Response: GDF15 Is a Novel Biomarker for Impaired Fasting Glucose (Diabetes Metab J 2014;38:472-9)
Article information
I'd like to express my deep appreciation for your interest and comments on our article entitled "GDF15 Is a Novel Biomarker for Impaired Fasting Glucose," which was published in Diabetes and Metabolism Journal [1].
In our study, we showed that the serum level of growth differentiation factor-15 (GDF15), which belongs to transforming growth factor-β (TGF-β) superfamily, increased in patients with prediabetes and type 2 diabetes mellitus (T2DM) compared with nondiabetic patients. Thus, GDF15 may be a novel biomarker for detecting impaired fasting glucose (IFG) in nondiabetic patients.
Our data are contradictory to the Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) trial, which suggested that serum level of GDF15 was not different between nondiabetic control and IFG [2]. As I mentioned in discussion session, the level of GDF15 in nondiabetic control was too high compared with other study's normal control population. Eligible individuals of XENDOS trial were composed of obese people with BMI ≥ 30 kg/m2 with a mean of 37.6 kg/m2. Furthermore, although there are controversial opinions, people with impaired glucose tolerance (IGT) are more insulin-resistant than those of IFG [3]. In our study, there were too few patients with IGT to compare the level of GDF15. Further study is required to validate the effect of GDF15 with patients with isolated IFG, isolated IGT, and both IFG and IGT.
Following your comment, we reanalyzed the factors that affect GDF15 using multivariate regression model. The serum level of GDF15 was affected by the change in fasting glucose, homeostasis model assessment-insulin resistance, and serum triglyceride (Table 1). These data showed that GDF15 is associated with insulin resistance, accompanying hypertriglyceridemia.
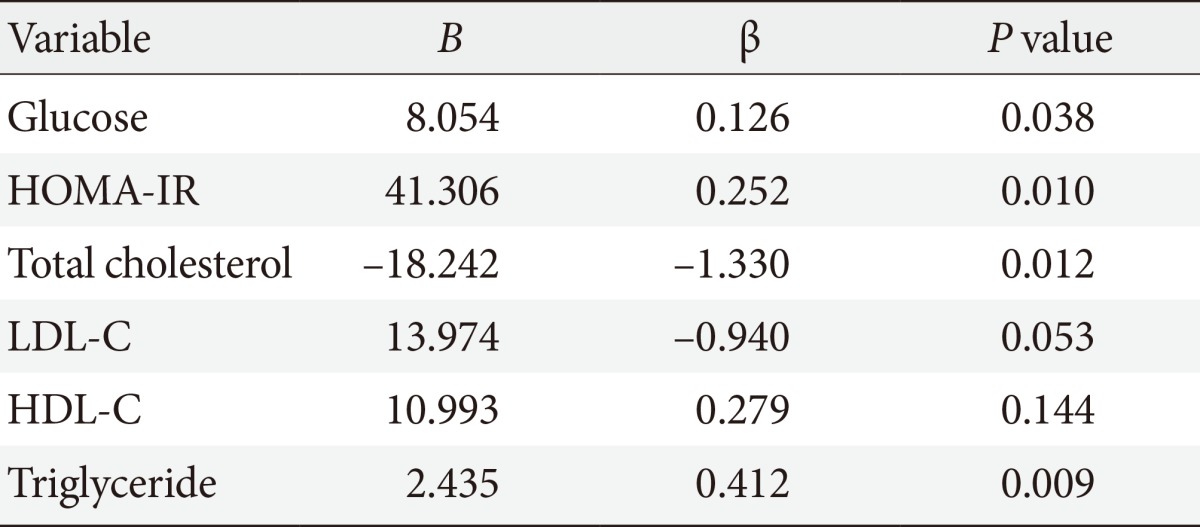
Association of growth differentiation factor-15 with glucose, HOMA-IR, lipid profiles by multivariate regression analysis
In human, GDF15 is prominently expressed in placenta and macrophages and to a lower extent in kidneys, pancreas, and prostate. However, the expression of GDF15 is highly increased by cytokines such as interleukine-1 and TGF-β [4]. In many clinical researches, the correlation of GDF15 with pathologic conditions was analyzed by the measurements of serum concentration, similarly to our study. The main source of GDF15 in metabolic diseases is not elucidated yet. Obesity is also known to be associated with elevated GDF15. However, GDF15 mRNA expression in adipose tissue did not show much difference between lean and nondiabetic obese patients [5]. According to recent research articles, it is proposed that elevated GDF15 level is closely associated with mitochondrial disease. Myopathy with mitochondrial depletion harboring mutation in the gene encoding thymidine kinase 2 (TK2) presented dramatic elevation of GDF15 (mean 3,562 pg/mL) in skeletal muscle biopsy compared with non mitochondrial muscle dystrophy (mean 320 pg/mL) [6]. According to Fujita et al. [7], serum GDF15 level was increased in patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). According to an in vitro study, secreted concentration of GDF15 was elevated in the culture of cybrid cells harboring a MELAS-causing mitochondrial gene mutation (m.3243 A>G) than wild type gene [7]. According to the articles above, GDF15 is closely correlated with mitochondrial diseases.
In the pathophysiology of T2DM, the aggravation of insulin resistance has a major contribution to the development of T2DM. Although the pathogenesis of insulin resistance is multifactorial, mitochondrial dysfunction of insulin-dependent organs is suggested as one of the major causes [8]. In the early phase of insulin resistance, mitochondrial dysfunction of skeletal muscle is prevalent in vivo, although it's not definite that mitochondrial dysfunction is the cause or result [9]. Insulin resistance and mitochondrial dysfunction may be in advance to the development of T2DM. Thus, measurement of GDF15 may be helpful for an early detection of patients with mitochondrial dysfunction at a risk of developing T2DM.
Measurement of hemoglobin A1c and performing the 75 g oral glucose tolerance test are definite gold standard tools to diagnose prediabetes and T2DM. Although there are not enough data about GDF15, it may be able to act as a novel biomarker for an early detection of prediabetes and T2DM as a representative considering the pathogenesis of insulin resistance and mitochondrial dysfunction. According to editorial of Hur [10], we will proceed to the prospective research to investigate the role of GDF15 in patients with prediabetes or T2DM. We would like to thank Dr. Koo for the interest in our study and for the considerate comments and Dr. Hur for the thoughtful and effective editorial.
Notes
No potential conflict of interest relevant to this article was reported.