2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Article information
Abstract
In May 2023, the Committee of Clinical Practice Guidelines of the Korean Diabetes Association published the revised clinical practice guidelines for Korean adults with diabetes and prediabetes. We incorporated the latest clinical research findings through a comprehensive systematic literature review and applied them in a manner suitable for the Korean population. These guidelines are designed for all healthcare providers nationwide, including physicians, diabetes experts, and certified diabetes educators who manage patients with diabetes or individuals at risk of developing diabetes. Based on recent changes in international guidelines and the results of a Korean epidemiological study, the recommended age for diabetes screening has been lowered. In collaboration with the relevant Korean medical societies, recently revised guidelines for managing hypertension and dyslipidemia in patients with diabetes have been incorporated into this guideline. An abridgment containing practical information on patient education and systematic management in the clinic was published separately.
INTRODUCTION AND SUMMARY OF REVISIONS
Diabetes is a common disease; however, as a chronic and progressive condition accompanied by various complications, it significantly increases individual and socioeconomic burdens. According to the “Diabetes fact sheet in Korea 2021” by the Korean Diabetes Association (KDA), 16.7% of adults over 30 years of age, which is about 5.7 million people, will have diabetes in 2020 [1]. Moreover, 44.3% of adults over 30 years of age have prediabetes with impaired fasting glucose or glycosylated hemoglobin (HbA1c) levels of 5.7% to 6.4% [1]. However, 35% of adults with diabetes, identified by an HbA1c level ≥6.5%, are undiagnosed, and only 25% of those achieve the HbA1c target of less than 6.5% [1]. Diabetes management involves improving individual health status and quality of life through the prevention and early detection of diabetes as well as the prevention or delay of complications via proper glycemic control. Simultaneously, this includes appropriate management of various comorbidities such as blood pressure (BP), lipids, and body weight. Since 1990, the KDA has continuously published treatment guidelines to achieve these treatment goals. These guidelines are targeted at adults with either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM), as well as children or adolescents with T2DM, and patients with gestational diabetes.
The Committee of Clinical Practice Guidelines of KDA has carried forward the “2021 Clinical practice guideline for diabetes (7th edition)” [2] and incorporated recent clinical evidence through systematic literature reviews to publish the revised “2023 Clinical practice guideline for diabetes (8th edition).” In this edition, we have organized the levels of evidence into four distinct categories based on the design of the key research that provides the evidence. These categories are: “randomized controlled trials (RCTs),” which include systematic reviews and/or meta-analyses of such trials; “non-randomized controlled studies (NRS)”; case series falling under “others”; and “expert opinions”. Additionally, the grade of recommendation is classified either as a “general recommendation (General)” applicable to the majority of subjects, or a “limited recommendation (Limited)” intended for specific subgroups within the target population.
In the “screenings for diabetes” section, guidelines were updated to recommend screening for diabetes in all adults aged ≥35 years or those aged ≥19 years who have risk factors, reflecting a recent position statement on screening for prediabetes and diabetes in Korean nonpregnant adults announced by the KDA [3]. In the section “glucose monitoring and glycemic target,” the use of continuous glucose monitoring (CGM) devices was emphasized, and target values of key metrics for CGM have been added. In the section on “pharmacologic treatment of T2DM,” the latest medications have been incorporated, and the algorithms, which were divided into four in the previous guidelines, have now been combined and displayed as one. In cases where intensified injectable therapy is required for people with T2DM who have not reached their glycemic target despite a combination of oral antidiabetic drugs, the recommendation has been changed to prioritize the use of glucagon-like peptide-1 receptor agonist (GLP-1RA) over basal insulin. In the sections “obesity, hypertension, and lipid management,” recent guidelines from other professional medical societies have been reflected [4-6]. The section “non-alcoholic fatty liver disease (NAFLD)” has been updated to include a new algorithm for evaluating NAFLD in people with T2DM. For the practical implementation and widespread use of these guidelines, we separately created an appendix compiling useful information and educational materials for people with diabetes and healthcare professionals.
SCREENING FOR DIABETES MELLITUS
Annual screening (Expert opinion, General) for diabetes should be conducted for adults aged ≥35 and adults aged ≥19 with risk factors (Others, General) using fasting plasma glucose, HbA1c, and 2-hour plasma glucose during a 75 g oral glucose tolerance test (NRS, General). The risk factors of T2DM are shown in Table 1, and abdominal obesity (waist circumference ≥90 cm for men, ≥85 cm for women) has been added compared to the previous guidelines. Owing to the recent increase in the prevalence of prediabetes, diabetes, obesity, and abdominal obesity among young adults under the age of 40 [1,7], there have been suggestions for a change in previous screening criteria for diabetes (adults aged ≥40 and adults aged ≥30 years with risk factors) [2]. The Committee of Clinical Practice Guidelines of the KDA conducted a cross-sectional study on the age for diabetes screening in adults aged ≥20 years using data from the Korean National Health and Nutrition Examination Survey (KNHANES, 2016 to 2020) and the Korean National Health Insurance Service sample cohort (2012 to 2017) [3]. In this study, when evaluating the number needed to screen (NNS) to identify one patient with diabetes according to age group, there was a significant change in NNS values in the 35 to 39 age group. Moreover, when evaluating the NNS for diabetes based on risk factors for T2DM in adults aged 20 to 34 using the KNHANES data, it was found that the NNS was lower at age 23 for abdominal obesity compared to an NNS of age 34 for general obesity (body mass index [BMI] ≥25 kg/m2). Based on these results, it was concluded that screening for diabetes is appropriate in adults aged ≥35 years and those aged ≥20 years with risk factors for T2DM. Applying the revised age criteria, we found that the number of missed participants in diabetes screening significantly decreased from 4.0% to 0.2%, without a significant increase in the NNS value, compared to the previous guideline standards.
GLUCOSE MONITORING AND GLYCEMIC TARGET
Strict glucose control is implemented to prevent microvascular and macrovascular complications in patients with diabetes (RCT, General) [8-10]. Therefore, the long-term maintenance of glycemic control within the near-normal range is critical for patients with diabetes [11]. The recommended optimal HbA1c target is <6.5% for patients with T2DM and <7.0% for those with T1DM (RCT, General) through lifestyle modifications and glucose-lowering agents, especially in recently diagnosed or young people without severe complications or hypoglycemia. However, the glycemic target should be individualized based on physical or psychological status, social circumstances, life expectancy, severity of comorbidities, or risk of hypoglycemia (NRS, General). In patients with a long duration of diabetes, a history of severe hypoglycemia, advanced diabetic complications, short life expectancy, or advanced age, consideration should be given to setting lower glycemic control targets [12-14].
In addition to HbA1c and blood glucose monitoring, real-time CGM is recommended to achieve better glycemic control and reduce the risk of hypoglycemia in adults with T1DM (RCT, General) and T2DM (RCT, Limited) treated with insulin injections [15-18]. In adults with diabetes receiving insulin therapy who cannot or do not wish to use real-time CGM continuously or in adults with T2DM receiving non-insulin therapy, real-time CGM can be used periodically for glycemic control (RCT, Limited) [19-21]. Intermittently scanned CGM may be used in patients with diabetes and indications for real-time CGM because it is also beneficial for glycemic control and preventing hypoglycemia [22-24] and is available at a lower cost. However, in a randomized controlled study that compared real-time CGM with intermittently scanned CGM, the time below range (below 70 mg/dL) and the time in range (70 to 180 mg/dL) were found to be better with real-time CGM [25]. Therefore, the use of real-time CGM is recommended in cases where insufficient benefits are obtained from intermittently scanned CGM. The recommended target values for the CGM metrics based on the type of diabetes are listed in Table 2.
PHARMACOLOGICAL TREATMENT FOR ADULTS WITH T2DM
Immediately upon diagnosis, actively educate patients on self-management methods and monitor whether it is maintained (RCT, General). When selecting drugs, consider the presence of comorbidities (established atherosclerotic cardiovascular disease [eASCVD], heart failure [HF], and chronic kidney disease [CKD]), glucose-lowering efficacy, effects on body weight, risk of hypoglycemia, side effects, treatment acceptability, age, value of life pursued by patients, and cost (Expert opinion, General) [26-38]. Insulin therapy is recommended for patients with severe hyperglycemia (HbA1c >9.0%) and hyperglycemic symptoms (polydipsia, polyuria, and weight loss) (Expert opinion, General) [39,40]. When initiating pharmacological therapy, monotherapy or combination therapy should be administered, taking into consideration the goal and current levels of HbA1c (RCT, General). Generally, the glucose-lowering efficacy (reduction in HbA1c) of monotherapy with an oral glucose-lowering agent is estimated to be 1.0%; therefore, if the current HbA1c level is 1.5% higher than the target HbA1c level, initial combination therapy is recommended [39,41]. However, early combination therapy should be actively considered from the beginning of the diagnosis to reduce the risk of glycemic control failure (RCT, Limited). The Vildagliptin Efficacy in combination with metfoRmIn for earlY treatment of type 2 diabetes (VERIFY) trial demonstrated that an early intervention strategy with combination therapy of vildagliptin and metformin in treatment-naïve patients with T2DM provided more durable long-term clinical benefits than metformin monotherapy with a traditional stepwise regimen [42]. According to a subgroup analysis of Korean patients with newly diagnosed T2DM among the VERIFY trial participants, early combination treatment significantly and consistently improved long-term glycemic durability compared to monotherapy with metformin [43]. Adherence to glucose-lowering agents is strongly associated with metabolic control in patients with T2DM. For each 10% increase in drug adherence, HbA1c levels decreased by 0.16% [44]. Therefore, medication adherence should be evaluated regularly, and medication adjustment should not be delayed if necessary (Expert opinion, General). If the target HbA1c level has not been reached within 3 to 6 months, uptitration of existing medications, combination therapy using drugs with different mechanisms of action, or injection therapy should be actively considered as soon as possible; however, dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1RAs should not be combined (RCT, General).
Metformin is recommended as a first-line glucose-lowering agent in patients with T2DM and is maintained if there are no contraindications or intolerable side effects (RCT, General). In the Practical Evidence of Antidiabetic Monotherapy (PEAM) study, the glucose-lowering efficacy of sulfonylureas, metformin, and thiazolidinediones as monotherapies administered for 48 weeks was similar in drug-naïve Korean patients with T2DM [45]. Metformin has adequate efficacy, a low-risk of hypoglycemia, weight neutrality, and cost-effectiveness. However, if there are contraindications or intolerable side effects of metformin, a different class of medication can be considered.
Injectable therapy, GLP-1RA or insulin, is recommended when potent glucose-lowering efficacy is required (RCT, General) [46]. When considering injectable-based combination therapy, GLP-1RAs are preferred over basal insulin (RCT, General) [47]. If the glycemic target is not achieved with GLP-1RA or basal insulin alone, the two drugs can be combined (RCT, Limited) [48-65]. If the glycemic target is not achieved with GLP-1RA or basal insulin, intensive insulin therapy such as a basal-plus, premixed, or basal-bolus regimen should be initiated (RCT, Limited) [66-72].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors with proven benefits for HF should be used first in patients with HF regardless of HbA1c levels and should be maintained as long as there are no contraindications or side effects (RCT, General). In the Dapagliflozin and Prevention of Adverse outcomes in Heart Failure (DAPA-HF) study, conducted in 4,744 patients with existing HF (New York Heart Association [NYHA] class II, III, or IV) and reduced ejection fraction (EF ≤40%) regardless of the presence of T2DM, dapagliflozin 10 mg reduced the risk of HF exacerbation or death from cardiovascular disease (CVD) by 26% (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65 to 0.85; P<0.001) over an average study period of 18.2 months and showed similar results in patients with or without diabetes [73]. Empagliflozin was also evaluated in patients with HF (NYHA class II, III, or IV HF and EF ≤40%) regardless of the presence of diabetes in the EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with reduced ejection fraction (EMPEROR-Reduced) study. Over an average study period of 16 months, the primary endpoint of death from CVD or hospitalization due to HF exacerbation was 25% less in the empagliflozin treatment group compared to the placebo group (HR, 0.75; 95% CI, 0.65 to 0.86; P<0.001). The effect of empagliflozin was evident regardless of the presence of diabetes [74]. The benefit of SGLT2 inhibitors in HF has also been demonstrated in patients with preserved EF. In the EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with preserved ejection fraction (EMPEROR-Preserved) study, which targeted patients with NYHA class II–IV HF and an EF of 40% or more, the primary endpoint of death from CVD or hospitalization due to HF exacerbation occurred 25% less in the empagliflozin treatment group compared to the placebo group (HR, 0.79; 95% CI, 0.69 to 0.90; P<0.001) over an average study period of 26 months [75].
SGLT2 inhibitors with proven renal benefits should be used primarily regardless of the HbA1c level in patients with albuminuria or reduced estimated glomerular filtration rate (eGFR) and should be continued unless contraindicated or side effects are present (RCT, Limited). The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) study confirmed a 39% reduction in renal endpoints (a decrease of 50% or more in eGFR, end-stage renal disease, or death due to renal disease or CVD) in patients with or without diabetes with CKD (eGFR 25 to 75 mL/min/1.73 m2, urine albumin/creatinine ratio [UACR] 200 to 5,000 mg/g) compared to placebo [76]. The Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY) included patients with CKD regardless of their diabetes status (eGFR 20 to 45 mL/min/1.73 m2, or if the eGFR is 45 to 90 mL/min/1.73 m2 and the UACR is 200 mg/g or more). The primary endpoint, a composite of worsening kidney function or death due to CVD, was reduced by 18% compared to placebo (HR, 0.72; 95% CI, 0.64 to 0.82; P <0.001). Notably, the EMPA-KIDNEY study included patients with decreased eGFR without proteinuria and confirmed beneficial results in renal protection [77].
GLP-1RAs or SGLT2 inhibitors, which have proven cardiovascular benefits, should be primarily used in patients with eASCVD (RCT, General). In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose (EMPA-REG OUTCOME) trial, a randomized controlled study that administered the SGLT2 inhibitor empagliflozin to 7,020 patients with T2DM with established CVD, it was observed that over an average of 3 years, the occurrence of 3-point major adverse cardiovascular diseases (MACE; including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke) decreased by 14% compared to the placebo group (HR, 0.86; 95% CI, 0.74 to 0.99; P=0.04) [26]. The Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results (LEADER) study was a double-blind study that randomly assigned liraglutide and placebo to 9,340 adult patients with T2DM. The 3-point MACE was decreased by 13% in the liraglutide group compared to placebo group (HR, 0.87; 95% CI, 0.78 to 0.97), with cardiovascular death (HR, 0.78; 95% CI, 0.66 to 0.93), asymptomatic/nonfatal/fatal myocardial infarction (HR, 0.86; 95% CI, 0.73 to 1.00), and nonfatal/fatal stroke (HR, 0.89; 95% CI, 0.71 to 1.06). All-cause mortality decreased by 15% in the liraglutide group compared to the placebo group (HR, 0.85; 95% CI, 0.74 to 0.97), mainly due to decreased cardiovascular death [27]. The Trial to Evaluate CV and Other Long-term Outcomes With Semaglutide in Subjects With T2D (SUSTAIN-6) was a double-blind study that randomly assigned semaglutide and placebo to 3,297 adult patients with T2DM. Of the patients, 72% had baseline atherosclerotic cardiovascular disease (ASCVD). The 3-point MACE was decreased by 26% in the semaglutide group compared to the placebo group (HR, 0.74; 95% CI, 0.58 to 0.95), with each element showing cardiovascular death (HR, 0.98; 95% CI, 0.65 to 1.48), nonfatal myocardial infarction (HR, 0.74; 95% CI, 0.51 to 1.08), and nonfatal stroke (HR, 0.61; 95% CI, 0.38 to 0.99). All-cause mortality in the semaglutide group compared to the placebo group had a HR of 1.05 (95% CI, 0.74 to 1.50) [78]. The Researching CV Events With a Weekly Incretin in Diabetes (REWIND) was a double-blind study that randomly assigned dulaglutide and placebo to 9,901 adult patients with T2DM. Unlike the two studies above, baseline ASCVD was present in only 31% of all patients, and most had risk factors for ASCVD but not for ASCVD [4]. The 3-point MACE was decreased by 12% in the dulaglutide group compared to the placebo group (HR, 0.88; 95% CI, 0.79 to 0.99), and cardiovascular death (HR, 0.91; 95% CI, 0.7 to 1.06), nonfatal/fatal myocardial infarction (HR, 0.96; 95% CI, 0.79 to 1.15) were not statistically significant; however, the risk of nonfatal/fatal stroke decreased by 24% (HR, 0.76; 95% CI, 0.62 to 0.94). These reductions in MACE were consistent regardless of the presence of baseline ASCVD. In the REWIND study, 69% of the participants were adults with T2DM who did not have underlying ASCVD, and MACE was also reduced even in these cases (HR, 0.87; 95% CI, 0.74 to 1.02 for both; P for interaction=0.97) [28]. Therefore, dulaglutide could be considered for both secondary and primary prevention. However, there are no other large-scale RCTs for patients without ASCVD at baseline; therefore, further research is needed (Fig. 1).

Pharmacologic treatment fOr Adults With Type 2 Diabetes Mellitus (T2dm). This Algorithm Stratifies The Glycemic Control Strategy In Patients With T2dm Based On A1c Levels And Underlying Comorbidities. Self-management Education And Monitoring For Diabetes Should Be Continuously Implemented From The Time Of Diagnosis In All Patients With T2dm. If The A1c Level Is >9.0% And Symptomatic Hyperglycemia Or Metabolic Decompensation Is Present, Insulin Therapy With Or Without Oral Antidiabetic Drugs (Oads) Is Recommended. If Established Atherosclerotic Cardiovascular Disease (Eascvd), Heart Failure (Hf), Or Chronic Kidney Disease (Ckd) Are Combined, Sodium-glucose Cotransporter 2 Inhibitor (Sglt2i) Or Glucagon-like Peptide-1 Receptor Agonist (Glp-1ra), Which Have Proven Benefits Under These Conditions, Are Preferred. If The A1c Difference Between The Current And Target A1c Level Is ≥1.5% Or The Current A1c Level Is ≥7.5%, Initial Combination Therapy Is Recommended. If The Current A1c Level Is <7.5%, Metformin Monotherapy Is The Preferred Option, Depending On The Patient’s Condition. However, Early Combination Therapy Should Be Considered To Reduce The Risk Of Treatment Failure. Injectable Therapy, Glp-1ra Or Insulin Is Recommended When Potent Glucose-lowering Efficacy Is Required. When Considering Injectable-based Combination Therapy, Glp-1ra Is Preferred. If The Glycemic Target Is Not Achieved With Glp-1ra Or Basal Insulin Alone, The Two Drugs Can Be Combined. If The Glycemic Target Is Not Achieved With Glp-1ra Or Basal Insulin, Intensive Insulin Therapy Such As Basal-plus, Premixed, Or Basal-bolus Regimen Should Be Initiated. Tzd, Thiazolidinedione; Dpp-4i, Dipeptidyl Peptidase-4 Inhibitor; Su, Sulfonylurea; Α-gi, Α-glucosidase Inhibitor. aEstablished Atherosclerotic Cardiovascular Disease: History Of Acute Coronary Syndrome Or Myocardial Infarction, Stable Or Unstable Angina, Coronary Heart Disease With Or Without Revascularization, Other Arterial Revascularization, Stroke, Or Peripheral Artery Disease Assumed To Be Atherosclerotic In Origin, bHf: Current Or Prior Symptoms Of Hf With Documented Hf With Reduced Ejection Fraction (Hfref, Left Ventricular Ejection Fraction [Lvef] ≤40) Or Hf With Preserved Ejection Fraction (Hfpef, Lvef >40), cChronic Kidney Disease: Estimated Glomerular Filtration Rate (Egfr) <60 Ml/Min/1.73 M2 Or Urine Albumin-creatinine Ratio ≥30 Mg/G, dDulaglutide, Liraglutide, Semaglutide, eDapagliflozin, Empagliflozin, fDapagliflozin, Empagliflozin, Ertugliflozin, gPioglitazone.
HYPERTENSION MANAGEMENT IN PATIENTS WITH DIABETES
BP should be measured at every clinic visit and at home (Expert opinion, General). Home BP monitoring is recommended in adults with diabetes and hypertension (RCT, General) [79]. The recommended target BP level is <140/90 mm Hg in adults with diabetes without CVD or its risk factors (RCT, General) [5]. The recommended target BP level is <130/80 mm Hg in adults with diabetes with CVD, target organ damage (albuminuria, CKD, retinopathy, and left ventricular hypertrophy [LVH]), or risk factors for CVD (RCT, General) [5,80-86]. Adults with diabetes and BP ≥120/80 mm Hg should undergo lifestyle modifications, including weight management, proper exercise, and healthy diets, to maintain a normal BP (systolic BP <120 mm Hg and diastolic BP <80 mm Hg) (RCT, General). Pharmacological therapy should be implemented if the target BP is not achieved. Adults with diabetes and hypertension can be prescribed any class of antihypertensive medication as a primary medication for BP control to achieve the target range (RCT, General). There was no difference in the CVD prevention effect between angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), calcium channel blockers, and β-blockers, which can all be used as first-line antihypertensive agents in T2DM [87,88]. ACE inhibitors or ARBs are preferred when hypertension is accompanied by albuminuria (RCT, General) [89-91] or coronary artery disease (RCT, General) [92]. If BP is not controlled by the primary antihypertensive medication, combination therapy using a drug with a different mechanism is recommended (RCT, General). However, the combination of ACE inhibitors and ARBs is not recommended (RCT, General). If BP exceeds 160/100 mm Hg, initial combination therapy with two or more medications is recommended with intensive lifestyle intervention (RCT, General) (Fig. 2) [85].
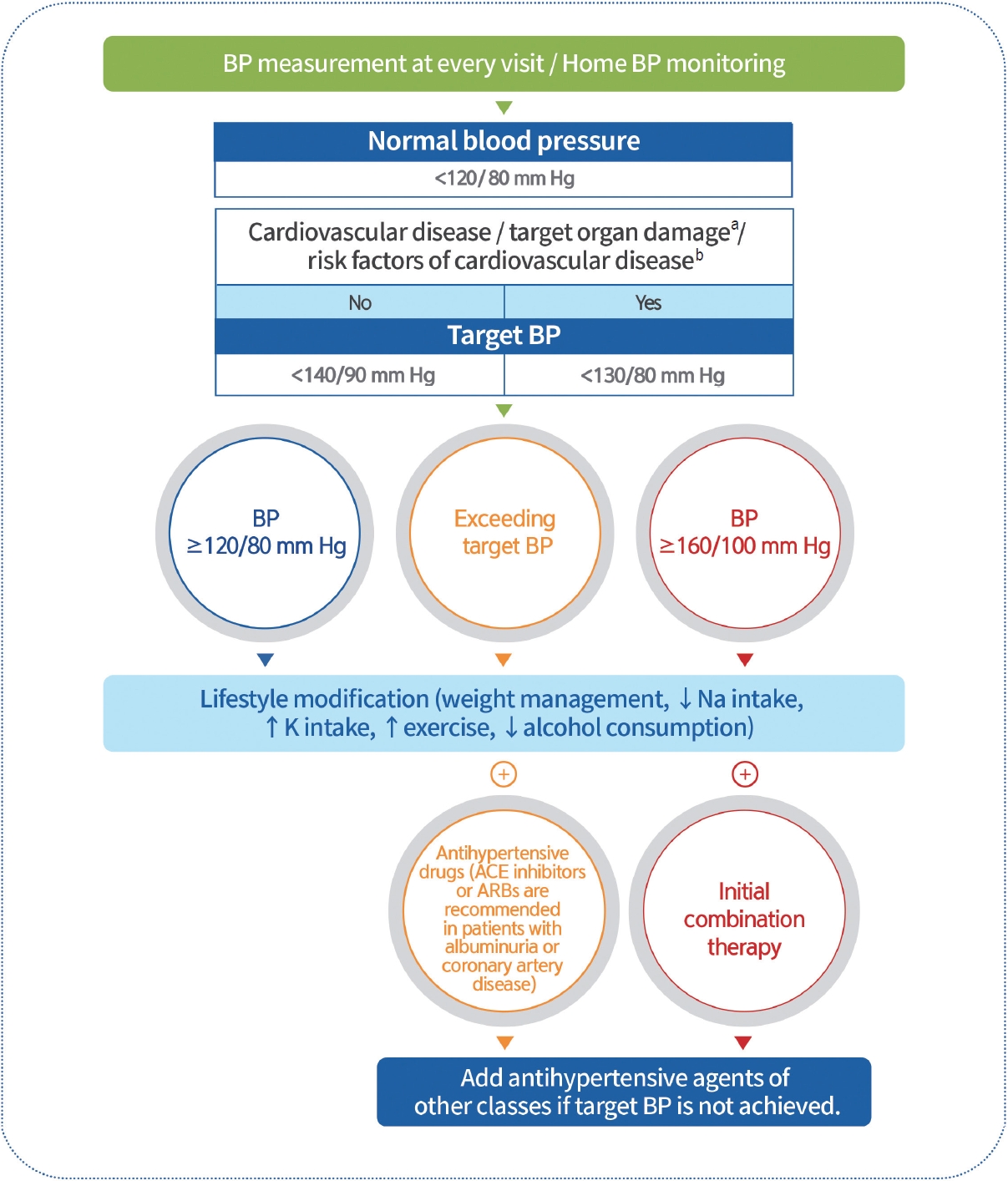
Hypertension management in patients with diabetes. Blood pressure (BP) should be measured at every clinic visit, and home BP monitoring is recommended. The recommended target BP level is <140/90 mm Hg in adults with diabetes without cardiovascular disease (CVD) or associated risk factors. The recommended target BP is <130/80 mm Hg in diabetic adults with CVD, target organ damage (albuminuria, chronic kidney disease, retinopathy, and left ventricular hypertrophy), or risk factors for CVD. Adults with diabetes and a BP >120/80 mm Hg should undergo lifestyle modifications. Pharmacological therapy should be implemented if the target BP is not achieved. Angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) are preferred when accompanied with albuminuria or coronary artery disease. If the BP exceeds 160/100 mm Hg, initial combination therapy is recommended. If BP is not controlled by primary antihypertensive medications, combination therapy using other drugs with different mechanisms is recommended. aAlbuminuria, chronic kidney disease, retinopathy, left ventricular hypertrophy, bAge (men ≥45 years, women ≥55 years), smoking, obesity, dyslipidemia, family history of early-onset coronary heart disease.
LIPID MANAGEMENT IN PATIENTS WITH DIABETES
To evaluate the risk of CVD, serum lipid profiling (total cholesterol, high-density lipoprotein cholesterol [HDL-C], triglycerides, and low-density lipoprotein cholesterol [LDL-C]) should be performed at the time of the initial diagnosis of diabetes and at least once a year thereafter (Expert opinion, General). The primary objective of lipid management is to control LDL-C levels (RCT, General). To determine the LDL-C goal, comorbidities including past history of CVD, target organ damage (albuminuria, eGFR <60 mL/min/1.73 m 2 , retinopathy, and LVH), major risk factors for CVD (age, family history of early-onset coronary artery disease, hypertension, smoking, and HDL-C <40 mg/dL), and duration of diabetes should be investigated first (Expert opinion, General). LDL-C goals are as follows. (1) For patients with CVD, LDL-C should be reduced to <55 mg/dL and at least 50% from baseline (RCT, General); (2) For those with a duration of diabetes ≥10 years or with the major risk factors for CVD or target organ damage, LDL-C should be reduced to <70 mg/dL (NRS, General); (3) For those who have target organ damage or three or more major risk factors for CVD, LDL-C can be reduced to <55 mg/dL (NRS, General); (4) For those whose duration of the disease is <10 years and who do not have major risk factors for CVD, LDL-C should be reduced to <100 mg/dL (RCT, General) [6,93-98]. Education on active lifestyle modifications and continuous monitoring of their implementation should be included in lipid management (RCT, general). Pharmacologic therapy should be implemented if LDL-C goals are not achieved as follows: (1) a statin should be used as first-line therapy (RCT, General); (2) ezetimibe should be added if the goal is not achieved with a maximum tolerable dose of statin therapy (RCT, Limited); (3) if patients with diabetes with CVD do not achieve the goal even after treatment with a statin adding ezetimibe, combination therapy with a statin and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors should be considered (RCT, Limited) [99-104]. Adults with hypertriglyceridemia (triglyceride levels ≥150 mg/dL) should be managed first with lifestyle modifications, such as stopping alcohol consumption and losing weight, as well as controlling secondary factors, such as glycemic control (RCT, General). Adults with severe hypertriglyceridemia (triglyceride levels ≥500 mg/dL) are treated with medications such as fenofibrate or omega-3 fatty acids to reduce the risk of acute pancreatitis (RCT, General) [105]. Four to 12 weeks after the initiation of lipid-lowering therapy, serum lipid tests must be performed to evaluate the response to the drug and patient compliance (Expert opinion, General) (Fig. 3).
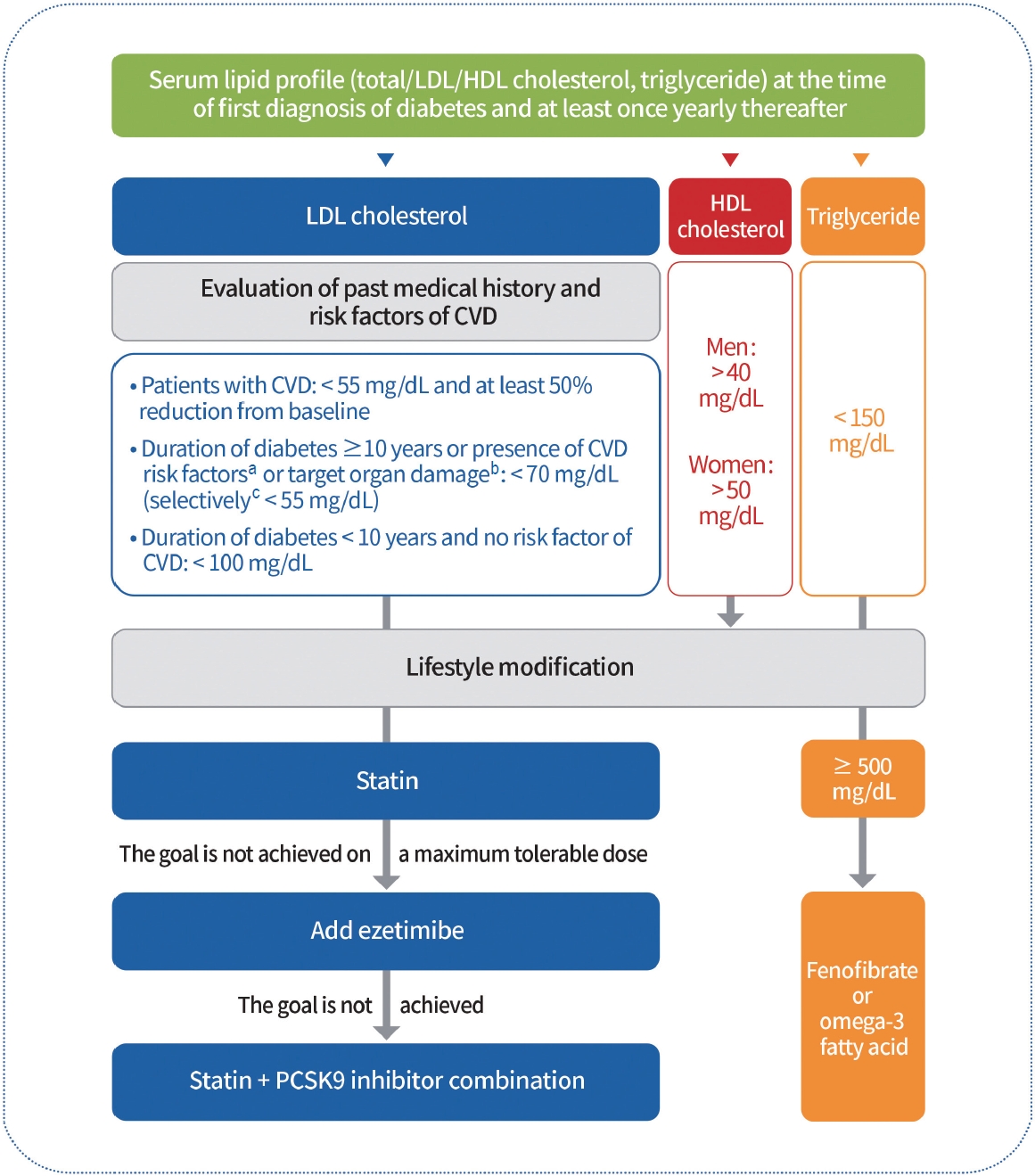
Lipid management in patients with diabetes. Serum lipid profiles should be obtained at the time of the first diagnosis of diabetes and at least once a year thereafter. Past history of cardiovascular disease (CVD) and target organ damage (albuminuria, estimated glomerular filtration rate <60 mL/min/1.73 m2, retinopathy, and left ventricular hypertrophy), the major risk factors for CVD (age, family history of early-onset coronary artery disease, hypertension, smoking, and high-density lipoprotein cholesterol [HDL-C] <40 mg/dL) and duration of diabetes should be investigated to determine the low-density lipoprotein cholesterol (LDLC) goal. Education on active lifestyle modifications should also be provided. Pharmacological therapy should be implemented if LDL-C goals are not achieved. Statins should be used as first-line therapy. Ezetimibe should be added if the goal is not achieved with the maximum tolerable dose of statin therapy. If diabetic patients with CVD do not achieve this goal even after treatment with a statin plus ezetimibe, combination therapy with a statin and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors should be considered. Adults with severe hypertriglyceridemia (triglyceride levels ≥500 mg/dL) were treated with fenofibrate or omega-3 fatty acids. aAge (men ≥45 years, women ≥55 years), family history of early-onset coronary heart disease, hypertension, smoking, HDL-C <40 mg/dL, bAlbuminuria, estimated glomerular filtration rate <60 mL/min/1.73 m2, retinopathy, and left ventricular hypertrophy, cTarget organ damage or three or more major risk factors for CVD.
DIABETIC KIDNEY DISEASE
UACR and eGFR should be evaluated in patients with T2DM at the time of diagnosis and at least once yearly thereafter (NRS, General) [106]. Blood glucose and BP should be optimally controlled to suppress the development and progression of diabetic kidney disease (DKD) (RCT, General) [107]. Patients with DKD should avoid excessively high or low ( ≤0.8 g/kg/day) consumption of protein (RCT, General) [108,109]. ACE inhibitors or ARBs should be prescribed to patients with diabetes with albuminuria and hypertension (RCT, General) [110-112]. To prevent DKD progression, ACE inhibitors or ARBs are not recommended for patients with normal BP (RCT, General) [113]. SGLT2 inhibitors with renal benefits should be used to inhibit the progression of DKD in patients with albuminuria or a reduced eGFR (RCT, General) [76,77]. SGLT2 inhibitors can be maintained until renal replacement therapies are initiated (RCT, General). Nonsteroidal mineralocorticoid receptor antagonists (e.g., finerenone) that have shown cardiac and renal benefits can be considered in T2DM patients with albuminuria, decreased eGFR, and normal blood potassium levels (RCT, General) [114-116]. GLP-1RAs that have shown cardiovascular and renal benefits can be considered to inhibit the progression of albuminuria in T2DM patients at a high-risk of CVD (RCT, General) [28,117,118]. Request a consultation with nephrologists about those with unknown causes of kidney diseases or progressed nephropathy (eGFR <30 mL/min/1.73 m2) (Expert opinion, General) (Fig. 4).
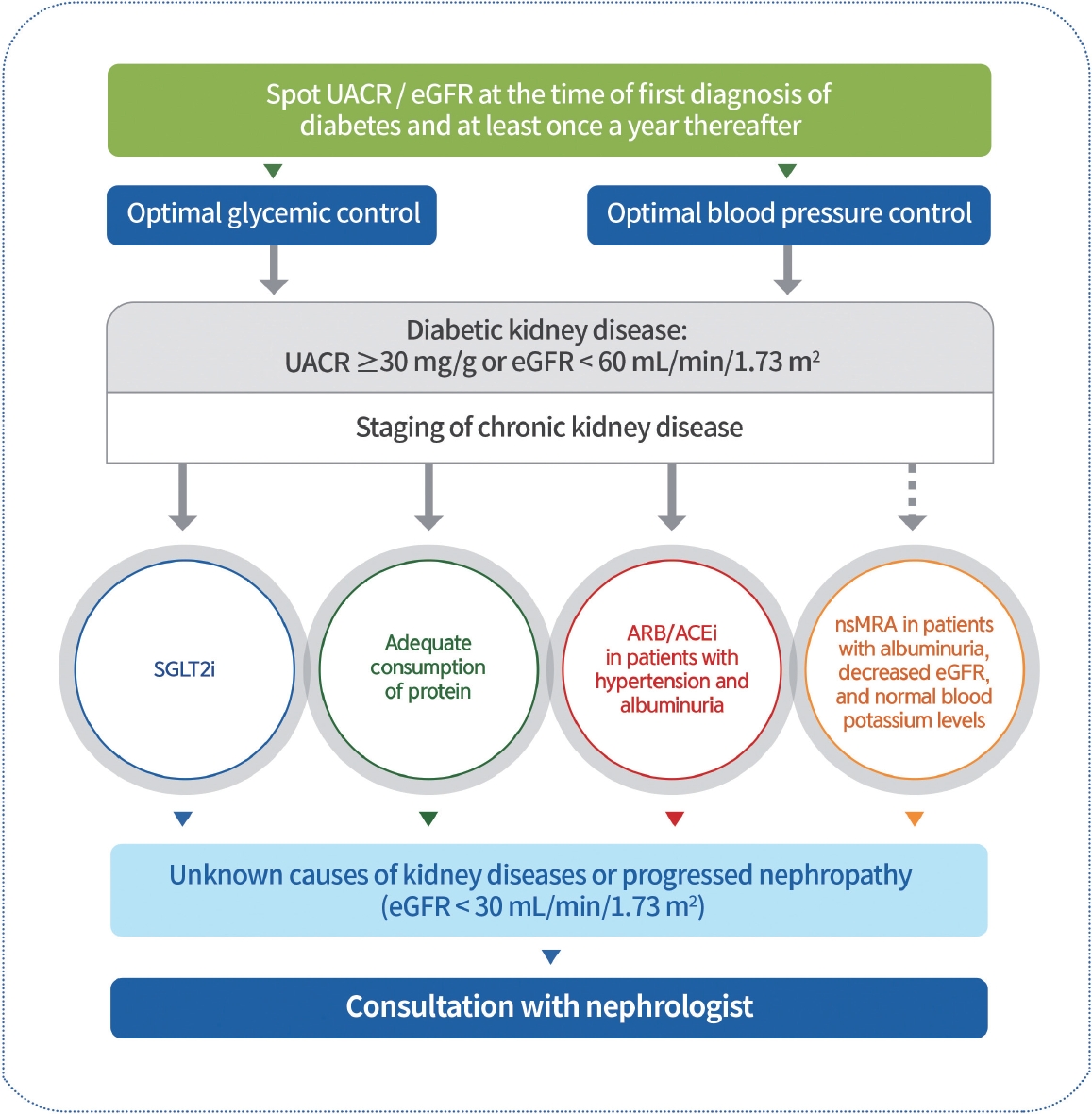
Management of diabetic kidney disease. Urine albumin/creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) should be evaluated in individuals with type 2 diabetes mellitus (T2DM) at the time of diagnosis and at least once yearly. Therefore, blood glucose levels and blood pressure should be optimally controlled. Sodium-glucose cotransporter 2 inhibitors (SGLT2is) that have shown renal benefits should first be used to inhibit diabetic kidney disease (DKD) progression in patients with albuminuria or a reduced eGFR. DKD patients consume adequate protein amounts. Angiotensin-converting enzyme inhibitors (ACEis) or angiotensin II receptor blockers (ARBs) should be prescribed for patients with diabetes, hypertension, and albuminuria. Non-steroidal mineralocorticoid receptor antagonists (nsMRAs), such as finerenone, which has shown cardiac and renal benefits, can be considered in patients with T2DM with albuminuria, decreased eGFR, and normal blood potassium levels. Request consultation with nephrologists for those with unknown causes of kidney disease or progressive nephropathy.
NON-ALCOHOLIC FATTY LIVER DISEASE IN PATIENTS WITH T2DM
All adults with T2DM should be evaluated for the presence of NAFLD (Expert opinion, General). Alanine aminotransferase or abdominal ultrasonography should be performed as primary screening to evaluate NAFLD and exclude secondary causes (Expert opinion, General) [119-122]. If NAFLD is confirmed, the evaluation of liver fibrosis is necessary. Non-invasive and relatively simple tests, such as the fibrosis index-4 (FIB-4) or NAFLD fibrosis score (NFS), can be utilized as markers. Compared to liver biopsy, these non-invasive diagnostic models have higher specificity but lower sensitivity [123-125]. Therefore, additional diagnostic strategies for liver fibrosis are necessary for the remainder of the group, excluding the low-risk group (FIB-4 <1.3, NFS <2.67) [126]. Vibration-controlled transient elastography should be considered for the evaluation of liver fibrosis in adults with T2DM with NAFLD (Expert opinion, Limited) (Fig. 5) [127,128]. Lifestyle modification is recommended to reduce the risk factors for CVD and treat fatty liver disease in adults with T2DM with NAFLD (RCT, General) [129-132]. Weight reduction should be performed by at least 7% of the body weight to improve intrahepatic inflammation in adults with T2DM with NAFLD and a BMI ≥23 kg/m2 (RCT, General) [132-134]. Thiazolidinediones can be used as first-line therapy for NAFLD in T2DM adults (RCT, General) [135,136]. GLP-1RAs that have shown effects can be used as first-line therapy for NAFLD in T2DM adults (RCT, Limited) [28-30]. Metformin, DPP-4 inhibitors, vitamin E, statins, ursodeoxycholic acid, and pentoxifylline are not used to treat NAFLD (RCT, General) [134,135,137,138]. Bariatric surgery can be considered for adults with T2DM with obesity (BMI ≥30 kg/m2) and NAFLD who have had non-surgical treatment but failed weight loss and had no improvement in the fatty liver (NRS, Limited) [139-142].
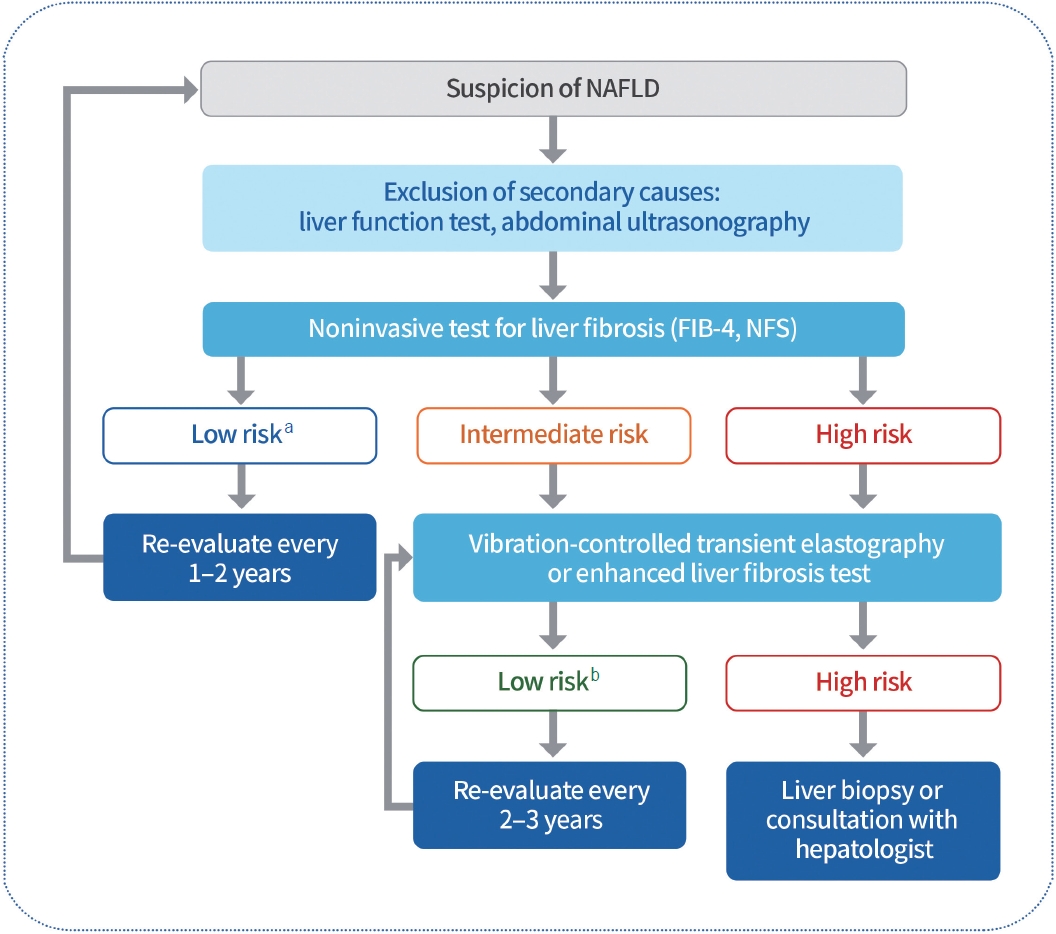
Evaluation of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes mellitus (T2DM). All adults with T2DM should be evaluated for the presence of NAFLD. Liver function tests or abdominal ultrasound should be performed to evaluate NAFLD and to exclude secondary causes. Non-invasive and simple tests, such as the fibrosis index-4 (FIB-4) or the NAFLD fibrosis score (NFS), can be used to evaluate liver fibrosis. Vibration-controlled transient elastography should be considered when evaluating liver fibrosis in T2DM adults, excluding those in low-risk groups. aLow risk: FIB-4 <1.3, NFS <2.67, bLow risk: liver stiffness measurement <8.0 kPa, Enhanced Liver Fibrosis (ELF) score <10.5.
CONCLUSIONS
Despite remarkable advances in pharmacological treatment, medical technology, and the ongoing updating of comprehensive guidelines for managing diabetes mellitus, the rate of achieving treatment goals remains unsatisfactory. Therefore, certified education by professional and multidisciplinary teams and constant monitoring of adherence to self-management, including nutrition and exercise, in people with diabetes should be emphasized. An appropriate choice, active titration, and adherence to antidiabetic medication while avoiding clinical inertia could lead to an individualized target glycemic goal. To achieve these goals, various supplementary methods, including information technology, can be helpful. In addition, regular monitoring and early detection of acute or chronic complications and comorbidities are urgently needed in patients with diabetes. The Clinical Practice Guidelines of KDA will provide timely, evidence-based clinical recommendations to support healthcare professionals in providing more advanced diabetes care in Korea.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the Korean Diabetes Association.
Acknowledgements
We thank the Research Groups and Committees of the Korean Diabetes Association (KDA), and external academic groups for their thoughtful peer review and endorsement of the guidelines: the KDA research group on Diabetic Neuropathy, Diabetic Nephropathy, Gestational Diabetes, Exercise, Diabetes in Old Age, Fatty Liver Disease; the KDA Committee of Education, Food and Nutrition, Patient Advocacy, Medical Practitioners; the Korean Association of Internal Medicine, Korean Endocrine Society, Korean Society for the Study of Obesity, Korean Association of Ophthalmology, Korean Society of Hypertension, Korean Society of Lipid and Atherosclerosis, Korean Society of Nephrology; Korean Association of Diabetes Dietetic Educators, Korean Association of Diabetes Nurse Educators, Korean Society of Social Workers for Diabetes Education. We also thank Hyun Jung Kim, a clinical guideline methodology expert; Seong Ouk Lee and Youngjin Lee, professional librarians; and Seung-Hyun Ko, Hyuk Sang Kwon, and Seak Ki Yun, advisory members along with Se Hee Min and Min Young Lee, members of the Committee of Clinical Practice Guidelines.


