Clinical and Lifestyle Determinants of Continuous Glucose Monitoring Metrics in Insulin-Treated Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Background
There was limited evidence to evaluate the association between lifestyle habits and continuous glucose monitoring (CGM) metrics. Thus, we aimed to depict the behavioral and metabolic determinants of CGM metrics in insulin-treated patients with type 2 diabetes mellitus (T2DM).
Methods
This is a prospective observational study. We analyzed data from 122 insulin-treated patients with T2DM. Participants wore Dexcom G6 and Fitbit, and diet information was identified for 10 days. Multivariate-adjusted logistic regression analysis was performed for the simultaneous achievement of CGM-based targets, defined by the percentage of time in terms of hyper, hypoglycemia and glycemic variability (GV). Intake of macronutrients and fiber, step counts, sleep, postprandial C-peptide-to-glucose ratio (PCGR), information about glucose lowering medications and metabolic factors were added to the analyses. Additionally, we evaluated the impact of the distribution of energy and macronutrient during a day, and snack consumption on CGM metrics.
Results
Logistic regression analysis revealed that female, participants with high PCGR, low glycosylated hemoglobin (HbA1c) and daytime step count had a higher probability of achieving all targets based on CGM (odds ratios [95% confidence intervals] which were 0.24 [0.09 to 0.65], 1.34 [1.03 to 1.25], 0.95 [0.9 to 0.99], and 1.15 [1.03 to 1.29], respectively). And participants who ate snacks showed a shorter period of hyperglycemia and less GV compared to those without.
Conclusion
We confirmed that residual insulin secretion, daytime step count, HbA1c, and women were the most relevant determinants of adequate glycemic control in insulin-treated patients with T2DM. In addition, individuals with snack consumption were exposed to lower times of hyperglycemia and GV.
INTRODUCTION
Individuals with diabetes and similar glycosylated hemoglobin (HbA1c) values may have different glycemic profiles, with different glycemic variability (GV), which is affected by lifestyle changes such as diet and exercise [1-3]. Reducing GV, as well as chronic hyperglycemia represented by HbA1c, has been an important target in various intervention trials [4].
Currently, continuous glucose monitoring (CGM) has been recommended as an indicator of glycemic control and for decision making in treatment regimens [5] and as a tool to assess GV [6,7]. Standardized CGM metrics were defined as the percentage time in range (TIR) of 70 to 180 mg/dL (TIR70–180), time above range (TAR) >180 mg/dL (TAR>180), TAR >250 mg/dL (TAR>250), time below range (TBR) <70 mg/dL (TBR<70), TBR <54 mg/dL (TBR<54), standard deviation (SD) and coefficient of variation (CV) representing hyperglycemia, hypoglycemia, and GV [5]. In this regard, the recommendations of experts on diabetes support the usefulness of CGM for patients with type 2 diabetes mellitus (T2DM) on insulin injections, as well as type 1 diabetes mellitus [5].
Although understanding the relationship between lifestyle habits and glucose fluctuation in an outpatient setting, which would offer real-world clinical data, could contribute to the optimal management of T2DM, to date, studies evaluating the association between detailed lifestyle habits and CGM metrics in patients with T2DM are scarce. Most previous studies conducted in T2DM did not include lifestyle factors [8,9], estimated lifestyle habits by self-reported questionnaire [2], and were conducted in a controlled environment [10]. Although there were two recent studies that focused on the influence of behavioral factors, the study subjects were nondiabetic individuals [11,12].
Thus, this study aimed to characterize the major determinants of standardized CGM metrics by implementing detailed measures of clinical, behavioral, and nutritional factors in insulin-treated patients with T2DM.
METHODS
Study participants and protocol
We analyzed the data set of an ongoing prospective observational study. We recruited Korean patients with T2DM taking both insulin and noninsulin glucose lowering medications (GLMs) between February 2021 and September 2022 at the outpatient clinic of the Korea University Ansan Hospital (Ansan). The inclusion criteria were as follows: (1) age between 19 and 80 years; (2) fasting C-peptide more than 0.4 ng/mL. Individuals were excluded under any of the following criteria: (1) those who had been hospitalized for hyperglycemic hyperosmolar syndrome, diabetic ketoacidosis, or cardiovascular disease (CVD) in the past 3 months; (2) patients with stage 4 or 5 chronic renal disease; (3) patients with hepatic dysfunction or symptomatic heart failure; (4) those who are currently receiving anticancer therapies or medicines that may affect blood glucose other than GLMs; (5) patients with uncontrolled hypertension (systolic blood pressure [BP] ≥180 mm Hg or diastolic BP ≥100 mm Hg); or (6) pregnant or lactating women.
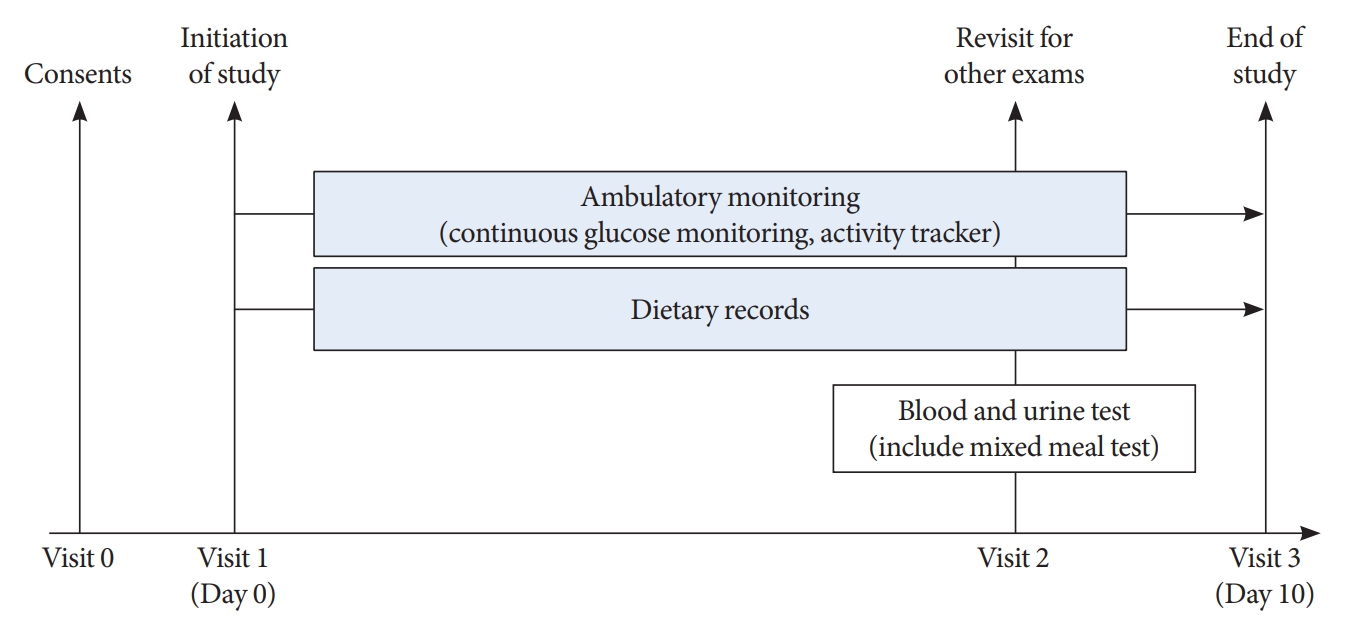
As shown in Fig. 1, all participants visited Korea University Ansan Hospital and were equipped with real-time CGM and wearable activity trackers during the 10-day study period. In addition, participants underwent a mixed meal test.
The present study was approved by the Institutional Review Board (IRB) of Korea University Ansan Hospital (IRB Nos. 2019AS0158 and 2021AS0099) and was conducted in accordance with the Helsinki Declaration of 1975. All participants provided written informed consent to use their data prior to participation. The study protocol was registered with the Clinical Research Information Service (CRiS) of the Ministry of Health and Welfare of the Republic of Korea (CRiS numbers: KCT0004320 and KCT0006171).
CGM metrics
Dexcom G6 (Dexcom Inc., San Diego, CA, USA), real-time CGM, was used for 10 days. To collect information about the lifestyle habits of the participants as is, only basic instruction was provided for the mobile application of Dexcom G6. All participants were advised to maintain their current insulin dose and health behaviors, without any education on diabetes management. The investigators obtained each participant’s CGM data via the Dexcom CLARITY for Clinics website (https://clarity.dexcom.eu/professional/).
Following the experts’ recommendations, the CGM-derived glycemic targets were defined, which were TIR70–180 >70%, TAR>180 <25%, TAR>250 <5%, TBR<70 mg/dL <4%, TBR<54 <1%, and %CV ≤36 [5]. We regarded the concurrent achievement of the above-mentioned targets as adequate glycemic control. Additionally, we calculated the SD.
Wearable activity tracker
We used the Fitbit Inspire 2 (from here on, termed simply Fitbit, Fitbit Inc., San Francisco, CA, USA) to collect information on step count, energy consumption, heart rate, and sleep time. As it contained a battery capable of lasting 10 days, the participants were instructed to wear Fitbit Inspire 2 all day, even while sleeping, without charging throughout the study period. Therefore, the caloric consumption measured by Fitbit included the basal metabolic rate during sleep, and movement around the house was also incorporated into the step count.
The data were transmitted to a Fitbit server using Bluetooth. We stratified the step counts into daytime and bedtime, as described below [13].
(1) Daytime: the period from sunrise to sunset.
(2) Bedtime: the period of 8 hours before sunrise of the next day.
Dietary records
Dietary data were investigated using the picture and dietary record method. Participants were instructed to maintain their eating habits and take pictures of the food twice (vertical and lateral photographs) before and after eating, and to describe the type and amount of food consumed, mealtime, results of self-monitoring of blood glucose, insulin unit, and GLMs in the diet diary. Each participant was educated on how to record the diet diary by a research dietitian, and examples of a diet diary and tableware (Slim Diet Tableware, Jinseoung C&T Co. Ltd., Sejong, Korea) were provided to accurately estimate the amount of a bowl of rice. The participants sent photos and diet diaries on a daily basis. At the end of the study, the research dietitian analyzed the dietary diary using the database of the Ministry of Food and Drug Safety’s integrated data collection of nutritional ingredients for eating out (2012 to 2017) [14] and the CAN-Pro 5.0 (Web version, The Korean Nutrition Society, Seoul, Korea). The calories and amounts of carbohydrates, proteins, lipids, and fibers in the main meals (breakfast, lunch, and dinner) and snack intake were calculated. Because diet information is often missing and underreported, we averaged the calories and nutrients for breakfast, lunch, dinner, and snacks during the study period. They were then summed to obtain the daily calorie and nutrient intake.
For an elaborate analysis of dietary records, the research dietitian scored the diet diary according to the following: the presence of dietary pictures of more than 70% of total meals (1 point), the accuracy of the quantity of intake (1 point) and the timing of intake (1 point). After calculating points of all three categories (0 to 3 points), we chose the participants with 2 or 3 points (n=91). Among the 91 participants who adhered with excellent fidelity to the food diary, we calculated the proportion of main meals and snacks with respect to total daily energy, carbohydrate, fat and protein intake. Snack consumption was defined as an average energy intake of ≥200 kcal, in addition to main meals during the 10-day-of study period [15]. The consumption of snacks at bedtime was defined as those eaten during bedtime and categorized as light (200 to 399 kcal) and heavy (≥400 kcal). Because there were few participants who consumed a snack at bedtime of more than ≥500 kcal, we adopted the cutoff of 400 kcal instead of 500 kcal [16].
Mixed meal test and laboratory measurements
After venous blood sampling with an overnight fast of at least 8 hours, they were asked to eat a mixed meal prepared by the investigators. A mixed meal is a precooked Korean food containing a carbohydrate content of 70 g (Michel food Inc., Seoul, Korea). Postprandial 1-hour and 2-hour blood samplings were performed. The participants were instructed to eat all the food, and after eating, the research dietitian estimated the remaining amount.
Fasting plasma glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol, blood urea nitrogen, serum creatinine, and the urinary albumin-to-creatinine ratio (uACR) were measured using an automated chemical analyzer (COBAS 8000 modular analyzer series, Roche, Basel, Switzerland). Low-density lipoprotein cholesterol levels were calculated using Friedewald’s formula. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula [17]. Dyslipidemia was defined as a total cholesterol level of ≥240 mg/dL or at least on the prescription of antihyperlipidemic agents under the International Classification of Diseases 10th Revision (ICD-10) code E78. HbA1c and hemoglobin concentrations were determined using high-performance liquid chromatography (Tosoh HLC-723 G11 analyzer, Tosoh, Tokyo, Japan) and flow cytometry (Sysmex XN-9000 analyzer, Sysmex, Kobe, Japan). Serum C-peptide levels were estimated using an automated radioimmunoassay analyzer (Gamma Pro, KaiEn, Seoul, Korea).
Insulin secretory capacity was calculated using fasting and postprandial glucose and C-peptide levels. The fasting C-peptide-to-glucose ratio (FCGR) and 1-hour and 2-hour postprandial C-peptide-to-glucose ratios (PCGR1 and PCGR2, respectively) were calculated as the fasting or postprandial C-peptide level (ng/mL), and fasting or postprandial glucose level (mg/dL)×100 [18].
Other covariates
The patients reported their clinical information, including the duration of diabetes and the prescribed medications. The body mass index (BMI) was calculated as their weight in kilograms divided by the square of their height in meters. Trained nurses measured BP in a sitting position using a standardized sphygmomanometer after 5 minutes of rest. Hypertension was defined as a systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or at least one prescription of antihypertensive medications per year under ICD-10 codes I10–I15. The smoking status and alcohol consumption were classified as never, former, or current. Regular exercise was defined as an exercise for >30 minutes at least three times a week during the previous month. A history of stroke and ischemic heart disease was self-reported.
Statistical analysis
To evaluate the association between CGM metrics and clinical, behavioral, and nutritional factors, Spearman correlation analysis was performed. To identify independent factors for the simultaneous achievement of six CGM-based targets, a multivariate logistic regression analysis was performed with covariates including age, sex, BMI, HbA1c level, duration of diabetes, PCGR2, eGFR, uACR, sleep duration, and daytime step counts. With adjustment for the above-mentioned covariates, we investigated the impact of use of sulfonylurea or meglitinide, inhibitor of dipeptidyl peptidase 4 (DPP-4 inhibitor), or inhibitors of sodium glucose cotransporter 2 (SGLT-2 inhibitors), total daily dose of insulin, two or three times injection of prandial insulin per day, the amount of calories, carbohydrate, fat, protein and fiber per day on adequate glycemic control separately in the regression model.
Furthermore, we performed a subgroup analysis according to the median of HbA1c, eGFR, uACR, the number of times of prandial insulin injection and the use of the DPP-4 inhibitor. The participants with eGFR <60 mL/min/1.73 m2 were only 18 patients. Therefore, we divided the participants according to an eGFR of 90 mL/min/1.73 m2 instead of 60 mL/min/1.73 m2. Furthermore, due to the small number of participants, we minimized the covariates.
As a sensitivity analysis in 91 participants with excellent fidelity of diet dairy, we explored the impact of the distribution of energy and nutrient intake among each meal and snack, and frequency of eating on CGM metrics using Spearman correlation analysis. Subsequently, we obtained the risk adjusted difference in the CGM metrics according to snack consumption using the analysis of covariance. Age, sex, BMI, eGFR, HbA1c, duration of diabetes, PCGR2, urine ACR ≥30 mg/g, daytime step counts, and daily sleep duration were used as covariates.
Finally, we compared the baseline characteristics according to snack consumption using a Student t-test and chi-square test. Data are presented as mean±SD, median (interquartile range), or the number of participants (percentage). Skewed variables were log-transformed for analysis. Statistical analyses were performed using Statistical Analysis System (SAS) version 9.1 for Windows (SAS Institute Inc., Cary, NC, USA). P values <0.05 were considered to be statistically significant.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
RESULTS
A total of 138 patients participated in this study. After excluding 16 participants whose activation rate of CGM was <50%, whose Fitbit data were not available, or with a lack of a diet diary, 122 participants were finally included in this analysis. The mean age was 53.4±12 years, and the proportion of the sexes was similar (Table 1). The mean duration of diabetes and mean HbA1c level measured at the end of CGM were 16±10.2 years and 8.1%±1.2%, respectively. Nearly 64% of participants (79 of 122) used both basal and prandial insulin as a premix or multiple daily injections. Degludec and aspart were the most common types of basal insulin and prandial insulin used, respectively. The most widely used oral GLMs were metformin, DPP-4 inhibitors, and SGLT-2 inhibitors. Information from Fitbit, diet diary, and CGM is also shown in Table 1. The mean CGM time was 232±33.1 hours (9.7±1.4 days).
Table 2 shows the results of the Spearman correlation analysis between five CGM metrics and various clinical, behavioral, and nutritional factors. Although there are some differences, BMI, HbA1c, duration of diabetes, uACR, and total daily insulin dose showed a negative association with TIR70‒180 and a positive association with TAR>250, TBR<70, SD, and CV in general. On the contrary, PCGR2 and eGFR exhibited an opposite direction of significance. Furthermore, fiber intake was positively related to TBR<70 and CV.

Association between clinical, behavioral, and nutritional factors and continuous glucose monitoring metrics in Spearman correlation analysis
In the multivariate-adjusted logistic regression analysis for the simultaneous achievement of the targets based on CGM (Table 3), women and participants with low HbA1c and high PCGR2 and daytime step count had a higher probability of good glycemic control estimated from CGM. However, total calorie intake, macronutrients, and fiber did not have any association with CGM-based glycemic targets. When we conducted the subgroup analyses according to HbA1c, eGFR, uACR, frequency of prandial insulin and DPP-4 inhibitors, (Supplementary Table 1), significant interaction was found in DPP-4 inhibitors and uACR with BMI and age, respectively.
In the sensitivity analysis conducted in 91 participants (Supplementary Table 2), the meal composition among energy and macronutrient intake is similar at about 26:32:33 with respect to breakfast, lunch, and dinner. In the analysis of the correlation between nutritional factors and CGM metrics, only the proportion of snacks among energy intake was negatively correlated with CV. Similarly, the snack composition among daily protein intake showed a positive association with TIR70–180 and a negative association with TAR>250 and SD.
When we compared the CGM metrics according to snack consumption after adjustment for several covariates (Table 4), the group eating snacks showed higher TIR70‒180, and lower TAR>250 and GV, and those consuming light snacks at night had lower TBR<70 compared to those without snack consumption. As shown in Supplementary Table 3, the snack consumption group was younger, included more men, had higher eGFR, burned more energy, although they ate more calories and fat, and consumed less fiber.
DISCUSSION
In this prospective observational study, we confirmed that factors such as residual insulin secretion capacity, daytime step count, female sex, and HbA1c were related to the high probability of simultaneous achievement of CGM-derived glycemic targets. In the nutritional aspect, snack intake was related to a lower exposure to hyperglycemia and GV, and light snack intake during bedtime was associated with a lower probability of hypoglycemia.
The limitation of HbA1c as a risk predictor is that it does not reflect the detailed events of hypoglycemia, hyperglycemia, or GV [19]. In contrast, the CGM metrics provide information not only for GV but also for the time spent in the target range, hypoglycemia, and hyperglycemia per day [6]. In the present study, HbA1c was correlated with TBR<70 and CV with a low degree (standardized β=–0.26 and 0.31, respectively) and showed slight significance in the logistic regression analysis for the achievement of CGM-derived glycemic targets.
Among behavioral components, we showed valid significance in step counts, especially during the daytime. Contrary to previous research demonstrating the clinical implications of step count on the risk of death, that measured step counts only during waking hours [20], participants in the present study wore Fitbit for 24 hours throughout the 10-day period, allowing us to understand the time-specific significance of step count. With reasonable reported accuracy [20], Fitbit, which is a consumer-grade accelerometer, has been suggested as an alternative to research-grade accelerometers because it is easy to use and relatively low cost [20]. Therefore, we chose Fitbit to consider generalizability.
We confirmed that PCGR was negatively associated with a high probability of adequate glycemic control and low GV, in agreement with previous research showing that enough residual insulin secretion was associated with low GV [21,22]. Instead of C-peptide levels, we adopted PCGR to estimate the β-cell function. The ratio of C-peptide to glucose has been considered a biomarker that reflects the absolute amount of endogenous insulin secretion in response to glucose levels [18,23]. And postprandial state (PCGR), in particular, is a more reliable marker that better reflects maximum β-cell function than fasting or unstimulated value, FCGR [18,23].
We did not find any implications for macronutrient composition or fiber intake for adequate glycemic control. Several diet interventions have shown that a low carbohydrate (<50 or <90 g/day) and a high fat diet (90 g/day) [24,25] or a high energy breakfast [26,27] is effective in reducing GV or postprandial hyperglycemia. However, our observational study design is inappropriate to draw conclusions on the usefulness of nutritional composition, due to the absence of energy restriction. Furthermore, in the present study, the macronutrient composition across the energy intake was 51.9%±7%, 28.3%±5.5%, and 17.4%±2.2%, which resembled those of the research investigating the high carbohydrate diet (53% carbohydrate, <30% lipid, and 17% protein) [25].
Although a previous study suggested an association between high fiber intake and a decreased risk of T2DM and CVD [28], we could not reveal any association between fiber intake and GV in the logistic regression analysis. This may be attributed to the relatively high fiber intake in the present study (the mean±SD of total dietary fiber intake was 29.8±8.6 g/day, comparable to the upper 20% of fiber intake [30.1±6.6 g/day] in the previous study) [28]. A rather positive correlation between fiber intake and TBR<70 or CV could be induced by the eating behavior of more fiber with a small amount of energy source in individuals with many episodes of hypoglycemia.
In detailed analysis on the nutritional aspects, we revealed the importance of snack consumption. A small number of studies have investigated the influence of snacks on glucose levels [10,29]. Timmer et al. [29] have shown that postprandial hyperglycemia after snacking was exacerbated more in the morning than in the evening among healthy adults. In a randomized trial in Canada, the consumption of low carbohydrate protein-rich bedtime snacks reduced fasting glucose compared to a high carbohydrate protein-matched snacks [10]. This could be explained by the fact that protein-rich snacks might enhance hepatic insulin sensitivity, resulting in suppressing hepatic glucose production [30]. Our findings were somewhat in line with previous evidence [10,29]. As shown in Supplementary Table 3, it was interesting to note that participants eating snacks tended to consume high calories and less fiber. The favorable characteristics of the participants with snack consumption could be speculated by high energy consumption and the high proportion of fat as an energy source. On the other aspect, those with snack consumption were younger and had better renal function than those without, which are important determinants of CGM metrics. However, we still demonstrated the significant association between snack consumption and CGM metrics even after age, sex, and eGFR. Therefore, we suggest that eating adequate snack can be helpful for glycemic control, and especially light night snack is important for reducing hypoglycemia.
Although the impact of GLMs on GV has been assessed in previous studies [8,9,22,31], the present study failed to demonstrate the influence of GLMs on study outcome. The only significant finding was a positive correlation between the total daily dose of insulin and hyperglycemia or GV (Table 2). An explanation for the discrepant findings might be the differences in participants’ characteristics. As the reimbursement could cover up to two classes of GLMs for insulin-treated patients with T2DM in South Korea, the majority of participants in the present study were taking metformin and DPP-4 inhibitors or SGLT-2 inhibitors, which are already proven GLMs that decrease GV [32]. Therefore, it may be difficult to demonstrate the implications of each class of GLMs in the present study.
To the best of our knowledge, this is the first attempt to integrate real-life information and CGM data from insulin-treated patients with T2DM. The participants were more homogeneous than those in prior studies performed on patients with various severities of T2DM.
However, this study had several limitations. First, the CGM metric data were derived from a mean period of 9.8 days, which might be insufficient to obtain an overall representative profile [5]. Second, as we employed a real-time CGM system in an outpatient setting, the participants could alter their lifestyle habits in response to the results of the CGM readings. However, since all participants had no prior experience with CGM, it seems difficult to skillfully control the insulin dose based on the CGM value after just one session of brief education [33]. A recall bias regarding meal information is also possible. Third, owing to the small sample size and cross-sectional design, it is unclear whether this association is causal. Finally, because all the participants were Koreans, it is difficult to generalize these findings to other ethnicities.
In conclusion, from the analysis of prospective observational studies with the environment reflecting the real-world, we found that residual insulin secretion, daytime step count, HbA1c, and women were the most relevant determinants of adequate glycemic control estimated from CGM in patients with T2DM receiving insulin injections. In addition, individuals with snack consumption were exposed to a shorter time of hyperglycemia and GV, and light snacks at night were associated with a low rate of hypoglycemia and low GV.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2022.0273.
Supplementary Table 1.
Subgroup analyses for the concurrent achievement of continuous glucose monitoring-based targetsa in the multivariate logistic regression analysisb
Supplementary Table 2.
Correlation between nutritional factors and continuous glucose monitoring metrics in 91 participants
Supplementary Table 3.
Comparison of clinical characteristics according to snack consumption
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: D.Y.L., N.K., S.Y.P., J.H.Y., S.M.P., N.H.K. Acquisition, analysis, or interpretation of data: D.Y.L., N.K., J.A.S., J.K., N.H.K., H.J.Y., S.G.K., K.M.C., S.H.B., S.M.P., N.H.K. Drafting the work or revising: D.Y.L., N.K., I.J.
Final approval of the manuscript: S.M.P., N.H.K.
FUNDING
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) (NRF-2019M3E5D3073102 and NRF-2019R1H1A 2039682), a National IT Industry Promotion Agency (NIPA) grant (No. S0252-21-1001, Development of AI Precision Medical Solution (Doctor Answer 2.0), the Basic Science Research Program through NRF funded by the Ministry of Education (NRF-2020R1I1A1A01071665) funded by the Korean government (MSIT), and a grant (Da Young Lee, 2019F-7) from the Korean Diabetes Association). However, the funders did not participate in the study design or reporting.
Acknowledgements
None