Incidence and Prevalence of Type 1 Diabetes Mellitus among Korean Children and Adolescents between 2007 and 2017: An Epidemiologic Study Based on a National Database
Article information
Abstract
Background
The incidence of type 1 diabetes mellitus (T1DM) among children is high in Europe and the USA and relatively low in Asia, including Korea. The present study aimed to investigate the incidence and prevalence of childhood-onset T1DM in Korea and examine trends in incidence.
Methods
This study was conducted using the national registry data provided by the Health Insurance Review and Assessment Service in Korea from 2007 to 2017. We included children aged 0 to 14 years who were newly registered with a T1DM diagnosis each year (code E10).
Results
A total of 29,013 children were registered. The overall incidence of T1DM was 4.45 per 100,000 persons (girls, 4.93; boys, 4.01). The overall incidence of childhood-onset T1DM in Korea increased from 3.70 in 2008 to 4.77 in 2016 (P=0.002). The incidence of T1DM increased from 3.07 in 2008 to 4.89 in 2016 (P<0.001) among boys. Although the incidence of the disease increased significantly among boys aged 5–9 and 10–14 years, it remained constant among girls (4.39 in 2008, 4.64 in 2016). The overall prevalence of childhood-onset T1DM in Korea increased from 32.85 in 2007 to 41.03 per 100,000 persons in 2017 (girls, 35.54 to 43.88; boys, 32.85 to 41.03).
Conclusion
We calculated relatively accurate incidence and prevalence of childhood-onset T1DM from a nation-based registry. The incidence increased by 3% to 4% every year from 2007 to 2017. The increasing trend is noteworthy compared with previous reports.
INTRODUCTION
Childhood-onset type 1 diabetes mellitus (T1DM) is a prevalent pediatric endocrine disease. The incidence and prevalence of T1DM among children are high in Europe (particularly in Scandinavian countries) and the United States of America and relatively low in Asia. The incidence of childhood-onset T1DM has remained less eminent in Asia than in Western countries owing to the paucity of long-term data obtained from population-based studies conducted in Asian countries, including Korea [1].
An increasing trend in the incidence of childhood-onset T1DM has been reported worldwide. The Diabetes Mondiale (DiaMond) Project Group reported an annual increase in T1DM incidence of 2.8% between 1990 and 1999 [2]. The European Diabetes (EURODIAB) study showed that the incidence of T1DM in Europe increased by approximately 3.9% annually between 1989 and 2003 [3]. The incidence and prevalence of T1DM and type 2 diabetes mellitus (T2DM) are increasing in many developing countries [4,5]. However, recent reports emanating from some countries suggest that this increasing trend has slowed. Studies conducted in Finland and Sweden demonstrated that the incidence of childhood-onset T1DM has ceased to increase in recent years [6,7]. In Norway, there was no significant increase in incidence between 2004 and 2012 [8]. Epidemiological data on T1DM among children and adolescents are still lacking for most of the global population, particularly in Africa, Asia, and South America [2]. Few comprehensive population-based studies of diabetes have investigated children and adolescents of diverse ethnic backgrounds. A few studies have forecasted that the prevalence of childhood-onset T1DM will increase mostly in developing countries, even though the incidence has remained low [9,10]. Surveillance of the incidence and prevalence of childhood-onset T1DM should be conducted not only to understand its complex etiology but also to recognize the implications for public health [11].
Although several epidemiologic studies have been conducted, it is unclear whether the estimates of incidence and prevalence of T1DM among children and adolescents are accurate and whether the incidence is increasing in Korea. The present study aimed to investigate the incidence and prevalence of childhood-onset T1DM in Korea using the nationwide registry data provided by the Health Insurance Review and Assessment Service (HIRA). We also investigated the annual trend in the incidence of childhood-onset T1DM during the past decade and analyzed the data according to age and sex.
METHODS
Patients and study design
The present retrospective population-based study aimed to investigate the incidence and prevalence of childhood-onset T1DM among Korean children through a complete enumeration survey using the HIRA claims data from January 1, 2007 to December 31, 2017. The study design and enrollment of subjects are shown in Fig. 1. Korea has a national health insurance system that provides medical insurance coverage to all its inhabitants, and each individual is registered with a health insurance identification number. Every resident is eligible regardless of nationality or profession. The system is funded by compulsory contributions from all residents and government subsidies. All Korean patients who visit a hospital are registered with a diagnosis according to the World Health Organization’s International Classifications of Diseases, Tenth Revision (ICD-10). The HIRA reviews the data to ensure proper diagnoses and management and to assess the quality of healthcare and medical fees.
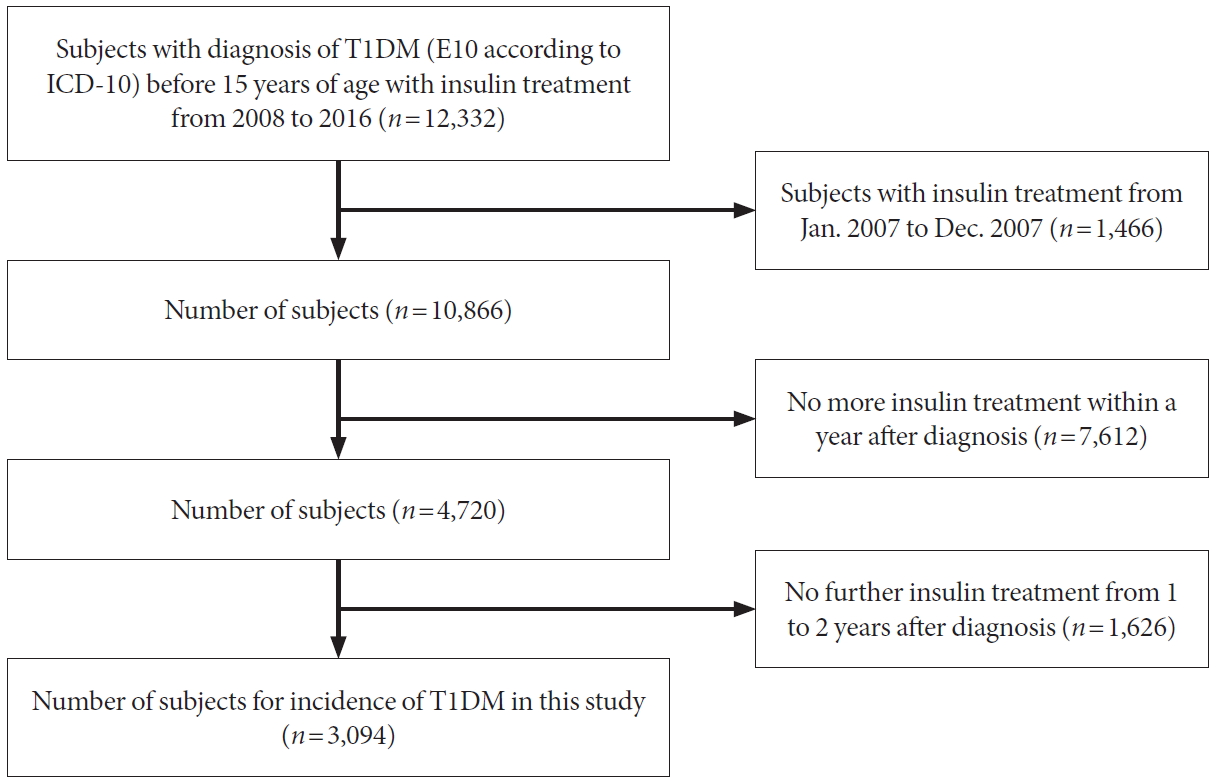
Study design and number of subjects for investigating the incidence of type 1 diabetes mellitus (T1DM) in Korean children. ICD-10, World Health Organization’s International Classifications of Diseases, Tenth Revision.
In calculating the incidence of T1DM, we included boys and girls who were newly registered with a diagnostic code of E10 according to the ICD-10 before the age of 15 years and were treated only with insulin, as done in other studies of the pediatric population. We excluded children who were injected with insulin before 2008 and patients who were not treated with insulin continuously for 2 consecutive years after diagnosis to rule out patients with T2DM. In estimating the prevalence, we included children who were registered with a diagnostic code of E10 before the age of 15 years and received at least two prescriptions of insulin over 12 months with continuous insulin treatment in the following year. The diagnosis of T1DM was confirmed with a thorough review of the medical records of all cases by medical personnel of the HIRA.
Ethics statement
The study adhered to the tenets of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Severance Hospital, Seoul, Korea, and written informed consents from the subjects were exempted by the IRB (no. 4-2019-0088).
Calculation of incidence and prevalence
We calculated the incidence of T1DM by dividing the number of children who were newly diagnosed with T1DM during a calendar year by the total number of children at risk living in Korea during that calendar year. Exact confidence intervals for incidence were calculated using the Ulm’s formula [12]. In addition, we calculated the prevalence of T1DM by dividing the number of children registered with a diagnosis of T1DM during a calendar year by the total number of children living in Korea during that calendar year. The 95% confidence intervals of the prevalence with a 0.05 significance level were also calculated using the formula
RESULTS
A total of 29,013 children were registered from January 2007 to December 2017. The annual incidences of T1DM according to gender from 2008 to 2016 are shown in Table 1. The overall incidence of T1DM during the study period was 4.45 per 100,000 persons (girls, 4.93; boys, 4.01). The overall incidence of childhood-onset T1DM in Korea increased significantly from 3.70 per 100,000 persons in 2008 to 4.77 per 100,000 persons in 2016 (P=0.002). The incidence of T1DM among boys increased from 3.07 per 100,000 persons in 2008 to 4.89 per 100,000 persons in 2016 (P<0.001). However, the incidence was not definite among girls during the study period (4.39 per 100,000 persons in 2008 and 4.64 per 100,000 persons in 2016). There was an increasing trend in gender-specific annual incidence of childhood-onset T1DM in Korea (Fig. 2). The incidence increased by approximately 3% to 4% every year from 2007 to 2017.
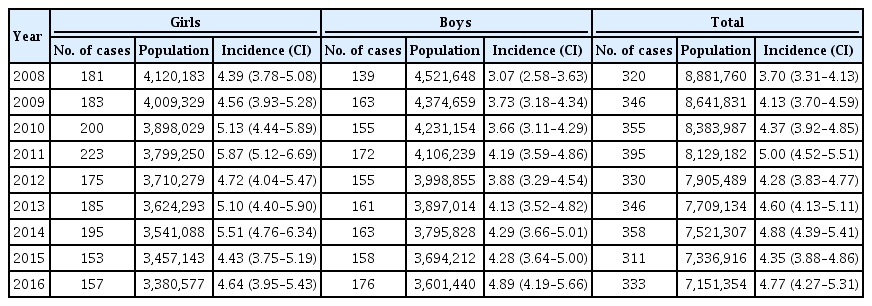
Gender-specific annual incidence (per 100,000 persons) of type 1 diabetes mellitus with confidence intervals among Korean children
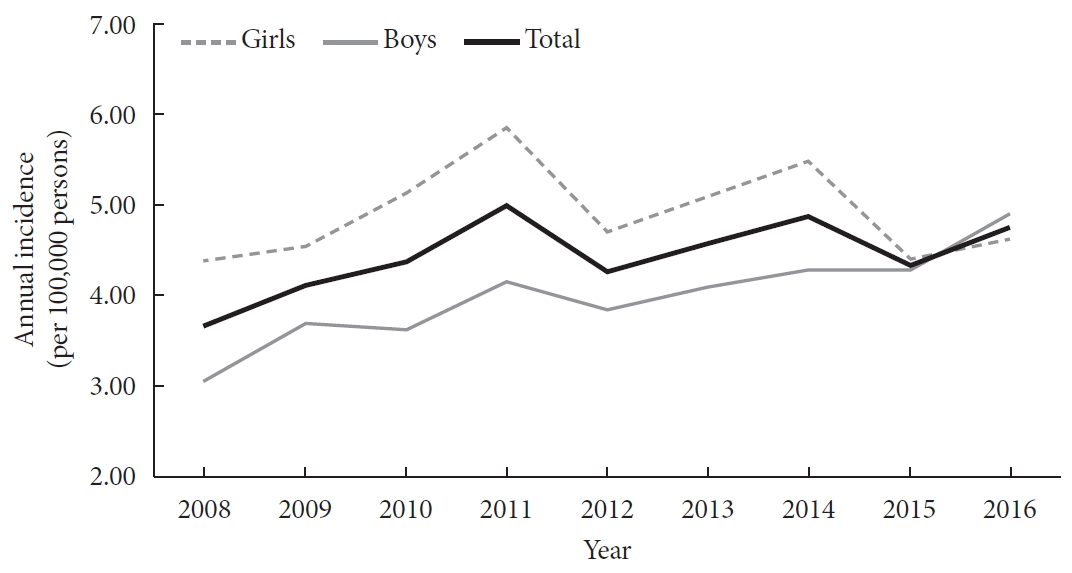
Gender-specific annual incidence (per 100,000 persons) of type 1 diabetes mellitus among Korean children.
The annual incidence of T1DM according to gender and age group is shown in Table 2. The overall incidence of childhood-onset T1DM increased significantly among children aged 10 to 14 years. The incidence increased significantly among boys aged 5–9 and 10–14 years; however, it was indefinite among boys aged 0 to 4 years. The incidence of T1DM increased from 1.16 to 3.72 per 100,000 persons among boys aged 5 to 9 years and from 5.53 to 8.90 per 100,000 persons among boys aged 10 to 14 years between 2008 and 2016 (P=0.040 and P<0.001, respectively). The incidence of T1DM among girls did not increase significantly in all age groups.

Gender-specific annual incidence (per 100,000 persons) of type 1 diabetes mellitus according to age group among Korean children
The distribution of the annual prevalence of T1DM according to age group is shown in Table 3. The overall prevalence of childhood-onset T1DM in Korea increased significantly from 32.85 to 41.03 per 100,000 persons between 2007 and 2017 (girls, 35.54 to 43.88 per 100,000 persons; boys, 32.85 to 41.03 per 100,000 persons). The annual prevalence increased rapidly over the last 2 years of the study period.

Gender-specific annual prevalence (per 100,000 persons) of type 1 diabetes mellitus with confidence intervals among Korean children
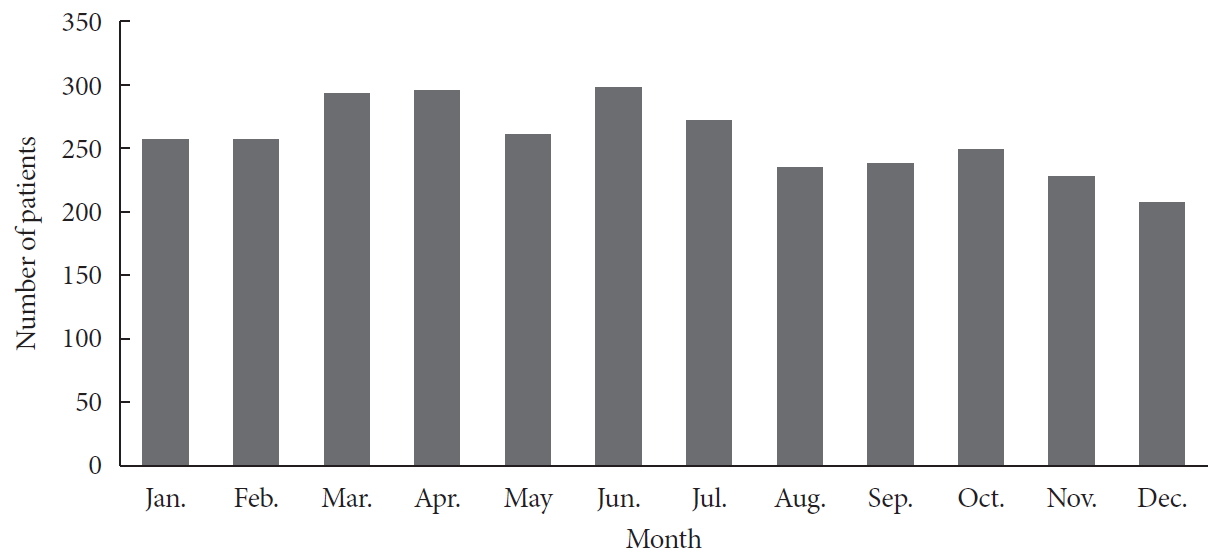
We analyzed the cumulative distribution of the incidence of disease according to months from 2008 to 2016. The incidence appeared to be relatively high in March, April, and June and low in November and December. However, this seasonal difference was not significant statistically (Fig. 3).
DISCUSSION
This work was a retrospective national registry-based epidemiologic study of the incidence and prevalence of childhood-onset T1DM in Korea from 2007 to 2017. The overall incidence of childhood-onset T1DM during the study period was 4.45 per 100,000 persons (girls, 4.93; boys, 4.01). The overall incidence of childhood-onset T1DM in Korea (per 100,000 persons) increased significantly from 3.70 in 2008 to 4.77 in 2016 and by approximately 3% to 4% annually during the study period. The overall prevalence (per 100,000 persons) increased significantly from 32.85 in 2007 to 41.03 in 2017 (girls, 35.54 to 43.88; boys, 32.85 to 41.03). We found that the incidence and prevalence of childhood-onset T1DM in Korea were higher than those determined in previous studies conducted in Korea and increased significantly during the study period.
The global incidence of diabetes among children and adolescents is increasing rapidly due to an increase in incidence of childhood obesity regardless of region. However, there are some variations in the incidence of T1DM according to ethnicity and region. Approximately 193,000 Americans younger than 20 years are estimated to have been diagnosed with diabetes, representing approximately 0.24% of that population [13]. A nationwide survey conducted in the USA from 2001 through 2016 demonstrated that 45,047 people aged 19 or younger who had T1DM were identified, representing a 5.4 to 79.6 prevalence rate per 100,000 person-years according to the states [13]. The DiaMond Project Group reported that a very high incidence was found in European countries and North America, and the lowest incidence was found in the populations from China and South America in 2000 [14]. The EURODIAB study reported the incidence of childhood-onset T1DM in the first (1989 to 1998) and second (1999 to 2008) halves of the 20-year period. The increase in annual incidence rates were similar (3.4% and 3.3%, respectively), suggesting that the incidence rate of childhood-onset T1DM continues to rise across Europe but not uniformly so among the regions [15].
Asian countries showed relatively lower incidences of childhood-onset T1DM than did Europe and the USA. These geographic differences in T1DM incidence may reflect population variations in the frequency of T1DM susceptibility or protective genes and/or environmental factors or their combination [16]. Although the incidence of T1DM is relatively lower in Asian countries, increasing trends in the incidence of T1DM have also been observed in Asian regions. In Japan, the annual incidence of childhood-onset T1DM was 1.5 per 100,000 persons from 1986 to 1990 [17]. A more recent study conducted in Japan reported that the incidence of T1DM from 2005 to 2010 was 2.25 per 100,000 persons, suggesting that the incidence is increasing in Japan [1]. In China, the incidence in the Beijing area was 1.81 per 100,000 population during 2003 to 2010 [18]. In reports of a Taiwanese nationwide epidemiologic study, the incidence of childhood-onset T1DM (per 100,000 persons) was 3.56 among boys and 5.88 among girls in 1999 to 2000 and increased significantly with an incidence of 4.42 among boys and 6.92 among girls in 2009 to 2010 [19]. The present study showed higher incidence of T1DM in Korea compared with that of Japan and China, and lower than that of Taiwan. The incidence and prevalence of T1DM would differ from country to country due to variable factors including change of lifestyle, increase of obesity, and environmental immune system [20,21].
It is known that the incidence of childhood-onset T1DM is exceptionally low in Korea. A limited number of studies have estimated the incidence and prevalence of childhood-onset T1DM in Korea. The first nationwide survey on the incidence of T1DM among Korean children and adolescents was conducted as part of the Diabetes Epidemiology Research International Study from 1985 to 1986 and showed an incidence of 0.5 per 100,000 population in both sexes [22]. Another study reported an incidence of 1.36 per 100,000 during 1995 to 2000 [23]. The most recent report on the epidemiology of childhood-onset T1DM in Korea was performed using the data obtained from the National Health Insurance Service during 2012 to 2014 [24]. The incidence was 3.19 per 100,000 (2.84 and 3.56 among boys and girls, respectively), and it was relatively higher than that reported in previous studies conducted in Korea. The present study demonstrated a higher incidence and prevalence than previously reported estimates in Korea and a significant increase in incidence of childhood-onset T1DM. We confirmed that the increasing trend in incidence of childhood-onset T1DM was sustained in Korea.
In the present study, we found a higher incidence and prevalence among girls than among boys. However, the increase in the rate of incidence was higher among boys. It is known that boys are predominantly affected by T1DM after puberty [25]. The overall ratio of incidence of T1DM among boys to that among girls was 1:1; however, a ratio of 1:7 was reported among children aged 13 years in a study conducted in Finland [26]. Some reports have shown that childhood-onset T1DM is more common among male children and adolescents in European countries [27-29]. Compared with that of Western countries, the Japanese cohort presents a preponderance of girls than boys [17]. A report emanating from China showed a preponderance of girls with newly diagnosed T1DM; however, a higher rate of increase occurred among boys between 1995 and 2010, similar to that observed in the present study [30]. A report showed that female predominance is associated with a lower incidence and ethnic group [27]. This implies that there should be specific considerations according to certain ethnicities or regions, although the reasons remain to be clarified.
In the present study, the incidences of childhood-onset T1DM appeared to be higher in March, April, and June; however, this seasonal difference was not significant statistically. It has been reported that the incidence of T1DM is higher in the fall or winter seasons [31,32]. However, the seasonality pattern appears to depend on the geographical location, at least as far as the hemisphere dichotomy is concerned [33]. A Japanese cohort study revealed no seasonal variation compared with that of Western countries [17].
We found that the incidence of childhood-onset T1DM in Korea increased rapidly over the past decade. It is unclear why the incidence of childhood-onset T1DM is increasing in Korea; nevertheless, it is not remarkably higher than that of Western countries. Factors contributing to the development of T1DM include genetic factors, which involve familial and ethnic patterns, and various environmental factors. A westernized lifestyle and national prosperity could reflect differences in environmental risk factors such as nutrition or lifestyle that affect the incidence rate [34]. The “accelerator hypothesis” tried to explain the loss of beta cells and the increasing incidence of both types of diabetes associated with the childhood obesity epidemic in some way [35]. An increasing prevalence of obesity among Korean children may also yield a possible association with the increasing rate of childhood-onset T1DM [36]. An assessment of the risk factors for T1DM in the general population and further efforts aimed at elucidating the causes underlying the increase in incidence of T1DM among Korean children are needed.
Our study had some limitations. First, some T1DM patients might not be registered for various reasons, such as emigration or studying abroad. Second, there is a possibility of misclassification of childhood T1DM or T2DM. For example, early treatments of T2DM often include insulin injections besides oral hypoglycemic agents. We tried to exclude patients who were not treated with insulin continuously for 2 consecutive years after diagnosis; nonetheless, misclassification might be a confounding factor in the present study. Third, the prevalence might be overestimated. We included children who were registered with a diagnostic code of E10 and were treated with only insulin continuously more than once; however, we could not perfectly exclude some cases of T2DM. Despite these limitations, we believe that the inclusion and exclusion criteria in the present study might be the most practical way to achieve the study aim and we were able to calculate relatively accurate incidences and prevalence of childhood-onset T1DM using the nationwide data.
In conclusion, we calculated relatively accurate incidences and prevalence of childhood-onset T1DM according to the data obtained from a nationwide registry from the HIRA. We found that the incidence and prevalence of childhood-onset T1DM increased significantly at a rate of about 3% to 4% every year from 2007 to 2017. Further long-term studies with investigation of environmental factors and other possible contributing factors are needed to elucidate the increasing trend in the occurrence of childhood-onset T1DM in Korea.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: G.H.S., H.S.K.
Acquisition, analysis, or interpretation of data: H.W.C., G.H.S., S.H., H.S.K.
Drafting the work or revising: H.W.C., K.S., H.S.C., J.S., A.K., H.S.K.
Final approval of the manuscript: H.W.C., G.H.S., K.S., H.S.C., J.S., A.K., S.H., H.S.K.
FUNDING
None
Acknowledgements
The authors thank the Yonsei Foreign Language Institute and Editage (www.editage.co.kr) for review and correction of the English version of the manuscript.