FTO Gene Variants Are Associated with PCOS Susceptibility and Hyperandrogenemia in Young Korean Women
Article information
Abstract
Background
The fat mass and obesity-associated (FTO) gene is associated with obesity and type 2 diabetes mellitus. Obesity and insulin resistance are also common features of polycystic ovary syndrome (PCOS). Therefore, the FTO gene might be a candidate gene for PCOS susceptibility. The aim of the present study was to evaluate the effects of FTO gene variants on PCOS susceptibility and metabolic and reproductive hormonal parameters.
Methods
We recruited 432 women with PCOS (24±5 years) and 927 healthy women with regular menstrual cycles (27±5 years) and performed a case-control association study. We genotyped the single nucleotide polymorphisms rs1421085, rs17817449, and rs8050136 in the FTO gene and collected metabolic and hormonal measurements.
Results
Logistic regression revealed that the G/G genotype (rs1421085, 1.6%), the C/C genotype (rs17817449, 1.6%), and the A/A genotype (rs8050136, 1.6%) were strongly associated with an increased risk of PCOS (odds ratio, 2.551 to 2.559; all P<0.05). The strengths of these associations were attenuated after adjusting for age and BMI. The women with these genotypes were more obese and exhibited higher free androgen indices (P<0.05) and higher free testosterone levels (P=0.053 to 0.063) compared to the other genotypes. However the significant differences disappeared after adjusting for body mass index (BMI). When we analyzed the women with PCOS and the control groups separately, there were no significant differences in the metabolic and reproductive hormonal parameters according to the FTO gene variants.
Conclusion
The rs1421085, rs17817449, and rs8050136 variants of the FTO gene were associated with PCOS susceptibility and hyperandrogenemia in young Korean women. These associations may be mediated through an effect of BMI.
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common female endocrinopathy. PCOS affects between 6% and 10% of premenopausal women [1,2] and is a heterogeneous disorder with uncertain pathophysiology. Many women with PCOS also exhibit insulin resistance and glucose intolerance [3]. Moreover, 38% to 88% of women with PCOS are overweight or obese [4,5]. Several family and twin studies have indicated a genetic contribution to the pathogenesis of PCOS [6,7].
A genome-wide association study led to the first major success in the field of obesity genetics with the discovery of the fat mass and obesity-associated (FTO) gene as an obesity-susceptibility gene [8]. The FTO gene is a very large gene whose nine exons span more than 400 kb on chromosome 16 [9]. The FTO gene is strongly conserved across various vertebrate species, is primarily expressed in the hypothalamus, and encodes the 2-oxoglutarate-dependent nucleic acid demethylase [10]. The FTO gene is associated with obesity and type 2 diabetes mellitus [11,12,13]. In a meta-analysis, two variants (rs9939609 and rs8050136) of the FTO gene were found to contribute to obesity and type 2 diabetes mellitus in an Asian population [11]. The roles of the A allele of the FTO rs9939609 variant in the risks for obesity and type 2 diabetes mellitus were confirmed in the French MONICA Study [12]. A genome-wide search for type 2 diabetes mellitus-susceptibility genes identified a common variant in the FTO gene that predisposes carries to diabetes via an effect on body mass index (BMI) [14].
The FTO gene affects adiposity and the development of type 2 diabetes mellitus. Obesity and PCOS are closely related, and type 2 diabetes mellitus and PCOS share common pathophysiological and epidemiological features. Therefore, the FTO gene variants might affect individuals' risks of PCOS. Recently published studies have shown that the FTO gene variants appear to have greater effects on obesity, glucose intolerance, and insulin resistance among those with PCOS phenotypes [15,16]. However, few studies have examined the associations of the FTO gene with PCOS susceptibility and PCOS phenotypes in Asian populations. The aim of the present study was to evaluate the role of the FTO gene in PCOS susceptibility and metabolic and reproductive hormonal parameters in Korean women.
METHODS
Subjects
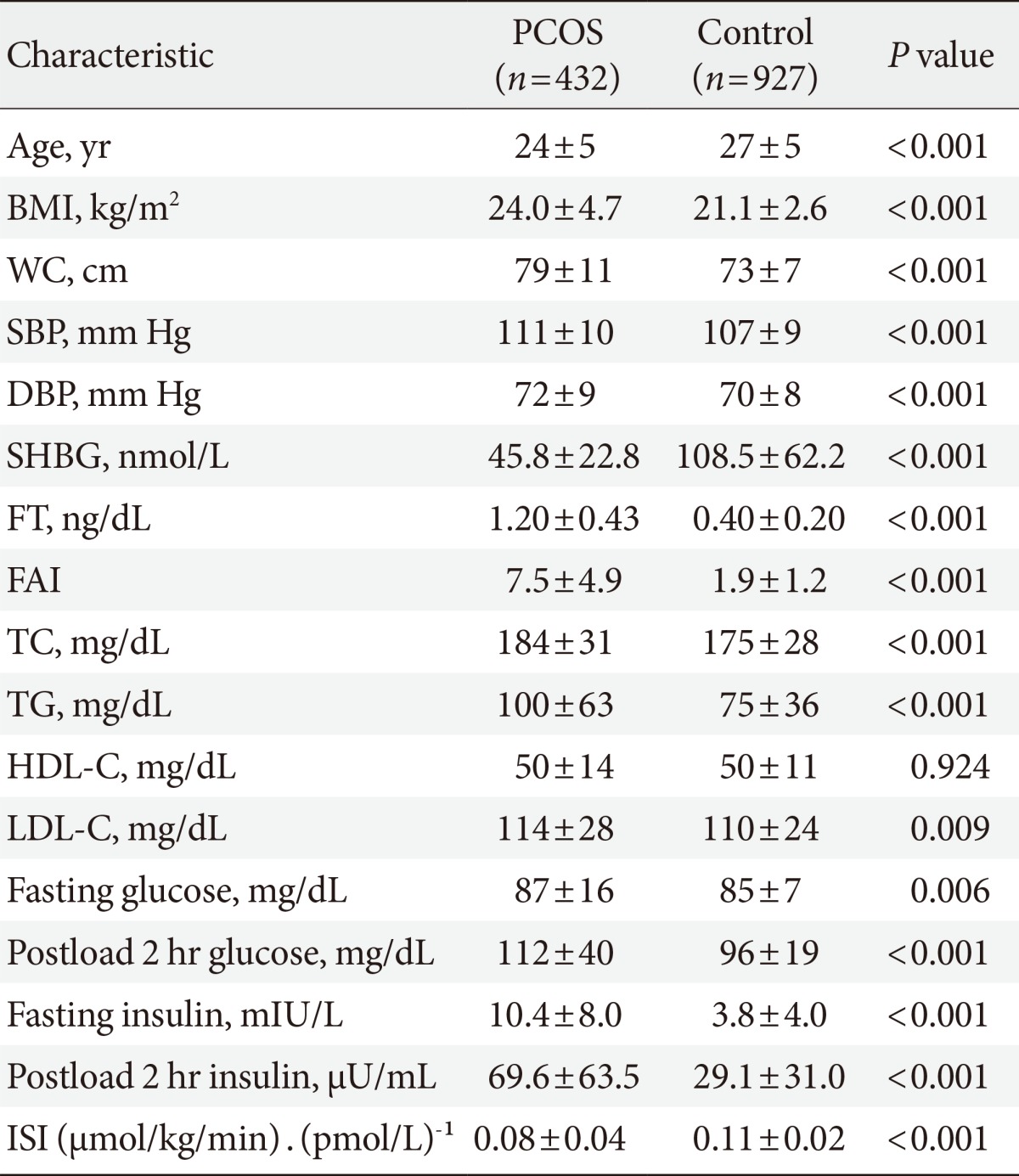
The present study was conducted using data from the "biospecimen collection from women with PCOS and control of infertility study" project. This study was performed at the Endocrinology Clinics at Ewha Womans University Mokdong Hospital between December 2008 and October 2010. Volunteers were recruited via local advertising. A trained nurse contacted the eligible candidates by telephone to determine whether they were both capable and willing to participate in the study. We invited volunteers to visit our hospital on the morning of the third day of their menstrual period following, an overnight fast of at least 8 h. We explained the purpose and characteristics of the study and obtained written informed consent. During this period, we recruited 432 women with PCOS (24±5 years) and 927 non-PCOS control women with regular menstrual cycles (27±5 years).
As proposed by the European Society for Human Reproduction and Embryology [17], PCOS was diagnosed when two or more of the following three criteria were met: oligo- or anovulation, hyperandrogenism, and polycystic ovaries. Oligomenorrhea was defined as fewer than 10 menstrual cycles per year. Biochemical hyperandrogenemia was defined as a total or free testosterone level above the 95th percentile (total testosterone ≥67 ng/dL or free testosterone ≥0.84 ng/dL) of the testosterone levels of 1,120 healthy, regular-cycling women [18]. Clinical hyperandrogenism was defined as the presence of hirsutism and a modified Ferriman-Gallwey score ≥8 [19]. The criteria for the diagnosis of polycystic ovaries required the visualization of ≥12 follicles/ovary that were 2 to 9 mm in diameter or an ovarian volume >10 cm3 by transvaginal ultrasonography or transabdominal ultrasonography with a distended bladder for virginal women. Patients with similar clinical presentations, such as congenital adrenal hyperplasia, androgen-secreting tumors, and Cushing syndrome, were excluded [17]. Written informed consent was obtained from all of the participants, and the institutional review board of Ewha Womans University Mokdong Hospital approved this study.
Methods
The height and weight of all subjects were measured, and BMI was calculated as weight (kg)/height (m)2. Waist circumference was measured in a standing position at the point midway between the lower costal margin and the iliac crest. Blood pressure was calculated as the mean of two manual sphygmomanometer readings with the patient in the seated position.
On the third day of the follicular phase of the menstrual cycle, a venous blood sample was taken from each subject after an overnight fast of at least 8 hours. Total testosterone levels were measured via the chemiluminescent immunoassay method using a commercially available kit (Siemens, New York, NY, USA; the mean inter- and intraassay CVs were 4.4% and 6.2%, respectively). Sex hormone-binding globulin (SHBG) levels were measured by immunoradiometric assay using a commercially available kit (DPC, Los Angeles, CA, USA; the mean inter- and intraassay CVs were 7.9% and 5.3%, respectively). Free testosterone levels were calculated using the formula available on the International Society for Study of the Aging Male website, which is based on the total testosterone, SHBG, and albumin levels in single samples from each subject [20]. The free androgen index was calculated as testosterone (in nanomoles per liter)/SHBG (in nanomoles per liter)×100.
The 75-g oral glucose tolerance test (OGTT) was performed in the morning after an overnight fast. After 30 minutes of supine rest, venous blood samples were drawn at baseline, 90 minutes, and 120 minutes after the administration of the 75-g glucose load. Plasma glucose levels were measured via the glucose oxidase method (Beckman Model Glucose Analyzer 2; Beckman Instruments, Fullerton, CA, USA), and insulin levels were measured by radioimmunoassay using a commercially available kit (BioSource, Nivelles, Belgium). Fasting serum levels of total cholesterol, triglyceride, and high density lipoprotein cholesterol were measured using an enzymatic assay on an automated analyzer (Hitachi 7150 Automatic Chemistry Analyzer; Hitachi, Tokyo, Japan). Insulin sensitivity was assessed as the insulin sensitivity index (ISI), which was calculated using the OGTT values [ISI (µmol/kg/min)·(pmol/L)-1=0.157-4.576×10-5×I120-0.00519×G90-0.000299×I0] [21].
We genotyped the single nucleotide polymorphisms rs1421085 (A/G), rs17817449 (A/C), and rs8050136 (C/A) in the FTO gene. Manual genomic DNA extraction from the peripheral blood was performed. The FTO gene variants were genotyped using Illumina Human Omni1 Quad version 1 (Illumina, San Diego, CA, USA). Genotyping, the success rates exceed 98%, and there was no deviation from the Hardy-Weinberg equilibrium (P>0.05).
Statistical analyses
The statistical analyses were performed using the SPSS version 18.0 (IBM Co., Armonk, NY, USA). Quantitative variables are reported as the means±the standard deviations. Comparisons of the different parameters between the women with PCOS and the controls were made using Student unpaired t-tests. Genetic associations with PCOS susceptibility were analyzed with a logistic regression analysis that was adjusted for age and BMI. The results are expressed as nominal P, odds ratios (ORs), and 95% confidence intervals (CIs). In a subanalysis, the differences in the metabolic and endocrine parameters between the subjects with different FTO gene variants were analyzed by analysis of variance followed by a Tukey honestly significant difference test for post hoc analysis. BMI was adjusted for with analysis of covariance (ANCOVA). P values <0.05 were considered significant.
RESULTS
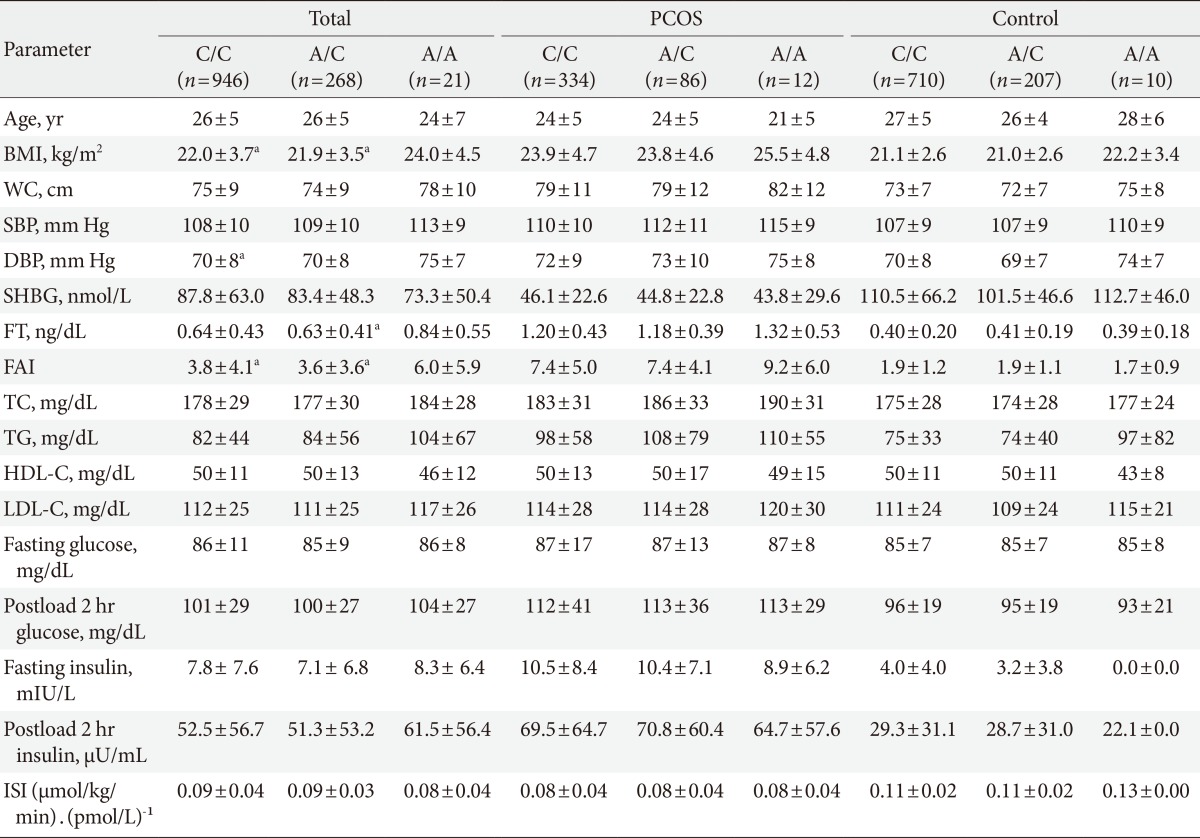
Table 1 shows the clinical and biochemical characteristics of the women with PCOS and the controls. The women with PCOS were younger and more obese than the controls (P<0.05). The women with PCOS exhibited higher systolic blood pressure, diastolic blood pressure, free testosterone, free androgen index, total cholesterol, triglycerides, low density lipoprotein cholesterol, fasting glucose, postload 2-hour glucose, fasting insulin, and postload 2-hour insulin values (all P<0.05) than did the controls. Moreover, the women with PCOS exhibited lower SHBG values and insulin sensitivity indices than did the controls.
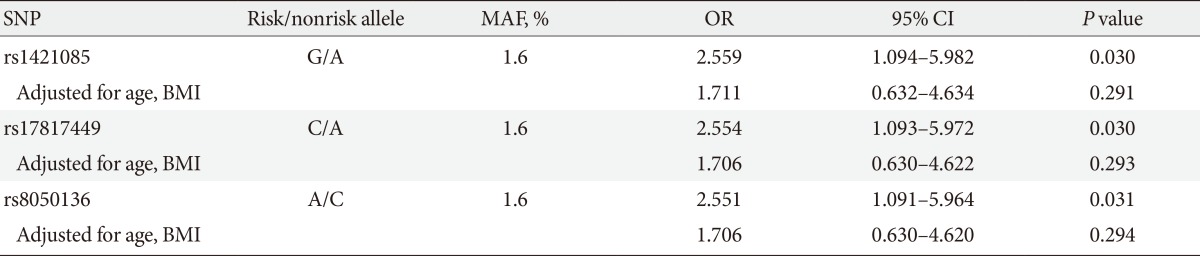
The minor allele frequency of rs1421085, rs17817449, and rs8050136 was 1.6%. The logistic regression analysis demonstrated that rs1421085, rs17817449, and rs8050136 were strongly associated with an increased risk of PCOS (ORs 2.559, 2.554, and 2.551, respectively; all P<0.05). The strengths of associations were attenuated and no longer significant after adjusting for age and BMI (ORs 1.711, 1.706, and 1.706, respectively) (Table 2).
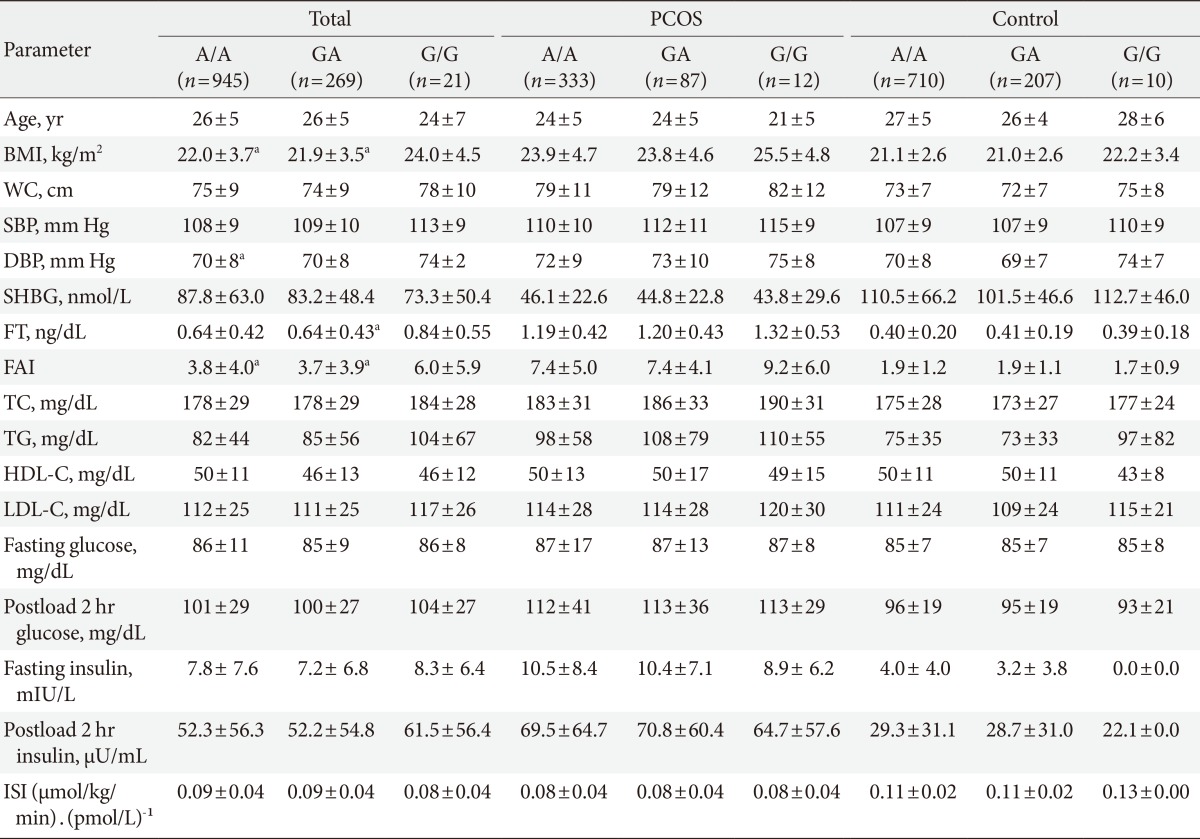
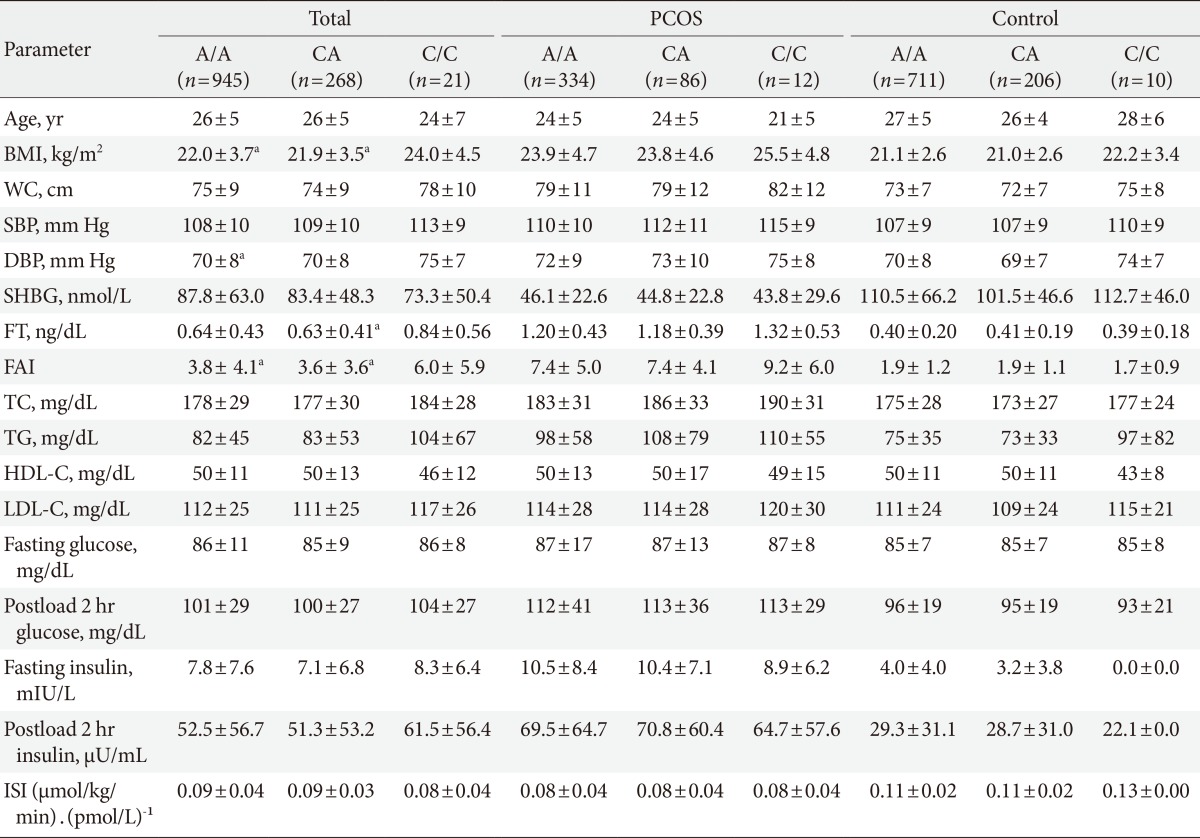
In all subjects, the women with the G/G genotype (rs1421085), the C/C genotype (rs17817449), and the A/A genotype (rs8050136) exhibited higher BMIs (P<0.05), higher free androgen indices (P<0.05), and higher free testosterone levels (P=0.041 to 0.065) compared to the women with the other genotypes (Tables 3,4,5). However, the significant differences in the free androgen index and free testosterone level according to FTO genotype disappeared after adjusting for BMI via ANCOVA (P=0.356, P=0.319, and P=0.319, respectively).
The metabolic and endocrine parameters according to the FTO gene variants were analyzed separately for the women with PCOS and the controls. There were no significant differences in the metabolic or endocrine parameters according to the FTO gene variants in this subanalysis (Tables 3,4,5).
DISCUSSION
In this study, the rs1421085, rs17817449, and rs8050136 variants of the FTO gene were associated with increased susceptibility to PCOS and hyperandrogenemia in young Korean women. Moreover, these effects might have been mediated by an effect of BMI.
There are few studies of the relationships between FTO gene variants and PCOS susceptibility. The rs9939609 variant of the FTO gene was reported to be associated with PCOS susceptibility in the UK [22] and the Chinese populations [23]. In our study, the rs1421085, rs17817449, and rs8050136 variants of the FTO gene were associated with increased susceptibility to PCOS. The results of the present study conflict with the results of a previously reported study in which the rs1421085 variant of the FTO gene was not a determinant of the development of PCOS in a Korean population [24]. In the logistic regression analysis, the associations of the rs1421085, rs17817449, and rs8050136 variants of the FTO gene with the risk of PCOS were attenuated and not significant after adjusting for BMI. These findings suggest that the FTO gene variants might have a positive effect on the development of PCOS via the mechanism of increased adiposity.
Several studies have examined the relationships of the FTO gene with other PCOS-associated-phenotypes, including obesity, glucose intolerance, and insulin resistance [15,16]. However, the association between the FTO gene and hyperandrogenemia remains controversial. In a study of women from a UK population, no relationship between FTO genotype and androgen levels was observed [22]. Moreover, in a Polish population, there were no differences in testosterone, SHBG, and free androgen index values according to the various genotypes [16]. However, in one study of 288 European women with PCOS, the rs9939609 variant of the FTO gene was reported to be associated with hyperandrogenemia [25]. In our study, the rs1421085, rs17817449, and rs8050136 variants of the FTO gene were associated with hyperandrogenemia. However, these associations were no longer significant after adjusting for BMI. These findings suggest that these FTO gene variants might positively affect hyperandrogenemia by a mechanism that is mediated through increased adiposity.
In a study of PCOS women from a Central European population, the FTO gene was found to be strongly influential on PCOS and to be correlated with various components of metabolic syndrome including obesity, impaired fasting glucose, glucose intolerance, and insulin resistance [15]. In a meta-analysis based on eight distinct cohorts, the effects of the FTO gene variants on obesity-related traits in PCOS seemed to be more than two times greater than those that had previously been reported in large population-based studies [26]. Several reports have demonstrated an association between BMI and rs9939609 in women with PCOS [25,27,28]. In our study, the FTO gene variants were associated with obesity, but there were no relationships between the FTO gene variants and insulin resistance or glucose intolerance in young Korean women. The results of the present study accord with those of an earlier study in a Korean population that reported that the rs734312 variant of the FTO gene is not related to the risk of type 2 diabetes mellitus [29]. These findings suggest that FTO gene variants might act differently according to the ethnicity.
In our study, we found no significant differences in metabolic or endocrine parameters according to the rs1421085, rs17817449, and rs8050136 variants of the FTO gene when these parameters were analyzed separately for the women with PCOS and the controls. In contrast to the results of our study, previous studies of European and Polish women with PCOS have found significant associations of the A allele of rs9939609 with increased BMI and impaired glucose tolerance [16,25]. Further studies in various ethnic groups are needed to determine the relationships of the variants of the FTO gene to the metabolic and endocrine parameters of women with PCOS.
The present study is the first to report a positive association between the FTO gene variants and PCOS susceptibility in Korean women. Moreover, the strength of our study is the relative large sample size.
The subjects of our study were younger (from 15 to 39 years old) than those of other studies of the general female population. Therefore, it is possible that some metabolic abnormalities had not been expressed in our population. Further studies of various age groups are needed to generalize the results of our study.
In summary, we found that there are significant associations between the rs1421085, rs17817449, and rs8050136 variants of the FTO gene and PCOS susceptibility and hyperandrogenemia in young Korean women and that these results were likely caused by the effects of obesity. To further confirm this association, additional studies in various age groups should be performed to elucidate the role of FTO gene variants in PCOS susceptibility and phenotypes.
Notes
No potential conflict of interest relevant to this article was reported.