Safety and Effectiveness of Empagliflozin in Korean Patients with Type 2 Diabetes Mellitus: Results from a Nationwide Post-Marketing Surveillance
Article information
Abstract
Background
To evaluate the safety and effectiveness of empagliflozin in routine clinical settings, we collected and assessed the clinical profiles of Korean patients with type 2 diabetes mellitus.
Methods
This was a post-marketing surveillance study of empagliflozin 10 and 25 mg. Information on adverse events and adverse drug reactions (ADRs) was collected as safety data sets. Available effectiveness outcomes, including glycosylated hemoglobin (HbA1c) level, fasting plasma glucose, body weight, and blood pressure, were assessed.
Results
The incidence rate of ADRs was 5.14% in the safety dataset (n=3,231). Pollakiuria, pruritis genital, and weight loss were the most common ADRs. ADRs of special interest accounted for only 1.18%, and there were no serious events that led to mortality or hospitalization. In the effectiveness data set (n=2,567), empagliflozin significantly reduced the mean HbA1c level and body weight during the study period by –0.68%±1.39% and –1.91±3.37 kg (both P<0.0001), respectively. In addition, shorter disease duration, absence of dyslipidemia, and higher baseline HbA1c levels were identified as the clinical features characteristic of a “responder” to empagliflozin therapy.
Conclusion
Empagliflozin is a safe and potent glucose-lowering drug in routine use among Korean patients with type 2 diabetes mellitus. It is expected to have better glycemic efficacy in Korean patients with poorly controlled type 2 diabetes mellitus.
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) in Korea is estimated to be 13.8% in adults older than 30 years [1], and it has increased about 5-fold over the past 30 years according to the Korea National Health and Nutrition Examination Survey (KNHANES III, 2005) [2]. The number of patients with T2DM is expected to increase dramatically from about 3.2 million in 2011 (8.8% of the national population) to about 4.25 million (11.1%) by 2030 [3].
Sodium-glucose cotransporter-2 inhibitors (SGLT2is) are a relatively new class of glucose-lowering drug (GLDs) that exert their effect through an insulin-independent mechanism of action by reducing renal glucose reabsorption, and are being increasingly used for cardiorenal protection, body weight reduction, and blood pressure-lowering in clinical practice [4,5]. While the safety profiles are generally acceptable, controversies still exist regarding the risk of mild to severe adverse events (AEs), which range from genital and urinary tract infection (UTI) [6] to diabetic ketoacidosis (DKA) [7], lower limb amputation [8], and bone fracture [9].
Empagliflozin was approved for use in Korea in 2014 and has been known as the first-in-class drug that has proven its benefits against major adverse cardiovascular events, including cardiovascular death and all-cause mortality, and showed advantages in hospitalization for heart failure and renal composite outcomes in the (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial [10]. This groundbreaking trial provided longterm safety data with an acceptable safety profile and revealed an increased risk of genital infection, which is commonly experienced by SGLT2i users compared to the placebo group. Recently, a meta-analysis of randomized controlled trials (RCTs) consistently confirmed the safety of empagliflozin in patients with diabetes [11]. Empagliflozin also significantly improved glycemic control in patients with T2DM [12], and greater efficacy was suggested in Asian patients than Caucasian patients based on a comparative meta-analysis [13].
Despite the evidence from previous studies, data on the safety and effectiveness of empagliflozin use in real-world settings are limited, especially in Korea. In addition, the clinical characteristics of patients who respond appropriately to empagliflozin treatment are not yet well defined. To address these questions, we assessed the safety and effectiveness of empagliflozin use in routine clinical settings in Korean patients with T2DM based on the results of the nationwide post-marketing surveillance (PMS).
METHODS
Study design and population
This observational, prospective, non-interventional, open-label, single-arm, multicenter, and national study (ClinicalTrials. gov, NCT02848833) investigated the safety and effectiveness of empagliflozin (Jardiance 10, 25 mg; Boehringer Ingelheim International GmbH & Co., Ingelheim, Germany) in Korean patients with T2DM in a routine clinical practice setting. Patients with T2DM were recruited by 67 investigators from 52 institutions in Korea and received empagliflozin 10 or 25 mg once daily as monotherapy or as combination therapy with other GLDs with the starting dose and dose escalation defined according to the local authorized label and at the discretion of the physician between August 12, 2014, and August 11, 2020 (6 years). Only patients who signed the data release consent form were enrolled, and patients with type 1 diabetes mellitus or those who were pregnant or who previously received empagliflozin were excluded.
The enrolled patients were followed up for 24 weeks or until discontinuation of empagliflozin. The primary endpoint of the study was safety, defined as the incidence rate of adverse drug reactions (ADRs). Other safety endpoints included in the analysis were serious ADRs and ADRs of special interest, including genital infection, increased urination, UTI, volume depletion, DKA , decreased renal function, hepatic injury, and lower limb amputation, as shown through incident rates or descriptively. The effectiveness endpoints of empagliflozin were evaluated by the changes from baseline to the last observation date in glycosylated hemoglobin (HbA1c) level, fasting plasma glucose (FPG) level, body weight, and systolic and diastolic blood pressure (SBP and DBP, respectively).
The diagnosis of comorbidities was based on the medical records of each patient or clinical judgment by the physician (as already described in the ‘Methods’ section), data which was used for analyzing the safety and effectiveness of empagliflozin, was collected by standard procedure. All reported AEs in patients who take at least one dose of empagliflozin was collected. Data for effectiveness analysis were collected within 1 month prior to baseline and after 12 and/or 24 weeks of treatment by standard procedure (Supplementary Table 1). Previous GLDs, except empagliflozin, were maintained, titrated, or switched in accordance with the physician’s decision during the study period. Lifestyle modification therapy and education for both diet and exercise were also provided in parallel with the medications.
This study was conducted in accordance with the Standard for Re-examination of New Drugs published by the Ministry of Food and Drug Safety in Korea. All institutions received ethical approval from their institutional review boards according to the rules of each institution. Informed consent was obtained from all patients for participation in this study, and physicians treated patients based on their own clinical judgment throughout the study.
Data assessment
Clinical data including demographic characteristics, baseline vital signs, and medical history such as the duration of T2DM, diabetic complications, comorbidities, concomitant medications, and renal function were assessed and recorded in electronic case report forms (CRFs) at the baseline visit. The diagnosis of comorbidities was based on the medical records of each patient or clinical judgment by the physician. Blood samples were obtained to measure HbA1c, FPG, and creatinine levels.
During the observation period, all reported AEs in patients who received at least one dose of empagliflozin were collected, regardless of whether there was causality with the drug. AEs were coded using the Medical Dictionary for Regulatory Activities version 20.0. Serious, unexpected AEs and AEs leading to discontinuation were noted. Concomitant treatments were coded according to the latest version of the Korean Index of Medical Specialties. Physicians reported the name of the AE, date of occurrence, intensity, causal relationship to empagliflozin, measures taken, and the outcome. The intensity and causal relationship to the drug were determined based on the physician’s medical and scientific judgment.
The safety outcomes of empagliflozin comprised data on ADRs, which were defined as AEs for which causality with empagliflozin could not be excluded. If the ADR was lifethreatening or the outcome was death, hospitalization, disability, or permanent damage, the ADR was defined as a serious ADR. If an ADR case needed any interventions to prevent serious ADRs, it was also included in the serious ADR.
The effectiveness of empagliflozin was assessed by differences in HbA1c level, FPG level, weight, SBP, and DBP measured at baseline and last visit or at the last measurement when available.
Statistical analysis
Continuous variables are presented as mean±standard deviation, and categorical variables are presented as numbers or percentages. In the effectiveness analysis, changes in HbA1c level, FPG level, weight, SBP, and DBP between the baseline and last observation were compared using a paired t-test. Multiple linear regression analysis was performed to identify independent variables that were significantly correlated with changes in HbA1c levels. The relative factors for the changes in weight were determined using univariate correlation analysis. All statistical analyses for calculations and graphics were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA), and results were considered statistically significant at P<0.05.
RESULTS
Study population characteristics
The study flowchart is presented in Fig. 1. During the study period, CRFs were retrieved from 3,368 subjects. Of these, 3,231 were included in the safety analysis set and 2,567 were included in the effectiveness analysis set. The baseline characteristics of the patients in the safety analysis set are presented in Supplementary Table 2. The mean age was 56.66±11.60 years, and the proportion of males was 57.29%. The average duration of diabetes was 7.37 years, and HbA1c was 7.98%± 1.40%. All patients with chronic kidney disease (CKD) were at stage 1 or 2 of disease, which met the criteria of the local label that did not allow initiation of the drug when the estimated glomerular filtration rate (eGFR) was below 60 mL/min/1.73 m2. Most patients (89.94%) were prescribed empagliflozin 10 mg. Metformin (85.45%) was the most commonly used oral GLD with empagliflozin. Of the patients, 38.94% reported cardiovascular disease (CVD), which were consisted of coronary artery disease (34.08%) and stroke (4.86%).

Flow diagram of the analyzed population in the Korean post-marketing surveillance. The safety analysis set was completed by excluding subjects who were duplicated (one case), subjects who did not take the study drug (11 cases), subjects with follow-up failure (six cases), and subjects with inclusion/exclusion criteria violations (119 cases). The effectiveness analysis set was completed by excluding subjects whose glycosylated hemoglobin (HbA1c) levels before or after administration of the study drug were not recorded (235 cases); subjects whose HbA1c levels before or after administration of the study drug were unknown (429 cases). eCRF, electronic case report form.
Safety of empagliflozin
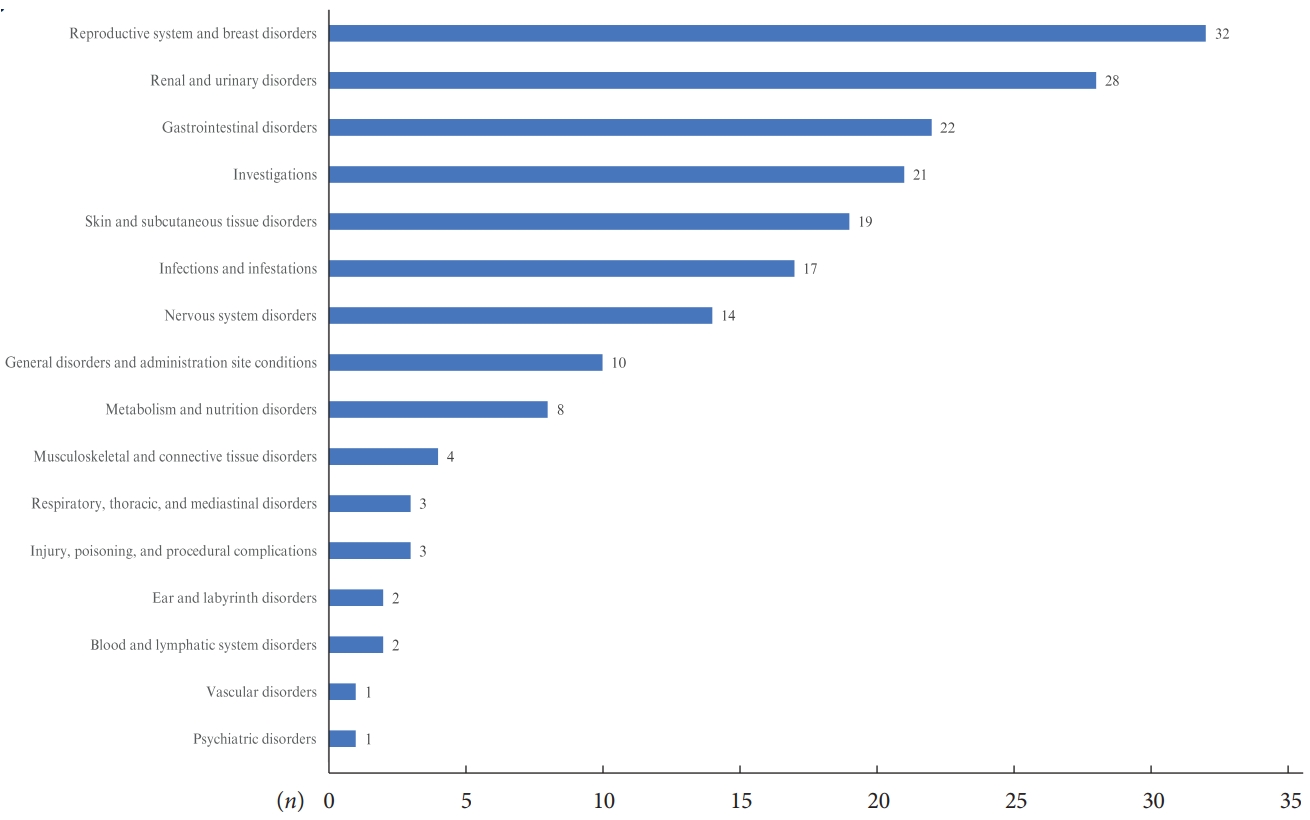
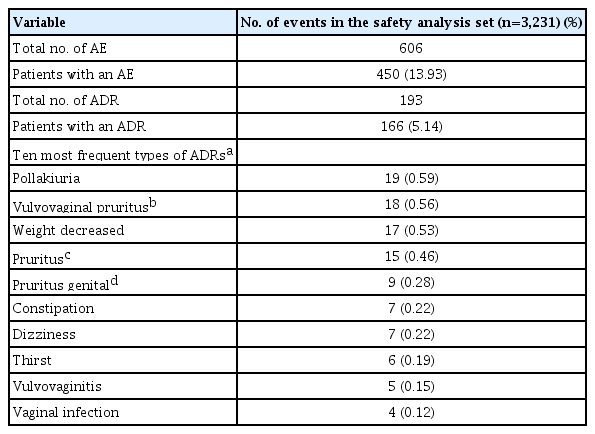
ADR reports of patients in the safety analysis set by system organ class are presented in Fig. 2 and Supplementary Table 3. Fig. 2 demonstrated the number of subjects who reported ADRs by system organ class, and ‘reproductive system and breast disorders,’ ‘renal and urinary disorders,’ and ‘gastrointestinal disorders’ were the three most common organ classes related to side effects of empagliflozin. In the safety analysis, the 10 most frequently reported ADRs by preferred term are summarized in the order of frequency in Table 1. A total of 606 AEs were reported in the safety analysis set, and 450 patients (13.93%) experienced AEs. Among them, 193 ADRs were reported, and 166 patients (5.14%) experienced ADRs. Pollakiuria (frequent urination) was the most common ADR, followed by vulvovaginal pruritus, weight loss, pruritus whole body, and pruritus genital. Overall, the reported incidence rates of all ADRs were less than 1%. Except for the three most common ADRs (pollakiuira, vulvovaginal pruritus, and weight loss with incidence rates 0.59%, 0.56%, and 0.53%, respectively), incidence rates for most ADRs were less than 0.5%. Regarding pre-defined ADRs of special interest, increased urination was the most commonly experienced ADR after empagliflozin use; however, only 22 patients (0.68%) reported this ADR. There were 13 (0.40%) and three (0.09%) patients with genital infections and UTIs, respectively, and no serious ADRs of special interest, such as DKA, kidney and hepatic injury, and limb amputation, were reported (Table 2). Only five serious ADRs were reported (acute pyelonephritis, ureterolithiasis, cervix inflammation, hyperglycemia, and hyponatremia) and are listed in Supplementary Table 4. Except for cervix inflammation, the other four serious ADRs required hospitalization and all patients recovered.
Effectiveness of empagliflozin
Baseline characteristics of participants included in the effectiveness analysis set were similar to those of the safety analysis set and are presented in Supplementary Table 5. The effectiveness of empagliflozin on glycemic control, weight, blood pressure, and renal outcomes are presented in Table 3 and Supplementary Table 6. Glycemic parameters, as well as weight, systolic and diastolic pressure, were improved after 12 weeks of empagliflozin treatment, while renal function and urine albumin/creatinine ratio (ACR) remained unchanged (Supplementary Table 6). Empagliflozin treatment significantly reduced the mean HbA1c level during the study period by −0.68%±1.39% (P<0.0001). In addition, 44.10% of patients achieved the glycemic control target (HbA1c <7%) and over half (51.23%) showed an A1c reduction greater than 0.5%. The mean FPG level significantly decreased by −25.59±59.84 mg/dL (P<0.0001). The average change in body weight was −1.91±3.37 kg (P<0.0001), and both SBP and DBP significantly decreased (−3.95±15.44 and −1.80±10.82 mm Hg, respectively; P<0.0001). Among the patients who were available for collecting renal outcomes, eGFR was slightly decreased during the treatment period (−1.22± 15.57 mL/min/1.73 m2, P=0.0365), while neither serum creatinine level nor urine ACR changed (Table 3).
Clinical factors associated with the effectiveness of empagliflozin
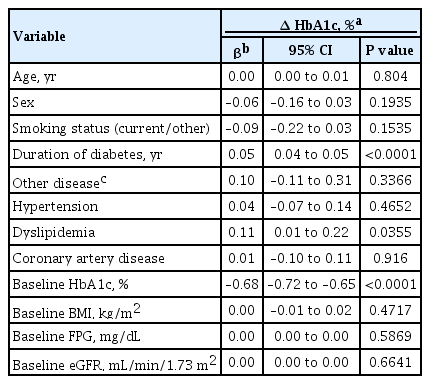
To identify the major factors associated with the response to empagliflozin, we analyzed the clinical factors relevant to efficacy in terms of glycemic control and weight reduction. In multiple regression analysis, disease duration, dyslipidemia, and baseline HbA1c levels were independently correlated with changes in the HbA1c levels (Table 4). Age, sex, baseline body mass index (BMI), and baseline eGFR were not statistically significant after adjustment. Consequently, a shorter duration of diabetes, absence of dyslipidemia, and higher baseline HbA1c levels were clinical factors associated with a greater reduction in HbA1c levels. Additionally, younger age, lower baseline HbA1c level, and higher baseline BMI were associated with weight reduction, as shown in Supplementary Fig. 1.
DISCUSSION
In this PMS, we found that empagliflozin is a safe, potent GLD that can be administered to Korean patients with T2DM. In this study, the incidence of ADRs was 5.14%. Most ADRs, such as pollakiuria, pruritus genital, and weight loss, are related to the mode of action of SGLT2is and can be managed with appropriate education in clinical practice. Only 1.18% patients reported ADRs of special interest, and there were no serious events related to mortality and hospitalization, such as DKA, acute kidney/hepatic injury, and lower limb amputation. Empagliflozin therapy showed potent glycemic efficacy, consistent with previous RCT results, and further added benefits of weight reduction and blood pressure-lowering effects. We identified the clinical features contributing to the definition of ‘responder’ to empagliflozin therapy: shorter disease duration, absence of dyslipidemia, and higher baseline HbA1c level.
Empagliflozin administration led to increased incidence of genital infections than placebo (6.4% vs. 1.8%), which was more frequently observed in women (10% vs. 2.6% in placebo, vs. 5% in male) in the EMPA-REG OUTCOME trial [10]. This tendency was consistent in the subgroup analysis of patients with baseline eGFR ≥60 mL/min/1.73 m2 [14] and with that observed in our study. In addition, a large meta-analysis showed a 4.7% event rate, and the risk of genital infection was over three times higher (relative risk [RR], 3.3; 95% confidence interval [CI], 2.74 to 3.99) compared with that due to control [15]. Among SGLT2is, the RR was lower for empagliflozin than for canagliflozin and dapagliflozin [15]. Another metaanalysis including only the East Asian population found that the risk of genital infection was also increased but relatively lower in the study population (RR, 1.73; 95% CI, 1.02 to 2.96) than in the overall population [16]. Symptoms related to genital infection (vaginal moniliasis, vulvovaginitis, balanitis, and other genital infection) in our study (0.4% as shown in Table 2) were evidently fewer than those reported in previous studies. This decrease might be attributed to the nature of the PMS; contrary to concerns, it implied that this ADR could be controlled with proper education and hygiene management in clinical settings.
UTIs have been a major concern after SGLT2i use, but large meta-analysis studies showed similar risk with a placebo [4,15,17]. A large United States population-based cohort study confirmed that the risk of UTI, regardless of severity, was not higher than that observed on use of other anti-diabetic drugs [18]. The incidence of UTIs in clinical trials is reported to range from 4% to 9% [15], whereas in our study, the incidence was <1%. This gap may be attributed to the different study durations and designs (RCT vs. PMS), but we hypothesize some possible factors contributing to this difference. Interestingly, a previous study showed that more UTIs were reported by patients from hot climate regions (typically year-round warm weather regions) than other regions after canagliflozin use (19.5% vs. 6.9%) [19]. A recent meta-analysis of safety profiles in Japanese patients, which is closely located to Korea, showed that SGLT2is were associated with risks of both genital infection and UTI. Based on these findings, empagliflozin can be used safely in the Korean population, as long as it is with caution regarding the risk of both genital infection and UTI.
In our study, pollakiuria and weight loss were the most common side effects. However, these events are closely related to the mode of action of SGLT2is. SGLT-2 inhibitors are an evidence-based therapeutic option for overweight/obese patients with T2DM [20]. For safety reporting, which is suggested to include unexpected efficacy of the drug, weight loss is reported as a side effect. Severe ADRs were rarely reported (only 0.15%) in our study, and there were no fatalities or hospitalization-required events, such as DKA, lower limb amputation, thromboembolism, acute kidney or hepatic injury, and fracture. These findings are consistent with those of previous meta-analyses [4,6,21,22]. Based on this evidence, empagliflozin can be used safely, and concerns about the risk of serious ADRs, especially in Korean patients with T2DM with good renal function, are negligible.
Empagliflozin, a potent GLD, has shown good glycemic efficacy in previous RCTs and observational studies. HbA1c level reduction at 28 weeks was approximately −0.6% to −0.7% in the EMPA-REG OUTCOME study, consistent the results of our study. HbA1c level reduction of −0.62% was confirmed in a meta-analysis of empagliflozin RCTs [11]. Analysis of 46 systemic reviews, comprising 175 RCTs, confirmed that SGLT2is significantly lowered HbA1c levels from −0.49% to −0.77% [12]. Interestingly, the glucose-lowering efficacy of SGLT2i was greater in Asian patients than in Caucasian patients [13]. Further studies are required to elucidate the reason why Asian patients respond better to SGLT2is than Caucasian patients. Meanwhile, we found the clinical factors related to the ‘responders’ to empagliflozin treatment, and shorter disease duration, higher baseline HbA1c level, and an absence of dyslipidemia were significantly associated with the glycemic efficacy of empagliflozin in Korean patients. These factors were similar to the surveillance study results of dapagliflozin in Korea [23]. Han et al. [23] showed that a recent diagnosis of diabetes and higher HbA1c level at baseline are characteristics of a favorable response in patients who received an add-on therapy of dapagliflozin. In addition, the glycemic efficacy of dapagliflozin was greater in subjects with renal hyperfiltration (eGFR ≥120 mL/min/1.73 m2). Thus, we suggest that empagliflozin would be more favorable in Korean patients with poorly controlled T2DM in the early phase after diagnosis.
We could not provide the exact mechanism and causality for an accidental result of why the absence of dyslipidemia is related to glycemic response, but some possibilities could be postulated. Dyslipidemia is frequently found in patients with T2DM as a component of metabolic syndrome closely associated with insulin resistance. When considering most of hypoglycemic agents are more effective in insulin-sensitive subjects, intuitively the responders might have less insulin resistant. In addition, it has been reported that SGLT-2 inhibitors paradoxically increase hepatic glucose production [24,25]. Hypothetically, SGLT-2 inhibitors enhance lipolysis via glucagon production due to glucose-lowering effect irrespective of insulin action [26]. If patient who doesn`t have dyslipidemia is taking empagliflozin, hepatic glucose production might be limited for source scarcity. Further evidence will be warranted which clinical factors are responders to empagliflozin in patients with diabetes.
This study has several limitations. First, this study has limitations by nature as the study was conducted in a routine clinical setting under market authorization, thus channeling bias due to preferential prescribing in relation to different risks for the events of interest, confounding due to outcome of interest, selected research questions, residual unmeasured confounding. Moreover, the nature of the PMS may tend to overestimate safety. ADRs in our study were reported at a significantly lower rate than in previous studies, which may be because physicians focused on safety while prescribing, due to selection bias. In addition, there may be AEs that are not actually reported because safety records usually depend on the patient’s subjective symptoms. However, the overall assessment is consistent with the safety of empagliflozin proven in a number of studies and confirms that our findings are reliable, despite the relatively short study period. Second, our study design did not allow safety or benefit assessment regarding CVD or renal outcomes in Korean patients with diabetes. Finally, these findings cannot be generalized to other ethnicities or countries. During the study period, empagliflozin was not prescribed to patients with moderate to severe renal impairment, following the guidelines by the Ministry of Food and Drug Safety policy. Health insurance coverage was also limited to metformin and sulfonylurea combinations. Despite these limitations, to the best of our knowledge, this study is the first PMS report on empagliflozin for routine use in Korea. The findings from a large-scale, prospective data collection in a real-world setting would provide clinical evidence and tips regarding the safety and effectiveness of empagliflozin use in Korean patients with T2DM.
In conclusion, empagliflozin is a safe, potent GLD that is routinely administered to Korean patients with T2DM. Empagliflozin is expected to show favorable glycemic efficacy in Korean patients with poorly controlled T2DM in the early stages of diabetes. Further prospective, well-designed, large studies are warranted to identify the clinical characteristics of ‘responders’ to empagliflozin.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2022.0106.
Study flow chart
Baseline characteristics (safety analysis set)
Adverse drug reactions (by system organ class)
Serious adverse drug reactions
Baseline characteristics of patients (effectiveness analysis set)
Effectiveness parameters in empagliflozin users: week 12
The relationships between the change in weight and the (A) age, (B) glycosylated hemoglobin (HbA1c), (C) body mass index, and (D) estimated glomerular filtration rate (eGFR) at baseline in the effectiveness analysis set.
Notes
CONFLICTS OF INTEREST
Kyu Chang Won has been honorary editor of the Diabetes & Metabolism Journal since 2020. In-Kyung Jeong was editor in chief of the Diabetes & Metabolism Journal from 2020 to 2021. Jun Sung Moon was editorial board member of the Diabetes & Metabolism Journal from 2020 to 2021. They were not involved in the review process of this article. Otherwise, there was no conflict of interest. Jinhong Cho, Dong Woo Lee, and Sun Woo Lee are employees of Boehringer Ingelheim, and the other authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Conception or design: J.S.M., J.C., D.W.L., S.W.L., K.C.W.
Acquisition, analysis, or interpretation of data: J.S.M., N.H.K., J.O.N., J.H.C., I.K.J., S.H.L., J.O.M., N.H.K., D.J.C., J.C., D.W.L., S.W.L., K.C.W.
Drafting the work or revising: J.S.M., N.H.K., J.O.N., I.K.J., S.H.L., D.J.C., J.C., D.W.L.
Final approval of the manuscript: J.S.M., N.H.K., J.O.N., J.H.C., I.K.J., S.H.L., J.O.M., N.H.K., D.J.C., J.C., D.W.L., S.W.L., K.C.W.
FUNDING
This study was funded by Boehringer Ingelheim Korea Ltd.
Acknowledgements
None