Enhancing Patient Outcomes: Prioritizing SGLT2is and GLP-1RAs in Diabetes with CVD
Article information
Recent worldwide diabetes treatment guidelines recommend prioritizing the prescription of sodium-glucose cotransporter 2 inhibitors (SGLT2is) or glucagon-like peptide 1 receptor agonists (GLP-1RAs) for diabetic people with established cardiovascular diseases (CVDs) because they have been proven to suppress CVD occurrence [1,2]. Considering this, Yang et al. [3] conducted a study to verify whether prescriptions were in accordance with the guidelines by investigating the extent to which SGLT2is and GLP-1RAs were prescribed to people with diabetes hospitalized for CVD. Although the study involved 2,050 participants with a relatively short follow-up period of 9.7 months, it included 13 secondary and tertiary hospitals. In Korea, patients with severe CVD are mostly admitted to secondary or tertiary hospitals upon first diagnosis, and the patients involved in this study represent Korean patients with diabetes and established CVD.
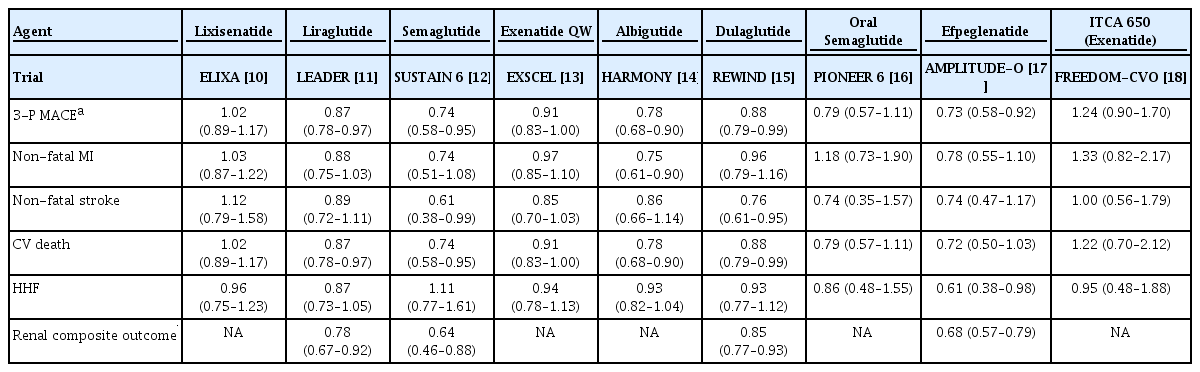
The results of the study can be summarized as follows: SGLT2is were prescribed to 25.7% of all patients, whereas GLP-1RAs were prescribed to only 1.8% of patients. When classified by disease, SGLT2i were prescribed to 31.3% of patients with ischemic heart disease (IHD) and GLP-1RAs to 1.8%. For heart failure (HF), 26.5% were prescribed SGLT2is and 1.5% were prescribed GLP-1RAs [3]. Most diabetes treatment guidelines recommend prioritizing the prescription of SGLT2is or GLP-1RAs for people with type 2 diabetes mellitus with established CVD because cardiovascular outcome trials (CVOTs) have demonstrated that SGLT2is or GLP-1RAs significantly suppress CVDs occurrence in people with type 2 diabetes mellitus with established CVD. CVOTs are clinical trials conducted to prove the cardiovascular (CV) safety of diabetes medications, involving randomized controlled trials with a primary endpoint of 3-point major adverse cardiovascular outcomes (3-P MACE), including myocardial infarction (MI), stroke, and CV death. The initial aim was to demonstrate that the new diabetes drugs do not increase the occurrence of 3-P MACE compared with a placebo. However, the actual findings indicated that several SGLT2is or GLP-1RAs significantly reduced the occurrence of 3-P MACE compared with the placebo. Although not a primary endpoint, they markedly decreased the rate of hospitalization for heart failure (HHF) and renal composite outcomes (Tables 1 and 2) [4-18].
When examining the results of CVOTs for SGLT2is in detail, the indicator of atherosclerotic cardiovascular disease (ASCVD), 3-P MACE, showed a significant reduction in four out of six SGLT2is CVOTs. However, the individual components of the 3-P MACE, such as nonfatal MI and stroke, did not show significant changes in most CVOTs. MI and stroke were significantly reduced only in the Effect of Sotagliflozin on Cardiovascular and Renal Events in Participants With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) trial; however, comparing the SCORED trial directly with other CVOTs is challenging because its primary endpoint was the total number of MACE, whereas the primary endpoint in other CVOTs was the first occurrence of MACE [9]. Additionally, in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial, a representative SGLT2is CVOT, one can see the separation of the cumulative curves for 3-P MACE and CV death over time, beginning early in the trial. This separation occurs much earlier than in clinical trials for statins, which inhibit atherosclerosis, suggesting that the CV protective effects of SGLT2is are not due to atherosclerosis inhibition, but rather to a hemodynamic mechanism [19]. The incidence of HHF resulting from hemodynamic mechanisms was significantly reduced in all six SGLT2is CVOTs. The reduction in the hazard ratio (HR) for HHF ranged from 27% to 39%, which was arithmetically larger than the reduction in HR for 3-P MACE, which was only 3% to 20%. The renal composite outcome showed significant reductions in four of the six CVOTs, but caution is advised in its interpretation due to the varying definitions of renal composite outcomes across CVOTs (Table 1). Notably, the definitions used in the Evaluation of Ertugliflozin EffIcacy and Safety Cardiovascular Outcomes Trial (VERTIS-CV) and SCORED trials, which did not show significant results, include “doubling of serum creatinine level” and “≥50% decrease in the estimated glomerular filtration rate (eGFR) from baseline,” respectively, differing from other CVOTs. However, it is mentioned that if the definition of renal composite outcome in VERTIS-CV, specifically “doubling of serum creatinine level,” is changed to “≥40% decrease in eGFR” similar to other CVOTs, the HR and 95% confidence interval (CI) for the occurrence of renal composite outcomes would be significant at 0.66 (95% CI, 0.50 to 0.88) [20]. Therefore, the renoprotective effect of SGLT2is can be considered as a class effect. Summarizing the cardiorenal outcomes of SGLT2is CVOTs, there is no prominent evidence to claim the effect of SGLT2is in preventing ASCVD occurrence as a class effect, since 3-P MACE did not show significant results across all CVOTs. However, because the HHF and renal composite outcomes showed meaningful results in most CVOTs, the effect of SGLT2is on preventing HF and kidney diseases can be considered a class effect.
Considering the outcomes of CVOTs for GLP-1RAs, 3-P MACE showed significant reductions in five out of nine GLP-1RAs CVOTs. Notably, CVOTs that demonstrated significant outcomes used long-acting formulations (liraglutide, semaglutide, albiglutide, dulaglutide, and efpeglenatide), whereas trials using short-acting formulations (lixisenatide and exenatide) did not yield significant results. Despite being a long-acting GLP-1RA, the Peptide Innovation for Early Diabetes Treatment 6 (PIONEER 6) trial of oral semaglutide did not show significant results. Notably, the trial was designed primarily to demonstrate CV safety, and it had the shortest median duration among the GLP-1RAs CVOTs at 15.9 months. It significantly reduced total mortality with HR of 0.51 (95% CI, 0.31 to 0.84) [16]. It is speculated that, if the PIONEER 6 trial had been conducted over a longer period, a significant reduction in 3-P MACE might have been observed. A 5-year Semaglutide Cardiovascular Outcomes Trial (SOUL) (NCT03914326) aimed at proving CV superiority is currently underway. The individual components of 3-P MACE, including MI, stroke, and CV death, also showed significant results in some GLP-1RA CVOTs involving the administration of long-acting GLP-1RAs. HHF did not show significant effects on most GLP-1RAs CVOTs (Table 2). A recent research article published in the New England Journal of Medicine reported that semaglutide may improve HF; however, the primary endpoint of this study was not a hard outcome but rather HF symptom scores [21]. Therefore, there is still no clear evidence that GLP-1RAs prevent the occurrence of HF. The renal composite outcome was not measured in all GLP-1RAs CVOTs. However, it was significantly reduced (15% to 36% reduction in HR) in all trials that analyzed it as a secondary endpoint, including the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER), Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes 6 (SUSTAIN 6), Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (HARMONY), Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND), and A cardiovascular outcomes study to evaluate the potential of efpeglenatide to reduce cardiovascular risk in adults with type 2 diabetes at high cardiovascular risk (AMPLITUDE-O) trials (Table 2).
When synthesizing the results of CVOTs for cardiorenal outcomes, SGLT2is significantly reduced 3-P MACE in some trials; however, there is no evidence to suggest that they prevent ASCVD. Long-acting GLP-1RAs significantly reduced 3-P MACE. HHF was consistently and markedly reduced by SGLT2is, whereas GLP-1RAs had little effect. Renal outcomes were significantly reduced by both SGLT2is and GLP-1RAs, with a slightly greater reduction in the HR observed with SGLT2is. In summary, SGLT2is and GLP-1RAs reduced various cardiorenal outcomes in CVOTs; however, the effect of reducing 3-P MACE was more evident in the long-acting GLP-1RAs than in SGLT2is, and the effect on suppressing HHF was significant with SGLT2is but minimal with GLP-1RAs. Reduced renal outcomes were observed with both SGLT2is and long-acting GLP-1RAs, with a slightly greater reduction in the HR observed with SGLT2is. Alternately, among the heart- and kidney-protective effects observed in CVOTs, the effect of SGLT2is on suppressing HHF is the most consistent and notable, whereas GLP-1RAs appear to have little effect on suppressing HHF. The effect on reducing renal outcomes was observed with both SGLT2is and long-acting GLP-1RAs, with SGLT2is having a slightly greater effect. An inhibitory effect on 3-P MACE was observed with some SGLT2is and long-acting GLP-1RAs, and the mechanism of action of SGLT2is is considered to be unrelated to atherosclerosis.
In conclusion, the compelling findings of the study by Yang et al. [3] underscore the critical gap between current clinical practice and the latest diabetes treatment guidelines, particularly in the management of patients with diabetes and established CVD, including ASCVD and HF. Despite clinical practice guidelines advocating the prioritization of SGLT2is and GLP-1RAs because of their significant cardiorenal benefits, the actual prescription patterns in Korea exhibit a notable discrepancy. SGLT2is, with robust evidence for reducing HHF, are underutilized in HF settings, whereas their use in IHD is more prevalent. Conversely, GLP-1RAs, despite their lower effect on HHF, are prescribed at similar frequencies for IHD and HF. This discrepancy highlights the critical need for guidelines to explicitly prioritize SGLT2 inhibitors in HF over ASCVD, and to refine the indications for GLP-1RAs to ensure their optimal use for ASCVD without undue emphasis on HF. Moreover, Yang et al. [3] called for a deeper exploration of the mechanisms of CV and renal protection afforded by SGLT2is and GLP-1RAs. Understanding these mechanisms could sharpen the focus on prescription recommendations in clinical guidelines, ensuring that these powerful medications are used more judiciously and effectively in patients with diabetes and CVD. Considering these insights, it is paramount to bridge the gap between guideline recommendations and clinical practice. This entails not only updating and clarifying the guidelines based on the latest evidence but also ensuring that these updates are communicated effectively to practitioners. This will enhance the quality of care for people with diabetes and established CVD, ultimately leading to improved clinical outcomes. The research by Yang et al. [3] is a call to the medical community to adapt and evolve in response to the growing body of evidence on the management of chronic conditions such as diabetes and CVD, ensuring that patients receive the most effective, evidence-based care.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.