Predictors of Incident Type 2 Diabetes Mellitus in Japanese Americans with Normal Fasting Glucose Level
Article information
Abstract
Background
Little is known about the natural course of normal fasting glucose (NFG) in Asians and the risk factors for future diabetes.
Methods
A total of 370 Japanese Americans (163 men, 207 women) with NFG levels and no history of diabetes, aged 34 to 75 years, were enrolled. Oral glucose tolerance tests were performed at baseline, 2.5, 5, and 10 years after enrollment.
Results
During 10 years of follow-up, 16.1% of participants met criteria for diabetes diagnosis, and 39.6% of subjects still had NFG levels at the time of diabetes diagnosis. During 5 years of follow-up, age (odds ratio [OR], 1.05; 95% confidence interval [CI], 1.01 to 1.10; P=0.026) and family history of diabetes (OR, 3.24; 95% CI, 1.42 to 7.40; P=0.005) were independently associated with future diabetes diagnosis; however, fasting glucose level was not an independent predictor. During 10 years of follow-up, family history of diabetes (OR, 2.76; 95% CI, 1.37 to 5.54; P=0.004), fasting insulin level (OR, 1.01; 95% CI, 1.00 to 1.02; P=0.037), and fasting glucose level (OR, 3.69; 95% CI, 1.13 to 12.01; P=0.030) were associated with diabetes diagnosis independent of conventional risk factors for diabetes.
Conclusion
A substantial number of subjects with NFG at baseline still remained in the NFG range at the time of diabetes diagnosis. A family history of diabetes and fasting insulin and glucose levels were associated with diabetes diagnosis during 10 years of follow-up; however, fasting glucose level was not associated with diabetes risk within the relatively short-term follow-up period of 5 years in subjects with NFG.
INTRODUCTION
The upper limit value for normal fasting glucose (NFG) has been redefined twice over the past 20 years by the American Diabetes Association. In 1997, it was set at 6.1 mmol/L, with values above but below the diabetes threshold defined as impaired fasting glucose (IFG). In 2003, the upper limit value for NFG was lowered from 6.1 to 5.6 mmol/L [1]. Following this revision, people with fasting plasma glucose (FPG) levels of 5.6 to 6.0 mmol/L have been additionally included as having IFG; thereby, identifying more individuals who may be at increased risk of diabetes [2].
It has been suggested; however, that higher FPG levels within the normoglycemic range are independently associated with an increased risk for type 2 diabetes mellitus (T2DM), and the annual incidence of diabetes has been reported to be approximately 0.3% to 0.6% [345]. For example, in a study performed with 13,163 young Israeli men with NFG, the risk for T2DM increased progressively within the normoglycemic range during a mean follow-up period of 5.7 years. In addition, although the absolute risk of diabetes is very low, measurement of either the body mass index (BMI) or triglyceride levels along with FPG levels helped to identify apparently healthy men with NFG who were at increased risk for T2DM [3].
However, it has been suggested that fasting and postchallenge hyperglycemia may be phenotypes with distinct natural histories in the development of T2DM [6]. In addition, impaired glucose tolerance (IGT) is a more common form of prediabetes than isolated IFG in Asians compared to Europeans [78]; thus, not measuring the 2-hour postload glucose (2PG) during an oral glucose tolerance test (OGTT) will therefore underestimate the prevalence of diabetes in Asians [910].
Therefore, the aims of this study were to determine (1) how many individuals with NFG already have abnormal glucose tolerance by OGTT, (2) how frequently individuals who have NFG develop T2DM, and (3) which demographic, lifestyle, clinical, and metabolic variables predict future diabetes diagnosis in Japanese Americans with NFG at baseline.
METHODS
Study subjects
The study received approval from the University of Washington Human Subjects Division and written informed consent was obtained from all subjects. The study population consisted of Japanese American men and women enrolled in the Japanese American Community Diabetes Study, a cohort of second- (Nisei) and third-generation (Sansei) Japanese Americans of 100% Japanese ancestry. A detailed description of the selection and recruitment of the study subjects has been published previously [11]. In brief, study participants were selected as volunteers from a community-wide comprehensive mailing list and telephone directory that included nearly 95% of the Japanese American population in King County, Washington. Among the total of 658 subjects in the original cohort, 126 subjects were excluded for having a history of diabetes at baseline, and then 162 subjects with FPG ≥5.6 mmol/L were further excluded. Finally, a total of 370 subjects (163 men, 207 women) with NFG levels, aged 34 to 75 years, were enrolled in this study. Among these 370 subjects, seven had 2PG levels ≥11.1 mmol/L during the baseline OGTT; however, since we were interested in the subsequent OGTT category of all individuals who would have been diagnosed as normal based solely upon a FPG measurement, they were included in the analysis of the occurrence of a future diabetes diagnosis. Subjects were followed up at 2.5 years (Nisei men only), 5 to 6 years, and 10 to 11 years after the baseline examination.
Clinical and laboratory examination
All evaluations were performed at the General Clinical Research Center, University of Washington. At baseline, a complete physical examination was performed, and personal medical history and lifestyle factors including cigarette smoking, alcohol consumption, and physical activity were determined through a standardized questionnaire. Two categories were used for the classification of smoking status (current smoker and past smoker or never smoker). A previous meta-analysis of prospective observational studies suggested that moderate alcohol consumption (6 to 48 g/day) reduces the risk of T2DM, so we used this criterion to define moderate alcohol consumption [12]. The Paffenbarger physical activity index questionnaire was used to determine the physical activity level (usual kilocalories expended weekly) [13], and regular physical activity was defined as that performed at a more than moderate intensity.
BMI was calculated as the weight in kilograms divided by the square of the height in meters. Waist circumference was measured at the level of the umbilicus. Blood pressure was measured with a mercury sphygmomanometer to the nearest 2 mm Hg with the subject in a recumbent position. Systolic blood pressure was determined by the first perception of sound, and diastolic blood pressure was determined at the disappearance of sounds (fifth-phase Korotkoff). Average blood pressure was calculated from the second and third of three consecutive measurements.
Biochemical measurements were performed on fresh samples at the time of sample collection as reported previously [14]. All blood samples were obtained following an overnight fast of 10 hours. Plasma glucose was measured by the hexokinase method on an autoanalyzer (Department of Laboratory Medicine, University of Washington, Seattle, WA, USA). Plasma insulin was measured by a radioimmunoassay (Immunoassay Core, Diabetes Research Center, University of Washington, Seattle, WA, USA). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the product of the fasting insulin (µIU/mL) and FPG (mmol/L) concentrations, divided by 22.5. The homeostasis model assessment of β-cell function (HOMA-B%) was calculated as (20×fasting insulin)/(FPG–3.5) and was used to represent β-cell function. The insulinogenic index, a marker of early-phase insulin secretion, was calculated as the ratio of the increment in insulin to the increment in glucose above fasting during the first 30 minutes of the OGTT [15]. Lipid and lipoprotein measurements were performed according to modified procedures of the Lipid Research Clinics (Northwest Lipid Research Laboratory, Seattle, WA, USA).
A 75-g OGTT was performed at baseline, 2.5 years (Nisei men only), 5 to 6 years, and 10 to 11 years after enrollment for the determination of diabetes status. In this study, T2DM was defined by the presence of one of the following: (1) fasting glucose level ≥7.0 mmol/L; (2) treatment involving oral hypoglycemic agents or insulin therapy; or (3) 2PG ≥11.1 mmol/L [16]. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or taking antihypertensive medications. The presence of cardiovascular disease was diagnosed by a clinical history of one of the following: (1) coronary artery disease (acute myocardial infarction, angina, coronary artery bypass graft, or coronary angioplasty); (2) cerebrovascular disease (transient ischemic attack, carotid endarterectomy, atherosclerotic stroke, or non-atherosclerotic stroke); (3) peripheral artery occlusive disease (claudication or bypass surgery in lower extremities); or (4) abdominal, thoracic, or other type of aortic aneurysm.
Statistical analyses
Data are expressed as mean±standard deviation for continuous measures or as proportions for categorical variables, except for skewed continuous variables, which are presented as the median (interquartile range). A variance inflation factor >3.0 was used as an indicator of multicollinearity. Multiple logistic regression analysis was used to identify independent associations of clinical and biochemical variables with future diabetes risk. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for the independent variables included in the logistic models, with a 1-SD increment used for OR calculations for continuous measurements. The presence of interaction was assessed in multivariate models through evaluation of the significance of first-order interaction terms. The presence of nonlinearity was assessed via insertion of the quadratic transformation of FPG into models that contained the linear term. All statistical analyses were performed with PASW version 18.0 (SPSS, Chicago, IL, USA). A P<0.05 was considered significant.
RESULTS
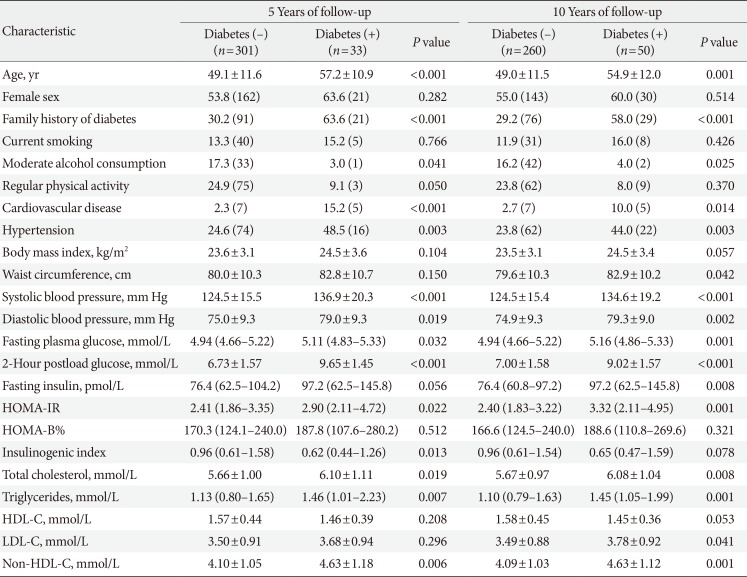
Tables 1 and 2 depict the baseline characteristics of the study subjects. The mean age was 50.0 years and 55.9% of the subjects were women. Approximately one-third of the study subjects had a family history of diabetes. In terms of personal history, 15.7% and 12.4% of the subjects were moderate alcohol drinkers and current smokers, respectively, and 24.1% of the subjects performed regular physical activity at a more than moderate intensity. At baseline, the mean FPG and 2PG levels were 4.9 and 7.0 mmol/L, respectively.
Over 5 years of follow-up, the status of T2DM could be assessed in 334 subjects, among whom a total of 33 met criteria for diabetes, leading to a cumulative rate of 9.9%. Over 10 years of follow-up, the cumulative rate of diabetes was 16.1% (50/310). Of the subjects diagnosed with diabetes over 5 years, two subjects were excluded from the classification of diabetes subtypes because they were already taking glucose-lowering medication at the time of the OGTT. Of the remaining 31 subjects with diabetes diagnosed during 5 years of follow-up, 17 subjects (54.8%) still had NFG levels with an elevated 2PG (≥11.1 mmol/L) at the time of diabetes diagnosis, while 10 subjects (32.3%) had IFG levels, and only four subjects (12.9%) were diagnosed with diabetes based on having FPG levels ≥7.0 mmol/L. Similarly, of the 48 subjects with diabetes diagnosed during 10 years of follow-up, 19 subjects (39.6%) had NFG at the time of diabetes diagnosis, 23 subjects (47.9%) had IFG levels, and only six subjects (12.5%) had FPG levels ≥7.0 mmol/L during 10 years of follow-up (Table 3).

Number of diagnosed cases of diabetes by fasting plasma glucose and 2-hour postload glucose concentrations at 5 and 10 years follow-up assessments
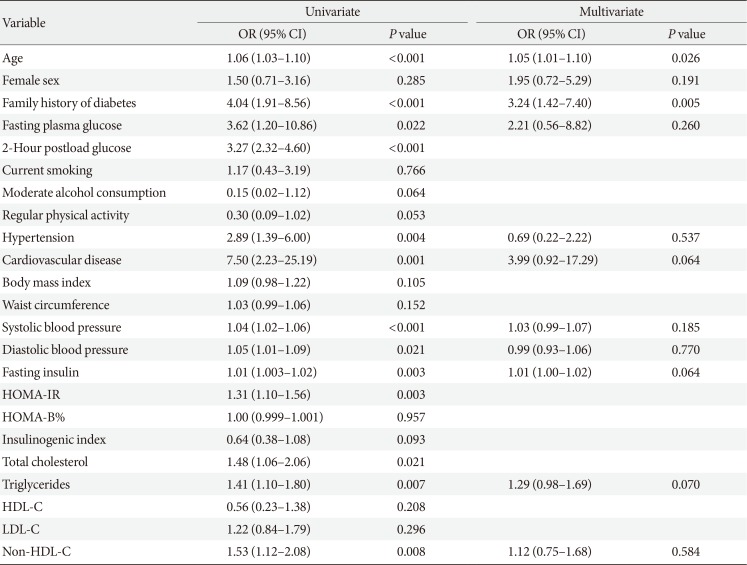
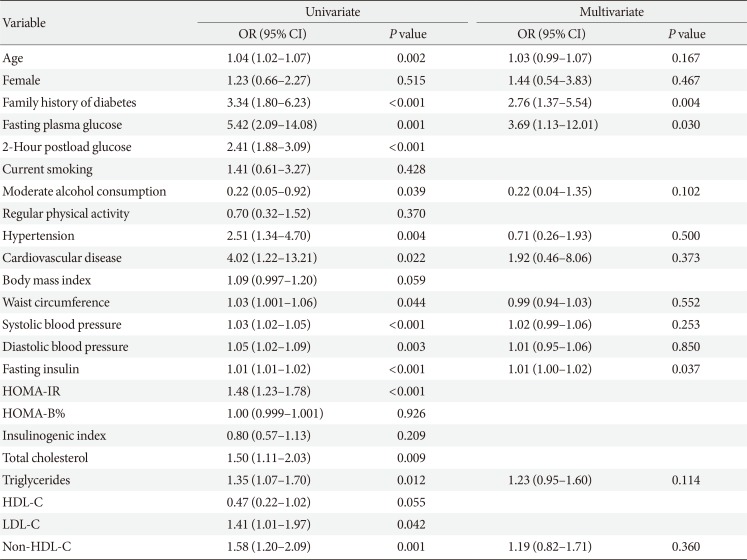
To determine which variables were independently associated with diabetes diagnosis, multiple logistic regression models were used by considering all the variables shown in the univariate models with the following exceptions. Total cholesterol, low density lipoprotein cholesterol, and non-high density lipoprotein cholesterol (non-HDL-C) were highly correlated with each other, but only non-HDL-C was included in the model due to its smaller P value in univariate analysis. Similarly, the inclusion of both HOMA-IR and fasting insulin in a model causes multicollinearity; thus, we selected fasting insulin. In univariate analysis, the 2PG level was the strongest predictor for the future diagnosis of T2DM. However, we did not include 2PG levels in the multivariate analysis because the OGTT is not performed routinely in real clinical practice. Age and family history of diabetes were independent predictors for diabetes diagnosis during 5 years of follow-up; however, FPG level was not (Table 4). During 10 years of follow-up, a family history of diabetes and fasting glucose and insulin levels were independently associated with increased risk of diabetes (Table 5).

Risk of diabetes diagnosis during 5 years of follow-up among participants with normal fasting glucose at baseline (<5.6 mmol/L)
DISCUSSION
In the current prospective study performed on Japanese American men and women with NFG at baseline, 16.1% of subjects were diagnosed with T2DM during 10 years of follow-up. However, a substantial number of these subjects still showed NFG levels at the time of diagnosis of diabetes (54.8% during 5 years of follow-up and 39.6% during 10 years of follow-up). On the other hand, only 12.5% of the diabetes cases were diagnosed based on FPG levels ≥7.0 mmol/L. Age, family history of diabetes, and fasting insulin level were independently associated with future diagnosis of T2DM during 10 years of follow-up, but an independent association between FPG levels and diabetes diagnosis risk was not evident during the first 5 years of follow-up.
Previous studies performed in subjects with NFG have consistently demonstrated that higher FPG levels are associated with future diabetes risk even within the normoglycemic range, although the absolute risk of diabetes was relatively low, with an annual incidence of approximately 0.3% to 0.6% [345]. However, our results contradicted those of previous studies by demonstrating that FPG levels within the NFG range did not independently predict future diabetes diagnosis during a relative short-term follow-up period of up to 5 years, but were a significant predictor for diabetes upon long-term follow-up (10 years). We do not know the reason for this discrepancy, but may offer the following explanations. First, T2DM in Asians has been suggested to differ from that in Caucasians [17]. T2DM is characterized by both deterioration of insulin sensitivity and β-cell dysfunction [18]. In many non-Asian individuals who have IGT, there is hyperinsulinemia to compensate for insulin resistance, but eventually insulin secretion becomes lower during the development of overt diabetes. Studies have shown that an inadequate insulin secretory capacity to compensate for insulin resistance is a key factor in the development of glucose intolerance in the Asians [19]. In a study of native Japanese, no compensatory hyperinsulinemia was observed, even in those with IGT status, and insulin levels declined rapidly after the development of overt diabetes [20]. Based upon these observations, we have postulated that Japanese Americans are individuals with diminished β-cell reserve and that diabetes develops when insulin resistance is superimposed [21]. In addition, it was suggested that postchallenge hyperglycemia is more common in Asians than in Caucasians [2223], and isolated IGT or an isolated high 2PG level of ≥11.1 mmol/L is closely related to defective early-phase insulin secretion, which is commonly seen in Asians, and is related to a lesser extent to insulin resistance [24]. Second, in previous studies [345] on this subject, non-Asian populations were evaluated, OGTTs were not performed, and only FPG levels and/or medical records were used to diagnose incident diabetes. Therefore, incident diabetes cases with high 2PG levels of ≥11.1 mmol/L could not be identified, so the incidence of diabetes was undoubtedly underestimated [345]. In support of our finding, the annual rate of diabetes was 1.6% during the 10 years of follow-up, while previous studies reported an annual incidence of approximately 0.3% to 0.6%.
Our study also has some limitations. First, the sample size was smaller than those of previous studies. Second, glycated hemoglobin levels were not available for the diagnosis of diabetes. Glycated hemoglobin level may be an early indicator of diabetes, especially for patients in whom an NFG level is available while OGTT is not, and thus the frequency of diabetes diagnosis might have been underestimated. Lastly, although it was an acceptably low level, 16.2% of subjects were lost to follow-up over the 10 years of the study period. This study also has several clinical implications. In clinical practice, physicians should not view NFG as a benign condition with low risk for future diabetes diagnosis and thus be complacent regarding patients who have NFG levels. This may be especially true for Asian patients. Moreover, FPG levels may have limited clinical relevance in the assessment of the risk of future diabetes diagnosis among subjects with NFG, at least within a short-term follow-up period. Instead, physicians should pay attention to age, degree of insulin resistance, and family history of diabetes when predicting future diabetes diagnosis in subjects with NFG levels. In addition, the value of the OGTT should not be underestimated as a way to detect diabetes earlier and thus provide an opportunity to institute measures to prevent diabetic complications in subjects with NFG.
In summary, our results suggest that a substantial proportion of Japanese American subjects with NFG progress to T2DM diagnosis over 10 years of follow-up. However, the actual level of FPG does not predict future diabetes diagnosis within a short-term follow-up period of 5 years. On the other hand, age, family history of diabetes, and fasting insulin level may be predictors of future diagnosis in subjects with NFG.
ACKNOWLEDGMENTS
We are grateful to the King County Japanese-American community for support and cooperation. VA Puget Sound Health Care System provided support for Drs. Boyko and Kahn's involvement in this research.
This work was supported by facilities and services provided by the Diabetes Research Center (DK-17047), Clinical Nutrition Research Unit (DK-35816), and the General Clinical Research Center (RR-00037) at the University of Washington. Funding was through National Institutes of Health grants DK-31170, HL-49293 and DK-017047. The funding entities had no role in the conduct of this study or interpretation of its results.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.