Determinants of Long-Term Durable Glycemic Control in New-Onset Type 2 Diabetes Mellitus
Article information
Abstract
Background
Long-term durable glycemic control is a difficult goal in the management of type 2 diabetes mellitus (T2DM). We evaluated the factors associated with durable glycemic control in a real clinical setting.
Methods
We retrospectively reviewed the medical records of 194 new-onset, drug-naïve patients with T2DM who were diagnosed between January 2011 and March 2013, and were followed up for >2 years. Glycemic durability was defined as the maintenance of optimal glycemic control (glycosylated hemoglobin [HbA1c] <7.0%) for 2 years without substitution or adding other glucose-lowering agents. Clinical factors and glycemic markers associated with glycemic durability were compared between two groups: a durability group and a non-durability group.
Results
Patients in the durability group had a higher baseline body mass index (26.1 kg/m2 vs. 24.9 kg/m2) and lower HbA1c (8.6% vs. 9.7%) than the non-durability group. The initial choice of glucose-lowering agents was similar in both groups, except for insulin and sulfonylureas, which were more frequently prescribed in the non-durability group. In multiple logistic regression analyses, higher levels of education, physical activity, and homeostasis model assessment of β-cell function (HOMA-β) were associated with glycemic durability. Notably, lower HbA1c (<7.0%) at baseline and first follow-up were significantly associated with glycemic durability (adjusted odds ratio [OR], 7.48; 95% confidence interval [CI], 2.51 to 22.3) (adjusted OR, 9.27; 95% CI, 1.62 to 53.1, respectively), after adjusting for confounding variables including the types of glucose-lowering agents.
Conclusion
Early achievement of HbA1c level within the glycemic target was a determinant of long-term glycemic durability in new-onset T2DM, as were higher levels of education, physical activity, and HOMA-β.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is considered a progressive metabolic disorder caused by two major pathophysiological defects: insulin resistance and β-cell dysfunction [1]. Large-scale, randomized controlled trials have proved that intensive glucose control decreases the risk of microvascular complications [23], and even the risks of cardiovascular events or mortality, as shown in long-term follow-up studies [45]. However, mainly because of progressive decline in β-cell function and clinical inertia, long-term durable glycemic control remains a difficult goal to attain in the management of T2DM [678].
During past decades, the development of novel glucose-lowering agents and strategies has demonstrated more positive impact on glycemic durability than previously observed. A report from the Swedish National Diabetes Register demonstrated that, in real clinical practice, metformin resulted in superior glycemic durability than sulfonylureas or meglitinides [9]. Similarly, in the A Diabetes Outcome Progression Trial (ADOPT) study, rosiglitazone proved to be a better choice in achieving glycemic durability than either sulfonylureas or metformin [6]. Among recently developed novel agents, some, but not all, sodium glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists have shown more tolerable glycemic control than metformin and sulfonylureas [1011]. Results of several trials have suggested that an early intensive insulin therapy can even lead to a complete disease resolution in patients with new-onset T2DM and help attain glycemic durability [1213]. However, these results are based on tightly regulated clinical trials comprising highly selective patients, which limit their applicability in real clinical practice. In fact, other studies have noted patient-related factors or characteristics other than the use of specific glucose-lowering agents, such as tolerability, patient acceptance, and costs, that can affect durable glycemic control [1415]. On the other hand, current treatment guidelines and recommendations have reached a consensus that tight glycemic control is more beneficial to patients with short-duration diabetes or those who are free of related complications [16].
Nonetheless, despite improved understanding of inter-patient differences affecting responses to therapy, little information is available on factors that are strongly associated with durable glycemic control in real clinical settings. Therefore, we aimed to assess the major determinants of durable glycemic control in new-onset T2DM using 2 years of observational data.
METHODS
Study population
We retrospectively reviewed clinical data of patients who were diagnosed with T2DM between January 2011 and March 2013 at the Korea University Anam Hospital. Among the 314 new-onset T2DM patients, 194 patients were followed up for at least 2 years, with the last follow-ups ended in March 2015, and were included in this study. All diagnoses were made in accordance with the American Diabetes Association (ADA) criteria [17]. All included subjects were aged ≥18 years and had not taken any glucose-lowering agents before their diagnosis.
The study subjects were classified into the durability group or the non-durability group based on their glycemic durability. Glycemic durability was defined as the maintenance of optimal glycemic control (glycosylated hemoglobin [HbA1c] <7.0%) over 6 months after diagnosis for 2 years, without substitution or adding other glucose-lowering agents. Subjects who did not maintain their HbA1c values at the desired level were included in the non-durability group.
This study was approved by the Institutional Review Board of Korea University Hospital (IRB number: ED16182).
Clinical and laboratory variables
A structured interview was conducted at the patients' first visit, and demographic characteristics and medical histories were recorded by two trained diabetes education nurses. Anthropometric parameters were also measured at this visit. Demographic information included age, sex, residential area, lifestyle, occupation, and education level. Medical information included any history of hypertension, dyslipidemia, cardiovascular disease, malignancy, and the use of medications for any of these conditions.
Patients' histories of smoking, alcohol consumption, physical activity, and education level were also recorded. For statistical analyses, these demographic data were stratified further into two or three groups as follows: smoking (never smokers, former smokers, or current smokers), alcohol consumption (yes or no), educational level (lower than middle school, high school, or higher than college), and physical activity (none, ≤twice per week, or ≥three times per week).
Anthropometric data including height, body weight, and waist circumference were measured by nurses. The initial laboratory tests included evaluations of fasting plasma glucose (mg/dL), 2-hour postprandial glucose (mg/dL), HbA1c (%), basal C-peptide (ng/mL), basal insulin (µIU/mL), serum creatinine (mg/dL), estimated glomerular filtration rate (mL/min/1.73 m2), and lipid profiles (total cholesterol, high density lipoprotein cholesterol, and triglycerides). The following equations were used to calculate insulin resistance and β-cell function: homeostasis model assessment of insulin resistance (HOMA-IR)=fasting insulin (µIU/mL)×fasting plasma glucose (mmol/L)/22.5; HOMA of β-cell function (HOMA-β)=20×fasting plasma insulin (µIU/mL)/[fasting plasma glucose (mmol/L)–3.5].
Follow-up measurements
Patients were followed up every 2 to 3 months over the 2-year period, and their HbA1c levels, fasting plasma glucose, systolic and diastolic blood pressure, and body weight were measured at each follow-up visit. The enrolled patients were educated on structured lifestyle modifications, including diet control and regular exercise. Physicians generally followed the current ADA/European Association for the Study of Diabetes (EASD) guidelines for the management of T2DM [16]; however, they were not obliged to select specific glucose-lowering agents. The determination of therapeutic options, including the selection of a specific class of glucose-lowering agents or regimens, or recommending observation without medications, was entirely at the physicians' discretion.
Statistical analyses
Patients were divided into two groups according to a prespecified definition of glycemic durability. A repeated measures logistic model for the longitudinal analysis of HbA1c over time was performed to compare mean HbA1c trajectories between the groups.
At baseline, the mean values of various laboratory findings were compared between the durability and the non-durability groups using a paired t-test and a Mann-Whitney U test. Categorical variables were compared using univariate analysis. Results were presented as numbers/percentage or mean±standard deviation values. Multiple logistic regression analysis was performed to investigate the clinical and laboratory factors associated with glycemic durability. The factors used in the multivariate analysis were adjusted for age, sex, body mass index (BMI), medication use, and the baseline HbA1c. A P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
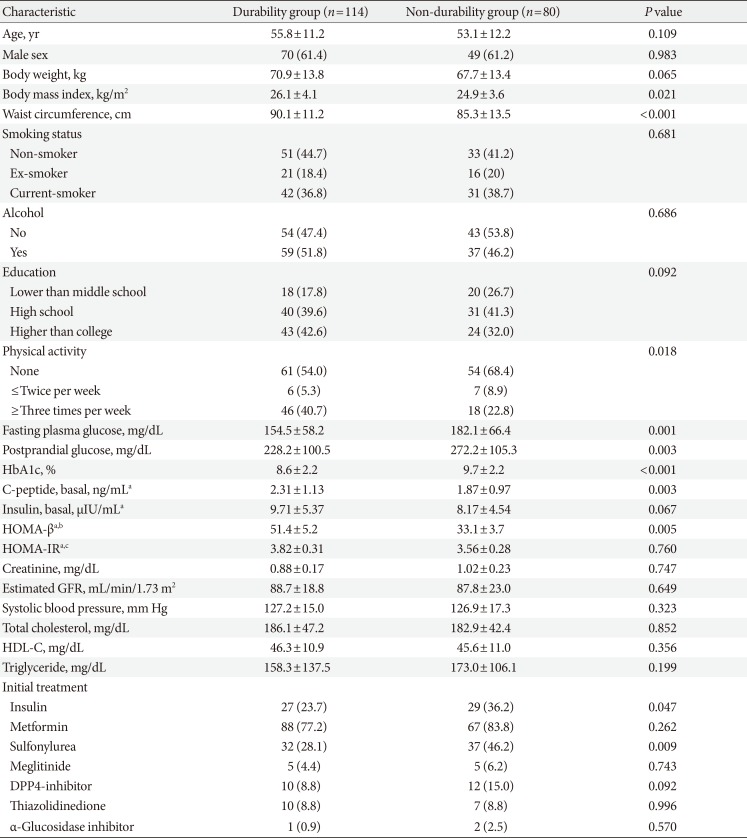
Subjects were divided into the durability group (n=114) and the non-durability group (n=80). Fig. 1 and Supplementary Fig. 1 shows the changes in the mean HbA1c levels in both groups during the 2-year follow-up period. The baseline HbA1c level was significantly higher in the non-durability group than in the durability group (mean, 9.7% vs. 8.6%). Three months after diagnosis, the difference in HbA1c between groups was 0.6%, however, the gap increased during folllow-up, producing ~1.3% difference in the mean HbA1c level 2 years after diagnosis (7.5% vs. 6.2%, P<0.01).
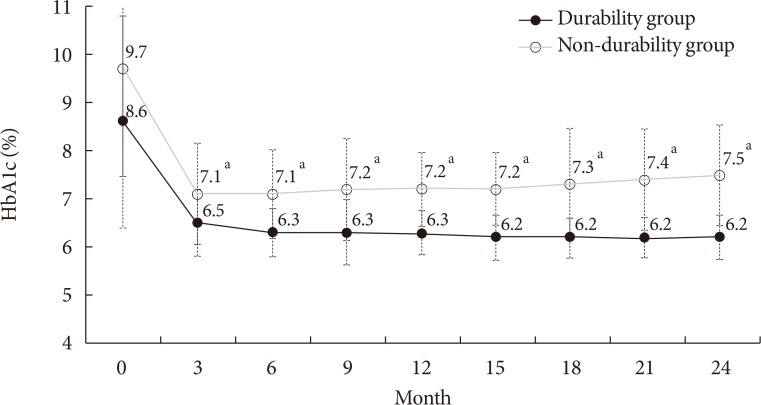
Changes in the mean glycosylated hemoglobin (HbA1c) levels during the 2-year follow-up period. aDifference between groups P<0.01.
The baseline patient characteristics are described in Table 1. Subjects in the durability group had a higher BMI (26.1 kg/m2 vs. 24.9 kg/m2, P=0.021), and waist circumference (90.1 cm vs. 85.3 cm, P<0.001) than those in the non-durability group. Although the lifestyle factors were comparable between the groups, a comparison of the level of physical activity indicated that subjects in the durability group were more physically active. The durability group also had higher HOMA-β levels than the non-durability group, although the HOMA-IR levels were comparable between the two groups.
Metformin was the most frequently prescribed initial glucose-lowering agent in both groups, followed by sulfonylureas. The difference in treatment regimen between the two groups was the use of insulin and sulfonylureas, which were used more frequently in the non-durability group than in the durability group (Table 1). The usage frequencies of each prescription glucose-lowering agent during the entire follow-up period are displayed in Supplementary Table 1.
Clinical and laboratory factors related to glycemic durability
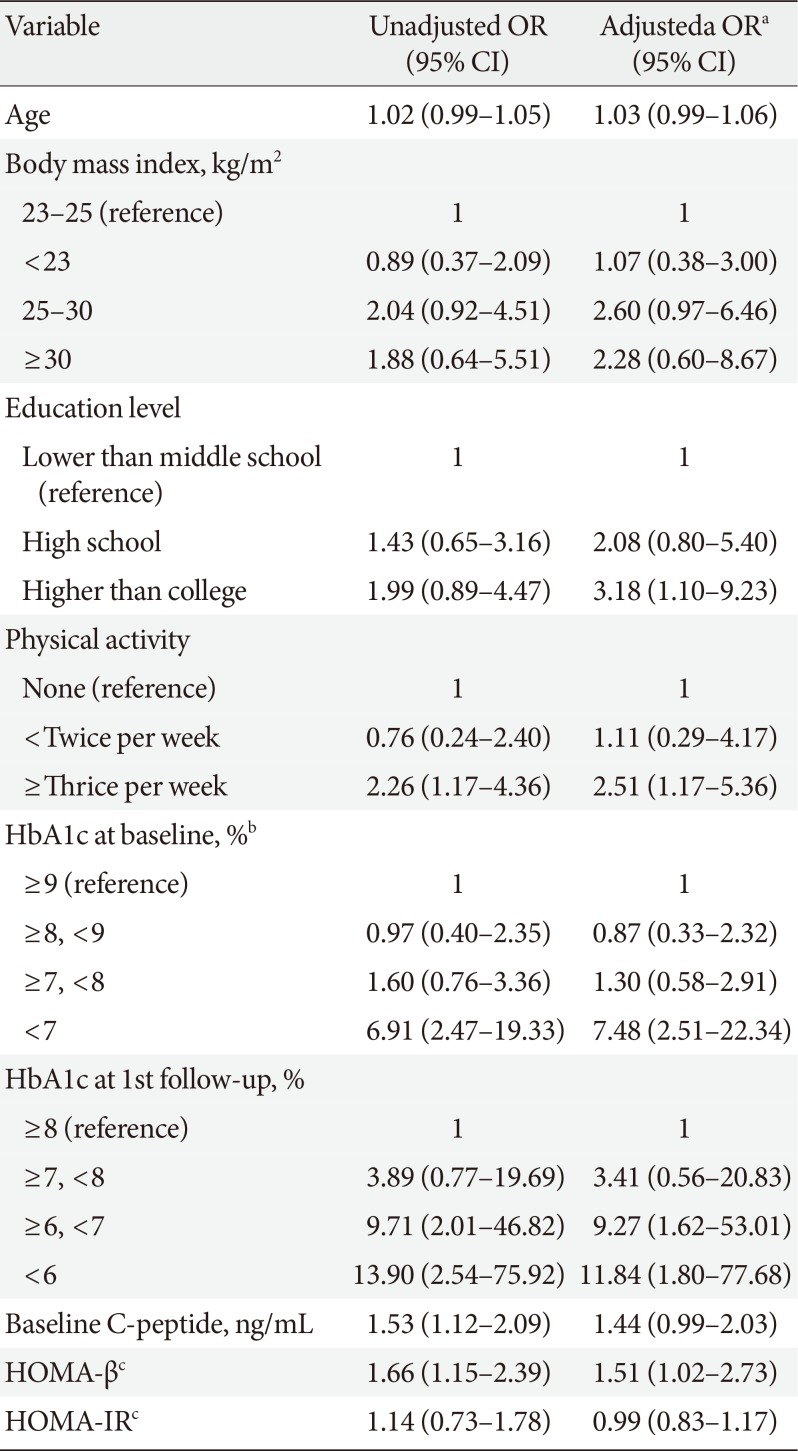
We selected candidate variables associated with glycemic durability based on the differences between the two study groups at baseline. Table 2 shows the variables and their glycemic durability predicting values, which we evaluated using multiple logistic regression analyses.
The result of an adjusted multiple regression model indicated that higher levels of education, physical activity, and baseline HOMA-β were significantly associated with an increased likelihood of glycemic durability than were lower values of these variables. However, age, BMI, and HOMA-IR did not show a significant effect on glycemic durability.
The HbA1c levels at both the baseline and the first follow-up visit were significantly associated with glycemic durability. Subjects with baseline HbA1c values <7.0% tended to maintain optimal glycemic control during the 2-year follow-up period, with an adjusted odds ratio (OR) of 7.48 (95% confidence interval, 2.51 to 22.3), compared to subjects with baseline HbA1c >9.0%. Further, in an adjusted regression model, lower HbA1c levels at the first follow-up visit (<6.0%, and between 6.0% and 7.0%) demonstrated significantly higher ORs for glycemic durability compared to a higher HbA1c level at this visit (OR, 11.84 and 9.27, respectively).
HbA1c level at early treatment phase as a determinant of long-term durable glycemic control
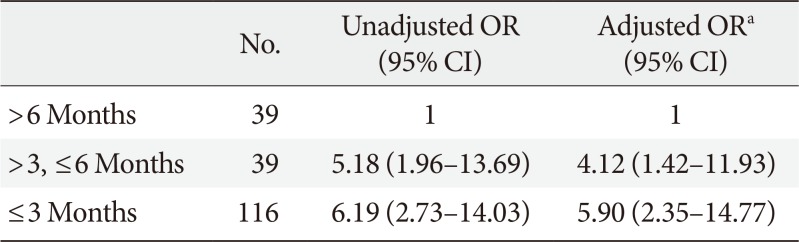
Based on the results of the multiple logistic regression analyses, we further analyzed the association between the HbA1c level at the early treatment phase following diagnosis and glycemic durability. As illustrated in Fig. 2, 86.8% of the patients with baseline HbA1c <7.0% maintained durable glycemic control during the 2-year follow-up period. However, only 51.1% of the patients with baseline HbA1c >9.0% were likely to maintain durable glycemic control. This pattern was more apparent for HbA1c levels noted at the first follow-up visit, which commonly measured 2 to 3 months after diagnosis (Fig. 3). For example, patients with HbA1c <6.0% at the first follow-up visit were 4 times more likely to maintain durable glycemic control than those with HbA1c ≥8.0% (73.5% vs. 16.7%, P<0.001). Table 3 shows the association between the duration required to reach the target HbA1c value (<7.0%) and glycemic durability. Compared to patients who reached the target HbA1c value 6 months after diagnosis, those who reached the target value in <3 months were ~6 times more likely to maintain durable glycemic control during the 2-year period.
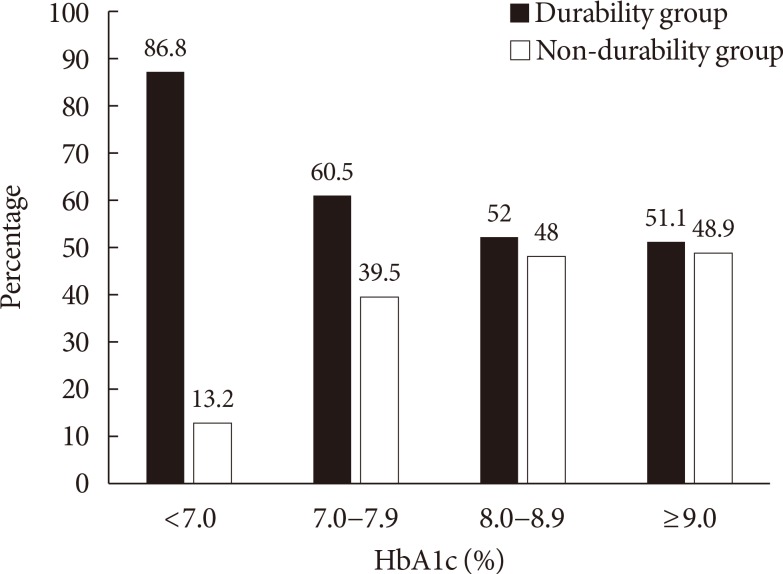
The frequency of glycemic durability and non-durability according to the baseline glycosylated hemoglobin (HbA1c) levels (P<0.005).
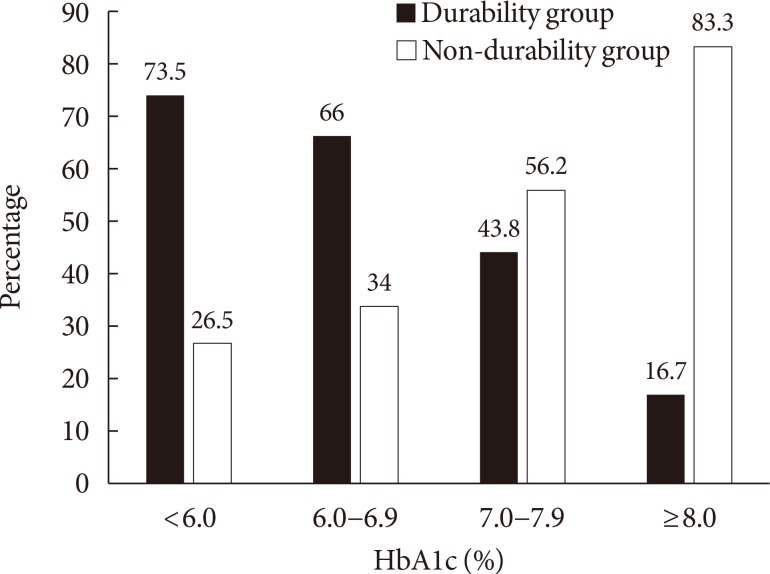
The frequency of glycemic durability and non-durability according to the glycosylated hemoglobin (HbA1c) level at the first follow-up visit (P<0.001).
DISCUSSION
This study showed that some clinical factors contribute to the maintenance of durable glycemic control in new-onset T2DM. HbA1c levels at the time of diagnosis, and at the first follow-up visit were important determinants of glycemic durability. In addition, higher levels of education, physical activity, and HOMA-β were associated with durable glycemic control during a 2-year follow-up period.
HbA1c is the most widely used marker of glycemic control, and reflects overall glycemic exposure for the previous 2 to 3 months [18]. A higher baseline HbA1c level in the non-durability group suggests long-term exposure to hyperglycemia before the diagnosis. Therefore, we assume that a higher glucotoxicity in the non-durability group may have impaired both insulin secretion and activity [19]; thereby, lowering the possibility of maintaining favorable glycemic control in these patients. A significantly lower HOMA-β level in the non-durability group further supported this explanation.
Interestingly, we observed that a lower HbA1c level at the first follow-up visit was a strong indicator of glycemic durability. The significance of this association was maintained even after adjusting for baseline HbA1c levels and the type of glucose-lowering agents. Two possible explanations, with divergent mechanisms, should be considered for this observation. First, a rapid correction of hyperglycemia may be responsible for the glycemic durability in patients with new-onset T2DM. In this context, several studies have reported that early and intensive therapy produced favorable outcomes, i.e., recovery and maintenance of β-cell function, in drug-naïve patients at the onset of T2DM. In a meta-analysis to assess the efficacy of intensive insulin therapy, approximately 42.1% of patients experienced drug-free remission of diabetes during a 2-year period with only 2 to 3 weeks of intensive insulin therapy at the onset of diabetes [12]. Intensive insulin therapy provides rest to the β-cells by decreasing the hepatic glucose production, and by reducing glucotoxicity and lipotoxicity [13]. Our results, although retrospectively analyzed, expanded on the concept that a rapid correction of hyperglycemia, with or without insulin, may aid in the long-term maintenance of hyperglycemia within the target range. Second, patients in the durability group may be “good responders” to the glucose-lowering treatments. When the analysis was done among patients who attained HbA1c <7.0% at 6 months, we also observed similar findings (Supplementary Tables 2 and 3). This may partly be due to relatively short-term exposure to hyperglycemia or due to their having genetically healthier β-cells than patients in the non-durability group. However, individual differences that may affect therapeutic responses remain unclear. More targeted studies will be required to recognize the degree to which alterations in specific aspects of glucose homeostasis will differ between individuals, and how an individual will respond to a specific medication in a real clinical setting [20].
Our results further demonstrated that T2DM patients' educational and physical activity levels were associated with long-term glycemic durability. Several studies have identified patient-specific factors that may influence the durability of glycemic responses, emphasizing the need to personalize therapies based on patient characteristics. In a Japanese study, the avoidance of weight gain contributed to the maintenance of better glycemic control [21]. In another study by Mamza et al. [7], female gender, smoking, longer duration of diabetes, and a higher baseline HbA1c level were associated with a poor durability of therapeutic efficacy.
Patients' education level and physical activity are factors that need to be considered as seriously as socioeconomic status and patient attitude [2223]. Patients with low socioeconomic status are more exposed to unhealthy lifestyles and adverse environmental factors such as obesity, physical inactivity, and smoking, as they are less likely to avail themselves of routine health checkups and health education compared to those with high socioeconomic status [24]. The European Diabetes (EURODIAB) Prospective Complications study reported that a healthy lifestyle was more prevalent among better-educated men and women with diabetes [22]. A previous study noted that exercise training, which promotes a higher physical activity level, could directly reduce HbA1c level in addition to reducing the risk of developing diabetic complications [25]. Another report identified patient adherence as an important determinant of response to treatment for T2DM [26]. Therefore, patients' attitude to treatment and their educational level should also be considered as predictive indicators of response to treatment and long-term glycemic control.
There are several limitations to this study. First, some factors varied because they were controlled by clinicians. Physicians in this study generally followed the current ADA/EASD guideline that recommends the following: HbA1c <7.0% as the glycemic target, metformin as the initial glucose-lowering agent, and lifestyle modifications. However, the glycemic targets were set individually, and the choice of glucose-lowering agent was solely dependent on the clinician's discretion. This is an inevitable limitation of a retrospective study design. Second, because we included only those patients who attended follow-up visits for at least 2 years, all our study subjects demonstrated good adherence to treatment, which may limit the applicability of our results to a broader patient spectrum.
In conclusion, our findings will help illuminate inter-individual differences in responses to therapy by providing evidence for various factors that can affect the durability of glycemic control. Above all, an early treatment response in terms of glycemic control was an important predictor of continuing durable glycemic control. Further well-controlled trials will be needed to confirm this hypothesis.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Proportion of glucose-lowering agents prescribed during the 2-year follow-up period
Supplementary Table 2
Baseline characteristics of patients who initially attained HbA1c goal (HbA1c <7%) at 6 months
Supplementary Table 3
Factors associated with glycemic durability among patients who initially attained HbA1c goal (HbA1c <7%) at 6 months
Supplementary Fig. 1
Mean change in glycosylated hemoglobin (HbA1c) from baseline to 24 months.