Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications
Article information
Abstract
By providing blood glucose (BG) concentration measurements in an almost continuous-time fashion for several consecutive days, wearable minimally-invasive continuous glucose monitoring (CGM) sensors are revolutionizing diabetes management, and are becoming an increasingly adopted technology especially for diabetic individuals requiring insulin administrations. Indeed, by providing glucose real-time insights of BG dynamics and trend, and being equipped with visual and acoustic alarms for hypo- and hyperglycemia, CGM devices have been proved to improve safety and effectiveness of diabetes therapy, reduce hypoglycemia incidence and duration, and decrease glycemic variability. Furthermore, the real-time availability of BG values has been stimulating the realization of new tools to provide patients with decision support to improve insulin dosage tuning and infusion. The aim of this paper is to offer an overview of current literature and future possible developments regarding CGM technologies and applications. In particular, first, we outline the technological evolution of CGM devices through the last 20 years. Then, we discuss about the current use of CGM sensors from patients affected by diabetes, and, we report some works proving the beneficial impact provided by the adoption of CGM. Finally, we review some recent advanced applications for diabetes treatment based on CGM sensors.
INTRODUCTION
Among the many glucose-sensing mechanisms tested to guarantee all the necessary requirements for long-term use of biosensor in free-living conditions, i.e., biocompatibility, lifetime, safety, sensitivity, and specificity, the most popular technique used for continuous glucose monitoring (CGM) systems relies on the glucose oxidation reaction [1]. Specifically, CGM devices based on this principle use a glucose-oxidase-doped platinum electrode deposited on a needle inserted in the subcutaneous tissue to ignite and catalyze glucose oxidation. This results in the production of gluconolactone, hydrogen peroxide, and an electrical current signal that is transformed, in the end, to a glucose concentration through a calibration process using a few self-monitoring of blood glucose (SMBG) samples collected by the patient [2].
The introduction of these “minimally invasive” needle CGM sensors in 1999 [3] revolutionized, de facto, blood glucose (BG) concentration monitoring in diabetes and opened new exciting scenarios in the daily management of diabetes [4]. CGM sensors deliver an almost continuous glucose trace providing BG readings every 1 to 5 minutes, mitigating the need of SMBG and greatly increasing the information on BG fluctuations and trend (which shows that CGM reveals hypoglycemic and hyperglycemic events not visible by SMBG) (Fig. 1A). Since the first prototype, CGM sensors have evolved remarkably. Nowadays, they are also able to provide patients with many smart features, such as arrows depicting the current glucose rate-of-change and smart alarms for impeding hypo-/hyperglycemic events, improving patient self-management. Although, mainly due to economic cost and patient acceptability of sensor devices, CGM users represent only a small part of total diabetes population, CGM sensors proved to be effective in improving patient glucose control [567] and enabling the possibility of designing new advanced technologies for diabetes management [89].

(A) Representative blood glucose (BG) monitoring data obtainable with self-monitoring of blood glucose (SMBG; in green) and with continuous glucose monitoring (CGM; in blue). Dotted circles denote hyperglycemic and hypoglycemic episodes that, using only SMBG measurements, are not detectable. (B) Assessment of the accuracy of a CGM sensor can be performed by comparing Yellow Spring Instruments Inc. (YSI) measurements (red stars) versus Dexcom G4 Platinum CGM (black solid line) measurements. For example, mean absolute relative difference can be calculated as the average ratio between the absolute difference between the CGM measurements and the YSI over the YSI.
The purpose of this paper is to (1) provide an overview of the latest advances on CGM system development and glucose sensing technologies, (2) discuss about the current use of CGM sensor from patient affected by diabetes, and (3) review some recent advanced applications for diabetes treatment based on CGM. To do so, based on our experience as researchers active in the diabetes technology field, we will focus on the current literature works that we think are able to provide a general overview on the many undergoing activities involving CGM.
HISTORY OF CGM SENSORS
The first CGM prototypes
CGM devices based on glucose-oxidase have been proposed starting from the 1999, when the U.S. Food and Drug Administration (FDA) approved the first professional CGM system to be used by healthcare professionals, thus enabling the possibility of analyzing retrospective user data for review [3]. This system, however, suffered from several limitations, the most important of which was poor accuracy, i.e., a concept quantifiable through some metrics. This system, however, suffered from several limitations, the most important of which was poor accuracy, which is usually assessed by comparing the CGM trace with very accurate and precise BG concentration values, commonly collected in a hospital setting by mean of laboratory-grade medical instruments, i.e., Yellow Spring Instruments Inc. (YSI, Yellow Spring, OH, USA) (Fig. 1B). Several metrics can be computed over these differences, such as %20/20-ISO boundaries, absolute relative difference, and mean absolute relative difference (MARD) [10]. Among these quantities, MARD is the most common metric currently used in the literature to assess CGM accuracy [11], and the one on which we will focus hereafter. In 2004, Medtronic (Medtronic Minimed, Northridge, CA, USA) introduced and successfully commercialized the first real-time CGM system for personal use: the Medtronic Real-Time Guardian. This system provided patients with a glucose concentration value every 5 minutes, lasted 3 days, and it was able to sound an alarm when the glucose concentration level became either too high or too low, helping users to improve glucose control. The Medtronic Real-Time Guardian's MARD was estimated to be 15%. Dexcom Inc. (San Diego, CA, USA) commercialized the Dexcom SEVEN Plus, which had longer lifetime, lasting up to 7 consecutive days. The accuracy of the Dexcon SEVEN Plus was 16.7% [12], slightly worst of that of the Medtronic Real-Time Guardian, but significantly better than that of its predecessor. The same year, the Abbott Freestyle Navigator (Abbott Diabetes Care, Alameda, CA, USA) was marketed, featuring a glucose sensor that could be worn up to 5 days and achieving an MARD of 12.8% [13]. Compared to SMBG, whose MARD falls between 5% and 10%, the low accuracy (i.e., elevated MARD) of these “first generation” CGM systems represented one of the major barrier for the early adoption of these devices by both users, who felt unsafe in adopting CGM, and many leading diabetologists, whose reluctance to integrate CGM sensors in the daily diabetes management greatly limited the spreading of this technology.
The most recent CGM systems
In the last decade, CGM manufacturers invested many efforts to overcome the problems of lack of accuracy of their first generation devices. The first new generation product was the Medtronic Enlite CGM system. This device, besides achieving a MARD of 13.6% [14], extended the wear time up to 6 days. In addition, it improved the sensor comfortability by reducing its size and weight, it was designed to be water-proof, and it allowed the memorization of BG up to 10 hours if the receiver-transmitter connection is interrupted for any reasons. The same year, Abbott launched the Freestyle Navigator II, a newly designed CGM system that provided BG readings every minute with a 12.3% MARD [15]. In 2012, Dexcom introduced the G4 Platinum, featuring a smaller sensor, lasting for 7 days, and reducing the MARD to 13% [16], later improved to 9% in 2014 thanks to new algorithms integrated directly within the sensor [17]. In 2015, Dexcom introduced the G5 Mobile [17] achieving a MARD of 9%, a 7 days wear time, now allowing BG data to be directly transmitted to the user's cell phone without the need of a dedicated receiver.
Later on, in 2016, Abbott commercialized the Freestyle Libre featuring a MARD of 11.4% [18]. This CGM system is the first that required no fingerstick testing during wear. In addition, it extended the wear time up to 14 days. Unlike Dexcom or Medtronic CGM devices, the Freestyle Libre does not sound any alarms if BG falls out of the safe glycemic range and it requires patient to wave the receiver over the transmitter in order to get BG information, and to do so at least one time every 8 hours in order not to lose data. For this reason, the Freestyle Libre is labelled as a flash glucose monitoring (FGM) device, i.e., a device that measures BG at continuous time but displays the measured values only when scanning the sensor with the receiver. The Freestyle Libre has been the first glucose monitoring device that required no calibrations, with the additional advantage of performing similarly in terms of both accuracy and BG control compared to CGM devices that require two or more calibrations per day, e.g., the G4 Platinum and the Freestyle Navigator II [19]. Following this technological trend, Dexcom launched in 2017 the G6 [20], a CGM system that can be used without in vivo calibrations, for 10 consecutive days, ensuring the same accuracy of the G5 Mobile. In the same year, Medtronic introduced the Guardian Sensor 3, whose accuracy was quantified as 10.6% and 9.1% MARD [21], when inserted in the abdomen and in the arm, respectively. This sensor is 80% smaller than the Enlite, and it ensures up to 7 days of sensor life as well as a shorter startup time.
To summarize, in the last decade, besides achieving accuracies close to, or even within, the SMBG accuracy range, CGM systems improved also in terms of features and comfort for the patient. In Fig. 2 we reported a graphical representation of the CGM system accuracy evolution through years. In Table 1, we summarized the main characteristics in terms of accuracy, features, and limitations of the most known CGM sensor devices.
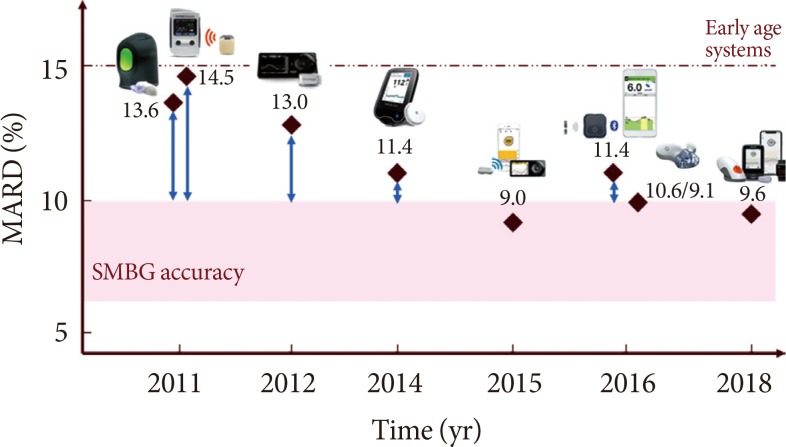
Accuracy evolution of state-of-the-art CGM systems through years. From the left: Medtronic Enlite, Abbott Freestyle Navigator, Dexcom G4 Platinum, Abbott Freestyle Libre, Dexcom G4 Platinum with 505 software, Senseonics Eversense, Dexcom G5, Dexcom G6. MARD, mean absolute relative difference; SMBG, self-monitoring of blood glucose.
Technological challenges of next generation CGM sensors
CGM systems are now accepted as standard tools for intensive glucose control in patients with type 1 diabetes mellitus (T1DM). However, several important limitations are still present. Indeed, glucose-oxidase based electrochemical sensors suffer from several limits such as their non-linear response within the biological relevant range, the possible interference with active agents (e.g., acetaminophen, ascorbate), and most importantly, their dependence of both sensitivity and specificity on the enzyme availability on the electrode surface. Moreover, BG readings provided by glucose-oxidase based CGM sensors are affected by delay artifacts, which range from 5 to 10 minutes, due to the time lag between glucose concentration in the interstitial fluid and BG. On one hand, delay is not important when analyzing retrospective glucose data, on the other, it can be critical when CGM is used for real-time decision making.
For this reason, further research is currently undergoing to address previously listed issues and designing new CGM sensors able to better meet technological requirements such as sensor size, lifetime, and capabilities.
From this perspective, in August 2015, Dexcom started a new collaboration with Verily (Verily Life Science LLC, San Francisco, CA, USA) to develop a new miniaturized, cheaper, patch CGM sensor designed to minimize its burden to the patient and to help people with type 2 diabetes mellitus (T2DM) managing their daily routine [22]. In 2018, Abbott released the Freestyle Libre 2, which successfully secured the Conformité Européene (CE) mark in October 2018, and improved the Libre by adding smart alarms [23].
Next generation CGM system development also involves the exploration of new glucose sensing technologies beyond glucose-oxidase. In this regard, glucose sensors based on optical sensing have been recently proposed. These sensors provide an interesting alternative to traditional electrochemical sensors since they have the benefit of being free from electromagnetic interference, simple to design and handling, and characterized by low manufacturing cost. These principles have been used to design non-invasive sensors based on near infrared detection and Raman spectroscopy [24], and fully implanted CGM systems based on fluorescence [25]. In 2016, Senseonics (Senseonics Inc., Germantown, MD, USA) launched the Eversense, the first implantable CGM system to receive the CE mark. As already mentioned, it is based on fluorescence sensing, featuring a lifetime of 90 days, and an accuracy of 11.4% MARD [26]. Of course, this approach is quite demanding for the patient, who is required to undergo a, even if simple, surgical procedure, but the sensor lifetime makes this system a good and appealing alternative.
Lastly, next generation CGM systems require guaranteeing data security. Indeed, being limited by low computing capabilities, CGM transmitters, and more in general wearable devices, have been proven to suffer from several security weaknesses that make user data relatively easy to potentially be hacked [27]. Even if, to the best of our knowledge, cyberattacks to CGM sensor devices have never been publicly reported yet, these potential security flaws need to be fixed to ensure data confidentiality and integration without undermining its availability. Recently, the Diabetes Technology Society established DTSec, i.e., a new consensus-based standard thought to provide a high security and assurance level among electronic devices used in diabetes treatment including, but not limited to, insulin pumps, SMBG devices, and, of course, CGM systems [28].
CURRENT USE OF CGM TECHNOLOGIES
Professional use
CGM sensors can be used as professional or personal devices. Professional CGM systems are owned by caregivers and intermittently prescribed to patients in blinded mode. Blinded CGM devices collect glucose concentration data continuously but do not display them in real-time to the user; data can only be reviewed retrospectively by the caregiver at the end of the monitoring. Blinded data collection allows tracking patients' glucose profiles without influencing their behavior. Examples of professional CGM devices for blinded data collection include the Abbott Freestyle Libre Pro system and the Medtronic iPro2 system. The retrospective analysis of CGM data allows the caregiver to extract glycemic variability metrics, identify previously unappreciated glucose patterns and adjust therapy regimens accordingly.
Glycemic variability represents how much BG fluctuates around the average value and is considered an important glycemic target, together with glycosylated hemoglobin (HbA1c), to reduce the risk of diabetes complications [29]. Notably, glycemic variability cannot be captured by sparse SMBG measurements, but it can be detected by CGM almost continuous-time profiles. Several CGM-based glycemic variability metrics have been proposed in the literature [30]. Recently an international panel of physicians, researchers and patients expert in CGM technologies defined the key metrics for CGM data analysis and reporting [31]. The panel also recommended the Ambulatory Glucose Profile (AGP) [32], a standardized single page report with summary statistics and daily glycemic patterns developed by the International Diabetes Center, as a standard for the visualization of CGM data. The AGP report has been included in many proprietary software for retrospective CGM data analysis, e.g., Dexcom CLARITY, Diasend-Glooko, Tidepool, LibreView, and Medtronic CareLink.
Personal use
In addition to the retrospective data analysis, CGM systems for personal use allow the individual to visualize in real-time information on current BG and trend on a portable receiver or a smartphone application. Most of personal CGM systems currently on the market, including the Dexcom G5 Mobile and G6, the Medtronic Enlite and Guardian, and the Senseonics Eversense, provide high and low BG alerts that help the patient to detect hypo/hyperglycemic events. Alerts are not available with the FreeStyle Libre, which, despite considered a glucose sensor for personal use, falls within the category of FGM devices. Alerts have been introduced in a new generation FGM device, i.e., the FreeStyle Libre 2. Devices for personal use also allow data sharing with third parties, such as parents, partners and caregivers. The Dexcom G5 Mobile and G6 and the Eversense sensors can share the data in real-time, a feature that is very useful for the pediatric population, as parents are enabled to check remotely their children's BG during school, physical activity or sleep. The Freestyle Libre and the Medtronic Enlite and Guardian sensors can share the data, but not in real-time.
Until a few years ago, all CGM devices for personal use were approved to be used in adjunct to SMBG, i.e., before making the treatment decisions patients were required to check CGM readings by confirmatory fingersticks. Indeed, as described in Section “History of CGM Sensors,” past CGM sensors suffered from accuracy problems due to plasma-interstitium kinetics, imperfect calibrations, compression artefacts and sensitivity to interfering substances, such as acetaminophen. Thanks to recent technological developments and enhancement of signal processing algorithms, the accuracy of CGM sensors has been remarkably improved during the last years, reaching the range of accuracy performance of SMBG devices (MARD <10%). These improvements led to the regulatory approval of CGM nonadjunctive use, i.e., the use of CGM readings to make treatment decisions without confirmatory fingersticks, whose safety and effectiveness have been proved by simulations [8] and a randomized non-inferiority clinical trial [33]. From 2014 to 2015, three CGM sensors received the nonadjunctive label in Europe: the FreeStyle Navigator II, the FreeStyle Libre and the Dexcom G5 Mobile. The approval of nonadjunctive use by the FDA came a couple of years later: the Dexcom G5 Mobile obtained the approval in 2016, followed by the FreeStyle Libre in 2017 and the Dexcom G6 in 2018. Despite the nonadjunctive label, sensor companies still recommend the use of confirmatory fingersticks when symptoms do not match sensor's readings or a trend arrow is not displayed. With the FreeStyle Libre, confirmatory fingersticks are also recommended when glucose is rapidly changing and in order to confirm hypoglycemia or impending hypoglycemia.
Real-time CGM sensors for personal use, in particular those approved for nonadjunctive use, can be used by the patients to make therapeutic decisions, e.g., insulin dosing. Compared to conventional SMBG devices, CGM also provides information on current glucose trend that can be exploited for a more accurate calculation of insulin boluses. Clinical experts in the field have proposed some guidelines that recommend the patient to increase or decrease the insulin dose by a certain amount (either proportional to the dose or inversely proportional to the patient's correction factor) when the CGM trend arrow indicates glucose concentration is rising or decreasing [34]. Nevertheless, these guidelines have never been assessed in clinical trials and survey data evidenced that patients actually perform much greater corrections to the insulin dose than those recommended by the guidelines [35]. A recent in silico study compared three different trend adjustment guidelines in simulation [36]; the results evidenced that none of the guidelines prevailed on the others for all the pre-meal scenarios, suggesting that these simple guidelines can be further improved.
CGM uptake
Historically CGM uptake was poor. From 2010 to 2012, only 7% of the T1D Exchange Registry participants were using CGM sensors [37]. A recent study reported that the most common barriers to CGM use were related to the high cost of the device, lack of insurance coverage, the hassle of wearing devices and the dislike of having devices on the body; the most common reasons for stopping CGM were cost, alarm fatigue and perceived sensor inaccuracy [38]. Nowadays, thanks to the recent developments in CGM technology, in particular the release of new more accurate sensors with reduced sensor size and calibration requirements, most of these barriers have been overcome. After the regulatory approval of CGM therapeutic use, the United States medical insurance company Medicare has announced reimbursement criteria for therapeutic CGM devices to all T1DM and T2DM patients on intensive insulin treatment [39]. Similarly, in many European countries CGM expenses are now covered by national healthcare systems, thus increasing the accessibility of such technology.
These changes has led to a growth of CGM use both in United States and in Europe. Most recent data from the T1D Exchange Registry reported that about 30% of participants have been using CGM in the period from 2016 to 2018 [40]. Abbott reported that about 800,000 people in 43 countries worldwide are currently using the FreeStyle Libre sensor [41]. In 2017, it was estimated that 18% of German/Austrian Diabetes Patienten Verlaufsdokumentation registry participants were using a CGM device [42]. Although the use of CGM sensors has increased, the CGM market is still moving slowly. Currently, only a small portion of well-trained T1DM individuals is using CGM, which represents less than 0.5% of the global diabetic population [43]. A possible reason for the low global uptake of CGM is that there are weak evidences that CGM is beneficial for T2DM patients not on intensive insulin treatment, which actually represent the majority of the diabetic population.
To whom it is recommended
Standards of diabetes care, like the American Diabetes Association and the American Association of Clinical Endocrinologists and American College of Endocrinology, recommend CGM use in conjunction with intensive insulin treatment for T1DM subjects who do not meet the glycemic target or suffer from hypoglycemia unawareness [4445]. The Endocrine Society also recommends intermittent use of personal CGM devices for T2DM patients with poor glycemic control who are able and willing to use the device [46]. T2DM individuals were identified as possible beneficiaries of CGM use also by an international consensus on CGM, which recommended the use of CGM in conjunction with HbA1c to assess the glycemic status and adjust therapy regimen in all T1DM and T2DM patients on intensive insulin treatments [31]. Apart from T1DM and T2DM people on intensive insulin treatment, other groups may benefit from CGM use. For example, some studies have demonstrated that CGM use improves the neonatal outcomes when used in pregnant women with diabetes or gestational diabetes [4748].
THE IMPACT OF CGM ON DAILY MANAGEMENT OF DIABETES
Nowadays, the beneficial impact brought by the integration of a CGM system in diabetes management has been proven [49]. Indeed, initial reluctance from both clinicians and patients has diminished through years thanks to the constant accumulation of clinical evidence from research over adult and pediatric populations with T1DM [29]. Just to mention a few, recent research proved CGM sensors to be effective for patients with frequent hypoglycemic events [7], sensor augmented pumps [50], and gestational diabetes [47], treated with either continuous subcutaneous insulin infusion (CSII) or a multiple daily injection (MDI) insulin regimen. Published randomized controlled trials (RCTs) have been devoted to study the impact of CGM sensor systems on (1) improving glycemic control, (2) mitigating hypoglycemic episodes, (3) reducing glycemic variability. In the following, some significant literature results proving the beneficial impact of CGM are reported.
Improvement of glycemic control
Numerous RCTs have demonstrated improved glucose control in terms of reduced HbA1c in individuals using CGM compared to those using SMBG. Two major studies by Bergenstal et al. [51] and Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group et al. [5] considered T1DM individuals undergoing both MDI and CSII therapies and assessed the ability of several CGM sensors in improving the glucose control. In particular, both studies showed a significant reduction of HbA1c of 0.64% and 0.53%, respectively, in subjects adopting CGM versus a control group employing standard therapy based on SMBGs. Moreover, both studies confirmed a direct relationship between sensor usage time and average HbA1c reduction. Specifically, Bergenstal et al. [51] showed that significant HbA1c reduction was possible only for users using CGM sensor between 41% and 60% of the time, which doubled in users who used CGM more than 80% of the time. Consistently, Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group et al. [5] reported that using CGM at least 6 days per week translate to an average reduction of 0.5% of HbA1c. Similar conclusions have been reported in many other studies, spanning different population categories, e.g., from children to adult, from individuals affected by T1DM to women with gestational diabetes, elevating CGM as a particularly useful tool to achieve effective glucose control in diabetes.
Mitigation of hypoglycemic episodes
Reduction of hypoglycemia has been shown to be one of the major advantages provided by CGM use. Indeed, thanks to its intrinsically superior time resolution, CGM enables users to capture glycemic fluctuations that are invisible using SMBG only. The real-time availability of glucose concentration values as well as visual/acoustic hypoglycemic alerts allow users to act in order to mitigate, or even avoid, hypoglycemia.
In a recent multicenter clinical study involving T1DM subjects having good starting glucose control, a 50% reduction of time spent in hypoglycemia has been shown, even if no significant reduction of HbA1c was reported [52]. Another study by Haak et al. [53] analyzed the impact of CGM use on individuals affected by T2DM in free-living conditions under either MDI or CSII therapy. Results showed a significant reduction of hypoglycemia of 53%. However, no significant reduction of HbA1c has been observed although the study participants were poorly controlled T2DM subjects. The reason behind this result probably lies behind the lack of optimal education from the patients. To support this thesis, according to a recent study by Hermanns et al. [54], the usage of FGM was effective to reduce HbA1c in participants receiving proper structured education, but FGM itself without education was not effective in poorly controlled T1DM and T2DM under MDI therapy.
Summarizing, thanks to the adoption of CGM in diabetes treatment, hypoglycemia can be successfully mitigated both improving individuals' quality of life and reducing the shortcoming of dangerous short-term complications.
Reduction of glycemic variability
Several studies appear to confirm that glycemic variability can play a key-role in the appearance of vascular damages [29] and in the set-up of hypoglycemic events [55]. CGM sensors are important also in reducing glycemic variability in individuals affected by diabetes. In Jamiolkowska et al. [56], 40 subjects aged 14.6 years have been recruited to evaluate whether the use of CGM could improve glycemic variability in terms of glucose standard deviation (SD) and area under the curve (AUC). Results showed a reduction of SD from 60.7 to 51.7 mg/dL as well as a decreasing of AUC with threshold fixed at 140 mg/dL from 41.2% of the control group to 21.2% of the group that accepted the adoption of CGM use. Another study from Tumminia et al. [57] compared 10 individuals with T1DM undergoing MDI therapy. Participants were monitored for 6 months while being randomized into two groups to compare SMBG versus CGM in terms of SD of the glucose profile and range of glycemic excursions (RGEs). Results showed a reduction of both SD and RGE when using CGM compared to SMBG (62.3 mg/dL vs. 75.5 mg/dL and 132.3 mg/dL vs. 175.3 mg/dL, respectively).
In summary, the use of CGM was shown to be crucial to allow people affected by diabetes reducing glycemic variability. As a result, this unlocks the possibility of mitigating the shortcoming of both hypo and hyperglycemia as well as dangerous micro/macrovascular complications.
ADVANCED APPLICATIONS BASED ON REAL-TIME CGM
Decision support systems
The increased amount of available information brought by wearable devices, such as CGM systems and physical activity monitoring bands, has led to the development of decision-making tools and applications that can enhance the management of the disease [58]. A decision support system (DSS) gives the possibility to support users with proactive and personalized decisions in any scenario of their daily living and allows to react at shorter time scales. Over the past few years, DSSs for diabetes have been an emerging concept in health care. By means of this new technology, data can be automatically collected, transmitted, aggregated with other physiological data, analyzed, stored, and presented to the patient. By integrating e-health and tele-monitoring systems, DSSs for T1DM have the potential to improve glycemic outcomes thanks to prevention of hypo- or hyperglycemic events, reducing uncertainty when making critical self-management decisions [59]. A DSS for diabetes treatment provides an alternative to the closed-loop system, the so-called artificial pancreas (AP). Indeed, a wide range of users do not feel confident with the use of AP systems, being concerned about errors occurring in the insulin pump, and they prefer an open-loop therapy, which can be assisted by DSSs. Most of DSSs already available in the literature are composed by a predictive glucose module (which alerts the user whenever its BG is predicted to fall outside the safe range in the next future), an insulin suspension module (which temporarily suspends basal insulin delivery to avoid hypoglycemia when BG is critically low in patient using insulin pumps), and an adaptive insulin bolus calculator (which provides users with a suggestion of the correct insulin dosage to compensate the BG fluctuation due to a meal).
Many literature studies showed that DSSs are viable tools for improving diabetes treatment. A prototype bolus calculator algorithm based on neural networks providing personalized insulin recommendations has been developed and preliminarily tested in silico by Cappon et al. [60]. Breton et al. [61] proposed a DSS with automated insulin titration and dosing, proving that the use of the system results in reduced glucose variability and improved protection against hypoglycemia. Moreover, DreaMed (DreaMed Diabetes Ltd., Petah Tikva, Israel) recently introduced the Advisor Pro, a responsive application that provides real-time automated analysis of patient specific behavior to come up with personalized estimates of the optimal insulin treatment plan [62], which received FDA approval to be marketed in the United States in 2018 [63]. Finally, Patient Empowerment through Predictive PERsonalised decision support (PEPPER), a project funded by the European Community under the Horizon 2020 research program, has recently entered its final test phase, after several preliminary studies showed that it is able to improve glucose control and reducing the incidence of hypoglycemic episodes [64].
Basal insulin attenuation/suspension
Since 2006, medical devices integrating CGM sensors and insulin pumps have become commercially available, the Medtronic MiniMed Paradigm REAL-time system being the first device allowing such integration. Later in 2009, Medtronic integrated systems have been equipped with the low glucose suspend (or threshold suspend) feature that allows to automatically suspend basal insulin infusion for up to 2 hours when CGM measurements fall below a user-defined low glucose threshold. This feature, implemented in the Medtronic Paradigm Veo and MiniMed 530G, was designed to mitigate hypoglycemic events in insulin pump therapy [65]. Most recent Medtronic systems, i.e., the MiniMed 640G and 630G, implement the SmartGuard feature, which also allows to suspend basal insulin infusion when CGM measurements are predicted to fall below a preset threshold in the next 30 minutes (prediction low glucose management) [66].
Basal insulin suspension algorithms have been intensively studied also by academic research groups. In particular, the group led by Dr. Bruce Buckingham have been very active in this research line since 2009, by testing several prediction algorithms and insulin suspension criteria in both inpatient and outpatient clinical trials. Their final algorithm performs 30-minute ahead glucose prediction by a Kalman filter and suspends basal insulin when the predicted glucose concentration fall below 80 mg/dL [67]. Basal insulin delivery is restarted as soon as CGM measurements start rising or after 2 consecutive hours of suspension.
The algorithms developed by Buckingham et al. [67], as well as those implemented in commercial devices, can only turn on or off basal insulin delivery. A different approach was adopted by the group led by Prof. Kovatchev at the University of Virginia, who proposed algorithms to attenuate, rather than suspend, basal insulin delivery in presence of hypoglycemia risk. Specifically, their “power brakes” algorithm performs 15-minute ahead glucose prediction using physiological model-based Kalman filtering and applies a BG risk function in order to calculate a basal insulin attenuation factor depending on predicted risk of hypoglycemia [68].
Several clinical trials were performed to assess the safety and effectiveness of basal insulin suspension algorithms both in clinic under controlled conditions [69707172] and at home under real-life conditions [67737475]. Evidences from these trials supported the effectiveness of these algorithms in reducing hypoglycemia, at the expenses of a slight increase in hyperglycemia. Nevertheless, the use of basal insulin suspension was not associated with a significant increase of HbA1c or occurrence of ketoacidosis.
For an exhaustive review of algorithms for basal insulin suspension/attenuation, their implementation in commercial devices and clinical evidence of their effectiveness and safety, we refer the reader to [76].
Closed-loop systems
Automatic CGM-based basal insulin suspension/attenuation represents the first step towards closed-loop systems, namely AP, in which a control algorithm automatically tune insulin pump injections based on CGM readings. Research on closed-loop systems has been very intense in the last 10 years [777879]. Several control algorithms were proposed and assessed in clinical trials, including proportional-integral-derivative controller [8081], model predictive control [82], and fuzzy logic controller [83]. Most of closed-loop systems adopt the hybrid approach, in which insulin boluses are manually administered by the user (meal amount required for meal bolus computation), while basal insulin rate is automatically tuned by the control algorithm. Recently, a hybrid closed-loop system has entered the market, as in 2017 Medtronic launched the MiniMed 670G, i.e., the first commercially available hybrid closed-loop system. A retrospective analysis of 3-month real-world glucose data has shown improved clinical outcomes in patients using the Auto Mode of the MiniMed 670G, compared to patients on Manual Mode [84]. Fully closed-loop systems that do not require patients to announce meals to the controller are also under development, though an increased risk for hypoglycemia has been reported in T1DM individuals with fully closed-loop control compared to hybrid closed-loop control [85]. A fully-closed loop system has been recently tested in adult inpatients with T2DM receiving non-critical care [8687]. Results demonstrated that the closed-loop insulin delivery can greatly improve the time spent in the target range (difference between closed-loop and control groups of up to 32% points) without increasing hypoglycemia. A recent pilot study suggested that hybrid AP can be beneficial also for non-hospitalized MDI-treated T2DM subjects [88], although further investigation is needed to evaluate the cost-effectiveness of AP in this population.
While research in AP is progressively increasing the safety and effectiveness of such devices, also exploring bi-hormonal systems allowing controlled delivery of both insulin and glucagon [89], patients have shown an increased interest for the AP technology. This gave rise to the OpenAPS community, a community of patients highly interested in directly improving diabetes technologies, who have designed their own open source AP system, also called do-it-yourself closed-loop system. Although no clinical trial has ever assessed the safety and effectiveness of such open source systems, OpenAPS users self-reported an improvement in HbA1c, time in range, glycemic variability and quality of life, while perceiving the OpenAPS system as safe [9091].
For an exhaustive review of algorithms for closed-loop control and AP technologies, we refer the reader to recent reviews [777879].
FUTURE PERSPECTIVES
The advent of CGM sensors has revolutionized the glucose monitoring in T1DM. The recent approval of CGM therapeutic use and the new reimbursement policies have contributed to increase the number of CGM users worldwide, which is expected to further rise in the next years when less obtrusive and cheaper sensors will become available. Indeed, major CGM companies, like Dexcom and Medtronic, have announced the development of new products designed to be smaller and less expensive than current state-of-the-art systems, which can target not only T1DM patients, but also the much larger market of people with T2DM [2292]. Furthermore, emerging companies are working on low-cost non-invasive CGM systems [9394], which may even bring CGM technology to the consumer market.
Important advances are expected also in terms of CGM interoperability with other devices, e.g., medical devices for diabetes therapy, activity trackers and other physiological wearable sensors. Indeed, the FDA has recently defined a new class of CGM devices, i.e., integrated continuous glucose monitoring (iCGM) systems, including devices to be used as part of an integrated system with other compatible medical devices and electronic interfaces. This will enable iCGM developers to bring their device to the market more rapidly. The first CGM sensor to receive FDA approval with the iCGM label is the Dexcom G6 device, launched in 2018 [95]. The integration of CGM data with data of insulin pump and other wearable sensors, like activity trackers, will allow improving algorithms for glucose prediction and automatic basal insulin modulation, as recent studies with basal insulin suspension [96] and AP during exercise [97] have suggested. CGM data integrated with other diabetes management data (e.g., insulin pumps, SMBG and mHealth apps data) and activity data will also allow to enhance diabetes DSSs, enabling a better understanding of the causes driving to abnormal glucose events and, finally, a better tailoring of diabetes therapy to the patient's lifestyle and habits.
Finally as more broadly discussed in [9], CGM data could be integrated with other clinical data sources, including clinical registries, electronic health records, prescription registries and biomarkers collected in laboratory tests, which will provide important clinical contextualization to CGM data. This will allow the generation of a digital ecosystem of diabetes data that could be exploited to extract novel insights on the mechanisms of diabetes progression and develop cutting-edge data analytics for personalized diabetes management and prevention of related complications.
ACKNOWLEDGMENTS
None
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.