27(3): 607. CrossRef - Pharmacotherapeutic options in pediatric obesity: an urgent call for further research
María Florencia González Bagnes, Claudio González, Valeria Hirschler, Guillermo Di Girolamo
Expert Opinion on Pharmacotherapy.2022; 23(8): 869. CrossRef - Russelioside B: a Pregnane Glycoside with Pharmacological Potential
Essam Abdel-Sattar, Dalia E. Ali
Revista Brasileira de Farmacognosia.2022; 32(2): 188. CrossRef - Pharmacotherapy in Childhood Obesity
Peter Kühnen, Heike Biebermann, Susanna Wiegand
Hormone Research in Paediatrics.2022; 95(2): 177. CrossRef - Effects of Vinegar/Acetic Acid Intake on Appetite Measures and Energy Consumption: Systematic Review
Faten O. Hasan, Kristen P. Hamilton, Siddhartha S. Angadi, Sibylle Kranz
Translational Journal of the American College of Sports Medicine.2022;[Epub] CrossRef - Renaming NAFLD to MAFLD: Advantages and Potential Changes in Diagnosis, Pathophysiology, Treatment, and Management
Fajuan Rui, Hongli Yang, Xinyu Hu, Qi Xue, Yayun Xu, Junping Shi, Jie Li
Infectious Microbes and Diseases.2022; 4(2): 49. CrossRef - Treatments for obesity in the context of nonalcoholic steatohepatitis and mental health
Aadi Sharma, Somaya Albhaisi, Arun J. Sanyal
Clinical Liver Disease.2022; 20(2): 48. CrossRef - A narrative review of anti-obesity medications for obese patients with osteoarthritis
Win Min Oo, Ali Mobasheri, David J Hunter
Expert Opinion on Pharmacotherapy.2022; 23(12): 1381. CrossRef - Sulfated Glucan from the Green Seaweed Caulerpa sertularioides Inhibits Adipogenesis through Suppression of Adipogenic and Lipogenic Key Factors
Gildacio Chaves Filho, Lucas Batista, Silvia de Medeiros, Hugo Rocha, Susana Moreira
Marine Drugs.2022; 20(8): 470. CrossRef - Clinical Impact of Semaglutide, a Glucagon-Like Peptide-1 Receptor Agonist, on Obesity Management: A Review
Nasser M Alorfi, Alanood S Algarni
Clinical Pharmacology: Advances and Applications.2022; Volume 14: 61. CrossRef - The Role of Gut Microbiota Modulation Strategies in Obesity: The Applications and Mechanisms
Lingyue Shan, Akanksha Tyagi, Umair Shabbir, Xiuqin Chen, Selvakumar Vijayalakshmi, Pianpian Yan, Deog-Hwan Oh
Fermentation.2022; 8(8): 376. CrossRef - Orlistat and ezetimibe could differently alleviate the high-fat diet-induced obesity phenotype by modulating the gut microbiota
Jin Jin, Jiani Wang, Ruyue Cheng, Yan Ren, Zhonghua Miao, Yating Luo, Qingqing Zhou, Yigui Xue, Xi Shen, Fang He, Haoming Tian
Frontiers in Microbiology.2022;[Epub] CrossRef - N-linoleyltyrosine ameliorates high-fat diet-induced obesity in C57BL/6 mice via cannabinoid receptor regulation
Zheng-yu Yang, Yi-ying Wu, Yi Zhou, Yun-qi Yang, Jia-hui Zhang, Tao He, Sha Liu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Anti-obesity pharmacotherapy for treatment of pediatric type 2 diabetes: Review of the literature and lessons learned from adults
Megan O. Bensignor, Aaron S. Kelly, Silva Arslanian
Frontiers in Endocrinology.2022;[Epub] CrossRef - Blockade of CXXC5-dishevelled interaction inhibits adipogenic differentiation, obesity, and insulin resistance in mice
Seol Hwa Seo, Dasung Lee, Soung-Hoon Lee, Kang-Yell Choi
Scientific Reports.2022;[Epub] CrossRef - Effect of the Melanocortin 4-Receptor Ile269Asn Mutation on Weight Loss Response to Dietary, Phentermine and Bariatric Surgery Interventions
Itzel G. Salazar-Valencia, Hugo Villamil-Ramírez, Francisco Barajas-Olmos, Martha Guevara-Cruz, Luis R. Macias-Kauffer, Humberto García-Ortiz, Omar Hernández-Vergara, David Alberto Díaz de Sandy-Galán, Paola León-Mimila, Federico Centeno-Cruz, Luis E. Gon
Genes.2022; 13(12): 2267. CrossRef - A Nephrologist Perspective on Obesity: From Kidney Injury to Clinical Management
Clara García-Carro, Ander Vergara, Sheila Bermejo, María A. Azancot, Joana Sellarés, Maria José Soler
Frontiers in Medicine.2021;[Epub] CrossRef - Phentermine-Induced Acute Kidney Injury Secondary to Uncontrolled Hypertension in a Patient with Weight Regain Post-bariatric Surgery
Marvin Wei Jie Chua, Boon Cheok Lai
Obesity Surgery.2021; 31(8): 3874. CrossRef - BMI Course Over 10 Years After Bariatric Surgery and Biopsychosocial Complexity Assessed with the INTERMED: a Retrospective Study
Yann Corminboeuf, Beate Wild, Catherine Zdrojewski, Dieter Schellberg, Lucie Favre, Michel Suter, Friedrich Stiefel
Obesity Surgery.2021; 31(9): 3996. CrossRef - The Role of Positron Emission Tomography in Bariatric Surgery Research: a Review
Jason Bini, Mathieu Norcross, Maija Cheung, Andrew Duffy
Obesity Surgery.2021; 31(10): 4592. CrossRef - Anti-Obesity Effect of Hot Water Extract of Barley Sprout through the Inhibition of Adipocyte Differentiation and Growth
Myeong-Jin Kim, Hye-Won Kawk, Sang-Hyeon Kim, Hyo-Jae Lee, Ji-Won Seo, Jong-Tae Kim, Seung-Hee Jang, Min-Jeong Kim, Young-Min Kim
Metabolites.2021; 11(9): 610. CrossRef - Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes
Wona Jee, Seung-Hyeon Lee, Hyun Min Ko, Ji Hoon Jung, Won-Seok Chung, Hyeung-Jin Jang
International Journal of Molecular Sciences.2021; 22(19): 10405. CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - Chlorogenic Acids Inhibit Adipogenesis: Implications of Wnt/β-Catenin Signaling Pathway
Mengting Liu, Jian Qin, Jing Cong, Yubin Yang, Muhittin Yurekli
International Journal of Endocrinology.2021; 2021: 1. CrossRef - Médicaments anti-obésité : leçons des échecs pour l’avenir
André J. Scheen
Médecine des Maladies Métaboliques.2021; 15(8): 734. CrossRef - Obesity and Eating Disorders in Children and Adolescents: The Bidirectional Link
Stella Stabouli, Serap Erdine, Lagle Suurorg, Augustina Jankauskienė, Empar Lurbe
Nutrients.2021; 13(12): 4321. CrossRef - MEK6 Overexpression Exacerbates Fat Accumulation and Inflammatory Cytokines in High-Fat Diet-Induced Obesity
Suyeon Lee, Myoungsook Lee
International Journal of Molecular Sciences.2021; 22(24): 13559. CrossRef - Skin and obesity in childhood: an update
Valeria Hirschler
AIMS Medical Science.2021; 8(4): 311. CrossRef - An Avocado Extract Enriched in Mannoheptulose Prevents the Negative Effects of a High-Fat Diet in Mice
Paul J. Pistell, Tadanobu Utsuki, Joseph Francis, Philip J. Ebenezer, Jennifer Terrebonne, George S. Roth, Donald K. Ingram
Nutrients.2021; 14(1): 155. CrossRef
- Shedding light on weight loss: A narrative review of medications for treating obesity
- Technology/Device
- Present and Future of Digital Health in Diabetes and Metabolic Disease
- Sang Youl Rhee, Chiweon Kim, Dong Wook Shin, Steven R. Steinhubl
- Diabetes Metab J. 2020;44(6):819-827. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0088
- 9,046 View
- 262 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The use of information and communication technology (ICT) in medical and healthcare services goes beyond everyday life. Expectations of a new medical environment, not previously experienced by ICT, exist in the near future. In particular, chronic metabolic diseases such as diabetes and obesity, have a high prevalence and high social and economic burden. In addition, the continuous evaluation and monitoring of daily life is important for effective treatment and management. Therefore, the wide use of ICTbased digital health systems is required for the treatment and management of these diseases. In this article, we compiled a variety of digital health technologies introduced to date in the field of diabetes and metabolic diseases.
-
Citations
Citations to this article as recorded by- Digital Health in Diabetes and Cardiovascular Disease
Dorothy Avoke, Abdallah Elshafeey, Robert Weinstein, Chang H. Kim, Seth S. Martin
Endocrine Research.2024; : 1. CrossRef - Weight Management Health Note, a Mobile Health Platform for Obesity Management Developed by the Korean Society for the Study of Obesity
Yujung Lee, Hyunji Sang, Sunyoung Kim, Doo Ah Choi, Sang Youl Rhee
Journal of Obesity & Metabolic Syndrome.2024; 33(1): 1. CrossRef - Effectiveness of a Social Networking Site Based Automatic Mobile Message Providing System on Glycemic Control in Patients with Type 2 Diabetes Mellitus
Kyuho Kim, Jae-Seung Yun, Joonyub Lee, Yeoree Yang, Minhan Lee, Yu-Bae Ahn, Jae Hyoung Cho, Seung-Hyun Ko
Endocrinology and Metabolism.2024; 39(2): 344. CrossRef - A data-driven approach to manage type 2 diabetes mellitus through digital health: The Klivo Intervention Program protocol (KIPDM)
Camila Maciel de Oliveira, Luiza Borcony Bolognese, Mercedes Balcells, Davi Casale Aragon, Roberto Luis Zagury, Clemente Nobrega, Chunyu Liu, Dured Dardari
PLOS ONE.2023; 18(2): e0281844. CrossRef - Public Health Framework for Smart Cities within the Comprehensive Approach to Sustainability in Europe: Case Study of Diabetes
Luís Velez Lapão, Jorge César Correia, Marija Jevtic
Sustainability.2023; 15(5): 4269. CrossRef - Lessons for Vietnam on the Use of Digital Technologies to Support Patient-Centered Care in Low- and Middle-Income Countries in the Asia-Pacific Region: Scoping Review
Leona Kosowicz, Kham Tran, Toan Tran Khanh, Thu Ha Dang, Van An Pham, Hue Ta Thi Kim, Hoang Thi Bach Duong, Tran Dong Nguyen, Anh Tuyet Phuong, Trong Hieu Le, Van Anh Ta, Nilmini Wickramasinghe, Penelope Schofield, John Zelcer, Tuan Pham Le, Tuan Anh Nguy
Journal of Medical Internet Research.2023; 25: e43224. CrossRef - Digital health, cardiometabolic disease and ethnicity: an analysis of United Kingdom government policies from 2010 to 2022
Zareen Thorlu-Bangura, Lydia Poole, Harpreet Sood, Nushrat Khan, Fiona Stevenson, Kamlesh Khunti, Paramjit Gill, Madiha Sajid, Wasim Hanif, Neeraj Bhala, Shivali Modha, Kiran Patel, Ann Blandford, Amitava Banerjee, Mel Ramasawmy
Journal of Public Health Policy.2023; 44(2): 179. CrossRef - Digital Behavior Change Interventions to Reduce Sedentary Behavior and Promote Physical Activity in Adults with Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Xiaoyan Zhang, Xue Qiao, Ke Peng, Shan Gao, Yufang Hao
International Journal of Behavioral Medicine.2023;[Epub] CrossRef - Exploring the underlying mechanisms of obesity and diabetes and the potential of Traditional Chinese Medicine: an overview of the literature
Yan-kun Chen, Ting-ting Liu, Farah Khameis Farag Teia, Meng-zhou Xie
Frontiers in Endocrinology.2023;[Epub] CrossRef - Stakeholders’ Perceptions Regarding Digital Therapeutics Reimbursement in South Korea: Qualitative Study
Boram Sim, Jin Han Ju, Byungsoo Kim, Jin Yong Lee
JMIR mHealth and uHealth.2023; 11: e47407. CrossRef - Research Trends in Motivation and Weight Loss: A Bibliometric-Based Review
Uroš Železnik, Peter Kokol, Jasmina Starc, Danica Železnik, Jernej Završnik, Helena Blažun Vošner
Healthcare.2023; 11(23): 3086. CrossRef - Analysis of the management and therapeutic performance of diabetes mellitus employing special target
Hong-Yan Sun, Xiao-Yan Lin
World Journal of Diabetes.2023; 14(12): 1721. CrossRef - Psychoeducational Interventions in Children and Adolescents with Type-1 Diabetes: A Systematic Review
Bárbara Luque, Joaquín Villaécija, Rosario Castillo-Mayén, Esther Cuadrado, Sebastián Rubio, Carmen Tabernero
Clínica y Salud.2022; 33(1): 35. CrossRef - Prevalence of Hyperuricemia Among Chinese Adults: Findings From Two Nationally Representative Cross-Sectional Surveys in 2015–16 and 2018–19
Mei Zhang, Xiaoxia Zhu, Jing Wu, Zhengjing Huang, Zhenping Zhao, Xiao Zhang, Yu Xue, Weiguo Wan, Chun Li, Wenrong Zhang, Linhong Wang, Maigeng Zhou, Hejian Zou, Limin Wang
Frontiers in Immunology.2022;[Epub] CrossRef - Blood Pressure Monitoring as a Digital Health Tool for Improving Diabetes Clinical Outcomes: Retrospective Real-world Study
Yifat Fundoiano-Hershcovitz, Dror Bacher, Marilyn D Ritholz, David L Horwitz, Omar Manejwala, Pavel Goldstein
Journal of Medical Internet Research.2022; 24(2): e32923. CrossRef - The effectiveness of a structured group education programme for people with established type 2 diabetes in a multi-ethnic population in primary care: A cluster randomised trial
Helen Dallosso, Panna Mandalia, Laura J. Gray, Yogini V. Chudasama, Sopna Choudhury, Shahrad Taheri, Naina Patel, Kamlesh Khunti, Melanie J. Davies
Nutrition, Metabolism and Cardiovascular Diseases.2022; 32(6): 1549. CrossRef - Efficacy of Personalized Diabetes Self-care Using an Electronic Medical Record–Integrated Mobile App in Patients With Type 2 Diabetes: 6-Month Randomized Controlled Trial
Eun Young Lee, Seon-Ah Cha, Jae-Seung Yun, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, Min Kyung Hyun, Seung-Hyun Ko
Journal of Medical Internet Research.2022; 24(7): e37430. CrossRef - Qatar Diabetes Mobile Application Trial (QDMAT): an open-label randomised controlled trial to examine the impact of using a mobile application to improve diabetes care in type 2 diabetes mellitus—a study protocol
Noor Suleiman, Meis Alkasem, Zaina Al Amer, Obada Salameh, Noora Al-Thani, Mohammad Khair Hamad, Khaled Baagar, Ibrahem Abdalhakam, Manal Othman, Ragae Dughmosh, Dabia Al-Mohanadi, Ali Al Sanousi, Mohammed Bashir, Odette Chagoury, Shahrad Taheri, Abdul-Ba
Trials.2022;[Epub] CrossRef - Digital environment: An evolutionary component in environmental health
Afiqah Syamimi Masrani, Nik Rosmawati Nik Husain
Journal of Public Health Research.2022; 11(2): 227990362211031. CrossRef - Innovations in Cardio-oncology Resulting from the COVID-19 Pandemic
Lavanya Kondapalli, Garima Arora, Riem Hawi, Efstathia Andrikopoulou, Courtney Estes, Nirav Patel, Carrie G. Lenneman
Current Treatment Options in Oncology.2022; 23(9): 1288. CrossRef - Effects of peer support and mobile application-based walking programme on physical activity and physical function in rural older adults: a cluster randomized controlled trial
Xue Cai, Shanhu Qiu, Dan Luo, Ruxue Li, Chengyu Liu, Yanhui Lu, Cuirong Xu, Mingzi Li
European Geriatric Medicine.2022; 13(5): 1187. CrossRef
- Digital Health in Diabetes and Cardiovascular Disease
- Type 1 Diabetes
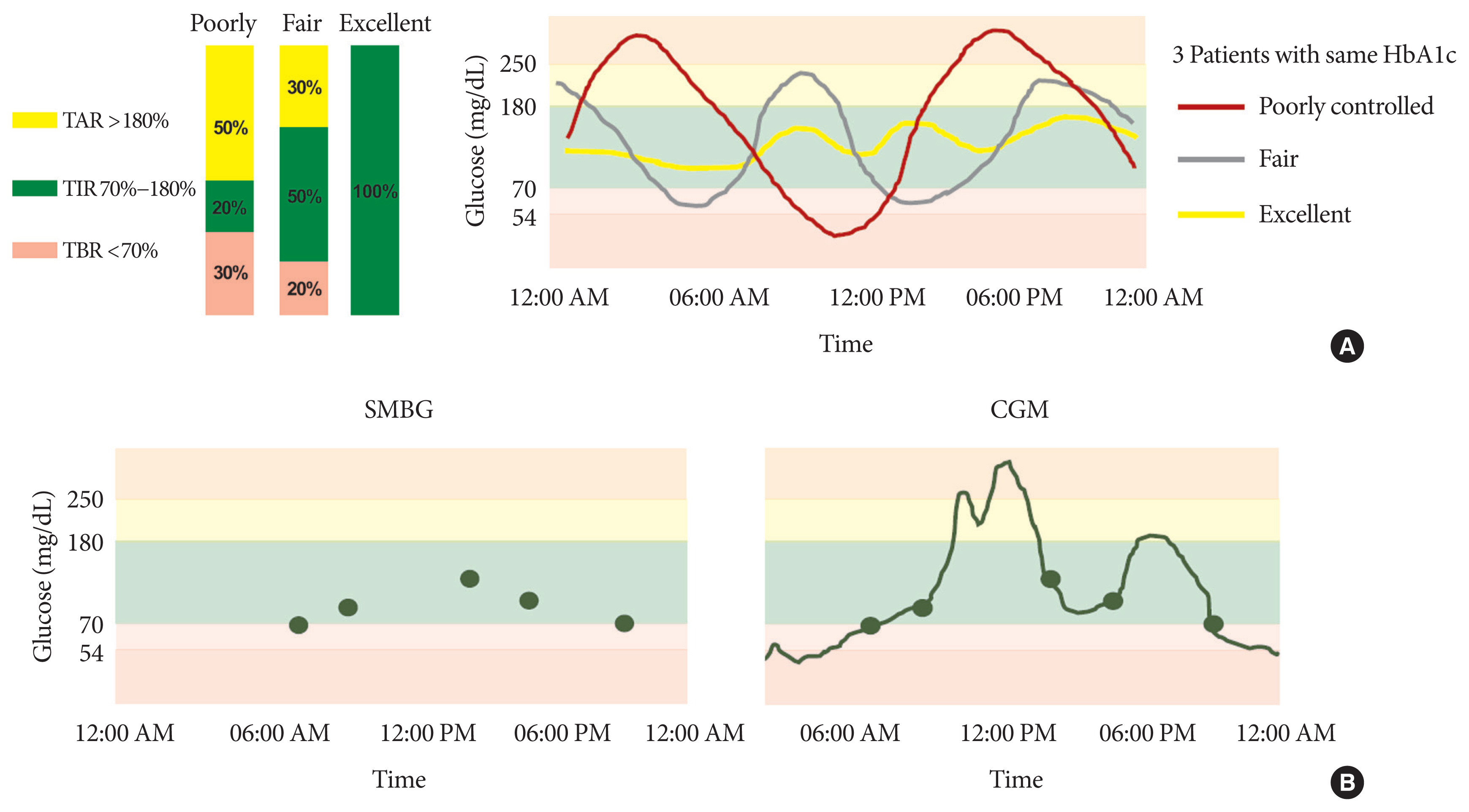
- Time in Range from Continuous Glucose Monitoring: A Novel Metric for Glycemic Control
- Jee Hee Yoo, Jae Hyeon Kim
- Diabetes Metab J. 2020;44(6):828-839. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0257
- Correction in: Diabetes Metab J 2021;45(5):795
- 9,822 View
- 467 Download
- 31 Web of Science
- 36 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Glycosylated hemoglobin (HbA1c) has been the sole surrogate marker for assessing diabetic complications. However, consistently reported limitations of HbA1c are that it lacks detailed information on short-term glycemic control and can be easily interfered with by various clinical conditions such as anemia, pregnancy, or liver disease. Thus, HbA1c alone may not represent the real glycemic status of a patient. The advancement of continuous glucose monitoring (CGM) has enabled both patients and healthcare providers to monitor glucose trends for a whole single day, which is not possible with HbA1c. This has allowed for the development of core metrics such as time spent in time in range (TIR), hyperglycemia, or hypoglycemia, and glycemic variability. Among the 10 core metrics, TIR is reported to represent overall glycemic control better than HbA1c alone. Moreover, various evidence supports TIR as a predictive marker of diabetes complications as well as HbA1c, as the inverse relationship between HbA1c and TIR reveals. However, there are more complex relationships between HbA1c, TIR, and other CGM metrics. This article provides information about 10 core metrics with particular focus on TIR and the relationships between the CGM metrics for comprehensive understanding of glycemic status using CGM.
-
Citations
Citations to this article as recorded by- Acute and Chronic Adverse Outcomes of Type 1 Diabetes
Rachel Longendyke, Jody B. Grundman, Shideh Majidi
Endocrinology and Metabolism Clinics of North America.2024; 53(1): 123. CrossRef - La plongée sous-marine en scaphandre autonome avec un diabète de type 1. Une belle histoire du dernier millénaire
Lise Dufaitre Patouraux, Agnès Sola-Gazagnes, Boris Lormeau, Corinne Lormeau
Médecine des Maladies Métaboliques.2024; 18(1): 67. CrossRef - S100A9 exerts insulin-independent antidiabetic and anti-inflammatory effects
Gloria Ursino, Giulia Lucibello, Pryscila D. S. Teixeira, Anna Höfler, Christelle Veyrat-Durebex, Soline Odouard, Florian Visentin, Luca Galgano, Emmanuel Somm, Claudia R. Vianna, Ariane Widmer, François R. Jornayvaz, Andreas Boland, Giorgio Ramadori, Rob
Science Advances.2024;[Epub] CrossRef - Hybrid Closed-Loop Versus Manual Insulin Delivery in Adults With Type 1 Diabetes: A Post Hoc Analysis Using the Glycemia Risk Index
Melissa H. Lee, Sara Vogrin, Timothy W. Jones, David N. O’Neal
Journal of Diabetes Science and Technology.2024;[Epub] CrossRef - Clinically relevant stratification of patients with type 2 diabetes by using continuous glucose monitoring data
Xiaopeng Shao, Jingyi Lu, Rui Tao, Liang Wu, Yaxin Wang, Wei Lu, Hongru Li, Jian Zhou, Xia Yu
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Effects of a 2-Week Kinect-Based Mixed-Reality Exercise Program on Prediabetes: A Pilot Trial during COVID-19
So Young Ahn, Si Woo Lee, Hye Jung Shin, Won Jae Lee, Jun Hyeok Kim, Hyun-Jun Kim, Wook Song
Journal of Obesity & Metabolic Syndrome.2024; 33(1): 54. CrossRef - Continuous glucose monitoring with structured education in adults with type 2 diabetes managed by multiple daily insulin injections: a multicentre randomised controlled trial
Ji Yoon Kim, Sang-Man Jin, Kang Hee Sim, Bo-Yeon Kim, Jae Hyoung Cho, Jun Sung Moon, Soo Lim, Eun Seok Kang, Cheol-Young Park, Sin Gon Kim, Jae Hyeon Kim
Diabetologia.2024;[Epub] CrossRef - Comparison between a tubeless, on-body automated insulin delivery system and a tubeless, on-body sensor-augmented pump in type 1 diabetes: a multicentre randomised controlled trial
Ji Yoon Kim, Sang-Man Jin, Eun Seok Kang, Soo Heon Kwak, Yeoree Yang, Jee Hee Yoo, Jae Hyun Bae, Jun Sung Moon, Chang Hee Jung, Ji Cheol Bae, Sunghwan Suh, Sun Joon Moon, Sun Ok Song, Suk Chon, Jae Hyeon Kim
Diabetologia.2024;[Epub] CrossRef - Anagliptin twice‐daily regimen improves glycaemic variability in subjects with type 2 diabetes: A double‐blind, randomized controlled trial
Yong‐ho Lee, Doo‐Man Kim, Jae Myung Yu, Kyung Mook Choi, Sin Gon Kim, Kang Seo Park, Hyun‐Shik Son, Choon Hee Chung, Kyu Jeung Ahn, Soon Hee Lee, Ki‐Ho Song, Su Kyoung Kwon, Hyeong Kyu Park, Kyu Chang Won, Hak Chul Jang
Diabetes, Obesity and Metabolism.2023; 25(5): 1174. CrossRef - Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes & Metabolism Journal.2023; 47(1): 27. CrossRef - Status of continuous glucose monitoring use and management in tertiary hospitals of China: a cross-sectional study
Liping Chen, Xiaoqin Liu, Qin Lin, Hongmei Dai, Yong Zhao, Zumin Shi, Liping Wu
BMJ Open.2023; 13(2): e066801. CrossRef - Real-world outcomes of continuous glucose monitoring in adults with diabetes mellitus attending an Irish tertiary hospital
Aoife Courtney, Diarmuid Smith, Hannah Forde
Irish Journal of Medical Science (1971 -).2023; 192(6): 2763. CrossRef - Insight into continuous glucose monitoring: from medical basics to commercialized devices
Ayman Chmayssem, Małgorzata Nadolska, Emily Tubbs, Kamila Sadowska, Pankaj Vadgma, Isao Shitanda, Seiya Tsujimura, Youssef Lattach, Martin Peacock, Sophie Tingry, Stéphane Marinesco, Pascal Mailley, Sandrine Lablanche, Pierre Yves Benhamou, Abdelkader Zeb
Microchimica Acta.2023;[Epub] CrossRef - Efficacy of polyethylene glycol loxenatide versus insulin glargine on glycemic control in patients with type 2 diabetes: a randomized, open-label, parallel-group trial
Shuo Zhang, Chuanyan Zhang, Jingxian Chen, Feiying Deng, Zezhen Wu, Dan Zhu, Fengwu Chen, Yale Duan, Yue Zhao, Kaijian Hou
Frontiers in Pharmacology.2023;[Epub] CrossRef - Impact of continuous glucose monitoring on glycemic control and its derived metrics in type 1 diabetes: a longitudinal study
So Hyun Cho, Seohyun Kim, You-Bin Lee, Sang-Man Jin, Kyu Yeon Hur, Gyuri Kim, Jae Hyeon Kim
Frontiers in Endocrinology.2023;[Epub] CrossRef - Association Between Continuous Glucose Monitoring-Derived Glycemia Risk Index and Albuminuria in Type 2 Diabetes
Jee Hee Yoo, Ji Yoon Kim, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2023; 25(10): 726. CrossRef - Acute Glycemic Variability and Early Outcomes After Cardiac Surgery:
A Meta-Analysis
Shuo Chang, Mian Xu, Yu Wang, Yanbo Zhang
Hormone and Metabolic Research.2023; 55(11): 771. CrossRef - Comparison of Glycemia Risk Index with Time in Range for Assessing Glycemic Quality
Ji Yoon Kim, Jee Hee Yoo, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2023; 25(12): 883. CrossRef - Correlação entre tempo no alvo e hemoglobina glicada de pessoas com diabetes mellitus: revisão sistemática
Rafael Aparecido Dias Lima, Daiane Rubinato Fernandes, Rute Aparecida Casas Garcia, Lucas Ariel da Rocha Carvalho, Renata Cristina de Campos Pereira Silveira, Carla Regina de Souza Teixeira
Revista Latino-Americana de Enfermagem.2023;[Epub] CrossRef - Correlación entre tiempo en rango y hemoglobina glicosilada en personas con diabetes mellitus: revisión sistemática
Rafael Aparecido Dias Lima, Daiane Rubinato Fernandes, Rute Aparecida Casas Garcia, Lucas Ariel da Rocha Carvalho, Renata Cristina de Campos Pereira Silveira, Carla Regina de Souza Teixeira
Revista Latino-Americana de Enfermagem.2023;[Epub] CrossRef - Correlation between time on target and glycated hemoglobin in people with diabetes mellitus: systematic review
Rafael Aparecido Dias Lima, Daiane Rubinato Fernandes, Rute Aparecida Casas Garcia, Lucas Ariel da Rocha Carvalho, Renata Cristina de Campos Pereira Silveira, Carla Regina de Souza Teixeira
Revista Latino-Americana de Enfermagem.2023;[Epub] CrossRef - Smart Insulin Pen: Managing Insulin Therapy for People with Diabetes in the Digital Era
Jee Hee Yoo, Jae Hyeon Kim
The Journal of Korean Diabetes.2023; 24(4): 190. CrossRef - Novel Glycemic Index Based on Continuous Glucose Monitoring to Predict Poor Clinical Outcomes in Critically Ill Patients: A Pilot Study
Eun Yeong Ha, Seung Min Chung, Il Rae Park, Yin Young Lee, Eun Young Choi, Jun Sung Moon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Hypoglycemic agents and glycemic variability in individuals with type 2 diabetes: A systematic review and network meta-analysis
SuA Oh, Sujata Purja, Hocheol Shin, Minji Kim, Eunyoung Kim
Diabetes and Vascular Disease Research.2022; 19(3): 147916412211068. CrossRef - Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus
Jeongmin Lee, Jae-Seung Yun, Seung-Hyun Ko
Nutrients.2022; 14(15): 3086. CrossRef - Influence of dipeptidyl peptidase-4 inhibitors on glycemic variability in patients with type 2 diabetes: A meta-analysis of randomized controlled trials
Shangyu Chai, Ruya Zhang, Ye Zhang, Richard David Carr, Yiman Zheng, Swapnil Rajpathak, Miao Yu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Glucose Profiles Assessed by Intermittently Scanned Continuous Glucose Monitoring System during the Perioperative Period of Metabolic Surgery
Kyuho Kim, Sung Hee Choi, Hak Chul Jang, Young Suk Park, Tae Jung Oh
Diabetes & Metabolism Journal.2022; 46(5): 713. CrossRef - Deterioration in glycemic control on schooldays among children and adolescents with type 1 diabetes: A continuous glucose monitoring-based study
Yu Ding, Wenhao Zhang, Xiumei Wu, Tian Wei, Xulin Wang, Xueying Zheng, Sihui Luo
Frontiers in Pediatrics.2022;[Epub] CrossRef - Effect of repeated bolus and continuous glucose infusion on a panel of circulating biomarkers in healthy volunteers
Roland Feldbauer, Matthias Wolfgang Heinzl, Carmen Klammer, Michael Resl, Johannes Pohlhammer, Klemens Rosenberger, Verena Almesberger, Florian Obendorf, Lukas Schinagl, Thomas Wagner, Margot Egger, Benjamin Dieplinger, Martin Clodi, Stephen L. Atkin
PLOS ONE.2022; 17(12): e0279308. CrossRef - Relationship between glycemic intraday variations evaluated in continuous glucose monitoring and HbA1c variability in type 2 diabetes: pilot study
Akemi Tokutsu, Yosuke Okada, Keiichi Torimoto, Yoshiya Tanaka
Diabetology & Metabolic Syndrome.2021;[Epub] CrossRef - Time-in-range for monitoring glucose control: Is it time for a change?
Virginia Bellido, Pedro José Pinés-Corrales, Rocío Villar-Taibo, Francisco Javier Ampudia-Blasco
Diabetes Research and Clinical Practice.2021; 177: 108917. CrossRef - Glucose Management Indicator for People with Type 1 Asian Diabetes Is Different from That of the Published Equation: Differences by Glycated Hemoglobin Distribution
Jee Hee Yoo, Seung Hee Yang, Gyuri Kim, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2021;[Epub] CrossRef - Health-Related Quality of Life, Family Conflicts and Fear of Injecting: Perception Differences between Preadolescents and Adolescents with Type 1 Diabetes and Their Mothers
Marta Tremolada, Maria Cusinato, Sabrina Bonichini, Arianna Fabris, Claudia Gabrielli, Carlo Moretti
Behavioral Sciences.2021; 11(7): 98. CrossRef - Daytime Glycemic Variability and Frailty in Older Patients with Diabetes: a Pilot Study Using Continuous Glucose Monitoring
Seung Min Chung, Yun Hee Lee, Chang Oh Kim, Ji Yeon Lee, Sang-Man Jin, Seung-Hyun Yoo, Jun Sung Moon, Kwang Joon Kim
Journal of Korean Medical Science.2021;[Epub] CrossRef - Benefits of a Switch from Intermittently Scanned Continuous Glucose Monitoring (isCGM) to Real-Time (rt) CGM in Diabetes Type 1 Suboptimal Controlled Patients in Real-Life: A One-Year Prospective Study §
Yannis Préau, Sébastien Galie, Pauline Schaepelynck, Martine Armand, Denis Raccah
Sensors.2021; 21(18): 6131. CrossRef - Recent Advances of Integrative Bio-Omics Technologies to Improve Type 1 Diabetes (T1D) Care
Nisha Karwal, Megan Rodrigues, David D. Williams, Ryan J. McDonough, Diana Ferro
Applied Sciences.2021; 11(24): 11602. CrossRef
- Acute and Chronic Adverse Outcomes of Type 1 Diabetes
- The Anti-Diabetic Drug Metformin from the Neuropathy Perspective
- Jong Chul Won
- Diabetes Metab J. 2020;44(6):840-841. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0252
- 3,511 View
- 154 Download
- Drug/Regimen
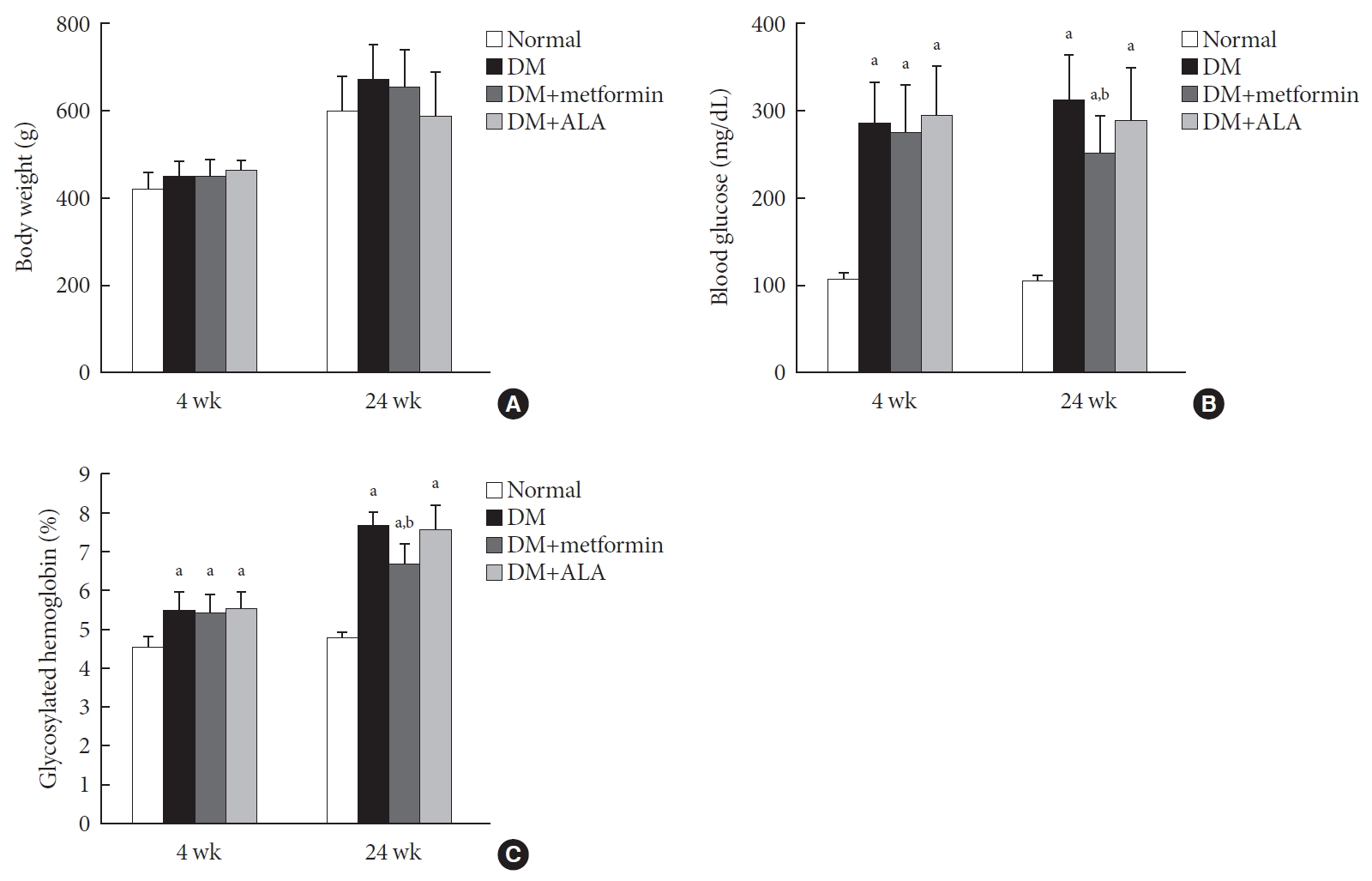
- Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats
- Sun Hee Kim, Tae Sun Park, Heung Yong Jin
- Diabetes Metab J. 2020;44(6):842-853. Published online May 28, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0190
- 6,144 View
- 177 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Metformin is widely marketed medication for the treatment of diabetes, but its pharmacological effect on diabetic peripheral neuropathy remains unclear. In this study, the effect of metformin on peripheral nerves in diabetic rats was investigated using diverse neuronal parameters of nerve fibers.
Methods Rats were assigned to one of four groups (
n =7 to 10 per group): normal, diabetes mellitus (DM), DM+metformin (100 mg/kg), and DM+alpha lipoic acid (ALA, 100 mg/kg). DM was induced by streptozotocin/high-fat diet (STZ/HFD). After 12 weeks, the sensory thresholds to mechanical and heat stimuli were assessed. Repeated sensory tests, immunofluorescence microscopic comparison of peripheral nerves, and biochemical blood analysis were performed after 24 weeks.Results Both DM+metformin and DM+ALA groups showed similar trends to diverse sensory tests at 24 weeks compared to DM group although the degree of change were different according to the stimulated senses. There was no significant difference in the comparison of the intraepidermal nerve fiber density (IENFD) of peripheral nerves between the DM+metformin and DM+ALA groups (11.83±0.07 fibers/mm vs. 12.37±1.82 fibers/mm, respectively). Both groups showed preserved IENFD significantly compared with DM group (8.46±1.98 fibers/mm,
P <0.05). Sciatic nerve morphology of the experimental animals showed a similar trend to the IENFD, with respect to axonal diameter, myelin sheath thickness, and myelinated fiber diameter.Conclusion Metformin has beneficial pharmacological effects on the preservation of peripheral nerves in diabetic rats and its effects are comparable to those of ALA.
-
Citations
Citations to this article as recorded by- Metformin improves diabetic neuropathy by reducing inflammation through up-regulating the expression of miR-146a and suppressing oxidative stress
Fengmin Liu, Fangqin You, Lihang Yang, Siyun Wang, Diya Xie
Journal of Diabetes and its Complications.2024; : 108737. CrossRef - Effect of Metformin on the Functional and Electrophysiological Recovery of Crush Injury-Induced Facial Nerve Paralysis in Diabetic Rats
Kyung Hoon Sun, Cheol Hee Choi, Gwang-Won Cho, Chul Ho Jang
Journal of Personalized Medicine.2023; 13(9): 1317. CrossRef - Is metformin neuroprotective against diabetes mellitus-induced neurodegeneration? An updated graphical review of molecular basis
Fatemeh Karami, Hamidreza Jamaati, Natalie Coleman-Fuller, Maryam Shokrian Zeini, A. Wallace Hayes, Mina Gholami, Mahsa Salehirad, Mohammad Darabi, Majid Motaghinejad
Pharmacological Reports.2023; 75(3): 511. CrossRef - Early Diagnosis through Estimation of Inflammatory Biomarkers and the Neuroprotective Role of Metformin in Diabetic Peripheral Neuropathy
Laxmi Sri, Prabhakar Orsu
International Journal of Pharmaceutical Sciences and Nanotechnology(IJPSN).2023; 16(2): 6427. CrossRef - Bidirectional association between diabetic peripheral neuropathy and vitamin B12 deficiency: Two longitudinal 9-year follow-up studies using a national sample cohort
Heung Yong Jin, Kyung Ae Lee, Yu Ji Kim, In Sun Gwak, Tae Sun Park, Sang Woo Yeom, Jong Seung Kim
Primary Care Diabetes.2023; 17(5): 436. CrossRef - An overview of painful diabetic peripheral neuropathy: Diagnosis and treatment advancements
Jonathan M. Hagedorn, Alyson M. Engle, Tony K. George, Jay Karri, Newaj Abdullah, Erik Ovrom, Jhon E. Bocanegra-Becerra, Ryan S. D'Souza
Diabetes Research and Clinical Practice.2022; 188: 109928. CrossRef - The role of MicroRNA networks in tissue-specific direct and indirect effects of metformin and its application
Qinzhi Yang, Gang Wang, Dan Fang, Xiaojun Gao, Yu Liang, Liqun Wang, Jianbo Wu, Min Zeng, Mao Luo
Biomedicine & Pharmacotherapy.2022; 151: 113130. CrossRef - Is metformin a possible treatment for diabetic neuropathy?
Juechun Wei, Yanling Wei, Meiyan Huang, Peng Wang, Shushan Jia
Journal of Diabetes.2022; 14(10): 658. CrossRef - Metformin as a potential therapeutic for neurological disease: mobilizing AMPK to repair the nervous system
Sarah Demaré, Asha Kothari, Nigel A. Calcutt, Paul Fernyhough
Expert Review of Neurotherapeutics.2021; 21(1): 45. CrossRef - Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats (Diabetes Metab J 2020;44:842-53)
Bo Kyung Koo
Diabetes & Metabolism Journal.2021; 45(1): 125. CrossRef - Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats (Diabetes Metab J 2020;44:842-53)
Sun Hee Kim, Tae Sun Park, Heung Yong Jin
Diabetes & Metabolism Journal.2021; 45(1): 127. CrossRef - Impacts of statin and metformin on neuropathy in patients with type 2 diabetes mellitus: Korean Health Insurance data
Hong Ki Min, Se Hee Kim, Jong Han Choi, Kyomin Choi, Hae-Rim Kim, Sang-Heon Lee
World Journal of Clinical Cases.2021; 9(33): 10198. CrossRef
- Metformin improves diabetic neuropathy by reducing inflammation through up-regulating the expression of miR-146a and suppressing oxidative stress
- Type 1 Diabetes
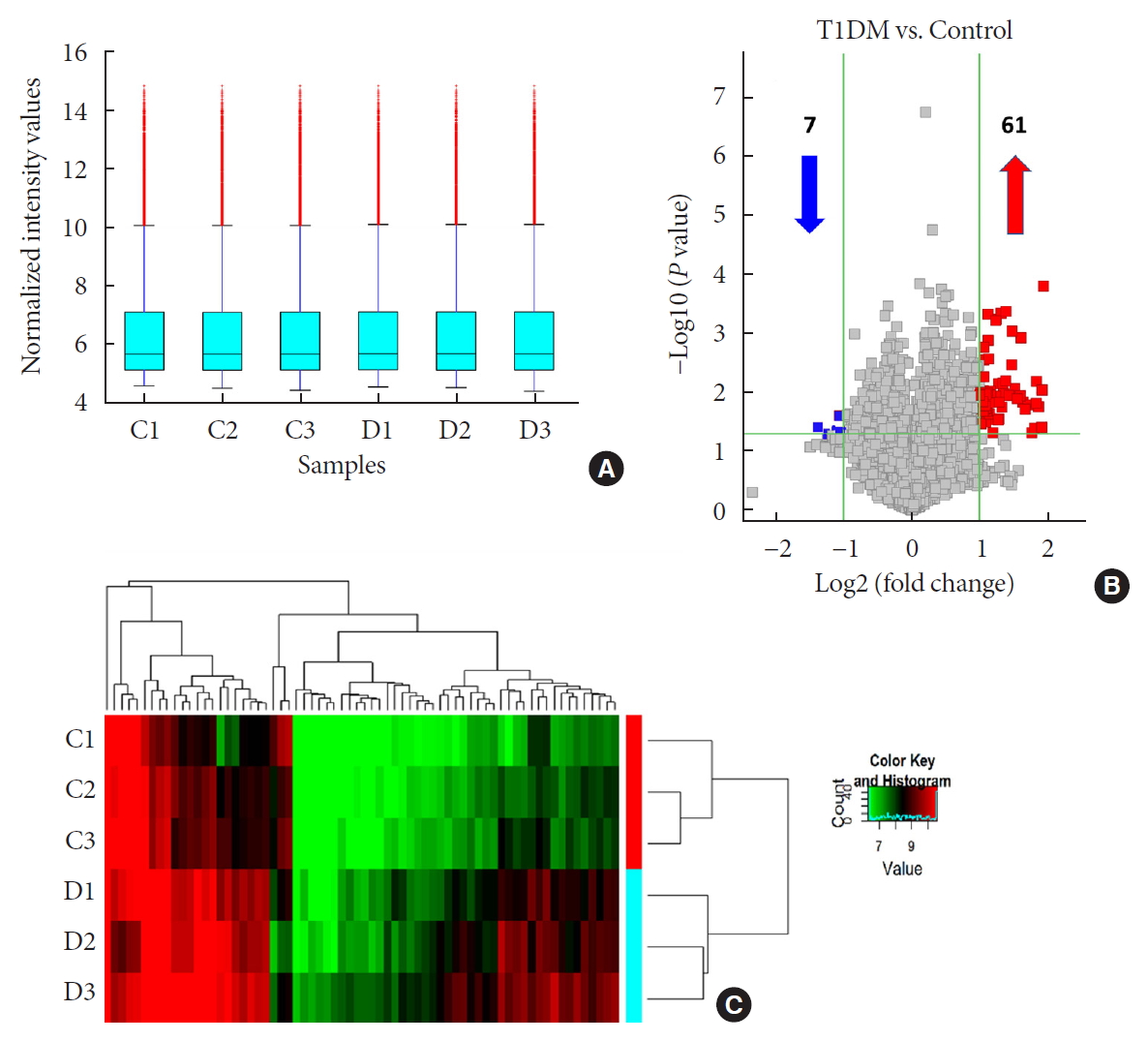
- Differential Profile of Plasma Circular RNAs in Type 1 Diabetes Mellitus
- Yangyang Li, Ying Zhou, Minghui Zhao, Jing Zou, Yuxiao Zhu, Xuewen Yuan, Qianqi Liu, Hanqing Cai, Cong-Qiu Chu, Yu Liu
- Diabetes Metab J. 2020;44(6):854-865. Published online July 13, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0151
- 6,152 View
- 131 Download
- 19 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background No currently available biomarkers or treatment regimens fully meet therapeutic needs of type 1 diabetes mellitus (T1DM). Circular RNA (circRNA) is a recently identified class of stable noncoding RNA that have been documented as potential biomarkers for various diseases. Our objective was to identify and analyze plasma circRNAs altered in T1DM.
Methods We used microarray to screen differentially expressed plasma circRNAs in patients with new onset T1DM (
n =3) and age-/gender-matched healthy controls (n =3). Then, we selected six candidates with highest fold-change and validated them by quantitative real-time polymerase chain reaction in independent human cohort samples (n =12). Bioinformatic tools were adopted to predict putative microRNAs (miRNAs) sponged by these validated circRNAs and their downstream messenger RNAs (mRNAs). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed to gain further insights into T1DM pathogenesis.Results We identified 68 differentially expressed circRNAs, with 61 and seven being up- and downregulated respectively. Four of the six selected candidates were successfully validated. Curations of their predicted interacting miRNAs revealed critical roles in inflammation and pathogenesis of autoimmune disorders. Functional relations were visualized by a circRNA-miRNA-mRNA network. GO and KEGG analyses identified multiple inflammation-related processes that could be potentially associated with T1DM pathogenesis, including cytokine-cytokine receptor interaction, inflammatory mediator regulation of transient receptor potential channels and leukocyte activation involved in immune response.
Conclusion Our study report, for the first time, a profile of differentially expressed plasma circRNAs in new onset T1DM. Further
in silico annotations and bioinformatics analyses supported future application of circRNAs as novel biomarkers of T1DM.-
Citations
Citations to this article as recorded by- Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: A narrative review
Yuhong Zhong, Juan Xia, Li Liao, Mohammad Reza Momeni
International Journal of Biological Macromolecules.2024; 259: 128182. CrossRef - Circular RNAs: Potential biomarkers and therapeutic targets for autoimmune diseases
Ren-Jie Zhao, Wan-Ying Zhang, Xing-Xing Fan
Heliyon.2024; 10(1): e23694. CrossRef - Research progress of circular RNA molecules in aging and age-related diseases
Zhidan Zhang, Yuling Huang, AYao Guo, Lina Yang
Ageing Research Reviews.2023; 87: 101913. CrossRef - CircRNAs and RNA-Binding Proteins Involved in the Pathogenesis of Cancers or Central Nervous System Disorders
Yuka Ikeda, Sae Morikawa, Moeka Nakashima, Sayuri Yoshikawa, Kurumi Taniguchi, Haruka Sawamura, Naoko Suga, Ai Tsuji, Satoru Matsuda
Non-Coding RNA.2023; 9(2): 23. CrossRef - Decrypting the circular RNAs does a favor for us: Understanding, diagnosing and treating diabetes mellitus and its complications
Zi Li, Yuanyuan Ren, Ziwei Lv, Man Li, Yujia Li, Xiaobin Fan, Yuyan Xiong, Lu Qian
Biomedicine & Pharmacotherapy.2023; 168: 115744. CrossRef - Circular RNA PIP5K1A Promotes Glucose and Lipid Metabolism Disorders and Inflammation in Type 2 Diabetes Mellitus
Ge Song, YiQian Zhang, YiHua Jiang, Huan Zhang, Wen Gu, Xiu Xu, Jing Yao, ZhengFang Chen
Molecular Biotechnology.2023;[Epub] CrossRef - Hsa_circRNA_405498 and hsa_circRNA_100033 Serve as Potential Biomarkers for Differential Diagnosis of Type 1 Diabetes
Ziwei Zhang, Shuoming Luo, Zilin Xiao, Wenfeng Yin, Xiajie Shi, Hongzhi Chen, Zhiguo Xie, Zhenqi Liu, Xia Li, Zhiguang Zhou
The Journal of Clinical Endocrinology & Metabolism.2023;[Epub] CrossRef - Circular RNA PIP5K1A act as microRNA-552-3p sponge to regulates inflammation, oxidative damage in glucolipotoxicity-induced pancreatic INS-1 β-cells via Janus kinase 1
Lei Ren
Bioengineered.2022; 13(3): 5724. CrossRef - Circular RNAs in diabetes mellitus and its complications
Wenqi Fan, Haipeng Pang, Zhiguo Xie, Gan Huang, Zhiguang Zhou
Frontiers in Endocrinology.2022;[Epub] CrossRef - Type 1 Diabetes Mellitus-Related circRNAs Regulate CD4+ T Cell Functions
Jianni Chen, Guanfei Jia, Xue Lv, Shufa Li, Christos K. Kontos
BioMed Research International.2022; 2022: 1. CrossRef - An intriguing role of circular RNA in insulin resistance and endothelial dysfunction: the future perspectives
Monisha Prasad, Selvaraj Jayaraman, Vishnu Priya Veeraraghavan
Hypertension Research.2022; 45(11): 1843. CrossRef - Circular RNAs in Diabetic Nephropathy: Updates and Perspectives
Miao Liu, Junli Zhao
Aging and disease.2022; 13(5): 1365. CrossRef - CircRNAs: Key molecules in the prevention and treatment of ischemic stroke
Zeyu Liu, Yanhong Zhou, Jian Xia
Biomedicine & Pharmacotherapy.2022; 156: 113845. CrossRef - Pro-Inflammatory Cytokines Promote the Transcription of Circular RNAs in Human Pancreatic β Cells
Simranjeet Kaur, Caroline Frørup, Aashiq H. Mirza, Tina Fløyel, Reza Yarani, Maikel L. Colli, Jesper Johannesen, Joachim Størling, Decio L. Eizirik, Flemming Pociot
Non-Coding RNA.2022; 8(5): 69. CrossRef - Differential Expression and Bioinformatics Analysis of Plasma-Derived Exosomal circRNA in Type 1 Diabetes Mellitus
Haipeng Pang, Wenqi Fan, Xiajie Shi, Shuoming Luo, Yimeng Wang, Jian Lin, Yang Xiao, Xia Li, Gan Huang, Zhiguo Xie, Zhiguang Zhou, Jinhui Liu
Journal of Immunology Research.2022; 2022: 1. CrossRef - Circular RNAs in diabetes and its complications: Current knowledge and future prospects
Wenfeng Yin, Ziwei Zhang, Zilin Xiao, Xia Li, Shuoming Luo, Zhiguang Zhou
Frontiers in Genetics.2022;[Epub] CrossRef - Circular RNA in autoimmune diseases: special emphasis on regulation mechanism in RA and SLE
Yurong Huang, Qiuyun Xue, Chenglong Cheng, Yuting Wang, Xiao Wang, Jun Chang, Chenggui Miao
Journal of Pharmacy and Pharmacology.2022;[Epub] CrossRef - Emerging roles of circular RNAs in systemic lupus erythematosus
Xin Wang, Rui Ma, Weimin Shi, Zhouwei Wu, Yuling Shi
Molecular Therapy - Nucleic Acids.2021; 24: 212. CrossRef - Understanding Competitive Endogenous RNA Network Mechanism in Type 1 Diabetes Mellitus Using Computational and Bioinformatics Approaches
Xuanzi Yi, Xu Cheng
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 3865. CrossRef
- Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: A narrative review
- Type 1 Diabetes
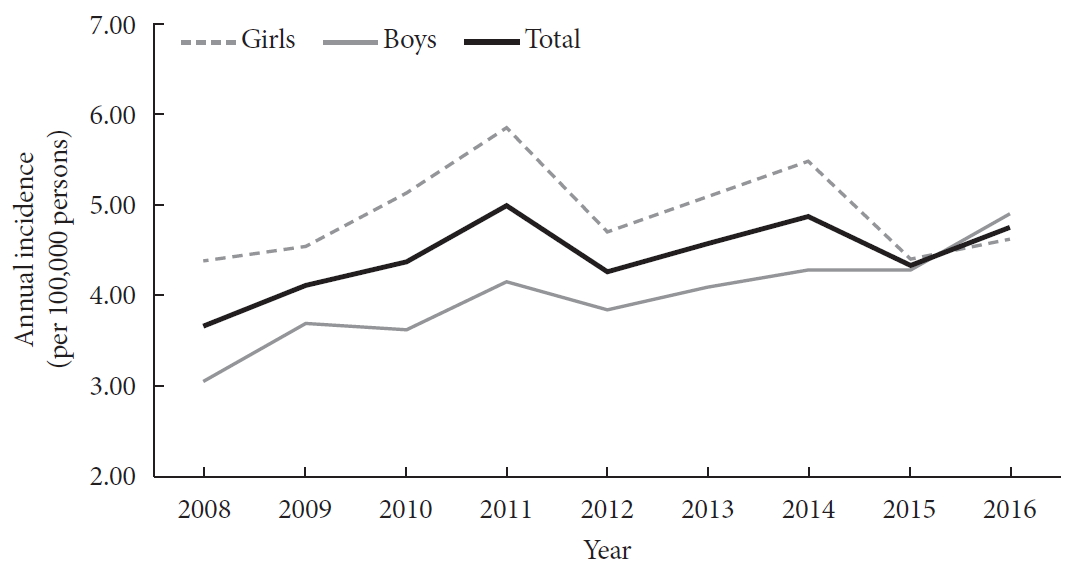
- Incidence and Prevalence of Type 1 Diabetes Mellitus among Korean Children and Adolescents between 2007 and 2017: An Epidemiologic Study Based on a National Database
- Hyun Wook Chae, Gi Hyeon Seo, Kyungchul Song, Han Saem Choi, Junghwan Suh, Ahreum Kwon, Sangmi Ha, Ho-Seong Kim
- Diabetes Metab J. 2020;44(6):866-874. Published online November 4, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0212
- 7,724 View
- 319 Download
- 25 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The incidence of type 1 diabetes mellitus (T1DM) among children is high in Europe and the USA and relatively low in Asia, including Korea. The present study aimed to investigate the incidence and prevalence of childhood-onset T1DM in Korea and examine trends in incidence.
Methods
This study was conducted using the national registry data provided by the Health Insurance Review and Assessment Service in Korea from 2007 to 2017. We included children aged 0 to 14 years who were newly registered with a T1DM diagnosis each year (code E10).
Results
A total of 29,013 children were registered. The overall incidence of T1DM was 4.45 per 100,000 persons (girls, 4.93; boys, 4.01). The overall incidence of childhood-onset T1DM in Korea increased from 3.70 in 2008 to 4.77 in 2016 (P=0.002). The incidence of T1DM increased from 3.07 in 2008 to 4.89 in 2016 (P<0.001) among boys. Although the incidence of the disease increased significantly among boys aged 5–9 and 10–14 years, it remained constant among girls (4.39 in 2008, 4.64 in 2016). The overall prevalence of childhood-onset T1DM in Korea increased from 32.85 in 2007 to 41.03 per 100,000 persons in 2017 (girls, 35.54 to 43.88; boys, 32.85 to 41.03).
Conclusion
We calculated relatively accurate incidence and prevalence of childhood-onset T1DM from a nation-based registry. The incidence increased by 3% to 4% every year from 2007 to 2017. The increasing trend is noteworthy compared with previous reports. -
Citations
Citations to this article as recorded by- Risk of non-thyroidal autoimmune diseases in patients with Graves’ disease: a nationwide retrospective cohort study
Seo Young Sohn, Jiyeon Ahn, Min Kyung Lee, Jae Hyuk Lee, Ji-Won Kwon, Ji-Min Kweon, Ju-Yeun Lee
Rheumatology.2024;[Epub] CrossRef - Effectiveness of Non-pharmacological Interventions for Adolescents With Type 1 Diabetes in the Last Five Years: A Systematic Review and Meta-analysis
DaeEun Lee, Haejung Lee, YoonYoung Shin, Gaeun Park
Asian Nursing Research.2024; 18(1): 51. CrossRef - Development and Testing of the School Healthcare Partnership Scale for Parents
Ju-Yeon Uhm, Suhee Kim
Western Journal of Nursing Research.2024; 46(3): 219. CrossRef - Trends in incidence rates of childhood type 1 diabetes mellitus: A retrospective study in Isfahan province, Iran
Mahin Hashemipour, Mohammadreza Maracy, Shaghayegh Haghjooy Javanmard, Farzane Zamaneh, Neda Mostofizadeh, Silva Hovsepian
Journal of Diabetes Investigation.2023; 14(3): 376. CrossRef - Incidence of Childhood Type 1 Diabetes in Beijing During 2011–2020 and Predicted Incidence for 2025–2035: A Multicenter, Hospitalization-Based Study
Yuchuan Li, Kun Qian, Di Wu, Xinli Wang, Hong Cui, Geheng Yuan, Jinfang Yuan, Lijun Yang, Liya Wei, Bingyan Cao, Chang Su, Xuejun Liang, Min Liu, Wenjing Li, Miao Qin, Jiajia Chen, Xi Meng, Rui Wang, Shan Su, Xiaobo Chen, Hui Chen, Chunxiu Gong
Diabetes Therapy.2023; 14(3): 519. CrossRef - Utilization of nutrition labels and related factors among patients with diabetes in Korea
So-Jung Lee, Mi Ah Han, Jong Park, So Yeon Ryu
Nutrition Research and Practice.2023; 17(2): 297. CrossRef - Long-term trends of pediatric type 1 diabetes incidence in Japan before and after the COVID-19 pandemic
Fumika Matsuda, Tomoyo Itonaga, Miwako Maeda, Kenji Ihara
Scientific Reports.2023;[Epub] CrossRef - The burdens faced by parents of preschoolers with type 1 diabetes mellitus: an integrative review
Sunyeob Choi, Hyewon Shin
Child Health Nursing Research.2023; 29(3): 166. CrossRef - Hypoglycemic Effect of an Herbal Decoction (Modified Gangsimtang) in a Patient with Severe Type 2 Diabetes Mellitus Refusing Oral Anti-Diabetic Medication: A Case Report
Sungjun Joo, Hyonjun Chun, Jisu Lee, Seungmin Seo, Jungmin Lee, Jungtae Leem
Medicina.2023; 59(11): 1919. CrossRef - Improving self-management and diabetes indicators in adolescents with type 1 diabetes through self-care education
Narges Asghari, Bahman Dashtebozorgi, Shahnaz Rostami, Saeed Ghanbari, Kourosh Riahi-Ghahfarokhi
Journal of Family Medicine and Primary Care.2023; 12(10): 2322. CrossRef - Механізм дії та особливості застосування холекальциферолу в дітей та підлітків на етапах розвитку цукрового діабету 1-го типу
V.V. Popova, N.V. Het´man, Ya.I. Labanets, H.V. Kulikovs´ka, O.V. Furmanova, K.P. Zak
Endokrynologia.2023; 28(1): 36. CrossRef - Comparison of Initial Presentation of Pediatric Diabetes Before and During the Coronavirus Disease 2019 Pandemic Era
Yoonha Lee, Minseung Kim, Kyeongeun Oh, Eungu Kang, Young-Jun Rhie, Jieun Lee, Yong Hee Hong, Young-Lim Shin, Jae Hyun Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Recent information on test utilization and intraindividual change in anti-glutamic acid decarboxylase antibody in Korea: a retrospective study
Rihwa Choi, Wonseo Park, Gayoung Chun, Jiwon Lee, Sang Gon Lee, Eun Hee Lee
BMJ Open Diabetes Research & Care.2022; 10(3): e002739. CrossRef - Comparison of the clinical characteristics and outcomes of pediatric patients with and without diabetic ketoacidosis at the time of type 1 diabetes diagnosis
Young-Jun Seo, Chang Dae Kum, Jung Gi Rho, Young Suk Shim, Hae Sang Lee, Jin Soon Hwang
Annals of Pediatric Endocrinology & Metabolism.2022; 27(2): 126. CrossRef - Prevalence trends of type 1 and type 2 diabetes in children and adolescents in North Rhine-Westphalia, the most populous federal state in Germany, 2002-2020
C. Baechle, A. Stahl-Pehe, N. Prinz, T. Meissner, C. Kamrath, R.W. Holl, J. Rosenbauer
Diabetes Research and Clinical Practice.2022; 190: 109995. CrossRef - Diabetic ketoacidosis in children with new-onset type 1 diabetes mellitus: demographics, risk factors and outcome: an 11 year review in Hong Kong
Sarah Wing-yiu Poon, Joanna Yuet-ling Tung, Wilfred Hing-sang Wong, Pik-to Cheung, Antony Chun-cheung Fu, Gloria Shir-wey Pang, Sharon Wing-yan To, Lap-ming Wong, Wai-yu Wong, Suk-yan Chan, Ho-chung Yau, Wing-shan See, Betty Wai-man But, Shirley Man-yee W
Journal of Pediatric Endocrinology and Metabolism.2022; 35(9): 1132. CrossRef - School Nurse–Parent Partnership in School Health Care for Children with Type 1 Diabetes: A Hybrid Method Concept Analysis
Ju-Yeon Uhm, Mi-Young Choi
Asian Nursing Research.2022; 16(5): 282. CrossRef - Increased Incidence of Pediatric Diabetic Ketoacidosis After COVID-19: A Two-Center Retrospective Study in Korea
Min Jeong Han, Jun Ho Heo
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 783. CrossRef - Age at Diagnosis and the Risk of Diabetic Nephropathy in Young Patients with Type 1 Diabetes Mellitus (Diabetes Metab J 2021;45:46-54)
Ye Seul Yang, Tae Seo Sohn
Diabetes & Metabolism Journal.2021; 45(2): 277. CrossRef - Relationships between emissions of toxic airborne molecules and type 1 diabetes incidence in children: An ecologic study
Agostino Di Ciaula, Piero Portincasa
World Journal of Diabetes.2021; 12(5): 673. CrossRef - Diagnosis and management of pediatric type 1 diabetes mellitus
Jieun Lee
Journal of the Korean Medical Association.2021; 64(6): 425. CrossRef - Diabetes in Adolescence, Appropriate Transition to Adult Clinic
Jieun Lee, Jae Hyun Kim
The Journal of Korean Diabetes.2021; 22(2): 77. CrossRef - Trajectories in glycated hemoglobin and body mass index in children and adolescents with diabetes using the common data model
Yun Jeong Lee, Sooyoung Yoo, Soyoung Yi, Seok Kim, Chunggak Lee, Jihoon Cho, Soyeon Ahn, Sunkyu Choi, Hee Hwang, Young Ah Lee, Choong Ho Shin, Hyung-Jin Yoon, Kwangsoo Kim, Eunhye Song, Jin Ho Choi, Han Wook Yoo, Young-Hak Kim, Ji Seon Oh, Eun-Ae Kang, Ga
Scientific Reports.2021;[Epub] CrossRef - What Affects Quality of Life for People with Type 1 Diabetes?: A Cross-Sectional Observational Study
Mi-Kyoung Cho, Mi-Young Kim
International Journal of Environmental Research and Public Health.2021; 18(14): 7623. CrossRef - The Relationship between Diabetes Family Conflict and Parental Conflict on Problem Recognition in Illness Self-Management among Individuals with Type 1 Diabetes Mellitus
Mi-Kyoung Cho, Mi Young Kim
International Journal of Environmental Research and Public Health.2021; 18(17): 8914. CrossRef - Relationship between Diabetes Family Conflicts or Problem Recognition in Illness Self-Management and Quality of Life of Adolescents with T1DM and Their Parents
Mi-Kyoung Cho, Mi Young Kim
International Journal of Environmental Research and Public Health.2021; 18(20): 10710. CrossRef - Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
Sun Joon Moon, Inha Jung, Cheol-Young Park
Diabetes & Metabolism Journal.2021; 45(6): 813. CrossRef - History of insulin treatment of pediatric patients with diabetes in Korea
Jae Hyun Kim, Choong Ho Shin, Sei Won Yang
Annals of Pediatric Endocrinology & Metabolism.2021; 26(4): 237. CrossRef - A Position Statement of the Utilization and Support Status of Continuous Glucose Monitoring in Korea
Won Jun Kim, Jae Hyun Kim, Hye Jin Yoo, Jang Won Son, Ah Reum Khang, Su Kyoung Kwon, Ji Hye Kim, Tae Ho Kim, Ohk Hyun Ryu, Kyeong Hye Park, Sun Ok Song, Kang-Woo Lee, Woo Je Lee, Jung Hwa Jung, Ho-Chan Cho, Min Jeong Gu, Jeongrim Lee, Dal Lae Ju, Yeon Hee
The Journal of Korean Diabetes.2021; 22(4): 225. CrossRef
- Risk of non-thyroidal autoimmune diseases in patients with Graves’ disease: a nationwide retrospective cohort study
- Complications
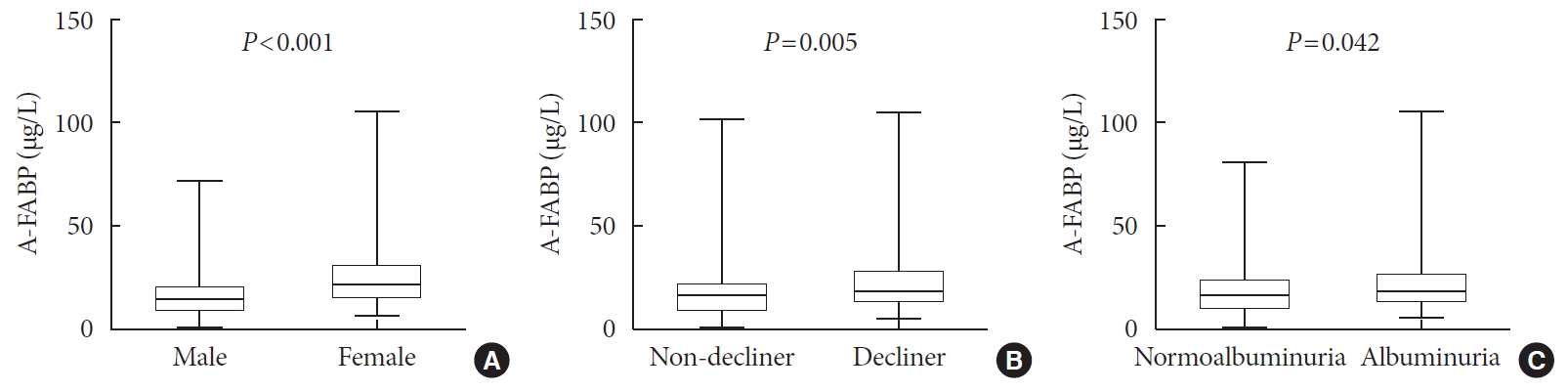
- Serum Levels of Adipocyte Fatty Acid-Binding Protein Are Associated with Rapid Renal Function Decline in Patients with Type 2 Diabetes Mellitus and Preserved Renal Function
- Da Hea Seo, Moonsuk Nam, Mihye Jung, Young Ju Suh, Seong Hee Ahn, Seongbin Hong, So Hun Kim
- Diabetes Metab J. 2020;44(6):875-886. Published online July 10, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0221
- 5,622 View
- 123 Download
- 10 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Recent studies have demonstrated that the levels of adipocyte fatty acid-binding protein (A-FABP) are closely associated with diabetic kidney disease (DKD) in patients with type 2 diabetes mellitus (T2DM). This study aimed to examine the association between serum A-FABP level and rapid renal function decline in patients with T2DM and preserved renal function.
Methods This was a prospective observational study of 452 patients with T2DM and preserved renal function who had serial measurements of estimated glomerular filtration rate (eGFR). Rapid renal function decline was defined as an eGFR decline of >4% per year. The association between baseline serum A-FABP level and rapid renal function decline was investigated.
Results Over a median follow-up of 7 years, 82 participants (18.1%) experienced rapid renal function decline. Median A-FABP levels were significantly higher in patients with rapid renal function decline, compared to non-decliners (20.2 ng/mL vs. 17.2 ng/mL,
P =0.005). A higher baseline level of A-FABP was associated with a greater risk of developing rapid renal function decline, independent of age, sex, duration of diabetes, body mass index, systolic blood pressure, history of cardiovascular disease, baseline eGFR, urine albumin creatinine ratio, total cholesterol, glycosylated hemoglobin, high-sensitivity C-reactive protein and use of thiazolidinedione, insulin, angiotensin-converting-enzyme inhibitors and angiotensin II-receptor blockers and statin (odds ratio, 3.10; 95% confidence interval, 1.53 to 6.29;P =0.002).Conclusion A high level of serum A-FABP is associated with an increased risk of rapid renal function decline in patients with T2DM and preserved renal function. This suggests that A-FABP could play a role in the progression of DKD in the early stages.
-
Citations
Citations to this article as recorded by- Serum fatty acid-binding protein 4 as a biomarker for early detection of diabetic nephropathy in type 2 diabetes
Amr M. Shaker, Maggie E. Mohamed, Tarek Ramzy, Mayssa I. Ali
The Egyptian Journal of Internal Medicine.2023;[Epub] CrossRef - Circulating thrombospondin-2 level for identifying individuals with rapidly declining kidney function trajectory in type 2 diabetes: a prospective study of the Hong Kong West Diabetes Registry
Chi-Ho Lee, David Tak-Wai Lui, Chloe Yu-Yan Cheung, Carol Ho-Yi Fong, Michele Mae-Ann Yuen, Wing-Sun Chow, Aimin Xu, Karen Siu-Ling Lam
Nephrology Dialysis Transplantation.2023;[Epub] CrossRef - Analysis of inflammatory cytokines and estimated glomerular filtration rate decline in Japanese patients with diabetic kidney disease: a pilot study
Yuka Sugawara, Yosuke Hirakawa, Koki Mise, Kosuke Kashiwabara, Ko Hanai, Satoshi Yamaguchi, Akihiro Katayama, Yasuhiro Onishi, Yui Yoshida, Naoki Kashihara, Yutaka Matsuyama, Tetsuya Babazono, Masaomi Nangaku, Jun Wada
Biomarkers in Medicine.2022; 16(10): 759. CrossRef - The role of statins in patients with early diabetic nephropathy
Xi Zhao, Shu Chun Zhou, Xiu Fang Wang, Hong Wu Liao
Medicine.2022; 101(24): e29099. CrossRef - Serum Adipocyte Fatty-Acid Binding Protein as an Independent Marker of Peripheral Artery Disease in Patients with Type-2 Diabetes Mellitus
Bang-Gee Hsu, Chin-Yee Mah, Du-An Wu, Ming-Chun Chen
International Journal of Environmental Research and Public Health.2022; 19(15): 9459. CrossRef - Fatty acid-binding protein 4 in kidney diseases: From mechanisms to clinics
Weijing Lai, Min Shi, Rongshuang Huang, Ping Fu, Liang Ma
European Journal of Pharmacology.2022; 931: 175224. CrossRef - Serum fatty acid-binding protein 4 levels and responses of pancreatic islet β-cells and α-cells in patients with type 2 diabetes
Hong Wang, Jie Cao, Jian-bin Su, Xue-qin Wang, Xing Wang, Dong-mei Zhang, Xiao-hua Wang
Diabetology & Metabolic Syndrome.2021;[Epub] CrossRef - The Low-Expression Variant of FABP4 Is Associated With Cardiovascular Disease in Type 1 Diabetes
Emma H. Dahlström, Jani Saksi, Carol Forsblom, Nicoline Uglebjerg, Nina Mars, Lena M. Thorn, Valma Harjutsalo, Peter Rossing, Tarunveer S. Ahluwalia, Perttu J. Lindsberg, Niina Sandholm, Per-Henrik Groop
Diabetes.2021; 70(10): 2391. CrossRef - White adipocyte-targeted dual gene silencing of FABP4/5 for anti-obesity, anti-inflammation and reversal of insulin resistance: Efficacy and comparison of administration routes
Jee Young Chung, Juhyeong Hong, Hyung-Jin Kim, Yoonsung Song, Seok-Beom Yong, Jieun Lee, Yong-Hee Kim
Biomaterials.2021; 279: 121209. CrossRef
- Serum fatty acid-binding protein 4 as a biomarker for early detection of diabetic nephropathy in type 2 diabetes
- Metabolic Risk/Epidemiology
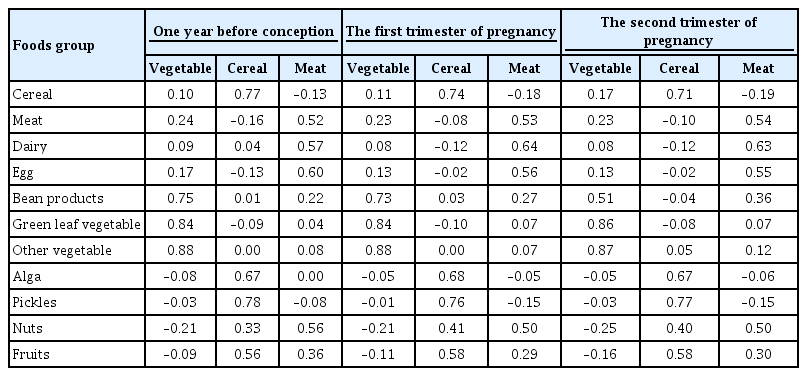
- A Vegetable Dietary Pattern Is Associated with Lowered Risk of Gestational Diabetes Mellitus in Chinese Women
- Qiong Chen, Weiwei Wu, Hailan Yang, Ping Zhang, Yongliang Feng, Keke Wang, Ying Wang, Suping Wang, Yawei Zhang
- Diabetes Metab J. 2020;44(6):887-896. Published online September 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0138
- 6,562 View
- 135 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Identification of modifiable dietary factors, which are involved in the development of gestational diabetes mellitus (GDM), could inform strategies to prevent GDM.
Methods
We examined the dietary patterns in a Chinese population and evaluated their relationship with GDM risk using a case-control study including 1,464 cases and 8,092 control subjects. Propensity score matching was used to reduce the imbalance of covariates between cases and controls. Dietary patterns were identified using factor analysis while their associations with GDM risk were evaluated using logistic regression models.
Results
A “vegetable” dietary pattern was characterized as the consumption of green leafy vegetables (Chinese little greens and bean seedling), other vegetables (cabbages, carrots, tomatoes, eggplants, potatoes, mushrooms, peppers, bamboo shoots, agarics, and garlic), and bean products (soybean milk, tofu, kidney beans, and cowpea). For every quartile increase in the vegetables factor score during 1 year prior to conception, the first trimester, and the second trimester of pregnancy, the GDM risk lowered by 6% (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.89 to 0.99), 7% (OR, 0.94; 95% CI, 0.88 to 0.99), and 9% (OR, 0.91; 95% CI, 0.86 to 0.96).
Conclusion
In conclusion, our study suggests that the vegetable dietary pattern is associated with lower GDM risk; however, the interpretation of the result should with caution due to the limitations in our study, and additional studies are necessary to explore the underlying mechanism of this relationship. -
Citations
Citations to this article as recorded by- Maternal dietary components in the development of gestational diabetes mellitus: a systematic review of observational studies to timely promotion of health
Victoria Lambert, Sonia Edith Muñoz, Carla Gil, María Dolores Román
Nutrition Journal.2023;[Epub] CrossRef - Fruit, vegetable, and fruit juice consumption and risk of gestational diabetes mellitus: a systematic review and meta-analysis
Yan-Ping Liao, Qing-Xiang Zheng, Xiu-Min Jiang, Xiao-Qian Chen, Xiao-Xia Gao, Yu-Qing Pan
Nutrition Journal.2023;[Epub] CrossRef - The effects of plant-based dietary patterns on the risk of developing gestational diabetes mellitus: A systematic review and meta-analysis
Yu Zhu, QingXiang Zheng, Ling Huang, XiuMin Jiang, XiaoXia Gao, JiaNing Li, RuLin Liu, Kent Lai
PLOS ONE.2023; 18(10): e0291732. CrossRef - Molecular pathways and nutrigenomic review of insulin resistance development in gestational diabetes mellitus
Patricia Guevara-Ramírez, Elius Paz-Cruz, Santiago Cadena-Ullauri, Viviana A. Ruiz-Pozo, Rafael Tamayo-Trujillo, Maria L. Felix, Daniel Simancas-Racines, Ana Karina Zambrano
Frontiers in Nutrition.2023;[Epub] CrossRef - Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—a systematic review and meta-analysis of 257,876 pregnancies
Swetha Sampathkumar, Durga Parkhi, Yonas Ghebremichael-Weldeselassie, Nithya Sukumar, Ponnusamy Saravanan
Nutrition & Diabetes.2023;[Epub] CrossRef - Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies
Elaine Luiza Santos Soares de Mendonça, Marilene Brandão Tenório Fragoso, Jerusa Maria de Oliveira, Jadriane Almeida Xavier, Marília Oliveira Fonseca Goulart, Alane Cabral Menezes de Oliveira
Antioxidants.2022; 11(1): 129. CrossRef - Ferulic acid targets ACSL1 to ameliorate lipid metabolic disorders in db/db mice
Jie Gao, Xue Gu, Manqian Zhang, Xingwang Zu, Fukui Shen, Xiaotao Hou, Erwei Hao, Gang Bai
Journal of Functional Foods.2022; 91: 105009. CrossRef - Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance
Jianping Wang, Zuoliang Xie, Peipei Chen, Yuhuan Wang, Baoqing Li, Fen Dai
Open Life Sciences.2022; 17(1): 202. CrossRef - Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women
Weijia Wu, Nu Tang, Jingjing Zeng, Jin Jing, Li Cai
Nutrients.2022; 14(8): 1623. CrossRef - Dietary Acid Load Is Positively Associated With Risk of Gestational Diabetes Mellitus in a Prospective Cohort of Chinese Pregnant Women
Rui Zhao, Leilei Zhou, Gang Lei, Shanshan Wang, Yan Li, Xuefeng Yang, Guoping Xiong, Liping Hao
Frontiers in Nutrition.2022;[Epub] CrossRef
- Maternal dietary components in the development of gestational diabetes mellitus: a systematic review of observational studies to timely promotion of health
- COVID-19
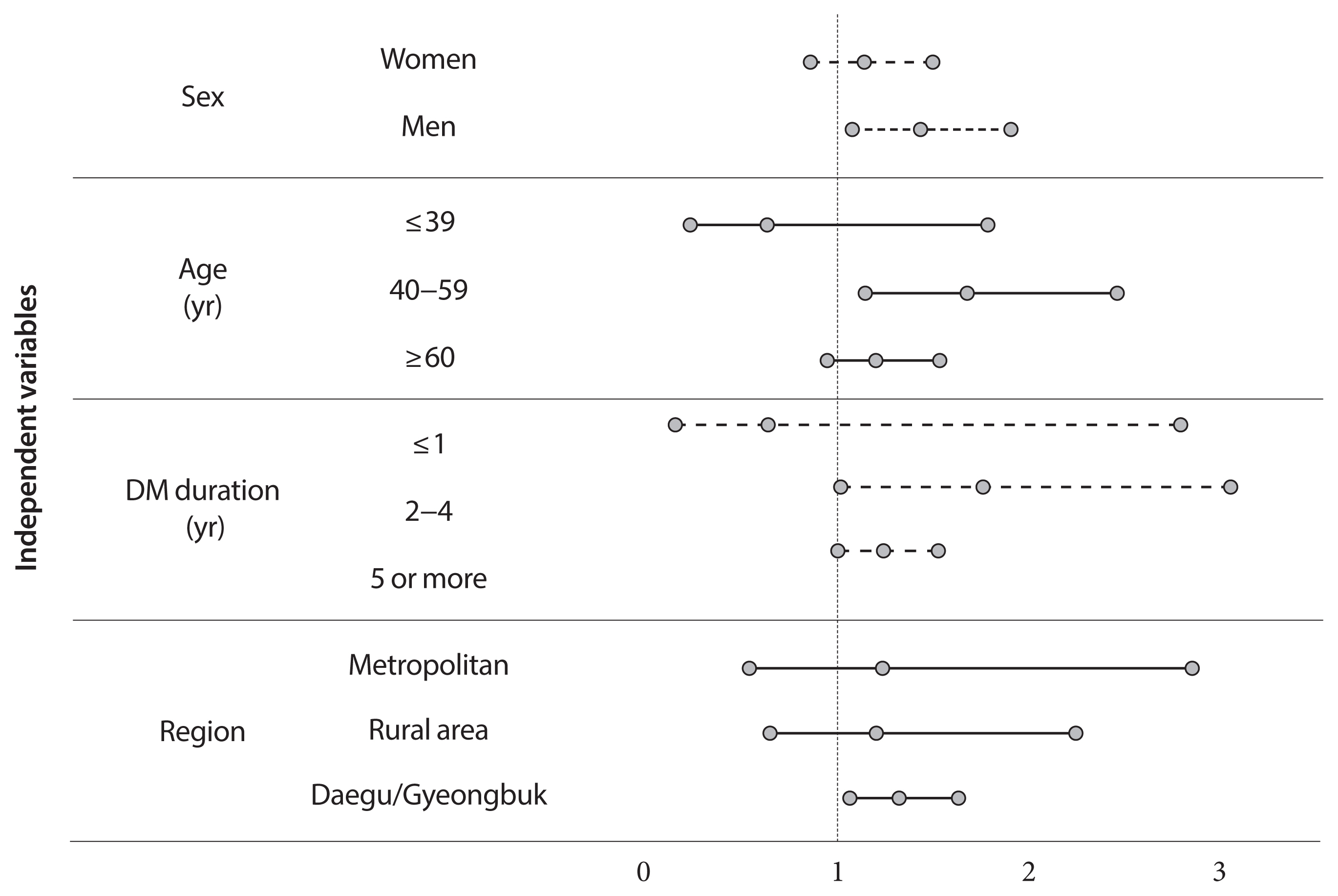
- Does Diabetes Increase the Risk of Contracting COVID-19? A Population-Based Study in Korea
- Sung-Youn Chun, Dong Wook Kim, Sang Ah Lee, Su Jung Lee, Jung Hyun Chang, Yoon Jung Choi, Seong Woo Kim, Sun Ok Song
- Diabetes Metab J. 2020;44(6):897-907. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0199
- 7,583 View
- 143 Download
- 8 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study aimed to determine the infection risk of coronavirus disease 2019 (COVID-19) in patients with diabetes (according to treatment method).
Methods
Claimed subjects to the Korean National Health Insurance claims database diagnosed with COVID-19 were included. Ten thousand sixty-nine patients with COVID-19 between January 28 and April 5, 2020, were included. Stratified random sampling of 1:5 was used to select the control group of COVID-19 patients. In total 50,587 subjects were selected as the control group. After deleting the missing values, 60,656 subjects were included.
Results
Adjusted odds ratio (OR) indicated that diabetic insulin users had a higher risk of COVID-19 than subjects without diabetes (OR, 1.25; 95% confidence interval [CI], 1.03 to 1.53; P=0.0278). In the subgroup analysis, infection risk was higher among diabetes male insulin users (OR, 1.42; 95% CI, 1.07 to 1.89), those between 40 and 59 years (OR, 1.66; 95% CI, 1.13 to 2.44). The infection risk was higher in diabetic insulin users with 2 to 4 years of morbidity (OR, 1.744; 95% CI, 1.003 to 3.044).
Conclusion
Some diabetic patients with certain conditions would be associated with a higher risk of acquiring COVID-19, highlighting their need for special attention. Efforts are warranted to ensure that diabetic patients have minimal exposure to the virus. It is important to establish proactive care and screening tests for diabetic patients suspected with COVID-19 for timely disease diagnosis and management. -
Citations
Citations to this article as recorded by- Risk factors for SARS-CoV-2 infection during the early stages of the COVID-19 pandemic: a systematic literature review
Matthew Harris, John Hart, Oashe Bhattacharya, Fiona M. Russell
Frontiers in Public Health.2023;[Epub] CrossRef - Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study
Brenda Eskenazi, Stephen Rauch, Enrico Iurlaro, Robert B. Gunier, Albertina Rego, Michael G. Gravett, Paolo Ivo Cavoretto, Philippe Deruelle, Perla K. García-May, Mohak Mhatre, Mustapha Ado Usman, Mohamed Elbahnasawy, Saturday Etuk, Raffaele Napolitano, S
American Journal of Obstetrics and Gynecology.2022; 227(1): 74.e1. CrossRef - The Role of Diabetes and Hyperglycemia on COVID-19 Infection Course—A Narrative Review
Evangelia Tzeravini, Eleftherios Stratigakos, Chris Siafarikas, Anastasios Tentolouris, Nikolaos Tentolouris
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - COVID-19 and Gestational Diabetes: The Role of Nutrition and Pharmacological Intervention in Preventing Adverse Outcomes
Ruben Ramirez Zegarra, Andrea Dall’Asta, Alberto Revelli, Tullio Ghi
Nutrients.2022; 14(17): 3562. CrossRef - A Comprehensive Analysis of Chinese, Japanese, Korean, US-PIMA Indian, and Trinidadian Screening Scores for Diabetes Risk Assessment and Prediction
Norma Latif Fitriyani, Muhammad Syafrudin, Siti Maghfirotul Ulyah, Ganjar Alfian, Syifa Latif Qolbiyani, Muhammad Anshari
Mathematics.2022; 10(21): 4027. CrossRef - The World-Wide Adaptations of Diabetic Management in the Face of COVID-19 and Socioeconomic Disparities: A Scoping Review
Jaafar Abou-Ghaida, Annalia Foster, Sarah Klein, Massah Bassie, Khloe Gu, Chloe Hille, Cody Brown, Michael Daniel, Caitlin Drakeley, Alek Jahnke, Abrar Karim, Omar Altabbakh, Luzan Phillpotts
Cureus.2022;[Epub] CrossRef - Dissection of non-pharmaceutical interventions implemented by Iran, South Korea, and Turkey in the fight against COVID-19 pandemic
Mohammad Keykhaei, Sogol Koolaji, Esmaeil Mohammadi, Reyhaneh Kalantar, Sahar Saeedi Moghaddam, Arya Aminorroaya, Shaghayegh Zokaei, Sina Azadnajafabad, Negar Rezaei, Erfan Ghasemi, Nazila Rezaei, Rosa Haghshenas, Yosef Farzi, Sina Rashedi, Bagher Larijan
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1919. CrossRef
- Risk factors for SARS-CoV-2 infection during the early stages of the COVID-19 pandemic: a systematic literature review
- Basic Research
- Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
- Mahesh Raj Nepal, Mi Jeong Kang, Geon Ho Kim, Dong Ho Cha, Ju-Hyun Kim, Tae Cheon Jeong
- Diabetes Metab J. 2020;44(6):908-918. Published online April 6, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0147
- 5,666 View
- 114 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Voglibose, an α-glucosidase inhibitor, inhibits breakdown of complex carbohydrates into simple sugar units in intestine. Studies showed that voglibose metabolism in the liver might be negligible due to its poor intestinal absorption. Numerous microorganisms live in intestine and have several roles in metabolism and detoxification of various xenobiotics. Due to the limited information, the possible metabolism of voglibose by intestinal microbiota was investigated
in vitro andin vivo .Methods For the
in vitro study, different concentrations of voglibose were incubated with intestinal contents, prepared from both vehicle- and antibiotics-treated mice, to determine the decreased amount of voglibose over time by using liquid chromatography-mass spectrometry. Similarly,in vivo pharmacodynamic effect of voglibose was determined following the administration of voglibose and starch in vehicle- and antibiotic-pretreated non-diabetic and diabetic mice, by measuring the modulatory effects of voglibose on blood glucose levels.Results The
in vitro results indicated that the remaining voglibose could be significantly decreased when incubated with the intestinal contents from normal mice compared to those from antibiotic-treated mice, which had less enzyme activities. Thein vivo results showed that the antibiotic pretreatment resulted in reduced metabolism of voglibose. This significantly lowered blood glucose levels in antibiotic-pretreated mice compared to the control animals.Conclusion The present results indicate that voglibose would be metabolized by the intestinal microbiota, and that this metabolism might be pharmacodynamically critical in lowering blood glucose levels in mice.
-
Citations
Citations to this article as recorded by- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
Liyang Jia, Shiqiong Huang, Boyu Sun, Yongguang Shang, Chunsheng Zhu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Phenolics from endophytic fungi as natural α-glucosidase inhibitors: A comprehensive review
Muhammad Imran Tousif, Saba Tauseef, Sadeer Nabeelah, Jugreet Sharmeen, Gokhan Zengin, Lesetja Legoabe, Muhammad Imran, Mohamad Fawzi Mahomoodally
Journal of Molecular Structure.2023; 1291: 135852. CrossRef - Ligand-targeted fishing of α-glucosidase inhibitors from Tribulus terrestris L. based on chitosan-functionalized multi-walled carbon nanotubes with immobilized α-glucosidase
Xin Meng, Hou Zong, Zhong Zheng, Junpeng Xing, Zhiqiang Liu, Fengrui Song, Shu Liu
Analytical and Bioanalytical Chemistry.2023; 415(14): 2677. CrossRef - Isolation, structure elucidation, and biological activities of sesquiterpenes and phthalides from two edible mushrooms Pleurotus species
Jewel C De Padua, Emi Fukushima-Sakuno, Kotomi Ueno, Thomas Edison E dela Cruz, Atsushi Ishihara
Bioscience, Biotechnology, and Biochemistry.2023; 87(12): 1429. CrossRef - Effects of Oral Glucose-Lowering Agents on Gut Microbiota and Microbial Metabolites
Dongmei Wang, Jieying Liu, Liyuan Zhou, Qian Zhang, Ming Li, Xinhua Xiao
Frontiers in Endocrinology.2022;[Epub] CrossRef - 18:0 Lyso PC, a natural product with potential PPAR-γ agonistic activity, plays hypoglycemic effect with lower liver toxicity and cardiotoxicity in db/db mice
Yiming Ma, Xinyi Du, Dandan Zhao, Kegong Tang, Xiaona Wang, Shaoting Guo, Xiaobei Li, Song Mei, Na Sun, Jiaqi Liu, Chengyu Jiang
Biochemical and Biophysical Research Communications.2021; 579: 168. CrossRef
- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
- Basic Research
- Hypoxia Increases β-Cell Death by Activating Pancreatic Stellate Cells within the Islet
- Jong Jin Kim, Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
- Diabetes Metab J. 2020;44(6):919-927. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0181
- 5,930 View
- 146 Download
- 15 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Hypoxia can occur in pancreatic islets in type 2 diabetes mellitus. Pancreatic stellate cells (PSCs) are activated during hypoxia. Here we aimed to investigate whether PSCs within the islet are also activated in hypoxia, causing β-cell injury.
Methods Islet and primary PSCs were isolated from Sprague Dawley rats, and cultured in normoxia (21% O2) or hypoxia (1% O2). The expression of α-smooth muscle actin (α-SMA), as measured by immunostaining and Western blotting, was used as a marker of PSC activation. Conditioned media (hypoxia-CM) were obtained from PSCs cultured in hypoxia.
Results Islets and PSCs cultured in hypoxia exhibited higher expressions of α-SMA than did those cultured in normoxia. Hypoxia increased the production of reactive oxygen species. The addition of N-acetyl-L-cysteine, an antioxidant, attenuated the hypoxia-induced PSC activation in islets and PSCs. Islets cultured in hypoxia-CM showed a decrease in cell viability and an increase in apoptosis.
Conclusion PSCs within the islet are activated in hypoxia through oxidative stress and promote islet cell death, suggesting that hypoxia-induced PSC activation may contribute to β-cell loss in type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- Effects of hypoxia in the diabetic corneal stroma microenvironment
Purnima Sharma, Jian-Xing Ma, Dimitrios Karamichos
Experimental Eye Research.2024; 240: 109790. CrossRef - Visualizing hypoxic modulation of beta cell secretions via a sensor augmented oxygen gradient
Kai Duan, Mengyang Zhou, Yong Wang, Jose Oberholzer, Joe F. Lo
Microsystems & Nanoengineering.2023;[Epub] CrossRef - Pancreatic stellate cells promote pancreatic β-cell death through exosomal microRNA transfer in hypoxia
Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
Molecular and Cellular Endocrinology.2023; 572: 111947. CrossRef - Pancreatic stellate cells in pancreatic cancer: as potential targets for future therapy
Zhengfeng Wang, Ru He, Shi Dong, Wence Zhou
Frontiers in Oncology.2023;[Epub] CrossRef - Recent advances in the development of bioartificial pancreas using 3D bioprinting for the treatment of type 1 diabetes: a review
Anushikha Ghosh, Arka Sanyal, Abhik Mallick
Exploration of Medicine.2023; : 886. CrossRef - Pancreas and islet morphology in cystic fibrosis: clues to the etiology of cystic fibrosis-related diabetes
Sarah S. Malik, Diksha Padmanabhan, Rebecca L. Hull-Meichle
Frontiers in Endocrinology.2023;[Epub] CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - HIF-1 and NRF2; Key Molecules for Malignant Phenotypes of Pancreatic Cancer
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Cancers.2022; 14(2): 411. CrossRef - Pancreatic Stellate Cells and Metabolic Alteration: Physiology and Pathophysiology
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Frontiers in Physiology.2022;[Epub] CrossRef - Exosomal miR-140–3p and miR-143–3p from TGF-β1-treated pancreatic stellate cells target BCL2 mRNA to increase β-cell apoptosis
Xiangyun Zhu, Dechen Liu, Guoqing Li, Mengmeng Zhi, Ji Sun, Liang Qi, Jingbo Li, Stephen J. Pandol, Ling Li
Molecular and Cellular Endocrinology.2022; 551: 111653. CrossRef - Mitochondria oxidative stress mediated nicotine-promoted activation of pancreatic stellate cells by regulating mitochondrial dynamics
Yue Yuan, Zhiren Li, Miaomiao Li, Tong Jin, Xiaoyun Zhang, Xinjuan Liu, Jianyu Hao
Toxicology in Vitro.2022; 84: 105436. CrossRef - Antioxidant Mitoquinone Alleviates Chronic Pancreatitis via Anti-Fibrotic and Antioxidant Effects
Miaomiao Li, Yue Yuan, Xue Han, Xinjuan Liu, Weizhen Zhang, Jianyu Hao
Journal of Inflammation Research.2022; Volume 15: 4409. CrossRef - Diabetic Ferroptosis and Pancreatic Cancer: Foe or Friend?
Le Li, Xing-jia Yu, Lei Gao, Long Cheng, Bei Sun, Gang Wang
Antioxidants & Redox Signaling.2022; 37(16-18): 1206. CrossRef - Melatonin Induces Apoptosis and Modulates Cyclin Expression and MAPK Phosphorylation in Pancreatic Stellate Cells Subjected to Hypoxia
Matias Estaras, Manuel R. Gonzalez-Portillo, Miguel Fernandez-Bermejo, Jose M. Mateos, Daniel Vara, Gerardo Blanco-Fernandez, Diego Lopez-Guerra, Vicente Roncero, Gines M. Salido, Antonio González
International Journal of Molecular Sciences.2021; 22(11): 5555. CrossRef - Integrated pancreatic microcirculatory profiles of streptozotocin‐induced and insulin‐administrated type 1 diabetes mellitus
Yuan Li, Bingwei Li, Bing Wang, Mingming Liu, Xiaoyan Zhang, Ailing Li, Jian Zhang, Honggang Zhang, Ruijuan Xiu
Microcirculation.2021;[Epub] CrossRef - Pancreatic stellate cells - rising stars in pancreatic pathologies
P Hrabák, M Kalousová, T Krechler, T Zima
Physiological Research.2021; (S4): S597. CrossRef
- Effects of hypoxia in the diabetic corneal stroma microenvironment
- Complications
- Diabetic Retinopathy and Related Clinical Practice for People with Diabetes in Korea: A 10-Year Trend Analysis
- Yoo-Ri Chung, Kyoung Hwa Ha, Kihwang Lee, Dae Jung Kim
- Diabetes Metab J. 2020;44(6):928-932. Published online July 10, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0096
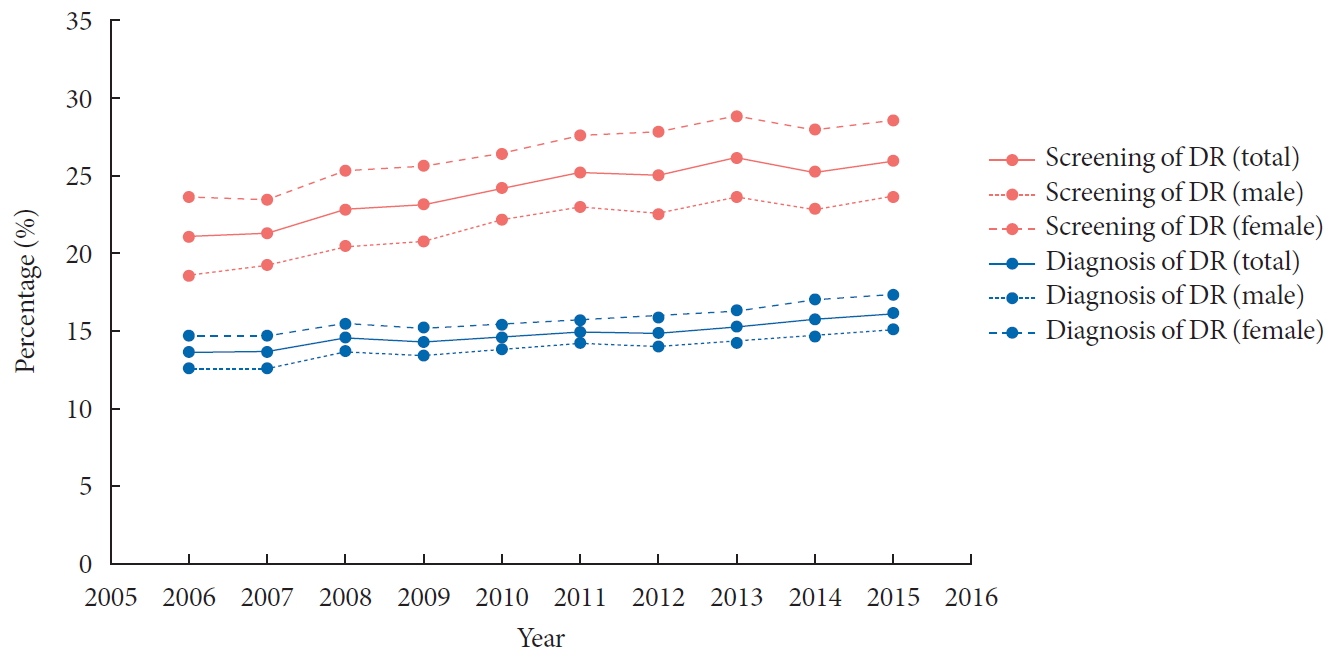
- 5,150 View
- 177 Download
- 5 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub We performed a retrospective cohort study including people diagnosed with diabetes from 2006 to 2015 according to the Korean National Health Insurance Service-National Sample Cohort database, to analyze the changes in the prevalence, screening rate, and treatment patterns for diabetic retinopathy (DR) over 10 years. The proportion of people who underwent fundus screening for DR steadily increased over the past decade. The prevalence of DR increased from 13.4% in 2006 to 15.9% in 2015, while that of proliferative DR steadily decreased from 1.29% in 2006 to 1.16% in 2015. The proportion of patients undergoing retinal photocoagulation constantly decreased. The prevalence of DR increased over the past decade, while its severity seemed to have improved, with a decreased rate of proliferative DR and retinal photocoagulation. A higher proportion of patients underwent ophthalmic screening using fundus examination, but still less than 30% of patients with diabetes underwent comprehensive examination in 2015.
-
Citations
Citations to this article as recorded by- Variations in Electronic Health Record-Based Definitions of Diabetic Retinopathy Cohorts
Jimmy S. Chen, Ivan A. Copado, Cecilia Vallejos, Fritz Gerald P. Kalaw, Priyanka Soe, Cindy X. Cai, Brian C. Toy, Durga Borkar, Catherine Q. Sun, Jessica G. Shantha, Sally L. Baxter
Ophthalmology Science.2024; 4(4): 100468. CrossRef - Present and future screening programs for diabetic retinopathy: a narrative review
Andreas Abou Taha, Sebastian Dinesen, Anna Stage Vergmann, Jakob Grauslund
International Journal of Retina and Vitreous.2024;[Epub] CrossRef - Chronic disease management program applied to type 2 diabetes patients and prevention of diabetic complications: a retrospective cohort study using nationwide data
Min Kyung Hyun, Jang Won Lee, Seung-Hyun Ko
BMC Public Health.2023;[Epub] CrossRef - Visual Acuity Outcomes in Diseases Associated with Reduced Visual Acuity: An Analysis of the National Health Insurance Service Database in Korea
Sang-Yeob Kim, Byeong-Yeon Moon, Hyun-Gug Cho, Dong-Sik Yu
International Journal of Environmental Research and Public Health.2022; 19(14): 8689. CrossRef - Prevalence of Diabetic Retinopathy in Undiagnosed Diabetic Patients: A Nationwide Population-Based Study
Han Na Jang, Min Kyong Moon, Bo Kyung Koo
Diabetes & Metabolism Journal.2022; 46(4): 620. CrossRef - Prevalence and Risk Factors of Diabetic Retinopathy in Diabetes People using Korean National Health and Nutrition Examination Survey VII
Ihn Sook Jeong, Chan Mi Kang
Journal of Korean Academy of Community Health Nursing.2022; 33(4): 408. CrossRef - Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study (Diabetes Metab J 2021;45:368-78)
Ja Young Jeon
Diabetes & Metabolism Journal.2021; 45(4): 613. CrossRef
- Variations in Electronic Health Record-Based Definitions of Diabetic Retinopathy Cohorts
- Complications
- Trends in the Incidence, Prevalence, and Mortality of End-Stage Kidney Disease in South Korea
- Min-Jeong Lee, Kyoung Hwa Ha, Dae Jung Kim, Inwhee Park
- Diabetes Metab J. 2020;44(6):933-937. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0156
- 5,815 View
- 239 Download
- 12 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Knowledge of the epidemiologic characteristics of end-stage kidney disease (ESKD) patients is essential. The trends in the prevalence, incidence, and mortality rates of ESKD were analyzed retrospectively using the Korean National Health Insurance ServiceNational Sample Cohort database between 2006 and 2015. From 2006 to 2015, the incidence of ESKD decreased from 28.6 to 24.0 per 100,000 people and showed a decreasing pattern with or without diabetes mellitus. However, the incidence of those aged ≥75 years increased, as did the mean age at the onset of ESKD. From 2007 to 2015, the prevalence of ESKD increased in all age groups, but particularly in those aged ≥75 years. The prevalence of ESKD differed by sex and diabetes mellitus status and this gap widened over time. Mortality rates in ESKD patients remained relatively constant throughout the study period. However, mortality rates in ESKD without diabetes decreased over the same period.
-
Citations
Citations to this article as recorded by- Kidney Health Plan 2033 in Korea: bridging the gap between the present and the future
Do Hyoung Kim, Young Youl Hyun, Jin Joo Cha, Sua Lee, Hyun Kyung Lee, Jong Wook Choi, Su-Hyun Kim, Sang Youb Han, Cheol Whee Park, Eun Young Lee, Dae Ryong Cha, Sung Gyun Kim, Chun Soo Lim, Sun-Hee Park
Kidney Research and Clinical Practice.2024; 43(1): 8. CrossRef - Remnant cholesterol is an independent risk factor for the incidence of chronic kidney disease in newly-diagnosed type 2 diabetes: A nationwide population-based study
Soo Yeon Jang, Minwoong Kang, Eyun Song, Ahreum Jang, Kyung Mook Choi, Sei Hyun Baik, Hye Jin Yoo
Diabetes Research and Clinical Practice.2024; 210: 111639. CrossRef - Waist circumference and end‐stage renal disease based on glycaemic status: National Health Insurance Service data 2009–2018
Yun Kyung Cho, Ji Hye Huh, Shinje Moon, Yoon Jung Kim, Yang‐Hyun Kim, Kyung‐do Han, Jun Goo Kang, Seong Jin Lee, Sung‐Hee Ihm
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(1): 585. CrossRef - Usefulness of continuous glucose monitoring of blood glucose control in patients with diabetes undergoing hemodialysis: A pilot study
Sua Lee, Soyoung Lee, Kyeong Min Kim, Jong Ho Shin
Frontiers in Medicine.2023;[Epub] CrossRef - Association between N-Terminal Prohormone Brain Natriuretic Peptide and Decreased Skeletal Muscle Mass in a Healthy Adult Population: A Cross-Sectional Study
Tae Kyung Yoo, Marie Yung-Chen Wu, Moon Soo Kim, Mi-Yeon Lee, Yong-Taek Lee, Kyung Jae Yoon, Chul-Hyun Park
Endocrinology and Metabolism.2023; 38(2): 269. CrossRef - Age at Mortality in Patients with Type 2 Diabetes Who Underwent Kidney Transplantation: An Analysis of Data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
Sun Ok Song, Eugene Han, Kang Ju Son, Bong-Soo Cha, Byung-Wan Lee
Journal of Clinical Medicine.2023; 12(9): 3160. CrossRef - Epigenome-wide association study of diabetic chronic kidney disease progression in the Korean population: the KNOW-CKD study
Hye Youn Sung, Sangjun Lee, Miyeun Han, Woo Ju An, Hyunjin Ryu, Eunjeong Kang, Yong Seek Park, Seung Eun Lee, Curie Ahn, Kook-Hwan Oh, Sue K. Park, Jung-Hyuck Ahn
Scientific Reports.2023;[Epub] CrossRef - Effects of Dapagliflozin in Asian Patients With Heart Failure and Reduced Ejection Fraction in DAPA-HF
Kieran F. Docherty, Inder S. Anand, Chern-En Chiang, Vijay K. Chopra, Akshay S. Desai, Masafumi Kitakaze, Subodh Verma, Pham N. Vinh, Silvio E. Inzucchi, Lars Køber, Mikhail N. Kosiborod, Felipe A. Martinez, Olof Bengtsson, Piotr Ponikowski, Marc S. Sabat
JACC: Asia.2022; 2(2): 139. CrossRef - Glomerular filtration rate as a kidney outcome of diabetic kidney disease: a focus on new antidiabetic drugs
Hyo Jin Kim, Sang Soo Kim, Sang Heon Song
The Korean Journal of Internal Medicine.2022; 37(3): 502. CrossRef - The risk of Parkinson's disease according to diabetic kidney disease status in a Korean population
Seung Eun Lee, Juhwan Yoo, Han Seok Choi, Kyungdo Han, Kyoung-Ah Kim
Parkinsonism & Related Disorders.2022; 100: 13. CrossRef - Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
Nam Hoon Kim, Nan Hee Kim
Diabetes & Metabolism Journal.2022; 46(4): 543. CrossRef - The Incidence and Risk Factors of Renal Insufficiency among Korean HIV infected Patients: The Korea HIV/AIDS Cohort Study
Jun Hyoung Kim, Heeseon Jang, Jung Ho Kim, Joon Young Song, Shin-Woo Kim, Sang Il Kim, Bo Youl Choi, Jun Yong Choi
Infection & Chemotherapy.2022; 54(3): 534. CrossRef - Sex difference in the association among nutrition, muscle mass, and strength in peritoneal dialysis patients
Jun Young Do, Seok Hui Kang
Scientific Reports.2022;[Epub] CrossRef - Additive interaction of diabetes mellitus and chronic kidney disease in cancer patient mortality risk
Seohyun Kim, Gyuri Kim, Jae Hyeon Kim
Scientific Reports.2022;[Epub] CrossRef
- Kidney Health Plan 2033 in Korea: bridging the gap between the present and the future

 KDA
KDA


 First
First Prev
Prev





